Abstract
Aging is characterized by a loss of bone marrow hematopoietic tissue, systemic chronic inflammation, and higher susceptibility to infectious and non-infectious diseases. We previously reported the tightly regulated kinetics and massive daily production of neutrophils during homeostasis in adult rhesus macaques aged 3 to 19 years old (equivalent to approximately 10 to 70 years of age in humans). In the current study, we observed an earlier release of recently-dividing neutrophils from bone marrow and greater in-group variability of neutrophil kinetics based on in vivo BrdU labeling in a group of older rhesus macaques of 20 – 26 years of age. Comparing neutrophil numbers and circulating cytokine levels in rhesus macaques spanning 2 to 26 years of age, we found a negative correlation between age and blood neutrophil counts and a positive correlation between age and plasma G-CSF levels. Hierarchical clustering analysis also identified strong associations between G-CSF with the pro-inflammatory cytokines, IL-1β and MIP-1α. Furthermore, neutrophils from older macaques expressed less myeloperoxidase and comprised higher frequencies of polymorphonuclear myeloid-derived suppressor cells (PMN-MDSCs) compared to the young adult macaques. In summary, we observed an earlier release from bone marrow and a reduced production of neutrophils despite the increased levels of plasma G-CSF in the especially elderly rhesus macaques. This lower neutrophil production capacity associated with increased production of pro-inflammatory cytokines as well as an earlier release of less mature neutrophils and PMN-MDSCs may contribute to the chronic inflammation and greater susceptibility to infectious and non-infectious diseases during aging.
Keywords: polymorphonuclear cells, myeloid-derived suppressor cells, aging, hematopoiesis
Graphical Abstract
Introduction
Neutrophils are short-lived myeloid cells that require constant replenishment from bone marrow. They function as frontline immune cells to clear pathogens and help mediate acute inflammation that is then resolved to restore homeostasis. During aging or other disease conditions, neutrophils also may contribute to chronic inflammation or inflammaging through interactions with other immune and non-immune cells for release of reactive oxygen species (ROS) and formation of neutrophil extracellular traps [1]. This is important because chronic inflammation during aging is associated with increased risk for diseases such as cardiovascular disease, Alzheimer’s and Parkinson’s diseases, insulin resistance associated with type 2 diabetes, atherosclerosis, and rheumatoid arthritis [2, 3].
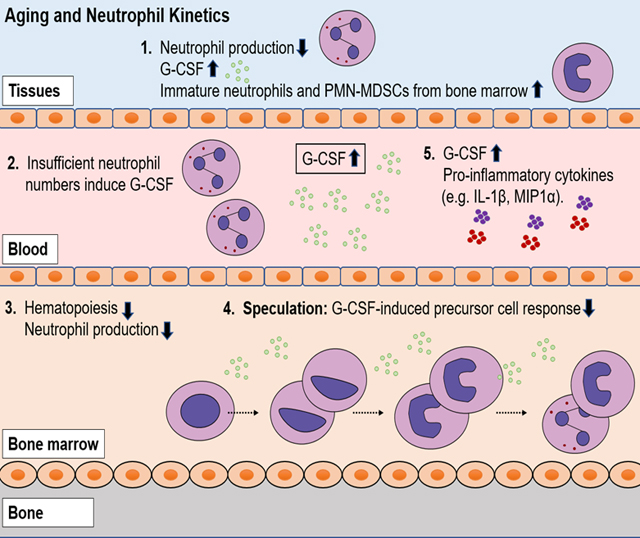
The production and release of neutrophils from bone marrow is mainly controlled by granulocyte colony-stimulating factor (G-CSF), a growth factor with hematopoietic mobilization and pro-inflammatory effects [4]. We previously reported that during homeostasis in adult rhesus macaques, neutrophil kinetics follow a consistent pattern characterized by 4–5 days of post-mitotic development in the bone marrow prior to transit into the blood and circulation for a half-life of 1.6 days [5]. More importantly, we and others demonstrated that the bone marrow produces massive numbers of neutrophils for release into the blood throughout one’s lifespan [5, 6]. During aging, however, hematopoietic bone marrow tissue volume and progenitor cell production capability decline [7, 8]. Studies about the effects of aging on neutrophils often focused on isolating circulating neutrophils from aged mice and humans to examine their functions ex vivo. Indeed, neutrophils isolated from older animals and humans compared to their younger counterparts, exhibited less phagocytosis activity [9, 10], lower reactive oxygen species (ROS) production [11], reduced neutrophil extracellular traps formation [12], and declining chemotaxis [13] (and also reviewed in [14, 15]). A gap in knowledge yet exists however, about how aging, which is characterized by chronic inflammation and bone marrow hematopoiesis deficiency, affects neutrophil kinetics and functions in vivo. This is of particular interest considering the constant massive daily replenishment of neutrophils from bone marrow throughout life and the rapid release of neutrophils in response to inflammatory stimuli.
In the studies presented here, we used rhesus macaques because they are genetically and physiologically similar to humans and serve as models to study immune responses during various human infectious diseases and aging [16, 17]. Given that a massive daily production of neutrophils is required during homeostasis in younger adults while bone marrow hematopoietic capabilities decline during aging, the purpose of this study was to further characterize the basis for altered neutrophil production and kinetics in especially older animals over 20 years of age (i.e. approximately equivalent to humans older than 70 years of age). The results suggested that in the especially older rhesus macaques, neutrophils begin to exhibit shorter bone marrow development time accompanied by earlier release into the blood, despite higher levels of circulating G-CSF, a major growth factor that promotes neutrophil production during homeostasis and emergency granulopoiesis [18]. Furthermore, in the older rhesus macaques, we observed a decreased expression of myeloperoxidase (MPO) in neutrophils and an increased frequency in polymorphonuclear myeloid-derived suppressor cells (PMN-MDSCs) reported to accumulate in cancers and under conditions of chronic inflammation in aged mice and humans [19–21]. The overall results suggest that during aging in rhesus macaques, dysregulation developed between declining neutrophil production despite increased G-CSF levels that was associated with elevated levels of other inflammatory cytokines, neutrophil function alterations, and increased frequency of PMN-MDSCs.
Materials and Methods
Rhesus macaques
Rhesus macaques of Indian-ancestry from the Tulane National Primate Research Center were used in this study (Supplemental Table I). The majority of animals were specific pathogen-free of Macacine herpesvirus 1 (i.e. herpes B virus), SIV, simian betaretrovirus (formerly simian retrovirus type D), and simian T-cell lymphotropic virus 1, as well as Mycobacterium tuberculosis. A few of the extremely older macaques were housed in the conventional animal colonies and may have tested seropositive for STLV and/or B virus exposures but were clinically normal at the time of sample collections. Eleven female rhesus macaques between 20 to 26 years old [Median, 95%CI: 24.8, (21.1–26.6)]) and housed indoors were used for neutrophil kinetics studies. Another 24 rhesus macaques (20 females and 4 males) aged 5 to 27 years old from indoor and outdoor housing were used for neutrophil MPO expression studies (Supplemental Table I). Blood and plasma samples obtained during routine bio-surveillance evaluations from an additional 126 outdoor-housed rhesus macaques (103 females and 23 males) between 2 to 23 years old were evaluated for hematology and cytokines analyses. All procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals [22] and after approval by the Tulane University Institutional Animal Care and Use Committee.
5-bromo-2’-deoxyuridine (BrdU) administration and blood collection
The thymidine analogue, BrdU (Catalogue number B5002–100G, Sigma-Aldrich, St. Louis, MO, USA), was prepared at 30 mg/ml in endotoxin-free PBS (Catalogue number TMS-012-A, Millipore Sigma, Burlington, MA), filter sterilized through a 0.2 μm polyethersulfone membrane (Steriflip, Catalogue number SCGP00525, Millipore Sigma, or Catalogue number 09–740-2A, ThemoFisher Scientific, Waltham, MA, USA), and administered intravenously at a dose of 60 mg/kg body weight. Blood samples were obtained approximately between 8 AM and 11 AM on collection days (approximately 24 hours after BrdU injections) to minimize effects of daily oscillations on the kinetics studies.
Flow cytometry and hematology analyses
Cellular immuno-phenotyping and staining for BrdU incorporation were performed as described [5, 23]. Briefly, 200 μl of EDTA–anticoagulated whole blood were washed with PBS (Catalogue number 21–040-CV, Corning, Corning, NY) containing 2% FBS (Catalogue number 16000044, Thermo Fisher Scientific, Vacaville, CA) and incubated with surface monoclonal antibodies (Supplemental Table 1) at room temperature for 20 minutes. RBCs were lysed with FACS lysing solution (10× stock, Catalogue Number 349202, BD Biosciences, San Jose, CA) prepared to 1× final concentration in deionized water, and the remaining cells were permeabilized using a three-step Cytofix/Cytoperm protocol per manufacturer instructions (BD Biosciences). For analysis of BrdU incorporation, cells were incubated with DNase I (Catalogue number DN25, Sigma-Aldrich) at 37°C for one hour and then stained with anti-BrdU antibody for 20 min at room temperature (Supplemental Table 1). After washing, cells were fixed in 250 μl of 1% paraformaldehyde in PBS. Samples were acquired with a LSRFortessa flow cytometer (BD Biosciences) and data were analyzed with FlowJo software version 10 (FlowJo, LLC, Ashland, OR).
Venipuncture and PBMC isolation for immunostaining
EDTA–anticoagulated whole blood cells were first washed with PBS containing 2% FBS. Two volumes of the washed blood cell suspension were layered over one volume Ficoll-Paque Plus (Catalogue number GE17–1440-03, GE Healthcare, Marlborough, MA) and centrifuged (1,825 × g at 20° C for 20 min using deceleration without braking). PBMCs were harvested from the Ficoll interface, resuspended in PBS containing 2% FBS, and counted. Approximately one million cells were used for immunostaining of surface markers and BrdU as described above.
Reactive oxygen species (ROS) production assay
Neutrophil ROS production was measured through the oxidization of non-fluorescent dihydrorhodamine 123 to fluorescent rhodamine 123 by ROS using the Neutrophil/Monocyte Respiratory Burst Assay Kit (Catalogue number 601130, Cayman Chemicals, Ann Arbor, MI) according to manufacturer’s instructions [24]. In brief, 100 μl EDTA–anticoagulated whole blood and 10 μl of 5 μg/ml dihydrorhodamine 123 working solution were added to each 5-ml round-bottom FACS tube (Catalogue number 352054, Corning), mixed well, and incubated in a 37°C water bath for 15 min. To stimulate ROS production, phorbol 12-myristate 13-acetate (PMA; Catalogue number 400145, Cayman Chemicals) in dimethyl sulfoxide (DMSO) was added to each cell suspension to reach a final concentration of 200 nM or as indicated in the dose-response assay, and the same volume of PBS was added to controls. After incubation in a 37° C water bath for 45 min, 3 ml PBS was added to each tube followed by centrifugation of the cells (700 × g for 5 minutes at room temperature). Cell pellets were resuspended in 200 μl PBS for immunostaining (CD66abce-PE) and live/dead detection via flow cytometry as described above for neutrophil phagocytosis. ROS production was measured by detection of rhodamine 123 signal in the FITC channel of live CD66abce+ cells.
Multiplex cytokine assay
Multiplex cytokine quantification was performed using the Cytokine 29-Plex Monkey Panel kit (Invitrogen Catalogue number LPC0005M; ThemoFisher Scientific) according to manufacturer’s instructions and as previously described [25]. In brief, EDTA-anticoagulated blood samples were centrifuged (14000×g for 5 min) and plasma aliquots were cryopreserved at −80 °C until used. Once-thawed plasma samples were filtered through an Ultrafree Centrifugal Filter (Millipore Sigma) just prior to assay. The reaction plates were read on a Bioplex-200 system instrument and the results were calculated using BioPlex software version 6.2 (BioRad, Hercules, CA).
Statistical analysis
Mann-Whitney U test was used to compare differences between two groups and Kruskal-Wallis test (with Dunn’s post test) was used to compare results of more than two groups. Correlations were assessed using the Spearman rank-order test. Multiplex cytokine data on 18 cytokines (IFN-γ, TNF-α, G-CSF, GM-CSF, IL-1β, IL-1ra, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12/23(p40), IL-15, MCP-1, MIP-1β, MIP-1α, and VEGF) were first scaled by z-scores (calculated by distance to mean divided by standard deviation for each cytokine values) and hierarchical clustering analysis was performed using Python (version 3.6.6) and library Scipy (version 1.1.0) with euclidean distance and Ward’s method. Cytokine values that were three standard deviations outside the mean were considered outliers and filtered before performing the analysis as described [26]. A standard scale color map value was calculated for each cytokine concentrations by subtraction of the minimum values for this cytokine and divided by the distance between the minimum and maximum values. Cluster dissimilarity was defined with Euclidean distance and Ward’s method was used for clustering linkage. A correlation matrix was computed with Spearman rank-order correlation coefficient (red, r > 0, positive correlation; blue: r < 0, negative correlation) and the P value to test for non-correlation (i.e. any correlation relationships with P > 0.05 were shown in white). Hierarchical clustering and correlation matrix figures were generated with Matplotlib (version 2.2.3) and Seaborn (version 0.9.0) in Python (version 3.6). All other graphs were prepared using Graphpad Prism version 8.1.2 (Graphpad Software, San Diego, CA), and P < 0.05 was considered significant.
Results
Shorter neutrophil post-mitotic transit time from bone marrow and greater variability among elderly rhesus macaques
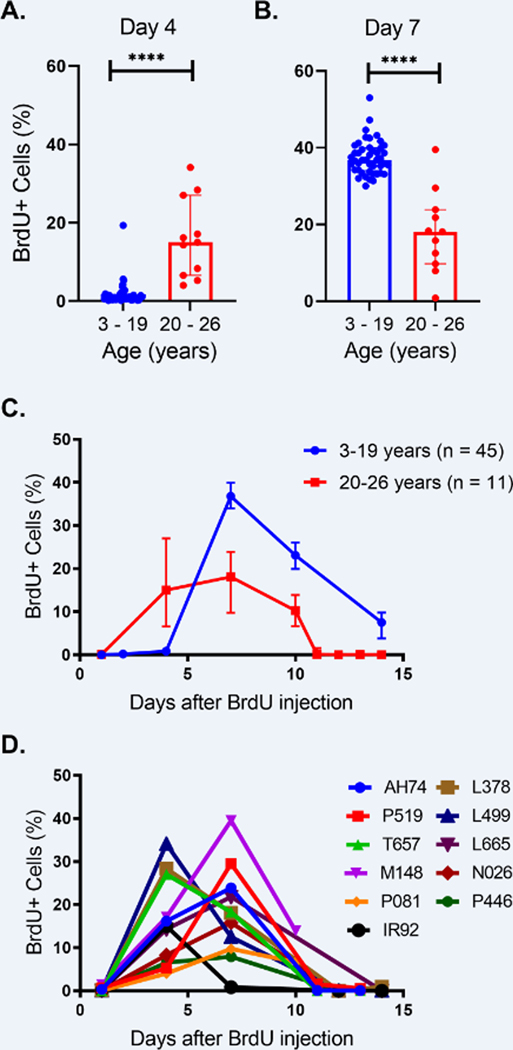
In earlier studies, rhesus macaques aged 3–19 years old (equivalent to approximately 10 – 65 years of age in humans) exhibited consistent within-group patterns in neutrophil kinetics related to bone marrow development and time until transit to blood. In addition, there was a significant decrease in the daily production of neutrophils over increasing age [5]. To expand on those results, we applied the same in vivo BrdU pulse-chase labeling method and gating strategy Supplemental Fig. 1) to next follow neutrophil kinetics in an older group of rhesus macaques aged 20–26 years old (equivalent to humans aged approximately 70–90 years old). Surprisingly, we found that in the 20–26 year-old macaques, the neutrophil kinetics patterns shifted whereby BrdU-incorporated (i.e. recently-dividing) neutrophils were released and detected in the circulation earlier than in the younger rhesus macaques aged 3–19 years old. Four days after BrdU administration into the older animals, approximately 15% of the neutrophils were labeled with BrdU in blood versus less than 2% BrdU-labeled neutrophils in the younger animals injected with BrdU (Fig. 1 A & B ). This indicated that neutrophils in the macaques older than 20 years underwent a shorter post-mitotic bone marrow development period with earlier transit time to blood. We also observed a lower median percentage of BrdU-labeled neutrophils 10–11 days after BrdU administration in the elderly animals compared to the younger animals, which is the time when BrdU-labeled neutrophils were leaving the circulation for tissues or clearance (Fig. 1C). In contrast to the internally-consistent BrdU kinetics among the 45 rhesus macaques in the 3–19 year-old group, the elderly group exhibited higher variability between the individuals (Fig. 1D).
FIGURE 1. Neutrophils exhibited shorter bone marrow post-mitotic development time, earlier transit into blood, and higher in-group variability among older rhesus macaques aged 20 – 26 years of age compared to younger adults aged 3 – 19 years of age.
Animals each received a single bolus of BrdU (60 mg/kg) intravenously and EDTA-treated blood samples were collected various days later for antibody staining and flow cytometry analysis. Neutrophils were gated from single cells, FSC/SSChigh/dim, HLA-DR-, CD3-, CD20-, CD123-, and the percentages of BrdU+ cells were gated from the neutrophil population. (A&B) Percentages of BrdU-positive neutrophils four days (A) and seven days (B) after administration of BrdU were compared between the two age groups using the nonparametric Mann-Whitney U test. (C) The kinetics of BrdU-labeled blood neutrophil kinetics were compared between rhesus macaques aged 20–26 years old (red line) and previously reported data from rhesus macaques between 3 to 19 years old (blue line) [5]. Medians and interquartile ranges were shown at different time points after BrdU injection. (D) BrdU-labeled blood neutrophil kinetics of individual rhesus macaques aged 20–26 years old demonstrates in-group variability in this age group. ****, P <0.0001. Medians and interquartile ranges were shown.
Paradoxical decline in blood neutrophil numbers despite elevated G-CSF levels in elderly rhesus macaques.
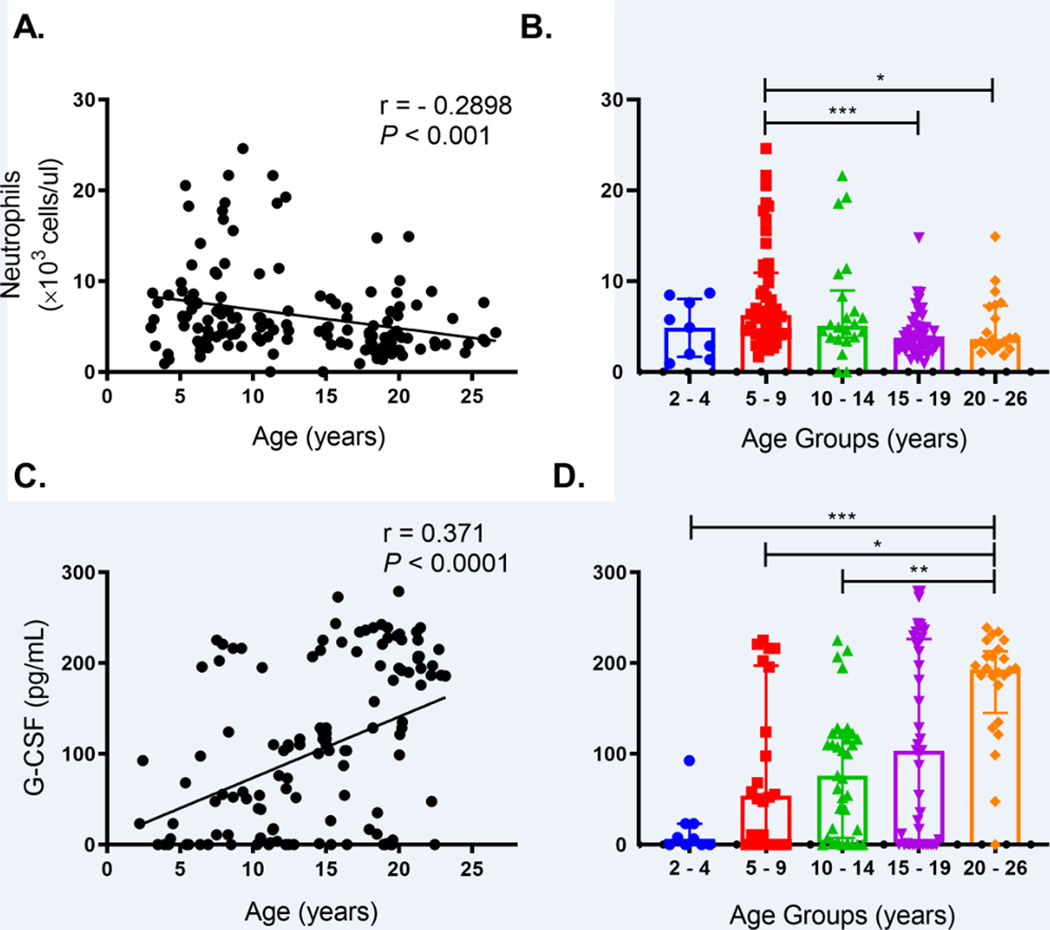
Consistent with our previous observations in animals aged 3 – 19 years old [5], the relationship between neutrophil counts in blood continued to be significantly negatively correlated with increasing chronological age after including animals 20 – 26 years old (Fig. 2A). Compared to the mean neutrophil levels in the group of young adults aged 5 – 9 years old, animals aged 15–19 years old exhibited significantly lower mean neutrophil counts (Fig. 2B). While the 20–26 year-old animals also exhibited a lower mean neutrophil count, this did not quite reach statistical significance, possibly due to the higher variability and relatively high standard deviation within this group (Fig. 2B).
FIGURE 2. Declining blood neutrophil numbers despite elevated plasma G-CSF levels were observed in elderly rhesus macaques.
(A) Blood neutrophil counts in 142 animals aged 3 – 26 years old were examined by nonparametric Spearman correlation. (B) Blood neutrophil counts and medians with interquartile range were plotted and compared between age groups of rhesus macaques by Kruskal-Wallis test followed by Dunn’s post-test for pairwise comparisons. (C) Plasma G-CSF levels (pg/ml) were measured in 132 samples from 126 rhesus macaques as well as 12 specimens collected from six animals at different ages. Results were analyzed by nonparametric Spearman correlation. (D) G-CSF levels in age groups of rhesus macaques were plotted along with medians with interquartile ranges and compared by Kruskal-Wallis test with Dunn’s post-test for pairwise comparisons. *, P < 0.05; **, P < 0.01; ***, P < 0.001. P < 0.05 was considered statistically significant.
The decrease in blood neutrophil numbers and earlier release into blood suggested a deficiency in bone marrow neutrophil production in the elderly rhesus macaques. We thus examined the animals for circulating G-CSF, a major growth factor that promotes neutrophil production during homeostasis and emergency granulopoiesis [18]. A multiplex cytokine quantification analysis was performed on plasma samples collected during routine clinical surveillance examinations from a cohort of 126 outdoor-housed 2–23 year-old rhesus macaques, and we observed that plasma G-CSF levels significantly positively correlated with age (Fig. 2C). Furthermore, mean levels of G-CSF in the group of rhesus macaques above 20 years old were significantly higher than in groups aged 2–4, 5–9 and 10–14 years of age (Fig. 2D). In 79 out of these 126 animals, the complete blood cell counts were available at the same time points when plasma samples were collected for analyzing cytokine levels. Similar trends were observed whereby G-CSF positively correlated with age yet conversely, neutrophil numbers negatively correlated with age (Supplemental Fig. 2A). Decreasing neutrophil counts with increased aging were observed overall but were only statistically significant in the females (Supplemental Fig. 2B). The correlation between increasing G-CSF plasma levels and age was also observed in both sexes (Supplemental Fig. 2C). Thus, despite the higher plasma G-CSF levels, the numbers of blood neutrophils were lower in elderly rhesus macaques.
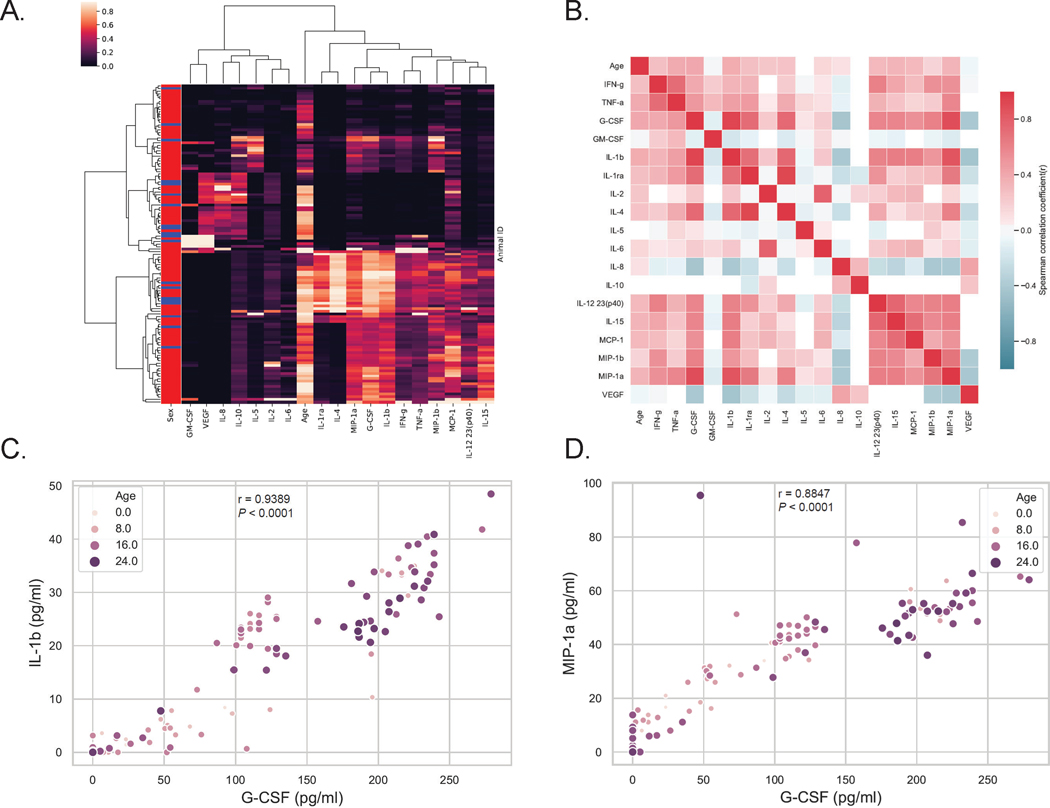
Hierarchical clustering and multiplex cytokine analyses revealed strong associations between G-CSF, IL-1β, and MIP-1α during aging in rhesus macaques
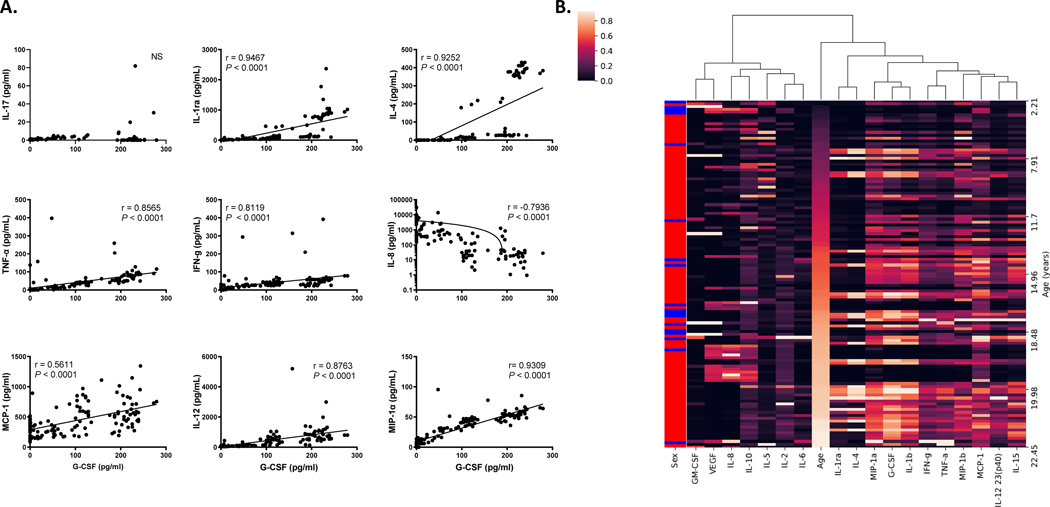
The higher circulating levels of G-CSF in elderly rhesus macaques appeared unable to induce neutrophil production by bone marrow to the levels observed in younger adults. We next determined if elevated concentrations of G-CSF were associated with shifts in other cytokines, especially those related to chronic inflammation of aging, i.e. “inflammaging”. To address this, we performed a hierarchical cluster analysis from the multiplex cytokine quantification data and observed that G-CSF most closely clustered with IL-1β and MIP-1α (Fig. 3A & Fig 4A). A correlation matrix heatmap then was prepared to compare each pair of cytokines as well as age to each cytokine (Fig. 3B). The results further demonstrated positive correlations (indicated by pink-red) between increasing levels of G-CSF, IL-1β (Fig. 3C) and MIP-1α (Fig. 3D), as well as additional circulating inflammatory cytokines (e.g. IFN-γ, TNF-α, IL-6, IL-12, and MCP-1) in relation to increasing age of the rhesus macaques. There also were statistically significant correlations between G-CSF and other inflammatory cytokines such as IL-1ra, IL-4, TNF-α, IFN-γ, IL-8, MCP-1, IL-12/23(p40), and MIP1α, but not with IL-17 (Figure 4A).
FIGURE 3. Hierarchical clustering analysis and pairwise correlation matrix of cytokine profiles and age revealed that G-CSF levels are closely associated with IL-1β and MIP-1α levels as well as with aging.
Multiplex cytokines profiles were measured in 123 rhesus macaques aged 2 – 24 years old. (A) Hierarchical clustering analysis was performed on 18 plasma cytokines levels. Sex; red = female and blue = male. (B) A pairwise Spearman correlation matrix was performed for cytokine levels and age. Red indicates positive correlations (r > 0) and blue indicates negative correlations (r < 0). P < 0.05 was considered statistically significant. Scatter plots were shown between G-CSF and IL-1β (C), MIP-1α (D) with the ages of the animals indicated by the color intensity and size of the dots. Spearman nonparametric correlation coefficient (r) and P value were calculated.
FIGURE 4. Cytokines that directly correlated with G-CSF levels in rhesus macaques aged 2 – 24 years old.
(A) Spearman nonparametric correlations and pairwise scatter plots between G-CSF and representative examples of cytokines are shown. Note the levels for IL-8 were plotted on a log scale. P < 0.05 was considered significant. NS, not significant. (B) Hierarchical clustering analysis of 18 plasma cytokines levels in 123 rhesus macaques that were sorted by age. (i.e. young to old: dark to light, Sex; red = female and blue = male)
Neutrophils from elderly macaques displayed lower intracellular myeloperoxidase expression
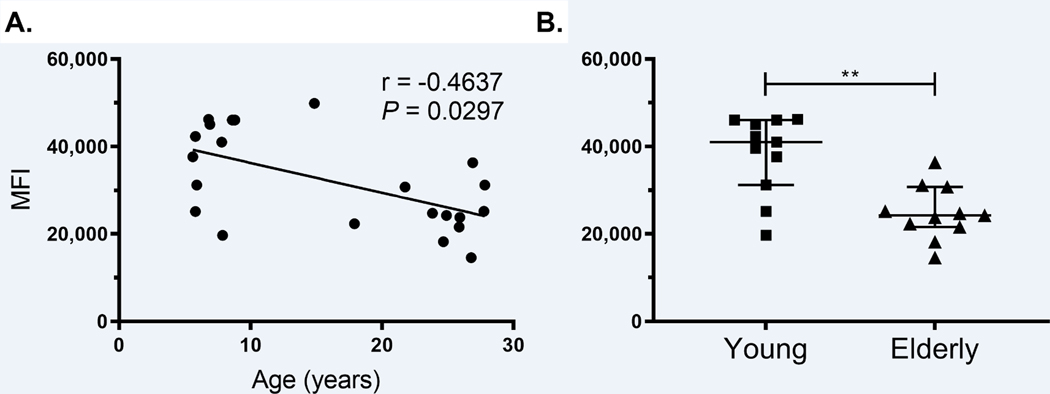
In a cross-sectional analysis of complete blood cell counts, we had reported that there were lower numbers of blood neutrophils in middle-aged rhesus macaques aged 15–20 years old compared to younger adult rhesus macaques aged 5–10 years old [5]. In elderly rhesus macaques over 20 years old, neutrophils exhibited significantly shorter bone marrow post-mitotic development and earlier transit time into blood. Next we sought to examine the function of neutrophils from these elderly monkeys. A major function of neutrophils is to produce MPO for performing antimicrobial activity. Whole blood specimens from rhesus macaques ranging from 5.6 – 27.8 years of age that were stained and analyzed by flow cytometry for cell phenotype were also examined for intracellular MPO expression measured as median fluorescent intensity (MFI). We observed that MPO expression negatively correlated with aging (Fig. 5A) and that the elderly rhesus macaques over 20 years old expressed significantly lower MPO compared to the young adults younger than 10 years old (Fig. 5B), suggesting that indeed, there was a decline in neutrophil functionality.
FIGURE 5.
Neutrophils in elderly rhesus macaques express lower levels of MPO.
Whole blood samples from rhesus macaques ranging in age from 5.6 – 27.8 years old were stained for surface and intracellular markers. MPO expression was measured as median florescent intensity (MFI) in neutrophils gated from single cells/live/CD45+/CD3-/CD20-/HLA-DR-/CD66abce+. (A) Spearman nonparametric correlation test was applied. (B) Unpaired Mann-Whitney U test was used to compare MPO MFI expression in the younger animals aged 5.6 – 8.8 years old with that in older animals aged 17.9 – 27.83 years old. ** P < 0.01.
CD33+ PMN-MDSC like cells increased in elderly rhesus macaques.
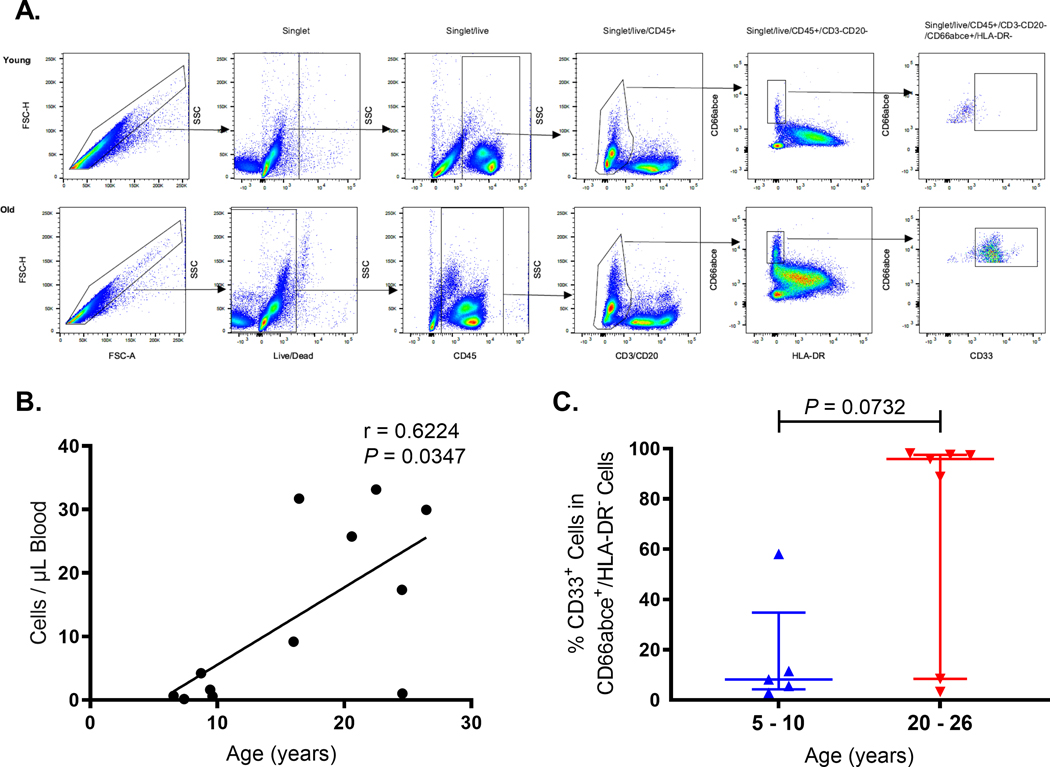
PMN-MDSCs are immature myeloid-lineage cells induced by chronic inflammation that resemble neutrophils, express lower levels of MPO, and exhibit T cell-like suppressor immune responses. Therefore, we hypothesized that the shorter post-mitotic bone marrow development time, earlier release into blood, and decreased MPO expression in blood neutrophils, despite the increased levels of G-CSF, may contribute to the generation and release of PMN-MDSCs from the bone marrow into the blood circulation of animals above 20 years of age so we examined blood from rhesus macaques ranging from 5.6 to 27.83 years of age (Supplemental Table 1). PMN-MDSCs comprise a subset of myeloid-derived suppressor cells with a similar phenotype to neutrophils in humans [27] and rhesus macaques [28–30]. Thus, the PMN-MDSC-like cells were defined as single/live/CD45+/CD3-/CD20-/HLA-DR-/CD66abce+/CD33+ cells in PBMCs (Fig. 6A) and were located at the Ficoll interface with PBMC after density gradient centrifugation compared to the more dense mature neutrophils that centrifuged to the surface of the RBC pellet as described previously [30]. The absolute numbers of PMN-MDSCs significantly positively correlated with age among all the animals (Fig. 6B). A trend in higher frequencies of CD33+ cells in the CD66abce+/HLA-DR- population also was observed in blood from the elderly rhesus macaques compared to that from the young adults (Fig. 6C). In addition to frequency, we measured the BrdU kinetics of neutrophils and PMN-MDSCs in the group of six elderly rhesus macaques over age 20 years. Four days after BrdU injection, higher percentages of labeled and recently dividing cells were identified among the PMN-MDSC population compared to neutrophils (Supplemental Fig. 3). Although this difference was not statistically significant, the results suggested that PMN-MDSCs arrive earlier into the blood circulation than neutrophils and may account for an increased frequency of PMN-MDSCs in the elderly rhesus macaques compared to the younger adult animals.
FIGURE 6. Increased frequency and absolute numbers of CD33+ PMN-MDSC-like cells were observed in elderly rhesus macaques over 20 years of age.
(A) A representative gating strategy is shown for PMN-MDSCs in PBMCs of younger (5–10 year-old) and elderly (20–26 year-old) rhesus macaques. PMN-MDSCs were gated as single/live/CD45+/CD3-/CD20-/HLA-DR-/CD66abce+/CD33+. (B) The absolute counts of CD33+ PMN-MDSCs were plotted against age of each animal and analyzed by Spearman correlation test. P < 0.05 was considered statistically significant. (C) The percentages of CD33+ PMN-MDSCs in CD66abce+ low-density neutrophils were plotted along with median and interquartile range for comparison between the younger and older rhesus macaques by Mann-Whitney U Test.
Discussion
Neutrophils develop in bone marrow, transit into the blood, and traffic to sites of injury where they promote acute inflammation in their innate immune responses against infections or other insults [31]. We previously reported that during homeostasis in young and middle-aged adult rhesus macaques, neutrophils undergo approximately 4–5 days of post-mitotic bone marrow development prior to their movement into blood where they have a half-life of just over 1.6 days [5]. Our earlier studies further demonstrated that in rhesus macaques 2 – 19 years old, equivalent to humans aged approximately 7 – 65 years old, there was a statistically significant correlation between increasing age and declining numbers of blood neutrophils. The data reported here build upon the earlier studies to examine neutrophils in older rhesus macaques over 20 years of age and possible mechanisms for their decline.
In rhesus macaques over age 20 years old, the bone marrow appeared to release neutrophils earlier than in the younger adults to compensate for the lower production or numbers of blood neutrophils. Evidence to support this was the shorter bone marrow post-mitotic development time, whereby BrdU-labeled recently-divided neutrophils appeared in the circulation four days after BrdU injection in elderly monkeys while virtually no BrdU-labeled neutrophils were detected in blood of younger adults at this time point. Another noticeable difference in the neutrophil kinetics patterns among the animals greater than 20 years of age was the higher in-group variability of the kinetics patterns compared to the younger animals that generated very similar in-group kinetics with low variability (i.e. small standard deviations of the mean). For example, some elderly animals exhibited a pattern more similar to the younger animals while others exhibited earlier and lower release from bone marrow suggesting differing rates of hematopoietic aging. Interestingly, the observed changes in kinetics only began to be observed in the rhesus macaques above 20 years old which corresponds to an approximate age of 70 years in humans, which is about the age at which a second decline of bone marrow hematopoietic tissues was reported to occur in humans [7]. Additional explanations to account for the higher in-group variability among the older animals could include different levels and rates of accumulated impacts from exposures to pathogens, environmental conditions, or other stressors (e.g. animal groupings and social structures) that had not yet affected younger animals [32]. This would be similar to current discussions about the impact of cytomegalovirus infections, for example, on human immune responses during later age [33]. Thus, future studies on various environmental exposures and stresses will contribute to better understanding their effects on immune status in the elderly.
In an effort to identify mechanisms to explain the decreased neutrophil production during aging, we examined levels of 29 plasma cytokines/chemokines in a cohort of 126 outdoor-housed rhesus macaques between 2 and 23 years of age. Unexpectedly, we found that G-CSF positively correlated with age despite the lower neutrophil production in the older animals. This may reflect a compensatory mechanism by which G-CSF was produced in response to deficient neutrophil numbers in elderly rhesus macaques in an attempt to induce increased neutrophil production by the bone marrow. G-CSF is the major growth factor that controls neutrophil production during homeostasis and emergency granulopoiesis [18] and it mobilizes bone marrow hematopoietic stem cells (HSCs) into peripheral blood [34]. Clinically, recombinant G-CSF has been used to treat chemotherapy-induced neutropenia and to mobilize bone marrow HSCs for transplantation [35]. In mice, G-CSF deficiency leads to neutropenia [36] and in return, the process of neutrophil clearance feedback regulates the production of G-CSF to maintain neutrophil homeostasis [37, 38]. Further studies, however, will be required to evaluate the proliferative capacity of neutrophil progenitor cells from these elderly macaques in response to G-CSF.
To gain some understanding about the relationship between G-CSF and other cytokines in the network, we performed hierarchical clustering and correlation matrix analyses of 18 cytokines in 123 animals from which all data points were available. G-CSF most strongly clustered with IL-1β and MIP-1α and also associated with other pro-inflammatory cytokines. As expected, aged animals in general exhibited higher inflammatory cytokine patterns and exhibited higher variability between individuals, as also previously reported [25]. Despite the higher levels of G-CSF, circulating neutrophil numbers in the older animals failed to reach levels observed in animals younger than 20 years. This suggested a dysregulated feedback mechanism whereby higher levels of G-CSF failed to compensate for the low neutrophil production in elderly rhesus macaques. A consequence of deficient hematopoietic bone marrow function thus appeared to result in earlier emigration of neutrophils in attempt to restore neutrophil blood cell numbers. The higher G-CSF levels in concert with cytokine feedback signaling may thus contribute to, or be a consequence of the chronic inflammation that further exacerbates the greater susceptibility to infectious and non-infectious diseases in the elderly.
The earlier transit of neutrophils from bone marrow to blood in the elderly rhesus macaques over 20 years old appeared to reflect declining or senescent hematopoietic bone marrow function that was insufficient to maintain the homeostatic requirements of neutrophil replenishment observed in younger adults. This further suggested that the neutrophils released earlier from the bone marrow may be less mature. Indeed, the neutrophils in the animals over 20 years old compared to younger rhesus macaques expressed less MPO, an antimicrobial factor present in neutrophil primary granules. ROS production and phagocytosis by neutrophils from middle-aged, 15–20 year-old, animals were not significantly different compared to neutrophils from younger 5–10 year-olds (data not shown). Sufficient volumes of fresh blood from the especially older animals over 20 years of age were not available for the additional ROS and phagocytosis studies in this report. Thus, future studies will be required to further evaluate and compare neutrophil functions among a broader, especially older age range of animals.
The earlier release of neutrophils prompted us also to characterize the phenotypes of neutrophils trafficking into blood in the animals over age 20 years. Myeloid-derived suppressor cells (MDSCs) are recently identified immature myeloid-lineage cells that exhibit immunosuppressive activity towards T cells [39]. PMN-MDSCs are a subset of MDSCs that express phenotypes similar to neutrophils (CD11b+HLA-DR-CD33+CD66abce+) in humans [27] and rhesus macaques [28–30] but are less dense and thus separate with PBMC rather than erythrocytes during gradient centrifugation procedures [40]. Originally described from studies in the cancer field, MDSCs (including PMN-MDSCs) were also reported to accumulate in chronic inflammatory conditions [19] including in aged mice [20] and humans [21]. Indeed, in our studies, both absolute numbers and frequencies of CD33+ PMN-MDSCs increased in the elderly rhesus macaques. In this study, however, the older animals were all females while the younger animals were males, and while we anticipate that there was minimal effect of sex on the age-related difference in CD33+PMN-MDSC frequencies, our use of animals in the study reflected the demographics of the animal colonies at the TNPRC in which females predominate at older age. A possible mechanism for the generation of PMN-MDSCs during aging is that the increased G-CSF levels were produced in attempt to induce neutrophil production but also mobilize bone marrow immature cells into circulation, including the release of PMN-MDSCs. Another possibility is that PMN-MDSCs could be a subset of earlier-derived neutrophils in response to inflammatory signals from the tissues. Thus, more work is needed to identify the effects of sex and the origin of PMN-MDSCs in their relationships with neutrophils, especially since the increased in PMN-MDSCs in the elderly animals may predict immune senescence or alternatively, a cause or consequence of chronic inflammation that could serve as intervention target.
Supplementary Material
Acknowledgement
We are grateful for the assistance of Toni P. Penny, Edith M. Walker, Erin M. Haupt, Jeanne M. Perkins, Kelly A. Goff, and Calvin R. Lanclos in the Division of Immunology, Robert Blair in the Division of Comparative Pathology, and Jason P. Dufour in the Division of Veterinary Medicine at Tulane National Primate Research Center. We also thank Mary Barnes, Stephanie Feely, Melissa Pattison, Elizabeth Scheef, and Coty Tatum of the Pathogen Detection and Quantification Core for assistance with the multiplex cytokine assays. This study was supported by research funding from the National Institutes of Health from grants AI097059, AI110163, and MH108458, to M.J.K, AG052349 to E.S.D., HL139278 to E.S.D. and M.J.K., GM103629 to N.R., and OD011104, OD010568, OD024282 to the TNPRC.
Abbreviations:
- BrdU
5-bromo-2’-deoxyuridine
- DMSO
Dimethyl sulfoxide
- G-CSF
Granulocyte colony-stimulating factor
- MPO
Myeloperoxidase
- PMA
Phorbol 12-myristate 13-acetate
- PMN-MDSCs
Polymorphonuclear myeloid-derived suppressor cells
- ROS
Reactive oxygen species
Footnotes
Summary sentence: Dysregulated neutrophil kinetics, increasing G-CSF levels, and higher frequency of PMN-MDSCs accompany chronic inflammation during aging in rhesus macaques.
Conflict-of-interest disclosure
The authors declare no competing financial interests.
References
- 1.Soehnlein O, Steffens S, Hidalgo A, Weber C. 2017. Neutrophils as protagonists and targets in chronic inflammation. Nat Rev Immunol 17, 248–261. [DOI] [PubMed] [Google Scholar]
- 2.Franceschi C. 2007. Inflammaging as a major characteristic of old people: can it be prevented or cured? Nutr Rev 65, S173–6. [DOI] [PubMed] [Google Scholar]
- 3.Ferrucci L. and Fabbri E. 2018. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nature Reviews Cardiology 15, 505–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eyles JL, Roberts AW, Metcalf D, Wicks IP 2006. Granulocyte colony-stimulating factor and neutrophils--forgotten mediators of inflammatory disease. Nat Clin Pract Rheumatol 2, 500–10. [DOI] [PubMed] [Google Scholar]
- 5.He Z, Allers C, Sugimoto C, Ahmed N, Fujioka H, Kim WK, Didier ES, Kuroda MJ 2018. Rapid Turnover and High Production Rate of Myeloid Cells in Adult Rhesus Macaques with Compensations during Aging. J Immunol 200, 4059–4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Price TH, Chatta GS, Dale DC 1996. Effect of recombinant granulocyte colony-stimulating factor on neutrophil kinetics in normal young and elderly humans. Blood 88, 335–40. [PubMed] [Google Scholar]
- 7.Hartsock RJ, Smith EB, Petty CS 1965. Normal Variations with Aging of the Amount of Hematopoietic Tissue in Bone Marrow from the Anterior Iliac Crest. A Study Made from 177 Cases of Sudden Death Examined by Necropsy. Am J Clin Pathol 43, 326–31. [DOI] [PubMed] [Google Scholar]
- 8.Geiger H, de Haan G, Florian MC 2013. The ageing haematopoietic stem cell compartment. Nat Rev Immunol 13, 376–89. [DOI] [PubMed] [Google Scholar]
- 9.Butcher SK, Chahal H, Nayak L, Sinclair A, Henriquez NV, Sapey E, O’Mahony D, Lord JM 2001. Senescence in innate immune responses: reduced neutrophil phagocytic capacity and CD16 expression in elderly humans. J Leukoc Biol 70, 881–6. [PubMed] [Google Scholar]
- 10.Wenisch C, Patruta S, Daxbock F, Krause R, Horl W. 2000. Effect of age on human neutrophil function. J Leukoc Biol 67, 40–5. [DOI] [PubMed] [Google Scholar]
- 11.Sauce D, Dong Y, Campillo-Gimenez L, Casulli S, Bayard C, Autran B, Boddaert J, Appay V, Elbim C. 2017. Reduced Oxidative Burst by Primed Neutrophils in the Elderly Individuals Is Associated With Increased Levels of the CD16bright/CD62Ldim Immunosuppressive Subset. J Gerontol A Biol Sci Med Sci 72, 163–172. [DOI] [PubMed] [Google Scholar]
- 12.Hazeldine J, Harris P, Chapple IL, Grant M, Greenwood H, Livesey A, Sapey E, Lord JM 2014. Impaired neutrophil extracellular trap formation: a novel defect in the innate immune system of aged individuals. Aging Cell 13, 690–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sapey E, Greenwood H, Walton G, Mann E, Love A, Aaronson N, Insall RH, Stockley RA, Lord JM 2014. Phosphoinositide 3-kinase inhibition restores neutrophil accuracy in the elderly: toward targeted treatments for immunosenescence. Blood 123, 239–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaw AC, Goldstein DR, Montgomery RR 2013. Age-dependent dysregulation of innate immunity. Nat Rev Immunol 13, 875–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tseng CW and Liu GY 2014. Expanding roles of neutrophils in aging hosts. Curr Opin Immunol 29, 43–8. [DOI] [PubMed] [Google Scholar]
- 16.Rivera A, Rais M, Barr T, Arnold N, Sureshchandra S, Messaoudi I. (2018) Nonhuman Primate Models of Immunosenescence. In Handbook of Immunosenescence 1–28. [Google Scholar]
- 17.Didier ES, MacLean AG, Mohan M, Didier PJ, Lackner AA, Kuroda MJ 2016. Contributions of Nonhuman Primates to Research on Aging. Vet Pathol 53, 277–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bugl S, Wirths S, Muller MR, Radsak MP, Kopp HG 2012. Current insights into neutrophil homeostasis. Ann N Y Acad Sci 1266, 171–8. [DOI] [PubMed] [Google Scholar]
- 19.Salminen A, Kauppinen A, Kaarniranta K. 2018. Myeloid-derived suppressor cells (MDSC): an important partner in cellular/tissue senescence. Biogerontology. [Google Scholar]
- 20.Enioutina EY, Bareyan D, Daynes RA 2011. A role for immature myeloid cells in immune senescence. J Immunol 186, 697–707. [DOI] [PubMed] [Google Scholar]
- 21.Verschoor CP, Johnstone J, Millar J, Dorrington MG, Habibagahi M, Lelic A, Loeb M, Bramson JL, Bowdish DM 2013. Blood CD33(+)HLA-DR(−) myeloid-derived suppressor cells are increased with age and a history of cancer. J Leukoc Biol 93, 633–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Research Council (U.S.). Committee for the Update of the Guide for the Care and Use of Laboratory Animals., Institute for Laboratory Animal Research (U.S.), National Academies Press (U.S.) 2011 Guide for the care and use of laboratory animals. National Academies Press,, Washington, D.C. xxv, 220 p. [Google Scholar]
- 23.Sugimoto C, Hasegawa A, Saito Y, Fukuyo Y, Chiu KB, Cai Y, Breed MW, Mori K, Roy CJ, Lackner AA, Kim WK, Didier ES, Kuroda MJ 2015. Differentiation Kinetics of Blood Monocytes and Dendritic Cells in Macaques: Insights to Understanding Human Myeloid Cell Development. J Immunol 195, 1774–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Y. and Junger WG 2012. Measurement of oxidative burst in neutrophils. Methods Mol Biol 844, 115–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Didier ES, Sugimoto C, Bowers LC, Khan IA, Kuroda MJ 2012. Immune correlates of aging in outdoor-housed captive rhesus macaques (Macaca mulatta). Immun Ageing 9, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wallner FK, Hultquist Hopkins M, Woodworth N, Lindvall Bark T, Olofsson P, Tilevik A. 2018. Correlation and cluster analysis of immunomodulatory drugs based on cytokine profiles. Pharmacol Res 128, 244–251. [DOI] [PubMed] [Google Scholar]
- 27.Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, Mandruzzato S, Murray PJ, Ochoa A, Ostrand-Rosenberg S, Rodriguez PC, Sica A, Umansky V, Vonderheide RH, Gabrilovich DI 2016. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun 7, 12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sui Y, Frey B, Wang Y, Billeskov R, Kulkarni S, McKinnon K, Rourke T, Fritts L, Miller CJ, Berzofsky JA 2017. Paradoxical myeloid-derived suppressor cell reduction in the bone marrow of SIV chronically infected macaques. PLoS Pathog 13, e1006395. [Google Scholar]
- 29.Sui Y, Hogg A, Wang Y, Frey B, Yu H, Xia Z, Venzon D, McKinnon K, Smedley J, Gathuka M, Klinman D, Keele BF, Langermann S, Liu L, Franchini G, Berzofsky JA 2014. Vaccine-induced myeloid cell population dampens protective immunity to SIV. J Clin Invest 124, 2538–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin A, Liang F, Thompson EA, Vono M, Ols S, Lindgren G, Hassett K, Salter H, Ciaramella G, Lore K. 2018. Rhesus Macaque Myeloid-Derived Suppressor Cells Demonstrate T Cell Inhibitory Functions and Are Transiently Increased after Vaccination. J Immunol 200, 286–294. [DOI] [PubMed] [Google Scholar]
- 31.Hidalgo A, Chilvers ER, Summers C, Koenderman L. 2019. The Neutrophil Life Cycle. Trends Immunol 40, 584–597. [DOI] [PubMed] [Google Scholar]
- 32.Oxford KL, Dela Pena-Ponce MGA, Jensen K, Eberhardt MK, Spinner A, Van Rompay KK, Rigdon J, Mollan KR, Krishnan VV, Hudgens MG, Barry PA, De Paris K. 2015. The interplay between immune maturation, age, chronic viral infection and environment. In Immunity & ageing : I & A, Volume 12 3. [Google Scholar]
- 33.Jergovic M, Contreras NA, Nikolich-Zugich J. 2019. Impact of CMV upon immune aging: facts and fiction. Medical microbiology and immunology. [Google Scholar]
- 34.Rutella S, Zavala F, Danese S, Kared H, Leone G. 2005. Granulocyte Colony-Stimulating Factor: A Novel Mediator of T Cell Tolerance. The Journal of Immunology 175, 7085–7091. [DOI] [PubMed] [Google Scholar]
- 35.Mehta HM, Malandra M, Corey SJ 2015. G-CSF and GM-CSF in Neutropenia. J Immunol 195, 1341–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lieschke GJ, Grail D, Hodgson G, Metcalf D, Stanley E, Cheers C, Fowler KJ, Basu S, Zhan YF, Dunn AR 1994. Mice lacking granulocyte colony-stimulating factor have chronic neutropenia, granulocyte and macrophage progenitor cell deficiency, and impaired neutrophil mobilization. Blood 84, 1737–46. [PubMed] [Google Scholar]
- 37.Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. 2005. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity 22, 285–94. [DOI] [PubMed] [Google Scholar]
- 38.Gordy C, Pua H, Sempowski GD, He YW 2011. Regulation of steady-state neutrophil homeostasis by macrophages. Blood 117, 618–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. 2012. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol 12, 253–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou J, Nefedova Y, Lei A, Gabrilovich D. 2018. Neutrophils and PMN-MDSC: Their biological role and interaction with stromal cells. Semin Immunol 35, 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.