Abstract
Objective:
The prevalence of sleep disorders during the perinatal period is high and large health administrative database surveys have shown that the use of exogenous melatonin in pregnant populations is quite common, about 4%. Much of the concern about using melatonin during pregnancy and breastfeeding stems from animal research. Thus, the objective of this article is to provide a critical review of human studies related to exogenous melatonin use during pregnancy and breastfeeding.
Methods:
The electronic databases Ovid, MEDLINE, Embase, and the Cochrane Library were searched using terms and keywords related to melatonin, pregnancy, and breastfeeding.
Results:
Fifteen studies were included in this review. Eight focused on melatonin use during pregnancy and seven focused on melatonin use during breastfeeding. There was a variety of study designs, including case reports, cohort studies, and clinical trials. There is a lack of randomized, controlled trials examining the efficacy and safety of melatonin as a treatment for sleep disorders during pregnancy or breastfeeding and, notably, insomnia was not the primary outcome measure in any of the studies included in this review. Clinical trials that used exogenous melatonin during pregnancy and breastfeeding for other clinical conditions have not suggested major safety concerns or adverse events.
Conclusion:
Contrary to what animal studies have suggested, evidence from clinical studies to date suggests that melatonin use during pregnancy and breastfeeding is probably safe in humans. This review further emphasizes the need for clinical studies on sleep disorders, including exogenous melatonin, during pregnancy and lactation.
Keywords: Melatonin, pregnancy, breastfeeding, clinical trials
Introduction
Sleep disturbances, including insomnia, are very common in pregnancy. Prevalence estimates of pregnant women with sleep disturbances range from 66 to 94%, with higher rates as pregnancy progresses into later trimesters.1,2 A variety of pregnancy-related hormonal and physiological changes could contribute to these sleep disturbances. Studies have shown that chronic sleep loss in pregnant women is associated with adverse pregnancy outcomes, including gestational diabetes, preeclampsia,3 and preterm birth,4 as well as adverse fetal outcomes.5 Increasing evidence also suggests that sleep disturbances may have significant consequences for mental health during pregnancy and the postpartum period. Insomnia during late pregnancy has been associated with co-morbid depressive symptoms,6 and poor sleep during both late pregnancy and the postpartum period have been found to be associated with postpartum depression symptom severity.7 In a systematic review of the relationship between sleep and postpartum depression, seven out of 10 studies that used subjective sleep assessments found a relationship between sleep disturbances in the 3rd trimester and the development of postpartum depression.8 Furthermore, 17 out of 20 studies that used subjective sleep assessments found a relationship between sleep disturbances in the postpartum period and the development of postpartum depression.8 Although the review found evidence for the relationship between self-reported sleep disturbances and postpartum depression, the evidence for the relationship between objectively assessed sleep and postpartum depression was mixed.8 Most of the research in this area has focused on postpartum depression, but one study also found an association between insomnia in late pregnancy and perinatal anxiety.9 Research on sleep during pregnancy and the postpartum period is of the utmost importance due to the high prevalence of sleep disturbances in this population. Moreover, this has a significant impact on other aspects of health, including postpartum psychiatric illness, which is one of the leading causes of maternal morbidity and mortality in the perinatal period.10
There is also evidence that treating sleep disturbances can prevent postpartum depressive symptoms. A randomized clinical trial treated insomnia during the 3rd trimester with either trazodone, diphenhydramine, or placebo, finding that trazodone and diphenhydramine significantly improved sleep and reduced postpartum depressive symptoms compared to placebo.11 As such, studying the safety and efficacy of treatments for disturbed sleep during pregnancy and breastfeeding is critical not only for improving sleep and thus quality of life in this population, but also for reducing postpartum depression. In addition, a number of non-pharmacological treatment options have been found to improve self-reported maternal sleep, including massage, exercise,12 and cognitive behavioral therapy for insomnia.13 There are also various pharmacotherapy options for insomnia and other sleep disorders, but human data regarding pregnancy and breastfeeding are largely lacking and, despite reassuring safety data for certain medications, potential concerns still include congenital anomalies and neonatal withdrawal.14
Melatonin is a hormone released by the pineal gland primarily to regulate the sleep-wake cycle. Exogenous melatonin supplementation is often used for short-term treatment of insomnia and sleep-wake cycle disturbances and is available over the counter in Canada and the United States, making it widely accessible. Melatonin use was reported by 0.9% of pregnant women in a psychiatrically ill population15 and, more generally, by about 4% of pregnant women aged 18 to 40 years old.16 Such high rates of use in pregnant populations highlight the importance of studying the safety and efficacy of melatonin use during pregnancy and breastfeeding. To date, the vast majority of the safety concerns about melatonin use during pregnancy have come from animal studies. For example, a study of antenatal melatonin treatment in pregnant sheep found potential adverse effects, including decreased birth weight and prolonged gestation.17 Another study of antenatal melatonin in rat dams found that it resulted in the mortality of most pups by 6 weeks of age.18 Conversely, other animal studies have found no adverse effects from maternal melatonin administration and instead show evidence of neuroprotective effects for offspring.19 Additionally, since endogenous melatonin plays a role in the development of fetal circadian rhythms by transferring information about maternal circadian rhythms through the placenta or breast milk,20 there are theoretical, but as yet unproven, concerns that exogenous melatonin use may alter this development in humans.21
Due to conflicting evidence from animal studies and the high rates of estimated use in pregnant women, there is a need to review and synthesize the available human studies of exogenous melatonin use during pregnancy and breastfeeding. The objective of this article is to provide a critical review of clinical studies related to exogenous melatonin use during pregnancy and breastfeeding.
Methods
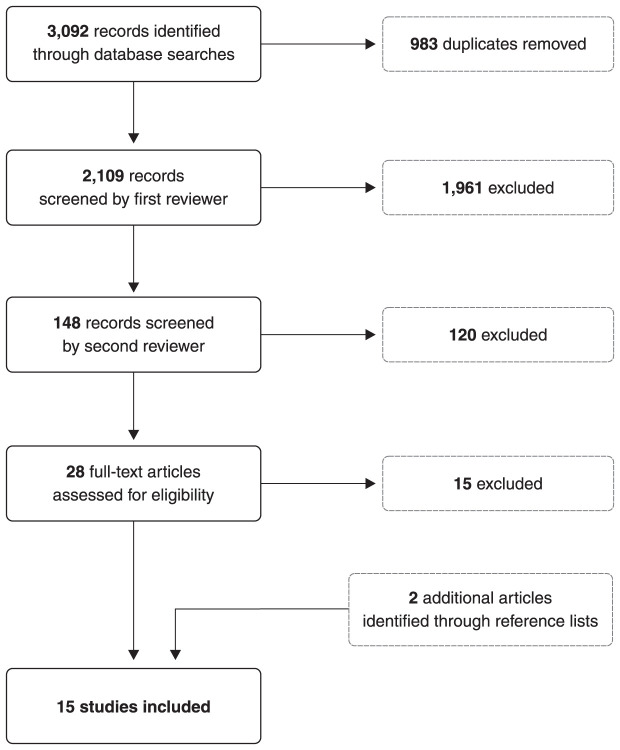
A literature review was conducted by searching the electronic databases Ovid MEDLINE, Embase, and Cochrane Library using appropriate Medical Subject Heading (MeSH) terms and keywords related to melatonin, pregnancy, and breastfeeding. The specific terms used for the search strategy included the MeSH descriptors “pregnancy or breast feeding or prenatal care or postnatal care” or keywords “pregnan* or prenatal or postnatal or breast feed* or breastfeed* or gestation*” and MeSH descriptor “melatonin” or keyword “melatonin”. Duplicates were removed from the database search results, and the records were screened by a first independent reviewer (TV), followed by a second independent reviewer (BF) (Figure 1). The selected articles were clinical studies that focused on melatonin use during pregnancy or breastfeeding. No study designs were excluded, so any relevant case reports, case series, cohort studies, cross-sectional studies, open label studies, and randomized controlled trials were included. The reference lists of articles that received a full-text review were also examined to identify any additional references that would be relevant to this review. Through this methodological approach, a total of 15 articles were included.
Figure 1. Flowchart of review methodology.
Results
Of the 15 studies included in this review, eight focused on melatonin use during pregnancy15,16,22-27 and seven focused on melatonin use during breastfeeding.28-34 Seven of the pregnancy studies and three of the lactation studies involved exogenous melatonin. The breakdown of study designs of the included studies is as follows: five cross-sectional studies, two randomized controlled trials, two experimental studies, two Phase I clinical trials, one case study, one dose-ranging study, one feasibility study, and one cohort study. Notably, insomnia was not the primary outcome in any of these studies. Three studies actively measured congenital anomalies, obstetric, and/or neonatal complications as outcomes.
Melatonin use during pregnancy
Two of the included studies focused on the epidemiology of melatonin use during pregnancy.15,16 A large U.S. national survey of 2,217,213 pregnant women aged 18 to 40 years old found that about 4% reported using melatonin in the past 12 months.16 Another study that surveyed 534 pregnant women in a psychiatrically ill population reported that 0.9% used melatonin.15
Three clinical trials have investigated the use of melatonin as an antioxidant treatment for various conditions during pregnancy.22-24 A randomized controlled trial compared the effects of melatonin and quercetin, a flavonoid supplement, in 180 pregnant women with hyperglycemia.22 Women in the melatonin group received 10 mg daily from the 15th week to the 33rd week of pregnancy.22 Overall, a composite of six neonatal complications was significantly lower in the melatonin group than the quercetin group.22 Three of the neonatal complications (hypoglycemia, stillbirth or neonatal death, and birth injury) were not observed in any neonates of the melatonin group.22 The other three complications (need for phototherapy, respiratory distress, and macrosomia) were seen in small percentages of the melatonin group neonates.22 There was also significantly better maternal glycemic control in women treated with melatonin.22 This study concluded that melatonin is clinically more efficacious than quercetin for treating pregnant women with hyperglycemia and did not find any safety concerns regarding the use of melatonin during pregnancy.22
Another study explored the use of melatonin as an antioxidant treatment for preeclampsia.23 After results from the in vitro placental explant model showed that melatonin reduced oxidative stress and enhanced antioxidant markers, a phase I clinical trial was initiated for 20 pregnant women with early-onset preeclampsia to receive 30 mg total of melatonin daily (taken as 10 mg three times per day) from diagnosis until delivery.23 This trial found that melatonin was safe for women with preeclampsia and their fetuses, and did not report any adverse events or adverse drug reactions in the mothers, fetuses, or neonates.23 The women did not report increased daytime drowsiness, which is another important safety finding.23 The trial also found that melatonin significantly extended the mean diagnosis-to-delivery interval by 6 days and decreased the need for antihypertensive medications compared to historical controls.23 The neonates of melatonin-treated women did have a higher rate of being small for gestational age at birth than historical controls; however, the fetuses also had a higher rate of being small for gestational age at time of recruitment, before the initiation of melatonin treatment, and no other neonatal outcomes differed from controls.23
The final study using melatonin as an antioxidant treatment was for intrauterine growth restriction (IUGR).24 This phase I clinical trial of 12 pregnant women with severe early onset IUGR involved a melatonin treatment group and a historical control cohort.24 Women in the melatonin group received 8 mg total daily (4 mg twice per day) from recruitment until delivery, and the trial reported no adverse maternal or fetal effects associated with melatonin administration.24 Furthermore, the trial found that in placentae collected at birth, the concentration of placental malondialdehyde, a marker of oxidative stress, was significantly lower in women treated with melatonin than controls.24
The three remaining studies of melatonin use during pregnancy focused on aspects of the placental transfer of melatonin.25-27 One study obtained placentae from three normal, term pregnant women at delivery, isolated a single cotyledon, and perfused it to create an isolated perfused single cotyledon model to investigate the transfer of antioxidants, including melatonin, across full term normal human placenta.25 Based on values seen in pregnancy, 200 pmol/L of melatonin was administered to the maternal compartment, and it crossed the placenta rapidly and similarly to the freely diffusible comparator marker.25 Equilibration of melatonin concentration between the maternal and fetal compartments was reached within 150 minutes, without significant metabolism of the melatonin.25
A study of serum melatonin concentrations included 30 women with normal singleton pregnancies, as well as 16 women with twin pregnancies, 19 preeclampsia pregnancies, 14 IUGR pregnancies, and seven non-pregnant controls.26 Nighttime serum melatonin levels were consistently higher than daytime levels throughout pregnancy, with nighttime levels of normal singleton pregnant women gradually increasing after 24 weeks to become significantly higher after 32 weeks, compared to non-pregnancy, < 24-week pregnancy, and puerperium values, which returned to non-pregnant levels by the 2nd day of puerperium.26 In twin pregnancies, nighttime melatonin levels were significantly higher after 28 weeks and, conversely, in IUGR pregnancies melatonin levels were generally (but not significantly) lower after 36 weeks, both compared to normal singleton pregnancies.26 In women with severe preeclampsia, melatonin levels were significantly lower than in women with mild preeclampsia or women with normal pregnancies after 32 weeks.26 Finally, samples were also collected from the umbilical arteries and veins after delivery, and melatonin levels tended to be higher in the umbilical artery than umbilical vein.26
Looking more specifically at maternal-fetal transfer of melatonin near term, another study collected samples from a maternal vein, the umbilical artery, and the umbilical vein at the time of birth.27 This study included 12 women who spontaneously delivered vaginally and did not receive any melatonin, as well as 33 women who delivered by elective caesarean section and received 3 mg of melatonin either 1, 2, 3, or 4 hours before the delivery.27 There was also a third group of pregnant women at term who received 3 mg of melatonin when they were not in pain and had blood samples taken every hour for the next 4 hours, which showed that oral melatonin administration led to increased serum melatonin levels that reached a maximum 2 hours after administration and then significantly decreased over the following 2 hours.27 The results from the women who delivered vaginally showed that serum melatonin levels in the maternal and umbilical veins did not differ significantly, and the concentration in the umbilical vein was significantly and closely correlated with the concentration in the maternal vein.27 In the women who received melatonin prior to caesarean section, changes in serum melatonin levels in the umbilical artery and vein were correlated with those in the maternal vein, suggesting that maternally administered melatonin is transferred easily and rapidly to fetal circulation at term.27
Melatonin use during breastfeeding
Two studies included in the present review investigated the rhythm of melatonin in human milk.28,29 The first study found that in 10 mothers of neonates sampled 3 to 4 days after delivery, melatonin was not present at a detectable level (< 43 pmol/L) in either blood or breast milk during the day, but the melatonin level rose to 280±34 pmol/L in serum and 99±26 pmol/L in breast milk at night.28 The melatonin concentration in breast milk was on average about 35% of the serum concentration.28 Individual profiles of 24-h breast milk melatonin levels collected within 3 months after delivery also demonstrated a pronounced daily rhythm, with slight timing differences between individuals.28 The second study of melatonin rhythm collected breast milk samples from 42 women at three different stages of maturity: postpartum day 3 (colostrum), day 10 (transitional milk), and day 30 (mature milk).29 Results for all three stages showed that the melatonin concentration was higher in nocturnal samples than diurnal samples.29 In diurnal samples, the melatonin concentration was significantly lower in mature milk than in colostrum or transitional milk.29 In nocturnal samples, the melatonin concentration was significantly higher in transitional milk than in colostrum.29
This review included one study that focused on the stability of melatonin in human milk.30 This study also found that melatonin levels were significantly higher in nighttime milk than daytime milk.30 Milk samples were collected from 13 women during the first 6 months of breastfeeding and were immediately frozen, then defrosted after about 4 months and assayed to mimic real-life conditions of freezing, storing, and defrosting milk.30 Melatonin levels in milk were not significantly different immediately after defrosting or after 1, 2, 3, 4, or 24 hours, although they had increased variability 24 hours after defrosting.30 This study concluded that melatonin is stable in breast milk for at least 4 hours (even up to 24 hours) after defrosting.30
Another study compared melatonin levels in the breast milk of 5 women and in 3 artificial formulas, finding that melatonin followed a clear circadian rhythm in breast milk but was undetectable in all artificial formulas.31 They also applied a questionnaire to 94 mothers, finding that exclusively breast-fed infants had a significantly lower incidence of colic attacks and a lower severity and frequency of irritability attacks than formula-fed infants.31 The breast-fed infants also trended toward longer nocturnal sleep duration, although this was not significant, and they aroused significantly more times during the night.31 Combining the questionnaire and the milk/formula analysis results, the authors suggested that the reason breastfeeding was more advantageous than formula regarding infantile colic may be the role breast milk plays in melatonin absorption through the infant’s gastrointestinal tract.31
Only one included study discussed exogenous melatonin use by a breastfeeding mother.32 This was a case study of an 18-month-old child who had a history of bleeding episodes since birth. The episodes were associated with a reduced platelet aggregation time after breastfeeding but a normal aggregation time in a fasting state.32 The child’s mother occasionally used melatonin during and after pregnancy (unspecified dose and frequency), so maternal melatonin intake was suspended. After 3 months, platelet aggregation returned to normal and there were no further bleeding episodes.32 The authors suggested that one potential side effect of maternal melatonin use was to induce non-fatal bleeding in infants through breastfeeding.32
The final two studies investigated the pharmacokinetics of melatonin in preterm neonates.33,34 In the first study, 18 preterm infants (born with less than 31 weeks of gestation and less than 7 days old) received intravenous infusions of melatonin in the following dose regimens: 0.1 µg kg-1 h-1 for 6 hours, 0.1 µg kg-1 h-1 for 2 hours, 0.02 µg kg-1 h-1 for 2 hours, and 0.01 µg kg-1 h-1 for 2 hours, and 0.04 µg kg-1 h-1 for 30 minutes.33 Baseline melatonin in the infants was largely undetectable, but the dose regimen of 0.1 µg kg-1 h-1 for 2 hours achieved melatonin concentrations closest to adult physiological concentrations.33 Regarding the pharmacokinetic results, melatonin clearance was 0.045 L/h, the volume of distribution was 1.098 L, and the half-life was 16.91 hours, with race and sex as significant covariates.33 No adverse events were reported.33 These results revealed that, compared to adults and older children, both the clearance and half-life of melatonin are prolonged and the volume of distribution is decreased in preterm infants, which may have implications for dosing in this population.33 The second study administered oral melatonin to 15 preterm infants (born less than 37 weeks’ gestation and 24-72 hours old) in the following dose regimens: one intragastric bolus of 0.5 mg kg-1, three intragastric boluses of 1 mg kg-1 at 24-hour intervals, and three intragastric boluses of 5 mg kg-1 at 24-hour intervals.34 Regarding the pharmacokinetic results, the half-life in plasma ranged from 7.98 to 10.94 hours, the area under the curve ranged from 10.48 to 118.17 µg mL-1 h-1, and the time to reach maximum concentration ranged from 2.91 to 4.70 hours.34 This study also concluded that the pharmacokinetic profile of melatonin in premature neonates is different than in adults, with a prolonged half-life and time to maximum concentration, and that a single oral melatonin dose repeated every 12 or 24 hours could be used to obtain and maintain high serum concentrations for therapeutic purposes in preterm infants.34
Discussion
Studies investigating melatonin use during pregnancy have revealed a number of findings. Firstly, the proportion of psychiatrically ill pregnant women who report melatonin use is quite significant (approximately 1%)15 and is even higher in the general pregnant population (approximately 4%).16 This highlights the importance of studying the safety and efficacy of exogenous melatonin use for both maternal and fetal outcomes. Secondly, there is evidence that maternally administered exogenous melatonin crosses the placenta easily and rapidly at or near term, similar to a freely diffusible marker and without significant metabolism.25,27 Serum melatonin levels peak 2 hours after administration27 and maternal and fetal concentrations reach equilibrium within 150 minutes.25 These findings about the placental transfer of melatonin demonstrate that maternal administration can affect the fetus, and thus this topic should be clinically studied. As for endogenous melatonin, serum nighttime levels significantly increase later in pregnancy before decreasing postpartum. These levels are even higher in twin pregnancies but, conversely, are significantly lower in severe preeclampsia pregnancies.26 Thus, serum melatonin levels vary throughout pregnancy, as well as between different conditions associated with pregnancy and, although the mechanism for this is not well understood, it may represent a potential therapeutic target.26
In terms of clinical trials, the present review found no trials whose primary outcomes were the safety or efficacy of melatonin for insomnia or other sleep disorders during pregnancy. All three clinical trials in this review used melatonin as an antioxidant treatment for conditions during pregnancy, specifically hyperglycemia,22 preeclampsia,23 and IUGR.24 Each of these trials reported that melatonin had some efficacy for each condition, although sample sizes were relatively small. Importantly, none of the three trials reported safety concerns or adverse maternal or fetal events related to melatonin administration during pregnancy.22-24 Of note, the dose of melatonin used in these trials ranged from 8 to 30 mg daily, whereas over-the-counter melatonin is typically available in tablets ranging from 1 to 10 mg, with 10 mg marketed as maximum strength. The lack of safety concerns in trials with higher doses suggests that safety concerns might be even less likely in over-the-counter doses. Furthermore, the trial with the highest daily dose (30 mg) reported no increased daytime drowsiness, which is another important safety finding about sleep disorder treatments.23 Overall, the results of the eight included studies on melatonin use during pregnancy revealed that it is common and that it crosses the placenta easily and rapidly, although it has not been associated with safety concerns or adverse events in clinical trials to date. However, there is a clear lack of clinical trials investigating the safety and efficacy of melatonin for sleep disorders during pregnancy, and this review highlights the need for well-powered clinical trials in this area.
Regarding melatonin and breastfeeding, melatonin in breast milk clearly follows a circadian rhythm, with higher levels at night and lower levels during the day, which has been reported by multiple studies.28-31 This rhythm is consistent over the stages of breast milk maturation, although there are slight differences between stages, notably that nocturnal levels are higher in transitional milk than colostrum.29 The concentration of melatonin in breast milk is about 35% of the serum concentration,28 and melatonin in breast milk is stable in real-life conditions of freezing, storing, and defrosting.30 Regarding potential benefits, one study suggested that the melatonin in breast milk may be associated with lower severity and frequency of infantile colic.31 Conversely, a case study of a breastfeeding mother using exogenous melatonin found a possible association with non-fatal bleeding episodes in the child.32 The present review found no clinical trials investigating safety or efficacy as an outcome of exogenous melatonin use during breastfeeding, revealing a significant gap in the literature. However, studies of exogenous melatonin administration directly to preterm infants reported no adverse events.33,34 Furthermore, the pharmacokinetic profile of melatonin in premature infants is different than in adults, with prolonged clearance, half-life, and time to maximum concentration, as well as decreased volume of distribution.33,34 This highlights why it is critical to study melatonin use in breastfeeding mothers and its potential effects on their infants.
Due to the scarcity of clinical trials of melatonin use during pregnancy and breastfeeding, especially trials with outcomes related to safety and efficacy in sleep disorders, no conclusions can be drawn from the available evidence. However, the present review does reveal that no major safety concerns or adverse events have been reported in clinical studies, with the exception of one case report.32 Thus, although we cannot strongly conclude that melatonin use is safe during pregnancy and breastfeeding, the available evidence from clinical studies suggests that melatonin use is probably safe in humans. The safety concerns about melatonin use in pregnancy originated from animal studies and include decreased birth weight,17 altered circadian rhythm development,21 and mortality.18 Since none of these concerns were identified in the clinical studies included in this review, there is no evidence to substantiate these concerns in humans. Furthermore, there have been multiple examples of medications that were teratogenic in animal models but not in humans.35 For example, although corticosteroids were found to cause oral clefts in mice,36 they are now commonly used in humans during both early and mid-to-late pregnancy.37 Therefore, the significance of animal studies should be kept in perspective, especially since the clinical literature about melatonin use during pregnancy and breastfeeding continues to grow. A recent review revealed that safety and efficacy data are largely lacking regarding various other pharmacotherapy options for insomnia during pregnancy and breastfeeding, and current evidence from animal and human studies is mixed.14 Ultimately, the current review serves to further emphasize the need for robust clinical studies of sleep disorders in pregnant and breastfeeding populations, including exogenous melatonin, given its prevalent use in the general population and in individuals with psychiatric disorders.
Disclosure
The authors report no conflicts of interest.
Footnotes
How to cite this article: Vine T, Brown GM, Frey BN. Melatonin use during pregnancy and lactation: a scoping review of human studies. Braz J Psychiatry. 2022;44:342-348. http://dx.doi.org/10.1590/1516-4446-2021-2156
References
- 1.Schweiger MS. Sleep disturbance in pregnancy. A subjective survey. Am J Obstet Gynecol. 1972;114:879–82. doi: 10.1016/0002-9378(72)90091-9. [DOI] [PubMed] [Google Scholar]
- 2.Suzuki S, DennersteinL, Greenwood KM, Armstrong SM, Satohisa E. Sleeping patterns during pregnancy in Japanese women. J Psychosom Obstet Gynaecol. 1994;15:19–26. doi: 10.3109/01674829409025625. [DOI] [PubMed] [Google Scholar]
- 3.Hayase M, Shimada M, Seki H. Sleep quality and stress in women with pregnancy-induced hypertension and gestational diabetes mellitus. Women Birth. 2014;27:190–5. doi: 10.1016/j.wombi.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Warland J, Dorrian J, Morrison JL, O’Brien LM. Maternal sleep during pregnancy and poor fetal outcomes: a scoping review of the literature with meta-analysis. Sleep Med Rev. 2018;41:197–219. doi: 10.1016/j.smrv.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Palagini L, Gemignani A, Banti S, Manconi M, Mauri M, Riemann D. Chronic sleep loss during pregnancy as a determinant of stress: impact on pregnancy outcome. Sleep Med. 2014;15:853–9. doi: 10.1016/j.sleep.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Dørheim SK, Bjorvatn B, Eberhard-Gran M. Insomnia and depressive symptoms in late pregnancy: a population-based study. Behav Sleep Med. 2012;10:152–66. doi: 10.1080/15402002.2012.660588. [DOI] [PubMed] [Google Scholar]
- 7.Park EM, Meltzer-Brody S, Stickgold R. Poor sleep maintenance and subjective sleep quality are associated with postpartum maternal depression symptom severity. Arch Womens Ment Health. 2013;16:539–47. doi: 10.1007/s00737-013-0356-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lawson A, Murphy KE, Sloan E, Uleryk E, Dalfen A. The relationship between sleep and postpartum mental disorders: a systematic review. J Affect Disord. 2015;176:65–77. doi: 10.1016/j.jad.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 9.Osnes RS, Roaldset JO, Follestad T, Eberhard-Gran M. Insomnia late in pregnancy is associated with perinatal anxiety: a longitudinal cohort study. J Affect Disord. 2019;248:155–65. doi: 10.1016/j.jad.2019.01.027. [DOI] [PubMed] [Google Scholar]
- 10.Austin MP, Kildea S, Sullivan E. Maternal mortality and psychiatric morbidity in the perinatal period: challenges and opportunities for prevention in the Australian setting. Med J Aust. 2007;186:364–7. doi: 10.5694/j.1326-5377.2007.tb00940.x. [DOI] [PubMed] [Google Scholar]
- 11.Khazaie H, Ghadami MR, Knight DC, Emamian F, Tahmasian M. Insomnia treatment in the third trimester of pregnancy reduces postpartum depression symptoms: a randomized clinical trial. Psychiatry Res. 2013;210:901–5. doi: 10.1016/j.psychres.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 12.Owais S, Chow CH, Furtado M, Frey BN, Van Lieshout RJ. Non-pharmacological interventions for improving postpartum maternal sleep: a systematic review and meta-analysis. Sleep Med Rev. 2018;41:87–100. doi: 10.1016/j.smrv.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Felder JN, Epel ES, Neuhaus J, Krystal AD, Prather AA. Efficacy of digital cognitive behavioral therapy for the treatment of insomnia symptoms among pregnant women: a randomized clinical trial. JAMA Psychiatry. 2020;77:484–92. doi: 10.1001/jamapsychiatry.2019.4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller MA, Mehta N, Clark-Bilodeau C, Bourjeily G. Sleep pharmacotherapy for common sleep disorders in pregnancy and lactation. Chest. 2020;157:184–97. doi: 10.1016/j.chest.2019.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freeman MP, Sosinsky AZ, Moustafa D, Viguera AC, Cohen LS. Supplement use by women during pregnancy: data from the Massachusetts General Hospital National pregnancy registry for atypical antipsychotics. Arch Womens Ment Health. 2016;19:437–41. doi: 10.1007/s00737-015-0586-0. [DOI] [PubMed] [Google Scholar]
- 16.Chung S, Yeh T, Wu CH. Trend and pattern of herb and supplement use among pregnant women in the United States: findings from the 2002, 2007, and 2012 US National Health Interview Surveys. Am J Obstet Gynecol. 2017;216:189–90. doi: 10.1016/j.ajog.2016.11.1019. [DOI] [PubMed] [Google Scholar]
- 17.González-Candia A, Veliz M, Araya C, Quezada S, Ebensperger G, Serón-Ferré M, et al. Potential adverse effects of antenatal melatonin as a treatment for intrauterine growth restriction: findings in pregnant sheep. Am J Obstet Gynecol. 2016;215:245.e1–7. doi: 10.1016/j.ajog.2016.02.040. [DOI] [PubMed] [Google Scholar]
- 18.Singh HJ, Keah LS, Kumar A, Sirajudeen KN. Adverse effects of melatonin on rat pups of Wistar-Kyoto dams receiving melatonin supplementation during pregnancy. Exp Toxicol Pathol. 2012;64:751–2. doi: 10.1016/j.etp.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 19.Rubio AP, Correa F, Aisemberg J, Dorfman D, Bariani MV, Rosenstein RE, et al. Maternal administration of melatonin exerts short- and long-term neuroprotective effects on the offspring from lipopolysaccharide-treated mice. J Pineal Res. 2017;63 doi: 10.1111/jpi.12439. [DOI] [PubMed] [Google Scholar]
- 20.Tamura H, Nakamura Y, Terron MP, Flores LJ, Manchester LC, Tan DX, et al. Melatonin and pregnancy in the human. Reprod Toxicol. 2008;25:291–303. doi: 10.1016/j.reprotox.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Naitoh N, Watanabe Y, Matsumura K, Murai I, Kobayashi K, Imai-Matsumura K, et al. Alteration by maternal pinealectomy of fetal and neonatal melatonin and dopamine D1 receptor binding in the suprachiasmatic nuclei. Biochem Biophys Res Commun. 1998;253:850–4. doi: 10.1006/bbrc.1998.9819. [DOI] [PubMed] [Google Scholar]
- 22.Fang JH, Zhang SH, Yu XM, Yang Y. Effects of quercetin and melatonin in pregnant and gestational diabetic women. Lat Am J Pharm. 2016;35:1420–5. [Google Scholar]
- 23.Hobson SR, Gurusinghe S, Lim R, Alers NO, Miller SL, Kingdom JC, et al. Melatonin improves endothelial function in vitro and prolongs pregnancy in women with early‐onset preeclampsia. J Pineal Res. 2018;65:e12508. doi: 10.1111/jpi.12508. [DOI] [PubMed] [Google Scholar]
- 24.Miller SL, Yawno T, Alers NO, Castillo‐Melendez M, Supramaniam VG, VanZyl N, et al. Antenatal antioxidant treatment with melatonin to decrease newborn neurodevelopmental deficits and brain injury caused by fetal growth restriction. J Pineal Res. 2014;56:283–94. doi: 10.1111/jpi.12121. [DOI] [PubMed] [Google Scholar]
- 25.Schenker S, Yang Y, Perez A, Acuff RV, Papas AM, Henderson G, et al. Antioxidant transport by the human placenta. Clin Nutr. 1998;17:159–67. doi: 10.1016/s0261-5614(98)80052-6. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura Y, Tamura H, Kashida S, Takayama H, Yamagata Y, Karube A, et al. Changes of serum melatonin level and its relationship to feto‐placental unit during pregnancy. J Pineal Res. 2001;30:29–33. doi: 10.1034/j.1600-079x.2001.300104.x. [DOI] [PubMed] [Google Scholar]
- 27.Okatani Y, Okamoto K, Hayashi K, Wakatsuki A, Tamura S, Sagara Y. Maternal‐fetal transfer of melatonin in pregnant women near term. J Pineal Res. 1998;25:129–34. doi: 10.1111/j.1600-079x.1998.tb00550.x. [DOI] [PubMed] [Google Scholar]
- 28.Illnerova H, Buresova M, Presl J. Melatonin rhythm in human milk. J Clin Endocrinol Metab. 1993;77:838–41. doi: 10.1210/jcem.77.3.8370707. [DOI] [PubMed] [Google Scholar]
- 29.Silva NA, Honorio-Franca AC, Giachini FR, Mores L, de Souza EG, Franca EL. Bioactive factors of colostrum and human milk exhibits a day-night variation. Am J Immunol. 2013;9:68–74. [Google Scholar]
- 30.Molad M, Ashkenazi L, Gover A, Lavie-Nevo K, Zaltsberg-Barak T, Shaked-Mishan P, et al. Melatonin stability in human milk. Breastfeed Med. 2019;14:680–2. doi: 10.1089/bfm.2019.0088. [DOI] [PubMed] [Google Scholar]
- 31.Engler AC, Hadash A, Shehadeh N, Pillar G. Breastfeeding may improve nocturnal sleep and reduce infantile colic: potential role of breast milk melatonin. Eur J Pediatr. 2012;171:729–32. doi: 10.1007/s00431-011-1659-3. [DOI] [PubMed] [Google Scholar]
- 32.Luciani M, Massoud M, Foligno S, Di Felice G, Pulcinelli F, Rapini N. Melatonin antiplatelets effect through breastfeeding: a sobering case. Haemophilia. 2019;25:138–9. [Google Scholar]
- 33.Merchant NM, Azzopardi DV, Hawwa AF, McElnay JC, Middleton B, Arendt J, et al. Pharmacokinetics of melatonin in preterm infants. Br J Clin Pharmacol. 2013;76:725–33. doi: 10.1111/bcp.12092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carloni S, Proietti F, Rocchi M, Longini M, Marseglia L, D’Angelo G, et al. Melatonin pharmacokinetics following oral administration in preterm neonates. Molecules. 2017;22:2115. doi: 10.3390/molecules22122115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brent RL. Utilization of animal studies to determine the effects and human risks of environmental toxicants (drugs, chemicals, and physical agents) Pediatrics. 2004;113:984–95. [PubMed] [Google Scholar]
- 36.Walker BE, Fraser FC. The embryology of cortisone-induced cleft palate. J Embryol Exp Morphol. 1957;5:201–9. [Google Scholar]
- 37.Kemp MW, Newnham JP, Challis JG, Jobe AH, Stock SJ. The clinical use of corticosteroids in pregnancy. Hum Reprod Update. 2016;22:240–59. doi: 10.1093/humupd/dmv047. [DOI] [PubMed] [Google Scholar]