Abstract
Background:
Self and informant (proxy or study partner) reports of everyday cognitive functioning have been shown to be associated with incipient neurodegenerative disease. The 20-item Cognitive Change Index (CCI) and the 39-item Measurement of Everyday Cognition (ECog) were each developed to characterize early subjective changes in cognitive function.
Objective:
We examined the relationship between CCI and ECog self and informant-based evaluations to determine content overlap and provide a co-calibration for converting between these widely used instruments.
Methods:
950 participants (57.1% female, mean age = 71.2 years) from ADNI and the Indiana ADRC with self-based evaluations and 279 participants (60.9% female, mean age = 71.8 years) with informant-based evaluations (Indiana ADRC) were included. Analyzed variables for the CCI and ECog included domain mean scores, memory domain total scores, and total scores for all items. Spearman correlations, regression analyses, and frequency distributions were used to assess the relationship between CCI and ECog. Sex, age, years of education, race/ethnicity, APOE ε4 carrier status, and baseline diagnosis were also analyzed as potentially relevant covariates.
Results:
CCI and ECog total scores were highly correlated for the self (r = 0.795, p < 0.001) and informant-based (r = 0.840, p < 0.001) versions, as expected. Frequency distributions of self and informant total scores were generated and plotted separately. Quadratic regressions for self (r2 = 0.626) and informant (r2 = 0.741) scores were used to create a translation table between the CCI and ECog total scores.
Conclusion:
Self and informant total scores can be harmonized and translated between the CCI and ECog to facilitate cross-study and longitudinal assessment of perceived cognitive change, an important patient-reported outcome.
Keywords: Alzheimer’s disease, co-calibration, cognitive assessment screening instrument, cognitive decline, harmonization, subjective cognitive decline
INTRODUCTION
Prior to the onset of objective cognitive impairment, individuals with preclinical Alzheimer’s disease (AD) often present with self reports of cognitive decline or reports of cognitive decline by someone who knows them well [1]. Subjective cognitive decline (SCD) is a term given to the period of preclinical dementia during which some decline in everyday cognitive functioning is subjectively recognized by an individual, but there is no evidence of a deficit on objective cognitive tests [2, 3]. The presence of significant subjective cognitive concerns can serve as an additional factor to identify individuals at increased risk of progressing to mild cognitive impairment (MCI) or dementia. In previous studies, SCD has been shown to be associated with relevant AD biomarker abnormalities [4–6] and later a diagnosis of MCI or AD [1]. These concerns are important for early detection of dementia because they have been shown to present as early as 15 years prior to diagnosis of MCI or AD [1].
A variety of cognitive assessment tools are available to screen for the early stages of neurodegenerative disease. The Mini-Mental State Examination (MMSE) [7] and Montreal Cognitive Assessment (MoCA) [8] are objective cognitive assessments commonly used for dementia screening; however, they have limitations in terms of detecting these early stages [9]. The 20-item Cognitive Change Index (CCI) [10] (Supplementary Table 1) and the 39-item Measurement of Everyday Cognition (ECog) [9] (Supplementary Table 2) are two widely used tools developed specifically to assess SCD. The 12-item CCI-12 is a shortened version, consisting of only the memory domain, that has also been used to evaluate SCD. The CCI and ECog emphasize ecological validity as they are directly relevant to activities of daily living. These instruments are complementary to performance based cognitive assessments such as the MMSE and MoCA. SCD as a clinical entity has multiple potential determinants and requires evaluation by a specialist for proper diagnosis. SCD has been shown to be associated with a higher likelihood of onset of neurodegenerative disease [11, 12], which is important, as it informs clinicians about possible risk factors. Both tools have subject (self) and informant (proxy or study partner) versions to evaluate the extent of cognitive concerns for memory, executive functioning, and language. The ECog has additional items to assess the visual-spatial domain. The CCI and ECog each have a different Likert scale and total number of items. There is currently no method for directly comparing or translating between CCI and ECog scores.
The aim of this study is to define the relationship between these tools and create a crosswalk to improve quantitative assessment of cognitive concerns. Crosswalk or translation tables are commonly employed to map scores from one cognitive instrument to another. For example, a crosswalk table was developed to translate between MMSE and MoCA scores to improve clinical care and advance research efforts related to cognitive decline. This cross-sectional study provides a means to co-calibrate data translating between the CCI and the ECog to improve research and future clinical assessments and promote future cross-study and longitudinal analyses.
MATERIALS AND METHODS
Participants
Data used in the preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative phases 2 and 3 (ADNI-2/3) and the Indiana Alzheimer’s Disease Research Center (IADRC) including participants from the Indiana Memory and Aging Study (IMAS) and the IADRC Clinical Core cohort.
ADNI was launched in 2003 by the National Institute on Aging (NIA), the National Institute of Biomedical Imaging and Bioengineering (NIBIB), the Food and Drug Administration (FDA), and private pharmaceutical companies and non-profit organizations. One of the primary goals of ADNI has been to test whether neuropsychological assessment can be used to measure the progression of MCI and early AD. See the ADNI website (https://adni.loni.usc.edu) for more details. The IADRC, including the IMAS phase and the IADRC Clinical Core cohort, included objective and subjective evaluation of cognitive function as part of longitudinal observational studies of older adults at risk for and with clinical AD. The IMAS phase included a subset of participants enrolled prior to the co-enrollment of participants from IMAS into the IADRC Clinical Core cohort. Informed consent for all included data was obtained by the respective study protocol according to the Declaration of Helsinki.
Data were acquired between 2012 and 2019, and included 639 participants from ADNI, 326 participants from IADRC cohort, and 20 participants from IMAS. At baseline, 615 participants were classified as cognitively normal (CN), 261 were considered to have MCI, and 109 were diagnosed with AD. Participants were included if they or their informant had completed equal to or more than 75% of the items on both the CCI and the ECog, and if the assessments were completed less than a year apart. In the ADNI protocol, the CCI is obtained during the screening visit, whereas the ECog is administered at baseline visit. On average, the difference in time was less than 2 months. In the IMAS and IADRC protocols, the assessments can be completed beforehand or at the time of visit, but only the visit date was recorded. 950 self-based assessments and 279 informant-based assessments were available for analysis. Sex, age, years of education, race/ethnicity, APOE ε4 carrier status, baseline diagnosis, and time difference between administration of the assessments were analyzed as potentially relevant covariates and did not have any significant impact on the results. This study was approved by the Indiana University (IU) Institutional Review Board for data access and analysis.
Clinical diagnosis of CN, MCI, or AD was determined based on the diagnostic criteria of each individual study which were highly similar. To summarize, CN participants were defined as having no significant cognitive concerns or psychometric deficits and had preserved functional status. MCI was defined as having cognitive concerns, cognitive impairment, no dementia, and generally preserved functional status [13]. Classification of AD as a clinical syndrome was based on the presence of dementia, history of progressive decline in memory, and absence of another primary explanatory neurological disorder. Definitive diagnosis of AD rather than another dementia or mixed pathology requires postmortem evaluation, but for the purposes of this study, the term AD was used to indicate probable AD dementia in participants who are still living. These clinical classifications were highly similar in ADNI and the IADRC samples. The ADNI database includes participants ages 55 to 90. Participants from the IADRC cohort and IMAS phase were included if their age was greater than 50. Participants were not included if they had a major neurological or psychiatric disorder such as major depressive disorder, bipolar disorder, or schizophrenia other than AD. Notably, minor depression and anxiety were not excluded.
Cognitive Change Index (CCI) and Measurement of Everyday Cognition (ECog)
The CCI consists of 20 items that ask participants and their informants to rate the participant’s cognitive status relative to the previous 5 years [10]. The self and informant evaluation forms are available from the authors (contact Dr. Saykin at E-mail asaykin@iupui.edu). Each item is rated on a Likert scale out of 5 possible points (1 = no change or better, 2 = minimal change or slight/occasional problem, 3 = some change or mild problem, 4 = clearly noticeable change or moderate problem, 5 = much worse or severe problem). There are 12 items in the episodic memory domain, 5 items in the executive functioning domain, and 3 items in the language domain. The CCI-12 consists of the memory domain only and has also been used to define SCD.
The Ecog includes 39 items that ask participants and their informants to rate the participant’s cognitive status relative to 10 years ago (contact Dr. Tomaszewski Farias at E-mail: sarah.farias@ucdmc.ucdavis.edu. for a copy of the 39 item Ecog) [9]. Each item is rated out of 4 possible points (1 = better or no change, 2 = questionable/occasionally worse, 3 = consistently a little worse, 4 = consistently much worse, 9 = don’t know). There are 8 items in the memory domain, 9 items in the language domain, 15 items in the executive function domain (in three sub-domains: planning, organization, and divided attention), and 7 items in the visual-spatial domain. Of note, the CCI does not have a visual-spatial domain.
For both the CCI and Ecog, a mean score for each domain, a total score, and a total memory score were calculated to determine which calculation had the highest correlation between the assessment tools. Domain mean scores were calculated by dividing the sum of each domain by the number of items. Total scores were determined by adding together the points for each item in the assessment. Total memory scores were calculated by adding the points for each item in the memory domain alone.
Statistical analysis
Descriptive statistics of participant characteristics were calculated including frequency for categorical variables and mean and standard deviation for continuous measures. Characteristics including age, sex, years of education, race/ethnicity, APOE ε4 carrier status, CCI scores, and Ecog scores were compared between ADNI, IADC, and IMAS self and informant-based reports. Since participants were included if they had completed at least 75% of both assessments, there was some item level missing data. Linear interpolation was performed using SPSS 27 to impute this missing data. One-way ANOVA multiple comparisons test was used to compare total scores for each measure between diagnostic groups. Post-hoc comparisons were performed using a Bonferroni test. Self and informant-based data were analyzed independently. For each of these groups, associations between CCI and ECog mean domain scores for the memory, executive function, and language domains were assessed using Spearman correlation. Spearman correlation was used because the self-based and informant-based datasets were determined to be non-parametric. Repeating the regression models after rank score transform-based normalization yielded little change supporting the validity of the regression models. Additionally, associations between CCI and ECog total scores for the memory domain were also assessed using Spearman correlation. Histograms of self-based and informant-based CCI and ECog total scores were plotted using a bin width of 10 and a bin center starting at 20 and 40 for the CCI and ECog respectively. Relationships were established between CCI and ECog total scores using polynomial regression, and it was determined that a quadratic model provided the greatest adjusted R-square value compared to linear and cubic models. Adjusted R-square values were used to adjust for the number of terms in each analysis model. The quadratic equation was then used to generate crosswalk tables by using the CCI to predict the ECog [14]. Statistical analyses were performed using SPSS 27 and GraphPad Prism 9, and plots were generated using GraphPad Prism 9.
RESULTS
Participant demographic characteristics
There were 639 participants from the ADNI cohort and 311 participants from the IADRC study and IMAS studies in the self-based assessment group. Demographic characteristics are shown in Table 1. The mean age of all participants in the self-based assessment group was 71.2 years (range: 52–93 years), with 57.1% female and 83.6% Non-Hispanic White. The mean education for participants in the self-based assessment group was 16.4 years (range: 7–23). The mean self-based total score using the imputed data was 60.7 ± 20.2 (39–144) for the ECog and 37.3 ± 14.5 (20–88) for the CCI.
Table 1.
Demographic Characteristics of the Cohorts
| A) Demographic Characteristics of Self-Based Assessment Cohort | |||||
|---|---|---|---|---|---|
|
| |||||
| CN (n = 612) | MCI (n = 255) | AD (n = 83) | p | Pairwise* | |
| Age (y) | 70.6 (6.8) | 72.1 (7.6) | 73.1 (9.3) | 0.001 | MCI, AD>CN |
| Education (y) | 16.5 (2.5) | 16.2 (2.6) | 16.0 (2.3) | 0.06 | None |
| Gender (M, F) | 228, 384 | 137, 118 | 43, 40 | < 0.001 | n/a |
| Race/Ethnicity (%NHW) | 85.1% | 80.4% | 82.0% | 0.53 | n/a |
| APOE Genotype (% ε4+)1 | 34.1% | 48.7% | 60.0% | < 0.001 | n/a |
| CDR-Sum of Boxes2 | 0.1 (0.3) | 1.5 (1.0) | 4.4 (1.9) | < 0.001 | AD > MCI > CN |
| NPI-Q Total3 | 1.1 (2.6) | 4.2 (6.0) | 7.9 (8.5) | < 0.001 | AD > MCI > CN |
| GDS Total4 | 1.0 (1.3) | 2.1 (2.0) | 1.9 (1.8) | < 0.001 | AD, MCI > CN |
| MoCA Total Score5 | 26.0 (2.7) | 22.6 (3.2) | 17.2 (4.7) | < 0.001 | CN > MCI > AD |
| Story Immediate Recall (z)6 | 0.2 (0.9) | −1.2 (1.1) | −2.6 (0.8) | < 0.001 | CN > MCI > AD |
| Story Delayed Recall (z)6 | 0.2 (0.9) | −1.1 (1.0) | −2.4 (0.7) | < 0.001 | CN > MCI > AD |
| List Learning Immediate (z)7 | −0.1 (1.0) | −1.1 (1.0) | −2.4 (0.8) | < 0.001 | CN > MCI > AD |
| List Learning Delayed (z)7 | −0.2 (1.2) | −1.4 (1.3) | −2.5 (0.7) | < 0.001 | CN > MCI > AD |
| Naming Score (z)8 | −1.0 (3.0) | −0.8 (2.2) | −2.5 (3.4) | < 0.001 | CN, MCI > AD |
|
| |||||
| B) Demographic Characteristics of Participants in Informant-Based Assessment Cohort | |||||
|
| |||||
| CN (n = 163) | MCI (n = 75) | AD (n = 41) | p | Pairwise* | |
|
| |||||
| Age (y) | 71.0 (8.3) | 73.0 (7.7) | 72.6 (11.6) | 0.20 | None |
| Education (y) | 15.9 (2.7) | 16.0 (2.8) | 15.3 (3.3) | 0.44 | None |
| Gender (M, F) | 63, 100 | 31, 44 | 15, 26 | 0.87 | n/a |
| Race/Ethnicity (%NHW) | 88.3% | 74.7% | 75.6% | 0.02 | n/a |
| APOE Genotype (% ε4+)9 | 33.3% | 61.3% | 61.0% | < 0.001 | n/a |
| CDR-Sum of Boxes10 | 0.2 (0.5) | 1.4 (1.0) | 6.1 (4.6) | < 0.001 | AD > MCI > CN |
| NPI-Q Total11 | 1.6 (2.5) | 3.6 (3.8) | 7.2 (6.2) | < 0.001 | AD > MCI > CN |
| GDS Total12 | 1.4 (1.7) | 3.0 (2.6) | 2.3 (2.5) | < 0.001 | AD, MCI > CN |
| MoCA Total Score13 | 26.0 (2.9) | 21.6 (3.3) | 14.9 (6.1) | < 0.001 | CN > MCI > AD |
| Story Immediate Recall (z)14 | 0.3 (1.0) | −1.2 (1.2) | −2.5 (1.0) | < 0.001 | CN > MCI > AD |
| Story Delayed Recall (z)14 | 0.2 (1.0) | −1.0 (1.3) | −2.1 (1.1) | < 0.001 | CN > MCI > AD |
| List Learning Immediate (z)15 | −0.1 (1.0) | −1.4 (0.9) | −2.4 (0.9) | < 0.001 | CN > MCI > AD |
| List Learning Delayed (z)15 | 0.1 (1.0) | −1.6 (1.0) | −2.5 (0.8) | < 0.001 | CN > MCI, AD |
| Naming Score (z)16 | −0.4 (2.3) | −1.1 (2.3) | −1.8 (2.7) | 0.001 | CN, MCI > AD |
Missing 62 participants (23 CN, 31 MCI, 8 AD).
Missing 3 participants (2 CN, 1 MCI).
Missing 22 participants (14 CN, 8 MCI).
Missing 1 participant (1 MCI).
Missing 16 participants (5 CN, 7 MCI, 4 AD).
Missing 4 participants (3 CN, 1 MCI).
Missing 33 participants (20 CN, 10 MCI, 2 AD).
Missing 7 participants (4 CN, 2 MCI, 1 AD).
Missing 1 participant (1 CN).
Missing 1 participant (1 CN).
Missing 4 participants (4 CN).
Missing 3 participants (3 AD).
Missing 5 participants (2 MCI, 3 AD).
Missing 5 participants (5 AD).
Missing 32 participants (10 CN, 7 MCI, 15 AD).
Missing 5 participants (5 AD).
Bonferroni corrected p < 0.05
There were 279 participants from the IADRC and IMAS studies in the informant-based assessment group. 244 of these reports were for individuals for whom self-based data was also included. The mean age of all participants in the informant-based assessment group was 71.8 years (range: 52–95 years), with 60.9% female and 82.8% Non-Hispanic White. The mean education for participants in the informant-based assessment group was 15.9 years (range: 4–22). The mean informant-based total score for the ECog was 66.5 ± 30.7 (range: 39–156) and for the CCI was 39.5 ± 21.0 (range: 20–100).
Total scores in each diagnostic group
The diagnostic groups differed significantly from each other in terms of total scores for the CCI and ECog self and informant-based assessments (Table 2). The CCI and ECog total scores were the lowest in the CN group as anticipated, with intermediate scores in MCI, and the highest scores in AD.
Table 2.
Total scores of ECog (39 items) and CCI (20 items) by diagnostic group
| CN | MCI | AD | p | ||
|---|---|---|---|---|---|
| Self | n | 612 | 255 | 83 | |
| ECog | 54.1 ± 14.1 | 71.9 ± 23.3 | 75.3 ± 24.6 | < 0.0001 between CN-AD and CN-MCI | |
| CCI-20 | 31.8 ± 10.4 | 46.9 ± 15.7 | 48.4 ± 14.6 | < 0.0001 between CN-AD and CN-MCI | |
| Spearman Correlation | 0.729 | 0.789 | 0.718 | ||
| p | 2.44e-102 | 1.61e-55 | 2.14e-14 | ||
| Informant | n | 163 | 75 | 41 | |
| ECog | 50.5 ± 16.0 | 76.6 ± 25.9 | 111.7 ± 30.9 | < 0.0001 between all groups | |
| CCI-20 | 27.4 ± 10.2 | 47.3 ± 15.2 | 73.7 ± 17.8 | < 0.0001 between all groups | |
| Spearman Correlation | 0.817 | 0.702 | 0.668 | ||
| p | 3.90e-10 | 1.18e-12 | 1.39e-22 |
CN, cognitively normal; MCI, mild cognitive impairment; AD, Alzheimer’s disease.
Associations between CCI and ECog scores
Spearman correlations (Table 3) showed that the CCI and ECog were significantly associated (range r = 0.604 to 0.840, all p < 0.001). The highest correlation for the self and the informant-based assessments was between the total scores.
Table 3.
Descriptive Statistics and Spearman correlations between CCI and ECog paired scores
| Domain | Self N = 950 |
Informant N = 279 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| CCI | ECog | Spearman Correlation | p | CCI | ECog | Spearman Correlation | p | ||
| Mean Domain Score | Memory | 2.02 ± 0.78 | 1.91 ± 0.71 | 0.752 | 1.64e-173 | 2.08 ± 1.10 | 1.98 ± 0.95 | 0.823 | 5.19e-70 |
| Language | 1.64 ± 0.76 | 1.60 ± 0.61 | 0.604 | 1.08e-95 | 1.91 ± 1.13 | 1.60 ± 0.75 | 0.721 | 5.46e-46 | |
| Executive function | 1.64 ± 0.77 | 1.41 ± 0.50 | 0.570 | 6.48e-83 | 1.70 ± 0.97 | 1.65 ± 0.81 | 0.693 | 3.32e-41 | |
| All domains | 1.89 ± 0.72 | 1.56 ± 0.52 | 0.786 | 2.27e-200 | 2.00 ± 1.06 | 1.71 ± 0.79 | 0.840 | 2.16e-75 | |
| Total Score | Memory | 24.1 ± 9.34 | 15.2 ± 5.66 | 0.753 | 1.52e-174 | 24.9 ± 13.2 | 15.8 ± 7.6 | 0.822 | 1.11e-69 |
| All domains | 37.3 ± 14.5 | 60.7 ± 20.2 | 0.795 | 1.19e-207 | 39.5 ± 21.1 | 66.5 ± 30.8 | 0.840 | 1.69e-75 | |
We evaluated frequency distributions of the 950 self-based assessment and the 279 informant-based assessment total scores (Fig. 1). Based on the histogram of self-based assessments, the highest number of entries fell between a score of 25 to 35 for the CCI and 45 to 55 for the ECog. For the informant, the highest number of entries fell between 20 to 25 for the CCI and 39 to 45 for the ECog. The difference between the CCI and ECog is explained by the 19-point difference in minimum total score. Quadratic regression analysis (Fig. 2) showed the best fit between the CCI and ECog total scores with adjusted r-square values of 0.626 for the self-based assessments and 0.741 for the informant-based assessments. The equation used for the self-based group was y = 26.04x2 + 0.7692x + 0.003757. The equation used for the informant-based group was y = 24.74x2 + 0.8556x + 0.003961. All diagnostic groups were included in the analysis. A quadratic regression equation was generated to create a conversion table between the CCI-20 and the ECog-39. Separate tables were made for the self-based (Table 4) and informant-based (Table 5) versions. Similar tables were made to translate between the CCI-12 and the ECog (Tables 6 and 7).
Fig. 1.
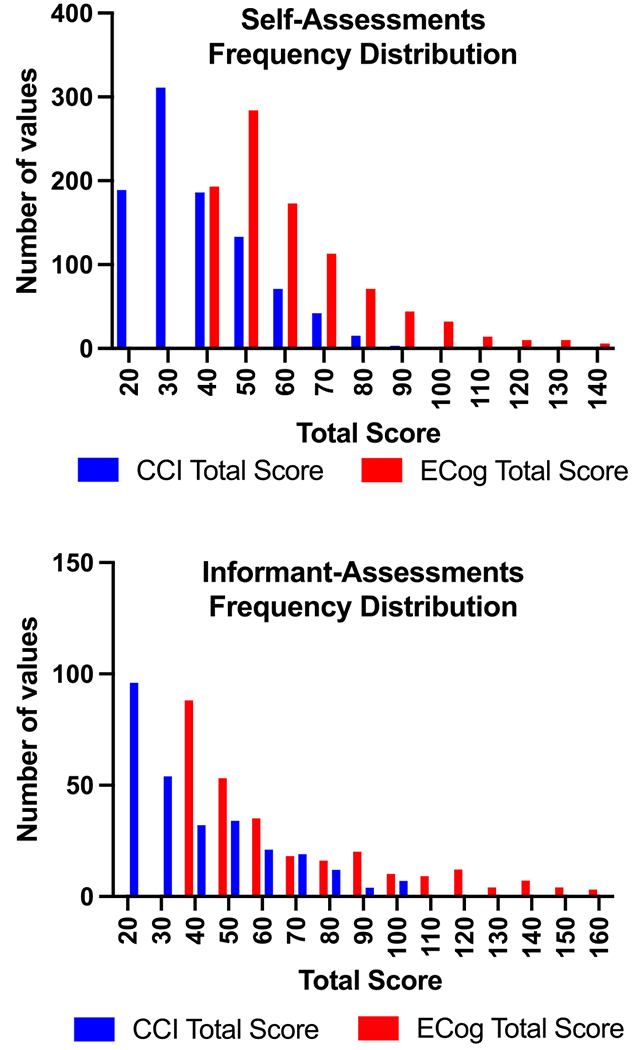
Frequency distributions showing the patterns between CCI 20-item and ECog 39-item total scores in the self and informant-based assessments. CCI, Cognitive Change Index; ECog, Measurement of Everyday Cognition.
Fig. 2.
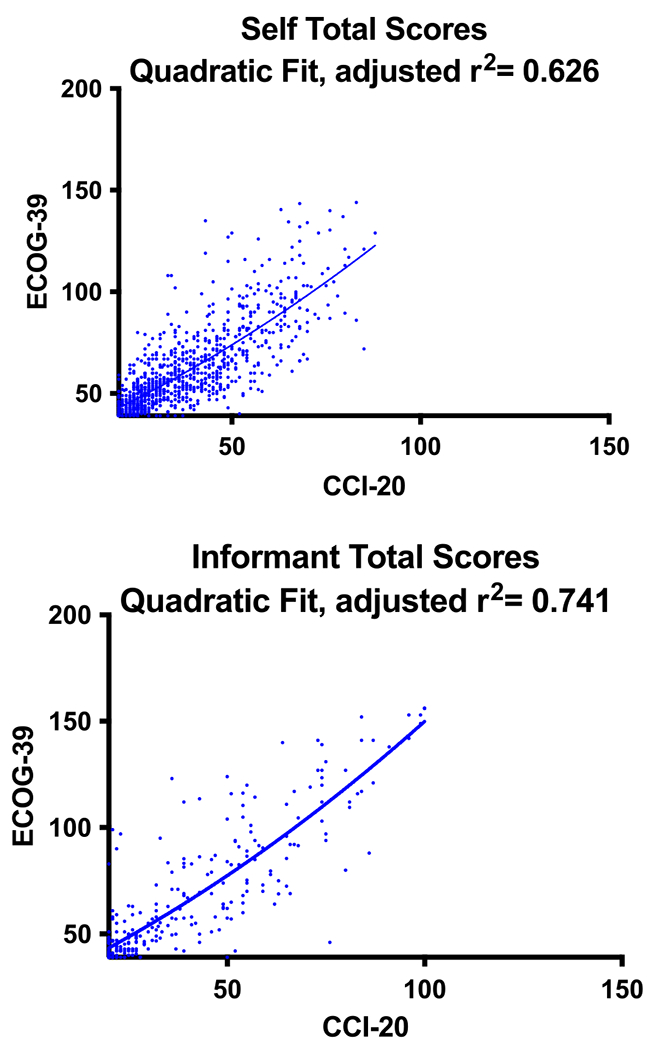
Quadratic regression showing the relationship between CCI 20-item and ECog 39-item total scores in the self and informant-based assessments. CCI, Cognitive Change Index; ECog, Measurement of Everyday Cognition.
Table 4.
CCI (20 items) self-based assessment mapped to ECog (39 items) self-based assessment using Quadratic Regression
| CCI-20 | ECog | CCI-20 | ECog | CCI-20 | ECog | CCI-20 | ECog |
|---|---|---|---|---|---|---|---|
| 20 | 43 | 41 | 64 | 62 | 88 | 83 | 116 |
| 21 | 44 | 42 | 65 | 63 | 89 | 84 | 117 |
| 22 | 45 | 43 | 66 | 64 | 91 | 85 | 119 |
| 23 | 46 | 44 | 67 | 65 | 92 | 86 | 120 |
| 24 | 47 | 45 | 68 | 66 | 93 | 87 | 121 |
| 25 | 48 | 46 | 69 | 67 | 94 | 88 | 123 |
| 26 | 49 | 47 | 70 | 68 | 96 | 89 | 124 |
| 27 | 50 | 48 | 72 | 69 | 97 | 90 | 126 |
| 28 | 51 | 49 | 73 | 70 | 98 | 91 | 127 |
| 29 | 52 | 50 | 74 | 71 | 100 | 92 | 129 |
| 30 | 52 | 51 | 75 | 72 | 101 | 93 | 130 |
| 31 | 53 | 52 | 76 | 73 | 102 | 94 | 132 |
| 32 | 55 | 53 | 77 | 74 | 104 | 95 | 133 |
| 33 | 56 | 54 | 79 | 75 | 105 | 96 | 135 |
| 34 | 57 | 55 | 80 | 76 | 106 | 97 | 136 |
| 35 | 58 | 56 | 81 | 77 | 108 | 98 | 138 |
| 36 | 59 | 57 | 82 | 78 | 109 | 99 | 139 |
| 37 | 60 | 58 | 83 | 79 | 110 | 100 | 141 |
| 38 | 61 | 59 | 85 | 80 | 112 | ||
| 39 | 62 | 60 | 86 | 81 | 113 | ||
| 40 | 63 | 61 | 87 | 82 | 114 |
Table 5.
CCI informant-based assessment (20 items) mapped to ECog informant-based assessment (39 items) using Quadratic Regression
| CCI-20 | ECog | CCI-20 | ECog | CCI-20 | ECog | CCI-20 | ECog |
|---|---|---|---|---|---|---|---|
| 20 | 43 | 41 | 66 | 62 | 93 | 83 | 123 |
| 21 | 44 | 42 | 68 | 63 | 94 | 84 | 125 |
| 22 | 45 | 43 | 69 | 64 | 96 | 85 | 126 |
| 23 | 47 | 44 | 70 | 65 | 97 | 86 | 128 |
| 24 | 48 | 45 | 71 | 66 | 98 | 87 | 129 |
| 25 | 49 | 46 | 72 | 67 | 100 | 88 | 131 |
| 26 | 50 | 47 | 74 | 68 | 101 | 89 | 132 |
| 27 | 51 | 48 | 75 | 69 | 103 | 90 | 134 |
| 28 | 52 | 49 | 76 | 70 | 104 | 91 | 135 |
| 29 | 53 | 50 | 77 | 71 | 105 | 92 | 137 |
| 30 | 54 | 51 | 79 | 72 | 107 | 93 | 139 |
| 31 | 55 | 52 | 80 | 73 | 108 | 94 | 140 |
| 32 | 56 | 53 | 81 | 74 | 110 | 95 | 142 |
| 33 | 57 | 54 | 83 | 75 | 111 | 96 | 143 |
| 34 | 58 | 55 | 84 | 76 | 113 | 97 | 145 |
| 35 | 60 | 56 | 85 | 77 | 114 | 98 | 147 |
| 36 | 61 | 57 | 86 | 78 | 116 | 99 | 148 |
| 37 | 62 | 58 | 88 | 79 | 117 | 100 | 150 |
| 38 | 63 | 59 | 89 | 80 | 119 | ||
| 39 | 64 | 60 | 90 | 81 | 120 | ||
| 40 | 65 | 61 | 92 | 82 | 122 |
Table 6.
CCI-12 (12 items) self-based assessment mapped to ECog self-based assessment using Quadratic Regression
| CCI-12 | ECog | CCI-12 | ECog | CCI-20 | ECog |
|---|---|---|---|---|---|
| 12 | 36 | 33 | 56 | 54 | 79 |
| 13 | 37 | 34 | 57 | 55 | 80 |
| 14 | 38 | 35 | 58 | 56 | 81 |
| 15 | 38 | 36 | 59 | 57 | 82 |
| 16 | 39 | 37 | 60 | 58 | 83 |
| 17 | 40 | 38 | 61 | 59 | 85 |
| 18 | 41 | 39 | 62 | 60 | 86 |
| 19 | 42 | 40 | 63 | ||
| 20 | 43 | 41 | 64 | ||
| 21 | 44 | 42 | 65 | ||
| 22 | 45 | 43 | 66 | ||
| 23 | 46 | 44 | 67 | ||
| 24 | 47 | 45 | 68 | ||
| 25 | 48 | 46 | 69 | ||
| 26 | 49 | 47 | 70 | ||
| 27 | 50 | 48 | 72 | ||
| 28 | 51 | 49 | 73 | ||
| 29 | 52 | 50 | 74 | ||
| 30 | 52 | 51 | 75 | ||
| 31 | 53 | 52 | 76 | ||
| 32 | 55 | 53 | 77 |
Table 7.
CCI-12 (12 items) informant-based assessment mapped to ECog informant-based assessment using Quadratic Regression
| CCI-12 | ECog | CCI-12 | ECog | CCI-20 | ECog |
|---|---|---|---|---|---|
| 12 | 36 | 33 | 57 | 54 | 83 |
| 13 | 37 | 34 | 58 | 55 | 84 |
| 14 | 37 | 35 | 60 | 56 | 85 |
| 15 | 38 | 36 | 61 | 57 | 86 |
| 16 | 39 | 37 | 62 | 58 | 88 |
| 17 | 40 | 38 | 63 | 59 | 89 |
| 18 | 41 | 39 | 64 | 60 | 90 |
| 19 | 42 | 40 | 65 | ||
| 20 | 43 | 41 | 66 | ||
| 21 | 44 | 42 | 68 | ||
| 22 | 45 | 43 | 69 | ||
| 23 | 47 | 44 | 70 | ||
| 24 | 48 | 45 | 71 | ||
| 25 | 49 | 46 | 72 | ||
| 26 | 50 | 47 | 74 | ||
| 27 | 51 | 48 | 75 | ||
| 28 | 52 | 49 | 76 | ||
| 29 | 53 | 50 | 77 | ||
| 30 | 54 | 51 | 79 | ||
| 31 | 55 | 52 | 80 | ||
| 32 | 56 | 53 | 81 |
DISCUSSION
The purpose of this study was to examine the relationship between the CCI and the ECog to facilitate future harmonized cross-study and longitudinal assessments of perceived cognitive change. Table 2 illustrates the relative ability of these instruments to differentiate between participants classified as CN, MCI, or AD. By definition, these groups differ in extent of cognitive concerns. We found high correlations between the CCI and ECog in self and informant-based assessments across all overlapping domains, which supported the development of a co-calibrated crosswalk to translate between scores on the two assessments. The highest correlations for self and informant-based assessments were between the ECog and CCI total scores. Due to the content differences between the instruments, which in the ECog includes visuospatial questions and the CCI does not, the high correlations despite these differences suggest that the visual-spatial items in the ECog do not have a large impact on the total score.
Histograms of all the self-based CCI and ECog and the informant-based CCI and ECog total scores were plotted to qualitatively assess the distribution of total scores (Fig. 1). While the highest number of entries in the informant report histogram included the minimum total score, the self-based histogram did not. This could suggest that participants more often report mild cognitive concerns compared to informants. However, there are limitations to this conclusion because different participants were included in the self and informant cohorts. Previous research has shown that informant reports are more strongly correlated to biomarkers of neurodegenerative disease [15]. Two tables were made to translate between the ECog and CCI: one for self-based assessments and one for informant-based assessments. We were able to map the CCI total score to an equivalent ECog total score using a quadratic equation. In the self-based data, a total score of 20 on the CCI did not map below 43 on the ECog, even though the minimum score is 39. A score of 100 on the CCI did not map above a score of 141 on the ECog, although the maximum score is 156 (Table 4). In the informant-based data, a score of 20 did not map below 43 and a score of 100 mapped to 150 (Table 5).
Limitations of this study include generalizability, due to the high mean education and limited portion of the sample from underrepresented groups. Other studies using these instruments are currently working on replication and extension in more diverse and representative community-based samples. Racial and ethnic categories were used in accordance with NIH terminology to compare population characteristics of the various study groups. In future research it is important to distinguish between race and ethnicity while assessing the relationship between ethnocultural factors and cognitive concern. Additionally, the CCI and ECog were not given at precisely the same time, which may have impacted our results. Despite these limitations, co-calibration and translation between scales is feasible and of practical value.
In summary, this study contributes analysis and harmonization methodology for two commonly used instruments to assess self-perceived and proxy-based cognitive concerns. This is important to foster additional research on varying AD prodromal stages, in which SCD concerns are among the first reported symptoms. The ADRD research field is intensifying the recent focus on harmonization efforts for cognitive, clinical, imaging and biomarker approaches, as these provide ability to aggregate data across studies, especially as quantitative phenotypes for genetic investigations. The NIH has recently invested in the creation of a phenotype harmonization consortium for AD genetics. Total scores of the CCI and ECog assessments show strong correlations and validate the harmonization of these tools to assess longitudinal change in cognitive concerns. These tools can be used by healthcare researchers and providers to add precision to the evaluation of SCD given their ability to detect mild perceived loss of everyday cognitive functioning. Future longitudinal research is needed to characterize the relationship between these assessments and long-term clinical outcomes and biomarker trajectories. This could include additional longitudinal analysis of measurement invariance using confirmatory factor analysis by domain and by diagnostic groups. Self and informant-based assessments should also be analyzed for other neurodegenerative diseases to better understand potential implications for differential diagnosis, longitudinal follow-up, and other clinical applications.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by grants from the Indiana CTSI (UL1 TR002529) and the National Institute on Aging (U01 AG024904, R01 AG19771, P30 AG010133, P30 AG072976, K01 AG049050, R01 AG067188, U01 AG068057, U01 AG072177 and U24 AG074855). Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). See the ADNI website (https://adni.loni.usc.edu) for more details.
Footnotes
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/21-5388r1).
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-215388.
REFERENCES
- [1].Reisberg B, Prichep L, Mosconi L, John ER, Glodzik-Sobanska L, Boksay I, Monteiro I, Torossian C, Vedvyas A, Ashraf N, Jamil IA, de Leon MJ (2008) The pre-mild cognitive impairment, subjective cognitive impairment stage of Alzheimer’s disease. Alzheimers Dement 4, S98–s108. [DOI] [PubMed] [Google Scholar]
- [2].Jessen F, Amariglio RE, van Boxtel M, Breteler M, Ceccaldi M, Chetelat G, Dubois B, Dufouil C, Ellis KA, van der Flier WM, Glodzik L, van Harten AC, de Leon MJ, McHugh P, Mielke MM, Molinuevo JL, Mosconi L, Osorio RS, Perrotin A, Petersen RC, Rabin LA, Rami L, Reisberg B, Rentz DM, Sachdev PS, de la Sayette V, Saykin AJ, Scheltens P, Shulman MB, Slavin MJ, Sperling RA, Stewart R, Uspenskaya O, Vellas B, Visser PJ, Wagner M, Subjective Cognitive Decline Initiative Working Group (2014) A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement 10, 844–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jessen F, Amariglio RE, Buckley RF, van der Flier WM, Han Y, Molinuevo JL, Rabin L, Rentz DM, Rodriguez-Gomez O, Saykin AJ, Sikkes SAM, Smart CM, Wolfsgruber S, Wagner M (2020) The characterisation of subjective cognitive decline. Lancet Neurol 19, 271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Risacher SL, Kim S, Nho K, Foroud T, Shen L, Petersen RC, Jack CR Jr., Beckett LA, Aisen PS, Koeppe RA, Jagust WJ, Shaw LM, Trojanowski JQ, Weiner MW, Saykin AJ (2015) APOE effect on Alzheimer’s disease biomarkers in older adults with significant memory concern. Alzheimers Dement 11, 1417–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jessen F, Wiese B, Bachmann C, Eifflaender-Gorfer S, Haller F, Kolsch H, Luck T, Mosch E, van den Bussche H, Wagner M, Wollny A, Zimmermann T, Pentzek M, Riedel-Heller SG, Romberg HP, Weyerer S, Kaduszkiewicz H, Maier W, Bickel H (2010) Prediction of dementia by subjective memory impairment: Effects of severity and temporal association with cognitive impairment. Arch Gen Psychiatry 67, 414–422. [DOI] [PubMed] [Google Scholar]
- [6].Saykin AJ, Wishart HA, Rabin LA, Santulli RB, Flashman LA, West JD, McHugh TL, Mamourian AC (2006) Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology 67, 834–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12, 189–198. [DOI] [PubMed] [Google Scholar]
- [8].Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H (2005) The Montreal Cognitive Assessment, MoCA: Abrief screening tool for mild cognitive impairment. J Am Geriatr Soc 53, 695–699. [DOI] [PubMed] [Google Scholar]
- [9].Farias S Tomaszewski, Mungas D, Reed B, Cahn-Weiner D, Jagust W, Baynes K, et al. (2008) The measurement of everyday cognition (ECog): Scale development and psychometric properties. Neuropsychology 22, 531–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rattanabannakit C, Risacher SL, Gao S, Lane KA, Brown SA, McDonald BC, Unverzagt FW, Apostolova LG, Saykin AJ, Farlow MR (2016) The Cognitive Change Index as a measure of self and informant perception of cognitive decline: Relation to neuropsychological tests. J Alzheimers Dis 51, 1145–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Reisberg B, Shulman MB, Torossian C, Leng L, Zhu W (2010) Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimers Dement 6, 11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Petersen RC, Morris JC (2005) Mild cognitive impairment as a clinical entity and treatment target. Arch Neurol 62, 1160–1163; discussion 1167. [DOI] [PubMed] [Google Scholar]
- [13].Petersen RC (2016) Mild cognitive impairment. Continuum (Minneap Minn) 22(2 Dementia), 404–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kolen MJ, Brennan RL (2006) Test equating, scaling and linking: Methods and practices, Springer, New York, NY. [Google Scholar]
- [15].Rueda AD, Lau KM, Saito N, Harvey D, Risacher SL, Aisen PS, Petersen RC, Saykin AJ, Farias ST, Alzheimer’s Disease Neuroimaging Initiative (2015) Self-rated and informantrated everyday function in comparison to objective markers of Alzheimer’s disease. Alzheimers Dement 11, 10801089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


