Abstract
Background.
High attack rates among vaccinated young adults reported during the 2006 mumps outbreak in the United States heightened concerns regarding mumps vaccine failure.
Methods.
Serum specimens from university students and staff were tested for mumps immunoglobulin (Ig) G by enzyme immunoassay (EIA). A subset of participants vaccinated for ⩽5 years and ⩾15 years were tested by neutralizing antibody (NA) assay. Persons seronegative by EIA were offered a third dose of measles-mumps-rubella vaccine (MMR3), and serum specimens were obtained 7–10 days and 2–3 months after its administration.
Results.
Overall, 94% (95% confidence interval [CI], 91%–96%) of the 440 participants were seropositive. No differences existed in seropositivity rates by sex, age, age at receipt of the second dose of MMR vaccine (MMR2), or time since receipt of MMR2 (P = .568). The geometric mean titer (GMT) of NA among persons vaccinated with MMR2 during the previous 1–5 years was 97 (95% CI, 64–148), whereas, among those vaccinated ⩾15 years before blood collection, the GMT was 58 (95% CI, 44–76) (P = .065). After MMR3, 82% (14/17) and 91% (10/11) seroconverted in 7–10 days and 2–3 months, respectively.
Conclusions.
Lower levels of NA observed among persons who received MMR2 ⩾15 years ago demonstrates antibody decay over time. MMR3 vaccination of most seronegative persons marked the capacity to mount an anamnestic response.
Mumps is an acute viral illness and is classically manifested by fever and inflammation of the salivary glands. Although usually a mild disease, mumps can cause complications (e.g., orchitis, encephalitis, and meningitis [1]). In the United States in 1967, the modified live Jeryl Lynn strain of mumps vaccine was licensed, and in 1977 the Advisory Committee on Immunization Practices recommended routine vaccination of all children aged ⩾12 months with combined measles-mumps-rubella (MMR) modified live vaccine [2]. In 1989, a 2-dose MMR vaccination schedule was recommended for school-aged children and college-aged students for measles control [3]. Under this 2-dose MMR schedule, most children and adolescents now receive 2 doses of mumps vaccine. Epidemiologic data indicate that the routine use of mumps vaccine has decreased the incidence of mumps by >99%, such that by 2003 a historic low was reached in the United States, with 231 cases reported [4].
Despite the achievement of vaccination coverage levels for at least 1 dose of mumps vaccine of ⩾90% among children aged 19–35 months since 1996 [5-7] and high vaccination coverage among schoolchildren, a large mumps outbreak (>5000 cases through the end of July 2006) recently occurred in the United States, affecting mainly Midwestern states [8, 9]. The highest attack rate was reported among persons aged 18–24 years, the majority of whom were college or university students who had been vaccinated with 2 doses of MMR. High attack rates among highly vaccinated young adults during this outbreak raised concerns regarding the mumps vaccine failure.
Antibody determinations are frequently used as surrogate measures of immunity to viral infections. Although the immune correlates of protection against mumps disease have not been defined, virus neutralizing antibody (NA) appears to be a reasonable marker. Vaccine failure can be classified as primary or secondary. Primary vaccine failure is the lack of an immunologic response appropriate to vaccination. The antibody response to mumps virus infection among such persons can be best characterized as that of an immunologically naive person; an IgM response and decreased levels of low-avidity IgG are expected [10]. Secondary vaccine failure is the failure to maintain effective immunity over time despite having an initial immune response. Among these persons, an IgM response after mumps virus infection might be present to varying degrees or it might be absent, and an increased level of high-avidity IgG is usually observed. IgG avidity testing can be used to differentiate between primary and secondary vaccine failure [10].
Although no more than 10 cases have been reported annually since 1975, Nebraska was one of multiple states in the Midwest affected by the outbreak in 2006. One Nebraska university with a 2-dose MMR immunization requirement since 2002 had not reported mumps cases during 2006. This university setting provided an excellent opportunity to evaluate the persistence of mumps antibodies induced by MMR vaccination and to document measurable declines in mumps antibody levels that might be indicative of waning immunity and, consequently, of increased susceptibility to mumps virus infection. Our objectives were (1) to evaluate the persistence of mumps antibody among persons aged 19–30 years by correlating antibody levels with time since the previous dose of MMR vaccine and (2) to characterize vaccine failure by evaluating, among seronegative persons, the serologic response after challenge with a third dose of MMR vaccine (MMR3).
SUBJECTS, MATERIALS, AND METHODS
Study population.
A convenience sample of volunteer university students and staff was included in the study. Because consent can be given by persons aged ⩾19 years in Nebraska and because persons aged >30 years were more likely to have antibody induced by wild mumps virus infection instead of vaccination, persons 19–30 years old were targeted for enrollment. Enrollment criteria included being a university student or staff aged 19–30 years and having a documented history of receipt of 2 doses of MMR vaccine.
Study design.
All university students and staff were notified of the study and enrollment criteria by a E-mail message. After informed consent was obtained, a blood specimen was collected from each study participant. Blood specimens were centrifuged at the study site, and the resultant serum specimens were shipped to the Centers for Disease Control and Prevention (CDC) for mumps IgG antibody testing by EIA. To examine IgG antibody levels by time since vaccination, a subset of specimens from participants vaccinated ⩽5 years and ⩾15 years before enrollment were tested at the Food and Drug Administration for NA levels by plaque reduction neutralization test (PRNT).
Participants without evidence of mumps antibody by EIA were offered MMR3, and blood specimens from vaccine recipients were obtained 7–10 days and 2–3 months after vaccination. Serum specimens were processed at the study site and forwarded to the CDC for mumps IgM and IgG antibody measurement by EIA.
Data collection.
At the time of enrollment, participants completed a questionnaire that included the following: name, date of birth, date and place of mumps-containing vaccination, country of birth, date of entry into the United States (if not born in the United States), history of mumps disease, race/ethnicity, and sex. The university vaccination records were used to obtain the vaccination history of each participant.
Laboratory testing.
For the detection and qualitative determination of IgG antibody to mumps virus in serum specimens, a commercially available indirect assay (Mumps IgG ELISA II Assay; Wampole Laboratories) was used. This EIA had been reported to favorably compare with PRNTs regarding sensitivity (70%) and specificity (96%) for detection of mumps antibody [11]. The cutoff points for mumps IgG antibodies based on index standard ratio (ISR) values were as follows: seronegative, ISR of ⩽0.90; indeterminate, ISR of 0.91–1.09; and seropositive, ISR of ⩾1.10.
For quality assurance, all samples with seronegative or indeterminate results for mumps IgG were retested with an equal number of randomly selected positive serum specimens. If retested specimens had positive results, they were reported as IgG seropositive. Serum specimens testing either negative or indeterminate were considered negative for our investigational purposes.
Serum specimens from recipients of MMR3 were tested for mumps IgM and IgG as a measure of primary and secondary antibody responses. A CDC in-house mumps IgM capture EIA, which uses recombinant mumps nucleocapsid protein as antigen, was used to detect mumps IgM. The format for the mumps assay was based on an IgM capture assay for measles that has been described elsewhere [12], except that the assay used a cell lysate containing baculovirus-expressed mumps nucleoprotein as the viral antigen and that the detector antibody was a monoclonal antibody directed against the mumps nucleoprotein (N5; gift of J. Wolinsky, University of Texas Medical School, Houston). Serum specimens were considered to be negative for mumps IgM if the ratio of optical density (OD) values for positive (P) mumps-antigen wells divided by negative (N) mump-santigen wells (P/N) was <3.0 and the OD value P – N was <0.10. Specimens were considered to be positive for mumps IgM if the ratio of P/N was ⩾3.0 and the P – N value was ⩾0.093. Serum specimens fulfilling only 1 of the 2 positivity criteria were considered to be indeterminate. As an additional control for antibody response after MMR vaccination, measles IgG antibody levels were measured using a Wampole Measles IgG ELISA (Wampole Laboratories). For certain analyses, the mumps IgG antibody level was stratified using the ISR values into 3 groups, as follows: low, ISR of 1.10–2.5; intermediate, ISR of 2.6–4.4; and high, ISR of ⩾4.5.
PRNT was used to measure neutralizing anti-mumps antibody levels. Briefly, serum was thawed at room temperature and heated at 56°C for 45 min to inactivate the complement. Two-fold serial dilutions of serum (or medium alone as a negative control) were mixed with equal volumes of ~30 pfu of the attenuated Jeryl Lynn mumps virus strain (the mumps component of MMR vaccine), to achieve a final dilution range of 1:4–1:512. The serum/virus mixtures were incubated at 37°C in 5% CO2 for 1 h and then placed on Vero cell monolayers in 24-well plates and incubated for 1 h at 37°C in 5% CO2. The serum/virus mixtures were removed by aspiration, and cell monolayers were rinsed with minimal essential medium (MEM) immediately before being covered with 0.75% Nobel agar in 2× MEM (Quality Biologicals) supplemented with 10% fetal bovine serum. Plates were then incubated at 37°C in 5% CO2 for 5 days. A second layer of agar containing 0.01% neutral red (Quality Biologicals) was added and incubated overnight to visualize the plaques produced by the remaining infectious virus. For each serum specimen, the NA level was determined as the highest dilution of serum capable of reducing the number of virus plaques by ⩾50%, compared with control values (from virus incubated with negative control serum). The cutoff point for seropositivity was a NA level of ⩾1:4. Levels of NA to the Jeryl Lynn strain of mumps virus were classified into 4 groups, as follows: negative, <1:4; borderline positive, 1:4–1:8; moderate positive, 1:16–1: 128; and highly positive, >1:128. Hereafter, titers are reported as reciprocal values.
Sample size and statistical analysis.
Sample size was calculated to evaluate, at a 5% significance level and 80% power, a 10% difference in the proportion of persons seronegative for IgG among the 2 groups—vaccinated persons who received the second dose of MMR vaccine (MMR2) ⩽5 years and ⩾15 years before blood collection. Risk factors evaluated for seronegative EIA results included sex, race/ethnicity, age, age at first dose of MMRvaccine (MMR1) and at MMR2, time between MMR1 and MMR2, and time since receipt of MMR2. In addition, the likelihood of seronegativity was assessed by controlling for age and years since receipt of MMR2. Arithmetic means were used to describe ISR values by time since vaccination. To describe NA levels, GMTs were calculated using log-transformed reciprocal levels and are reported as back-transformed values. For bivariate analysis, the tests used were the χ2 test for categorical variables, the Cochran-Armitage test for trend for ordinal variables, and the t test and Wilcoxon rank-sum test for continuous variables. Association between ISR and NA levels was examined via Pearson correlation. P < .05 was considered to indicate statistical significance. Data were analyzed using SAS (version 9.1) [13].
RESULTS
A total of 446 persons were enrolled in the study. Of these, 6 (1.3%) were excluded as a result of a history of mumps virus infection (2 students) or vaccination outside the United States (4 students). Of the 440 persons included in the analysis, 62% were female, 97% were white, and 98% were born in the United States. The median age of the participants was 21 years (range, 19–30 years). The median age at MMR1 vaccination was 1 year (range, 1–27 years) and at MMR2 vaccination was 12 years (range, 1–28 years).
EIA testing.
The presence of serum mumps IgG antibody was determined for all 440 participants. The overall mumps IgG seropositivity rate was 94% (95% confidence interval [CI], 91%–96%). In contrast, all 440 participants (100%) were seropositive for measles IgG. No differences existed in mumps seropositivity rates by sex or race/ethnicity (table 1). Mumps seropositivity rates did not differ by age group, age at receipt of MMR1 or MMR2, time interval between MMR1 and MMR2, or time since receipt of MMR2. The likelihood of seropositivity was not associated with increased time since receipt of MMR2, even after adjustment for age (table 2).
Table 1.
Seropositive EIA results for mumps IgG among university study participants, by characteristic—Nebraska, 2006.
| IgG seropositive | ||||
|---|---|---|---|---|
| Variable | Tested, no. | No. | % (95% CI) | P |
| Sex | .193 | |||
| Male | 169 | 155 | 92 (88–96) | |
| Female | 271 | 257 | 95 (92–98) | |
| Race/ethnicity | .366 | |||
| White | 425 | 397 | 93 (91–96) | |
| Other | 15 | 15 | 100 (100–100) | |
| Age | .276 | |||
| 19–20 years | 171 | 160 | 94 (90–97) | |
| 21–22 years | 175 | 167 | 95 (92–98) | |
| ⩾23 years | 94 | 85 | 90 (84–96) | |
| Age at MMR1 | .243 | |||
| 1 year | 235 | 225 | 96 (93–98) | |
| 2 year | 165 | 151 | 92 (87–96) | |
| ⩾3 years | 40 | 36 | 90 (81–99) | |
| Age at MMR2a | .797 | |||
| 1–6 years | 59 | 56 | 95 (89–100) | |
| 7–14 years | 338 | 315 | 93 (91–96) | |
| ⩾15 years | 42 | 40 | 95 (89–100) | |
| Years between MMR1 and MMR2a | .515 | |||
| 1–5 years | 71 | 67 | 94 (89–100) | |
| ⩾6 years | 368 | 344 | 93 (91–96) | |
| Years since receipt of MMR2a | .265 | |||
| 1–10 years | 293 | 277 | 95 (92–97) | |
| ⩾11 years | 146 | 134 | 92 (87–96) | |
NOTE. CI, confidence interval; MMR1, first dose of MMR vaccine; MMR2, second dose of MMR vaccine.
Data for age at time of administration of MMR2 was unavailable for 1 student.
Table 2.
Seropositive EIA results for mumps IgG among university study participants, by age and time since receipt of the second dose of measles-mumps-rubella vaccine (MMR2)—Nebraska, 2006.
| Variable | IgG seropositive, % |
ORa (95%CI) | P |
|---|---|---|---|
| Age | |||
| 19–20 years | 94 | Referent | |
| 21–22 years | 95 | 1.45 (0.57–3.71) | .238 |
| ⩾23 years | 90 | 0.74 (0.25–2.15) | .325 |
| Years since receipt of MMR2 | |||
| 1–10 years | 95 | Referent | |
| ⩾11 years | 90 | 0.80 (0.32–2.01) | .629 |
NOTE. CI, confidence interval; OR, odds ratio.
Adjusted for age and years since receipt of MMR2.
Although higher mean ISR values were identified among participants vaccinated with MMR2 ⩽5 years ago, the association between mean ISR values and time since receipt of MMR2 was borderline significant (P = .051) (figure 1).
Figure 1.
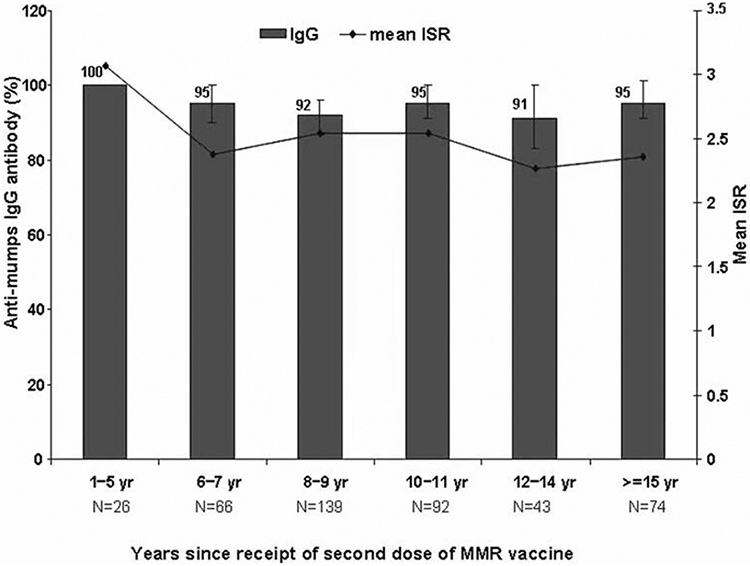
Prevalence of mumps seropositive EIA results and index standard ratio (ISR) values for mumps antibody among 440 university study participants, by years since receipt of a second dose of measlesmumps-rubella vaccine—Nebraska, 2006.
NA levels.
Levels of NA to the Jeryl Lynn strain of mumps virus were measured for 91 persons, who had received MMR2 1–5 years (n = 23) or ⩾15 years (n = 68) before blood collection. The overall GMT of NA was 66 (95% CI, 52–85); the titers did not differ by sex, age at receipt of MMR1 or MMR2, or time interval between MMR1 and MMR2 (table 3). The titer to the Jeryl Lynn strain of mumps virus was higher among participants who had received MMR2 within the previous 1–5 years (GMT, 97 [95% CI, 64–148]), compared with that among participants who received MMR2 ⩾15 years earlier (GMT, 58 [95% CI, 44–76]); however, this difference was only borderline significant (P = .065) (figure 2).
Table 3.
Geometric mean titers (GMTs) of mumps neutralizing antibody among university study participants who received a second dose of measles-mumps-rubella vaccine (MMR2) ⩽5 and ⩾15 years before blood collection (n = 91)—Nebraska, 2006.
| Variable | Tested, no. | GMT (95%CI) | P |
|---|---|---|---|
| Sex | .561 | ||
| Male | 49 | 62 (43–89) | |
| Female | 42 | 72 (51–100) | |
| Age at MMR1 | .314 | ||
| 1 year | 48 | 67 (47–95) | |
| 2 years | 30 | 53 (35–80) | |
| ⩾3 years | 13 | 109 (59–203) | |
| Age at MMR2 | .610 | ||
| 1–6 years | 48 | 58 (42–80) | |
| 7–14 years | 18 | 69 (36–113) | |
| ⩾15 years | 25 | 84 (53–135) | |
| Years between MMR1 and MMR2 | .244 | ||
| 1–5 years | 53 | 62 (46–85) | |
| ⩾6 years | 38 | 73 (49–109) |
NOTE. CI, confidence interval; MMR1, first dose of MMR vaccine.
Figure 2.
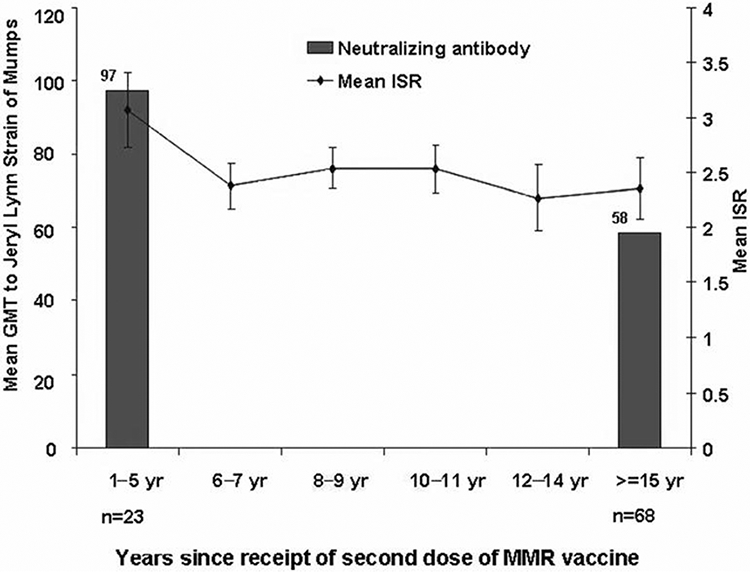
Geometric mean titers (GMTs) of mumps neutralizing antibody and index standard ratio (ISR) values for mumps antibody among 91 university study participants, by years since receipt of a second dose of measles-mumps-rubella vaccine—Nebraska, 2006.
Correlation between EIA and NA.
Eighty-six (95%) of the 91 specimens tested by both EIA and PRNT were seropositive for mumps IgG by EIA. Of these, 83 (97%) had NA titers higher than the borderline positive cutoff (i.e., >8), and 3 (3%) had borderline positive NA results. Among the 3 specimens with borderline positive results, 2 had low and 1 had intermediate ISR values. Among the 86 specimens seropositive by EIA, the median ISR value was 3, and the GMT of NA was 70. Correlation between NA levels and ISR values was significant, but the association was imperfect (ratio, 0.45; P < .01). Among the 5 participants with seronegative EIA results, none had high NA levels; the median ISR value was 1, and by PRNT the GMT of NA was 24.
Antibody response after MMR3.
Nineteen of the 28 persons who had seronegative EIA results (i.e., negative or indeterminate) for mumps IgG received MMR3 1–5 months after the initial blood collection (mean, 3.5 months). Seventeen persons (89%) provided a blood specimen 7–10 days after receipt of MMR3. Of these 17 persons, 3 had seronegative results for IgG and IgM. Seropositive results for IgG were detected in 14 (82%) of the 17 persons, and seropositive results for IgM were identified in 2 (14%) of the 14 persons (figure 3).
Figure 3.
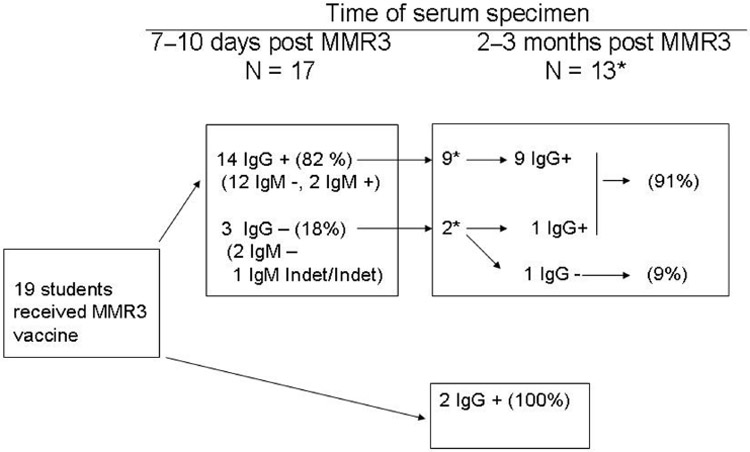
Description of mumps seropositive results among 17 seronegative persons after receipt of a third dose of MMR (MMR3) vaccine—Nebraska, 2006. Indet, indeterminate.
*All participants tested 2–3 months post MMR3 were IgM −
At 2–3 months after receipt of MMR3, 13 participants also provided a third blood specimen. All 9 persons who had been IgG positive at 7–10 days remained positive at 2–3 months after MMR3, and 1 of the 2 seronegative persons at 7–10 days had measurable IgG levels. One of the 2 participants who had been IgG seronegative at 7–10 days remained negative at 2–3 months. For these 11 participants, the overall seropositivity rate at 2–3 months was 91% (10/11); all were IgM seronegative. Two participants who had provided only a blood specimen 2–3 months after MMR3 were IgM seronegative and IgG seropositive. The mean ISR values for participants with paired specimens were 1.3 (95% CI, 1.2–1.5) at 7–10 days and 2.1 (95% CI, 1.6–2.7) at 2–3 months after receipt of MMR3.
DISCUSSION
The majority (94%) of study participants had seropositive EIA results for mumps IgG, and the seropositivity rate did not differ by age, sex, race/ethnicity, age at vaccination, or time since vaccination. The majority of seronegative persons vaccinated with MMR3 were seropositive 2–3 months after its receipt. The IgG serologic response, without detectable IgM at 7–10 days after MMR3 and the increased IgG levels 2–3 months after vaccination, indicates a B cell memory response in most instances. Thus, no major evidence for primary vaccine failure was observed among the seronegative persons.
Information regarding long-term persistence of mumps antibodies after a second dose of MMR vaccination is limited. Evidence for secondary vaccine failure can be demonstrated through epidemiologic investigation of outbreaks by assessing the vaccination status of case patients and by serologic studies demonstrating a decline in IgG antibody levels over time. Previous studies have yielded conflicting findings regarding the association between vaccine failure and time since vaccination, with some reporting no association during certain outbreaks [14-17] and others reported a positive correlation between risk for mumps virus infection and increasing interval since vaccination [18-20]. Existing data indicate that 1 dose of MMR vaccine can provide persistent antibodies to mumps virus ⩽5 years after vaccination [21]. A study in Finland identified a rapid decline in mumps antibody levels within the first year after 1 dose of mumps vaccine and a lesser decline after a second dose, with an overall seropositivity rate of 86% at 9 years after a second dose of mumps vaccine [22].
We did not identify any factor associated with seronegative results. Although a higher proportion of females with mumps were reported during outbreaks in the 1980s [14-16] and in 2006 [8, 9], we did not identify statistically significant differences in seropositive results or antibody levels by sex that might explain the preponderance of female patients during the 2006 outbreak.
Postlicensure studies conducted in the United States during the 1970s and 1980s documented that 1 dose of mumps virus–containing vaccine was ~80% effective in preventing mumps with parotitis [23]. Although fewer data are available on the effectiveness of 2 doses of mumps vaccine, estimates of effectiveness range from 88% to 95% [24-28]. The Jeryl Lynn mumps modified live vaccine virus and prior mumps virus infection does not seem to be as effective as the modified live measles vaccine virus in inducing immunity. Mumps virus infection has been documented among persons with previous mumps virus infection [29] and among 2-dose recipients of mumps vaccine. The 2006 US outbreak demonstrated that mumps transmission and outbreaks can occur among populations with high vaccination coverage with 2 MMR doses [8, 9].
Although infection with measles virus has been documented among recipients of 2 doses [30], high population vaccination coverage levels have been successful in preventing sustained measles transmission due to the high effectiveness of the measles vaccine [31]. The majority of the US population is vaccinated with MMR vaccine, and measles is considered more infectious than mumps [32, 33]. Therefore, the difference in mumps disease patterns cannot be explained by differences in vaccination coverage levels or infectivity. The difference in seropositive results between measles and mumps identified in our study (i.e., 100% for measles vs. 94% for mumps) confirms the differences in antibody response patterns. However, we cannot assume that the presence of antibody levels necessarily correlates with protection. Unlike measles, for which NA level seems to be a useful correlate of protection [34], a similar antibody-measurement correlate for mumps immunity is unclear. A prospective study determined that levels >8 by PRNT were protective against clinical mumps [35]. In our study, NA levels were not assessed among all participants; however, among the 91 participants with serum tested by both EIA and PRNT, 97% who were seropositive by EIA had levels >8, indicating the likelihood of IgG antibody levels >8 among this university population. In addition, the 6% EIA-based seronegativity rate identified during this study is similar to the failure rate reported among college students who had received 2 doses of MMR and who had been exposed to a roommate with mumps during the 2006 outbreak (CDC, unpublished data).
All but one person mounted an IgG response after administration of MMR3 to seronegative persons. This anamnestic response was typified by the rapid appearance of mumps virus–specific IgG and a general absence of a detectable IgM response [10, 36-38] among the majority of participants. This antibody response was similar to that observed during the mumps outbreak in 2006, thus making laboratory confirmation of clinically diagnosed mumps challenging because of the lack of IgM and the presence of IgG at the time when patients sought health care [9]. A rapid IgG response was observed at 7–10 days after MMR3, but peak antibody activity was not present until sometime between the 7–10-day and the 2–3-month intervals. Whether these levels of mumps antibody were maintained after 2–3 months or represent antibody levels that provide protection against mumps virus infection is unknown.
Our study has potential limitations. The students and staff participating in the study represent a convenience sample; therefore, results might not be generalizable to other populations. The possibility of IgG boosting caused by natural mumps virus infection among the study subjects is unknown. Although the university was not affected by the mumps outbreak, at the time of the study, 3 possible (clinically compatible but laboratory negative) mumps-like cases were reported at the university, and some cases were reported in the surrounding community. We could not assess the effect of exposure to such cases on the rate of seropositive results. The NA test in this study used the Jeryl Lynn vaccine virus strain; however, preliminary data from another study determined that higher antibody levels might be needed for neutralization of the 2006 mumps outbreak strain relative to the Jeryl Lynn strain [39]. We did not conduct IgG avidity testing to differentiate between primary and secondary vaccine failure.
The determinants of vaccine-induced mumps immune protection have not been fully determined. Two doses of mumps vaccine seem to induce a higher antibody response than 1 dose. In a mixture model applied to a serological survey in England and Wales, cohorts eligible for 2 doses of mumps vaccine presented higher antibody titers than did those eligible for 1 dose [40]. However, the optimal protective antibody level that might be required to protect against mumps disease is unknown. Understanding the protective mumps antibody level is critical to assessing waning immunity and to estimating the proportion of susceptible persons among the population. More studies are needed to confirm the decline of mumps antibody levels over time after MMR2 vaccination. The failure of the available mumps vaccine in preventing disease transmission among populations with high 2-dose vaccination coverage levels raises a question regarding the necessity of an additional mumps vaccine booster or the development of a more effective vaccine. Our results demonstrated seroconversion among seronegative persons after receipt of a third dose; however, more research is needed to understand the importance of a third dose and its relevance for future planning of specific strategies for mumps prevention.
Acknowledgments
We thank Kathryn Nickel, Lois Flagstad, Marilee Malcom, Susan Pederson, Peg Nyffeler, and other staff at the University of Nebraska at Kearney as well as Amy Elwood and other staff at the Two Rivers Public Health Department for their assistance and support during the study. We also thank Kris Bisgard, Anne O’Keefe, and Tom Török for their guidance and comments regarding the manuscript.
Financial support:
National Vaccine Program Office (grant to the study), which is administered by the Oak Ridge Institute for Science and Education through an interagency agreement (OPHS-7-039) between the US Department of Energy and the US Food and Drug Administration.
No official support or endorsement of this article by the Food and Drug Administration is intended or should be inferred. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Footnotes
Potential conflicts of interest: none reported.
Presented in part: 45th annual meeting of the Infectious Diseases Society of America, San Diego, 4-7 October 2007 (poster 860).
References
- 1.Galazka AM, Robertson SE, Kraigher A. Mumps and mumps vaccine: a global review. Bull World Health Organ 1999; 77:3–14. [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Recommendation of the Public Health Service Advisory Committee on Immunization Practices: mumps vaccine. MMWR Morb Mortal Wkly Rep 1977; 26:393–4. [Google Scholar]
- 3.Centers for Disease Control and Prevention. Measles prevention: recommendations of the Immunization Practices Advisory Committee. MMWR Morb Mortal Wkly Rep 1989; 38 (Suppl 9):1–18.2491906 [Google Scholar]
- 4.Centers for Disease Control and Prevention. Summary of notifiable diseases—United States, 2003. MMWR 2005; 52:1–85. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. National, state, and urban area vaccination coverage levels among children aged 19–35 months—United States, 1999. MMWR Morb Mortal Wkly Rep 2000; 49:585–9. [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. National, state, and urban area vaccination coverage levels among children aged 19–35 months—United States, 2003. MMWR Morb Mortal Wkly Rep 2004; 53:658–61. [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. National, state, and urban area vaccination coverage levels among children aged 19–35 months—United States, 2005. MMWR Morb Mortal Wkly Rep 2006; 55:988–93. [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Update: multistate outbreak of mumps—United States, Jan 1–May 2, 2006. MMWR Morb Mortal Wkly Rep 2006; 55:559–63. [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Update: mumps activity—United States, Jan 1–Oct 7. MMWR Morb Mortal Wkly Rep 2006; 55: 1152–3. [PubMed] [Google Scholar]
- 10.Narita M, Matsuzono Y, Takekoshi Y, et al. Analysis of mumps vaccine failure by means of avidity testing for mumps virus-specific immunoglobulin G. Clin Diagn Lab Immunol 1998; 5:799–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mauldin J, Carbone K, Hsu H, Yolken R, Rubin S. Mumps virus-specific antibody titers from pre-vaccine era sera: comparison of the plaque reduction neutralization assay and enzyme immunoassays. J Clin Microbiol 2005; 43:4847–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hummel KB, Erdman DD, Heath J, Bellini W. Baculovirus expression of the nucleoprotein gene of measles virus and utility of the recombinant protein in diagnostic enzyme immunoassays. J Clin Microbiol 1992; 30: 2874–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.SAS Institute. Getting started guide for SAS® 9.1.3 Cary, NC: SAS Institute, 2006. [Google Scholar]
- 14.Wharton M, Cochi SL, Hutcheson RH, Bistowish JM, Schaffner W. A large outbreak of mumps in the postvaccine era. J Infect Dis 1988; 158: 1253–60. [DOI] [PubMed] [Google Scholar]
- 15.Cheek JE, Baron R, Atlas H, Wilson DL, Crider RD Jr. Mumps outbreak in a highly vaccinated school population: evidence for large-scale vaccination failure. Arch Pediatr Adolesc Med 1995; 149:774–8. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan KM, Halpin TJ, Marks JS, Kim-Farley R. Effectiveness of mumps vaccine in a school outbreak. Am J Dis Child 1985; 139:909–12. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. Efficacy of mumps vaccine—Ohio. MMWR Morb Mortal Wkly Rep 1983; 32:391–2. [PubMed] [Google Scholar]
- 18.Briss PA, Fehrs LJ, Parker RA, et al. Sustained transmission of mumps in a highly vaccinated population: assessment of primary vaccine failure and waning vaccine-induced immunity. J Infect Dis 1994; 169:77–82. [DOI] [PubMed] [Google Scholar]
- 19.Hersh BS, Fine PE, Kent WK, et al. Mumps outbreak in a highly vaccinated population. J Pediatr 1991; 119:187–93. [DOI] [PubMed] [Google Scholar]
- 20.Vandermeulen C, Roelants M, Vermoere M, Roseeuw K, Goubau P, Hoppenbrouwers K. Outbreak of mumps in a vaccinated child population: a question of vaccine failure? Vaccine 2004; 22:2713–6. [DOI] [PubMed] [Google Scholar]
- 21.Miller E, Hill A, Morgan-Capner P, Forsey T, Rush M. Antibodies to measles, mumps and rubella in UK children 4 years after vaccination with different MMR vaccine. Vaccine 1995; 13:799–802. [DOI] [PubMed] [Google Scholar]
- 22.Davidkin I, Valle M, Julkunen I. Persistence of anti-mumps virus antibodies after a two dose MMR vaccination. Vaccine 1995; 13:1617–22. [DOI] [PubMed] [Google Scholar]
- 23.Plotkin S, Orenstein W. Vaccines. 4th ed. Philadelphia, PA: Saunders, 2004:441–469 (chapter 20). [Google Scholar]
- 24.Harling R, White JM, Ramsay ME, Macsween KF, van den Bosch C. The effectiveness of the mumps component of the MMR vaccine: a case control study. Vaccine 2005; 23:4070–4. [DOI] [PubMed] [Google Scholar]
- 25.Sartorius B, Penttinen P, Nilsson J, et al. An outbreak of mumps in Sweden, February–April 2004. Euro Surveill 2005; 10:191–9. [PubMed] [Google Scholar]
- 26.Cohen C, White JM, Savage EJ, et al. Vaccine effectiveness estimates, 2004–2005 mumps outbreak, England. Emerg Infect Dis 2007; 13:12–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schaffzin JK, Pollock L, Schulte C, et al. Effectiveness of previous mumps vaccination during a summer camp outbreak. Pediatrics 2007; 120: e862–8. [DOI] [PubMed] [Google Scholar]
- 28.Sartorius B, Penttinen P, Nilsson J, et al. An outbreak of mumps in Sweden, February–April 2004. Euro Surveill 2005; 10:191–9. [PubMed] [Google Scholar]
- 29.Gut JP, Lablanche C, Behr S, Kim A. Symptomatic mumps virus reinfections. J Med Virol 1995; 45:17–23. [DOI] [PubMed] [Google Scholar]
- 30.Yeung LF, Lurie P, Dayan G, et al. A limited measles outbreak in a highly vaccinated US boarding school. Pediatrics 2005; 116:1287–91. [DOI] [PubMed] [Google Scholar]
- 31.Parker AA, Staggs W, Dayan GH, et al. Implications of a 2005 measles outbreak in Indiana for sustained elimination of measles in the United States. N Engl J Med 2006; 355:447–55. [DOI] [PubMed] [Google Scholar]
- 32.Simpson RE. Infectiousness of communicable diseases in the household (measles, chickenpox, and mumps). Lancet 1952; 2:549–54. [DOI] [PubMed] [Google Scholar]
- 33.Anderson RM, May RM. Infectious disease of humans: dynamics and control. New York: Oxford University Press; 1992:70. [Google Scholar]
- 34.Chen RT, Markowitz LE, Albrecht P, et al. Measles antibody: reevaluation of protective titers. J Infect Dis 1990; 162:1036–42. [DOI] [PubMed] [Google Scholar]
- 35.Ennis FA. Immunity to mumps in an institutional epidemic: correlation of insusceptibility to mumps with serum plaque neutralizing and hemagglutination-inhibiting antibodies. J Infect Dis 1969; 119:654–7. [DOI] [PubMed] [Google Scholar]
- 36.López Hernández B, Martín Vélez RM, Román García C, Peñalver Sánchez I, López Rosique JA. An epidemic outbreak of mumps: a study of vaccine efficacy. Aten Primaria 2000; 25:148–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanz JC, Mosquera MM, Echevarría JE, et al. Sensitivity and specificity of immunoglobulin G titer for the diagnosis of mumps virus in infected patients depending on vaccination status. APMIS 2006; 114:788–94. [DOI] [PubMed] [Google Scholar]
- 38.Bloom S, Wharton M. Mumps outbreak among young adults in UK. BMJ 2005; 331:E363–4. [DOI] [PubMed] [Google Scholar]
- 39.Rubin S, Li Q, Audet S, et al. Mumps virus strain-specific antibody neutralization and waning immunity following vaccination [abstract 851]. In: Scientific program and abstracts of the 26th Annual Meeting of the American Society for Virology (Corvallis, Oregon). 2007. [Google Scholar]
- 40.Vyse AJ, Gay NJ, Hesketh LM, et al. Interpreting serological surveys using mixture models: the seroepidemiology of measles, mumps and rubella in England and Wales at the beginning of the 21st century. Epidemiol Infect 2006; 134:1303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]


