Abstract
BACKGROUND:
Penile duplex doppler ultrasound (PDDU) is a minimally invasive tool to evaluate erectile hemodynamics in patients with erectile dysfunction (ED). Despite decades of use, there is still a large variability in PDDU protocols and a high rate of false diagnosis is reported.
AIM:
Review of PDDU methodology in the published literature addressing protocol heterogeneity, technical and interpretation challenges.
METHODS:
A PubMed literature was performed using the search terms “penile doppler ultrasound”, “penile duplex ultrasound” or “penile ultrasound” and “Erectile dysfunction”. Studies were analyzed for the presence of the following elements in reporting of the PDDU protocol: (i) intracavernosal vasoactive agents used, (ii) use of a re-dosing protocol, (iii) means of rigidity assessment (iv) report of at-home best quality erection (BQE) (v) normative criteria for peak systolic velocity (PSV) and end-diastolic velocity (EDV) and (vi) use of time-based hemodynamics assessment. Inclusion criteria were studies available in English, from 2005 onwards and with full text available. Exclusion criteria were review, descriptive or short communication articles, animal studies and studies in populations other than those with ED.
OUTCOMES:
A critical review of the heterogeneity in published literature was used to guide a structured discussion of methodological challenges and to create a list of recommendations
RESULTS:
Significant heterogeneity was seen in key methodological aspects. 50% of studies reported the use of prostaglandin E1 only and 12% of studies did not mention the agent used. Redosing as part of the PDDU protocol was mentioned in only 26% of studies. The majority (56%) did not mention any form of rigidity assessment. The most frequently used grading system was the Erection Hardness Score (14%). Overall, the majority of studies (59%) used a timed-base protocol for hemodynamic assessment. No clear consensus was defined for normative criteria for peak systolic velocity (PSV) and end-diastolic velocity (EDV), 39% defining a normal PSV as ≥30cm/s, and 57% using EDV values ≤5cm/sec as normal.
CLINICAL IMPLICATIONS:
The absence of standardization has led to inadequate reporting of key factors which has rendered data interpretation and comparison between studies challenging.
STRENGTHS AND LIMITATIONS:
Our strengths include an extensive review of literature, with a structured analysis of the impact of each methodological pitfall. Our main limitation is the fact that protocol reporting, and not its application, was assessed.
CONCLUSION:
Despite its widespread use, analysis of the literature on PDDU use in the ED population shows marked protocol heterogeneity, rendering data interpretation a problem.
Keywords: Penile doppler ultrasound, Penile duplex ultrasound, Penile ultrasound, False diagnose, Venous leak, Erectile dysfunction
INTRODUCTION
Erectile dysfunction (ED) is defined as the persistent inability to achieve or maintain an erection sufficient for satisfactory sexual activity. 1 It is one of most common sexual dysfunction complaint in males seeking treatment 2, affecting 50-100% of men in their 70s and 80s. 3 In addition to being a prevalent condition, ED is known to have great impact on patient, partner and couples’ quality of life, with rates of depression in ED patients reported as high as 56%. 1, 4, 5
While for many years there was a belief that ED was primarily a psychogenic disorder, advances in the understanding of erection physiology over the past 3-4 decades have allowed the appreciation of organic causes, with current belief being that more than 80% of the ED patients have some underlying organic etiology. Among organic causes, vasculogenic ED is the most common and encompasses both insufficient arterial inflow and corporal venocclusive dysfunction (CVOD), also known as venous leak. 1
In the post-PDE5i era, while not all patients will need a detailed workup for ED, 6, 7 sexual medicine clinicians are commonly challenged with patients in whom investigation may be indicated. 7 In those cases, defining if the ED is vasculogenic may be helpful to guide patients and clinicians in the discussion for treatment options and prognosis.
Role of Penile Doppler Ultrasonography
Lue and colleagues, in 1985, first described penile duplex doppler ultrasound (PDDU) after a pharmacologically-induced erection as an alternative to assessing blood flow during erection. 8 Technological improvements over decades has made PDDU a frequently used technique, being a less invasive means of erectile hemodynamics assessment compared to the more invasive fashion dynamic infusion cavernosometry and cavernosography (DICC) and selective internal pudendal arteriography (SIPA). The classic hemodynamic parameters that are measured are: peak systolic velocity (PSV), that provides direct assessment of the arterial supply, end-diastolic velocity (EDV) and resistive Index (RI), that indirectly evaluate the venocclusive mechanism. 9
Despite decades having passed since it was first described and efforts at standardizing the procedure, there remains great heterogeneity in PDDU protocols published in the literature, including different intracavernosal vasoactive agents used, variability in the use of re-dosing schedules, different means of assessing erectile rigidity, timing of hemodynamic assessment and different hemodynamic parameter cutoffs. 10 These discrepancies are a source of significant concern in view of the high rates of false diagnosis of both CVOD and arterial insufficiency. For instance, in a study performed in patients with CVOD diagnosis based on a prior PDDU performed at an outside center, Teloken et al demonstrated a false diagnosis rate of 47%. 11 Thus, comparing such literature is a significant challenge.
METHODS
Literature Review
A PubMed literature search looking for studies using PDDU in patients with ED was performed. Search terms included “penile doppler ultrasound”, “penile duplex ultrasound” or “penile ultrasound” and “erectile dysfunction”. Studies were analyzed by three urologists with expertise in sexual medicine. Inclusion criteria were studies available in English, from 2005 onwards and with full text available. Exclusion criteria were review or descriptive articles, short communication articles, animal studies and studies in populations other than those with ED. Studies with same authorship were analyzed and if PDDU protocol was explained only as “described elsewhere”, the study was also excluded.
Evaluated Criteria
Each study was evaluated for the presence of the following elements in reporting of the PDDU technique: (i) intracavernosal vasoactive agents used, (ii) use of a redosing protocol, (iii) means of rigidity assessment (iv) report of at-home best quality erection (BQE) (v) normative criteria for peak systolic velocity (PSV) and end-diastolic velocity (EDV) and (vi) use of time-based hemodynamics assessment.
RESULTS AND DISCUSSION
From a total of 195 studies, 86 were considered eligible for this analysis. From the studies considered ineligible, 43 were review or descriptive articles, 49 were non-ED studies and 17 were for excluded for other reasons (Figure 1). All studies included are listed in the supplemental table.
Figure 1.
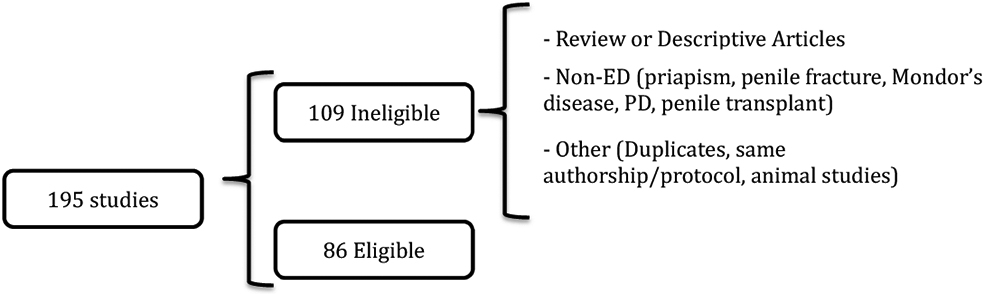
Study selection
A truism in sexual medicine is that a PDDU is never falsely normal as long as the cavernosal artery is being scanned. If a patient has a rigid erection with normal hemodynamic values after pharmacologically induced erection, ED due to macrovascular disease can be excluded. However, the same it is not true for abnormal exams and the most common cause of falsely abnormal exam involves inadequate smooth muscle relaxation most commonly due to low arousal level or high levels of adrenaline during the procedure. 11, 12
Adrenaline is known to promote smooth muscle contraction, limiting cavernosal artery relaxation, maintaining the cavernosal smooth muscle in a hypertonic state and preventing complete function of the venocclusive mechanism. 11, 13, 14 While in the bedroom setting, this translates into difficulty achieving an erection, loss of sustaining or a staccato type of erection, during a PDDU, such failure to achieve excellent smooth muscle relaxation leads to elevated EDV values suggesting venous leak. Therefore, complete smooth muscle relaxation (CSMR) is imperative to avoid false, adrenaline-mediated diagnosis of CVOD during PDDU.
Vasoactive Agents & Redosing
With regard to vasoactive agent of choice, 50% of studies used prostaglandin E1 only, 16% papaverine, 12% trimix, 7% bimix, 3% used different agents and 12% of the included studies did not mention the agent used (Table 1). The starting dose was also very variable and, from studies using PGE1, 35% used 10mcg, 5% 25mcg, 2% 15mcg, 44% used 20mcg, 2% 40mcg and 12% did not mention the dose. In studies using papaverine there was less dose heterogeneity and 64% used a starting dose of 60mg, however, 21% did not mention the dose utilized. In studies using trimix, 60% did not mention the starting dose, 20% used 50 units, 10% 25 units, and 10% 10 units. From studies using bimix the starting dose was similarly variable, with 33% failing to mention the dose and the remaining studies having starting doses varying from 10-50u. The use of a redosing protocol as part of PDDU was mentioned in only 26% of studies, with 11% using a second dose, 6% a third dose and the remaining 9% stating the possibility of redosing but not making clear the maximum number of doses permitted (Table 1).
Table 1.
Overview of current literature using PDDU – Intracavernosal agent, dose and rigidity assessment
| Number of studies (Percentage of total) | ||
|---|---|---|
| Intracavernosal Agent | Prostaglandin | 43 (50%) |
| Papaverine | 14 (16.3%) | |
| Trimix | 10 (11.6%) | |
| Bimix | 6 (7%) | |
| Multiple | 3 (3.5%) | |
| NR | 10 (11.6%) | |
| Re-dosing protocol | Yes | 22 (25.6%) |
| No | 64 (74.4%) | |
| Max number of doses | 2 | 9 (10.5%) |
| 3 | 5 (5.8%) | |
| NR | 72 (83.7%) | |
| Rigidity Assessment | None | 48 (55.8% ) |
| EHS | 12 (13.9%) | |
| 0-10 | 4 (4.6%) | |
| 0-5 | 3 (3.5%) | |
| Method NR | 19 (22.1%) | |
| BQE mentioned | Yes | 9 (10.5% ) |
| No | 77 (89.5%) |
NR = Not reported, EHS = Erection Hardness Score, BQE = Best quality erection
Comment:
Aversa et al. in 1996 showed that the combination of agents (PGE1 and alpha-blockers) allowed better erectile response in young men undergoing PDDU compared to PGE1 alone 13. Also, trimix (mix of papaverine, PGE1 and phentolamine) appears to be more effective than PGE1 in promoting complete smooth-muscle relaxation. 10, 12 Nevertheless, our review showed that the majority of studies (50%) used alprostadil as the agent of choice. Of note, the ISSM standard operating protocol (SOP) document (2013) suggests alprostadil 10mcg as a reasonable agent and starting dose. As shown in our analysis, there is marked heterogeneity in the agent and dose utilized (Table 1). However, neither the agent nor the starting dose are critical to the excellent conduct of a PDDU. Ultimately, maximal CCSM relaxation is the goal, and the possibility of redose may be a key point.
The evidence that redosing improves the accuracy of penile hemodynamic evaluation is robust. In a study from 2001, Mulhall et al. 15 tested the impact of a redosing schedule in patients undergoing DICC. if abnormal hemodynamics were evident, redosing was performed. The authors showed that 70% of patients warranted redosing and that in approximately one third of them, the diagnosis was changed from one of CVOD to normal. Importantly, half of the patients whose CVOD diagnosis was changed required three injections.
The impact of redosing on PDDU sensitivity is also very clear. 11, 16, 17 In a study by Teloken et al. 11, by following a PDDU protocol that utilized a redosing schedule, the authors demonstrated normalization of a CVOD diagnosis in more than a quarter of patients. Of note, in this study the mean number of injections per patient was 2.2 and best quality at-home erection (BQE) was achieved in 74% of exams, as compared to a mean number of injections of 1.2 and a BQE rate of 32% in the prior outside PDDU. The optimal BQE is a nocturnal erection if present, and is ultimately, a surrogate marker for complete smooth muscle relaxation, as discussed further in the review.
Furthermore, Gontero and colleagues 17 showed in patients with an initial EDV>5cm/sec after PGE1 injection that over 50% had the EDV normalized after addition of phentolamine. Also, Aversa et al 16 showed a change in the final diagnosis in 35% of patients by redosing with a combination of PGE1 and phentolamine.
Therefore, appropriate redosing seems an effective way to increase the chances of achieving complete CCSM relaxation and ensuring accuracy of the hemodynamic diagnosis. Nevertheless, our literature review showed that only 26% of studies cited redosing in their PDDU protocols, raising significant concerns regarding the veracity of the PDDU results.
Rigidity Grading:
The majority of studies (56%) did not mention any form of rigidity assessment. The most frequently used grading system was the Erection Hardness Score (14%) followed by 0-10 score (5%) and 0-5 score (3%), with the remaining 22% mentioning that rigidity assessment failed to mention what methodology or grading system was utilized. (Table 1).
Comment:
As previously stated, the goal of administration of vasoactive agents is to promote optimal smooth muscle relaxation (SMR), which allows the development of a rigid erection in patients without vasculogenic ED. Therefore, rigidity alone is an important factor to be reported. However, there are cases in cases in which erectile rigidity can be even more relevant, being an important guidance in the decision making process to redose a patient or not, with a significant impact on PDDU reliability as discussed above.
In patients with true CVOD, even with optimal SMR, a rigid erection may not develop. In such cases, the clinical surrogate to infer complete CCSM relaxation was achieved is the development of patient’s best quality at-home erection (BQE) during PDDU. BQE is the best erection grade a patient has experienced under any circumstances at home. This could be a masturbatory erection, sex erection or a nocturnal erection – scenarios with potentially less anxiety than the PDDU settings. If a BQE is not achieved, unless the hemodynamics are normal, it is advisable to administer further vasoactive medication.
The 2 most comprehensive studies using BQE did so as a means of deciding if redosing was needed. Aversa and colleagues 16 considered patients failing to achieve a BQE after 10mcg of PGE1 as “non-responders”, and all underwent redosing. Similarly, Teloken and colleagues 11 in their protocol, defined the need for re-dosing based on the ability to obtain a BQE. By repeating the vascular testing with this protocol in a group of men diagnosed with CVOD by an outside PDDU, Teloken et al demonstrated completely normal hemodynamics in 47% 11
Therefore, having a reliable way to assess rigidity and inquiring about BQE before the exam commences may be helpful to the clinician in defining if a patient needs redosing or if the abnormal hemodynamic parameters are seen under adequate CCSM relaxation. Despite that, the majority of studies (56%) failed to mention any rigidity assessment, and the report of BQE was done in only 10% of studies (Table 1).
Timed-Based Protocol
Overall, the majority of studies (59%) used a timed-base protocol for hemodynamic assessment. Among those, the vast majority (84%) used multiple time assessments after intracavernosal injection, the most common approach being to measure 3 times: after 5, 10 and 20 minutes. There were 8 studies (16%) assessing hemodynamics values based on a single time point after ICI (Table 2).
Table 2.
Overview of current literature using PDDU – Protocol and normative hemodynamic values
| Number of studies (Percentage) | ||
|---|---|---|
| Time-Based Protocol | Yes | 51 (59,3%) |
| No | 35 (40,7%) | |
| Total | 86 | |
| Time-Based Schedule | Multiple Meas | 43 (84,3%) |
| Single Meas | 8 (15,7%) | |
| Total | 51 | |
| PSV cutoff | <35 cm/sec | 13 (15.1%) |
| <30 cm/sec | 32 (37.2%) | |
| <25 cm/sec | 20 (23.3%) | |
| NR | 21 (24.4%) | |
| EDV cutoff | >5cm/sec | 49 (57%) |
| >6cm/sec | 2 (2,3%) | |
| >3 cm/sec | 1 (1,2%) | |
| NR | 34 (39,5%) |
PSV = Peak Systolic Velocity; EDV = End Diastolic Velocity; NR = Not reported
Comment:
The ISSM SOP recommends time-based PDDU, with hemodynamic parameter assessment at several time points up to 30 minutes. 10 We observed a great variety in the interval between assessments and length of measurements after ICI, with some authors tracking hemodynamic changes for up to 45 minutes.18
The other approach to hemodynamics assessment is rigidity-based, where time is not the guiding factor but rather the patient’s erection rigidity. We believe that this approach has the advantage of being a better indicator of complete CCSM relaxation, as discussed above. The idea that patients should be scanned at timed intervals, for example every 10 minutes, makes little sense if the erection rigidity is poor. Thus, while the clinician should allow enough time for the vasoactive agent to take effect, it is the erection rigidity which should define the best moment to assess hemodynamic parameters.
Irrespective of which protocol is used, time versus rigidity, a number of caveats exist. It is known that peak systolic velocity can be suppressed in the final phase of erection while intracavernosal pressure can be greater than systolic blood pressure resulting in obstruction of the cavernosal arterial inflow. 19 On such occasions, it might be impossible to tell a patient the exact PSV, but they can be informed that their venocclusive mechanism is intact.
Hemodynamics Parameter Cut-offs
Great variability in hemodynamic parameter cut-offs used was also observed in our literature analysis. 37% of studies considered peak systolic velocity (PSV) abnormal if <30cm/s, 23% <25cm/s, 15% <35cm/s and 24% did not mention which PSV cut-off was used. With regard to end diastolic velocity (EDV) cutoffs, 57% considered abnormal EDV values >5cm/sec, with just 4% using >6cm/sec and a single study (2%) using >3cm/sec as the cutoff. Finally, 40% of studies did not mention which EDV cut-off value was used to define CVOD (Table 2).
Comment:
It is worthwhile remembering that in situations where cut-offs are used, the process of finding the cut-off involves the choice of a value with optimal sensitivity and specificity. 20 Choosing a cutoff for normal PSV of >35cm/s instead of >25cm/s will increase the sensitivity for diagnosing cavernosal artery insufficiency but, at the same time, will decrease the specificity. This behavior is similar for all tests with continuous results and it is ultimately defined using a ROC curve (Receiver Operating Characteristics) – a tradeoff between test sensitivity and specificity. 20 In a study from Quam et al 21comparing PDDU with SIPA and DICC results, they found that all 5 patients with abnormal arteriography had a PSV <25 cm/sec (sensitivity 100%), with 6 of the 7 patients with normal arteriography showing a PSV ≥25cm/sec (specificity, 85.7%). In regards to venoclusive integrity, authors found that an EDV cutoff of >5cm/sec could identify patients with CVOD with a sensitivity of 90% and a specificity of 56%,
The International Society for Sexual Medicine through the Standard Operating Procedure publication (ISSM SOP) discusses that normal values for hemodynamic parameters should be a range and not a specific single value. Accepted normal intervals cited are from 25-30cm/sec for PSV and 3-6cm/sec for EDV. 10 There are, however, other authors suggesting normal hemodynamic values ranging from 25-35cm/sec and 5-7cm/sec. 22 in our review, we saw much smaller heterogeneity in EDV cut-offs, with majority (57%) of studies reporting a cutoff used <5cm/s for normalcy (Table 2).
Another interesting point to be discussed in hemodynamic parameters is the development of negative EDV. End-diastolic velocity (EDV) is the measurement of cavernosal arterial blood flow velocity at the end of diastole.23 Corpus cavernosal pressure rises as a consequence of venous outflow obstruction after complete CCSM relaxation is achieved and emissary veins are compressed against tunica albuginea. 24 When intracavernosal pressure (ICP) rises above diastolic pressure, negative EDV occurs. As a consequence, negative EDV can be used as a sign of the achievement of complete CCSM relaxation. 25
Need for Reversal
It is essential that any clinic in which PDDU is performed have the appropriate resources to promptly treat a prolonged erection after PDDU. Literature shows a prolonged erection and priapism rate of up to 10% with Trimix and Bimix (mix of papaverine and phentolamine), 2.7% with papaverine and close to 1% with PGE1 alone. 10, 26-28 The frequency of patients needing reversal was described in only 7% of studies.
Comment:
Priapism can be safely avoided with appropriate pharmacologic reversal with an alpha-agonist.10, 16, 29 In a study from Jiang and colleagues 29 in the population with Peyronie’s disease and using a protocol with re-dosing based on erectile response, reversal with phenylephrine was performed if patients showed sustained erection for longer than 45-60min. Authors reported that reversal was performed in 57% of patients, but they had no cases of priapism. This result resembles those in our practice and we believe that any patient with prolonged (longer than 1 hour) or painful erections should be offered reversal.
Overall, therefore, it seems unreasonable to decrease intracavenosal agent dosing for the “safety” of the exam, especially in the view of the discussion above linking under-dosing to falsely abnormal diagnoses. Furthermore, the rates of erection reversal are seldomly reported in the literature and we considered this inadequate reporting.
Clinical Implications
The major implications from our review are two-fold: firstly, that there is no rigorous standardization of measurement guidelines; secondly, the failure to have a standardized reporting checklist makes interpretation of individual studies and comparison of different studies challenging. Mean PSV and EDV values are very attractive as they are easily reported and allow a generally straightforward statistical analysis. However, the percentage of men falling outside normative values is very important to understand the frequency of abnormal hemodynamics. Furthermore, without information on important factors, such as, the use of a vasoactive agent redosing schedule and rigidity during the study, full interpretation of the data is impossible.
Strengths and Limitations
Our strengths were the fact that an extensive review of current literature was performed, with a comprehensive discussion on the impact of each methodological factor on study interpretation.
RECOMMENDATIONS
Based on the discussion above and the lack of consensus in the literature pertaining the performance and interpretation of PDDU, we would like to make the following recommendations:
Training – We believe that PDDU should only be conducted by clinicians who have received formal training in the performance of PDDU and the analysis of the data generated. Furthermore, all clinicians should be facile with the reversal of any prolonged erection. Clinicians should be capable of performing a full informed consent discussion regarding all aspects of the procedure.
Equipment – Clinicians should use appropriate equipment to visualize penile structures, measure erectile hemodynamics and interpret the data appropriately. We believe that PDDU is best performed using a machine capable of color Doppler waveform analysis and a high frequency (10-15 MHz) probe capable of evaluating the structure of the erectile tissue and the tunica albuginea, as well as delineating cavernosal arteries and recording the erectile hemodynamics.
Redosing – Given that the ultimate goal of the administration of vasoactive agents is the achievement of complete cavernosal smooth muscle relaxation, we encourage all clinicians performing PDDU to use a redosing schedule to optimize the reliability and reproducibility of the hemodynamic data acquired
Rigidity Assessment – We believe that rigidity-based hemodynamic assessment is preferable over time-based assessment. To this end, clinicians should record the rigidity at the time of scanning the penis, and this rigidity should be recorded separately for right and left cavernosal arteries during the hemodynamic assessment.
Hemodynamic Criteria – The normative criteria used during the study for PSV and EDV depends on the sensitivity and specificity that the clinician is trying to achieve. While the range between 25-35cm/sec has been cited as a definition for cavernosal artery insufficiency, in an effort to achieve global consensus, we encourage clinicians to use a PSV value < 30cm/sec to diagnose arterial inflow insufficiency. Likewise, while normative EDV values have been cited between 3-7cm/sec, we recommend a value greater or equal 5cm/sec tp diagnose venous leak.
Reversal – We strongly discourage clinicians from discharging patients from their offices until the patient has detumesced below penetration hardness. Furthermore, all patients should be given explicit information as to the steps that should be taken should a delayed prolonged erection occur.
Clinicians Judgement – As with nearly all functional tests in medicine, PDDU is not 100% accurate. Therefore, the clinicians should always use their clinical judgement in the interpretation of erectile hemodynamic.
CONCLUSION
Despite its generalized use, analysis of literature using PDDU in ED population shows marked protocol heterogeneity, challenging result interpretation. Common problems include lack of rigidity reporting (56% of studies), absence of a re-dosing schedule (74% of studies) and hemodynamic cut-off heterogeneity. Clinicians should be aware of the impact those factors have on the accuracy of results.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest and Disclosure Statement: None
References
- [1].Yafi FA, Jenkins L, Albersen M, et al. Erectile dysfunction. Nat Rev Dis Primers. 2016;2: 16003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Albersen M, Shindel AW, Mwamukonda KB, Lue TF. The future is today: emerging drugs for the treatment of erectile dysfunction. Expert Opin Emerg Drugs. 2010;15: 467–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lewis RW, Fugl-Meyer KS, Corona G, et al. Definitions/epidemiology/risk factors for sexual dysfunction. J Sex Med. 2010;7: 1598–607. [DOI] [PubMed] [Google Scholar]
- [4].Malavige LS, Jayaratne SD, Kathriarachchi ST, Sivayogan S, Ranasinghe P, Levy JC. Erectile dysfunction is a strong predictor of poor quality of life in men with Type 2 diabetes mellitus. Diabet Med. 2014;31: 699–706. [DOI] [PubMed] [Google Scholar]
- [5].Nelson CJ, Mulhall JP, Roth AJ. The association between erectile dysfunction and depressive symptoms in men treated for prostate cancer. J Sex Med. 2011;8: 560–6. [DOI] [PubMed] [Google Scholar]
- [6].Hatzichristou D, Hatzimouratidis K, Bekas M, Apostolidis A, Tzortzis V, Yannakoyorgos K. Diagnostic steps in the evaluation of patients with erectile dysfunction. J Urol. 2002;168: 615–20. [PubMed] [Google Scholar]
- [7].Lobo JR, Nehra A. Clinical evaluation of erectile dysfunction in the era of PDE-5 inhibitors. Urol Clin North Am. 2005;32: 447–55, vi. [DOI] [PubMed] [Google Scholar]
- [8].Lue TF, Hricak H, Marich KW, Tanagho EA. Vasculogenic impotence evaluated by high-resolution ultrasonography and pulsed Doppler spectrum analysis. Radiology. 1985;155: 777–81. [DOI] [PubMed] [Google Scholar]
- [9].Aversa A, Sarteschi LM. The role of penile color-duplex ultrasound for the evaluation of erectile dysfunction. J Sex Med. 2007;4: 1437–47. [DOI] [PubMed] [Google Scholar]
- [10].Sikka SC, Hellstrom WJ, Brock G, Morales AM. Standardization of vascular assessment of erectile dysfunction: standard operating procedures for duplex ultrasound. J Sex Med. 2013;10: 120–9. [DOI] [PubMed] [Google Scholar]
- [11].Teloken PE, Park K, Parker M, Guhring P, Narus J, Mulhall JP. The false diagnosis of venous leak: prevalence and predictors. J Sex Med. 2011;8: 2344–9. [DOI] [PubMed] [Google Scholar]
- [12].Ghanem H, Shamloul R. An evidence-based perspective to commonly performed erectile dysfunction investigations. J Sex Med. 2008;5: 1582–9. [DOI] [PubMed] [Google Scholar]
- [13].Aversa A, Rocchietti-March M, Caprio M, Giannini D, Isidori A, Fabbri A. Anxiety-induced failure in erectile response to intracorporeal prostaglandin-E1 in non-organic male impotence: a new diagnostic approach. Int J Androl. 1996;19: 307–13. [DOI] [PubMed] [Google Scholar]
- [14].Nehra A, Goldstein I, Pabby A, et al. Mechanisms of venous leakage: a prospective clinicopathological correlation of corporeal function and structure. J Urol. 1996;156: 1320–9. [DOI] [PubMed] [Google Scholar]
- [15].Mulhall JP, Abdel-Moneim A, Abobakr R, Goldstein I. Improving the accuracy of vascular testing in impotent men: correcting hemodynamic alterations using a vasoactive medication re-dosing schedule. J Urol. 2001;166: 923–6. [DOI] [PubMed] [Google Scholar]
- [16].Aversa A, Bonifacio V, Moretti C, Frajese G, Fabbri A. Re-dosing of prostaglandin-E1 versus prostaglandin-E1 plus phentolamine in male erectile dysfunction: a dynamic color power Doppler study. Int J Impot Res. 2000;12: 33–40. [DOI] [PubMed] [Google Scholar]
- [17].Gontero P, Sriprasad S, Wilkins CJ, Donaldson N, Muir GH, Sidhu PS. Phentolamine re-dosing during penile dynamic colour Doppler ultrasound: a practical method to abolish a false diagnosis of venous leakage in patients with erectile dysfunction. Br J Radiol. 2004;77: 922–6. [DOI] [PubMed] [Google Scholar]
- [18].Safarinejad MR, Lashkari MH, Babaei A, Dadkhah F, Kolahi AA. Penile vascular indices in surgically treated and conservatively treated penile fracture: does conventional immediate repair matter? Int Urol Nephrol. 2012;44: 1631–40. [DOI] [PubMed] [Google Scholar]
- [19].Jung DC, Park SY, Lee JY. Penile Doppler ultrasonography revisited. Ultrasonography. 2018;37: 16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Habibzadeh F, Habibzadeh P, Yadollahie M. On determining the most appropriate test cut-off value: the case of tests with continuous results. Biochem Med (Zagreb). 2016;26: 297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Quam JP, King BF, James EM, et al. Duplex and color Doppler sonographic evaluation of vasculogenic impotence. AJR Am J Roentgenol. 1989;153: 1141–7. [DOI] [PubMed] [Google Scholar]
- [22].Terris MK, Klaassen Z. Office-based ultrasound for the urologist. Urol Clin North Am. 2013;40: 637–47. [DOI] [PubMed] [Google Scholar]
- [23].Berookhim BM. Doppler Duplex Ultrasonography of the Penis. J Sex Med. 2016;13: 726–31. [DOI] [PubMed] [Google Scholar]
- [24].Dean RC, Lue TF. Physiology of penile erection and pathophysiology of erectile dysfunction. Urol Clin North Am. 2005;32: 379–95, v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Pagano MJ, Stahl PJ. Variation in Penile Hemodynamics by Anatomic Location of Cavernosal Artery Imaging in Penile Duplex Doppler Ultrasound. J Sex Med. 2015;12: 1911–9. [DOI] [PubMed] [Google Scholar]
- [26].Metawea B, El-Nashar AR, Gad-Allah A, Abdul-Wahab M, Shamloul R. Intracavernous papaverine/phentolamine-induced priapism can be accurately predicted with color Doppler ultrasonography. Urology. 2005;66: 858–60. [DOI] [PubMed] [Google Scholar]
- [27].Kilic M, Serefoglu EC, Ozdemir AT, Balbay MD. The actual incidence of papaverine-induced priapism in patients with erectile dysfunction following penile colour Doppler ultrasonography. Andrologia. 2010;42: 1–4. [DOI] [PubMed] [Google Scholar]
- [28].The long-term safety of alprostadil (prostaglandin-E1) in patients with erectile dysfunction. The European Alprostadil Study Group. Br J Urol. 1998;82: 538–43. [PubMed] [Google Scholar]
- [29].Jiang P, Christakos A, Fam M, Sadeghi-Nejad H. Prophylactic phenylephrine for iatrogenic priapism: a pilot study with Peyronie's patients. Korean J Urol. 2014;55: 665–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


