Abstract
Approximately 7% of preterm infants receive an ASD diagnosis. Yet, there is a significant gap in the literature in identifying prospective markers of neurodevelopmental risk in preterm infants. The present study examined two electroencephalography (EEG) parameters during infancy, absolute EEG power and aperiodic activity of the power spectral density (PSD) slope in association with subsequent autism risk and cognitive ability in a diverse cohort of children born preterm in South Africa. Participants were 71 preterm infants born between 25 and 36 weeks gestation (34.60 ± 2.34 weeks). EEG was collected during sleep between 39 and 41 weeks postmenstrual age adjusted (40.00 ± 0.42 weeks). The Bayley Scales of Infant Development and Brief Infant Toddler Social Emotional Assessment (BITSEA) were administered at approximately three years of age adjusted (34 ± 2.7 months). Aperiodic activity, but not the rhythmic oscillatory activity, at multiple electrode sites was associated with subsequent increased autism risk on the BITSEA at three years of age. No associations were found between the PSD slope or absolute EEG power and cognitive development. Our findings highlight the need to examine potential markers of subsequent autism risk in high-risk populations other than infants at familial risk.
Keywords: Electroencephalography (EEG), Preterm birth, Aperiodic EEG, Autism risk, Infants, Neuronal oscillations
Introduction
Autism Spectrum Disorder (ASD) is a heterogenous neurodevelopmental condition characterized by core difficulties in social communication and the presence of stereotypic behaviors or restricted interests. In the United States (US), approximately 1 in every 54 children meets diagnostic criteria for ASD with the median age of diagnosis ranging from 50 – 56 months (Baio et al., 2018). In low and middle income countries (LMICs) such as South Africa, there are significant infrastructure barriers and racial disparities that affect ASD screening and diagnosis (Franz et al., 2018; Springer, van Toorn, Laughton, & Kidd, 2013). Given that early intervention can improve long-term outcomes across multiple domains including symptom severity, academics, and quality of life, identifying objective early life markers of emerging neurodevelopmental risk is a significant public health priority (Anderson, Liang, & Lord, 2014; Clark, Vinen, Barbaro, & Dissanayake, 2018; Fuller & Kaiser, 2020; Pickles et al., 2016). Prospective studies of early life markers have predominately focused on infants at familial risk of ASD in high-resource samples (McDonald & Jeste, 2021). Preterm birth is also associated with increased risk of ASD with a recent meta-analysis reporting a prevalence rate of 7% across 18 studies (95% CI: 4% to 9%) (Agrawal, Rao, Bulsara, & Patole, 2018). Globally, approximately 11% of infants are born preterm (< 37 weeks’ gestation) with higher rates in LMICs (Walani, 2020). Yet, there is a significant gap in the literature in identifying prospective neural markers of ASD risk in preterm infants and within LMIC samples.
Both preterm birth and low birth weight are associated with increased risk of ASD, with decreasing gestational age and lower birth weight accounting for the greatest increases in risk (Agrawal et al., 2018; Jois, 2019; Talmi, Mankuta, & Raz, 2020). Prior studies have implemented developmental screening measures to examine emerging ASD risk in preterm infants. Infants born ≤ 30 weeks gestation are more likely to screen positive on the Modified Checklist for Autism in Toddlers (M-CHAT) than term-infants (Gray, Edwards, O’Callaghan, & Gibbons, 2015). Others studies have found moderate and late preterm infants born 32 – 36 weeks gestation have increased risk of delayed social competence on Brief Infant and Toddler Social Emotional Assessment (BITSEA) at 2 years of age (adjusted) compared to children born term (Johnson et al., 2015). However, the authors did not examine the derived BITSEA autism risk score. Premature infants are additionally at increased risk of cognitive and motor delays with an estimated prevalence of 16.9% (95% CI: 10.4% to 26.3%) and 20.6% (95% CI: 13.9% to 29.4%) respectively (Pascal et al., 2018). To our knowledge, there are no prior studies of EEG markers of neurodevelopmental or ASD risk in preterm infants.
Prenatal neurodevelopment is shaped by the emergence of neuronal oscillations and their differentiation into distinct frequencies, which can be considered indirect markers of brain development (Haegens & Zion Golumbic, 2018; Samaha, Iemi, Haegens, & Busch, 2020; Schaworonkow & Voytek, 2021; Spitzer & Haegens, 2017). Electroencephalography (EEG) is a non-invasive measure of cortical function reflecting electrical activity generated by spatially aligned excitatory and/or inhibitory post-synaptic potentials in cortical pyramidal cells (Clarke, Barry, McCarthy, & Selikowitz, 2001; Dustman, Shearer, & Emmerson, 1999; Matousek & Petersen, 1974; Somsen, vantKlooster, vanderMolen, vanLeeuwen, & Licht, 1997). Absolute EEG power systematically decreases with increasing EEG frequencies (e.g. from delta through gamma). Developmental trajectories are not linear from infancy through adolescence, however as children age the general consensus points to a developmental decrease in low frequency neural oscillations such as delta and theta and a developmental increase in high frequency oscillations such as beta and gamma as the brain undergoes synaptic pruning (Clarke et al., 2001; Dustman et al., 1999; Matousek & Petersen, 1974; Saby & Marshall, 2012; Somsen et al., 1997). There is also a developmental increase in alpha, which emerges at 3 – 4 months postnatally (Marshall, Bar-Haim, & Fox, 2002; Schaworonkow & Voytek, 2021). Salient low frequency oscillations (delta and theta) during infancy may be optimal for early sensory learning and memory whereas developmental changes in high frequency activity could be reflective of changes in cognitive perceptual abilities such as discrimination (Haegens & Zion Golumbic, 2018; Saby & Marshall, 2012).
Several neural metrics can be derived from resting state EEG but the majority of EEG studies to date examining group differences between autistic individuals* and age-matched peers have utilized absolute or relative EEG power. Absolute power is the total neural activity integrated over a frequency band of interest independent of neural activity in other bands whereas relative power is neural activity within a frequency band of interest divided by the activity in all other frequency bands (Wang et al., 2013). A review article determined that despite significant heterogeneity in sample demographics such as age of participants and the presence or absence of comorbid intellectual disability, autistic individuals demonstrate a “U shaped” electrophysiological profile of increased absolute or relative low-frequency EEG power (delta and theta), reduced absolute or relative alpha EEG power, and increased absolute or relative high-frequency EEG power (beta and gamma) (Wang et al., 2013). This electrophysiological profile may be linked to increased GABAergic activity underlying a shift in the excitatory/inhibitory (E/I) balance (Nelson & Valakh, 2015; Wang et al., 2013).
Significant evidence supports the notion that developmental differences in neural oscillations measured via resting state EEG during infancy may be both predictive of subsequent cognitive development and a suitable biomarker of subsequent ASD diagnosis (Bhat, McDonald, Eilbott, & Pelphrey, 2019; W. Bosl, Tierney, Tager-Flusberg, & Nelson, 2011; W. J. Bosl, Tager-Flusberg, & Nelson, 2018; Brito et al., 2019; Dickinson et al., 2021; Dickinson, Varcin, Sahin, Nelson, & Jeste, 2019; L. Gabard-Durnam, Tierney, Vogel-Farley, Tager-Flusberg, & Nelson, 2015; L. J. Gabard-Durnam et al., 2019; Haartsen et al., 2019; Levin, Varcin, O’Leary, Tager-Flusberg, & Nelson, 2017; Orekhova et al., 2014; Righi, Tierney, Tager-Flusberg, & Nelson, 2014; Riva et al., 2019; Wilkinson et al., 2020). Given that ASD heritability is approximately 50% with sibling recurrence risk ranging between 5% and 20% (Sandin et al., 2014), the majority of prior studies in this domain have examined prognostic EEG markers in infant’s at familial risk of ASD on the basis of having a first-degree sibling with ASD. Although several prior studies have demonstrated differences in measures of neural development between low-risk controls and high-risk siblings with or without an ASD diagnosis, some evidence suggests children at familial risk of ASD may represent a distinct phenotype (Dalton, Nacewicz, Alexander, & Davidson, 2007). The largest and most recent study of EEG power in infants at familial risk of ASD utilized 432 unique participants (n=222 at familial risk of ASD). Results revealed familial risk status, but not subsequent ASD diagnosis, was associated with reduced absolute power at 3-months of age and a steeper increase in absolute power from 3 to 36 months (Huberty et al., 2021). This finding emphasizes the need to examine quantitative neural markers of autism risk in the context of simplex ASD and other prenatal risk factors including preterm birth.
Another EEG parameter of interest is the log-frequency log-PSD (power spectral density) slope. The PSD slope is an index of the entire EEG power spectrum (PSD(f) ~1/fα). EEG data has a negative slope which is affected by behavioral state, age, and level of consciousness (sleep versus wakefulness) (Gao, Peterson, & Voytek, 2017; Robertson et al., 2019; Voytek et al., 2015). As the absolute value of the PSD slope decreases, the amplitude of higher frequencies is smaller relative to lower frequencies (Colombo et al., 2019). Preclinical and clinical data from electrocorticography (ECoG), intracranial electroencephalography (iEEG) local field potential studies, and computational models suggests the PSD slope reflects both the neural signal-to-noise ratio and the E/I ratio in neural circuitry (Freeman & Zhai, 2009; Gao et al., 2017; Robertson et al., 2019; Voytek et al., 2015). Although to our knowledge prior studies have not examined the PSD slope during infancy in association with subsequent ASD risk, a recent longitudinal study examined the development of the EEG power spectrum over the first year of life and found a progressive flattening of PSD slopes between 1 – 7 months postnatally (Schaworonkow & Voytek, 2021). Other studies have found associations between the PSD slope and stimulant treatment (Ostlund, Alperin, Drew, & Karalunas, 2021; Robertson et al., 2019) and response inhibition (Pertermann, Bluschke, Roessner, & Beste, 2019) in attention deficit hyperactivity disorder (ADHD) (Robertson et al., 2019). Two studies have also examined the PSD slope in association with symptom severity in neurodevelopmental genetic conditions, Rett Syndrome (Roche et al., 2019) and Fragile-X syndrome (Wilkinson & Nelson, 2021). Both studies found a steeper PSD slope in Rett Syndrome (Roche et al., 2019) and Fragile-X syndrome respectively compared to controls (Wilkinson & Nelson, 2021).
The objective of the current analysis was to examine absolute EEG spectral power and the PSD slope in preterm infants in association with subsequent ASD risk and cognitive development at three years of age. Based on previous findings, we hypothesized increased oscillatory activity (EEG power) in the lower frequencies (delta and theta) and a steeper low-frequency PSD slope would be associated with increased autism risk scores. We also hypothesized increased high-frequency oscillatory activity (beta and gamma) would be associated with increased cognitive ability scores.
Methods
Participants.
Participants include preterm infants from a low socioeconomic status residential area in the Western Cape Province of South Africa with available neonatal EEG data who participated in a follow-up study to examine ASD risk at three years of age. This specific community has a preterm birth rate of 13.8% (Brink, Gebhardt, Mason, Groenewald, & Odendaal, 2019). After exclusions for equipment failure or inability to fall asleep during the resting state EEG study (n=15), the final sample consisted of 71 preterm infants born between 25 and 36 weeks gestation (35 females; gestational age at birth: 34.60 ± 2.34 weeks) (Table 1). EEG was acquired at corrected term age between 39 and 41 weeks postmenstrual age (40.00 ± 0.42 weeks). Infants had no prenatal exposure to psychiatric medications (selective serotonin reuptake inhibitors, anti-depressants, classic antipsychotics, atypical antipsychotics, mood stabilizers, stimulants, anti-anxiety, or anticonvulsants). Maternal and newborn eligibility case report forms and abstracted maternal-infant medical charts were used to obtain maternal age at delivery, gestational age at birth, mode of delivery, the infant’s biological sex, and birthweight. Developmental follow-up assessments were administered between 31 and 36 months of age adjusted for prematurity (34.0 ± 2.7 months).
Table 1.
Participant Demographic Information
| Participants (n=71) | |
|---|---|
| Gestational Age at Birth (weeks) | |
| Mean (SD) | 34.6 (2.34) |
| Median [Min, Max] | 35.4 [25.6, 36.9] |
| Preterm Categories | |
| Extremely Preterm (< 28 weeks) | 2 (3%) |
| Very Preterm (≥ 28 to < 32 weeks) | 5 (7%) |
| Moderate Preterm (≥ 32 to < 34 weeks) | 14 (20%) |
| Late Preterm (≥ 34 to < 37 weeks) | 50 (70%) |
| Sex | |
| Male (%) | 36 (50.7%) |
| Female (%) | 35 (49.3%) |
| Race | |
| Mixed Ancestry (%) | 71 (100%) |
| Birth Weight (grams) | |
| Mean (SD) | 2160 (585) |
| Median [Min, Max] | 2210 [640, 4440] |
| Birth Weight Categories | |
| Very Low Birthweight (< 1500 grams) | 8 (11%) |
| Low Birthweight (< 2500 grams) | 42 (59%) |
| Normal Birthweight (≥ 2500 grams) | 21 (30%) |
| Mode of Delivery | |
| Vaginal Spontaneous | 52 (73%) |
| Vaginal Operative | 2 (3%) |
| Cesarean | 17 (24%) |
| Maternal Parity | |
| Mean (SD) | 1.56 (1.48) |
| Median [Min, Max] | 1.00 [0, 6.00] |
| Maternal Age at Delivery | |
| Mean (SD) | 26.7 (6.92) |
| Median [Min, Max] | 25.0 [16.0, 43.0] |
Written informed consent to record infant brain activity using EEG and to abstract medical charts was part of the consent for the NIH Safe Passage Study. Separate informed consent to participate in the developmental follow-up assessments was obtained from a parent or legal guardian of each participant for follow-up studies. Ethical approval was obtained for both time points from Stellenbosch University and the New York State Psychiatric Institute.
Neonatal EEG Acquisition and Processing.
EEG data were acquired during natural sleep using a 32-lead high-impedance electrode net with 28 active electrodes (Figure 1) (Electrical Geodesics) and a miniature amplifier and recording device (ATES, Colognola ai Colli, Italy). EEG data collection and processing were previously described (Brito et al., 2019; Shuffrey et al., 2020). In brief, EEG was acquired at a sampling rate of 250 Hz for 10 minutes as part of a 33 minute protocol. All EEG data were processed in MATLAB. During recording, the EEG voltage from each lead referenced to the vertex electrode was recorded through a low-pass antialiasing filter (cut off frequency equal to 96 Hz) and digitized with 16 bits per sample at a rate of 250 samples per second. Prior to screening data for artifact for each second using the thresholds described below, raw data for the entire minute were filtered for line noise and any ECG artifact was subtracted from each channel. A 16,000 point finite-impulse response 4 Hz wide notch filter was applied at the line noise frequency and its first harmonic (50 and 100 Hz) with 36 to 100 dB power reduction within the notches. ECG artifact was removed from each channel using a recently developed method that mimics the procedure for ballistocardiogram removal from EEG recorded during MRI (Allen, Polizzi, Krakow, Fish, & Lemieux, 1998). Ballistocardiogram artifact is removed by using a simultaneously recorded ECG signal to precisely identify the times of each R wave peak, and then to signal average the EEG over small windows centered on those times, deriving a channel-specific template that is then subtracted from each channel. EEG power spectra were then computed for 60-second epochs using the Welch method, averaging over fast Fourier transforms (FFTs) taken each second (Bendat, 1982). Data were demeaned and a Hanning window was applied prior to computing the FFT for each second. To determine the leads and times contaminated by movement-related or other sources of electrical artifact, we applied multiple criteria on a second by second basis to data from each lead. Criteria were as follows: standard deviation of voltage less than 50 μV and greater than 0.001 μV; sample-to-sample change less than 50 μV; absolute value of voltage less than 300 μV; log-log spectral slope of raw data between 20 and 120 Hz less than −0.1 (to screen for muscle artifact). If more than 5 leads had artifact during any one second, that second was excluded. Remaining data were re-referenced to the average over all leads at each sample. Finally, minute by minute power was the average of the squared FFT’s over the accepted seconds, requiring at least 30 acceptable seconds per minute for each lead as a minimum inclusion threshold.
Figure 1.
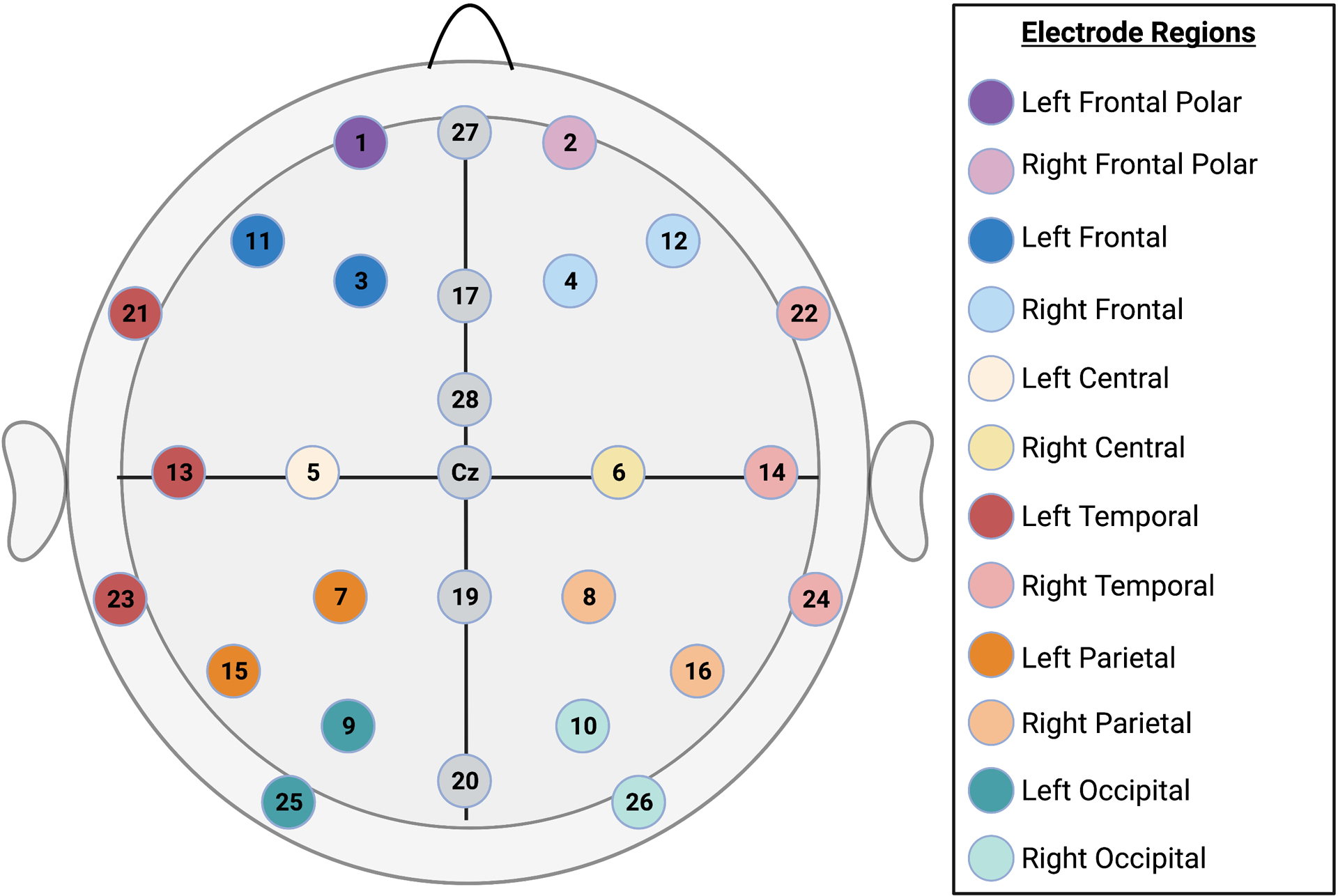
Electrode Map. Topographic map depiction of a 32-channel EGI Geodesic Sensor Net with 28 active channels.
Sleep State Coding.
Respiration waveforms were collected through a respiratory inductance belt and were digitized at 20 Hz. A previously published quantitative method to determine sleep state was utilized (Isler, Thai, Myers, & Fifer, 2016).
EEG Features.
Minute by minute EEG power was aligned with simultaneous sleep state codes and averaged over active sleep (AS) and quiet sleep (QS) minutes within each study.
EEG Power:
Absolute EEG power was computed in the following frequency bins: 1 – 3 Hz (i.e. delta), 4 – 6 Hz (i.e. theta), 7 – 9 Hz (i.e. low alpha), 10 – 12 Hz (i.e. high alpha), 13 – 30 Hz (i.e. beta), 31 – 45 Hz (i.e. gamma) for 12 scalp regions (left frontal-polar, right frontal-polar, left frontal, right frontal, left parietal, right parietal, left central, right central, left temporal, right temporal, left occipital, and right occipital) in AS and QS (separately). As newborns spend less time on average in quiet sleep, QS data were only available for a subset of n=46 participants (64% of the total sample). Figure 1 depicts an electrode map of scalp regions.
PSD Slope:
The rhythmic as well as the scale free components of EEG power can be summarized using a single index derived from the broadband power spectrum, namely the PSD slope. The PSD slope is a measure of the PSD background decay across all frequencies modelled following the power law: PSD(f) ~1/fα. The 1/f-like PSD slope was computed using the fitting oscillations & one over f (FOOOF) algorithm to obtain the aperiodic fit for low (1 – 20 Hz) and high (21 – 40 Hz) frequencies separately using the most recent methodology (Donoghue et al., 2020) (Figure 2). This approach allowed the computation of the PSD slope at each channel and for different frequency ranges, fitting the log-log least-square line after removing peaks and background activity associated to rhythmic oscillatory components (such as theta, alpha, beta, and gamma). The low- and high-frequency PSD slopes were computed in the frequency range 1 – 20 Hz and 21 – 40 Hz (separately) based on the inflection point visually identified in the neural spectral parametrization implemented by FOOOF (Figure 2). To statistically confirm the validity of the illustrated approach, the differences between low- and high-frequency slopes were tested at the population level using a Bland–Altman plot was used to test the bias of the two estimates and assess their relationship. Slopes were computed for each scalp region (Figure 1) in AS and the subset of n=46 participants in QS (separately).
Figure 2.
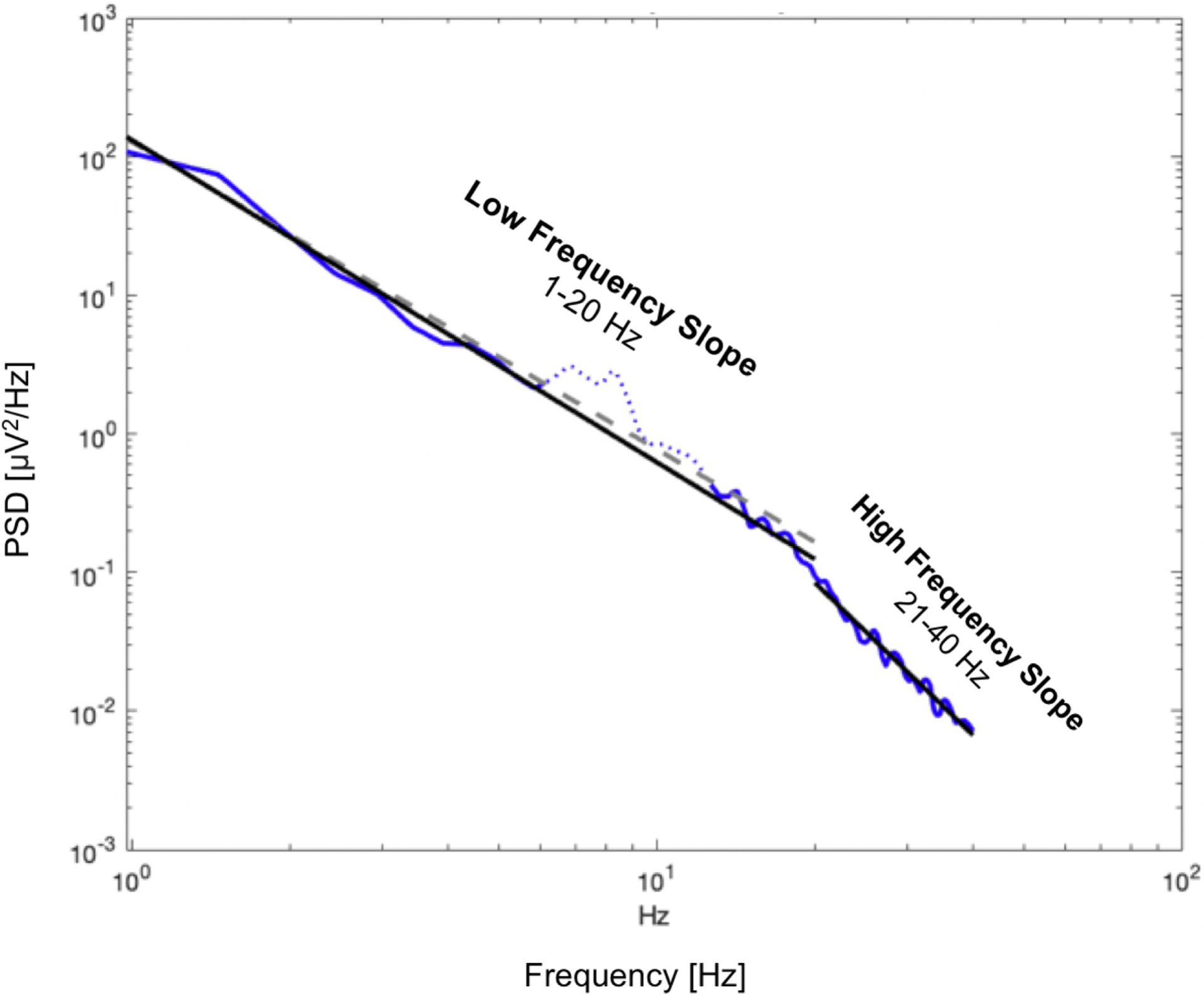
Power Spectral Density (PSD) Slope Example. Illustrative example of low and high frequency slope (solid black line) computation on EEG power spectral density (PSD) (solid blue line). Firstly, the PSD is fit with an estimated aperiodic component (dashed gray line). The estimated aperiodic portion of the signal is subtracted from the raw PSD. The residuals portion of the spectrum are assumed to be a mix of periodic oscillatory peaks and noise. The identified peaks (which are found above the noise threshold calculated from the standard deviation of the residuals), are fitted with a Gaussian distribution and remove via an iterative process (dotted blue line). Once these components are removed, based on the number of peaks above the noise threshold, multi-Gaussian fitting is performed on the aperiodic-adjusted signal to derive the spectral slopes.
Toddler Developmental Assessments.
Brief Infant Toddler Social Emotional Assessment (BITSEA). The BITSEA is a 42-item parental report measure of social-emotional development, behavioral problems, and delays in competence (Gowan, Carter, & Carter, 2006). Domains assessed within the BITSEA include: externalizing, internalizing, dysregulation, and competence (Gowan et al., 2006). The BITSEA demonstrated excellent test-retest reliability and good inter-rater agreement between parents of socioeconomically and ethnically diverse backgrounds (Gowan et al., 2006). The BITSEA contains 10 items consistent with ASD problem behaviors (Giserman Kiss, Feldman, Sheldrick, & Carter, 2017). The BITSEA ASD problem scale has moderate-to-high discriminative power, sensitivity, and specificity, to differentiate children with and without ASD using item level questions that directly relate to autism specific behaviors (Giserman Kiss, Feldman, Sheldrick, & Carter, 2017; Gowan et al., 2006; Kruizinga et al., 2014). For this analysis, we utilized the BITSEA ASD problem scale.
Bayley Scales of Infant Development III (Bayley-III) Screening Test. The Bayley-III screening test was designed as a rapid assessment of cognitive, language, and motor functioning in infants and young children to determine if a child’s development is within normal limits and identify risk for developmental delay. The Bayley-III screening test has high test-retest reliability: Cognitive (0.85), Receptive Language (0.88), Expressive Language (0.88), Fine Motor (0.82), and Gross Motor (0.86) (Bayley, 2006). The Bayley-III has been validated and is used throughout South Africa (Ballot et al., 2017; Rademeyer & Jacklin, 2013). For this analysis, we utilized the Bayley-III cognitive total score.
Statistical Analyses.
All statistical analyses were conducted in R version 4.0.2. Analyses for examining the association between EEG parameters in newborns during active sleep and subsequent autism risk and cognitive development controlled for sex, gestational age at birth, postmenstrual age at the EEG study, and postnatal age at the follow-up assessment. BITSEA ASD risk total scores and Bayley-III cognitive scores were winsorized at the 5th and 95th percentiles using the ‘Winsorize’ function prior to running analyses. A false discovery rate correction (FDR) was implemented to correct for multiple comparisons within frequency bin to account for each scalp location using the ‘padjust’ function (12 statistical comparisons per outcome measure) (Benjamini & Hochberg, 1995). Analyses were repeated within the subset of infants with available newborn EEG data in the QS state (n=46). Secondary analyses examined the effect of low birth weight (<2500g) without adjustment for gestational age at birth.
EEG Power:
Analyses consisted of individual linear regression models to examine the association between absolute EEG power within each frequency bin and brain region in predicting BITSEA ASD risk total scores and Bayley-III cognitive scores (separately). A total of 72 linear regression models were run with the BITSEA ASD risk score as the outcome measure, and 72 linear regression models were run with the Bayley-III cognitive total score as the outcome measure.
PSD Slope:
Analyses consisted of individual linear regression models to examine the association between the low frequency (1 – 20 Hz) and the high frequency (21 – 40 Hz) PSD slope in each brain region (same as in EEG power) and the BITSEA ASD problem total score and the Bayley-III cognitive score (separately). A total of 24 linear regression models were run with the BITSEA ASD risk score as the outcome measure, and another set of 24 linear regression models were run with the Bayley-III cognitive total score as the outcome measure.
Results
EEG Power
Autism Risk.
There were modest associations between 10 – 12 Hz EEG power in the active sleep state and BITSEA ASD problem scores, however after the FDR correction for multiple comparisons, there were no significant associations between EEG power in the active sleep state in any frequency bin (1 – 3 Hz, 4 – 6 Hz, 7 – 9 Hz, 10 – 12 Hz, 13 – 30 Hz, 31 – 45 Hz) within each scalp location (left frontal-polar, right frontal-polar, left frontal, right frontal, left parietal, right parietal, left central, right central, left temporal, right temporal, left occipital, and right occipital) and BITSEA ASD problem scores (p’s > .05). There were also no significant associations between EEG power in the quiet sleep state in any frequency bin (1 – 3 Hz, 4 – 6 Hz, 7 – 9 Hz, 10 – 12 Hz, 13 – 30 Hz, 31 – 45 Hz) within each scalp location (left frontal-polar, right frontal-polar, left frontal, right frontal, left parietal, right parietal, left central, right central, left temporal, right temporal, left occipital, and right occipital) and BITSEA ASD problem scores (p’s > .05). Secondary analyses revealed low birth weight was not a significant predictor in any model (p’s > .05).
Cognitive Development.
After correction for multiple comparisons, there were no significant associations between EEG power in the active sleep state in any frequency bin (1 – 3 Hz, 4 – 6 Hz, 7 – 9 Hz, 10 – 12 Hz, 13 – 30 Hz, 31 – 45 Hz) within each scalp location (left frontal-polar, right frontal-polar, left frontal, right frontal, left parietal, right parietal, left central, right central, left temporal, right temporal, left occipital, and right occipital) and cognitive scores on the Bayley-III (p’s > .05). There were also no significant associations between EEG power in the quiet sleep state in any frequency bin (1 – 3 Hz, 4 – 6 Hz, 7 – 9 Hz, 10 – 12 Hz, 13 – 30 Hz, 31 – 45 Hz) within each scalp location (left frontal-polar, right frontal-polar, left frontal, right frontal, left parietal, right parietal, left central, right central, left temporal, right temporal, left occipital, and right occipital) and Bayley-III cognitive scores (p’s > .05). Secondary analyses revealed low birth weight was not a significant predictor in any model (p’s > .05).
PSD Slope
Figure 3 illustrates the average spectrum and associated spectral fit separately for the low- and high-frequencies. Two independent Bland–Altman tests were run to compare the low- and high-frequency slope estimates. In AS, the minimum and maximum average of slope pairs were −2.8339 and −0.8383, respectively. The associated minimum and maximum differences were −3.6323 and 1.0967. The resulting bias between the two measures was −0.5395 ± 0.7547. This result signifies that estimates of low-frequency slope were significantly steeper (−2.2528 ± 0.2232) compared to the high-frequency slope (−1.7130 ± 0.6989); p-value <0.001. Also, a significant negative relationship between the mean of low- and high- frequency slopes and their difference was reported, such that the more negative their means, the smaller their differences. Analogous trends were obtained in QS. The minimum and maximum average of slope pairs were −2.9280 and −0.4641, respectively. The associated minimum and maximum differences were −3.870 and 1.1811. The resulting bias between the two measures was −0.5395 ± 0.8206. This result signifies that estimates of low-frequency slope were significantly steeper (−2.3163 ± 0.2647) compared to the high-frequency slope (−1.8092 ± 0.7487); p-value<0.001. A significant negative relationship between the mean of low- and high- frequency slopes and their difference was reported, such that the more negative their means, the smaller their differences. Based on these differences, we utilized the low-frequency (1 – 20 Hz) and high-frequency (21 – 40 Hz) slopes separately in subsequent analyses).
Figure 3.
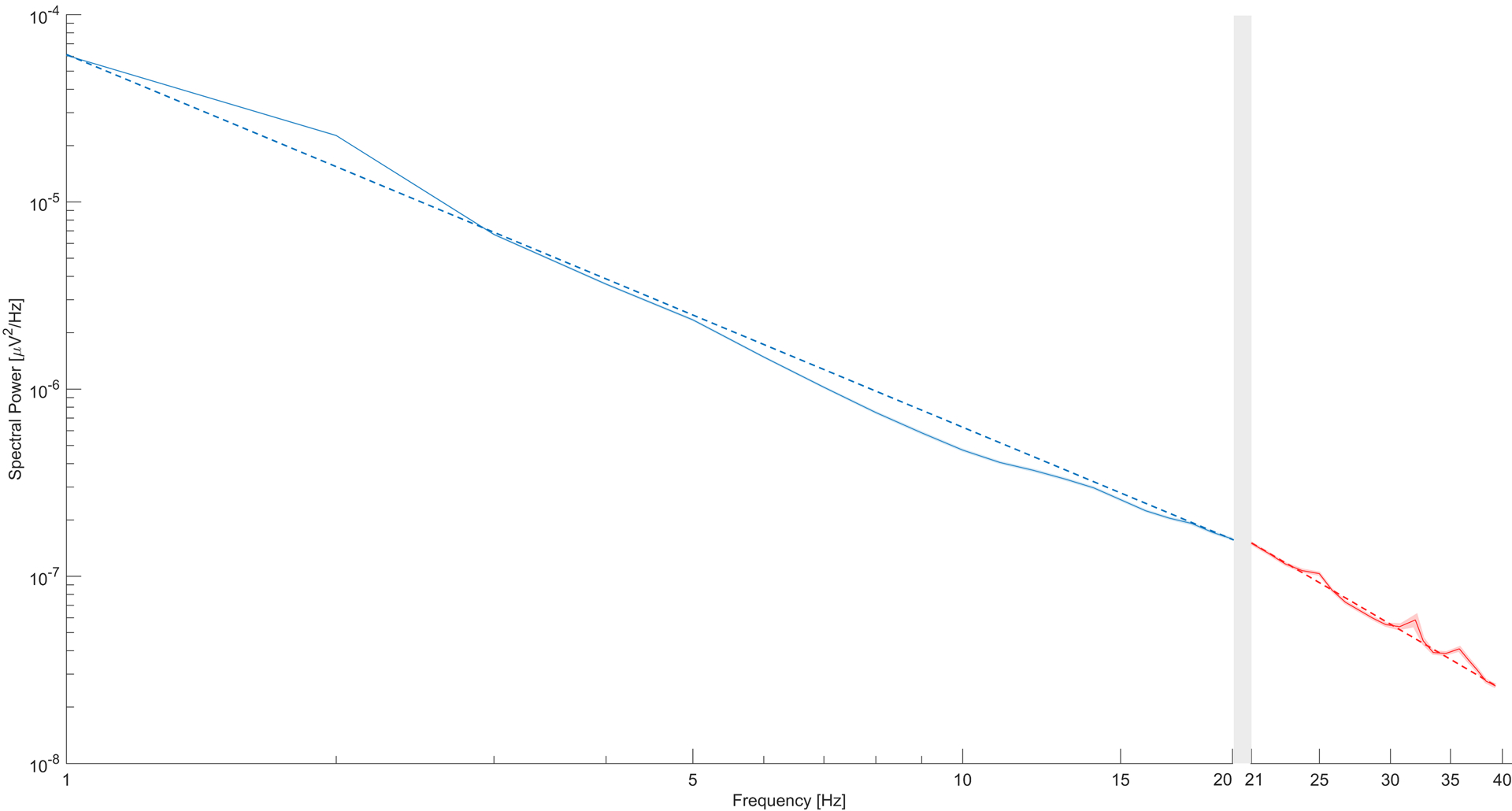
The average spectrum (solid lines), standard error of the mean (shaded area), and associated spectral fit lines (dashed lines) obtained by averaging spectra in AS across participants. The separation between the low- (1–20 Hz in blue) and high- (21–40 Hz in red) frequency portions of the average spectrum is accentuated by the gray-shadowed area.
Autism Risk.
After correction for multiple comparisons, there was a significant association between the low frequency power spectral density slope (1 – 20 Hz) in active sleep in the left frontal (F(1, 63) = 3.37, p < .01, adj R2 = 0.12), left central (F(1, 63) = 3.37, p < .05, adj R2 = 0.08), right central (F(1, 63) = 2.21, p < .05, adj R2 = 0.06), right parietal (F(1, 63) = 3.22, p < .05, adj R2 = 0.11), and left occipital (F(1, 63) = 2.33, p < .05, adj R2 = 0.07) regions and BITSEA ASD problem scores (Table 2). A steeper low-frequency (1 – 20 Hz) PSD slope in active sleep in these scalp locations was associated with increased BITSEA ASD problem scores (Figure 4). After correction for multiple comparisons, there was no significant association between the low-frequency PSD slope in active sleep in the left or right frontal polar, right frontal, left parietal, right occipital, and left or right parietal region and BITSEA ASD problem scores (p’s > .05). There were no significant associations between the high-frequency PSD slope (21 – 40 Hz) in active sleep in any scalp location (left frontal-polar, right frontal-polar, left frontal, right frontal, left parietal, right parietal, left central, right central, left temporal, right temporal, left occipital, and right occipital) and BITSEA ASD problem scores. Gestational age, sex, postmenstrual age at the EEG study, age at follow-up and were not significant predictors in any models (p’s > .05). There were also no significant associations between the low or high frequency PSD slope in the quiet sleep state in any scalp location (left frontal-polar, right frontal-polar, left frontal, right frontal, left parietal, right parietal, left central, right central, left temporal, right temporal, left occipital, and right occipital) and BITSEA ASD problem scores (p’s > .05). Secondary analyses revealed low birth weight was not a significant predictor in any model (p’s > .05).
Table 2.
Association Between the PSD Slope and Autism Risk by Brain Region
| Brain Region | T-statistic | p-value | FDR adjusted p-value |
|---|---|---|---|
| Left Frontal | −3.18 | 0.002 | 0.017 |
| Left Central | −2.66 | 0.009 | 0.039 |
| Right Central | −2.38 | 0.019 | 0.047 |
| Right Parietal | −3.09 | 0.002 | 0.017 |
| Left Occipital | −2.48 | 0.015 | 0.046 |
Figure 4.
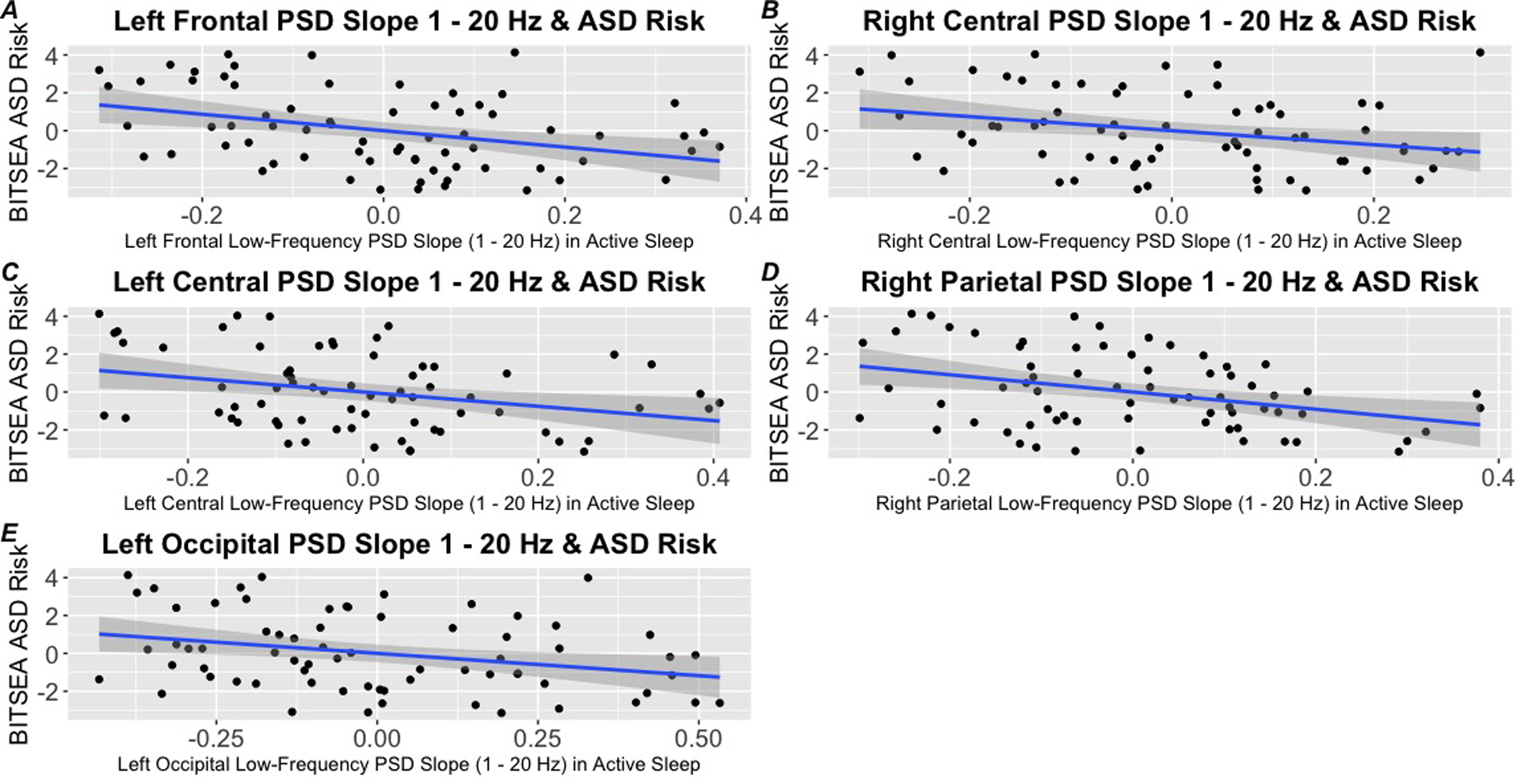
Association between the PSD Slope during the neonatal period during active sleep and subsequent autism risk at age 3. A steeper low-frequency (1 – 20 Hz) PSD slope in active sleep in the left frontal (Panel A), right central (Panel B), left central (Panel C), right parietal (Panel D), and left occipital (Panel E) regions are associated with increased BITSEA ASD problem scores
Cognitive Development.
After correction for multiple comparisons, there were no significant associations between the low (1 – 20 Hz) or high (21 – 40 Hz) frequency PSD slope in active sleep in any scalp location (left frontal-polar, right frontal-polar, left frontal, right frontal, left parietal, right parietal, left central, right central, left temporal, right temporal, left occipital, and right occipital) and cognitive scores on the Bayley-III (p’s > .05). There were also no significant associations between low (1 – 20 Hz) or high (21 – 40 Hz) frequency PSD slope in the quiet sleep state in any scalp location (left frontal-polar, right frontal-polar, left frontal, right frontal, left parietal, right parietal, left central, right central, left temporal, right temporal, left occipital, and right occipital) and Bayley-III cognitive scores (p’s > .05). Secondary analyses revealed low birth weight was not a significant predictor in any model (p’s > .05).
Discussion
To our knowledge, the present report is the only study to date to examine both the PSD slope and absolute EEG power (independently) in preterm infants as potential markers of subsequent autism risk and cognitive development. Further, this is the first study to examine early neurophysiological markers of neurodevelopmental risk in a sample of preterm infants born in a LMIC. To summarize, we found that aperiodic activity in multiple brain regions, but not oscillatory activity, was associated with subsequent autism risk at three years of age in a sample of preterm infants. Specifically, we found a steeper, more negative 1 – 20 Hz PSD slope during active sleep at 40 weeks adjusted postmenstrual age in the left frontal, left and right central, right parietal, and left occipital regions was associated with increased autism risk on the BITSEA at three years of age. However, no associations were found between absolute EEG power and autism risk. Additionally, no associations were found between absolute EEG power or the PSD slope and cognitive ability. Our null findings specific to EEG power are in part consistent with (Huberty et al., 2021) who demonstrated familial risk status, but not subsequent ASD diagnosis, was associated with developmental changes in EEG power. Conversely, other studies have shown links between resting-state EEG power in neonates and subsequent autism risk in term-infants (Brito et al., 2019) and cognitive ability in preterm infants (Scher, Steppe, & Banks, 1996). Our null findings in this domain are likely due to differences in the sample population (preterm vs. term), study design (matched-controls), or differences in EEG frequencies computed. It is also possible that some of prior findings linking EEG power to autism risk (Bhat et al., 2019; W. Bosl et al., 2011; W. J. Bosl et al., 2018; Brito et al., 2019; Dickinson et al., 2021; Dickinson et al., 2019; L. Gabard-Durnam et al., 2015; L. J. Gabard-Durnam et al., 2019; Haartsen et al., 2019; Levin et al., 2017; Orekhova et al., 2014; Righi et al., 2014; Riva et al., 2019; Wilkinson et al., 2020) or cognitive development (Scher et al., 1996) are in part driven by aperiodic activity which is inherently present in oscillatory activity.
Prior ECoG, iEEG, local field potential and computational studies suggest the PSD slope is driven by the E/I ratio in neural circuitry where increasing the E/I ratio would result in a steeper slope (Freeman & Zhai, 2009; Gao et al., 2017; Robertson et al., 2019; Voytek et al., 2015). Specifically, a prior study demonstrated associations between the PSD slope and the E/I ratio across CA1 layers and theta oscillations in the rat hippocampus (Gao et al., 2017).
Research from the ECoG literature on age-related cognitive decline has put forth the neural noise hypothesis, which suggests the effective signal-to-noise ratio of neural communication declines with increasing age (Voytek et al., 2015). In this framework, increased neural noise would be reflected by a flatter PSD slope associated with asynchronous neural firing at the local field potential level potentially driven by the E/I ratio (Katzner et al., 2009; Musall, von Pfostl, Rauch, Logothetis, & Whittingstall, 2014; Pertermann et al., 2019; Voytek et al., 2015). On the contrary, simultaneous spiking would be associated with a more negative PSD slope (Katzner et al., 2009; Musall et al., 2014; Pertermann et al., 2019; Voytek et al., 2015). Although it is difficult to draw comparisons between early developmental changes in the PSD slope and age-related changes in late adulthood, at least one prior study suggests cognitively optimal spectral slopes may vary by developmental stages (Voytek et al., 2015). A recent longitudinal study on the development of the EEG power spectrum demonstrated a progressive flattening of PSD slopes computed from 1 – 10 Hz between 1 – 7 months postnatally, which the authors attributed to developmental changes in the E/I ratio (Schaworonkow & Voytek, 2021). Given the developmental flattening of the low-frequency PSD slope in the first few months of life (Schaworonkow & Voytek, 2021), a steeper low-frequency PSD slope during early infancy may be linked to developmental changes in the GABAergic switch from excitatory to inhibitory.
Neuronal homeostasis of the E/I ratio consists of GABAergic inhibition being two to six times the strength of glutamatergic excitation (Gao et al., 2017). Shifts in the E/I ratio towards hyperexcitation from altered GABAergic signaling are hypothesized mechanisms implicated in several neurodevelopmental conditions including ASD (Kolesnik et al., 2019; Nelson & Valakh, 2015), genetic syndromes associated with ASD (Nelson & Valakh, 2015; Roche et al., 2019), and ADHD (Robertson et al., 2019).
A prior study found medication naïve 3 – 7 year old children with ADHD had steeper PSD slopes and increased alpha EEG power compared to age-matched peers without ADHD (Robertson et al., 2019). Stimulant treated children with ADHD had flatter slopes compared to non-stimulant treated children with ADHD and similar slopes compared to age-matched peers (controls) across all electrode regions, suggestive of normalization of the E/I ratio from stimulant treatment (Robertson et al., 2019). This study also found an association between the theta/beta ratio and the PSD slope. However, the PSD slope may be a superior metric of electrophysiological activity since it is not influenced by peak frequencies or narrow-band power (Levin et al., 2020; Robertson et al., 2019).
A separate study among school age children with ADHD and controls found children with ADHD had flatter PSD slopes in frontal electrode regions compared to their peers during a Go/NoGo task when engaged in response inhibition during NoGo trials (Pertermann et al., 2019). Additionally, stimulant treatment normalized differences in the PSD slope between children with ADHD and controls (Pertermann et al., 2019). A large study of adolescents with ADHD (n=87) and controls without ADHD (n=97) found adolescents with ADHD had a flatter PSD slope across an average of all electrode sites (Ostlund et al., 2021), which is consistent with (Pertermann et al., 2019), but not with (Robertson et al., 2019). Divergent results from these three studies which included children from different age ranges further suggests optimal spectral slopes may vary by developmental stages (Voytek et al., 2015). However, differences could also in part be driven by different methodologies used to compute the PSD slope (FOOOF vs. others), study design (task based vs. resting EEG), and electrodes included in analyses.
Although to our knowledge the PSD slope has not yet been examined during infancy in association with subsequent autism risk, two studies have utilized this parameter to examine links between the PSD slope and phenotype within specific genetic neurodevelopmental conditions associated with ASD. A cross-sectional study of girls with Rett syndrome, a progressive genetic neurodevelopmental condition caused by a mutation in the MECP2 gene, examined the PSD slope as a potential marker of cortical excitation and disease severity (Roche et al., 2019). EEG data were collected between 23 and 131 months of age. Mouse models of Rett syndrome have demonstrated altered GABAergic signaling from a loss of MECP2 resulting in cortical hyperexcitability (Dani et al., 2005; Zhang, Peterson, Beyer, Frankel, & Zhang, 2014). Therefore the PSD slope could serve as a potential marker of an increased E/I ratio. Results revealed that participants with Rett syndrome demonstrated a steeper PSD slope than the control group across all brain regions, which was more pronounced in the developmental regression subgroup (Roche et al., 2019). Within children diagnosed with Rett syndrome, a flatter (less negative) PSD slope was associated with improved performance on visual receptive, receptive and expressive language, and fine motor subscales (Roche et al., 2019).
A recent study of male children with Fragile X Syndrome (FXS), a genetic neurodevelopmental condition caused by an expansion of the CGG triplet related FMR1 gene on the X chromosome resulting in a FMRP protein deficiency, examined potential differences in aperiodic and oscillatory activity between children with FXS and controls (Wilkinson & Nelson, 2021). EEG data were collected between 33 and 78 months of age (Wilkinson & Nelson, 2021). FXS is also associated with an E/I imbalance and mouse models have reversed phenotypes in FMR1 knockout mice through the administration of GABA agonists (Wilkinson & Nelson, 2021). Similar to children or adults with ASD (Wang et al., 2013), children with FXS demonstrated increased oscillatory activity from 20 – 50 Hz across multiple brain regions (Wilkinson & Nelson, 2021). Additionally, children with FXS demonstrated a steeper PSD slope in frontal and central regions (Wilkinson & Nelson, 2021), potentially reflective of an altered E/I balance.
Although we cannot draw direct comparisons to the existing literature due to differences in sample characteristics, study design, and outcome measures, our findings are in general agreement with (Roche et al., 2019) and (Wilkinson & Nelson, 2021) who both reported a steeper PSD slope in children with genetic neurodevelopmental conditions. Additionally, we similarly reported associations in the frontal and central electrode sites (Wilkinson & Nelson, 2021). Given the existing literature in this domain and our finding that a steeper PSD slope was associated with subsequent autism risk at age three, the PSD slope is both potentially reflective of disruptions in the E/I balance and a marker of subsequent autism risk in preterm infants.
Limitations
Since we are the first to report an association between aperiodic activity in preterm neonates and subsequent autism risk outcomes and our analysis has a modest sample size, this association should be examined in independent cohorts to determine generalizability. Several children from this cohort study were referred for clinical evaluations, but ASD specific outcomes are not available to us. This limits our ability to examine neural activity associated with autism risk versus ASD diagnostic outcomes. However, given the lack of studies in this domain, our results are still a meaningful preliminary report. EEG has poor spatial resolution therefore it is difficult to draw conclusions regarding the underlying brain regions represented by the electrode sites in our current findings. Future studies should consider source localization to determine if there are regional differences in aperiodic activity. There are several differences in EEG processing pipelines across the developmental and adult literature, which is a significant limitation in the present report and across the entire field of electrophysiological research. Our findings may be influenced by frequency bins computed or subtle differences in artifact thresholds such as muscle artifacts, which may vary by neurodevelopmental risk status. Lastly, EEG is sleep state dependent, therefore our sample is reduced in the quiet sleep state (64% of the total sample) leaving us unable to determine if the lack of effects in the quiet sleep are related to the diminished sample size or state dependent effects.
Conclusions
Acquiring resting state EEG data from neonates during natural sleep is highly feasible. Even in a low-resource setting 82.5% of the neonates provided usable data in active sleep. In the current study, we found links between aperiodic activity in preterm infants and subsequent ASD risk scores at three years of age. Expected associations between EEG power with subsequent ASD risk and cognitive ability were not found within this sample. Our findings highlight the need to examine markers of autism risk in high-risk populations other than infants at familial risk. The resulting studies would help contribute to our understanding of early brain development in children with simplex ASD, idiopathic ASD, or ASD linked to specific genetic etiologies. Most importantly, this research domain may contribute to early identification of ASD, which could improve long-term symptom severity and quality of life outcomes for autistic individuals (Anderson et al., 2014; Mason et al., 2018; Pickles et al., 2016)
Acknowledgments:
The authors would like to thank Daianna Rodriguez for administrative support, J. David Nugent for data management support, and the families who participated in this research study.
Funding:
This research was supported by the Bill and Melinda Gates Foundation awarded to Dr. William Fifer (follow-up studies) and the grants associated with the NIH Safe Passage Study: U01HD055154, U01HD045935, U01HD055155, U01HD045991 and U01AA016501 issued by the National Institute on Alcohol Abuse and Alcoholism, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institute on Deafness and Other Communication Disorders. Dr. Lauren Shuffrey is supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD) award K99HD103910. Dr. Ayesha Sania is supported by UH3OD023279-05S1, re-entry supplement from Office of the Director, NIH, and Office of Research on Women Health (ORWH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest: The authors have no conflicts of interest to declare.
We have opted for identity-first language in the manuscript based on recent surveys from self-advocacy groups.
Data Availability:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Agrawal S, Rao SC, Bulsara MK, & Patole SK (2018). Prevalence of Autism Spectrum Disorder in Preterm Infants: A Meta-analysis. Pediatrics, 142(3). doi:ARTN e20180134 10.1542/peds.2018-0134 [DOI] [PubMed] [Google Scholar]
- Anderson DK, Liang JW, & Lord C (2014). Predicting young adult outcome among more and less cognitively able individuals with autism spectrum disorders. J Child Psychol Psychiatry, 55(5), 485–494. doi: 10.1111/jcpp.12178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, … Dowling NF (2018). Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. MMWR Surveill Summ, 67(6), 1–23. doi: 10.15585/mmwr.ss6706a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballot DE, Ramdin T, Rakotsoane D, Agaba F, Davies VA, Chirwa T, & Cooper PA (2017). Use of the Bayley Scales of Infant and Toddler Development, Third Edition, to Assess Developmental Outcome in Infants and Young Children in an Urban Setting in South Africa. Int Sch Res Notices, 2017, 1631760. doi: 10.1155/2017/1631760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayley N (2006). Bayley scales of infant and toddler development: Bayley-III (Vol. 7). San Antonio, Texas, USA: Harcourt Assessment, Psych. Corporation. [Google Scholar]
- Bendat JS (1982). Citation Classic - Random Data - Analysis and Measurement Procedures. Current Contents/Engineering Technology & Applied Sciences(25), 16–16. [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B-Statistical Methodology, 57(1), 289–300. [Google Scholar]
- Bhat AN, McDonald NM, Eilbott JE, & Pelphrey KA (2019). Exploring cortical activation and connectivity in infants with and without familial risk for autism during naturalistic social interactions: A preliminary study. Infant Behav Dev, 57, 101337. doi: 10.1016/j.infbeh.2019.101337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosl W, Tierney A, Tager-Flusberg H, & Nelson C (2011). EEG complexity as a biomarker for autism spectrum disorder risk. BMC Med, 9, 18. doi: 10.1186/1741-7015-9-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosl WJ, Tager-Flusberg H, & Nelson CA (2018). EEG Analytics for Early Detection of Autism Spectrum Disorder: A data-driven approach. Sci Rep, 8(1), 6828. doi: 10.1038/s41598-018-24318-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brink LT, Gebhardt GS, Mason D, Groenewald CA, & Odendaal HJ (2019). The association between preterm labour, perinatal mortality and infant death (during the first year) in Bishop Lavis, Cape Town, South Africa. S Afr Med J, 109(2), 102–106. doi: 10.7196/SAMJ.2019.v109i2.13438 [DOI] [PubMed] [Google Scholar]
- Brito NH, Elliott AJ, Isler JR, Rodriguez C, Friedrich C, Shuffrey LC, & Fifer WP (2019). Neonatal EEG linked to individual differences in socioemotional outcomes and autism risk in toddlers. Dev Psychobiol. doi: 10.1002/dev.21870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MLE, Vinen Z, Barbaro J, & Dissanayake C (2018). School Age Outcomes of Children Diagnosed Early and Later with Autism Spectrum Disorder. J Autism Dev Disord, 48(1), 92–102. doi: 10.1007/s10803-017-3279-x [DOI] [PubMed] [Google Scholar]
- Clarke AR, Barry RJ, McCarthy R, & Selikowitz M (2001). Age and sex effects in the EEG: development of the normal child. Clinical Neurophysiology, 112(5), 806–814. doi:Doi 10.1016/S1388-2457(01)00488-6 [DOI] [PubMed] [Google Scholar]
- Colombo MA, Napolitani M, Boly M, Gosseries O, Casarotto S, Rosanova M, … Sarasso S (2019). The spectral exponent of the resting EEG indexes the presence of consciousness during unresponsiveness induced by propofol, xenon, and ketamine. Neuroimage, 189, 631–644. doi: 10.1016/j.neuroimage.2019.01.024 [DOI] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Alexander AL, & Davidson RJ (2007). Gaze-fixation, brain activation, and amygdala volume in unaffected siblings of individuals with autism. Biol Psychiatry, 61(4), 512–520. doi: 10.1016/j.biopsych.2006.05.019 [DOI] [PubMed] [Google Scholar]
- Dani VS, Chang Q, Maffei A, Turrigiano GG, Jaenisch R, & Nelson SB (2005). Reduced cortical activity due to a shift in the balance between excitation and inhibition in a mouse model of Rett syndrome. Proc Natl Acad Sci U S A, 102(35), 12560–12565. doi: 10.1073/pnas.0506071102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson A, Daniel M, Marin A, Gaonkar B, Dapretto M, McDonald NM, & Jeste S (2021). Multivariate Neural Connectivity Patterns in Early Infancy Predict Later Autism Symptoms. Biol Psychiatry Cogn Neurosci Neuroimaging, 6(1), 59–69. doi: 10.1016/j.bpsc.2020.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson A, Varcin KJ, Sahin M, Nelson CA 3rd, & Jeste SS (2019). Early patterns of functional brain development associated with autism spectrum disorder in tuberous sclerosis complex. Autism Res, 12(12), 1758–1773. doi: 10.1002/aur.2193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue T, Haller M, Peterson EJ, Varma P, Sebastian P, Gao R, … Voytek B (2020). Parameterizing neural power spectra into periodic and aperiodic components. Nat Neurosci, 23(12), 1655–1665. doi: 10.1038/s41593-020-00744-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustman RE, Shearer DE, & Emmerson RY (1999). Life-span changes in EEG spectral amplitude, amplitude variability and mean frequency. Clinical Neurophysiology, 110(8), 1399–1409. doi:Doi 10.1016/S1388-2457(99)00102-9 [DOI] [PubMed] [Google Scholar]
- Franz L, Adewumi K, Chambers N, Viljoen M, Baumgartner JN, & de Vries PJ (2018). Providing early detection and early intervention for autism spectrum disorder in South Africa: stakeholder perspectives from the Western Cape province. J Child Adolesc Ment Health, 30(3), 149–165. doi: 10.2989/17280583.2018.1525386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman WJ, & Zhai J (2009). Simulated power spectral density (PSD) of background electrocorticogram (ECoG). Cogn Neurodyn, 3(1), 97–103. doi: 10.1007/s11571-008-9064-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller EA, & Kaiser AP (2020). The Effects of Early Intervention on Social Communication Outcomes for Children with Autism Spectrum Disorder: A Meta-analysis. J Autism Dev Disord, 50(5), 1683–1700. doi: 10.1007/s10803-019-03927-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabard-Durnam L, Tierney AL, Vogel-Farley V, Tager-Flusberg H, & Nelson CA (2015). Alpha asymmetry in infants at risk for autism spectrum disorders. J Autism Dev Disord, 45(2), 473–480. doi: 10.1007/s10803-013-1926-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabard-Durnam LJ, Wilkinson C, Kapur K, Tager-Flusberg H, Levin AR, & Nelson CA (2019). Longitudinal EEG power in the first postnatal year differentiates autism outcomes. Nat Commun, 10(1), 4188. doi: 10.1038/s41467-019-12202-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R, Peterson EJ, & Voytek B (2017). Inferring synaptic excitation/inhibition balance from field potentials. Neuroimage, 158, 70–78. doi: 10.1016/j.neuroimage.2017.06.078 [DOI] [PubMed] [Google Scholar]
- Giserman Kiss I, Feldman MS, Sheldrick RC, & Carter AS (2017). Developing Autism Screening Criteria for the Brief Infant Toddler Social Emotional Assessment (BITSEA). J Autism Dev Disord, 47(5), 1269–1277. doi: 10.1007/s10803-017-3044-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowan B, Carter MJ, & Carter AS (2006). ITSEA/BITSEA: Infant-Toddler and Brief Infant-Toddler Social and Emotional Assessment. The Psychological Corporation. [Google Scholar]
- Gray PH, Edwards DM, O’Callaghan MJ, & Gibbons K (2015). Screening for autism spectrum disorder in very preterm infants during early childhood. Early Hum Dev, 91(4), 271–276. doi: 10.1016/j.earlhumdev.2015.02.007 [DOI] [PubMed] [Google Scholar]
- Haartsen R, Jones EJH, Orekhova EV, Charman T, Johnson MH, & team, B. (2019). Functional EEG connectivity in infants associates with later restricted and repetitive behaviours in autism; a replication study. Transl Psychiatry, 9(1), 66. doi: 10.1038/s41398-019-0380-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegens S, & Zion Golumbic E (2018). Rhythmic facilitation of sensory processing: A critical review. Neurosci Biobehav Rev, 86, 150–165. doi: 10.1016/j.neubiorev.2017.12.002 [DOI] [PubMed] [Google Scholar]
- Huberty S, Carter Leno V, van Noordt SJR, Bedford R, Pickles A, Desjardins JA, … Elsabbagh M (2021). Association between spectral electroencephalography power and autism risk and diagnosis in early development. Autism Res, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isler JR, Thai T, Myers MM, & Fifer WP (2016). An automated method for coding sleep states in human infants based on respiratory rate variability. Developmental Psychobiology, 58(8), 1108–1115. doi: 10.1002/dev.21482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S, Matthews R, Draper ES, Field DJ, Manktelow BN, Marlow N, … Boyle EM (2015). Early Emergence of Delayed Social Competence in Infants Born Late and Moderately Preterm. J Dev Behav Pediatr, 36(9), 690–699. doi: 10.1097/DBP.0000000000000222 [DOI] [PubMed] [Google Scholar]
- Jois RS (2019). Understanding long-term neurodevelopmental outcomes of very and extremely preterm infants: A clinical review. Aust J Gen Pract, 48(1–2), 26–32. doi: 10.31128/AJGP-04-18-4545 [DOI] [PubMed] [Google Scholar]
- Katzner S, Nauhaus I, Benucci A, Bonin V, Ringach DL, & Carandini M (2009). Local origin of field potentials in visual cortex. Neuron, 61(1), 35–41. doi: 10.1016/j.neuron.2008.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesnik A, Begum Ali J, Gliga T, Guiraud J, Charman T, Johnson MH, … Team, B. (2019). Increased cortical reactivity to repeated tones at 8 months in infants with later ASD. Transl Psychiatry, 9(1), 46. doi: 10.1038/s41398-019-0393-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruizinga I, Visser JC, van Batenburg-Eddes T, Carter AS, Jansen W, & Raat H (2014). Screening for autism spectrum disorders with the brief infant-toddler social and emotional assessment. PLoS One, 9(5), e97630. doi: 10.1371/journal.pone.0097630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin AR, Naples AJ, Scheffler AW, Webb SJ, Shic F, Sugar CA, … Senturk D (2020). Day-to-Day Test-Retest Reliability of EEG Profiles in Children With Autism Spectrum Disorder and Typical Development. Front Integr Neurosci, 14, 21. doi: 10.3389/fnint.2020.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin AR, Varcin KJ, O’Leary HM, Tager-Flusberg H, & Nelson CA (2017). EEG power at 3 months in infants at high familial risk for autism. J Neurodev Disord, 9(1), 34. doi: 10.1186/s11689-017-9214-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall PJ, Bar-Haim Y, & Fox NA (2002). Development of the EEG from 5 months to 4 years of age. Clinical Neurophysiology, 113(8), 1199–1208. doi:Pii S1388-2457(02)00163-3 Doi 10.1016/S1388-2457(02)00163-3 [DOI] [PubMed] [Google Scholar]
- Mason D, McConachie H, Garland D, Petrou A, Rodgers J, & Parr JR (2018). Predictors of quality of life for autistic adults. Autism Res, 11(8), 1138–1147. doi: 10.1002/aur.1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matousek M, & Petersen I (1974). Automatic Evaluation of Eeg Background Activity by Means of Age-Dependent Quotients. Electroencephalography and Clinical Neurophysiology, 36(1), 95–95. [DOI] [PubMed] [Google Scholar]
- McDonald NM, & Jeste SS (2021). Beyond Baby Siblings-Expanding the Definition of “High-Risk Infants” in Autism Research. Curr Psychiatry Rep, 23(6), 34. doi: 10.1007/s11920-021-01243-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musall S, von Pfostl V, Rauch A, Logothetis NK, & Whittingstall K (2014). Effects of neural synchrony on surface EEG. Cereb Cortex, 24(4), 1045–1053. doi: 10.1093/cercor/bhs389 [DOI] [PubMed] [Google Scholar]
- Nelson SB, & Valakh V (2015). Excitatory/Inhibitory Balance and Circuit Homeostasis in Autism Spectrum Disorders. Neuron, 87(4), 684–698. doi: 10.1016/j.neuron.2015.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orekhova EV, Elsabbagh M, Jones EJ, Dawson G, Charman T, Johnson MH, & Team B (2014). EEG hyper-connectivity in high-risk infants is associated with later autism. J Neurodev Disord, 6(1), 40. doi: 10.1186/1866-1955-6-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund BD, Alperin BR, Drew T, & Karalunas SL (2021). Behavioral and cognitive correlates of the aperiodic (1/f-like) exponent of the EEG power spectrum in adolescents with and without ADHD. Dev Cogn Neurosci, 48, 100931. doi: 10.1016/j.dcn.2021.100931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascal A, Govaert P, Oostra A, Naulaers G, Ortibus E, & Van den Broeck C (2018). Neurodevelopmental outcome in very preterm and very-low-birthweight infants born over the past decade: a meta-analytic review. Dev Med Child Neurol, 60(4), 342–355. doi: 10.1111/dmcn.13675 [DOI] [PubMed] [Google Scholar]
- Pertermann M, Bluschke A, Roessner V, & Beste C (2019). The Modulation of Neural Noise Underlies the Effectiveness of Methylphenidate Treatment in Attention-Deficit/Hyperactivity Disorder. Biol Psychiatry Cogn Neurosci Neuroimaging, 4(8), 743–750. doi: 10.1016/j.bpsc.2019.03.011 [DOI] [PubMed] [Google Scholar]
- Pickles A, Le Couteur A, Leadbitter K, Salomone E, Cole-Fletcher R, Tobin H, … Green J (2016). Parent-mediated social communication therapy for young children with autism (PACT): long-term follow-up of a randomised controlled trial. Lancet, 388(10059), 2501–2509. doi: 10.1016/S0140-6736(16)31229-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademeyer V, & Jacklin L (2013). A study to evaluate the performance of black South African urban infants on the Bayley Scales of Infant Development III. South African Journal of Child Health, 7(2), 54–59. [Google Scholar]
- Righi G, Tierney AL, Tager-Flusberg H, & Nelson CA (2014). Functional connectivity in the first year of life in infants at risk for autism spectrum disorder: an EEG study. PLoS One, 9(8), e105176. doi: 10.1371/journal.pone.0105176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva V, Marino C, Piazza C, Riboldi EM, Mornati G, Molteni M, & Cantiani C (2019). Paternal-but Not Maternal-Autistic Traits Predict Frontal EEG Alpha Asymmetry in Infants with Later Symptoms of Autism. Brain Sci, 9(12). doi: 10.3390/brainsci9120342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson MM, Furlong S, Voytek B, Donoghue T, Boettiger CA, & Sheridan MA (2019). EEG power spectral slope differs by ADHD status and stimulant medication exposure in early childhood. J Neurophysiol, 122(6), 2427–2437. doi: 10.1152/jn.00388.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche KJ, LeBlanc JJ, Levin AR, O’Leary HM, Baczewski LM, & Nelson CA (2019). Electroencephalographic spectral power as a marker of cortical function and disease severity in girls with Rett syndrome. J Neurodev Disord, 11(1), 15. doi: 10.1186/s11689-019-9275-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saby JN, & Marshall PJ (2012). The utility of EEG band power analysis in the study of infancy and early childhood. Dev Neuropsychol, 37(3), 253–273. doi: 10.1080/87565641.2011.614663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaha J, Iemi L, Haegens S, & Busch NA (2020). Spontaneous Brain Oscillations and Perceptual Decision-Making. Trends Cogn Sci, 24(8), 639–653. doi: 10.1016/j.tics.2020.05.004 [DOI] [PubMed] [Google Scholar]
- Sandin S, Lichtenstein P, Kuja-Halkola R, Larsson H, Hultman CM, & Reichenberg A (2014). The familial risk of autism. JAMA, 311(17), 1770–1777. doi: 10.1001/jama.2014.4144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaworonkow N, & Voytek B (2021). Longitudinal changes in aperiodic and periodic activity in electrophysiological recordings in the first seven months of life. Dev Cogn Neurosci, 47, 100895. doi: 10.1016/j.dcn.2020.100895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher MS, Steppe DA, & Banks DL (1996). Prediction of lower developmental performances of healthy neonates by neonatal EEG-sleep measures. Pediatr Neurol, 14(2), 137–144. doi: 10.1016/0887-8994(96)00013-6 [DOI] [PubMed] [Google Scholar]
- Shuffrey LC, Myers MM, Isler JR, Lucchini M, Sania A, Pini N, … Network, P. (2020). Association Between Prenatal Exposure to Alcohol and Tobacco and Neonatal Brain Activity: Results From the Safe Passage Study. JAMA Netw Open, 3(5), e204714. doi: 10.1001/jamanetworkopen.2020.4714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somsen RJM, vantKlooster BJ, vanderMolen MW, vanLeeuwen HMP, & Licht R (1997). Growth spurts in brain maturation during middle childhood as indexed by EEG power spectra. Biological Psychology, 44(3), 187–209. doi:Doi 10.1016/S0301-0511(96)05218-0 [DOI] [PubMed] [Google Scholar]
- Spitzer B, & Haegens S (2017). Beyond the Status Quo: A Role for Beta Oscillations in Endogenous Content (Re)Activation. eNeuro, 4(4). doi: 10.1523/ENEURO.0170-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer PE, van Toorn R, Laughton B, & Kidd M (2013). Characteristics of children with pervasive developmental disorders attending a developmental clinic in the Western Cape Province, South Africa. South African Journal of Child Health, 7(3). doi:doi.org/ 10.7196/sajch.530 [DOI] [Google Scholar]
- Talmi Z, Mankuta D, & Raz R (2020). Birth weight and autism spectrum disorder: A population-based nested case-control study. Autism Res, 13(4), 655–665. doi: 10.1002/aur.2260 [DOI] [PubMed] [Google Scholar]
- Voytek B, Kramer MA, Case J, Lepage KQ, Tempesta ZR, Knight RT, & Gazzaley A (2015). Age-Related Changes in 1/f Neural Electrophysiological Noise. J Neurosci, 35(38), 13257–13265. doi: 10.1523/JNEUROSCI.2332-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walani SR (2020). Global burden of preterm birth. Int J Gynaecol Obstet, 150(1), 31–33. doi: 10.1002/ijgo.13195 [DOI] [PubMed] [Google Scholar]
- Wang J, Barstein J, Ethridge LE, Mosconi MW, Takarae Y, & Sweeney JA (2013). Resting state EEG abnormalities in autism spectrum disorders. J Neurodev Disord, 5(1), 24. doi: 10.1186/1866-1955-5-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson CL, Gabard-Durnam LJ, Kapur K, Tager-Flusberg H, Levin AR, & Nelson CA (2020). Use of longitudinal EEG measures in estimating language development in infants with and without familial risk for autism spectrum disorder. Neurobiol Lang (Camb), 1(1), 33–53. doi: 10.1162/nol_a_00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson CL, & Nelson CA (2021). Increased aperiodic gamma power in young boys with Fragile X Syndrome is associated with better language ability. Mol Autism, 12(1), 17. doi: 10.1186/s13229-021-00425-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Peterson M, Beyer B, Frankel WN, & Zhang ZW (2014). Loss of MeCP2 from forebrain excitatory neurons leads to cortical hyperexcitation and seizures. J Neurosci, 34(7), 2754–2763. doi: 10.1523/JNEUROSCI.4900-12.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


