Significance
Antigen-specific immunotherapy may be improved by focusing on epitopes that are disease-relevant and known to be presented on an individual’s human leukocyte antigen (HLA) haplotype, while targeting T cells across multiple antigens and including specific neoepitopes that are not present in protein antigens and/or not produced beyond inflamed sites. Here, we provide proof of principle that such a strategy applied to tolerogenic DNA vaccination is effective in a preclinical model of autoimmune diabetes, paving the way for precision medicine using endogenously encoded epitopes. It takes a minimum number of regular treatments to achieve a level of tolerance and regulation that is needed to limit insulitis and provide sustained protection before treatment may be discontinued or reduced in frequency.
Keywords: DNA vaccine, epitope, autoimmune diabetes, tolerance, precision medicine
Abstract
Antigen-specific immunotherapy involves the delivery of self-antigens as proteins or peptides (or using nucleic acids encoding them) to reestablish tolerance. The Endotope platform supports the optimal presentation of endogenously expressed epitopes on appropriate major histocompatibility complex (MHC) class I and II molecules. Using specific epitopes that are disease-relevant (including neoepitopes and mimotopes) and restricted to the subject’s MHC haplotypes provides a more focused and tailored way of targeting autoreactive T cells. We evaluated the efficacy of an Endotope DNA vaccine tailored to the nonobese diabetic (NOD) mouse in parallel to one expressing the Proinsulin protein, a central autoantigen in NOD mice, and assessed the influence of several parameters (e.g., route, dosing frequency, disease stage) on diabetes prevention. Secretion of encoded peptides and intradermal delivery of DNA offered more effective disease prevention. Long-term weekly treatments were needed to achieve protection that can persist after discontinuation, likely mediated by regulatory T cells induced by at least one epitope. Although epitopes were presented for at least 2 wk, weekly treatments were needed, at least initially, to achieve significant protection. While Endotope and Proinsulin DNA vaccines were effective at both the prediabetic normoglycemic and dysglycemic stages of disease, Proinsulin provided better protection in the latter stage, particularly in animals with slower progression of disease, and Endotope limited insulitis the most in the earlier stage. Thus, our data support the possibility of applying a precision medicine approach based on tailored epitopes for the treatment of tissue-specific autoimmune diseases with DNA vaccines.
Antigen-based approaches to treat autoimmune diseases have the attractive advantage of selectively blocking disease-driving lymphocytes without impairing our overall immunity to pathogens and malignancies. There is a clear yet unmet clinical need for such therapies capable of effectively reestablishing tolerance to targeted autoantigens, but despite an excellent safety profile, they have not yet achieved this goal in patients.
Antigen-specific immunotherapies (ASITs) in type 1 diabetes (T1D) are primarily targeted to diabetogenic CD4+ and CD8+ T cells that are reactive to multiple β-cell antigens (1, 2). Targeting is achieved by delivery and presentation of one or more of these antigens in a manner that results in deletion, regulation, anergy, and/or exhaustion of these autoreactive T cells. Selected autoantigens have been administered to patients in the form of proteins or peptides via parenteral, oral, or nasal routes, or in the form of protein-encoding DNA plasmids (DNA vaccines) (1, 3). A wide variety of delivery vehicles have been developed and tested to funnel these antigens to specific cell types and/or anatomical locations, including various micro- and nanoparticles (4) and, more recently, soluble antigen arrays (5). A DNA-based approach allows the patient’s own cells to 1) endogenously express the protein(s) of interest, which may sometimes be challenging to produce recombinantly (e.g., proinsulin), 2) apply natural posttranslational modifications (PTMs), some of which are playing a critical role in the disease process (6, 7), and 3) possibly allow the antigen(s) to persist longer than exogenous antigens (with the exception of nanoparticles designed to achieve slow antigen release).
Choosing the right antigen(s) to treat T1D is difficult (8). Historically, insulin/proinsulin and GAD65 have been favored due to successes in preclinical studies in nonobese diabetic (NOD) mice, the prevalence of antiinsulin and GAD65 autoantibodies, and the identification of many T cell clones reactive to these antigens in T1D patients. In recent years, new and more complex islet autoantigens have emerged, including PTM versions and other neoantigens such as hybrid peptides (6, 9, 10).
At the same time, a better understanding of the heterogeneity of T1D patients in disease progression, immune profile, and genetic risk factors has led to their stratification into subgroups termed “endotypes” (11, 12). Another parameter considered in the stratification of patients is their responsiveness to particular types of therapies. In ASITs, the fact that responsiveness is limited to a subset of patients in clinical trials [e.g., DPT-1 oral insulin (13, 14)] indicates that there is no “one-antigen-fits-all” option and, once responsiveness can be linked to an endotype, it becomes possible to subsequently screen patients that are more likely to benefit from the treatment. Most recent clinical trials have narrowed their eligibility criteria to include immune markers (e.g., the presence of a specific autoantibody such as GADA in NCT02387164) or specific human leukocyte antigen (HLA) haplotypes (in the case of peptide-based therapy, for example HLA-DRB1*0401 in NCT02620332).
Several studies suggest that presentation of pertinent epitopes with appropriate major histocompatibility complex (MHC) restriction can circumvent the need to use full proteins, in part because of linked/bystander suppression (15–18). For specific HLA haplotypes, several immunodominant or immunoprevalent epitopes have been identified across multiple antigens (9, 19–21).
We have designed constructs (Endotope platform) that achieve optimal presentation of endogenously expressed epitopes to CD4+ and CD8+ T cells (22). We previously reported that these constructs encoding both native epitopes and mimotopes could achieve engagement of the corresponding antigen-specific T cells in vitro (22) and in vivo (23) and significantly delay the onset of diabetes in NOD mice (23). Here, we assessed how multiple parameters influence the efficacy of Endotope plasmid DNA (pDNA) with epitopes tailored to the NOD mouse in preventing/delaying disease for preclinical optimization of this approach, including the localization of the expressed polypeptides, route of inoculation, duration and frequency of treatment, and stage of disease at treatment initiation. We also determined the effects on CD4+ T cells specific to one of the encoded epitopes after prolonged treatment. We compared its efficacy with the Proinsulin pDNA (24), used here as the gold standard because it constitutes the basis of the current phase II SUNRISE clinical trial (NCT03895437). In phase I studies, intramuscular inoculation of Proinsulin pDNA led to delayed loss of C peptide and reduction of insulin-reactive CD8 T cells while showing an excellent safety profile (25).
Results
Secretion of Endotope Polypeptide Is Required for Protection by a DNA Vaccine.
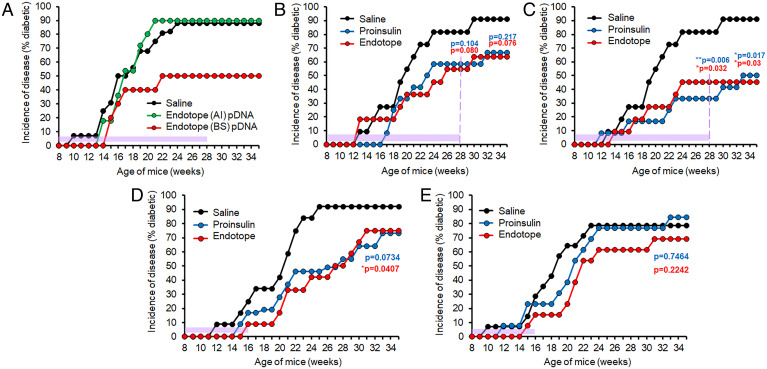
We previously reported that both "A" intracellular (AI) and "B" secreted (BS) constructs (SI Appendix, Fig. S1) elicit antigen-specific T cell responses (albeit stronger with BS) in the draining lymph nodes after intramuscular (i.m.) and intradermal (i.d.) treatments, and that delivery of a mix of AI- and BS-containing pDNA significantly delayed diabetes onset in NOD mice after an 8-wk i.m. treatment, similar to the Proinsulin DNA vaccine (23). To follow up on these studies, we evaluated constructs AI versus BS separately to ascertain their relative contribution to disease protection, this time testing the i.d. route. The secreted form of Endotope appears to be the one mediating the protection from T1D, as the intracellular form had no effect on incidence (Fig. 1A). As a result, only the BS variant of Endotope was used in subsequent experiments.
Fig. 1.
Parameters affecting the efficacy of NOD disease prevention by Endotope and Proinsulin pDNA vaccination. (A) Antigen dissemination. Secreted, but not intracellular, pDNA-encoded Endotope polypeptide reduced disease incidence in NOD mice. Female NOD mice were treated i.d. weekly for 20 wk, starting at week 8 of age (n = 16 mice for saline, 11 for AI, and 10 for BS). Saline vs. BS: P = 0.079. (B and C) Routes of vaccination. Proinsulin and Endotope pDNA vaccines have comparable efficacy, with i.d. administration of pDNA more effective. Female NOD mice were treated i.m. (B) or i.d. (C) for 20 wk starting at 8 wk of age (n = 11 mice for saline, 12 for Proinsulin pDNA i.m. and i.d., and 11 for Endotope pDNA i.m. and i.d.). (D and E) Treatment discontinuation and loss of durable protection. Female NOD mice were treated i.m. (D) or i.d. (E) for 8 wk starting at 8 wk of age (n = 12 mice for saline and Endotope and 11 for Proinsulin in i.m. treatment cohort; n = 14 for saline, 13 for Proinsulin, and 13 for Endotope in i.d. treatment cohort). For all graphs, the period of treatment is indicated with purple shading. Log-rank test was used to determine P values (against saline control) at the end of follow-up (and in B and C, at the end of treatment, as indicated by the dashed purple line).
Longer Endotope or Proinsulin pDNA Treatments with the Intradermal Route Confer the Best Protection.
We next compared our selected Endotope (BS) pDNA with the Proinsulin pDNA, applied in continuous weekly treatment. Using i.m. administration, both treatments reduced the incidence of disease, although it did not reach significance in this instance with the number of mice used (Fig. 1B). Using i.d. administration, the reduced incidence of disease was significant for Endotope and Proinsulin, both at the time of treatment discontinuation and at end point, 7 wk later (Fig. 1C). Thus, the mice appeared protected somewhat durably, without a precipitous increase in onset in remaining mice, when treatment was discontinued after long treatments. To determine whether stable control of disease could be achieved with fewer treatments, mice were treated weekly for 8 wk with Endotope or Proinsulin pDNA, either i.m. (Fig. 1D) or i.d. (Fig. 1E). In contrast to the previous long-term treatments, most mice rapidly developed T1D soon after treatment discontinuation in both groups and with both routes, indicating that longer continuous DNA delivery is necessary to achieve stable, long-lasting protection.
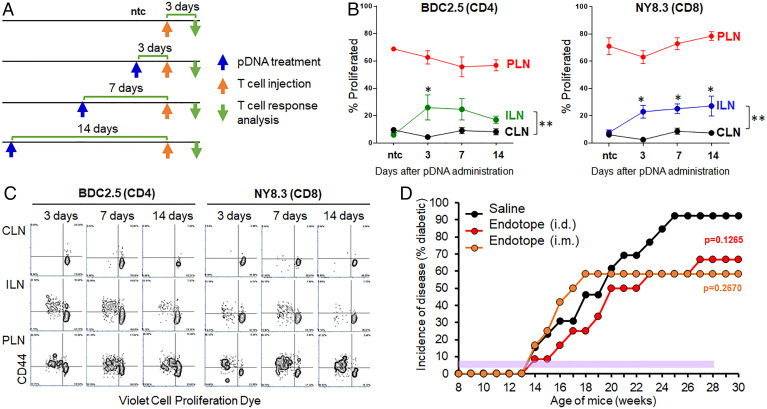
Persistence of Antigen Presentation Does Not Inform Treatment Frequency Requirements.
We previously showed that expression of the pDNA-encoded gene in vivo, after either i.m. or i.d. delivery, lasts for at least 1 wk based on luciferase signal measurements. Here, we determined whether antigen presentation can persist even longer after pDNA administration. To this end, we used antigen-specific CD4+ and CD8+ T cells from BDC2.5 and NY8.3 mice, respectively, which respond to two epitopes expressed by the Endotope pDNA (SI Appendix, Fig. S1A). These T cells were labeled with a proliferation dye and injected into i.d.-treated NOD mice at several time points (Fig. 2A). The proliferative response of the T cells, reflective of antigen recognition, was analyzed 3 d after transfer. The nontreated control shows the background proliferation of the T cells (i.e., no proliferation in lymph nodes except in the pancreatic lymph nodes [PLNs] where the antigen is naturally present, draining from the islets) (Fig. 2 B and C and SI Appendix, Fig. S2). Proliferation of the T cells in the inguinal lymph nodes (ILNs; draining the sites of pDNA inoculation) at all time points indicated that antigen was still presented as late as 14 d after treatment (Fig. 2 B and C and SI Appendix, Fig. S2). Based on these results, we postulated that although continuous treatment was important to achieve significant protection, weekly injections may be too frequent if antigen presentation persists at least 2 wk. Thus, we treated NOD mice i.m. and i.d. for 20 wk, this time every other week. Under these conditions, Endotope pDNA offered only partial and nonsignificant protection from T1D (Fig. 2D), suggesting that late presentation of antigens (beyond 1 wk) may not contribute to disease protection.
Fig. 2.
Persistence of Endotope’s epitope presentation in vivo after pDNA inoculation and effect of reducing the frequency of treatment on disease incidence. (A) Four groups of female NOD mice (n = 5 mice per group; except nontreated control [ntc], n = 2) were used as recipients of TCR-Tg T cells from BDC2.5 and NY8.3 mice. They were treated by i.d. injection as indicated by the blue arrows; T cells were adoptively transferred as indicated by the orange arrows and, 3 d after transfer, lymph nodes from these mice were analyzed for antigen-specific T cell responses. (B) Proliferation of BDC2.5 CD4+ and NY8.3 CD8+ T cells (based on % T cells having divided at least once) in ILNs draining the inoculation site, cervical LNs (CLNs) serving as internal negative control, and PLNs serving as positive control. Data show the mean ± SEM. Asterisks indicate the significance of difference between CLNs and ILNs at each time point (multiple t test comparison); *P < 0.05. In addition, two-way ANOVA with Sidak correction was applied across the time points, indicating a significant difference between CLNs and ILNs for BDC2.5 CD4+ T cells (**P = 0.002) and NY8.3 CD8+ T cells (**P = 0.0015). Data are reflective of two separate experiments. (C) Representative dot plots illustrating the data shown in B. (D) Incidence of disease in mice treated biweekly with i.m. or i.d. injections of Endotope pDNA. Female NOD mice (n = 13 mice for saline, 12 for Endotope pDNA i.d., and 12 for Endotope pDNA i.m.) were treated every other week for 20 wk starting at 8 wk of age (the period of treatment is indicated with purple shading). Log-rank test: The P values (against saline control) at the end of follow-up (30 wk of age) are indicated.
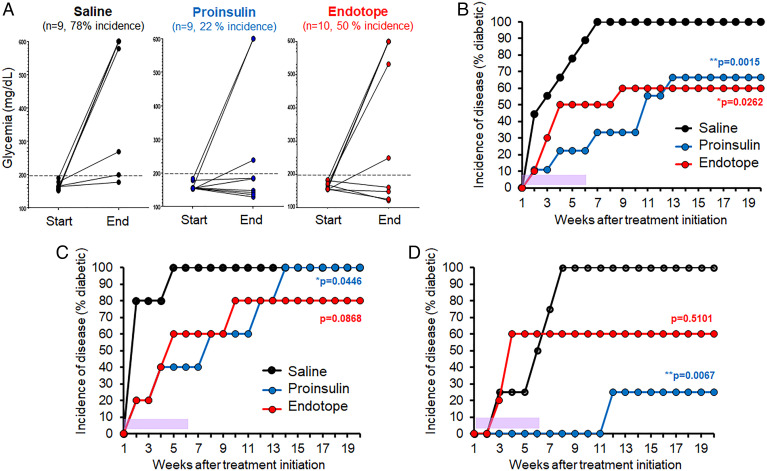
Proinsulin pDNA Provides Better Protection When Administered at a Stage Immediately Preceding the Onset of Diabetes, the Dysglycemic Stage.
Proinsulin pDNA vaccination was previously reported to be particularly effective at blocking the onset of disease when NOD mice start to develop dysglycemia (150 to 250 mg/dL range), on their way to hyperglycemia (24). We tested whether Endotope pDNA had the same capacity. When mice reached the 150 to 200 mg/dL range, they were treated twice a week for 6 wk. At the end of this treatment period, 78% of saline-treated mice had developed diabetes (glycemia > 250 mg/dL), as compared with 50% for the Endotope group and 22% for the Proinsulin group (Fig. 3A). We continued to follow these mice for another 14 wk after the treatment ended (Fig. 3B). Mice treated with Endotope pDNA that had not developed diabetes remained protected long-term, whereas mice treated with Proinsulin pDNA started to develop diabetes after the treatment ended to ultimately reach the same level of protection as Endotope. While diabetes onset typically occurs after 12 wk of age, dysglycemia may be detected anywhere between 8 and 20 wk of age. In contrast to stage 1 prevention studies, mice in this experimental setup for stage 2 prevention are not all treated at the same age. Mice that progress to dysglycemia later likely have a milder form of disease that may be easier to treat. Thus, we stratified each group of mice into two subsets: the one half that developed dysglycemia the earliest (before 12 wk approximately; Fig. 3C) and the other half that developed dysglycemia the latest (after 12 wk; Fig. 3D). Interestingly, the protective effect of Proinsulin pDNA was significantly more pronounced in the second half group, suggesting that this vaccine is particularly effective at blocking the milder form of disease, while Endotope pDNA had a modest effect in both aggressive and mild settings.
Fig. 3.
Efficacy of Endotope pDNA compared with Proinsulin pDNA in preventing progression of disease during the dysglycemic stage. Female NOD mice (n = 9 mice for saline, 9 for Proinsulin pDNA, and 10 for Endotope pDNA) were enrolled when glycemia reached the 150 to 200 mg/dL range and treated i.d. for 6 wk (the period of treatment is indicated with purple shading), with two injections per week, totaling 12 doses. (A) Blood glucose of mice at the start (first dose) and end (last dose) of the treatment. (B–D) Incidence of disease of the whole cohort (B), of the mice that developed dysglycemia before 12 wk of age (n = 5 mice per group) (C), and of the mice that developed dysglycemia after 12 wk of age (n = 4 mice for saline and Proinsulin and 5 mice for Endotope) (D). Log-rank test: The P values (against saline control) at the end of follow-up (20 wk of age) are indicated, and for Proinsulin pDNA comparing subgroups in C and D is P = 0.0218.
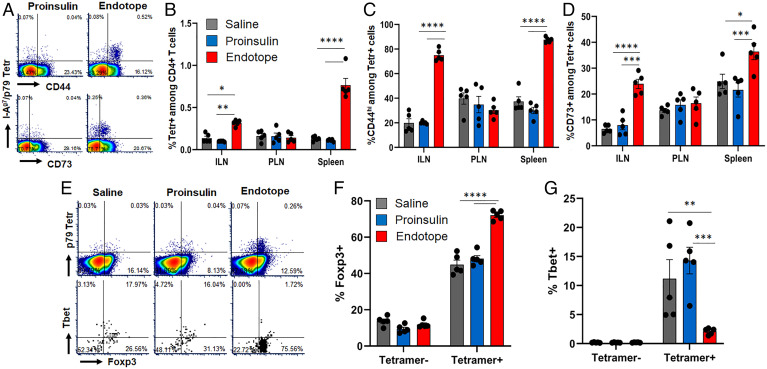
Endotope pDNA Expands CD4+ T Cells with Regulatory and/or Anergic Phenotype.
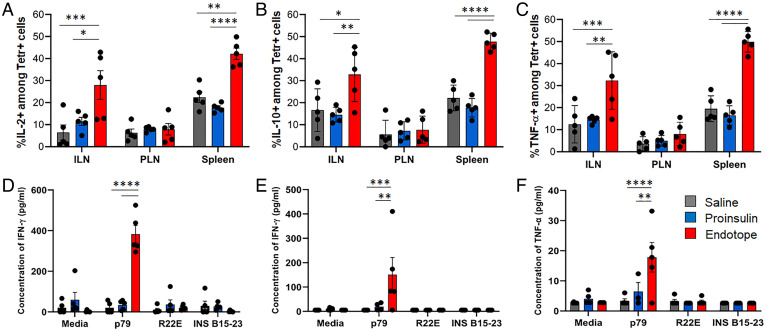
As Endotope constructs are designed to optimally engage CD4+ T cells, we assessed the phenotype of an endogenous CD4+ T cell population reactive to one of the encoded epitopes (p79) (SI Appendix, Fig. S1A), detected by the MHC tetramer, at a time the pDNA prevention curves flatten and diverge from the saline treatment curve (Fig. 1C), that is, approximately after 10 wk of weekly treatments. At this time, p79-reactive CD4+ T cells had significantly expanded (Fig. 4 A and B) and up-regulated CD44 (Fig. 4 A and C and SI Appendix, Fig. S3A) and CD73 (Fig. 4 A and D and SI Appendix, Fig. S3B) in both ILNs and spleen, but no effect was seen in PLNs. The frequency of CD73+ FR4+ anergic T cells was increased in ILNs but reduced in spleen (SI Appendix, Fig. S3C). PD-1 was up-regulated only in ILNs (SI Appendix, Fig. S3D), and KLRG1 expression was unchanged (SI Appendix, Fig. S3E). Importantly, the frequency of Foxp3+ and Foxp3+ CD25+ cells (only assessed in spleen due to insufficient material from other tissues) was significantly increased (Fig. 4 E and F and SI Appendix, Fig. S4) while that of Tbet+ cells was reduced (Fig. 4 E and G and SI Appendix, Fig. S4A), indicating a shift from effector to regulatory phenotype. As expected, the Proinsulin pDNA treatment had no effect on p79-reactive T cells, like the saline treatment. Significant up-regulation of intracellular interleukin-2 (IL-2), IL-10, and tumor necrosis factor α (TNF-α) was detected in p79-reactive CD4+ T cells (Fig. 5 A–C and SI Appendix, Fig. S5 A and B), while intracellular interferon γ (IFN-γ) (SI Appendix, Fig. S5 B and C) and IL-17A (SI Appendix, Fig. S5D) could barely be detected. In contrast, restimulation of ILNs and spleen cells with peptides ex vivo led to low/moderate levels of IFN-γ production (Fig. 5 D and E), as well as some TNF-α by ILN cells (Fig. 5F), but other cytokines tested (IL-2 [SI Appendix, Fig. S5 E and F], IL-4, IL-5, IL-6, IL-10, and IL-13) were not detected above background. No cytokine responses to InsB9–23 R22E and InsB15–23, despite being present in the Endotope construct (SI Appendix, Fig. S1A), were detected ex vivo.
Fig. 4.
Effect of Endotope pDNA on the phenotype of epitope-specific CD4+ T cells. Female NOD mice (n = 5 mice per group) were treated i.d. and weekly for 10 wk. (A) Representative dot plots showing expression of CD44 and CD73 on p79-reactive tetramer (Tetr)+ CD4+ T cells from spleen after Proinsulin or Endotope pDNA treatment (more plots are in SI Appendix, Fig. S2). (B) Percentage (mean ± SEM) of p79-reactive (Tetr+) among CD4+ T cells. (C and D) Percentage of CD44hi cells (C) and CD73+ cells (D) among Tetr+ CD4+ T cells. Tissues: ILNs, PLNs, and spleen. (E–G) Expression of Foxp3 and Tbet in p79-reactive CD4+ T cells from spleen: representative expression of Foxp3 in Tetr+ and Tetr− CD4+ T cells (E, Top) and Foxp3 and Tbet in Tetr+ CD4+ T cells (E, Bottom) (more plots are in SI Appendix, Fig. S3), and percentage (mean ± SEM) of Foxp3+ cells (F) and Tbet+ cells (G) among Tetr+ and Tetr− CD4+ T cells. Significant differences were determined by two-way ANOVA with Tukey’s multiple comparisons (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001).
Fig. 5.
Effect of Endotope pDNA on the cytokine profile of epitope-specific CD4+ T cells. Female NOD mice (n = 5 mice per group) were treated i.d. and weekly for 10 wk. (A–C) Percentage (mean ± SEM) of IL-2+ cells (A), IL-10+ cells (B), and TNF-α+ cells (C) among Tetr+ CD4+ T cells, assessed by intracellular staining. Tissues: ILNs, PLNs, and spleen. (D–F) Levels (mean ± SEM) of IFN-γ (D and E) and TNF-α (F) produced 3 d after restimulation of splenocytes (D) or ILN cells (E and F) with p79, InsB9–23 R22E, or InsB15–23 peptides. Significant differences were determined by two-way ANOVA with Tukey’s multiple comparisons (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001).
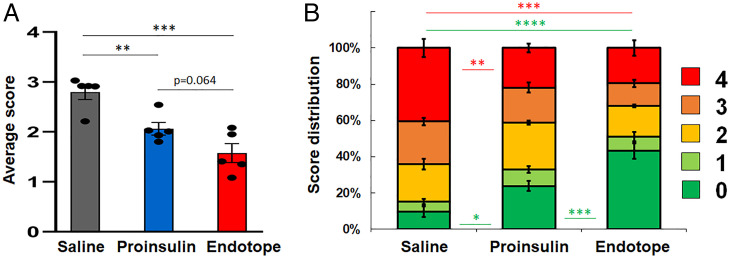
Endotope pDNA Limits Insulitis.
Consistent with the divergence of disease protection seen between control and pDNA treatments after 10 wk, insulitis was significantly reduced with both pDNA treatments (Fig. 6A and SI Appendix, Fig. S6) and Endotope pDNA–treated mice had significantly more islets without any immune cell infiltration (Fig. 6B).
Fig. 6.
Reduced insulitis with Endotope pDNA treatment. Female NOD mice (n = 5 mice per group) were treated weekly (i.d.) for 10 wk. (A) Average insulitis score (mean ± SEM). One-way ANOVA: P = 0.0005. Shown in the graphs are the results of unpaired t tests. (B) Relative distribution of all islets within each insulitis score (mean percentage ± SEM). Significant differences between treatments for each score are indicated using the score’s color (two-way ANOVA with Tukey correction for multiple comparisons; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001).
Discussion
In addition to the genetic heterogeneity that characterizes T1D patients, diversity in immune profiles (predominant autoantibodies, immunoprevalent autoreactive T cells) contributes to the establishment of disease endotypes, which can help stratify patients based on shared features (12). Responsiveness to ASITs is likely linked to this immune profile. For example, responsiveness to oral insulin in the DPT-1 study was limited to patients with the highest levels of insulin autoantibodies (13, 14). Accordingly, patients recruited for ASIT trials are increasingly selected based on the presence of at least the autoantibody corresponding to the administered antigen (e.g., GAD65 in the DiAPREV-IT2 trial, NCT02387164). More clinical studies and retrospective analyses are required to determine what responders have in common and how the choice of antigens and delivery modalities and routes should be made for these subpopulations in subsequent treatments (1, 8). Like in humans, the disease in NOD mice is influenced by genetic, environmental, and stochastic factors that affect the rate of spontaneous disease occurrence (26–28) and responsiveness to treatment. Although this inbred strain is genetically homogeneous and may only represent a fraction of the human population in its pathogenesis and response to treatment, NOD mice nonetheless provide an adequate model for precision medicine studies like this one, because our treatment is customized for this strain and can be customized differently for groups of human patients based on HLA haplotype and immune profile.
Epitope (peptide)-based therapies, including the Endotope platform, shift the focus from a single antigen to selected epitopes that can encompass multiple antigens and to which T cell reactivity is well-documented. Important disease-driving epitopes now include various neoepitopes (6, 7, 9, 29), which can be incorporated into the Endotope design. Mimotopes can also be used to mimic specific PTMs (e.g., deamidation) or force binding on MHC in particular registers that improve recognition by autoreactive T cells (30). With the Endotope platform, epitopes are endogenously produced by the patient’s own cells, which also increases the chance for these peptides to be endowed with relevant PTMs. When translated to patients, this approach will require a more detailed analysis of the immune profile and disease-relevant immunopeptidome, an area that is still in its infancy despite recent progress in profiling patients with comprehensive combinatorial MHC multimer panels (19–21, 31). It will also require selection of patients based on HLA haplotype to ensure proper presentation of the encoded epitopes, as done in trials involving the injection of peptide mixtures (NCT02620332) or peptide-pulsed dendritic cells (NCT04590872) (32). To its advantage, the full protein can produce peptides that can collectively cover a wide variety of HLA haplotypes, but delivery of more than one protein in a single plasmid is technically challenging. Because another major advantage of DNA vaccination is the ability to design and manufacture plasmids cheaply and in large quantities, it is an attractive modality when it comes to producing multiple versions for groups of patients. Thus, Endotope constructs would be particularly suitable for a precision medicine approach to treating T1D using ASITs.
Next is the question of how many epitopes are required. This depends on the mechanism of tolerance: Fewer epitopes are needed if regulation is involved due to bystander suppression of other specificities, whereas more epitopes/antigens are required if presentation leads primarily to deletion and/or anergy/exhaustion. The advantage of Proinsulin pDNA in treating T1D in stage 2 of the disease may be related to broader epitope coverage within Proinsulin if the initial transition from periinsulitis to insulitis primarily coincides with intramolecular epitope spreading within Proinsulin. It is unclear how many of the Endotope-encoded epitopes significantly contribute to the protection achieved, but we showed that regulation is likely involved in T cell responses to the p79 mimotope. Previous studies have expressed a single epitope from pDNA: Expression of the InsB9–23 peptide (33) or the p31 BDC2.5 mimotope (also targeted to the MHC-II pathway though differently from Endotope) (17) significantly reduced diabetes incidence. However, in these two studies, treatment was started at 3 to 4 wk of age, at a time when there is barely any insulitis and epitope spreading [and the InsB9–23 peptide is the very epitope known to initiate the disease (34)] and it would be difficult to find patients at such early stage for treatment. Treatment with a single InsB9–23 (R22E) mimotope in soluble form also significantly prevented the onset of disease in NOD mice, whether it was started at 4 to 6 or 12 to 14 wk of age (35). More recently, we showed that two peptides in combination (p79 mimotope and hybrid insulin peptide [2.5HIP]) delivered by soluble antigen array starting later, at 8 wk of age, protected 70% of mice (5). Presentation of a few selected epitopes on MHC molecules displayed on nanoparticles in Pere Santamaria’s successful studies on stage 2 disease also leads to tolerance that spread to other epitopes of the same antigen or others (16, 36). Collectively, these studies illustrate the successful transition of ASITs from antigens to a few epitopes. This type of approach is also needed if important epitopes are not found in a single protein, as in the case of hybrid peptides. Moreover, it is possible that hybrid peptides and PTM peptides are produced exclusively in the periphery under specific (possibly inflammatory) conditions and may not be found in the thymus to mediate negative selection. Thus, special consideration should be given to these neoepitopes in ASITs (7).
The mechanism of action appears to be connected to the duration and frequency of antigen exposure. We previously reported that presentation of CD4 epitopes from Endotope pDNA can result in a significant increase in antigen-specific Foxp3+ regulatory T cells (Tregs) after one to three doses (23). However, after 10 weekly doses, the expression of Foxp3 and IL-10 by intracellular staining has become more prominent than that of IFN-γ and T-bet in epitope-reactive CD4+ T cells, although ex vivo recall responses to the peptide only revealed some IFN-γ and TNF-α secretion. The more pronounced regulatory phenotype after long-term treatment may explain the significantly larger proportion of protected islets and why disease does not rapidly resurge after cessation of treatment, unlike what was seen with shorter (8 wk) treatments. Thus, reestablishing tolerance is a lengthy process in which the phenotype of specific T cells is gradually altered over repeated exposures to autoantigens. The response to pDNA-encoded p79 was similar, albeit less pronounced, to the response to p79-carrying soluble antigen arrays (5). Induction of p79-reactive CD4+ T cells with anergic (CD73+ FR4+) and suppressive (IL-10+) phenotype was seen with both approaches. It is possible that long-term repeated antigen exposure converts some of these anergic cells to Tregs (37). However, all epitopes delivered together may not have the same mechanism of action (5). Moreover, the limited stability of Tregs in both NOD mice and T1D patients (38, 39) remains a major issue and it is possible that, once this population is established, continued treatment, perhaps with less frequent dosing, will be required to maintain this population. However, it was encouraging that, after both long-term weekly treatments (20 wk) initiated in stage 1 and short-term (6 wk) but more frequent treatments initiated in stage 2, mice that remain normoglycemic appear to be protected relatively long-term after cessation of treatment.
The duration of antigen exposure is determined by both the frequency of administration and the persistence of antigen presentation in vivo. Relative to other delivery modalities, DNA vaccines may accomplish prolonged antigen exposure as transfected pDNA can reside in cells in episomal form. We therefore addressed how long would antigens be presented after a single pDNA injection (two epitopes, one presented on MHC-II and the other on MHC-I, were tested). Despite significant stimulation of antigen-specific T cells in lymph nodes draining the vaccination site being measured at least 2 wk later, reducing the treatment frequency from weekly to every 2 wk resulted in substantial reduction in therapeutic efficacy. Similarly, Pagni et al. (40) achieved better protection when increasing the frequency from once to three times a week. Thus, the production/persistence of antigen for at least 2 wk, while adequate to induce T cell stimulation, was not sufficient to build and/or maintain tolerance. It is possible that a substantially higher dose of antigen is needed for tolerance than for proliferation and that the required amount of antigen becomes too low after a few days. However, the dose and/or frequency requirements may be lowered once a sufficient tolerance state is established, based on our observations after cessation of treatment, and identifying biomarkers for this tolerance state would help in managing the treatment.
An important insight from these studies is the subcellular localization of the expressed antigens. We found that the secreted form of the Endotope polypeptide (with all epitopes), and not the intracellular form, had therapeutic benefit, even though both secreted and nonsecreted forms induced immune responses in draining lymph nodes (23). This is consistent with another DNA vaccine study in which the long GAD65190–315 peptide was more protective when secreted than the full nonsecreted GAD65 protein in NOD mice (41). This is, however, in contrast to the nonsecreted Proinsulin pDNA used, which was reported to be more effective than the secreted Preproinsulin version (24). Both Endotope and GAD65 contain epitopes that are not normally found extracellularly, and their secretion allows their dissemination and presentation at sites that are more conducive of tolerance (i.e., other than the inflamed islets and PLNs). Conversely, Proinsulin does not contribute new antigens aside from epitopes from the A–C and C–B chain junctions, as insulin and C peptide are already widely present in the circulation. Another possible explanation is that Preproinsulin localizes more efficiently to the endoplasmic reticulum and can lead to unwanted CD8+ T cell responses that can exacerbate disease (42). Thus, careful subcellular targeting of antigens can make a significant difference between immunogenic and tolerogenic responses. It is also possible that directly transfected antigen-presenting cells and those acquiring secreted antigens differ in lineage and in their tolerogenic capabilities.
The present study serves as preclinical proof of concept that a selection of relevant epitopes from various antigens recognized by dominant T cell clones and customized for the NOD mouse may be as effective as a single disease-driving protein antigen (Proinsulin) in limiting insulitis and the onset of diabetes in late stage 1 of disease. Given the complexity of the human disease, it is as yet unclear what fraction of the T1D patient population will benefit from the Proinsulin DNA vaccine, and the current phase II SUNRISE (NCT03895437) and phase I TOPPLE T1D (NCT04279613) trials are expected to provide further insights into the mechanisms of action and the factors influencing responsiveness. Proinsulin is certainly an excellent antigen choice for the NOD mouse, given its key role in disease initiation (34, 43). Interestingly, prevention by Proinsulin pDNA alone (i.m. route) was found by two independent laboratories not to reach significance when given at stage 1 of disease (24, 44) but was more effective in stage 2 (24). We also observe no significant effect with this route and treatment stage although, using the i.d. route, it is possible to achieve significant protection with Proinsulin pDNA in both stage 1 and stage 2 disease. In other preclinical studies, the subcutaneous route slightly outperformed the i.m. route (40) and was selected for the subsequent TOPPLE T1D study. Interestingly, a difference between Proinsulin and Endotope DNA vaccines was more evident when mice were treated upon entering the dysglycemic phase (stage 2). The effect of Endotope was the same whether the mice entered stage 2 early or late, whereas Proinsulin had a major impact on those entering stage 2 late. Mice that develop dysglycemia later have a milder form of disease that appears particularly responsive to Proinsulin pDNA treatment and other treatments such as with IL-4–overexpressing dendritic cells (45). In humans, individuals who develop 2+ autoantibodies at a younger age progress to diabetes at the fastest rate (46), so it will be interesting to determine whether Proinsulin pDNA is more effective in individuals who developed diabetes at an older age.
Overall, these studies provide important insights into the use of tolerogenic pDNA vaccines to treat autoimmunity, including proof of principle that expression of selected epitopes tailored to groups of patients (or a specific strain of mouse in this case) can achieve protection comparable to that of a full antigen known to drive the disease. However, issues of Treg stability and antigen persistence remain major hurdles to overcome for the successful treatment of T1D.
Materials and Methods
Plasmid Constructs.
All constructs used in these studies were previously described (23) and expressed in pBHT568 (originally containing Proinsulin) from Lawrence Steinman and Peggy Ho, Stanford University, Stanford, CA (24). The epitopes expressed (SI Appendix, Fig. S1A) have all been well-described to engage diabetogenic T cells in NOD mice (22, 23). Construct AI contains the endosome targeting signal (Ii1–80) selected from a previous study (22) and a T2A cleavage site. This construct generates two polypeptides that are differentially targeted to MHC class II or MHC class I (SI Appendix, Fig. S1B) (22). Construct BS contains the albumin secretion signal, and all epitopes are secreted as one polypeptide to be disseminated to other APCs (SI Appendix, Fig. S1B) (23).
Mice.
All mouse strains were purchased from The Jackson Laboratory and bred in our barrier facility: NOD (001976), NOD.CD45.2 (014149), and T cell receptor transgenic (TCR-Tg) mice BDC2.5 (004460) and NY8.3 (005868). TCR-Tg T cells from BDC2.5 and NY8.3 mice, respectively, recognize the p79/2.5 mimotope presented on MHC class II (I-Ag7) and the IGRP206–214 epitope presented on MHC class I (Kd), all encoded by our constructs (SI Appendix, Fig. S1A). Female TCR-Tg mice were used as donors at 8 to 16 wk of age. Female NOD mice were used at 8 to 10 wk of age as indicated, or upon demonstrating dysglycemia after 10 wk of age. All studies were approved by Columbia University’s Institutional Animal Care and Use Committee.
Disease Prevention Studies: Stage 1.
NOD females (8 wk of age) were treated by either i.m. or i.d. injection, weekly or biweekly for a duration of 8 or 20 wk as indicated in the figures. Each dose consisted of 50 μg of pDNA, encoding either Proinsulin or an Endotope construct, dissolved in 100 μL of saline. Control groups, used to determine the normal incidence of disease, received 100 μL of saline. At the time of treatment, mice were normoglycemic but are known to have periinsulitis and autoantibodies at this age (26); this corresponds to stage 1 disease in humans (47). The i.m. injection was split between the two quadriceps, while the i.d. administration was split between the two flanks of the abdominal area after shaving. Mice from different groups were mixed in cages to minimize cage variability. Blood glucose was monitored weekly (up to 35 wk of age) using a Prodigy glucometer and test strips. Mice were diagnosed as diabetic after two consecutive blood glucose levels greater than 250 mg/dL and killed upon diagnosis or at the end of the observation period if normoglycemic.
Antigen Persistence Study.
NOD female mice (8 to 10 wk of age), used as recipients, were immunized i.d. with 50 μg of Endotope (BS) pDNA at days 14, 7, and 3 before adoptive transfer of TCR-Tg T cells from BDC2.5 and NY8.3 donor female mice (8 to 16 wk of age). Spleen and pooled lymph nodes were collected from donor CD45.2+ BDC2.5 and NY8.3 mice, and CD4+ CD25− and CD8+ T cells were purified using the MojoSort Mouse CD4 Kit (supplemented with biotinylated anti-CD25) and CD8 T Cell Isolation Kit (BioLegend), respectively. Cells were then labeled with Violet Cell Proliferation Dye (eBioscience) and 0.5 to 1 × 106 T cells were injected intravenously into recipient NOD (CD45.1+) mice of all groups on day 0. Antigen-specific congenic CD4+ and CD8+ T cells were analyzed by flow cytometry (BD Fortessa) 3 d later. Data were analyzed with FCS Express 7.
Disease Stabilization Studies: Stage 2.
NOD female mice were monitored for blood glucose levels from 10 wk of age and were considered dysglycemic when in the range of 150 to 200 mg/dL [equivalent to stage 2 disease in humans (47)] and subsequently enrolled in the vaccination study. Following 2 doses weekly for a period of 6 wk (12 doses total), groups were randomly assigned on a rotating basis to i.d. treatment with saline or Proinsulin or Endotope pDNA until all animals were enrolled. Mice were diagnosed as diabetic after two consecutive blood glucose levels greater than 250 mg/dL and killed when reaching >500 mg/dL over two consecutive readings or at the end of the observation period if normoglycemic.
T Cell Phenotyping and Cytokine Profile.
Endogenous T cell responses were assessed 3 d after 10 weekly treatments (started at 8 wk of age) with saline, Proinsulin pDNA, or Endotope (BS) pDNA via i.d. injection, as in the disease prevention studies. Cells from ILNs, PLNs, and spleen were stained with I-Ag7/p79 tetramer (AAAAVRPLWVRMEAA; from the NIH Tetramer Core Facility) for 1 h at 37 °C followed by extracellular and/or intracellular staining for 30 min at 4 °C. Intracellular cytokine staining was done following a 4-h incubation with phorbol myristate acetate (0.1 µg/mL), ionomycin (40 µg/mL), brefeldin A (1.5 µg/mL), and monensin (1 µmol/L) using the CytoFix/CytoPerm Kit (BD Biosciences). Intracellular staining for Foxp3 and T-bet was performed on leftover spleen cells using the True-Nuclear Factor Kit (BioLegend) following the manufacturer’s instructions. See SI Appendix, Table S1 for details of the antibodies and tetramers used. Ex vivo cytokine recall responses were assessed by culturing ILN cells (2 × 105 per well) or splenocytes (3 × 105 per well) from control or treated NOD mice in the presence of p79 (100 nM), InsB9–23 R22E (1 μM), or InsB15–23 (10 μM). Culture supernatant was collected after 3 d and assessed for IFN-γ, TNF-α, IL-2, IL-4, IL-5, IL-6, IL-10, and IL-13 levels using the LEGENDPlex Mouse Th1/Th2 Cytokine Panel Kit (BioLegend) following the manufacturer’s instructions.
Histopathology.
Pancreas from NOD female mice treated for 10 wk (as above) was fixed in 10% buffered formalin and embedded in paraffin. Sections were stained with hematoxylin and eosin. All pancreatic islets from six different sections per mouse were scored as follows: 0, no visible infiltration; 1, periinsulitis; 2, up to 1/3 of islet infiltrated; 3, 1/3 to 2/3 of islet infiltrated; 4, >2/3 of islet infiltrated (scores were independently determined by two individuals and averaged).
Statistical Analysis.
All statistical testing was performed using GraphPad Prism 4.0. The log-rank (Mantel–Cox) test was used for diabetes incidence studies. Two-way ANOVA with Sidak correction or Tukey’s multiple-comparisons, one-way ANOVA, and/or unpaired t tests were performed in other studies as indicated in the legends. The threshold for statistical significance was set to P < 0.05.
Supplementary Material
Acknowledgments
J.P.-F. and R.F.-F. were funded by postdoctoral fellowships from the American Diabetes Association (1-18-PDF-151 and 1-19-PMF-022, respectively). The study was supported by the Translational Therapeutics Accelerator program funded by the National Center for Advancing Translational Sciences, NIH, through Grant UL1TR001873. Studies involved the use of the Advanced Tissue Pathology and Imaging Core, supported by the Diabetes Research Center (Grant P30DK063608), the Molecular Pathology Shared Resource, supported by the Herbert Irving Comprehensive Cancer Center (Grant P30CA013696), and the Columbia Center for Translational Immunology Flow Cytometry Core, supported by both P30 grants. We thank the NIH Tetramer Core Facility, supported by Contract HHSN272201300006C from the National Institute of Allergy and Infectious Diseases, for the MHC tetramer used in these studies.
Footnotes
Competing interest statement: R.J.C. is the inventor of the patented Endotope platform.
This article is a PNAS Direct Submission.
See online for related content such as Commentaries.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2110987119/-/DCSupplemental.
Data Availability
Raw data reported in this article have been deposited in Mendeley Data and are available at https://data.mendeley.com/datasets/zbksm23r8k/1. All study data are included in the article and/or SI Appendix.
References
- 1.Roep B. O., Wheeler D. C. S., Peakman M., Antigen-based immune modulation therapy for type 1 diabetes: The era of precision medicine. Lancet Diabetes Endocrinol. 7, 65–74 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Serra P., Santamaria P., Antigen-specific therapeutic approaches for autoimmunity. Nat. Biotechnol. 37, 238–251 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Loaiza Naranjo J. D., Bergot A. S., Buckle I., Hamilton-Williams E. E., A question of tolerance-antigen-specific immunotherapy for type 1 diabetes. Curr. Diab. Rep. 20, 70 (2020). [DOI] [PubMed] [Google Scholar]
- 4.Kwiatkowski A. J., Stewart J. M., Cho J. J., Avram D., Keselowsky B. G., Nano and microparticle emerging strategies for treatment of autoimmune diseases: Multiple sclerosis and type 1 diabetes. Adv. Healthc. Mater. 9, e2000164 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Firdessa-Fite R., et al. , Soluble antigen arrays efficiently deliver peptides and arrest spontaneous autoimmune diabetes. Diabetes 70, 1334–1346 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.James E. A., Pietropaolo M., Mamula M. J., Immune recognition of β-cells: Neoepitopes as key players in the loss of tolerance. Diabetes 67, 1035–1042 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen H., Guyer P., Ettinger R. A., James E. A., Non-genetically encoded epitopes are relevant targets in autoimmune diabetes. Biomedicines 9, 202 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ludvigsson J., Autoantigen treatment in type 1 diabetes: Unsolved questions on how to select autoantigen and administration route. Int. J. Mol. Sci. 21, 1598 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.James E. A., Mallone R., Kent S. C., DiLorenzo T. P., T-cell epitopes and neo-epitopes in type 1 diabetes: A comprehensive update and reappraisal. Diabetes 69, 1311–1335 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baker R. L., et al. , Hybrid insulin peptides are autoantigens in type 1 diabetes. Diabetes 68, 1830–1840 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ilonen J., Lempainen J., Veijola R., The heterogeneous pathogenesis of type 1 diabetes mellitus. Nat. Rev. Endocrinol. 15, 635–650 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Battaglia M., et al. , Introducing the endotype concept to address the challenge of disease heterogeneity in type 1 diabetes. Diabetes Care 43, 5–12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skyler J. S., et al. , Effects of oral insulin in relatives of patients with type 1 diabetes: The Diabetes Prevention Trial—Type 1. Diabetes Care 28, 1068–1076 (2005). [DOI] [PubMed] [Google Scholar]
- 14.Skyler J. S.; Type 1 Diabetes TrialNet Study Group, Update on worldwide efforts to prevent type 1 diabetes. Ann. N. Y. Acad. Sci. 1150, 190–196 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith E. L., Peakman M., Peptide immunotherapy for type 1 diabetes—Clinical advances. Front. Immunol. 9, 392 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clemente-Casares X., et al. , Expanding antigen-specific regulatory networks to treat autoimmunity. Nature 530, 434–440 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Rivas E. I., et al. , Targeting of a T cell agonist peptide to lysosomes by DNA vaccination induces tolerance in the nonobese diabetic mouse. J. Immunol. 186, 4078–4087 (2011). [DOI] [PubMed] [Google Scholar]
- 18.Umeshappa C. S., et al. , Suppression of a broad spectrum of liver autoimmune pathologies by single peptide-MHC-based nanomedicines. Nat. Commun. 10, 2150 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Culina S., et al. ; ImMaDiab Study Group, Islet-reactive CD8+ T cell frequencies in the pancreas, but not in blood, distinguish type 1 diabetic patients from healthy donors. Sci. Immunol. 3, eaao4013 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalez-Duque S., et al. , Conventional and neo-antigenic peptides presented by β cells are targeted by circulating naïve CD8+ T cells in type 1 diabetic and healthy donors. Cell Metab. 28, 946–960.e6 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Azoury M. E., et al. , Peptides derived from insulin granule proteins are targeted by CD8+ T cells across MHC class I restrictions in humans and NOD mice. Diabetes 69, 2678–2690 (2020). [DOI] [PubMed] [Google Scholar]
- 22.Dastagir S. R., et al. , Efficient presentation of multiple endogenous epitopes to both CD4+ and CD8+ diabetogenic T cells for tolerance. Mol. Ther. Methods Clin. Dev. 4, 27–38 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Postigo-Fernandez J., Creusot R. J., A multi-epitope DNA vaccine enables a broad engagement of diabetogenic T cells for tolerance in type 1 diabetes. J. Autoimmun. 98, 13–23 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Solvason N., et al. , Improved efficacy of a tolerizing DNA vaccine for reversal of hyperglycemia through enhancement of gene expression and localization to intracellular sites. J. Immunol. 181, 8298–8307 (2008). [DOI] [PubMed] [Google Scholar]
- 25.Roep B. O., et al. ; BHT-3021 Investigators, Plasmid-encoded proinsulin preserves C-peptide while specifically reducing proinsulin-specific CD8+ T cells in type 1 diabetes. Sci. Transl. Med. 5, 191ra82 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson M. S., Bluestone J. A., The NOD mouse: A model of immune dysregulation. Annu. Rev. Immunol. 23, 447–485 (2005). [DOI] [PubMed] [Google Scholar]
- 27.Markle J. G., et al. , Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 339, 1084–1088 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Mathews C. E., et al. , Acute versus progressive onset of diabetes in NOD mice: Potential implications for therapeutic interventions in type 1 diabetes. Diabetes 64, 3885–3890 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitchell A. M., et al. , T-cell responses to hybrid insulin peptides prior to type 1 diabetes development. Proc. Natl. Acad. Sci. U.S.A. 118, e2019129118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crawford F., et al. , Specificity and detection of insulin-reactive CD4+ T cells in type 1 diabetes in the nonobese diabetic (NOD) mouse. Proc. Natl. Acad. Sci. U.S.A. 108, 16729–16734 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.James E. A., et al. ; Immunology of Diabetes Society T Cell Workshop Committee, Combinatorial detection of autoreactive CD8+ T cells with HLA-A2 multimers: A multi-centre study by the Immunology of Diabetes Society T Cell Workshop. Diabetologia 61, 658–670 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Nikolic T., et al. , Safety and feasibility of intradermal injection with tolerogenic dendritic cells pulsed with proinsulin peptide—For type 1 diabetes. Lancet Diabetes Endocrinol. 8, 470–472 (2020). [DOI] [PubMed] [Google Scholar]
- 33.Urbanek-Ruiz I., et al. , Immunization with DNA encoding an immunodominant peptide of insulin prevents diabetes in NOD mice. Clin. Immunol. 100, 164–171 (2001). [DOI] [PubMed] [Google Scholar]
- 34.Nakayama M., et al. , Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature 435, 220–223 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daniel C., Weigmann B., Bronson R., von Boehmer H., Prevention of type 1 diabetes in mice by tolerogenic vaccination with a strong agonist insulin mimetope. J. Exp. Med. 208, 1501–1510 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Serra P., Santamaria P., Peptide-MHC-based nanomedicines for the treatment of autoimmunity: Engineering, mechanisms, and diseases. Front. Immunol. 11, 621774 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kalekar L. A., et al. , CD4(+) T cell anergy prevents autoimmunity and generates regulatory T cell precursors. Nat. Immunol. 17, 304–314 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou X., et al. , Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat. Immunol. 10, 1000–1007 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hull C. M., Peakman M., Tree T. I. M., Regulatory T cell dysfunction in type 1 diabetes: What’s broken and how can we fix it? Diabetologia 60, 1839–1850 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pagni P. P., et al. , Multicomponent plasmid protects mice from spontaneous autoimmune diabetes. Diabetes 71, 157–169 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu X., et al. , Vaccination with a co-expression DNA plasmid containing GAD65 fragment gene and IL-10 gene induces regulatory CD4(+) T cells that prevent experimental autoimmune diabetes. Diabetes Metab. Res. Rev. 32, 522–533 (2016). [DOI] [PubMed] [Google Scholar]
- 42.Stifter K., et al. , Preproinsulin designer antigens excluded from endoplasmic reticulum suppressed diabetes development in NOD mice by DNA vaccination. Mol. Ther. Methods Clin. Dev. 12, 123–133 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakayama M., Insulin as a key autoantigen in the development of type 1 diabetes. Diabetes Metab. Res. Rev. 27, 773–777 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sarikonda G., et al. , Transient B-cell depletion with anti-CD20 in combination with proinsulin DNA vaccine or oral insulin: Immunologic effects and efficacy in NOD mice. PLoS One 8, e54712 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Creusot R. J., et al. , A short pulse of IL-4 delivered by DCs electroporated with modified mRNA can both prevent and treat autoimmune diabetes in NOD mice. Mol. Ther. 18, 2112–2120 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leete P., et al. , The effect of age on the progression and severity of type 1 diabetes: Potential effects on disease mechanisms. Curr. Diab. Rep. 18, 115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Greenbaum C. J., et al. , Strength in numbers: Opportunities for enhancing the development of effective treatments for type 1 diabetes—The TrialNet experience. Diabetes 67, 1216–1225 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data reported in this article have been deposited in Mendeley Data and are available at https://data.mendeley.com/datasets/zbksm23r8k/1. All study data are included in the article and/or SI Appendix.