Abstract
Bipolar AQ1 AQ2 AQ3 disorder shares symptoms and pathological pathways with other neurodegenerative diseases, including frontotemporal dementia (FTD). Since TAR DNA-binding protein 43 (TDP-43) is a neuropathological marker of frontotemporal dementia and it is involved in synaptic transmission, we explored the role of TDP-43 as a molecular feature of bipolar disorder (BD). Homogenates were acquired from frozen hippocampus of postmortem brains of bipolar disorder subjects. TDP-43 levels were quantified using an ELISA-sandwich method and compared between the postmortem brains of bipolar disorder subjects and age-matched control group. We found higher levels of TDP-43 protein in the hippocampus of BD (n = 15) subjects, when compared to controls (n = 15). We did not find associations of TDP-43 with age at death, postmortem interval, or age of disease onset. Our results suggest that protein TDP-43 may be potentially implicated in behavioral abnormalities seen in BD. Further investigation is needed to validate these findings and to examine AQ4 the role of this protein during the disease course and mood states.
Keywords: Bipolar disorder, TAR DNA-binding protein 43, Frontotemporal dementia, Neurodegeneration, Post-mortem brain
Introduction
Bipolar disorder is a chronic and current psychiatric illness, associated with high burden and suicide rates (Carvalho et al. 2020). Although there have been several biological pathways implicated in BD, such as inflammation and mitochondrial dysfunction, we still lack the full picture of its physiopathology. Most studies investigated molecular findings in peripheral tissues, but the results vary widely (Passos et al. 2016). Unfortunately, studies with postmortem brain tissues, which is the ideal site to reflect the clinical aspects of BD, are not as frequent. Taken together, this suggests a potential benefit for identifying novel molecular features in postmortem brain tissue that may implicate in BD pathophysiology.
BD and neurodegenerative disorders present multiple similarities both in symptomatology and neuropathology (Kim et al. 2016; Nascimento et al. 2019; Taylor et al. 2018). Patients with a behavioral subtype of frontotemporal dementia (bv-FTD) can be misdiagnosed as BD due to specific psychiatric symptoms such as euphoria, disinhibition, impulsivity, and compulsive behaviors (Taylor et al. 2018; Woolley et al. 2011). Likewise, cognitive decline, present in later stages of bv-FTD, is an important clinical feature of a subgroup of BD patients as well (Cullen et al. 2017). However, in BD, periods of mood and behavioral recovery (euthymic) are distinctive (Vieta et al. 2018), whereas in frontotemporal dementia (FTD), these symptoms follow a progressive course (Bott et al. 2014).
Although BD and bv-FTD have distinct patterns of clinical progression, similarities between them raise the question as to whether common molecular pathways may contribute to the behavioral and cognitive alterations seen in both disorders. In fact, inflammation and oxidative stress have been implicated in both BD and frontotemporal dementia (FTD) (Nascimento et al. 2019).
The inherent progressive nature of neurodegenerative diseases has probably allowed the identification of their molecular features comparatively easier than psychiatric disorders. For example, biochemical modifications in transactive response DNA-binding Protein 43 (TDP-43) are verified as aggregates in the nucleus and cytoplasm of neurons and glial cells of FTD patients (Neumann et al. 2006). TDP-43 is a protein implicated in multiple crucial activities of the central nervous system, including stress granule formation, axonal transport of target mRNAs, and mRNA translation (Ratti and Buratti 2016). Disruption on the physiological role of TDP-43 causes synaptic dysfunction, oxidative stress, and stress granules’ formation (Cohen et al. 2011; Heyburn et al. 2016; Lee et al. 2018). Synaptic dysfunction and oxidative stress are also present in BD (Berk et al. 2011; Lee et al. 2018).
Clinical and molecular similarities between bv-FTD and BD made us to question whether a protein that has been related to bv-FTD could also play a role in BD pathophysiology. With this in mind, our study aimed to explore levels of TDP-43 in the hippocampus of BD subjects. We focused on this brain region, since it has been proven to be consistently affected in BD in morphological (Cao et al. 2017) and biochemical studies (Darby et al. 2016; Schubert et al. 2015).
Materials and methods
Participants
This study was conducted in deceased subjects submitted to autopsy at the Sao Paulo Autopsy Service (SPAS) between 2009 and 2016, whose family voluntarily donated the brain to the Biobank of Aging Studies (BAS). SPAS is a community-based autopsy service responsible for issuing death certificates for subjects who died from natural causes within Sao Paulo city. Death certificates are mandatory in Brazil when the cause of natural death was not well determined. After agreeing to participate in the study, the next of kin signed the informed consent, reported the clinical history and donated the brain of the deceased subject. Detailed BAS methodological procedures have been described elsewhere (Suemoto et al. 2017). The University of São Paulo research committee approved this study.
Our inclusion criteria were subjects above 18 years old with a non-traumatic cause of death and postmortem interval less than 24 h. Cases with no reliable informant, medical history of advanced chronic disease, significant cerebral lesions, or prolonged agonal state were excluded. We used a sample of psychiatric cases (de Oliveira et al. 2012) and we included all subjects with BD clinical diagnosis. Controls were selected according to sex, age (± 10 years) and educational background (± 5 years).
Clinical postmortem evaluation
A validated semi-structured interview of the BAS protocol was conducted with a knowledgeable informant, who had at least a weekly contact with the deceased (Suemoto et al. 2017). The interview contained several instruments to collect information about the clinical and functional status of the deceased subject. Cognitive function was assessed using the Clinical Dementia Rating (CDR) (Morris 1993) based on the informant (Harrison et al. 2016) and validated for postmortem use (Ferretti et al. 2010). Cognitive impairment was considered when CDR > 0.5. Information on the presence of neuropsychiatric symptoms was collected using the neuropsychiatric inventory (NPI) (Cummings 1997). In addition, demographic, death-related conditions, previous medical history, and medical treatments were evaluated. Medical history included: hypertension, diabetes mellitus, coronary artery disease, current alcohol and tobacco use. Body mass index (BMI in kg/m2) was calculated after measuring weight and height in the supine position and without clothes. Psychiatric history was assessed through the informant interview of the Structured Clinical Interview for Axis I DSM-IV Disorders (SCID) (Spitzer et al. 1995). If subjects fulfilled any criteria for psychiatric disorder, according to SCID, an additional interview was conducted by a psychiatrist to obtain detailed information regarding the clinical course (including mood episodes, remission, disease duration and age at onset), pharmacological treatments, suicide attempts, and hospitalization among other clinical variables. Information regarding postmortem interval (PMI) was collected through the coroner in charge of recognizing the time of death.
Diagnosis of BD
The diagnosis of BD was stablished according to DSM-5 criteria (American Psychiatry Association 2013) using information collected through the SCID informant interview and medical records, when available. Diagnosis of BD was then confirmed based on the final consent between two additional psychiatrists who were blinded to the initial diagnostic hypothesis—consisting of the best-estimate diagnosis, as previously described (de Oliveira et al. 2012). Participants in the control group did not have any mood episodes during their lifetime or any psychiatric diagnosis as screened through the SCID. To differentiate BD from FTD, we only included BD cases presenting with typical mood fluctuations including remission, especially for those cases in which disease onset was > 50 years.
TDP-43 measurements
During brain processing, different regions from one hemisphere were isolated and frozen at −80 °C for biochemical studies. After thawing, samples from the hippocampus were mechanically homogenized and total protein was extracted using EpiQuik Whole Cell Extraction Kit (Epigentek, Farmingdale, NY, USA) with one modification. Reagents from the extraction buffer of the EpiQuik Whole Cell Extraction Kit interfered in the Enzyme-Linked Immunosorbent Assay (ELISA) readings and were replaced by a phosphate buffer solution (PBS, Sigma-Aldrich, Sao Paulo, Brazil).
Protein concentration was measured using Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA USA). Brain homogenates were loaded at a concentration of 500 μg/ml. Quantitative levels of TDP-43 were measured using the Sandwich ELISA Human TDP-43/TARDBP ELISA Kit (LS-F5278-Lifespan Biosciences, Seattle, Washington, EUA), with modifications from the manufacturer’s instructions. Detection range of this kit is 62.5–4000 pg/ml, sensitivity is 24.6 pg/ml and the coefficient of variation is < 10%. Briefly, samples and standards were incubated overnight at 4 °C, following by incubation with a detection reagent for 2 h at 37 °C. The wells were then washed and incubated with an enzyme substrate for 45 min at 37 °C. Finally, a stop solution was added and the spectrophotometric readings were performed at 450 nm.
Statistical analysis
Descriptive analysis was conducted to compare demographic characteristics (age, sex, ethnicity), clinical variables (neuropsychiatric symptoms, cognitive impairment, BMI, alcoholism, hypertension, diabetes mellitus, coronary artery disease), and PMI for BD and control groups. Group differences were evaluated using Fisher’s exact test for categorical variables and unpaired t-test for quantitative variables.
For TDP-43 levels, we conducted the Kolmogorov–Smirnov test to evaluate whether the data were normally distributed. As TDP-43 levels did not follow a normal distribution, therefore, for all analysis including TDP as the independent variable, we conducted a Mann–Whitney test to assess between-group differences according to diagnostic.
We investigated independent associations between TDP-43 levels and age at death using Spearman’s rank correlation test. As an exploratory analysis, we analyzed potential associations of TDP-43 with alcoholism, hypertension, diabetes mellitus, and coronary artery disease, as well as correlations with BMI and NPI total scores. These analyses were performed separately for controls and BD subjects. For association analyses, we used the Mann–Whitney test and for correlation analyses Spearman correlation test. For the BD group, to search for markers of disease severity, we investigated the association between TDP-43 levels and the presence or absence of hospitalization history and disease duration. For disease duration, since we had a continuous variable, both correlational analysis and stratification were tested according to the average number of years with the disease (< or > 22 years). The level of significance was set at 0.05. Statistical analysis was performed using Statistical Package for Social Sciences (SPSS) version 23.0 (Inc., Chicago, Illinois, EUA).
Protein to protein interaction network for TAR DNA-binding protein 43 and relationship with bipolar disorder
Since TDP-43 binds to several different proteins, we decided to explore whether the proteins that are known to interact with TDP-43, were also related to BD. For this, we built a protein–protein interaction (PPI) network using STRING v11 (accessed 2020 September 3rd) using TDP-43 as the seed. The basic settings to build the PPI were as follows: (1) meaning of network edges by evidence; (2) active interactions sources were text mining (MEDLINE database), experiments (The Proteomics Standards Initiative—Molecular Interaction database), co‑occurrence (using the SVD-phy algorithm (Franceschini et al. 2016) and co‑expression (ProteomeHD database); (3) minimum required interaction score with highest confidence (0.9); (4) no more than 10 maximum number of interactors. Using the same parameters, we also AQ5 looked at the biological process (GO: Gene Ontology) and pathways (KEGG: Kyoto Encyclopedia of Genes and Genomes) enriched in the network. Then, using the MEDLINE database, we used “protein/gene name AND bipolar disorder” to investigate whether these proteins had been previously associated with BD.
Results
Subject characteristics
Cases were collected from the BAS (2009–2016). Out of 76 subjects identified with a psychiatric history, 15 had a history of BD. The remaining cases had schizophrenia, major depressive disorder, obsessive–compulsive disorder, panic disorder, generalized anxiety disorder, alcohol or drug dependence.
BD (n = 15) and control groups (n = 15) were similar for demographic characteristics, clinical history and PMI, as seen in Table 1. Groups did not differ in relation to age, BMI, PMI, ethnicity, sex, current alcohol use, hypertension, diabetes mellitus and coronary artery disease. BD group had more neuropsychiatric symptoms 3 months before death compared to the control group, as measured by higher NPI total scores (p = 0.0001).
Table 1.
Comparison of bipolar disorder cases and control group.
| Characteristic | BD (n = 15) | Control (n = 15) | p |
|---|---|---|---|
| Age (years), mean (SD)a | 65.8 (13.8) | 63.0 (11.6) | 0.50 |
| Female, n (%)b | 11 (73.3) | 11 (73.3) | 1.00 |
| BMI (kg/m2), mean (SD)a | 24.2 (5.4) | 24.1 (5.9) | 0.81 |
| Ethnicity, n (%)b | |||
| White/mixed | 10 (66.6) | 8 (53.3) | |
| Black | 5 (33.3) | 7 (46.6) | 0.58 |
| Cognitive impairment, n (%)b | 2 (13.4) | 0 (100.0) | 0.48 |
| NPI total score, mean (SD)a | 54.8 (33.1) | 1.0 (2.4) | 0.0001 |
| PMI (hours), mean (SD)a | 13.9 (2.52) | 15.7 (5.0) | 0.20 |
| Current alcohol use, n (%)b | 7 (46.6) | 6 (40.0) | 1.00 |
| Hypertension, n (%)b | 8 (53.3) | 9 (60.0) | 1.00 |
| Diabetes mellitus, n (%)b | 6 (40.0) | 2 (13.3) | 0.21 |
| Coronary artery disease, n (%)b | 4 (26.6) | 4 (26.6) | 1.00 |
| TDP-43 (pg/mL), mean (SD)a | 865.7 (177.8) | 617.9 (211.9) | 0.004 |
BD bipolar disorder; SD standard deviation; BMI body mass index; NPI neuropsychiatric inventory; PMI postmortem interval; TDP-43 TAR DNA-binding protein 43; N number of cases %: percentage; CDR cognitive dementia rating
Mann–Whitney test
Fisher exact test
Regarding BD subjects, one presented a history of suicide attempt and nine cases underwent psychiatric hospitalization.
TDP-43 levels in the hippocampus for BD and control groups
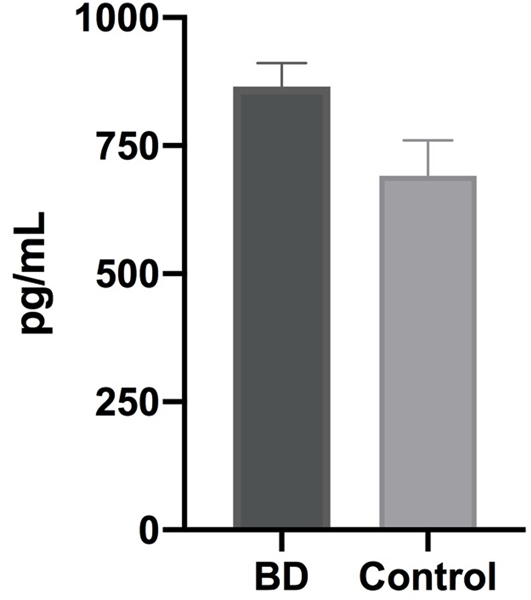
There was a significant group difference between BD and control subjects for TDP-43 levels in the hippocampus (Table 1, Fig. 1). Higher levels of the TDP-43 were found in BD when compared to controls (865.7 ± 177.8 pg/ml versus 617.9 ± 211.9 pg/ml; p = 0.004). Since we had two cognitively impaired subjects in the BD group, we repeated the analysis excluding these cases. The exclusion of these cases did not change the results, and the between-group difference remained significant, with a higher level of TDP-43 in BD compared to the control group (894.0 ± 170.8 pg/ml versus 655.7 ± 213.2 pg/ml; p = 0.007). We did not find an association between TDP-43 levels and age at death (ρ = 0.04, p = 0.82) when all subjects were included in the analysis.
Fig. 1.
Levels of TAR DNA-binding protein 43 in the hippocampus of subjects with a history of bipolar disorder. Data obtained from bipolar disorder subjects (n = 15), compared to controls with non-psychiatric symptoms (n = 15). Between groups difference with a p-value = 0.004*. Bars represent the median interval and SD ± 2
TDP-43 levels and the clinical variables in BD and control groups
In the BD group, TDP-43 levels were negatively correlated with BMI (ρ = −0.57, p = 0.03) and NPI total score (ρ = −0.65, p = 0.01). We did not find associations between TDP-43 and alcoholism, hypertension, diabetes mellitus or coronary artery disease (Table 2 ). TDP-43 was not associated with reported history of hospitalization (i.e., yes or no, 857.14 ± 157.29 versus 878.46 ± 220.64) and mean disease duration (i.e., < or > than 22 years, 846.64 ± 202.51 versus 887.42 ± 157.94, p = 0.64). For disease duration, we also used correlation analysis and found non-significant results (ρ = 0.193, p = 0.49).
Table 2.
Investigation of TAR DNA-binding protein 43 levels in the clinical variables in bipolar disorder cases and control group.
| Characteristic | TDP-43 (pg/ml) | |||
|---|---|---|---|---|
| BD (n = 15) | p | Control (n = 15) | p | |
| Current alcohol use, mean (SD)a | ||||
| Yes | 903.0 (165.7) | 0.54 | 612.5 (232.2) | 0.78 |
| No | 818.9 (251.9) | 658.7 (273.5) | ||
| Hypertension, mean (SD)a | ||||
| Yes | 815.9 (154.6) | 0.28 | 686.1 (258.0) | 0.72 |
| No | 922.5 (197.0) | 660.1 (262.2) | ||
| Diabetes mellitus, mean (SD)a | ||||
| Yes | 768.7 (131.5) | 0.09 | 987.0 (200.0) | 0.04 |
| No | 930.3 (181.1) | 597.94 (199.0) | ||
| Coronary artery disease, mean (SD)a | ||||
| Yes | 835.1 (222.2) | 0.69 | 780.4 (317.1) | 0.15 |
| No | 876.7 (170.2) | 637.7 (227.5) | ||
BD bipolar disorder; SD standard deviation; TDP-43 TAR DNA-binding protein 43
Mann–Whitney test
For the control group, we found an association between TDP-43 levels and hypertension (Table 2). Regarding alcoholism, hypertension, diabetes mellitus, or coronary artery disease, no significant differences were found (Table 2). Likewise, correlational analyses did not show significant results for BMI (ρ = − 0.17, p = 0.55) or NPI (ρ = 0.41, p = 0.12).
Protein to protein interaction network for TAR DNA-binding protein 43 reveals proteins previously associated with bipolar disorder
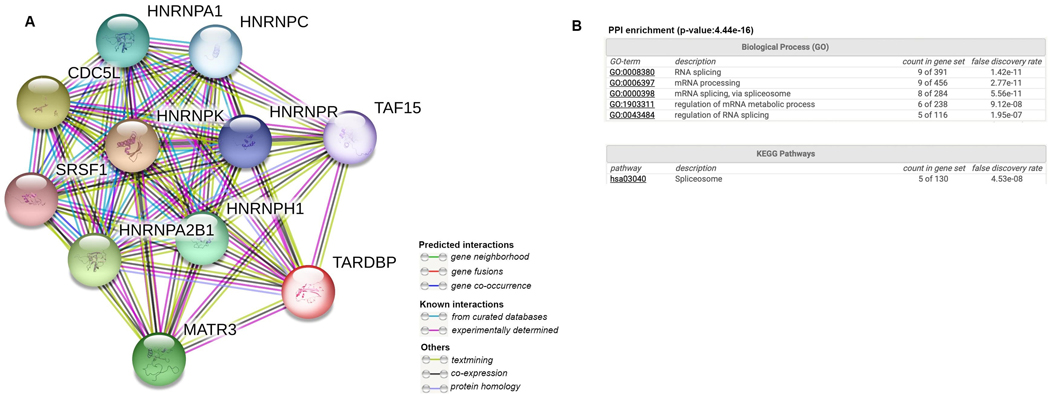
We found ten genes (SRSF1, HNRNPA1, HNRNPA2B1, HNRNPH1, MATR3, CDC5L, HNRNPR, HNRNPK, HNRNPC, and TAF15) associated with TDP-43 in previous studies (Fig. 2 a). All the proteins (nodes) were found to be co-expressed with TDP-43 (black edges) and all the interactions were previously experimentally determined (pink edges). Two (HNRNPC and TAF15) out of ten genes had been associated with BD in previous studies (Arloth et al. 2015; Clelland et al. 2013; Fries et al. 2017; Witt et al. 2014). Enrichment network analysis showed that RNA splicing (p = 1.42e−11), mRNA processing via spliceosome (p = 5.56e−11) and regulation of mRNA metabolic process (p = 9.12e−08) were among the biological processes, and spliceosome (p = 4.53e−08) was among the pathways associated with TDP-43 (Fig. 2 b).
Fig. 2.
Protein–protein interaction (PPI) network generated with TAR DNA-binding protein gene (TARDBP) as a seed (a), as well as network PPI enrichment (b)
Discussion
Here, we explored the levels of TDP-43 protein, a neuropathological marker of bv-FTD, as a molecular feature for BD. Our rationale for this investigation was similar symptoms for BD and bv-FTD, and the implication of TDP-43 in synaptic dysfunction and oxidative stress (Heyburn et al. 2016; Lee et al. 2018). We found higher levels of the TDP-43 protein in the postmortem hippocampus of BD group compared to controls. Interestingly, proteins that are known to interact with TDP-43 had also been previously associated with BD.
To the best of our knowledge, this is the first study to investigate TDP-43 protein levels in the hippocampus of individuals with BD. No previous studies examined levels of dementia-related neuropathological markers in the brain of BD compared to unaffected controls.
A molecular link between psychiatric and neurodegenerative disorders has been proposed based on their clinical similarities (Kim et al. 2016; Nascimento et al. 2019). For example, patients with bv-FTD are frequently diagnosed with BD (Woolley et al. 2011), especially in the earlier stages of the disease, since they present similar psychiatric symptoms (such as loss of social awareness, disinhibition, disillusions, and psychosis) before the appearance of cognitive symptoms. Moreover, BD, similar to other psychiatric conditions such as major depressive disorder (MDD) and schizophrenia (SCZ), shows a progressive course and a measurable decline in cognitive and functional status (Bauer et al. 2017; Kapczinski et al. 2014). For this reason, other than only focusing on the known pathways related to psychiatric disorders (for example, inflammation (Passos et al. 2016), studies have focused on identifying novel neuropathological markers, including the investigation of the presence of proteins related to neurodegeneration. Increased levels of TDP-43, the major neuropathological marker of bv-FTD, were found in peripheral blood of MDD patients (Ichikawa et al. 2019). In the brain, a morphological study showed changes in subcellular localization of TDP-43 in the hippocampus of SCZ and BD psychotic patients (Velakoulis et al. 2009).
In animal models, overexpression of TDP-43 was shown to cause social and memory deficits (Endo et al. 2018; Heyburn et al. 2016). TDP-43 might be playing a role in the emotional and cognitive regulation in BD. Molecularly, these phenotypic changes may be secondary to the suppression of critical pre-synaptic proteins, affecting the release of neurotransmitters in the synaptic cleft (Heyburn et al. 2016). TDP-43 overexpression was found to cause synaptic dysfunction in rodent models (Endo et al. 2018; Handley et al. 2017; Heyburn et al. 2016). These findings suggest that TDP-43 may be playing a role in synaptic dysfunction in BD, a pathway consistently reported in this disease (Lee et al. 2018). Furthermore, mechanistic studies showed that overexpression of TDP-43 was similarly associated with increased oxidative stress (Heyburn et al. 2016), another biological pathway strongly implicated in BD pathophysiology (Berk et al. 2011).
We did not find an association between TDP-43 levels and age at death in our study, which suggests that an increase in TDP-43 levels may be related to BD symptoms, and not age. Furthermore, the fact that the exclusion of two subjects with cognitive impairment did not change the results, reinforces the role of TDP-43 as a potential molecular feature for BD. In the BD group, we found a negative correlation between TDP-43 and BMI, as well as NPI total score and, in the controls, an association with hypertension. These findings are exploratory due to our small sample size and need replication in a larger sample. These results may be fruitful in understanding the role of TDP-43 in BD as to our knowledge, there have not been previous studies examining the role of TDP-43 in BMI, neuropsychiatric symptoms, and hypertension. Although previous work in animal models linked the role of TDP-43 expression in weight loss, fat deposition, and glucose metabolism (Chiang et al. 2010; Stallings et al. 2013). We did not find an association between TDP-43 levels and other clinical variables, such as history of hospitalization and disease duration. The lack of association with clinical variables may be related to the small sample size or the limited clinical information provided by the informant. Another possibility is that TDP-43 could be a trait marker for BD, not affected by dynamic variables (such as mood state) and also not detected in a small sample size. Future studies using larger sample sizes would be useful to elucidate whether TDP-43 can reflect the dynamic and/or progressive changes in patients with BD, such as mood states or cognitive decline.
Interestingly, RNA-binding proteins heterogeneous nuclear ribonucleoprotein C (HNRNPC) and TATA-Box Binding Protein-Associated Factor 15 (TAF15) are co-expressed with TDP-43 as identified from the PPI network (accessed September 3, 2020 (Szklarczyk et al. 2019), Fig. 2 ). Previous studies (Arloth et al. 2015; Clelland et al. 2013; Fries et al. 2017; Witt et al. 2014) found the same association in BD. More specifically, a single-nucleotide polymorphism from the HNRNPC gene is one of the top ten associated (3.9 × 10−2) genes from genome-wide association study (GWAS) between BD and controls (Witt et al. 2014). Moreover, the association was mediated by a transcriptional response to glucocorticoid receptor (GR) activation (Arloth et al. 2015). TAF15 was also reported to be differentially expressed in lymphoblastic cell lines of patients with BD and their first-degree relatives compared to controls, suggesting an involvement in the pathophysiology of BD (Fries et al. 2017). High sensitivity and specificity prediction of BD included TAF15, among a 10-gene panel (Clelland et al. 2013). Furthermore, using STRING (Szklarczyk et al. 2019), both HNRNPC and TAF15 were significantly enriched for RNA splicing pathways, a mechanism recently reported to play a role in the pathophysiology of BD (Gandal et al. 2018). Our results, together with previously published findings, suggest a potential role of TDP-43 on synaptic dysfunction and decreased neuroplasticity in BD, possibly mediated by the disruption of mRNA processing.
The use of samples from postmortem brains provides an invaluable opportunity to examine molecular alterations in brain regions involved in the pathophysiology of a disorder. Moreover, postmortem brains could be an ideal model for the exploration of novel molecular features of psychiatric disorders. However, there are limitations inherent to using brain samples. First, the retrospective nature of the study design and reliance on informants for the collection of demographic and clinical information. Furthermore, our small sample size limited our ability to explore the effects of clinical (including mood state at the time of death) and demographic variables. However, between-group differences are understated when using previously validated scales, well-established methods of brain collection, minimizing potential confounding effects of PMI, pH, and demographic variables. We believe that the results presented here open new avenues for further exploration of the role of TDP-43 in BD.
In conclusion, our findings suggest increased TDP-43 protein levels in postmortem hippocampus of individuals with BD, potentially affecting the disease pathophysiology. While exploratory, our findings offer novel opportunities to discuss further this protein as potentially involved in cognitive and psychiatric alterations seen in BD. Further investigation is needed to validate these findings and examine the role of TDP-43 in the disease course and mood states. Morphological analysis of the hippocampus (including of the dentate gyrus CA1, 2 and 3) could help to interpret the differences in TDP-43 levels detected in the present study at the protein level and compare with previous findings (Velakoulis et al. 2009). Functional studies using animal or cell models would offer an opportunity to explore the role of TDP-43 in specific molecular pathways involved in behavioral and cognitive manifestations of BD, and its associations with known TDP-43 pathobiological process (stress granules, mRNA metabolism and synapse functioning).
Acknowledgements
This study was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) [grant number 466763/2014-0] and by a generous private donation from Suzana and Carlos Melzer to the USP Bipolar Disorder Research Program (PROMAN). We would like to thank the families of the subjects that donated the brains and provided all information. Camila Nascimento was supported by a post-doctoral scholarship from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) [grant number 2017/07089-8].
Funding
This study was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) [grant number 466763/2014-0] and by a generous private donation from Suzana and Carlos Melzer to the USP Bipolar Disorder Research Program (PROMAN). Camila Nascimento was supported by a post-doctoral scholarship from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) [grant number 2017/07089-8].
Footnotes
Availability of data and material
Data have not been published elsewhere and available on request from the authors.
Code availability
Not applicable.
Declarations
Conflict of interest The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethics approval Approval was obtained from the ethics committee of University of Sao Paulo. The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Consent to participate Informed consent was obtained from all next of kin of the individual participants included in the study.
Consent for publication Next of kin of all participants signed informed consent regarding publishing data generated in the brain tissue.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Camila Nascimento, Department of Psychiatry, University of Sao Paulo Medical School, Sao Paulo, SP, Brazil.
Paula V. Nunes, Department of Psychiatry, University of Sao Paulo Medical School, Sao Paulo, SP, Brazil
Helena K. Kim, Department of Psychiatry, University of Toronto, Toronto, Canada
Renata E. P. Leite, Brazilian Biobank of Aging Studies, University of Sao Paulo Medical School, Sao Paulo, SP, Brazil
Roberta D. Rodriguez, Brazilian Biobank of Aging Studies, University of Sao Paulo Medical School, Sao Paulo, SP, Brazil
Katia Cristina De Oliveira, Federal University of ABC, Sao Bernardo do Campo, SP, Brazil.
Helena P. Brentani, Department of Psychiatry, University of Sao Paulo Medical School, Sao Paulo, SP, Brazil
Wilson Jacob-Filho, Brazilian Biobank of Aging Studies, University of Sao Paulo Medical School, Sao Paulo, SP, Brazil.
Ricardo Nitrini, Brazilian Biobank of Aging Studies, University of Sao Paulo Medical School, Sao Paulo, SP, Brazil.
Carlos A. Pasqualucci, Brazilian Biobank of Aging Studies, University of Sao Paulo Medical School, Sao Paulo, SP, Brazil
Lea T. Grinberg, Brazilian Biobank of Aging Studies, University of Sao Paulo Medical School, Sao Paulo, SP, Brazil Memory and Aging Center, University of California, San Francisco, USA.
Claudia K. Suemoto, Brazilian Biobank of Aging Studies, University of Sao Paulo Medical School, Sao Paulo, SP, Brazil
Beny Lafer, Department of Psychiatry, University of Sao Paulo Medical School, Sao Paulo, SP, Brazil.
References
- American Psychiatry Association (2013) Diagnostic and statistical manual of mental disorders, 5th edn. APA, Washington, DC [Google Scholar]
- Arloth J, Bogdan R, Weber P, Frishman G, Menke A, Wagner KV, Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium, P. G. C. (2015) Genetic differences in the immediate transcriptome response to stress predict risk-related brain function and psychiatric disorders. Neuron 86(5):1189–1202. 10.1016/j.neuron.2015.05.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer IE, Soares JC, Selek S, Meyer TD (2017) The link between refractoriness and neuroprogression in treatment-resistant bipolar disorder. Mod Trends Pharmacopsychiatry 31:10–26. 10.1159/000470803 [DOI] [PubMed] [Google Scholar]
- Berk M, Kapczinski F, Andreazza AC, Dean OM, Giorlando F, Maes M, Malhi GS (2011) Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neurosci Biobehav Rev 35(3):804–817. 10.1016/j.neubiorev.2010.10.001 [DOI] [PubMed] [Google Scholar]
- Bott NT, Radke A, Stephens ML, Kramer JH (2014) Frontotemporal dementia: diagnosis, deficits and management.Neurodegener Dis Manage 4(6):439–454. 10.2217/nmt.14.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B, Passos IC, Mwangi B, Amaral-Silva H, Tannous J, Wu MJ, Soares JC (2017) Hippocampal subfield volumes in mood disorders. Mol Psychiatry 22(9):1352–1358. 10.1038/mp.2016.262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho AF, Firth J, Vieta E (2020) Bipolar disorder. N Engl J Med 383(1):58–66. 10.1056/NEJMra1906193 [DOI] [PubMed] [Google Scholar]
- Chiang PM, Ling J, Jeong YH, Price DL, Aja SM, Wong PC (2010) Deletion of TDP-43 down-regulates Tbc1d1, a gene linked to obesity, and alters body fat metabolism. Proc Natl Acad Sci USA 107(37):16320–16324. 10.1073/pnas.1002176107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clelland CL, Read LL, Panek LJ, Nadrich RH, Bancroft C, Clelland JD (2013) Utilization of never-medicated bipolar disorder patients towards development and validation of a peripheral biomarker profile. PLoS ONE 8(6):e69082. 10.1371/journal.pone.0069082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen TJ, Lee VM, Trojanowski JQ (2011) TDP-43 functions and pathogenic mechanisms implicated in TDP-43 proteinopathies. Trends Mol Med 17(11):659–667. 10.1016/j.molmed.2011.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen B, Smith DJ, Deary IJ, Evans JJ, Pell JP (2017) The “cognitive footprint” of psychiatric and neurological conditions: cross-sectional study in the UK Biobank cohort. Acta Psychiatr Scand 135(6):593–605. 10.1111/acps.12733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JL (1997) The Neuropsychiatric Inventory: assessing psychopathology in dementia patients. Neurology 48(5 Suppl 6):S10–16 [DOI] [PubMed] [Google Scholar]
- Darby MM, Yolken RH, Sabunciyan S (2016) Consistently altered expression of gene sets in postmortem brains of individuals with major psychiatric disorders. Transl Psychiatry 6(9):e890. 10.1038/tp.2016.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira KC, Nery FG, Ferreti RE, Lima MC, Cappi C, Machado-Lima A, Grinberg LT (2012) Brazilian psychiatric brain bank: a new contribution tool to network studies. Cell Tissue Bank 13(2):315–326. 10.1007/s10561-011-9258-0 [DOI] [PubMed] [Google Scholar]
- Endo R, Takashima N, Nekooki-Machida Y, Komi Y, Hui KK, Takao M, Tanaka M (2018) TAR DNA-binding protein 43 and disrupted in schizophrenia 1 coaggregation disrupts dendritic local translation and mental function in frontotemporal lobar degeneration. Biol Psychiatry. 10.1016/j.biopsych.2018.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti REL, Damin AE, Brucki SMD, Morillo LS, Perroco TR, Campora F, Nitrini R (2010) Post-mortem diagnosis of dementia by informant interview. Dement Neuropsychol 4(2):138–144. 10.1590/S1980-57642010DN40200011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschini A, Lin J, von Mering C, Jensen LJ (2016) SVD-phy: improved prediction of protein functional associations through singular value decomposition of phylogenetic profiles. Bioinformatics 32(7):1085–1087. 10.1093/bioinformatics/btv696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries GR, Quevedo J, Zeni CP, Kazimi IF, Zunta-Soares G, Spiker DE, Soares JC (2017) Integrated transcriptome and methylome analysis in youth at high risk for bipolar disorder: a preliminary analysis. Transl Psychiatry 7(3):e1059. 10.1038/tp.2017.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandal MJ, Zhang P, Hadjimichael E, Walker RL, Chen C, Liu S, Geschwind DH (2018) Transcriptome-wide isoform-level dysregulation in ASD, schizophrenia, and bipolar disorder. Science 362(6420):eaat8127. 10.1126/science.aat8127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handley EE, Pitman KA, Dawkins E, Young KM, Clark RM, Jiang TC, Blizzard CA (2017) Synapse dysfunction of layer V pyramidal neurons precedes neurodegeneration in a mouse model of TDP-43 proteinopathies. Cereb Cortex 27(7):3630–3647. 10.1093/cercor/bhw185 [DOI] [PubMed] [Google Scholar]
- Harrison JK, Stott DJ, McShane R, Noel-Storr AH, Swann-Price RS, Quinn TJ (2016) Informant questionnaire on cognitive decline in the elderly (IQCODE) for the early diagnosis of dementia across a variety of healthcare settings. Cochrane Database Syst Rev 11:CD011333. 10.1002/14651858.CD011333.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyburn L, Hebron ML, Smith J, Winston C, Bechara J, Li Z, Moussa CE (2016) Tyrosine kinase inhibition reverses TDP-43 effects on synaptic protein expression, astrocytic function and amino acid dis-homeostasis. J Neurochem 139(4):610–623. 10.1111/jnc.13763 [DOI] [PubMed] [Google Scholar]
- Ichikawa T, Baba H, Maeshima H, Shimano T, Inoue M, Ishiguro M, Arai H (2019) Serum levels of TDP-43 in late-life patients with depressive episode. J Affect Disord 250:284–288. 10.1016/j.jad.2019.03.024 [DOI] [PubMed] [Google Scholar]
- Kapczinski F, Magalhaes PV, Balanza-Martinez V, Dias VV, Frangou S, Gama CS, Berk M (2014) Staging systems in bipolar disorder: an International Society for Bipolar Disorders Task Force Report. Acta Psychiatr Scand 130(5):354–363. 10.1111/acps.12305 [DOI] [PubMed] [Google Scholar]
- Kim HK, Nunes PV, Oliveira KC, Young LT, Lafer B (2016) Neuropathological relationship between major depression and dementia: a hypothetical model and review. Prog Neuropsychopharmacol Biol Psychiatry 67:51–57. 10.1016/j.pnpbp.2016.01.008 [DOI] [PubMed] [Google Scholar]
- Lee Y, Zhang Y, Kim S, Han K (2018) Excitatory and inhibitory synaptic dysfunction in mania: an emerging hypothesis from animal model studies. Exp Mol Med 50(4):12. 10.1038/s12276-018-0028-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC (1993) The clinical dementia rating (CDR): current version and scoring rules. Neurology 43(11):2412–2414 [DOI] [PubMed] [Google Scholar]
- Nascimento C, Nunes PV, Rodriguez RD, Takada L, Suemoto CK, Grinberg LT, Lafer B (2019) A review on shared clinical and molecular mechanisms between bipolar disorder and frontotemporal dementia. Progr Neuropsychopharmacol Biol Psychiatry. 10.1016/j.pnpbp.2019.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Lee VM (2006) Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314(5796):130–133. 10.1126/science.1134108 [DOI] [PubMed] [Google Scholar]
- Passos IC, Mwangi B, Vieta E, Berk M, Kapczinski F (2016) Areas of controversy in neuroprogression in bipolar disorder. Acta Psychiatr Scand 134(2):91–103. 10.1111/acps.12581 [DOI] [PubMed] [Google Scholar]
- Ratti A, Buratti E (2016) Physiological functions and pathobiology of TDP-43 and FUS/TLS proteins. J Neurochem 138(Suppl 1):95–111. 10.1111/jnc.13625 [DOI] [PubMed] [Google Scholar]
- Schubert KO, Focking M, Cotter DR (2015) Proteomic pathway analysis of the hippocampus in schizophrenia and bipolar affective disorder implicates 14–3–3 signaling, aryl hydrocarbon receptor signaling, and glucose metabolism: potential roles in GABAergic interneuron pathology. Schizophr Res 167(1–3):64–72. 10.1016/j.schres.2015.02.002 [DOI] [PubMed] [Google Scholar]
- Spitzer R, Gibbon M, Williams J (1995) Structured clinical interview for axis I DSM-IV disorders (SCID). American Psychiatric, Washington, DC [Google Scholar]
- Stallings NR, Puttaparthi K, Dowling KJ, Luther CM, Burns DK, Davis K, Elliott JL (2013) TDP-43, an ALS linked protein, regulates fat deposition and glucose homeostasis. PLoS ONE 8(8):e71793. 10.1371/journal.pone.0071793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suemoto CK, Ferretti-Rebustini RE, Rodriguez RD, Leite RE, Soterio L, Brucki SM, Grinberg LT (2017) Neuropathological diagnoses and clinical correlates in older adults in Brazil: a cross-sectional study. PLoS Med 14(3):e1002267. 10.1371/journal.pmed.1002267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, Mering CV (2019) STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res 47(D1):D607–D613. 10.1093/nar/gky1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JB, Prager LM, Quijije NV, Schaefer PW (2018) Case 21–2018: a 61-year-old man with grandiosity, impulsivity, and decreased sleep. N Engl J Med 379(2):182–189. 10.1056/NEJMcpc1712229 [DOI] [PubMed] [Google Scholar]
- Velakoulis D, Walterfang M, Mocellin R, Pantelis C, Dean B, McLean C (2009) Abnormal hippocampal distribution of TDP-43 in patients with-late onset psychosis. Aust N Z J Psychiatry 43(8):739–745. 10.1080/00048670903001984 [DOI] [PubMed] [Google Scholar]
- Vieta E, Berk M, Schulze TG, Carvalho AF, Suppes T, Calabrese JR, Grande I (2018) Bipolar disorders. Nat Rev Dis Primers 4:18008. 10.1038/nrdp.2018.8 [DOI] [PubMed] [Google Scholar]
- Witt SH, Juraeva D, Sticht C, Strohmaier J, Meier S, Treutlein J, Rietschel M (2014) Investigation of manic and euthymic episodes identifies state- and trait-specific gene expression and STAB1 as a new candidate gene for bipolar disorder. Transl Psychiatry 4:e426. 10.1038/tp.2014.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley JD, Khan BK, Murthy NK, Miller BL, Rankin KP (2011) The diagnostic challenge of psychiatric symptoms in neurodegenerative disease: rates of and risk factors for prior psychiatric diagnosis in patients with early neurodegenerative disease. J Clin Psychiatry 72(2):126–133. 10.4088/JCP.10m06382oli [DOI] [PMC free article] [PubMed] [Google Scholar]