Abstract
Introduction:
In preparation for the introduction of a rotavirus vaccine into the routine immunization program of Bangladesh in 2018, we report data and highlight evolving genotypes from five years of active hospital-based rotavirus surveillance which began in July 2012.
Methods:
We enrolled and collected fresh stool from every fourth child < 5 years admitted with acute gastroenteritis (AGE) at 8 participating surveillance hospitals. Rotavirus infections were detected by enzyme immune assay. Twenty-five percent of rotavirus isolates were genotyped using reverse transcription polymerase chain reaction.
Results:
We found that 64% (4832/7562) of children < 5 years of age admitted with AGE had evidence of rotavirus infection. The majority (57%) of patients with rotavirus infection were <12 months of age. The most common strains were G1P[8] (43%), G12P[8] (15%) and G9P[8] (9%); 11% of children had mixed infection.G3P[8], which has not been reported in Bangladesh since 2001, was documented for the first time in our surveillance system.
Conclusions:
The high burden of rotavirus-associated hospitalizations highlights the potential value of rotavirus vaccination in Bangladesh. Continued surveillance is important for monitoring the impact of vaccination as well as monitoring evolving genotypes.
Keywords: Bangladesh, Rotavirus, Hospital based surveillance, Rotavirus vaccination
1. Introduction
Rotavirus gastroenteritis remains a significant contributor to childhood morbidity and mortality globally, with nearly 2 million hospitalizations and 215,000 deaths reported annually among children under 5 years of age [1,2]. The routine use of 2 commercially available rotavirus vaccines (RVV) – the monovalent Rotarix (GSK) and pentavalent Rotateq (Merck) – in national immunization programs has been associated with decreases in rotavirus-related hospitalizations, all-cause diarrheal hospitalizations, and diarrheal deaths among this age group in numerous settings [3-6]. RVV implementation has lagged in Asia compared with other regions. Bangladesh plans to incorporate a RVV into the national immunization program in 2018. Previously, we reported trends in rotavirus hospitalizations from July 2012 to June 2015 in Bangladesh [7]. Here, we highlight the evolving genotypes in our surveillance system, as well as provide two additional years updating the epidemiology of rotavirus in Bangladesh. These data immediately precede the period of planned rotavirus vaccine introduction in Bangladesh, and provide suitable baseline data to monitor vaccine impact.
2. Methods
Surveillance sites:
Surveillance began in July 2012 at three sentinel sites (Dhaka, Rajshahi, and Sylhet), expanding to two others (Chittagong and Rangpur), in February 2013, and two more sites in August 2013 (Khulna and Barisal), for a total of 7 sentinel sites, located in each of Bangladesh’s seven geographic divisions. Due to the low number of acute gastroenteritis (AGE) hospitalizations in the original Rangpur site, we discontinued surveillance there in July 2015 and started surveillance activities at a different site in Rangpur starting in January 2016.
Case definition, enrollment, and specimen testing:
A detailed description of the surveillance system is available elsewhere [7]. Briefly, surveillance staff trained by the International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b) prospectively identified children aged < 5 years of age admitted with AGE, defined as the occurrence of ≥3 watery or looser-than-normal stools or ≥1 episode of forceful vomiting within a 24 h period, with symptoms lasting ≤ 7 days. Surveillance staff recorded demographic and clinical information and obtained a stool specimen from every fourth child admitted who met the AGE case definition. Children’s outcomes at discharge (cured, deceased, transferred to a different hospital) were also recorded. Rotavirus was identified from stool specimens using enzyme immune assay (EIA) (Prospect™, Oxoid Diagnostics Ltd, United Kingdom). Quarterly, 20–25% of isolated rotaviruses were selected for genotyping using previously described methods [8]. In brief, genomic RNA from stool specimens was extracted using the QIAamp Viral RNA minikit (Qiagen/Westburg, Leusden, the Netherlands). Multiplex reverse transcription polymerase chain reaction (RT-PCR) with type-specific primers was performed with Qiagen One-Step RT-PCR Kit (Qiagen, Hilden, Germany). Genotypes were further confirmed by sequencing using dideoxynucleotide chain termination method with the ABI PRISM® BigDye Terminator Cycle Sequencing Reaction kit v3.1 (Perkin-Elmer Applied Biosystems, Foster City, CA) in an automated genetic analyzer (ABI 3500xL).
Informed written consent was obtained from the enrolled children’s parents or guardians. This study protocol was reviewed and approved by the ethical committee of icddr,b.
Data analysis:
We report the number of children hospitalized with AGE and tested for rotavirus, the number of rotavirus positive specimens, the proportion of AGE hospitalizations due to rotavirus, as well as the genotypes identified over the surveillance period and by surveillance year. Surveillance years were defined as July of one year through June of the next year. We also report the age distribution of rotavirus positive cases by age groups (0–2, 3–5, 6–11, 12–23, and 24–59 months). We obtained a gastroenteritis severity score for each child using the 20-point Vesikari scale calculated from clinical information obtained at enrolment. Severe gastroenteritis was defined by a score of 11 or more [9]. χ2 and Cochran-Armitage trend tests were used to compare the proportions, and the Wilcoxon rank sum test was used to compare continuous variables. P-values <0.05 were considered statistically significant.
3. Results
During July 2012–June 2017, a total of 250,133 children aged less than five years were admitted to the sentinel sites. Among these children, 29,991 (12%) were hospitalized with AGE (Table 1) and 7562 (25%) were enrolled in the surveillance system and tested for rotavirus. Of these enrollees, 64% (4,832/7562) tested positive for rotavirus, with the annual proportion positive ranging from 61 to 66%. By site, the proportion ranged from a low of 56% in Khulna to a high of 68% in Rangpur (data not shown).
Table 1.
Proportion of hospitalized children aged <5 years with acute gastroenteritis (AGE) with evidence of rotavirus infection, by surveillance year, admitted to 8 sentinel hospitals in Bangladesh, July 2012–June 2017.
| Surveillance year | All Hospitals | |||
|---|---|---|---|---|
| No. of children admitted for AGE | No. of children enrolled in RV surveillancea | Children with RV- positive specimensb |
||
| No. | % | |||
| July 2012–June 2013 | 3150 | 780 | 483 | 62 |
| July 2013–June 2014 | 5603 | 1464 | 943 | 64 |
| July 2014–June 2015 | 6061 | 1539 | 1006 | 65 |
| July 2015–June 2016 | 7389 | 1839 | 1114 | 61 |
| July 2016–June 2017 | 7788 | 1940 | 1286 | 66 |
| Total | 29,991 | 7562 | 4832 | 64 |
Note. No., number; AGE, acute gastroenteritis; RV, rotavirus.
All enrolled patients submitted stool specimen for rotavirus testing.
Stool specimens that tested positive by enzyme immunoassay.
The proportion of gastroenteritis hospitalizations attributed to rotavirus ranged from 17% in May 2013 to 86% in January 2017 (Fig. 1). Seasonal peaks were seen during the coldest months of the year in Bangladesh: November–March. Approximately 80% of gastroenteritis hospitalizations were due to rotavirus in these months. Forty-seven percent of the children with AGE (3575) were aged 6–11 months with a median age of 10 months. Among children with rotavirus infection, 48% (2314) were aged 6–11 months.
Fig. 1.
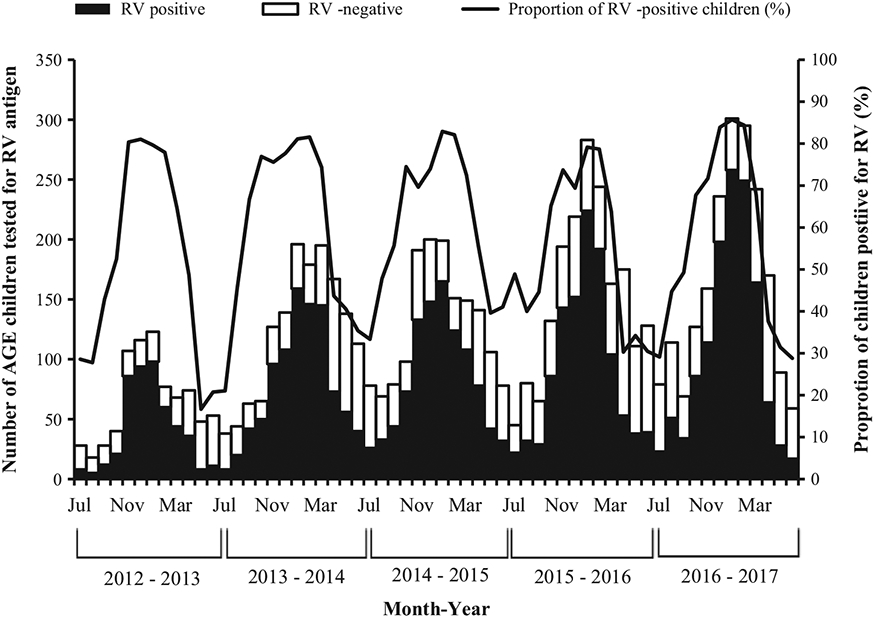
Seasonality of hospitalization for rotavirus (RV) acute gastroenteritis (AGE) among children aged <5 years at 8 sentinel hospitals in Bangladesh, July 2012–June 2017.
Clinical data indicated that 73% of children with rotavirus-confirmed AGE had severe clinical illness as defined by the Vesikari scale, with the median score of 12 compared to a median score of 11 in children with rotavirus negative AGE (P < 0.001). Similarly, children with rotavirus positive AGE had a higher frequency of diarrhoeal episodes per 24 h (median, 26 vs 19, P < .001), higher vomiting episodes per 24 h (median, 5 vs 4, P < .001), some sign of dehydration (67% vs 62%, P < .001) and a longer duration of days of illness (median, 6 vs 5, P < .001) as compared to children with rotavirus negative AGE.
Over the surveillance period, there were 10 deaths among the hospitalized children with AGE who were enrolled in the surveillance system. These deaths were evenly distributed among children with rotavirus positive and negative AGE, with five occurring in each group. All deaths among children with rotavirus-confirmed AGE had severe dehydration during hospitalization, as compared to 60% of those among children with rotavirus-negative AGE.
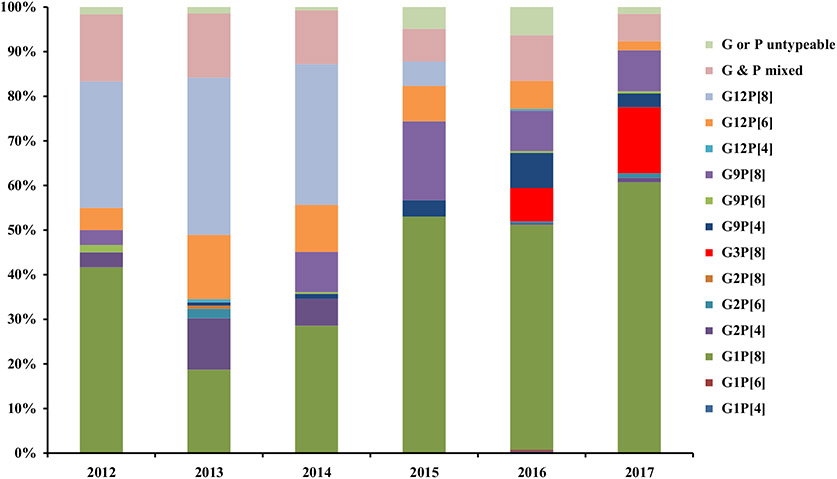
One thousand seventy-nine rotavirus positive specimens were genotyped. Among these, five G genotypes (G1, G2, G3, G9 and G12) and three P genotypes (P4, P6 and P8) were identified (Table 2). The most common strains were G1P[8] (43%), G12P[8] (15%), and G9P[8] (9%). Eleven percent of children had infections with more than one rotavirus strain. Similar trends were observed among the G & P genotypes in each of the seven divisions of Bangladesh, with G1P[8] and G12P[8] comprising the largest proportion of detected genotypes (Table 3). G3P[8] emerged in six of seven surveillance sites in2016 (see Fig. 2).
Table 2.
Distribution of rotavirus G and P genotypes isolated in samples from children aged <5 years with rotavirus acute gastroenteritis admitted to 8 sentinel hospitals in Bangladesh, July 2012–June 2017.
| G and P type | No. (%) of strains | |||||
|---|---|---|---|---|---|---|
| P[4] | P[6] | P[8] | Mixeda | P untypeable | Total | |
| G1 | 1(.1) | 1(.1) | 461(43) | 4(.4) | 7 (.7) | 474 (44) |
| G2 | 37(3) | 6(.6) | 1(.1) | 5(.5) | – | 49 (5) |
| G3 | – | – | 48(5) | – | 1(.1) | 49 (5) |
| G9 | 36(3) | 4(.4) | 96(9) | 9(.8) | 2 (.2) | 147 (14) |
| G12 | 2(.2) | 84(8) | 159(15) | 2(.2) | – | 247 (23) |
| Mixedb | 3(.3) | 1(.1) | 69(6) | 18(2)c | 3(.3) | 94 (9) |
| G untypeable | 1(.1) | 1(.1) | 16(2) | – | 2(.2) | 20 (2) |
| Total | 80 (7) | 97 (9) | 850 (79) | 38 (4) | 14 (2) | 1079 (1 0 0) |
Note: Data for the two most common strains are shown in bold face.
The mixed P types isolated included:3 G1P[4]P[8],5 G2P[4]P[8], 7 G9P[4]P[8], 2 G9P[6]P[8], one G1P[6]P[8], one G12P[6]P[8] and one G12 P[4]P[6]P[8].
The mixed G types isolated included:14 G1G9P[8], 24 G1G12P[8], 13 G2G9 P[8],8 G1G2G9 P[8], 7 G9G12 P[8], 3 G1G9G12 P[8], 2 G1G12P[0], 2 G2G9P[4], one G2G12P[4] and one G2G9P[6].
The combined mixed G & P types isolated included:4 G2G9P[4]P[8], 2 G9G12P[6]P[8], 2 G1G12P[4]P[8],2 G1G9 P[4]P[8], one G1G2 P[4]P[8], one G2G2P[6]P[8], one G1G12P[6]P[8], one G1G2G9 P[4]P[8], one G1G2G9 P[6]P[8], one G1G2G12P[6]P[8], one G2G12P[6]P[8] and one G9G12P[4]P[6]P[8].
Table 3.
Proportion of rotavirus genotype among the rotavirus positive EIA cases, by geographic location, July 2012–June 2017 (N = 1079).
| Rotavirus genotypes | Location & no. (%) of strains | ||||||
|---|---|---|---|---|---|---|---|
| Dhaka n = 183 |
Rajshahi n = 222 |
Sylhet n = 164 |
Rangpur n = 117 |
Chittagong n = 114 |
Khulna n = 112 |
Barisal n = 168 |
|
| G1P[4] | – | – | 1(1) | – | – | – | – |
| G1P[6] | – | – | – | – | – | – | 1(1) |
| G1P[8] | 61(33) | 129(58) | 63(38) | 50(43) | 27(24) | 54(48) | 77(48) |
| G2P[4] | 6(3) | 10(5) | 6(4) | 2(1) | 3(3) | 4(4) | 6(4) |
| G2P[6] | – | 1(.5) | 2(1) | – | – | 3(3) | – |
| G2P[8] | – | – | – | 1(1) | – | – | – |
| G3P[8] | 9(5) | 16(7) | 4(3) | 4(3) | – | 6(5) | 9(5) |
| G9P[4] | 13(7) | 7(3) | 2(1) | 3(3) | 2(2) | 4(4) | 5(3) |
| G9P[6] | – | 1(.5) | 1(1) | 1(1) | – | – | 1(1) |
| G9P[8] | 11(7) | 7(3) | 27(17) | 15(13) | 14(12) | 8(7) | 14(8) |
| G12P[4] | – | – | – | 1(1) | – | – | 1(1) |
| G12P[6] | 8(5) | 12(5) | 9(6) | 10(9) | 14(12) | 13(12) | 18(11) |
| G12P[8] | 40(22) | 21(10) | 28(17) | 18(15) | 32(28) | 8(7) | 12(7) |
| G & P mixed | 31(17) | 16(7) | 16(8) | 10(9) | 18(16) | 7(6) | 16(9) |
| G or P untypeable | 4(2) | 2(1) | 5(3) | 2(1) | 4(3) | 5(4) | 7(4) |
Fig. 2.
Distribution of rotavirus genotypes by year at 8 sentinels hospitals in Bangladesh, July 2012–June 2017.
4. Discussion
Rotavirus AGE was responsible for 64% of annual childhood AGE admissions, and roughly 8% of all pediatric admissions, in sentinel sites in Bangladesh during the surveillance period. Although enrolment numbers increased over time, there were only minor fluctuation in the proportion of rotavirus-confirmed AGE cases from year to year, and a consistent peak in rotavirus activity during November–March each year. Compared to the rotavirus detection rate of 40% among children under 5 years of age with AGE seen in 36 countries reporting to the Global Rotavirus Surveillance Network [10], and that of 45% among children with AGE from 8 countries reporting to the Asian Rotavirus Surveillance network [11], the rotavirus prevalence observed in sentinel sites in Bangladesh is one of the highest worldwide.
The age distribution of children with rotavirus positive AGE remained unchanged compared to our prior findings [7], with over half of infections occurring among infants. Administration of RVVs in the first few years of life has the potential to prevent most cases of rotavirus-AGE.
Changes in the genotype distribution of rotavirus were detected among our surveillance population. The G3P[8] strain was documented for the first time in our nation-wide surveillance network. This strain has also recently reported to have re-emerged at a separate hospital in Dhaka in 2016 (personal communication). G3P[8] is a significant contributor to rotavirus hospitalizations in neighboring India and Pakistan [12,13], but has not been reported in Bangladesh since 2001 [7,8,14]. Additionally, although G1P[8] and G12P[8] remained the predominant genotypes detected in our population, the proportion of G1P[8]increased by 12% and that of G12P[8] decreased by 14% overall compared to the prior report. These secular changes in rotavirus genotypes have been reported in Bangladesh and elsewhere [15] and although RVVs are effective against rotavirus genotypes that share vaccine strains (e.g. homotypic) as well as those that have strains different from the vaccine types (e.g. heterotypic) [16], continued surveillance is warranted to monitor for any new or sustained changes in genotype diversity after RVV introduction.
Our findings highlight the significant continued burden of rotavirus AGE and the potential value of rotavirus vaccination in Bangladesh. While reduced efficacy of rotavirus vaccines has been noted in randomized controlled trials from lower-income settings as compared to higher-income settings [17,18], given the high burden of disease documented in Bangladesh, the benefits of a lower efficacy vaccine would still be projected to be substantial. Additionally, given that the number of children suffering from severe illness necessitating hospitalization currently far exceeds the availability of hospital beds in Bangladesh; by introducing routine RVV, and reducing the number of children hospitalized for rotavirus, hospital beds can be freed for use by these other ill children [19].
5. Conclusion
Rotavirus AGE contributes significantly to childhood hospitalizations for diarrhea in Bangladesh and genotypic changes occurred during our surveillance period. Our surveillance system will allow for continued analysis of epidemiologic and genotypic trends in rotavirus activity, and provides a strong baseline of rotavirus disease burden for future measurement of the impact of RVV introduction.
Acknowledgements
We thank all the study participants for their time and support. We also grateful to our implementation partner, surveillance hospital sites and The Institute of Epidemiology, Disease Control and Research (IEDCR) under the Ministry of Health and Family Welfare of Bangladesh Government. Our technical assistance partner, US Centers for Disease Control and Prevention (CDC).
Funding statement
Financial support for this evaluation was provided by Gavi, the Vaccine Alliance through the CDC Foundation.
Financial support
This work was supported by U.S. Agency for International Development (USAID) through US Centers for Disease Control and Prevention (CDC) cooperative agreement no. 1U51GH001209-01. icddr,b is also grateful to the government of Bangladesh, Canada, Sweden, and the United kingdom for providing core/unrestricted support.
Disclaimer
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Potential conflicts of interest
There is no conflict of interest.
References
- [1].Parashar UD, Hummelman EG, Bresee JS, Miller MA, Glass RI. Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis 2003;9(5):565–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tate JE, Burton AH, Boschi-Pinto C, Parashar UD. World health organization-coordinated global rotavirus surveillance N. Global, regional, and national estimates of rotavirus mortality in children <5 years of age, 2000–2013. Clin Infect Dis 2016;62(Suppl 2):S96–S105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Enane LA, Gastanaduy PA, Goldfarb DM, Pernica JM, Mokomane M, Moorad B, et al. Impact of rotavirus vaccination on hospitalizations and deaths from childhood gastroenteritis in Botswana. Clin Infect Dis 2016;62(Suppl 2):S168–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mpabalwani EM, Simwaka CJ, Mwenda JM, Mubanga CP, Monze M, Matapo B, et al. Impact of rotavirus vaccination on diarrheal hospitalizations in children aged <5 years in Lusaka, Zambia. Clin Infect Dis 2016;62(Suppl 2):S183–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Burnett E, Jonesteller CL, Tate JE, Yen C, Parashar UD. Global impact of rotavirus vaccination on childhood hospitalizations and mortality from diarrhea. J Infect Dis 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Inchauste L, Patzi M, Halvorsen K, Solano S, Montesano R, Iniguez V. Impact of rotavirus vaccination on child mortality, morbidity, and rotavirus-related hospitalizations in Bolivia. Int J Infect Dis 2017;61:79–88. [DOI] [PubMed] [Google Scholar]
- [7].Satter SM, Gastanaduy PA, Islam K, Rahman M, Rahman M, Luby SP, et al. Hospital-based surveillance for rotavirus gastroenteritis among young children in Bangladesh: defining the potential impact of a rotavirus vaccine program. Pediatr Infect Dis J 2017;36(2):168–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Rahman M, Sultana R, Ahmed G, Nahar S, Hassan ZM, Saiada F, et al. Prevalence of G2P[4] and G12P[6] rotavirus, Bangladesh. Emerg Infect Dis 2007;13(1):18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ruuska T, Vesikari T. Rotavirus disease in Finnish children: use of numerical scores for clinical severity of diarrhoeal episodes. Scand J Infect Dis 1990;22(3):259–67. [DOI] [PubMed] [Google Scholar]
- [10].Centers for Disease C, Prevention. Rotavirus surveillance–worldwide, 2001–2008. MMWR Morb Mortal Wkly Rep 2008;57(46):1255–7. [PubMed] [Google Scholar]
- [11].Nelson EA, Bresee JS, Parashar UD, Widdowson MA, Glass RI. Asian Rotavirus Surveillance N. Rotavirus epidemiology: the Asian Rotavirus Surveillance Network. Vaccine 2008;26(26):3192–6. [DOI] [PubMed] [Google Scholar]
- [12].Umair M, Salman M, Alam MM, Rana MS, Zaidi SSZ, Bowen MD, et al. Rotavirus Surveillance in Pakistan during 2015–2016 reveals high prevalence of G12P[6]. J Med Virol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mohanty E, Dwibedi B, Kar SK, Acharya AS. Epidemiological features and genetic characterization of virus strains in rotavirus associated gastroenteritis in children of Odisha in Eastern India. Infect Genet Evol 2017;53:77–84. [DOI] [PubMed] [Google Scholar]
- [14].Afrad MH, Hassan Z, Farjana S, Moni S, Barua S, Das SK, et al. Changing profile of rotavirus genotypes in Bangladesh, 2006–2012. BMC Infect Dis 2013;13:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Leite JP, Carvalho-Costa FA, Linhares AC. Group A rotavirus genotypes and the ongoing Brazilian experience: a review. Mem Inst Oswaldo Cruz 2008;103(8):745–53. [DOI] [PubMed] [Google Scholar]
- [16].Leshem E, Lopman B, Glass R, Gentsch J, Banyai K, Parashar U, et al. Distribution of rotavirus strains and strain-specific effectiveness of the rotavirus vaccine after its introduction: a systematic review and metaanalysis. Lancet Infect Dis 2014;14(9):847–56. [DOI] [PubMed] [Google Scholar]
- [17].Zaman K, Dang DA, Victor JC, Shin S, Yunus M, Dallas MJ, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet 2010;376(9741):615–23. [DOI] [PubMed] [Google Scholar]
- [18].Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, Abate H, Breuer T, Clemens SC, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med 2006;354(1):11–22. [DOI] [PubMed] [Google Scholar]
- [19].Saha S, Santosham M, Hussain M, Black RE, Saha SK. Rotavirus vaccine will improve child survival by more than just preventing diarrhea: evidence from Bangladesh. Am J Trop Med Hyg 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]