In 1907, Alois Alzheimer, a German psychiatrist and neuropathologist, described the case of Auguste Deter, a 51-year-old woman who was closely monitored for 5 years for symptoms of progressive presenile dementia. After her death, necropsy revealed several abnormalities in her brain, including cortical atrophy, arteriosclerotic changes in vascular tissues, and the abnormal deposition of “special substances” inside and outside of neurons, now identified as hyperphosphorylated tau tangles and amyloid-β (Aβ) plaques, respectively.1 Presently, Alzheimer's disease (AD) is considered the leading cause of dementia worldwide and is rapidly becoming one of the most costly, deadly, and burdensome diseases.2 Unfortunately, more than 100 years after its first description, we do not entirely understand how AD is initiated and what factors modulate its progression. At present, we know that sporadic AD (accounting for over 95% of all AD cases) can be linked with unmodifiable and modifiable risk factors. Aging is the most notorious risk factor for AD. Female sex and certain ethnicities have also been associated with a higher risk of developing this type of dementia. More recently, several pathological conditions such as type 2 diabetes, hypertension, and others have been linked with AD at physiological and molecular levels. Among them, an emerging risk factor for AD includes microbial infections.3 Basic and clinical data support the role of microbial infection in AD. Some of this evidence is discussed below.
One example found in the literature includes a multiscale analysis of three distinct patient cohorts demonstrating that human herpesvirus (HHV)-6A and HHV-7 disrupt molecular, genetic, and clinical networks more severely in AD patients than in non-demented individuals.4 Another study showed that individuals recently diagnosed with herpes simplex virus (HSV, n=8,362) had a risk ratio of 2.564 to develop dementia compared to non-HSV controls (n=25,086). Notably, this risk ratio decreased to 0.092 when the HSV-infected patients received anti-herpetic treatment,5 suggesting that the removal of pathogens results in an improved prognosis.
In the preclinical field, growing evidence suggests that infections might be associated with the accumulation of amyloid plaques in the brain, one of the hallmarks and perhaps the triggering event in AD. One example includes experiments involving transgenic Caenorhabditis elegans expressing the human Aβ1-42 residue. These animals, developing Aβ deposits over time, displayed reduced mortality compared to their non-transgenic counterparts when infected with Candida albicans.6 Additional support comes from experiments involving the intracerebral injection of Salmonella Typhimurium in transgenic 5XFAD mice, an animal model of accelerated amyloid pathology. Treated mice exhibited increased brain amyloid pathology compared to sham-treated mice. Moreover, Aβ deposits in Salmonella-treated 5XFAD co-localized with the invading bacteria, suggesting a direct interaction between both pathogenic entities (amyloid plaques and bacteria). Interestingly, the mortality rate induced by Salmonella sp. administration was reduced in 5XFAD mice, consistent with previous in vitro and in vivo evidence suggesting an antimicrobial role for Aβ aggregates.6 Corroborating the involvement of infection in AD pathogenesis, we have demonstrated that pneumococcal meningitis increased the expression of the receptor for advanced glycation endproducts (RAGE), a known contributor for Aβ production by enhancing the activity of secretases and neuroinflammation. We found that infection with Streptococcus pneumoniae also enhanced the generation of Aβ1-42, an Aβ fragment associated with increased fibrillogenesis, microglial cell activation, and memory impairment.7
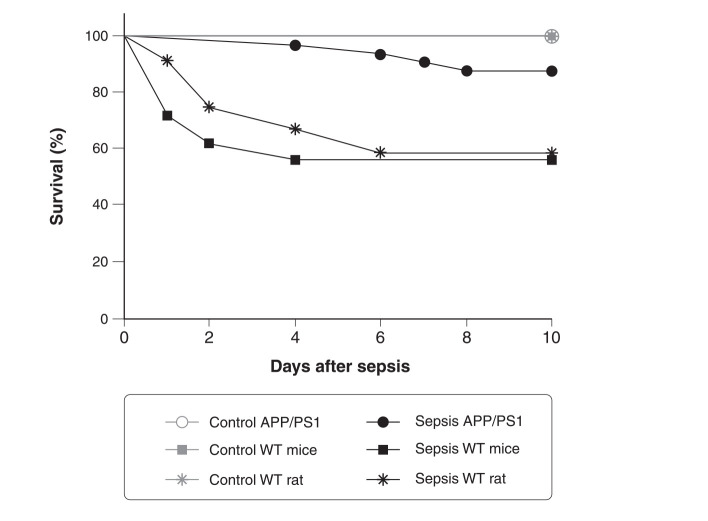
Similar results were observed in models of peripheral infection triggered by polymicrobial sepsis using cecal ligation and puncture (CLP) surgery, the gold-standard model of polymicrobial sepsis. These animals displayed an increase in Aβ production and higher Ser-202-phosphorylated Tau (p-TauSer-202, another indicator of AD pathology) and RAGE followed by cognitive impairment.8 In another study, APP/PS1 double transgenic mice that express human amyloid-β precursor protein (APP) and a mutant human presenilin (PS1) associated with early-onset AD, were subjected to CLP and their survival was measured over time. Usually, CLP triggers sepsis of moderate severity, with mortality rate in rodents of about 40%.8,9 Some crucial variables that influence the mortality rate of the animals include fluid resuscitation procedures, CLP conditions, the position of the ligation, the postoperative antibiotic regimen, and parenteral nutrition.9 Surprisingly, we found that APP/PS1 mice subjected to polymicrobial sepsis had a mortality rate of only 12.12%, while the mortality of CLP in wild-type mice was 44% (Figure 1). This was also observed in a rat model of sepsis, where wild-type Wistar rats had a mortality rate of 41.6% compared with the respective controls. In summary, our data show that wild-type C57BL/6 mice and Wistar rats had a threefold increase in mortality compared to APP/PS1 mice. Our results are in agreement with those found in the literature, demonstrating that Aβ provides protection during infection.
Figure 1. Kaplan-Meier survival curve of APP/PS1 mice, WT C57BL/6 mice, and Wistar rats subjected to experimental polymicrobial sepsis triggered by CLP. Control groups subjected to sham surgery were included for comparison. The animals were followed for 10 days and mortality was recorded (APP/PS1, sepsis n=32 vs. control n=16, p = 0.153; C57BL/6 mice, sepsis n=50 vs. control n=50, p < 0.001; Wistar rats, sepsis n=12 vs. control n=10, p = 0.02). The APP/PS1 mice subjected to sepsis had an 87.88% survival rate, WT mice had a survival rate of 56%, and Wistar rats had a survival rate of 58.33%. APP/PS1 = amyloid-β precursor protein/presenilin 1; CLP = cecal ligation and puncture.
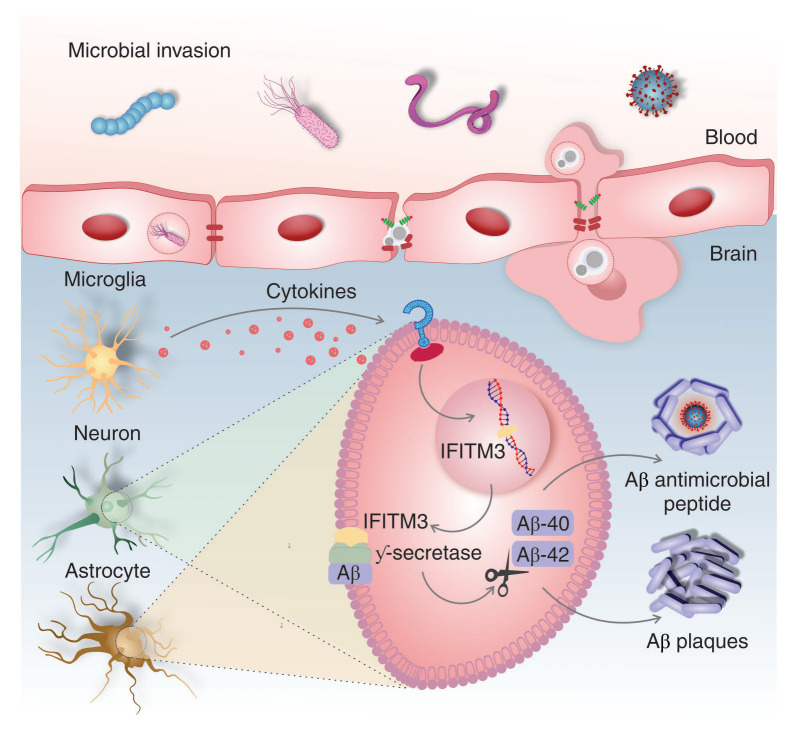
Neuroinflammation is a central component of AD pathogenesis; it contributes to disease development and neurodegeneration.2,3 Infection or inflammatory conditions derived from aging, as well as stroke, have been identified as AD risk factors,3 triggers of pro-inflammatory cytokine production in the brain, and drivers of neuroinflammation. Recently, an essential mechanism of cell signaling that associates neuroinflammation to Aβ production was identified. In the brain, insults or infection lead to production of pro-inflammatory cytokines, which in turn promote the formation of interferon-induced transmembrane protein 3 (IFITM3) in neurons and astrocytes. IFITM3 binds to γ-secretase, increasing its activity, and upregulates Aβ production.10Figure 2 provides a summary of the molecular cascade associated with this pathway.
Figure 2. Infection-induced inflammation as a potential trigger of Alzheimer’s disease pathology. Infection or inflammation activates microglial cells, triggering the release of pro-inflammatory cytokines. In sequence, the pro-inflammatory cytokines promote the formation of interferon-induced transmembrane protein 3 (IFITM3) in neurons and astrocytes, which binds to γ-secretase, increasing its activity and upregulating Aβ production. Released Aβ peptides have antimicrobial effects, preventing pathogen development by forming amyloid plaques. Brain Aβ deposition exacerbates the cerebral inflammatory response, accelerating AD pathological cascades. Aβ = amyloid-β; AD = Alzheimer’s disease.
Overall, several mechanisms link microbial infections and AD, including but not limited to Aβ misfolding and neuroinflammation. Additional research will help us clarify the specific molecular links between pathological events, explore the specificity of certain microbes with pathological variations in AD, and identify new preventive and therapeutic strategies to combat this fatal form of dementia.
Disclosure
The authors report no conflicts of interest.
Acknowledgements
This work was supported by The University of Texas Health Science Center at Houston. TB has received a grant from the Alzheimer's Association (AARGDNTF-19-619645). TB and RM have received grants from the National Institutes of Health/National Institute on Aging (NIH/NIA grant 1RF1AG072491).
Footnotes
How to cite this article: Barichello T, Giridharan VV, Comim CM, Morales R. What is the role of microbial infection in Alzheimer’s disease? Braz J Psychiatry. 2022;44:245-247. http://dx.doi.org/10.1590/1516-4446-2021-0037
References
- 1.Alzheimer A, Stelzmann RA, Schnitzlein HN, Murtagh FR. An English translation of Alzheimer's 1907 paper, “Uber eine eigenartige Erkankung der Hirnrinde.”. Clin Anat. 1995;8:429–31. doi: 10.1002/ca.980080612. [DOI] [PubMed] [Google Scholar]
- 2.Georges J, Miller O, Bintener C. Estimating the prevalence of dementia in Europe. Report N: ISBN 978-99959-995-9-9. Alzheimer Europe. 2020 Feb doi: 10.13140/RG.2.2.16880.81923. DOI: [DOI] [Google Scholar]
- 3.Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chételat G, Teunissen CE, et al. Alzheimer's disease. Lancet. 2021;397:1577–90. doi: 10.1016/S0140-6736(20)32205-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Readhead B, Haure-Mirande JV, Funk CC, Richards MA, Shannon P, Haroutunian V, et al. Multiscale analysis of independent Alzheimer's cohorts finds disruption of molecular, genetic, and clinical networks by human herpesvirus. Neuron. 2018;99:64–82.e7. doi: 10.1016/j.neuron.2018.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tzeng NS, Chung CH, Lin FH, Chiang CP, Yeh CB, Huang SY, et al. Anti-herpetic medications and reduced risk of dementia in patients with herpes simplex virus infections-a nationwide, population-based cohort study in Taiwan. Neurotherapeutics. 2018;15:417–29. doi: 10.1007/s13311-018-0611-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar DK, Choi SH, Washicosky KJ, Eimer WA, Tucker S, Ghofrani J, et al. Amyloid-β peptide protects against microbial infection in mouse and worm models of Alzheimer's disease. Sci Transl Med. 2016;8:340ra72. doi: 10.1126/scitranslmed.aaf1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giridharan VV, Generoso JS, Collodel A, Dominguini D, Faller CJ, Tardin F, et al. Receptor for Advanced Glycation End Products (RAGE) mediates cognitive impairment triggered by pneumococcal meningitis. Neurotherapeutics. 2021;18:640–53. doi: 10.1007/s13311-020-00917-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gasparotto J, Girardi CS, Somensi N, Ribeiro CT, Moreira JC, Michels M, et al. Receptor for advanced glycation end products mediates sepsis-triggered amyloid-β accumulation, Tau phosphorylation, and cognitive impairment. J Biol Chem. 2018;293:226–44. doi: 10.1074/jbc.M117.786756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4:31–6. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hur JY, Frost GR, Wu X, Crump C, Pan SJ, Wong E, et al. The innate immunity protein IFITM3 modulates γ-secretase in Alzheimer's disease. Nature. 2020;586:735–40. doi: 10.1038/s41586-020-2681-2. [DOI] [PMC free article] [PubMed] [Google Scholar]