Purpose
Vaccination against coronavirus disease 2019 (COVID-19) is currently under worldwide deployment. The consequences of this vaccination can be seen in radiology and nuclear medicine explorations with visualization of axillary lymph nodes (LNs), as observed on ultrasonography, MRI, or 18F-FDG PET/CT.
We aimed to evaluate on PET/CT the incidence of vaccine-related LNs and their characteristics after COVID-19 vaccination, using several radiopharmaceuticals different from 18F-FDG.
Patients and Methods
Between February and July 2021, all consecutive patients undergoing a whole-body PET/CT for any indication using a different radiopharmaceutical from 18F-FDG were eligible for inclusion if they had received at least 1 dose of the COVID-19 vaccine. The radiopharmaceutical administered and vaccine type were recorded for each patient. The incidence of positive vaccine-related axillary and supraclavicular LNs on PET/CT was our primary finding, along with the nodes characteristics. Statistical analyses were performed for patients with prostate cancer (PCa) to determine certain interaction factors that were associated with the detection of vaccine-related LNs.
Results
Of the 226 patients in our cohort study, 120 patients underwent an 18F-fluorocholine PET/CT, 79 a 68Ga-PSMA-11 PET/CT, 6 an 18F-FDOPA PET/CT, and 21 a 68Ga-DOTATOC PET/CT. A total of 67.3% of patients (152/226) received BNT162b2mRNA (Pfizer-BioNTech), 26.5% (60/226) ChAdOx1-S (AstraZeneca), 4.9% (11/226) mRNA-1273 (Moderna), and 1.3% (3/226) Ad26.COV2.S (Janssen). The incidence of positive vaccine-related axillary and supraclavicular LNs was 42.5% (51/120 patients) on PET/CT using 18F-fluorocholine and 12.7% (10/79 patients) with 68Ga-PSMA-11. None of our patients undergoing 18F-FDOPA or 68Ga-DOTATOC PET/CT presented any vaccine-related lymphadenopathy. Vaccine-related LNs were statistically associated with the nature of the radiopharmaceutical (P < 10−4), with the number of vaccine doses received (P = 0.041), with a short delay between vaccination and PET/CT realization (P < 10−5), and with a higher prostate-specific antigen level for patients with PCa (P = 0.032), but not with age or vaccine type. The vaccine-related nodes appeared in 85% of the cases, in the 30 days after vaccine injection, were limited in size and uptake, and were most often limited to the axilla level 1 area.
Conclusions
Detecting positive LNs after COVID-19 vaccination is not an exclusive 18F-FDG PET/CT pattern but is common on 18F-fluorocholine and possible on 68Ga-PSMA-11 PET/CT. Confronting PET/CT findings with clinical data (such as date and site of injection) seems essential in the current pandemic context, just as it does for the radiopharmaceuticals used in PCa to avoid PET/CT misinterpretation and incorrect patient treatment. For 18F-FDOPA or 68Ga-DOTATOC PET/CT, this seems to have a lesser impact.
Key Words: COVID-19, vaccination, 18F-fluorocholine, 68Ga-PSMA-11, PET/CT, pitfalls
Vaccination against coronavirus disease 2019 (COVID-19) is currently ongoing1 in France with the aim of covering most of its population, thanks to 4 authorized vaccines, the BNT162b2mRNA (Pfizer-BioNTech), mRNA-1273 (Moderna), ChAdOx1-S (AstraZeneca), and Ad26.COV2.S (Janssen) vaccines.2 The vaccination campaign started with patients older than 75 years or with severe comorbidities, then the adult population as a whole, and now covers teenagers. In this way, more than 74.7% of the population has received at least 1 dose of vaccine, and 66.3% are fully vaccinated, at the time of writing (October 2021).3
Local and systemic reactions are described after COVID-19 vaccines,4 and a commonly reported adverse effect after administration in the deltoid muscle is an ipsilateral swollen or sensitive axillary lymph node (LN).5–7 Several cases were encountered rapidly in imaging departments, with visualization of axillary and supraclavicular LNs. This was noted during breast cancer screening in patients undergoing breast ultrasound8 or MRI,9 leading to recommendations to guide interpretation.10 This was also reported during 18F-FDG PET/CT.11–14 This type of hypermetabolism had previously been described after other vaccinations, such as those against human papillomaviruses15 or H1N1 influenza A virus,16 so this could have been expected due to the lack of specificity of 18F-FDG, particularly in a context of inflammation.
More surprisingly, a case of left axillary lymphadenopathy detected on an 18F-fluorocholine (18F-FCH) PET/CT was reported in a 75-year-old patient monitored for biochemical recurrence of his prostate cancer (PCa), 3 days after receiving a dose of ChAdOx1-S (AstraZeneca) vaccine in his left arm.17
The aim of this study was to evaluate the incidence of vaccine-related LNs after COVID-19 vaccination and to describe their PET/CT pattern with several radiopharmaceuticals different from 18F-FDG such as 18F-FCH, 68Ga-PSMA-11, 18F-FDOPA, and 68Ga-DOTATOC.
PATIENTS AND METHODS
Patient Recruitment
All consecutive patients undergoing a whole-body PET/CT between February and July 2021, for any indication and with a radiopharmaceutical different from 18F-FDG, were eligible for inclusion in the study if they had received at least 1 dose of COVID-19 vaccine before their PET/CT. Patients were excluded in case of missing data, a site of administration different from the deltoid muscle (eg, gluteal muscle), known malignant involvement of axillary and supraclavicular LNs, or if the second dose was not with the same vaccine as the first (eg, ChAdOx1-S [AstraZeneca] followed by BNT162b2mRNA [Pfizer-BioNTech]).
All patients were informed and gave their consent for access to their data, and none objected to these data being used for research purposes. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was examined and approved on July 6, 2021 by the local ethics committee of the institutional review board and registered under number 2021-114.
Data Collection
Our study consisted of analyzing a cohort of patients whose data were collected prospectively. All patients were interviewed about their vaccination status on the day of their PET/CT. The data collected for each patient included sex, age, oncological status, current oncologic treatment, number of doses of COVID-19 vaccine already received, date(s) of administration, side(s) of administration, type of vaccine, and tumor marker if relevant. Data collection also included the examination date, indication, and radiopharmaceutical administered.
PET/CT Analysis
All imaging was performed on a PET/CT Vision 450 (Siemens Erlangen, Germany). Images were acquired (3 minutes per step) 1 or 2 hours after injection, depending on the radiopharmaceuticals. Low-dose CT 3D acquisition without contrast product injection was performed considering the system’s voltage and current adaptation. Four hundred forty pixel-wide PET images were reconstructed using the TrueX algorithm (Siemens) and time-of-flight (4 iterations, 35 subsets) and Gaussian postfiltering (2-mm full-width at half-maximum). Finally, the images were sent to our picture archive and communication system.
All PET/CT images were interpreted by senior nuclear medicine physicians and reviewed by one of them. The number of axillary and supraclavicular LNs with a radiopharmaceutical uptake was recorded, as well as their location and size.
An LN was taken into consideration if its SUVmax was greater than mediastinum uptake and/or if the diameter of its smaller axis was greater than or equal to 10 mm.
Its location was determined as axilla level 1 (below the pectoralis minor), axilla level 2 (beside the pectoralis minor), or axilla level 3 (above pectoralis minor) according to the Berg classification.18 Its size was measured directly on the CT images from the PET/CT.
These positive LNs were considered vaccine-related if they were on the same side as the injection and if involvement with the primitive tumor was very unlikely. This likelihood was assessed on a clinical basis, depending on patient disease stage, on whether or not there was a progression, on the presence of other lymphatic areas involved, and knowing the natural spread of the cancer afflicting pelvic and retroperitoneal nodes in the first place for PCa for instance, and this translated in practice in the conclusion of our examination interpretation by the absence of needing complementary (imaging or pathological) examinations to explore these nodes. Cases of LNs related to proven or potential tumor involvement were excluded from the cohort study; those cases were mainly PCa with extended nodal involvement at diagnosis or PCa with nodal metastatic progression. Patients presenting positive vaccine-related axillar or supraclavicular LNs made up our case group, whereas patients without vaccine-related LNs served as a comparison group. The incidence on PET/CT of positive vaccine-related axillary and supraclavicular LNs was our primary finding.
Statistical Analysis
Categorical variables were reported as frequencies, and continuous variables as medians with the first and third quartiles (Q1–Q3 range).
As the number of PET/CT using 18F-FDOPA and 68Ga-DOTATOC as radiopharmaceuticals was small, those cases were not included in the statistical analysis. We thus decided to limit our statistical analysis to patients with PCa, which was the largest number of patients, for better comparability.
The χ2 test was used to compare proportions between groups.
Logistic regression models were used to find a correlation between positive axillary and supraclavicular LNs and independent variables such as sex, age, oncological status, current oncologic treatment, number of doses of COVID-19 vaccine already received, date(s) of administration, side(s) of administration, type of vaccine, and prostate-specific antigen (PSA) level.
We considered as statistically significant a P value strictly less than 0.05.
RESULTS
Patient Characteristics
Of all the patients who had a PET/CT in our nuclear medicine department and who were screened with regard to our inclusion and exclusion criteria, 226 patients were included in our study cohort. The selection process is described in a flowchart (Fig. 1). Patients underwent PET/CT using 18F-FCH for 120 of them, 68Ga-PSMA-11 for 79 patients, 18F-FDOPA for 6, and 68Ga-DOTATOC for 21 patients.
FIGURE 1.
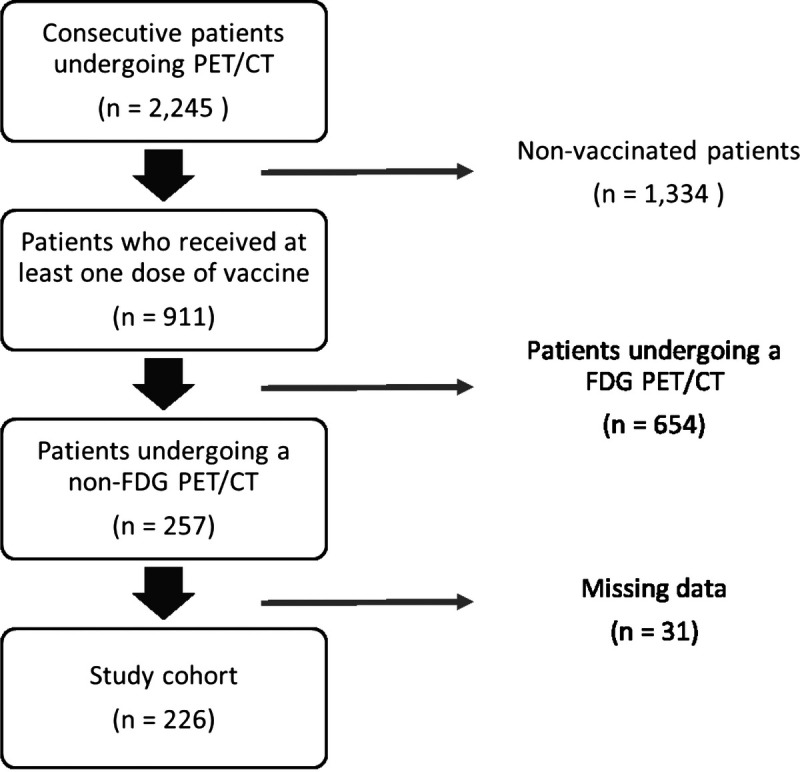
Patient flowchart.
With 18F-FCH, the indications were mainly biochemical recurrence of PCa in 82.5% (99/120) of patients, then initial assessment in 17.5% (21/120) of patients, and exploration of parathyroid nodules in 1.7% (2/120) of patients.
With 68Ga-PSMA-11, the indications were limited by the French Regulation Agency and limited to biochemical recurrence of PCa after noncontributive 18F-FCH PET/CT.
With 18F-FDOPA and 68Ga-DOTATOC, the indications were the initial assessment or follow-up of neuroendocrine tumors.
As described in Table 1, we observed a majority of patients with PCa, hence a high proportion of men (212/226), with only 14 women in our cohort. Patients’ ages ranged from 46 to 89 years. All patients were vaccinated with 1 of the 4 vaccines that received authorization in France. Most patients received the BNT162b2mRNA (Pfizer-BioNTech) vaccine, approximately one fourth received the ChAdOx1-S (AstraZeneca) vaccine, and very few received the mRNA-1273 (Moderna) or Ad26.COV2.S (Janssen) vaccines. A total of 54.9% (124/226) of patients had fully completed their vaccination schedule with 2 doses (or only 1 for the Janssen vaccine) before their PET/CT.
TABLE 1.
Patient Characteristics
| Total (n = 226) | Cases (n = 61) | Controls (n = 165) | |
|---|---|---|---|
| Median age in years (Q1–Q3) | 71 (67–76) | 72 (67.5–77) | 71 (65–75) |
| Male (frequency) | 212 (93.8%) | 62 (98.4%) | 150 (92.0%) |
| Radiotracers (frequency) | |||
| 18F-Fluorocholine | 120 (53.1%) | 51 (83.6%) | 69 (41.8%) |
| 68Ga-PSMA-11 | 79 (35.0%) | 10 (16.4%) | 69 (41.8%) |
| 18F-FDOPA | 6 (2.7%) | 0 (0%) | 6 (3.6%) |
| 68Ga-DOTATOC | 21 (9.2%) | 0 (0%) | 21 (12.8%) |
| Vaccines (frequency) | |||
| BNT162b2mRNA (Pfizer-BioNTech) | 152 (67.3%) | 46 (75.4%) | 106 (64.2%) |
| ChAdOx1-S (AstraZeneca) | 60 (26.5%) | 12 (19.7%) | 48 (29.1%) |
| mRNA-1273 (Moderna) | 11 (4.9%) | 2 (3.3%) | 9 (5.5%) |
| Ad26.COV2.S (Janssen) | 3 (1.3%) | 1 (1.6%) | 2 (1.2%) |
| Fully vaccinated (frequency) | 124 (54.9%) | 30 (49.2%) | 94 (57%) |
| Median interval between last dose and PET/CT in days (Q1–Q3) | 26 (14–51) | 15 (7–28) | 32 (18–60) |
Cases: patients with positive vaccine-related axillary or supraclavicular LNs.
Controls: patients with no vaccine-related LNs.
Positive Vaccine-Related Axillary or Supraclavicular LNs
In 18F-FCH PET/CT, 51/120 patients (42.5%) presented with positive vaccine-related LNs. For these patients, the time between the last dose of vaccine received and the PET/CT ranged from 1 to 86 days, with a median of 15 days, and approximately 85% of vaccine-related LNs cases were found to be in the 30 days after an injection.
Of the 61 patients who had received only the first dose of vaccine, 27 (44.3%) presented with vaccine-related LNs. Of the 59 fully vaccinated patients, 24 (40.7%) presented with vaccine-related LNs. Distribution of positive LNs according to the type of vaccine is shown in a chart (Fig. 2).
FIGURE 2.
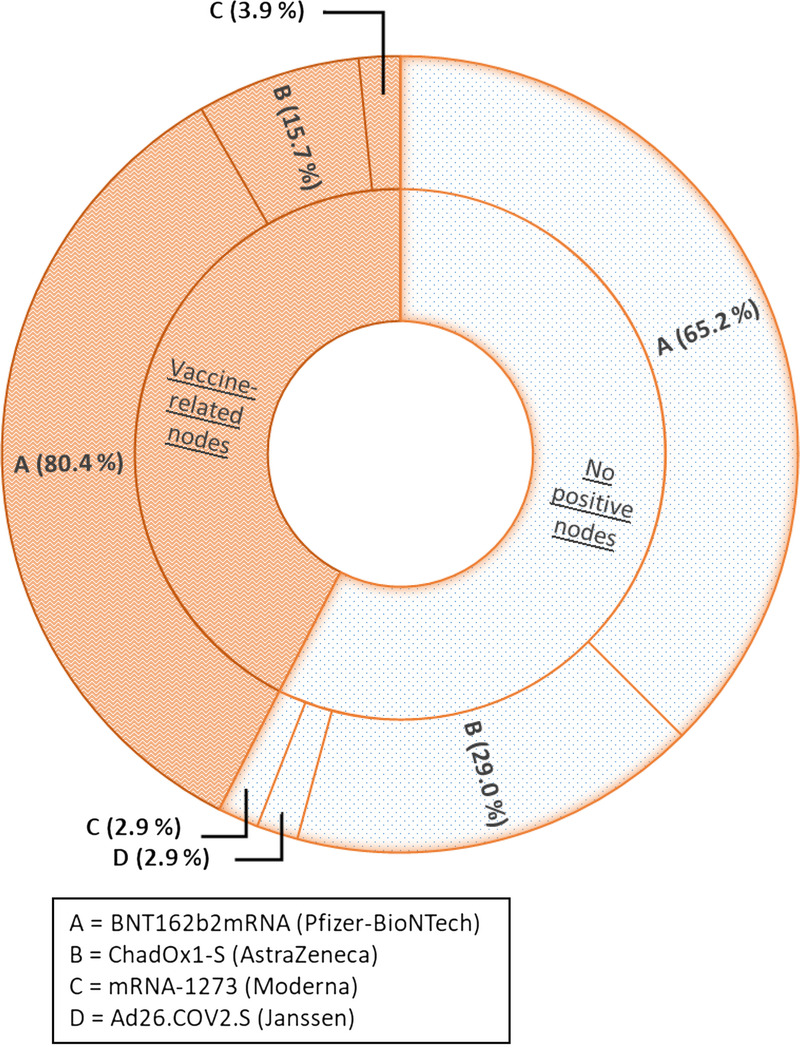
Distribution of vaccines administered in patients with 18F-fluorocholine PET/CT according to the presence of vaccine-related LNs.
In 68Ga-PSMA-11 PET/CT, 10/79 patients (12.7%) presented with positive vaccine-related LNs. For these 10 patients, the time between the last dose of vaccine received and the PET/CT ranged from 7 to 62 days, with a median of 18.5 days. Of the 24 patients who had received only the first dose of vaccine, 5 (20.8%) presented with vaccine-related LNs. Of the 55 fully vaccinated patients, 5 (9.1%) presented with vaccine-related LNs. Distribution of positive LNs according to the type of vaccine is shown in a chart (Fig. 3).
FIGURE 3.
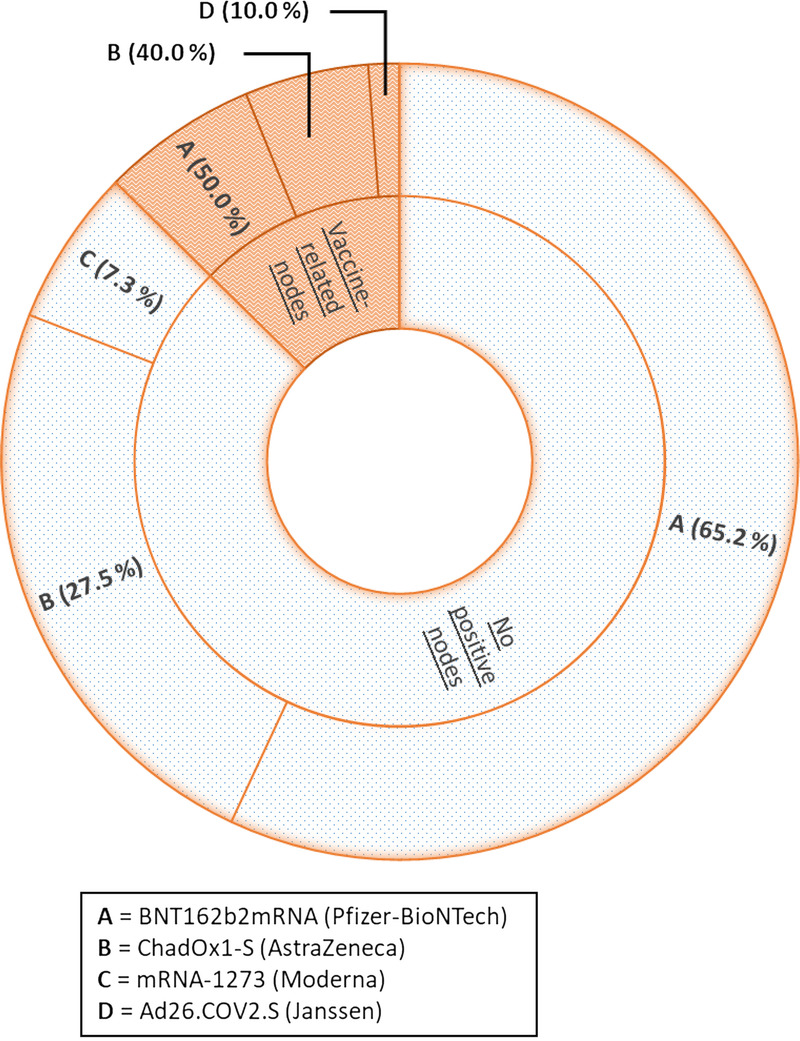
Distribution of vaccines administered in patients with 68Ga-PSMA-11 PET/CT according to the presence of vaccine-related LNs.
In 18F-FDOPA and 68Ga-DOTATOC PET/CT, no patients (0/6 and 0/21 patients, respectively) presented any vaccine-related LNs.
To illustrate the appearance of vaccine-related LNs on the PET/CT images, we present 2 cases where vaccine-related LNs were detected on PET/CT using 18F-FCH (Fig. 4) and 68Ga-PSMA-11 (Fig. 5).
FIGURE 4.
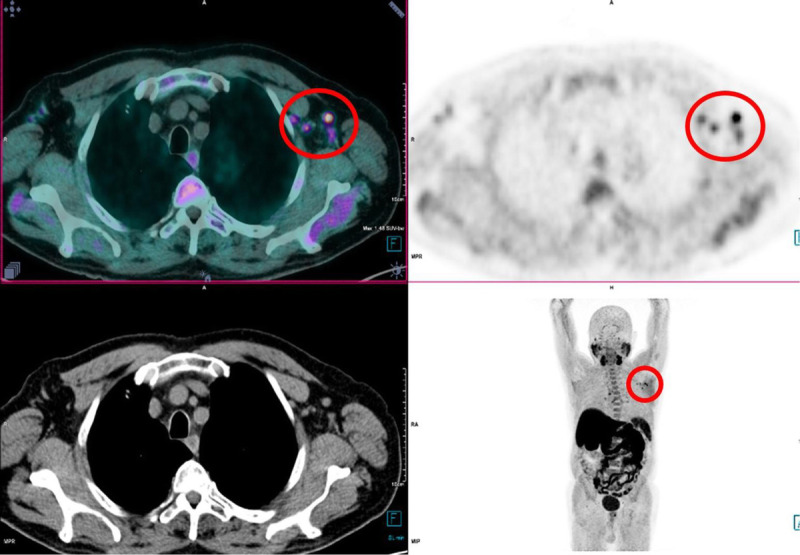
An 81-year-old man underwent an 18F-fluorocholine PET/CT for rising PSA after PCa radiotherapy. Multiple left axillary LNs (red circle) with higher uptakes than the mediastinum were observed. The man received the first and second doses of the BNT162b2mRNA (Pfizer-BioNTech) vaccine, respectively, 24 and 3 days before undergoing this PET/CT.
FIGURE 5.
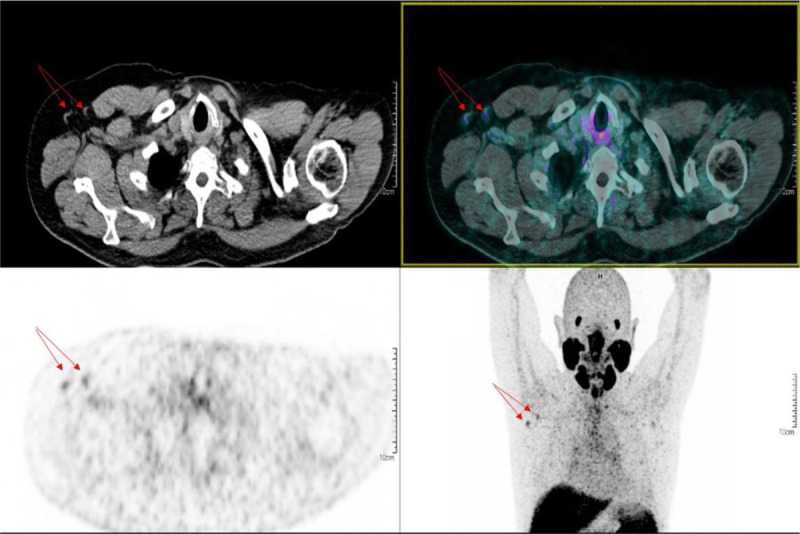
A 74 year-old man underwent a 68Ga-PSMA-11 PET/CT for rising PSA after radical treatment. Two right axillary LNs (red arrows) with higher uptake than the mediastinum were detected. The man received the first and second doses of the ChAdOx1-S (AstraZeneca) vaccine, respectively, 80 and 19 days before undergoing this PET/CT.
Data analysis showed some interaction factors for positive vaccine-related LNs in patients with PCa. Patients had a higher probability of having vaccine-related LNs with 18F-FCH PET/CT compared with 68Ga-PSMA-11 PET/CT, with an odds ratio of 5.1 (P < 10−4) and a 95% confidence interval (2.4–10.9).
Fully vaccinated patients seemed to present vaccine-related LNs less frequently compared with those receiving only 1 dose, but this difference was barely significant (odds ratio, 0.51; P = 0.041; 95% confidence interval, 0.28–0.93). However, we found a strong statistical association between LN positivity and a shorter time between the last dose of vaccine and the date of the PET/CT (P < 10−5).
We also found a slightly significant association between LN positivity and a higher level of PSA (P = 0.032), meaning that patients with higher PSA levels had vaccine-related LNs detected more frequently on their PET/CT, as described in Figure 6.
FIGURE 6.
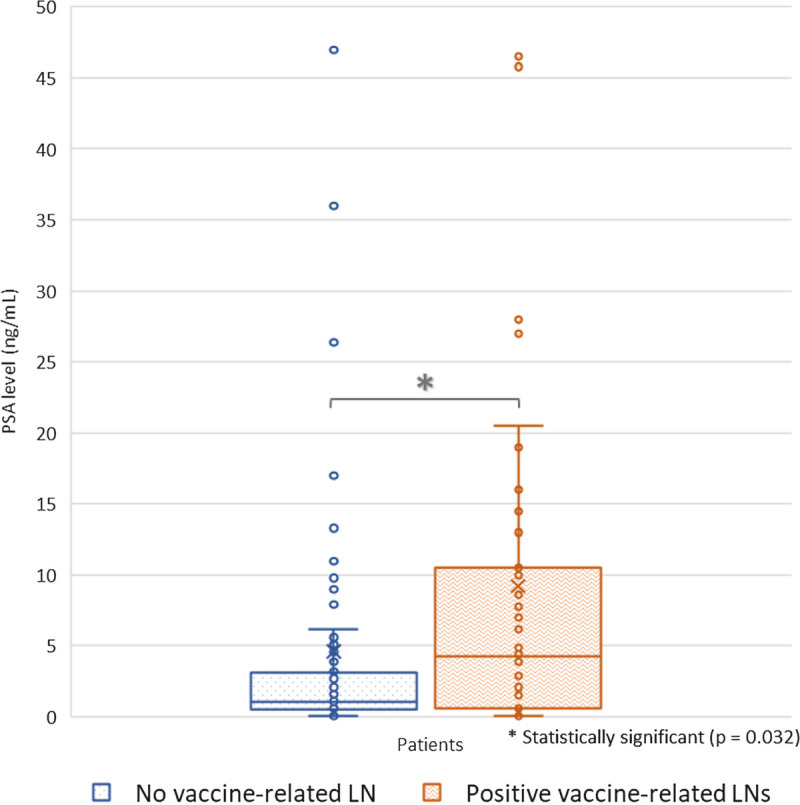
Detection of vaccine-related LNs according to PSA level.
On the contrary, there was no association with patient age (P = 0.60). Moreover, patients receiving the BNT162b2mRNA (Pfizer-BioNTech) vaccine did not have a significantly higher prevalence of vaccine-related LNs in comparison with the other 3 vaccines (odds ratio, 1.64; P = 0.14; 95% confidence interval, 0.83–3.24).
Intensity, Number, Size, and Location of the Vaccine-Related LNs
Most of the LNs that we retained as vaccine-related LNs had an SUVmax that was greater than the mediastinum uptake, but the intensity uptake remained moderate and less intense than the liver level.
Patients presented between 1 and 12 positive LNs, with a median of 3 LNs. Positive LNs ranged from 4 mm to 15 mm on the small axis. Few patients (14/61) presented LNs of more than 10 mm on the small axis.
Most patients (43/61) presented positive LNs limited to axilla level 1 only. Fifteen of 61 patients (24.6%) and 6/61 patients (9.8%) nonetheless presented positive LNs located at axilla level 2 and axilla level 3, respectively.
In additional statistical analyses for exploratory purposes only, neither the number of positive LNs nor the size of the LNs were significantly associated with number of doses received, patient age, type of vaccine, or PSA level.
DISCUSSION
In the context of the current pandemic and ongoing vaccination campaign, our study plays a part in emphasizing the influence of COVID-19 vaccines on PET/CT interpretation. To our knowledge, this study is the largest cohort to include non-FDG PET/CT and showed several distinctive features.
First, a notable highlight was finding that patients receiving 2 doses of vaccine presented vaccine-related LNs less frequently than patients receiving a single dose. This finding was unexpected because the clinical trials for vaccine authorizations had reported higher rates of local and systemic adverse effects after the second dose of the BNT162b2mRNA (Pfizer-BioNTech) or mRNA-1273 (Moderna) vaccines,4,19 and contrasted with other articles in the literature related to COVID-19 vaccination and 18F-FDG PET/CT, where patients presented vaccine-related LNs more frequently after the second booster dose.20,21 One hypothesis could be the high age of the patients (median age, 71 years in our cohort due to the pathology, which affects the elderly, compared with 18F-FDG cohorts with a wider range of diseases with younger median ages). How can this be explained? This result could be consistent with the fact that elder patients present fewer immune responses after vaccine administration. Impaired antibody response and lesser efficacy after influenza vaccination has already been demonstrated in older adults,22 and this phenomenon can be called “immunosenescence.” It describes several hallmarks of immune aging, such as a decreased number of naive CD8+ T-lymphocytes.23 More recently, similar observations questioning a lesser immune response in older people with regard to COVID-19 vaccination have started to be reported.24,25 To corroborate this finding, collecting patients’ serological status and antibody titration after each dose of vaccine could be relevant in future studies.
Otherwise, observations in 18F-FCH PET/CT had similarities with those in 18F-FDG PET/CT, starting with comparable incidence (42.5%) of vaccine-related LNs. For example, we can cite 2 cohort studies that were conducted in Israel between December 2020 and February 2021, including almost a thousand of patients who had received at least 1 dose of a COVID-19 vaccine and who underwent 18F-FDG PET/CT,20,21 and in both studies, approximately 45% of the patients presented hypermetabolic lymphadenopathies that were associated with the vaccine. Such observations led to advise to check the vaccine injection site and dates of the doses before interpreting the examination but also to avoid the administration in a potential confounding site (eg, left deltoid muscle in case of an ipsilateral breast cancer).26 This is a reminder that 18F-FCH, such as 18F-FDG, is not specific for cancer cells. In fact, 18F-FCH is a marker of phospholipid biosynthesis for cell membrane renewal. Its uptake has already been described in both benign tumors27 and malignancies,28,29 as well as in inflammatory sites, which were by far the most frequent situations.30–32 This may be explained by an increased need for membrane synthesis in case of intense proliferation of immune cells, as generally observed in activated LNs,33,34 leading to an increased uptake of 18F-FCH.35,36
Our cohort managed to find some rare cases of vaccine-related LNs detected in 68Ga-PSMA-11 PET/CT. They were less expected due to the properties of 68Ga-PSMA-11, which has a transmembrane enzyme used for its high uptake in PCa, although there can be uptake in benign and malignant diseases, as well as in a context of inflammation.37 The detailed mechanisms concerning PSMA uptake have yet to be elucidated, but neovasculature seems to play a preponderant role.38 This leads us to think that in our cases the vaccination mimics a SARS-CoV-2 infection a minima and generates inflammation, which is fertile ground for neovasculature, thus explaining the uptake. This is further supported by some reported cases of lung injuries related to COVID-19 detected in 68Ga-PSMA-11 PET/CT39 and by the observation of increased plasma levels of vascular cell adhesion molecule and vascular endothelial growth factor on account of SARS-CoV-2 infection.40
Our study aimed to compare different COVID-19 vaccines. The incidence rate of vaccine-related LNs was higher but not statistically significant with the BNT162b2mRNA (Pfizer-BioNTech) vaccine. This might be due to a lack of power in the test for showing a significant difference, due to a limited number of patients. It is now well described that the frequency and nature of adverse effects can vary depending on the type of vaccine,41 and one study showed a different frequency of vaccine-related LNs in 18F-FDG PET/CT after the mRNA-1273 (Moderna) vaccine compared with the BNT162b2mRNA (Pfizer-BioNTech) vaccine.42
Our statistical analyses highlighted an association between LN positivity and a higher level of PSA in patients with recurrent PCa. We may then hypothesize that a higher level of PSA reflects a more aggressive form of the disease, leading to more systemic inflammation and thus immunogenicity, explaining a higher propensity to react to vaccination, but the difference is quite slight, and these results need to be confirmed at a larger scale.
Furthermore, definitions of positive vaccine-related LNs are variable and arbitrarily chosen, thus limiting the comparability between the various publications. Some authors considered an LN as positive if the ratio between the SUVmax in the ipsilateral and contralateral reference sites was greater than or equal to 1.5, as described by Thomassen et al,43 whereas others classified LNs by uptake intensity.20 Our definition of positive vaccine-related LNs was a semiquantitative scale similar to the Deauville score as it was convenient for its high level of proof in hematological malignancies for example44 and for its good reproducibility, whereas quantitative SUVs can vary considerably depending on multiple parameters.
Although our study added some new information to our knowledge as far as the impact of COVID-19 vaccination is concerned, collecting more data concerning less studied populations would be welcome, as would a more precise description of the pathophysiological mechanisms involved.45,46 This seems even more relevant given that the vaccination campaign is ongoing and will continue, with the recommendation for a third booster dose of the vaccine, freshly supported by the positive results for efficacy and tolerance in phase III trials.47
Besides, we found no cases of vaccine-related LNs in 18F-FDOPA or 68Ga-DOTATOC PET/CT. First, the low number of patients for these 2 radiopharmaceuticals may explain the absence of vaccine-related nodes. We might also find an explanation through their respective targeting mechanisms. Imaging 18F-FDOPA uses the property of taking up amino acids, transforming them into biogenic amines by means of decarboxylation, and storing them in the vesicles of tumor cells.48 This targeting probably explains the absence of vaccine-related nodes. 68Ga-DOTATOC targets somatostatin receptors, which are overexpressed in activated macrophages and T-lymphocytes, and are used in inflammation-related diseases such as autoimmune mechanisms, but not in relation to infection of either viral or bacterial origin.49 It could be nonetheless noted that cases of vaccine-related axillary LNs were reported in the literature in 68Ga-DOTATATE PET/CT indicated for pulmonary50 and rectal51 neuroendocrine neoplasms.
Finally, our study has a number of limitations. It indeed a retrospective analysis, although our data were collected prospectively from consecutive patients. Definition of vaccine-related LNs as exposed in our methodology was based on clinical data, so identified LNs were not certain to be inflammatory due to the lack of pathological examination, but were very likely to be. Cases of LNs suspicious for malignancy, for instance PCa with extended nodal involvement at diagnosis or PCa with nodal metastatic progression, were discussed for complementary examinations such as ultrasounds at 6 to 12 weeks or nodal biopsies and were not reported for our present study.
Most of the patients in our cohort were men of advanced age due to the pathology and could not reflect patients with other pathologies or younger in age. In addition, we had a small number of patients undergoing 18F-FDOPA and 68Ga-DOTATOC PET/CT, limiting our conclusions with regard to these patients. We also tried to compare 4 different vaccines, but in practice, few patients were vaccinated with the mRNA-1273 (Moderna) or Ad26.COV2.S (Janssen) vaccines, which also limits our conclusions.
CONCLUSIONS
Thanks to a cohort of more than 200 patients, including several radiopharmaceuticals and different types of vaccine, we show that detecting positive LNs after COVID-19 vaccination is not limited to 18F-FDG PET/CT, but is common in 18F-FCH PET/CT and is also possible in 68Ga-PSMA-11 PET/CT. The vaccine-related nodes were limited in size and intensity, and were often limited to axilla level 1 and were closely associated with the time between the dose of vaccine and the date of the PET/CT. Understanding these pitfalls, combined with particularities in vaccine data, such as vaccination date and vaccination side, seems essential regarding the current pandemic context and the recent vaccine recommendations in elderly patients, to avoid misinterpreting images and adopting incorrect patient therapeutic protocols.
ACKNOWLEDGMENTS
The authors would like to thank the patients in the study and the nuclear medicine technologists at the cancer center. We also thank the French National Agency for Research (Investissements d’Avenir), LabexIRON and EquipexArronaxPlus for their moral support and assistance.
Footnotes
Conflicts of interest and sources of funding: none declared.
Ethical approval and consent to participate: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee, and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
This work was examined and approved on July 6, 2021 by the local ethics committee of the institutional review board, and registered under number 2021-114. All patients were informed of access to their data and none objected to their data being used for research purposes.
Consent for publication: All patients were informed and gave written consent for scientific publications.
Availability of data and material: The data sets used and/or analyzed during the current study are available from the corresponding author on request.
Grants and funding: No grant and other financial support were reported according to this work.
Contributor Information
Ludovic Ferrer, Email: ludovic.ferrer@ico.unicancer.fr.
Bruno Maucherat, Email: bruno.maucherat@ico.unicancer.fr.
Vincent Fleury, Email: vincent.fleury@ico.unicancer.fr.
Maelle Le Thiec, Email: maelle.lethiec@ico.unicancer.fr.
Daniela Rusu, Email: daniela.rusu@ico.unicancer.fr.
Caroline Rousseau, Email: caroline.rousseau@ico.unicancer.fr.
REFERENCES
- 1.Mathieu E Ritchie H Ortiz-Ospina E, et al. A global database of COVID-19 vaccinations. Nat Hum Behav. 2021;5:947–953. [DOI] [PubMed] [Google Scholar]
- 2.Info coronavirus COVID 19 - Vaccins. Gouvernement.fr. Available at: https://www.gouvernement.fr/info-coronavirus/vaccins. Accessed August 20, 2021.
- 3.Coronavirus (COVID-19) Vaccinations—Statistics and Research. Our World in Data. Available at: https://ourworldindata.org/covid-vaccinations. Accessed October 10, 2021.
- 4.Polack FP Thomas SJ Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reactions and Adverse Events of the Pfizer-BioNTech COVID-19 Vaccine | CDC. 2021. Available at: https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html. Accessed August 20, 2021. [DOI] [PMC free article] [PubMed]
- 6.Local Reactions, Systemic Reactions, Adverse Events, and Serious Adverse Events: Janssen COVID-19 Vaccine (J&J) | CDC. Available at: https://www.cdc.gov/vaccines/covid-19/info-by-product/janssen/reactogenicity.html. Accessed August 20, 2021.
- 7.Local Reactions, Systemic Reactions, Adverse Events, and Serious Adverse Events: Moderna COVID-19 Vaccine | CDC. Available at: https://www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html. Accessed August 20, 2021.
- 8.Mehta N Sales RM Babagbemi K, et al. Unilateral axillary adenopathy in the setting of COVID-19 vaccine. Clin Imaging. 2021;75:12–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edmonds CE, Zuckerman SP, Conant EF. Management of unilateral axillary lymphadenopathy detected on breast MRI in the era of COVID-19 vaccination. AJR Am J Roentgenol. 2021;217:831–834. [DOI] [PubMed] [Google Scholar]
- 10.Becker AS Perez-Johnston R Chikarmane SA, et al. Multidisciplinary recommendations regarding post-vaccine adenopathy and radiologic imaging: radiology scientific expert panel. Radiology. 2021;300:E323–E327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahn RW Mootz AR Brewington CC, et al. Axillary lymphadenopathy after mRNA COVID-19 vaccination. Radiol Cardiothorac Imaging. 2021;3:e210008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Özütemiz C Krystosek LA Church AL, et al. Lymphadenopathy in COVID-19 vaccine recipients: diagnostic dilemma in oncologic patients. Radiology. 2021;300:E296–E300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Avner M Orevi M Caplan N, et al. COVID-19 vaccine as a cause for unilateral lymphadenopathy detected by 18F-FDG PET/CT in a patient affected by melanoma. Eur J Nucl Med Mol Imaging. 2021;48:2659–2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McIntosh LJ Bankier AA Vijayaraghavan GR, et al. COVID-19 vaccination-related uptake on FDG PET/CT: an emerging dilemma and suggestions for management. AJR Am J Roentgenol. 2021;217:975–983. [DOI] [PubMed] [Google Scholar]
- 15.Coates EE Costner PJ Nason MC, et al. Lymph node activation by PET/CT following vaccination with licensed vaccines for human papillomaviruses. Clin Nucl Med. 2017;42:329–334. [DOI] [PubMed] [Google Scholar]
- 16.Burger IA Husmann L Hany TF, et al. Incidence and intensity of F-18 FDG uptake after vaccination with H1N1 vaccine. Clin Nucl Med. 2011;36:848–853. [DOI] [PubMed] [Google Scholar]
- 17.Nawwar AA Searle J Singh R, et al. Oxford-AstraZeneca COVID-19 vaccination induced lymphadenopathy on [18F]choline PET/CT—not only an FDG finding. Eur J Nucl Med Mol Imaging. 2021;48:2657–2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berg JW. The significance of axillary node levels in the study of breast carcinoma. Cancer. 1955;8:776–778. [DOI] [PubMed] [Google Scholar]
- 19.Baden LR El Sahly HM Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen D Krauthammer SH Wolf I, et al. Hypermetabolic lymphadenopathy following administration of BNT162b2 mRNA Covid-19 vaccine: incidence assessed by [18F]FDG PET-CT and relevance to study interpretation. Eur J Nucl Med Mol Imaging. 2021;48:1854–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eifer M Tau N Alhoubani Y, et al. Covid-19 mRNA vaccination: age and immune status and its association with axillary lymph node PET/CT uptake. J Nucl Med. 2022;63:134–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao X Hamilton RG Weng N, et al. Frailty is associated with impairment of vaccine-induced antibody response and increase in post-vaccination influenza infection in community-dwelling older adults. Vaccine. 2011;29:5015–5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pawelec G. Age and immunity: what is “immunosenescence”? Exp Gerontol. 2018;105:4–9. [DOI] [PubMed] [Google Scholar]
- 24.Soiza RL, Scicluna C, Thomson EC. Efficacy and safety of COVID-19 vaccines in older people. Age Ageing. 2021;50:279–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Y Klein SL Garibaldi BT, et al. Aging in COVID-19: vulnerability, immunity and intervention. Ageing Res Rev. 2021;65:101205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delgado Bolton RC Calapaquí Terán AK Erba PA, et al. Medical imaging in times of pandemic: focus on the cornerstones of successful imaging. Eur J Nucl Med Mol Imaging. 2021;48:1724–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmad Saad FF, Zakaria MH, Appanna B. PET/CT analysis of 21 patients with breast cancer: physiological distribution of 18F-choline and diagnostic pitfalls. J Int Med Res. 2018;46:3138–3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beheshti M Haroon A Bomanji JB, et al. Fluorocholine PET/computed tomography: physiologic uptake, benign findings, and pitfalls. PET Clin. 2014;9:299–306. [DOI] [PubMed] [Google Scholar]
- 29.Goineau A Colombié M Rousseau C, et al. Incidental detection of a Hodgkin lymphoma on 18F-choline PET/CT and comparison with 18F-FDG in a patient with prostate cancer. Clin Nucl Med. 2015;40:670–671. [DOI] [PubMed] [Google Scholar]
- 30.Schillaci O Calabria F Tavolozza M, et al. 18F-choline PET/CT physiological distribution and pitfalls in image interpretation: experience in 80 patients with prostate cancer. Nucl Med Commun. 2010;31:39–45. [DOI] [PubMed] [Google Scholar]
- 31.Calabria F, Chiaravalloti A, Schillaci O. (18)F-choline PET/CT pitfalls in image interpretation: an update on 300 examined patients with prostate cancer. Clin Nucl Med. 2014;39:122–130. [DOI] [PubMed] [Google Scholar]
- 32.Calabria F Chiaravalloti A Cicciò C, et al. PET/CT with 18F-choline: physiological whole bio-distribution in male and female subjects and diagnostic pitfalls on 1000 prostate cancer patients: 18F-choline PET/CT bio-distribution and pitfalls. A southern Italian experience. Nucl Med Biol. 2017;51:40–54. [DOI] [PubMed] [Google Scholar]
- 33.Shukla GS Olson WC Pero SC, et al. Vaccine-draining lymph nodes of cancer patients for generating anti-cancer antibodies. J Transl Med. 2017;15:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shah S Wagner T Nathan M, et al. COVID-19 vaccine-related lymph node activation—patterns of uptake on PET-CT. BJR Case Rep. 2021;7:20210040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wyss MT Weber B Honer M, et al. 18F-choline in experimental soft tissue infection assessed with autoradiography and high-resolution PET. Eur J Nucl Med Mol Imaging. 2004;31:312–316. [DOI] [PubMed] [Google Scholar]
- 36.Snider SA Margison KD Ghorbani P, et al. Choline transport links macrophage phospholipid metabolism and inflammation. J Biol Chem. 2018;293:11600–11611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Galiza Barbosa F Queiroz MA Nunes RF, et al. Nonprostatic diseases on PSMA PET imaging: a spectrum of benign and malignant findings. Cancer Imaging. 2020;20:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salas Fragomeni RA Amir T Sheikhbahaei S, et al. Imaging of nonprostate cancers using PSMA-targeted radiotracers: rationale, current state of the field, and a call to arms. J Nucl Med. 2018;59:871–877. [DOI] [PubMed] [Google Scholar]
- 39.Karasah Erkek B Ömür Ö Özkök S, et al. COVID-19 lung findings detected by 68Ga-PSMA PET/CT for staging purposes in patients with prostate cancer. Clin Nucl Med. 2022;47:e17–e19. [DOI] [PubMed] [Google Scholar]
- 40.Kristensen MK Plovsing RR Berg RMG, et al. Cell adhesion molecules and vascular endothelial growth factor at the systemic and alveolar level in coronavirus disease 2019 acute respiratory distress syndrome. J Infect Dis. 2021;224:1101–1103. [DOI] [PubMed] [Google Scholar]
- 41.Meo SA Bukhari IA Akram J, et al. COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna vaccines. Eur Rev Med Pharmacol Sci. 2021;25:1663–1669. [DOI] [PubMed] [Google Scholar]
- 42.Skawran S Gennari AG Dittli M, et al. [18F]FDG uptake of axillary lymph nodes after COVID-19 vaccination in oncological PET/CT: frequency, intensity, and potential clinical impact. Eur Radiol. 2022;32:508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomassen A Lerberg Nielsen A Gerke O, et al. Duration of 18F-FDG avidity in lymph nodes after pandemic H1N1v and seasonal influenza vaccination. Eur J Nucl Med Mol Imaging. 2011;38:894–898. [DOI] [PubMed] [Google Scholar]
- 44.Barrington SF Qian W Somer EJ, et al. Concordance between four European centres of PET reporting criteria designed for use in multicentre trials in Hodgkin lymphoma. Eur J Nucl Med Mol Imaging. 2010;37:1824–1833. [DOI] [PubMed] [Google Scholar]
- 45.Lim J Broughan J Crowley D, et al. COVID-19’s impact on primary care and related mitigation strategies: a scoping review. Eur J Gen Pract. 2021;27:166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alshammary AF, Al-Sulaiman AM. The journey of SARS-CoV-2 in human hosts: a review of immune responses, immunosuppression, and their consequences. Virulence. 2021;12:1771–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bar-On YM Goldberg Y Mandel M, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. 2021;385:1393–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoegerle S Altehoefer C Ghanem N, et al. Whole-body 18F Dopa PET for detection of gastrointestinal carcinoid tumors. Radiology. 2001;220:373–380. [DOI] [PubMed] [Google Scholar]
- 49.Velikyan I. Prospective of 68Ga radionuclide contribution to the development of imaging agents for infection and inflammation. Contrast Media Mol Imaging. 2018;2018:9713691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brophy J, Henkle G, Rohren EM. DOTATATE uptake in an axillary lymph node after COVID-19 vaccination. Clin Nucl Med. 2021;47:174–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shah HJ Halpern JI Watane GV, et al. COVID lessons continue: postvaccination somatostatin receptor-positive axillary nodes on DOTATATE imaging. Clin Nucl Med. 2022;47:e56–e58. [DOI] [PubMed] [Google Scholar]


