eTOC blurb
Johnson et al. show that orbitofrontal cortex (OFC) lesions reduce working memory for temporal order but not spatial position, and individual behavioral deficits are commensurate with lesion size. Findings suggest that OFC supports understanding of the order of events.
Keywords: orbitofrontal cortex, lateral prefrontal cortex, spatial cognition, temporal cognition, working memory
Correspondence
How do we think about time? Converging lesion and neuroimaging evidence indicates that orbitofrontal cortex (OFC) supports the encoding and retrieval of temporal context in long-term memory1, which may contribute to confabulation in individuals with OFC damage2. Here, we reveal that OFC damage diminishes working memory for temporal order, that is, the ability to disentangle the relative recency of events as they unfold. OFC lesions reduced working memory for temporal order but not spatial position, and individual deficits were commensurate with lesion size. Comparable effects were absent in patients with lesions restricted to lateral prefrontal cortex (PFC). Based on these findings, we propose that OFC supports understanding of the order of events. Well-documented behavioral changes in individuals with OFC damage2 may relate to impaired temporal-order understanding.
Examining patients with brain lesions permits inference about the necessity of a brain region for a particular function, and lesion studies play an essential role in neuroscience2,3. In this study, 56 adults performed a spatiotemporal working memory task, nine with lesions to OFC (Figures 1A, S1, Table S1). We hypothesized that OFC lesions would disrupt working memory for temporal order. To test this hypothesis, we included both a within-subjects control condition (spatial) and two control groups, patients with lateral PFC lesions not involving OFC (n = 14) and non-lesioned controls (NL; n = 33). Motivating the spatial control, extant literature reports that OFC lesions diminish long-term memory for temporal context, but not spatial context1 or standardized spatial working memory4. Motivating the PFC lesion control, we previously found that lateral PFC lesions underpinned domain-general deficits across identity, spatial, and temporal working memory trials on the same task5. Use of a PFC lesion control is also consistent with other research groups4,6.
Figure 1. OFC lesions specifically reduce working memory for temporal order.
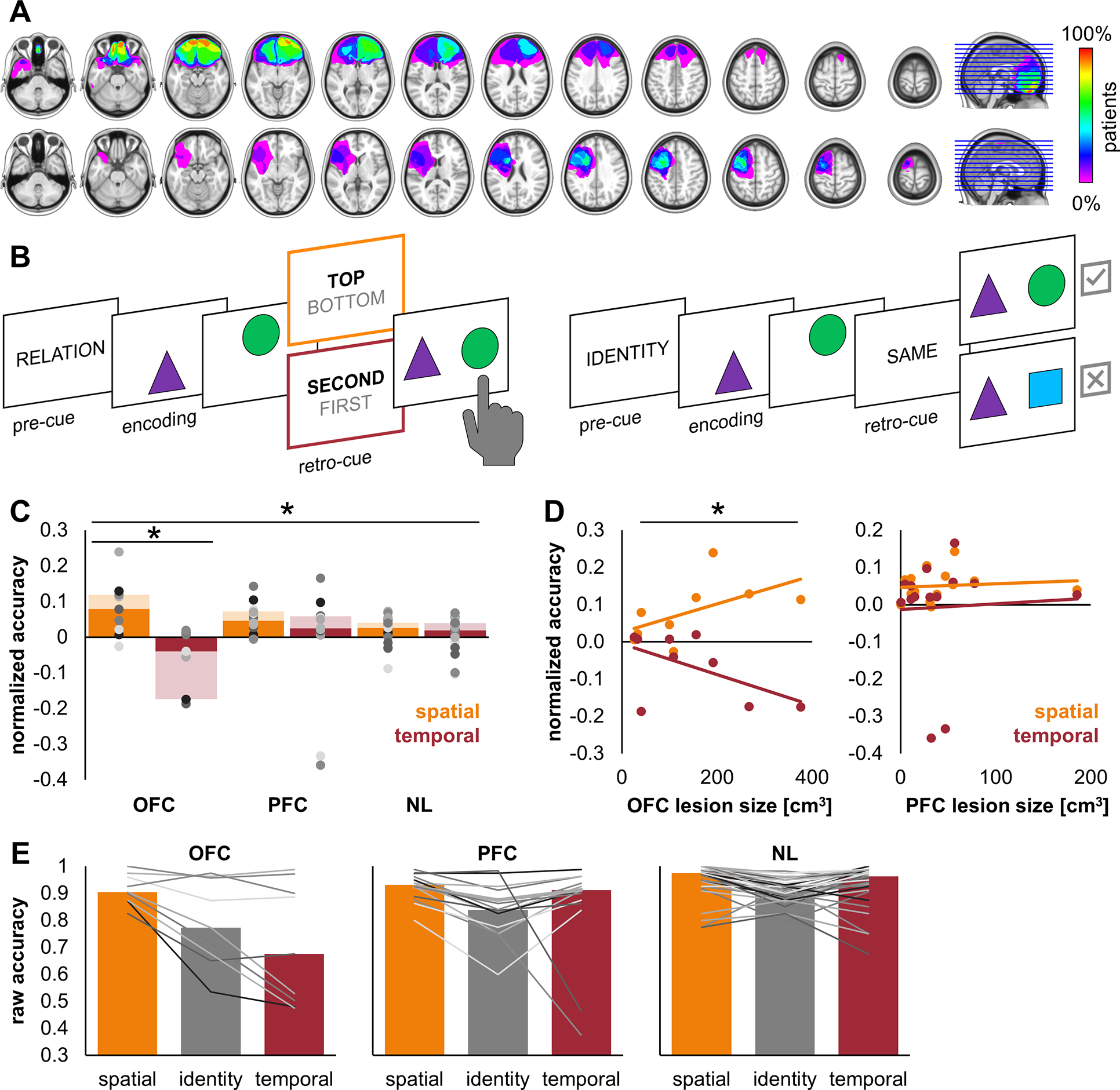
A) Lesion overlap for OFC (top; n = 9) and lateral PFC (bottom; n = 14) patients. PFC lesions are normalized to the left hemisphere5. Color scale, percentage of overlap across patients.
B) On each trial, subjects encoded a pair of stimuli in a specific spatiotemporal orientation and working memory was assessed in a two-alternative forced choice test (0.5 chance). The pre-cue and retro-cue designated trial type. On spatiotemporal relation trials (left), subjects indicated (by left or right keypress) which of two stimuli was in the top/bottom spatial or first/second temporal position. On identity trials (right), subjects indicated (by up/’yes’ or down/’no’ keypress) whether the pair was the same pair they just studied. Encoding stimuli were presented for 200 ms each, separated by 200 ms inter-stimulus fixation. The retro-cue was presented mid-delay, with 900–1,150 ms fixation before and after (not shown). The subsequent test was self-paced. Spatial, temporal, and identity trials were interspersed in random order.
C) OFC patients showed reduced accuracy for temporal order (red) but not spatial position (orange), compared to both PFC patients and NL controls. Points represent individual data and bars represent group median data (solid) ± 1 quartile spread (shaded). *, p < 0.001.
D) OFC lesion size predicted reduced accuracy for temporal order (red) but not spatial position (orange; left). PFC lesion size did not relate to individual accuracy (right). *, p < 0.001.
E) Data in (C) and (D) shown without normalization. Raw accuracy (proportion correct) for all trial types. Lines represent individual data and bars represent group median data.
The task required subjects to memorize pairs of shapes in a specific spatiotemporal orientation in preparation for an immediate test (Figure 1B)5. On spatiotemporal relation trials, the retro-cue directed subjects to identify which shape had been presented in the top/bottom spatial or first/second temporal position. On identity trials, the retro-cue directed subjects to maintain a representation of the identity of both shapes regardless of spatiotemporal orientation. We normalized individual spatial and temporal data against the identity control for analysis. Normalized accuracy indicates the relative benefit or cost of associating an item with its spatial or temporal context in working memory, holding item identity performance constant. This procedure also addresses systematic confounds of inter-individual and inter-group variability (e.g., in lesion etiology; Table S1)3. To further control for inherent limitations of variability not related to lesion focus, we performed all analyses using linear mixed-effects models with individual age included as a fixed effect and subjects as random intercepts (see Supplemental Experimental Procedures).
Normalized accuracy data from all subjects were submitted to a model with fixed factors of condition (temporal, spatial) and group (OFC, PFC, NL). There was a significant main effect of condition (temporal < spatial, F(1,105) = 28.89, p = 5×10−7) and a condition by group interaction (F(2,105) = 9.03, p = 2×10−4). The interaction was driven by condition differences in OFC patients (t(105) = −3.52, p = 6×10−4; Cohen’s d = −1.31), who showed a selective deficit in temporal working memory (Figure 1C). No other effects reached significance (p’s > 0.51).
Planned post-hoc analysis investigated whether OFC patients’ deficits in temporal working memory were related to lesion size. OFC patient and NL control data were submitted to a model with fixed factors of condition and lesion size. There was a significant condition by lesion size interaction (F(1,79) = 41.63, p = 8×10−9; Figure 1D, left). Larger OFC lesions were associated with decreased temporal accuracy. No other effects reached significance (age p = 0.35, other p’s > 0.05). PFC lesion size, in contrast, was not significantly related to individual accuracy (interaction F(1,89) = 1.21, p = 0.27; other p’s > 0.11; Figure 1D, right).
Our findings demonstrate that OFC damage diminishes working memory for temporal order. Comparable deficits were not observed in working memory for spatial position or with lateral PFC damage not involving OFC. Indeed, the opposite pattern in OFC patients’ spatial working memory confirms a temporal-specific deficit that cannot be attributed to generalized context performance deficits from larger lesions. We propose that OFC supports temporal-order understanding.
First, unlike lateral PFC2,5, OFC supports working memory for temporal order independent of working memory in general. Whereas deficits have been detected on the n-back task, which assesses memory for the item which appeared 2- or 3-back in a sequence, extant literature reports intact performance on tasks of pure maintenance and manipulation (e.g., digit and spatial span)6. Second, OFC supports temporal processing independent of long-term memory. Whereas our results corroborate evidence that OFC lesions diminish memory for temporal, but not spatial, context1, our task had no long-term component. Third, OFC may support processing of the past, present, and future. Indeed, OFC supports ‘reality filtering’, the ability to relate past and present7. Other influential theories link OFC to prediction, forming abstract cognitive maps, and signaling value8, all of which implicate representations of the future. OFC has also been shown to support judgments of temporal duration4.
Finally, behavioral changes in individuals with OFC damage may relate to impaired temporal-order understanding. Exemplified by patient Phineas Gage, whose personality changed dramatically after a penetrating head injury to bilateral OFC (note additional medial frontal pole, anterior cingulate, and white matter damage9), OFC damage is associated with newfound inflexibility, impulsivity, confabulation, and problems with emotional and social control2. Perhaps, as confabulation reflects diminished filtering of past and present7, impulsivity reflects diminished representations of what could come next, contributing to a myopic discounting of future reward. Although impaired temporal-order understanding is not the only explanation for such changes (cf. confabulation and impaired strategic retrieval10), it is a parsimonious one that links otherwise disparate behaviors to a common neurocognitive substrate. Future research should consider OFC in the framework of temporal cognition.
Supplementary Material
Acknowledgements
We thank J. Parvizi, I. Strieter, J. Lubell, H. Zhang, and C. M. Perry. This work was funded by grants from the National Institute of Neurological Disorders and Stroke (R00NS115918, R01NS021135, R37NS21135, U01NS108916, U19NS107609), Research Council of Norway (240389/F20), Research Council of Norway through its Centres of Excellence scheme (262762 RITMO), and RITPART International Partnerships for RITMO (274996).
Footnotes
Declaration of interests
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Duarte A, Henson RN, Knight RT, Emery T, and Graham KS (2010). Orbito-frontal cortex is necessary for temporal context memory. Journal of Cognitive Neuroscience 22, 1819–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Szczepanski SM, and Knight RT (2014). Insights into Human Behavior from Lesions to the Prefrontal Cortex. Neuron 83, 1002–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaidya AR, Pujara MS, Petrides M, Murray EA, and Fellows LK (2019). Lesion Studies in Contemporary Neuroscience. Trends in Cognitive Sciences 53, 653–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berlin HA, Rolls ET, and Kischka U (2004). Impulsivity, time perception, emotion and reinforcement sensitivity in patients with orbitofrontal cortex lesions. Brain 127, 1108–1126. [DOI] [PubMed] [Google Scholar]
- 5.Johnson EL, Dewar CD, Solbakk A-K, Endestad T, Meling TR, and Knight RT (2017). Bidirectional frontoparietal oscillatory systems support working memory. Current Biology 27, 1829–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barbey AK, Koenigs M, and Grafman J (2011). Orbitofrontal contributions to human working memory. Cerebral Cortex 21, 789–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schnider A (2013). Orbitofrontal reality filtering. Frontiers in Behavioral Neuroscience 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou J, Gardner MP, and Schoenbaum G (2021). Is the core function of orbitofrontal cortex to signal values or make predictions? Current Opinion in Behavioral Sciences 41, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Damásio H, Grabowski T, Frank R, Galaburda AM, and Damásio AR (1994). The return of Phineas Gage: Clues about the brain from the skull of a famous patient. Science 264, 1102–1105. [DOI] [PubMed] [Google Scholar]
- 10.Gilboa A, Alain C, Stuss DT, Melo B, Miller S, and Moscovitch M (2006). Mechanisms of spontaneous confabulations: A strategic retrieval account. Brain 129, 1399–1414. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


