Abstract
The existence of a viable but nonculturable (VBNC) state has been described for Campylobacter jejuni as it had been for a number pathogenic bacteria. Three C. jejuni human isolates were suspended in surface water and subsequently entered the VBNC state. After starvation for 30 days, VBNC cells were inoculated in the yolk sacs of embryonated eggs. Culturable cells were detected in a large proportion of the embryonated eggs inoculated with VBNC C. jejuni cells. Recovered cells kept their adhesion properties.
Campylobacter jejuni is an enteropathogenic and food-borne agent which causes diarrhea and enteritis in humans. In recent years, a marked increase in the incidence of enteric campylobacteriosis has been reported in many countries (29). The ability to enter a viable but nonculturable (VBNC) state has been described for several enteric pathogens, including Salmonella enteritidis (26), enterotoxigenic Escherichia coli (12), Vibrio vulnificus (21), Vibrio cholerae (31), and C. jejuni (25). VBNC cells cannot be detected by standard culture methods. The VBNC state represents a survival strategy in response to environmental stress, with VBNC bacteria being capable of retaining virulence (8, 9, 17, 25). Thus, enteropathogenic VBNC bacteria can be a potential public health threat. The VBNC state of C. jejuni was first described by Rollins and Colwell (25), who showed that these bacteria enter a nonculturable state in response to environmental conditions not conducive to active growth and cell division. Several studies have been conducted to explore recovery of VBNC C. jejuni cells to active growth. However, the pathogenicity of C. jejuni nonculturable cells remains controversial. Some authors have described the possibility of recovering VBNC cells of C. jejuni by animal passage (15, 27, 28). Other investigators were unable to recover VBNC C. jejuni cells after animal passage and regarded these cells as degenerative forms, without any role in the environmental transmission of C. jejuni (2, 18, 30).
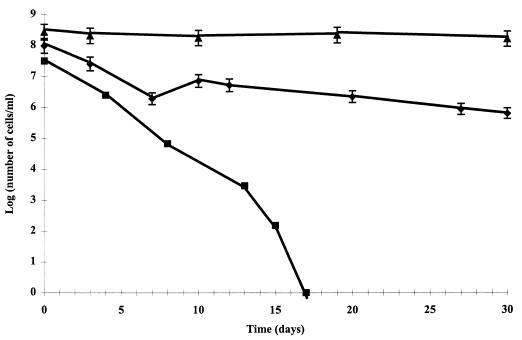
Three human isolates of C. jejuni, Bf, 79, and 85, identified as C. jejuni subsp. jejuni, were used in this study. These strains were chosen from among 36 strains tested for entering the VBNC state when they were incubated in filtered, sterilized surface water (6). The microcosm water system described by Rollins and Colwell (25) was used to obtain VBNC cells of C. jejuni. Cells were grown on Columbia agar, collected, and immediately suspended in 1-liter bottles containing 500 ml of filter-sterilized surface water adjusted to a pH of 6 ± 0.1 (mean ± standard deviation) to obtain a final concentration of 108 bacteria ml−1 as determined by acridine orange direct counting. At fixed times, samples from the C. jejuni suspensions were collected for culturable-, total-, and active-cell counting. Culturability was assayed by spread plate counting on 5% lysed horse blood–Columbia agar. After 48 h of incubation at 42°C in a microaerobic atmosphere, CFU at appropriate dilutions were counted and compared with numbers of CFU of the original sample. When culturable counts were below 10 CFU ml−1, culturability was assayed by the enrichment method of Park and Sanders (22). One milliliter of the bacterial suspension was added to 9 ml of Park and Sanders buffer without antibiotic supplement. After 48 h of incubation at 37°C under a microaerobic atmosphere, 0.1 ml was spread on the agar of Karmali et al. (16) and Columbia agar and incubated 1 to 5 days at 37°C under a microaerobic atmosphere. Total and active cells were counted after double staining with 5-cyano-2,3-ditolyl tetrazolium chloride (CTC) and 4′,6 diamino-2-phenylindole (DAPI), as previously described (7). All three strains entered the VBNC state (Fig. 1). Plate counts rapidly decreased below detection levels (<1 CFU/ml) after 15, 17, and 18 days for strains 79, 85, and Bf, respectively, while CTC-reducing-cell counts remained around 106 cells ml−1. After 30 days of starvation, no culturable cells were detected in 10 ml of microcosm water by the Park and Sanders enrichment method. In an initial experiment, 1 ml from each of the 30-day-old microcosm water samples was collected and used to inoculate an embryonated hen’s egg. In order to avoid inoculating culturable cells, in a second experiment, 30-day-old microcosm water samples were diluted to obtain a final concentration of 25 VBNC cells ml−1 (i.e., CTC-reducing cells). Seven-day-old embryonated eggs from specific-pathogen-free chickens, strain Isa-Brown, were purchased from the Centre Nationale d’Etude Vétérinaire et Alimentaire (Ploufragan, France). One milliliter of each Campylobacter suspension was injected into a yolk sac with a 1-ml syringe (needle dimensions, 0.9 by 40 mm). Negative-control eggs were inoculated with sterilized distilled water. The eggs were then incubated at 37°C. After incubation for 12, 48, and 96 h, the egg shells were broken. The vitellus fluid was harvested with a syringe, and 0.2 ml was spread on Columbia agar supplemented with 5% lysed horse blood. These plates were incubated 48 h at 37°C in a microaerobic atmosphere. Colonies were identified as C. jejuni and submitted to restriction enzyme analysis with the restriction enzyme SmaI (Boehringer Mannheim, Meylan, France) and CHEF DR II pulsaphor electrophoresis (Bio-Rad, Ivry-sur-Seine, France) to confirm that the strains recovered were the same as those inoculated. Table 1 shows the numbers of embryonated eggs from which C. jejuni was isolated after inoculation with the VBNC suspension. Viable Campylobacter organisms were successfully recovered from 33 of 40, 31 of 40, and 35 of 40 isolates of strains Bf, 79, and 85, respectively, in the embryonated eggs inoculated with 30-day-starved C. jejuni cells (106 VBNC cells ml−1). The percentage recovery was independent of the strain under study and of the incubation time of the inoculated eggs. The restriction enzyme analysis curves confirmed that the C. jejuni strains that were recovered after embryonated-egg passage were the same as those inoculated. Table 2 shows the efficacies of embryonated-egg passage for the three recoveries of each Campylobacter strain, relative to the number of VBNC cells. After inoculation of 10, 15, or 25 VBNC cells ml−1, all three Campylobacter strains were recovered from each of the four inoculated eggs. When the number of viable cells was approximately 1, as defined by the CTC reduction method, recovery was observed in 0, 25, and 75% of isolates of strains Bf, 79, and 85, respectively. When the number of CTC-positive cells was below 10, the recovery percentage decreased (Table 2). Recovery of VBNC C. jejuni cells after intestinal passage in mice (15, 27) or in 1-day-old chicks (28) has already been described. In contrast, Medema et al. (18) and Van de Giessen et al. (30) were unable to recover VBNC cells in animal models. The embryonated-egg model has been successfully used to recover VBNC Legionella pneumophila cells (14), but recovery of VBNC C. jejuni cells by embryonated-egg passage has not been described previously. According to results presented here, this model creates favorable conditions for VBNC Campylobacter cell recovery. Previous work showed that these same strains of C. jejuni in the VBNC state can also recover their culturability within a chick or mouse after intestinal passage (5), but the results observed with the embryonated-egg model were much better. The embryonated-egg model can be considered an animal model in which the animals have reduced defenses. In contrast, Medema et al. (18) were unable to recover viable cells from their strain of VBNC C. jejuni cells after embryonated-egg passage, but their experiment differed from that reported here in several significant aspects. First, the VBNC C. jejuni cells were obtained by nutrient deprivation at a higher temperature than that employed in the experiments reported here, i.e., 15 and 25°C versus 4°C. At the higher temperatures, VBNC cells occur sooner, but when incubation is at 4°C, the response rate is lower (25). Second, the VBNC cells were injected into the allantoic cavity, where the pH, presence of lysozyme, etc., are not favorable to bacterial survival and growth. The vitelline cavity contains nutrients and growth factors effective in sustaining growth of Campylobacter.
FIG. 1.
Growth of a C. jejuni 85 cell suspension in microcosm water over a 30-day incubation period. Culturable-cell counts were obtained by spread plate counting. Active-cell counts were obtained by the CTC and DAPI staining technique, with cells being counted with an epifluorescence microscope. ■, culturable-cell count; ⧫, active-cell count; ▴, total-cell count.
TABLE 1.
Numbers of embryonated eggs colonized by C. jejuni after inoculation with a VBNC cell suspension or sterilized distilled water (negative-control eggs)
| Inoculant | No. of embryonated eggs colonized/no. of eggs inoculated at time after inoculation (h):
|
Total | ||
|---|---|---|---|---|
| 12 | 48 | 96 | ||
| C. jejuni | ||||
| Bf | 6/8 | 16/19 | 11/13 | 33/40 |
| 79 | 6/8 | 15/19 | 10/13 | 31/40 |
| 85 | 7/8 | 17/19 | 11/13 | 35/40 |
| Sterilized distilled water | 0/4 | 0/4 | 0/4 | 0/12 |
TABLE 2.
Numbers of embryonated eggs colonized by C. jejuni per number of embryonated egg inoculated with various numbers of VBNC and culturable cells
| State of cells | C. jejuni strain | No. of viable cells inoculated per egg | No. of eggs colonized/no. of eggs inoculated |
|---|---|---|---|
| VBNC (CTC reductive) | Bf | 10 | 4/4 |
| 1 | 0/4 | ||
| 79 | 15 | 4/4 | |
| 2 | 1/4 | ||
| 85 | 25 | 4/4 | |
| 2 | 3/4 | ||
| Culturable | Bf | 8 | 4/4 |
| 79 | 1 | 4/4 | |
| 85 | 5 | 4/4 |
In all VBNC cell recovery experiments, the main difficulty is to verify that no culturable cells are inoculated into the animal system, in order to ensure that the cells that are recovered are not the result of cell division and growth of only a few culturable cells remaining in the sample. Dilution appears to be the best way to rule out the presence of culturable cells in the inoculum (19). The dilution performed in the study reported here reduced significantly the probability of culturable cells being present in the inoculum. On the day of inoculation (day 30), the culturable cell counts were below 1 CFU ml−1 and the viable cell counts, determined by reduction of CTC, remained above 106 cells ml−1. With dilution of the inoculum to 10−5 and 10−6, no culturable cells remained and the numbers of viable cells were ca. 1 to 25 cells ml−1. However, Ravel et al. (24) suggested that a part of the cell population is present in a nonculturable state on solid medium but that it can grow and divide in liquid medium after a temperature increase. Moreover, such cells are able to grow at a high rate as the nutrient level increases. Inoculum dilution, therefore, reduces the possibility of culturable cells being present, although it cannot absolutely preclude it. Dilutions also permit verification of the effectiveness of cell recovery after embryonated-egg passage, with respect to the number of inoculated cells able to reduce CTC. When the viable cell count determined by CTC reduction approached one cell per ml in the inoculum, the recovered-cell count decreased (Table 2). When one or two VBNC cells were inoculated, no viable Campylobacter organisms were recovered from eggs inoculated with strain Bf and viable cells were recovered from only one egg inoculated with strain 79 and from three of four eggs inoculated with strain 85. Certainly, some VBNC cells may be unable to reduce CTC, and perhaps the CTC-reducing population does not reflect precisely the VBNC population. So, in the study of VBNC cells, it may be preferable to use several methods to detect various metabolic activities of VBNC cells, such as direct viable counting or radioisotope incorporation (3). For culturable cells of C. jejuni, the proportion of colonized eggs was always 100%, even when a single cell was inoculated into the vitelline cavity, showing that culturable cells respond quickly and that VBNC cells require a longer response time.
In order to test the pathogenicity of VBNC C. jejuni cells, attachment indices with HeLa cells were determined for each strain in both the culturable and the VBNC state. According to Fauchère et al. (11), that colonization factor can be used for predicting the pathogenicity of a given strain of C. jejuni. HeLa 229 cells (Eurobio, Les Ullys, France) were maintained in a minimum essential medium. Listeria monocytogenes F48 pal was used as control attachment strain, and E. coli HB101 was used as control nonattaching strain. Each bacterial suspension was resuspended in nonsupplemented minimum essential medium and adjusted to 108 bacteria per ml. VBNC suspensions were adjusted to 108 viable (i.e., CTC-positive) bacteria. A portion of 300 μl of a bacterial suspension was added to the cell monolayer, and the mixture was incubated for 1 h at 37°C in a 5% CO2 atmosphere to permit bacterial adhesion to the cells. After cells were stained with a 0.025% acridine orange solution, the attachment indices were determined by counting the number of bacteria attached to each of 200 cells, calculated with the following formula: Σ(N · b)/Σ(N), where N is the number of cells associated with b bacteria (11). All tests were performed in duplicate. The results presented in Table 3 are means of results of duplicate determinations. In the culturable state, strains Bf and 79 exhibited an attachment index greater than 2, and according to Fauchère et al. (11), these two strains are enteropathogenic. Strain 85 exhibited an attachment index below 2 when cells were in the culturable state. In the VBNC state and 30 days after inoculation into the microcosm water, all the C. jejuni strains exhibited a lower attachment index, indicating that entry into the VBNC state was accompanied by a loss of the adhesion property. This loss is transient, because after chick and mouse passage, the attachment indices of all three strains increased. For strains Bf and 79, the attachment index again increased to more than 2 and both strains recovered their adhesion properties. In the VBNC state, our strains exhibited a much lower attachment index, corresponding most probably to the loss of enteropathogenicity. Such a transient loss of pathogenicity has already been highlighted for VBNC C. jejuni cells (27). The C. jejuni cells used were toxigenic and caused fluid accumulation in the rat gut. In the nonculturable state, they caused little or no fluid accumulation. After recovery by successive rat gut passages, fluid accumulation induced by the bacteria increased to the baseline level. In other bacteria, VBNC cells retain pathogenicity. Oliver and Bockian (19) showed that injections of VBNC V. vulnificus cells into mice killed the animals and concluded that VBNC V. vulnificus cells remain virulent, at least for some time after entry into the VBNC state, and are capable of causing fatal infection after recovery in vivo. In animal-model experiments, it is difficult to appreciate whether this is due to the VBNC cells or to the multiplication of a few cells remaining culturable. Other authors have shown that VBNC E. coli cells retain pathogenicity, with cells being able to produce enterotoxin (1, 10, 23) and maintain virulence plasmids (4, 13).
TABLE 3.
HeLa cell attachment indices (averages from duplicate experiments) determined for L. monocytogenes F48 pal (associative strain), E. coli HB101 (nonassociative strain), and C. jejuni Bf, 79, and 85
| Strain | HeLa cell attachment index
|
||
|---|---|---|---|
| Culturable cellsa | VBNC cellsb | VBNC cells recovered after egg passagea | |
| L. monocytogenes F48 pal | 11.35 | ||
| E. coli HB101 | 0.76 | ||
| C. jejuni Bf | 6.22 | 0.74 | 4.2 |
| C. jejuni 79 | 5.13 | 0.58 | 3.62 |
| C. jejuni 85 | 1.26 | 0.11 | 0.74 |
The HeLa cell monolayer was inoculated with 108 culturable cells.
The HeLa cell monolayer was inoculated with 108 active but VBNC (CTC-positive) cells.
Our results show that VBNC C. jejuni cells can recover from the VBNC state after egg passage. An important feature of culturable Campylobacter cells recovered after egg passage was that these cells regained attachment indices above those of VBNC cells inoculated into eggs and subsequently recovered. In particular, strains Bf and 79 were reclassified as pathogenic and cells recovered following inoculation in the VBNC state into embryonated eggs and maintenance of adhesion potential indicated that the VBNC state of Campylobacter does, in fact, constitute a public health concern.
Acknowledgments
Special thanks go to AERIAL for restriction enzyme analysis and to D. Woodward, LCDC, Ottawa, Canada, for confirmation of the identification of Campylobacter strains used in this study. We are also indebted to F. Jugiau and F. Rama for helpful technical support.
This research was funded by a grant from INRA.
REFERENCES
- 1.Barcina I, Gonzalez J M, Iriberri J, Egea L. Survival strategy of Escherichia coli and Enterococcus faecalis in illuminated fresh and marine systems. J Appl Bacteriol. 1990;68:189–198. doi: 10.1111/j.1365-2672.1990.tb02565.x. [DOI] [PubMed] [Google Scholar]
- 2.Beumer R R, De Vries J, Rombouts F M. Campylobacter jejuni non-culturable coccoid cells. Int J Food Microbiol. 1992;15:153–163. doi: 10.1016/0168-1605(92)90144-r. [DOI] [PubMed] [Google Scholar]
- 3.Braux A S, Minet J, Tamanai-Shacoori Z, Riou G, Cormier M. Direct enumeration of injured Escherichia coli cells harvested onto membrane filters. J Microbiol Methods. 1997;31:1–8. [Google Scholar]
- 4.Byrd J J, Colwell R R. Maintenance of plasmids pBR322 and pUC8 in nonculturable Escherichia coli in the marine environment. Appl Environ Microbiol. 1990;56:2104–2107. doi: 10.1128/aem.56.7.2104-2107.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cappelier J M, Magras C, Jouve J L, Federighi M. Recovery of viable but non-culturable Campylobacter jejuni cells in two animal models. Food Microbiol. 1999;16:375–383. [Google Scholar]
- 6.Cappelier J M, Federighi M. Mise en évidence de l’état viable non cultivable chez Campylobacter jejuni. Rev Med Vet. 1998;149:319–326. [Google Scholar]
- 7.Cappelier J M, Lazaro B, Rossero A, Fernandez-Astorga A, Federighi M. Double staining (CTC-DAPI) for detection and enumeration of viable but non-culturable Campylobacter jejuni cells. Vet Res. 1997;28:547–555. [PubMed] [Google Scholar]
- 8.Colwell R R, Brayton P, Herrington D, Tall B, Huq A, Levine M M. Viable but non-culturable Vibrio cholerae O1 revert to a cultivable state in the human intestine. World. J Microbiol Biothechnol. 1996;12:28–31. doi: 10.1007/BF00327795. [DOI] [PubMed] [Google Scholar]
- 9.Colwell R R, Tamplin M L, Brayton P R, Gauzens A L, Tall B D, Herrington D, Levine M M, Hall S, Huq A, Sack D A. Environmental aspects of Vibrio cholerae in transmission of cholera. In: Sack R B, Zinnaka Y, editors. Advances on cholera and related diarrheas. Vol. 7. Tokyo, Japan: KTK Scientific; 1990. pp. 327–343. [Google Scholar]
- 10.Davies C M, Evison L M. Sunlight and the survival of enteric bacteria in natural waters. J Appl Bacteriol. 1991;70:265–274. doi: 10.1111/j.1365-2672.1991.tb02935.x. [DOI] [PubMed] [Google Scholar]
- 11.Fauchère J-L, Rosenau A, Veron M, Moyen E N, Richard S, Pfister A. Association with HeLa cells of Campylobacter jejuni and Campylobacter coli isolated from human feces. Infect Immun. 1986;54:283–287. doi: 10.1128/iai.54.2.283-287.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flint K P. The long-term survival of Escherichia coli in river water. J Appl Bacteriol. 1987;63:261–270. doi: 10.1111/j.1365-2672.1987.tb04945.x. [DOI] [PubMed] [Google Scholar]
- 13.Grimes D J, Colwell R R. Viability and virulence of Escherichia coli suspended by membrane chamber in semitropical ocean water. FEMS Microbiol Lett. 1986;34:161–165. [Google Scholar]
- 14.Hussong D, Colwell R R, O’Brien M, Weiss E, Pearson A D, Weimer R M, Burge W D. Viable Legionella pneumophila not detectable by culture on agar media. Bio/Technology. 1987;5:947–950. [Google Scholar]
- 15.Jones D M, Sutcliffe E M, Curry A. Recovery of viable but nonculturable Campylobacter jejuni. J Gen Microbiol. 1991;137:2477–2482. doi: 10.1099/00221287-137-10-2477. [DOI] [PubMed] [Google Scholar]
- 16.Karmali M A, Simor A E, Rocoe M, Fleming P C, Smith S S, Lane J. Evaluation of a blood-free, charcoal-based, selective medium for the isolation of Campylobacter organisms from feces. J Clin Microbiol. 1986;23:456–459. doi: 10.1128/jcm.23.3.456-459.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKay A M. Viable but non-culturable forms of potentially pathogenic bacteria in water. Lett Appl Microbiol. 1992;14:129–135. [Google Scholar]
- 18.Medema G J, Schets F M, Van de Giessen A W, Havelaar A. Lack of colonization of one day old chicks by viable, non-culturable Campylobacter jejuni. J Appl Bacteriol. 1992;72:512–516. doi: 10.1111/j.1365-2672.1992.tb01868.x. [DOI] [PubMed] [Google Scholar]
- 19.Oliver J D, Bockian R. In vivo resuscitation, and virulence towards mice, of viable but nonculturable cells of Vibrio vulnificus. Appl Environ Microbiol. 1995;61:2620–2623. doi: 10.1128/aem.61.7.2620-2623.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oliver J D, Hite F, McDougald D, Andon N L, Simpson L M. Entry into, and resuscitation from, the viable but nonculturable state by Vibrio vulnificus in an estuarine environment. Appl Environ Microbiol. 1995;61:2624–2630. doi: 10.1128/aem.61.7.2624-2630.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oliver J D, Wanucha D. Survival of Vibrio vulnificus at reduced temperatures and elevated nutrient. J Food Saf. 1989;10:79–86. [Google Scholar]
- 22.Park C E, Sanders G W. A sensitive enrichment procedure for the isolation of Campylobacter jejuni from frozen foods. In: Riuz-Palacios G M, Calva F, Ruiz-Palacios B R, editors. Campylobacter V. Proceedings of the 5th International Workshop on Campylobacter Infections. Puerto Vallenta, Mexico: National Institute of Nutrition; 1991. p. 102. [Google Scholar]
- 23.Pommepuy M, Butin M, Derrien A, Gourmelon M, Colwell R R, Cormier M. Retention of enteropathogenicity by viable but non culturable Escherichia coli exposed to seawater and sunlight. Appl Environ Microbiol. 1996;62:4621–4626. doi: 10.1128/aem.62.12.4621-4626.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ravel J, Knight I T, Monahan C E, Hill R T, Colwell R R. Temperature-induced recovery of Vibrio cholerae from the viable but nonculturable state: growth or resuscitation? Microbiology. 1995;141:377–383. doi: 10.1099/13500872-141-2-377. [DOI] [PubMed] [Google Scholar]
- 25.Rollins D M, Colwell R R. Viable but nonculturable stage of Campylobacter jejuni and its role in survival in the natural aquatic environment. Appl Environ Microbiol. 1986;52:531–538. doi: 10.1128/aem.52.3.531-538.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roszak D B, Grimes D J, Colwell R R. Viable but nonrecoverable stage of Salmonella enteritidis in aquatic systems. Can J Microbiol. 1984;30:334–338. doi: 10.1139/m84-049. [DOI] [PubMed] [Google Scholar]
- 27.Saha S K, Saha S, Sanyal S C. Recovery of injured Campylobacter jejuni cells after animal passage. Appl Environ Microbiol. 1991;57:3388–3389. doi: 10.1128/aem.57.11.3388-3389.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stern N J, Jones D M, Wesley I V, Rollins D M. Colonization of chicks by non-culturable Campylobacter spp. Lett Appl Microbiol. 1994;18:333–336. [Google Scholar]
- 29.Taylor D N. Campylobacter infections in developing countries. In: Nachamkin I, Blaser M J, Tompkins L S, editors. Campylobacter jejuni: current status and future trends. Washington, D.C.: American Society for Microbiology; 1992. pp. 20–30. [Google Scholar]
- 30.Van de Giessen A W, Heuvelman C J, Abee A, Hazeleger W C. Experimental studies on the infectivity of non culturable forms of Campylobacter spp. in chicks and mice. Epidemiol Infect. 1996;117:463–470. doi: 10.1017/s0950268800059124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu H S, Robert N, Singleton F L, Attwell R W, Grimes D J, Colwell R R. Survival and viability of non culturable Escherichia coli and Vibrio cholerae in the estuarine and marine environment. Microb Ecol. 1982;8:313–323. doi: 10.1007/BF02010671. [DOI] [PubMed] [Google Scholar]