Abstract
Background:
The aim of this study was to evaluate the association between age and disease specific mortality (DSM) among adults diagnosed with medullary thyroid cancer (MTC).
Method:
Surveillance, Epidemiology, and End Results (SEER-18) was used to analyze adult MTC patients stratified by age (18–64, 65–79, ≥80 years). Associations between patient demographics, tumor size, nodal status, metastatic disease, and extent of surgery on DSM was assessed with multivariable Cox regression.
Results:
Among 1457 patients with MTC, 1008 (69.2%) were younger adults, 371 (25.5%) older adults, and 78 (5.4%) were super-elderly. A significantly higher proportion of older adults and super-elderly had less than the recommended operation for MTC. On multivariable analysis, older adults and super-elderly were 2.9 and 6.7 times more likely to have an increased DSM (HR:2.91, 95% CI: 1.83–4.63; p < 0.001 and HR: 6.70, 95%CI: 3.69–12.20; p < 0.001). Extent of surgery or lymphadenectomy did not affect DSM.
Conclusions:
Increased age is an independent predictor of DSM in patients with MTC.
Keywords: Medullary thyroid cancer, Disease-specific mortality, Aging, Elderly, Older adults, Survival
Introduction
An estimated 52,070 new cases of thyroid cancer were diagnosed in the United States in 2019, with an estimated 2170 cancer related deaths.1 Although medullary thyroid cancer (MTC) only accounts for approximately 5% of these new diagnoses, its incidence is rising.2 It represents approximately 13% of all thyroid cancer related deaths with an estimated 10-year survival of 65%–71% overall and 40%–44% with distant metastasis.2,3 The standard minimum surgical management of MTC without evidence of nodal involvement includes a total thyroidectomy with a bilateral prophylactic central neck dissection.4 However, variation in practice patterns exists regarding extent of surgery and lymphadenectomy for MTC despite the presence of guidelines.5 Factors associated with this variation, including patient age, are unclear. Interestingly, over time, disease specific mortality (DSM) among all MTC patients has improved, although it is unknown if this is also seen across all patient age groups.2
The combination of a rise in the proportion of older patients (age ≥65 years) in the US, an increase incidence of thyroid cancer, and advances in surgical techniques will lead to more thyroidectomies being performed in older adults.6–9 Additionally, older patients tend to present at advanced stages and with more aggressive histology.10,11 Therefore, the improvement in prognosis must be balanced against risk of surgery in an older patient with comorbidities. While the relationship between increasing age and prognosis among patients with differentiated thyroid cancer (DTC) is well established and incorporated into the American Joint Committee on Cancer (AJCC) staging system,12,13 the impact of age on MTC is less clear. Therefore, the aim of this study was to evaluate the association between age and DSM among adults diagnosed with MTC.
Methods
Data source and study population
The National Cancer Institute’s (NCI) Surveillance, Epidemiology, and End Results (SEER-18) database was queried for thyroid cancer cases (primary site: thyroid, ICD-O-3: C73–9) diagnosed from 2004 to 2015.14 SEER captures approximately 28% of the United States population in 18 geographic regions and broadly represents the US population. These geographic regions include Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, and Utah, Greater California, Greater Georgia, Kentucky, Louisiana (except cases July–December 2005), and New Jersey. Patients with MTC, according to the International Classification of Diseases (ICD) for Oncology-3 codes 8345 and 8510, with tumor size ≥1.0 cm were included. Patients were excluded if their age was <18 years, if thyroid cancer was diagnosed at autopsy only or present on death certificate, or if their histologic subtype or tumor stage (TNM) was missing or classified as unknown. Consequently, this study was exempt from review by an institutional review board because it is a limited data set subject to the requirements of a data use agreement.
Data collection
SEER*Stat version 8.3.5 was used to abstract the data routinely collected by SEER, which included patient demographics, tumor characteristics, stage of disease, treatment, and outcomes including vital status, cause of death, and survival time. Demographic data included patient age at diagnosis, which was stratified into three groups consistent with prior studies evaluating age associations with well differentiated thyroid cancer15–17: 18–64 (younger adults), 65–79 (older adults), and ≥80 years (super-elderly), gender (male or female), race (white, black, or other), and Hispanic origin. Clinical and pathologic variables included tumor stage, nodal status, and presence of metastases using the 7th edition of the American Joint Committee on Cancer (AJCC) staging system. Thyroid procedure was classified as none, lobectomy, or total thyroidectomy. ‘Lymph nodes removed’ categories were predefined through the SEER database.
Statistical analysis
The association among the three categories of age with gender, race, tumor size, grade, nodal status, and type of surgical intervention was assessed using Pearson Chi-square (χ2) for categorical variables and t-test for continuous variables. Survival was calculated as time in months after the diagnosis until either death or the end of the study period. Kaplan-Meier curve and 2-sided log rank tests were used to evaluate disease-specific survival as a function of age. Two-sided significance level was set as (α = 0.05). Univariable Cox regression assessed the association between co-variables and DSM. The proportional hazards assumption was checked using survival function plots. Multivariable Cox regression was utilized to assess associations between patient demographics, tumor size, nodal status, presence of metastatic disease, and extent of surgery on DSM. All analyses were performed using Stata 14.0/MP for Linux (College Station, Texas).
Results
Characteristics and treatment patterns of entire cohort
Our study cohort consisted of 1457 patients with MTC (Table 1). The majority of patients were female (59.8%). The majority of patients were of non-Spanish-Hispanic-Latino (SHL) (85.3%) ethnicity and white race (85.2%), followed by black (7.7%), and other (7.1%). The majority of patients were from the Pacific coast (46.8%), East coast (40.4%), Northern plains (8.2%), Southwest (4.4%), and Alaska (0.2%).
Table 1.
Patient demographics by age group.
| All Patients | Younger Adults (<65 years) | Older Adults (65–79 years) | |||
|---|---|---|---|---|---|
|
| |||||
| Age | 1457 | 1008 (69.2%) | 371 (25.5%) | 78 (5.4%) | |
| Gender | |||||
| Female | 871 (59.8%) | 611 (60.6%) | 210 (56.6%) | 50 (64.1%) | 0.293 |
| Race | |||||
| White | 1241 (85.2%) | 847 (84.0%) | 322 (86.8%) | 72 (92.3%) | 0.270 |
| Black | 112 (7.7%) | 85 (8.4%) | 24 (6.5%) | 3 (3.9%) | |
| Other | 104 (7.1%) | 76 (7.5%) | 25 (6.7%) | 3 (3.9%) | |
| Ethnicity | |||||
| Non-Hispanic | 1243 (85.3%) | 840 (83.3%) | 331 (89.2%) | 72 (92.3%) | 0.005 |
| Diagnosis Period | |||||
| 2004 – 2006 | 293 (20.1%) | 211 (20.9%) | 67 (18.1%) | 15 (19.2%) | 0.246 |
| 2007 – 2009 | 356 (24.4%) | 260 (25.8%) | 77 (20.8%) | 19 (24.4%) | |
| 2010 – 2012 | 408 (28.0%) | 271 (26.9%) | 112 (30.2%) | 25 (32.1%) | |
| 2013 – 2015 | 200 (27.5%) | 266 (26.4%) | 115 (31.0%) | 19 (24.4%) | |
| Region | |||||
| Alaska | 3 (0.2%) | 3 (0.3%) | 0 (0.0%) | 0 (0.0%) | 0.603 |
| East | 588 (40.4%) | 157 (42.3%) | 157 (42.3%) | 28 (35.9%) | |
| Northern Plains | 120 (8.2%) | 37 (10.0%) | 37 (10.0%) | 7 (9.0%) | |
| Pacific Coast | 682 (46.8%) | 161 (43.4%) | 161 (43.4%) | 41 (52.6%) | |
| Southwest | 64 (4.4%) | 16 (4.3%) | 16 (4.3%) | 2 (2.6%) | |
Total thyroidectomy (89.3%) was the most common procedure followed by thyroid lobectomy (6.2%), and non-operative management (4.5%) (Table 2). Majority of the patients in the cohort (n = 1457) underwent lymphadenectomy with removal of ≥4 lymph nodes (61.7%) or removal of 1–3 nodes (10.9%). However 24.5% did not have any lymph nodes removed with the index thyroidectomy. The majority of our cohort (n = 1457) had T1 and T2 (65.8%), N0 (55.9%), and M0 (92.1%) disease. Most patients had solitary focus of MTC (72.9%). There were 229 (15.7%) patient deaths over the study period, of which, 109 (47.5%) were attributable to MTC.
Table 2–
Surgical intervention, tumor staging, and disease specific mortality stratified by age group.
| All Patients | Younger Adults (<65 years) | Older Adults (65–79 years) | Super-Elderly (≥80 years) | P-value | |
|---|---|---|---|---|---|
|
| |||||
| Staging | |||||
| Tumor Size | |||||
| T1 | 486 (33.3%) | 316 (31.4%) | 145 (39.1%) | 25 (32.1%) | 0.043 |
| T2 | 474 (32.5%) | 354 (35.1%) | 95 (25.6%) | 25 (32.1%) | |
| T3 | 374 (25.7%) | 254 (25.2%) | 98 (26.4%) | 22 (28.2%) | |
| T4 | 123 (8.4%) | 84 (8.3%) | 33 (8.9%) | 6 (7.7%) | |
| Nodal Status | |||||
| N0 | 815 (55.9%) | 541 (53.7%) | 228 (61.5%) | 46 (59.0%) | 0.066 |
| N1a | 268 (18.4%) | 136 (13.5%) | 50 (13.5%) | 11 (14.1%) | |
| N1b | 374 (25.7%) | 331 (32.8%) | 93 (25.1%) | 21 (26.9%) | |
| Metastasis | |||||
| M0 | 1342 (92.1%) | 924 (91.7%) | 348 (93.8%) | 70 (89.7%) | 0.312 |
| M1 | 115 (7.9%) | 84 (8.3%) | 23 (6.2%) | 8 (10.3%) | |
| Focality | |||||
| Solitary | 1062 (72.9%) | 700 (69.4%) | 296 (79.8%) | 66 (84.6%) | 0.000 |
| Multifocal | 368 (25.3%) | 289 (28.7%) | 67 (18.1%) | 12 (15.4%) | |
| No Primary | 3 (0.2%) | 0 (0.0%) | 3 (0.8%) | 0 (0.0%) | |
| Unknown | 24 (1.7%) | 19 (1.9%) | 5 (1.4%) | 0 (0.0%) | |
| Surgical Intervention | |||||
| None | 66 (4.5%) | 39 (3.9%) | 18 (4.9%) | 9 (11.5%) | 0.006 |
| Lobectomy | 90 (6.2%) | 55 (5.5%) | 27 (7.3%) | 8 (10.3%) | |
| Total Thyroidectomy | 1301 (89.3%) | 914 (90.7%) | 326 (87.8%) | 61 (78.2%) | |
| Lymph Nodes Removed | |||||
| None | 357 (24.5%) | 207 (20.5%) | 116 (31.3%) | 34 (43.6%) | 0.000 |
| 1 – 3 | 159 (10.9%) | 113 (11.2%) | 37 (10.0%) | 9 (11.5%) | |
| ≥ 4 | 899 (61.7%) | 659 (65.4%) | 207 (55.8%) | 33 (42.3%) | |
| Other | 42 (2.9%) | 29 (2.9%) | 11 (3.0%) | 2 (2.6%) | |
| Disease Specific Mortality | 109 (7.5%) | 60 (6.0%) | 33 (8.9%) | 16 (20.5%) | 0.000 |
Characteristics and treatment patterns stratified by age
Of the 1457 patients with MTC, 69.2%, 25.5%, and 5.4% were younger adults (18–64 years), older adults (65–79 years), and super-elderly (≥80 years), respectively (Table 1). Demographic characteristics in each age group were reflective of the entire cohort, however, the proportions of non-SHL among the three age groups varied significantly (83.3% vs 89.2% vs 92.3%, in younger adults, older adults, super elderly respectively, p = 0.005). There were no other significant differences in cohort demographics when stratified by age.
A significant difference among the three age groups was observed in regards to surgical management including extent of thyroidectomy and extent of lymphadenectomy (Table 2). Younger patients underwent total thyroidectomy more frequently than the super-elderly (90.7% vs 78.2%, p = 0.006). Thyroid lobectomy was more prevalent in the super-elderly group than in younger adults (10.3% vs 5.5%, p = 0.006). Additionally, a greater proportion of the super-elderly underwent non-operative management compared to older and younger adults (11.5% vs 4.9% vs 3.9%, respectively; p = 0.006). Lymphadenectomy with removal of ≥4 lymph nodes was more frequent among those <65 years old compared to those 65–79 years old and ≥80 years old (65.4% vs 55.8% vs 42.3%, p = 0.000). Among the super-elderly and older adults, a significantly lower proportion underwent lymphadenectomy compared to the younger cohort (53.8% and 65.8% vs 76.6%, p = 0.000).
In regards to tumor size, there was a significant difference in the distribution of T staging among the three age groups (Fig. 1). T1 disease was the most common presentation in older adults compared to the super-elderly and younger adult groups (T1: 39.1% vs 32.1% and 31.4%), whereas higher proportion of T2 disease was observed among younger adults compared to older adults and super-elderly (T2: 35.1% vs 25.6% and 32.5%, p = 0.043). T3 disease presentation was more common in the super-elderly group (T3: 28.2% vs 26.4% older adults and 25.2% younger adults, p = 0.043) and T4 was more common in older adults (T4: 8.9% vs 7.7% super elderly and 8.3% younger adults, p = 0.043). Interestingly, no significant difference among age groups was observed in presence of nodal or distant metastases. Unifocal disease was more common in super elderly patients than the younger and older adult groups (84.6% vs 69.4% and 79.8%, respectively).
Fig. 1.
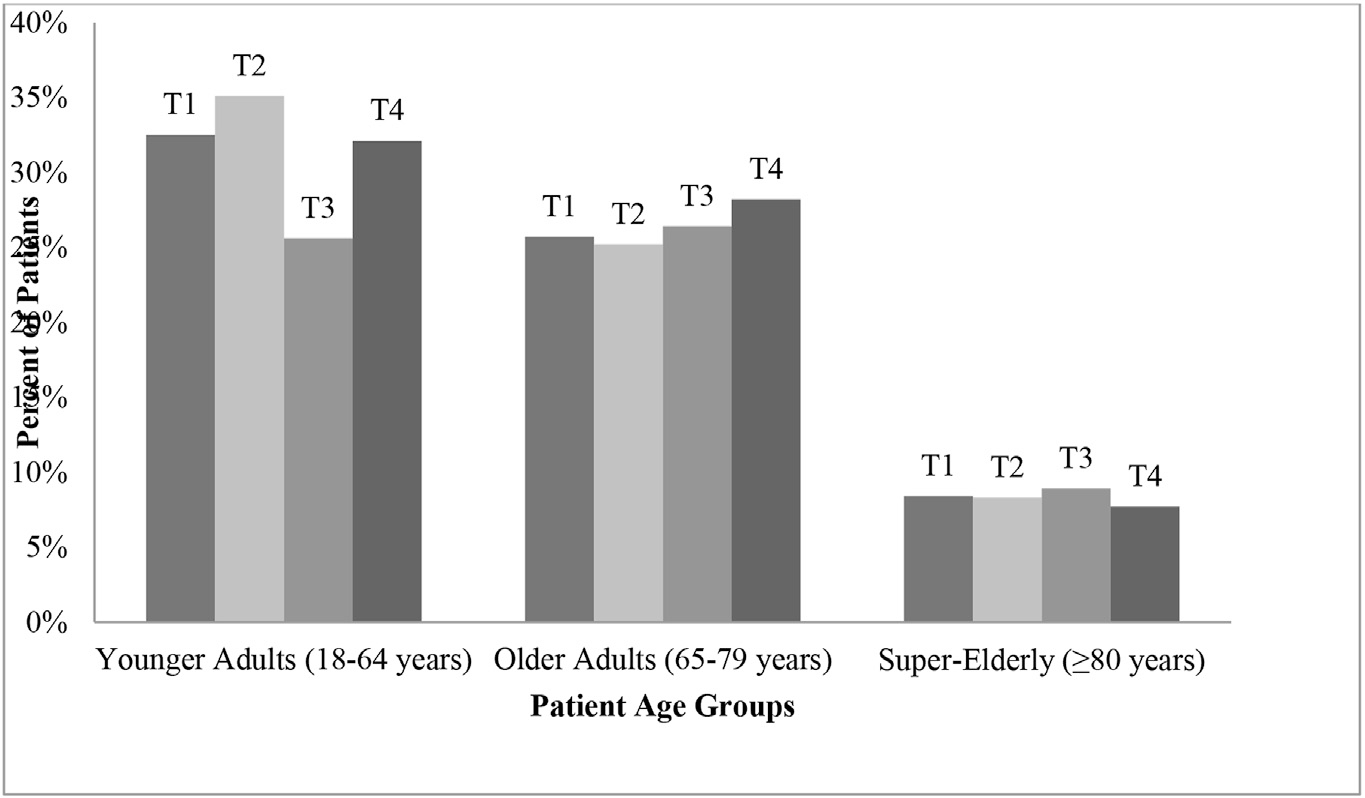
Distribution of tumor stage among each patient age group.
Of the 229 deaths that occurred over the study period, 44.1%, 41.0%, and 14.8% occurred in younger adults, older adults, and the super-elderly (p = 0.000). Overall survival at 5 years was 90.3%, 76.8%, and 57.8% for younger adults, older adults, and super-elderly, respectively (p = 0.000). Overall survival at 10-years was 89.9%, 74.7%, and 56.4% for younger adults, older adults, and super-elderly, respectively (p = 0.000). Of the 109 deaths attributable to MTC, 6.0%, 8.9%, and 20.5% occurred in younger, older, and super-elderly groups, respectively (p = 0.000). Disease-specific survival at 10-years was 90.5%, 81.8%, and 61.6% among younger, older, and super-elderly groups, respectively (p = 0.000).
Disease-specific survival analysis
On univariable analysis, DSM was associated with advancing age, ‘other’ race, male gender, T3 and T4 disease, lymph node and distant metastases, and non-operative management (Table 3, Fig. 2). Older age was associated with a higher DSM when adjusted for surgical intervention and for each T stage (Figs. 3 and 4).
Table 3.
Factors associated with disease-specific mortality on univariable analysis.
| Hazard Ratio | 95% CI | P-value | ||
|---|---|---|---|---|
|
| ||||
| Age | ||||
| <65 | Ref | |||
| 65–79 | 1.787 | 1.166 | 2.740 | 0.008 |
| 80+ | 4.801 | 2.754 | 8.372 | 0.000 |
| Race | ||||
| White | Ref | |||
| Black | 1.225 | 0.656 | 2.286 | 0.525 |
| Other | 0.131 | 0.182 | 0.936 | 0.043 |
| Gender | ||||
| Female | Ref | |||
| Male | 2.279 | 1.552 | 3.345 | 0.000 |
| Ethnicity | ||||
| Non-Hispanic | Ref | |||
| Hispanic | 0.800 | 0.439 | 1.459 | 0.467 |
| Multifocal | ||||
| No | Ref | |||
| Yes | 1.220 | 0.915 | 1.628 | 0.175 |
| Diagnosis Period | ||||
| 2004 – 2006 | Ref | |||
| 2007 – 2009 | 0.796 | 0.478 | 1.325 | 0.381 |
| 2010 – 2012 | 1.058 | 0.620 | 1.803 | 0.836 |
| 2013 – 2015 | 1.033 | 0.516 | 2.071 | 0.927 |
| Tumor Size | ||||
| T1 | Ref | |||
| T2 | 1.787 | 0.900 | 3.547 | 0.097 |
| T3 | 4.053 | 2.159 | 7.610 | 0.000 |
| T4 | 12.336 | 6.525 | 23.323 | 0.000 |
| Nodal Status | ||||
| N0 | Ref | |||
| N1a | 3.636 | 1.980 | 6.676 | 0.000 |
| N1b | 6.157 | 3.827 | 9.905 | 0.000 |
| Metastasis | ||||
| M0 | Ref | |||
| M1 | 19.716 | 13.449 | 28.903 | 0.000 |
| Surgical Intervention | ||||
| Total Thyroidectomy | Ref | |||
| Lobectomy | 0.482 | 0.152 | 1.526 | 0.215 |
| No surgery | 11.092 | 6.947 | 17.711 | 0.000 |
| Lymph Nodes Removed | ||||
| None | Ref | |||
| 1–3 | 1.356 | 0.727 | 2.528 | 0.338 |
| 4 + | 0.985 | 0.623 | 1.559 | 0.950 |
| Other | 1.327 | 0.463 | 3.804 | 0.598 |
Fig. 2.
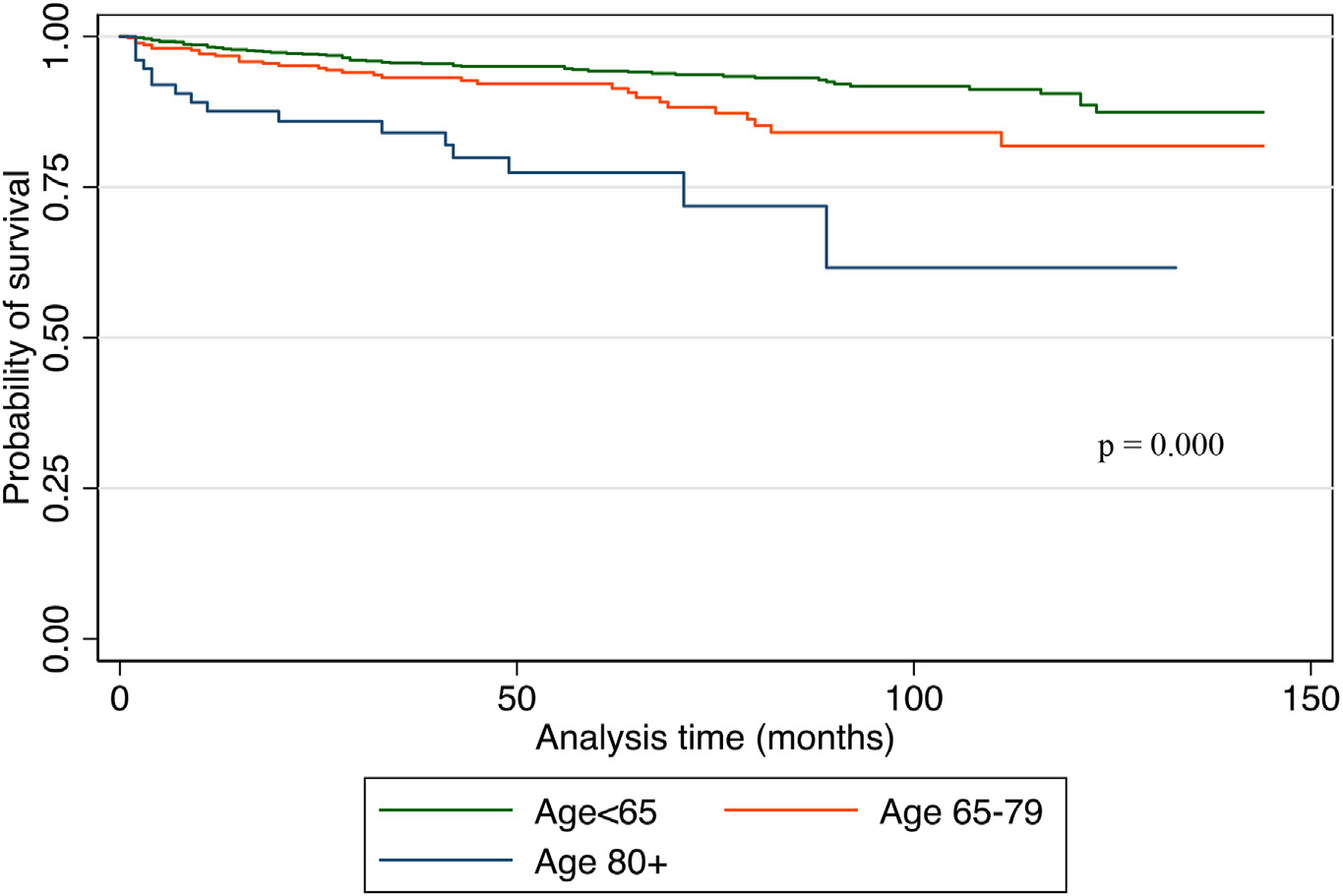
Kaplan Meier curve for disease-specific survival among three age categories.
Fig. 3.
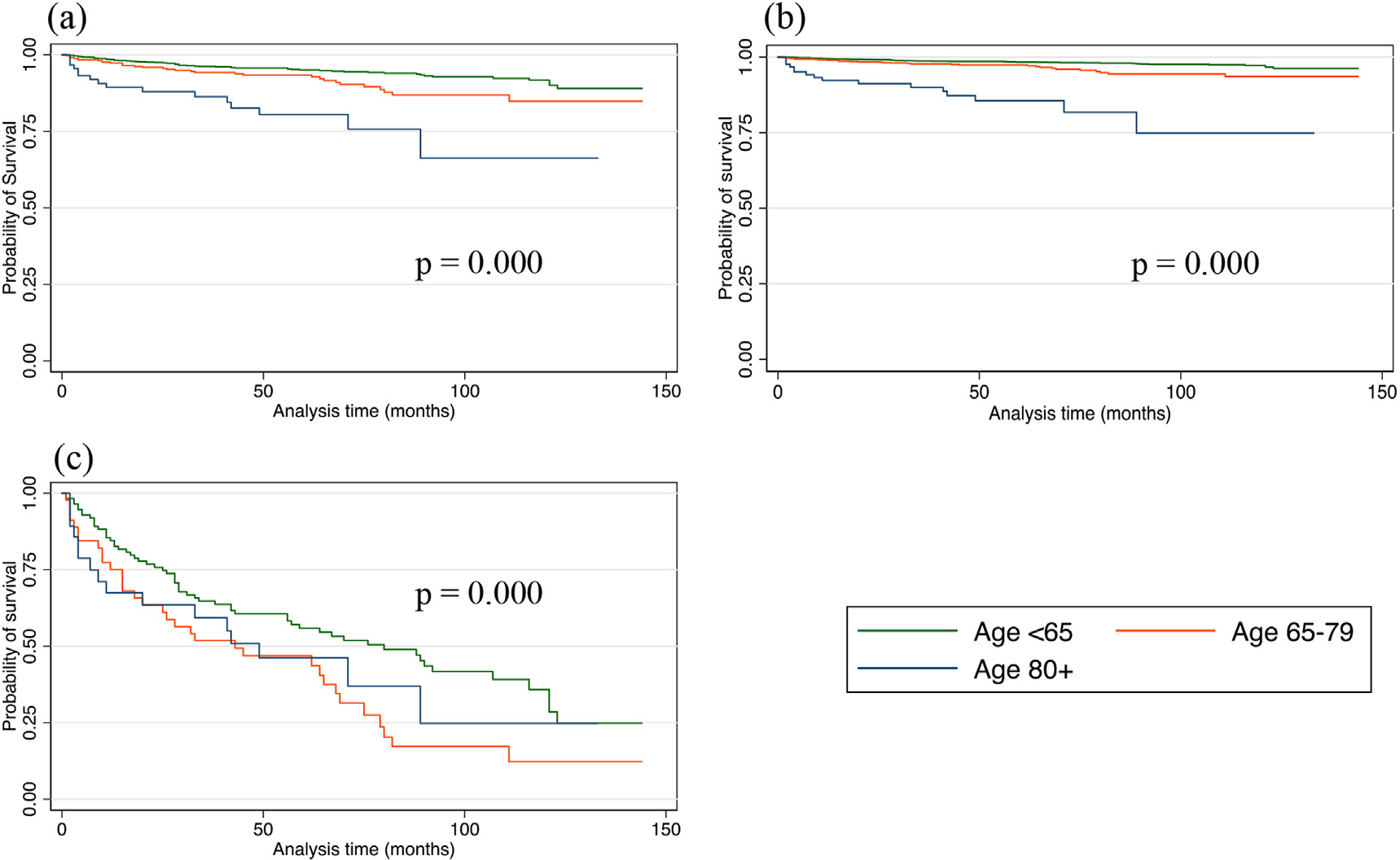
Kaplan Meier disease-specific survival among three age categories (a) after total thyroidectomy (b) after lobectomy (c) after non-operative management.
Fig. 4.
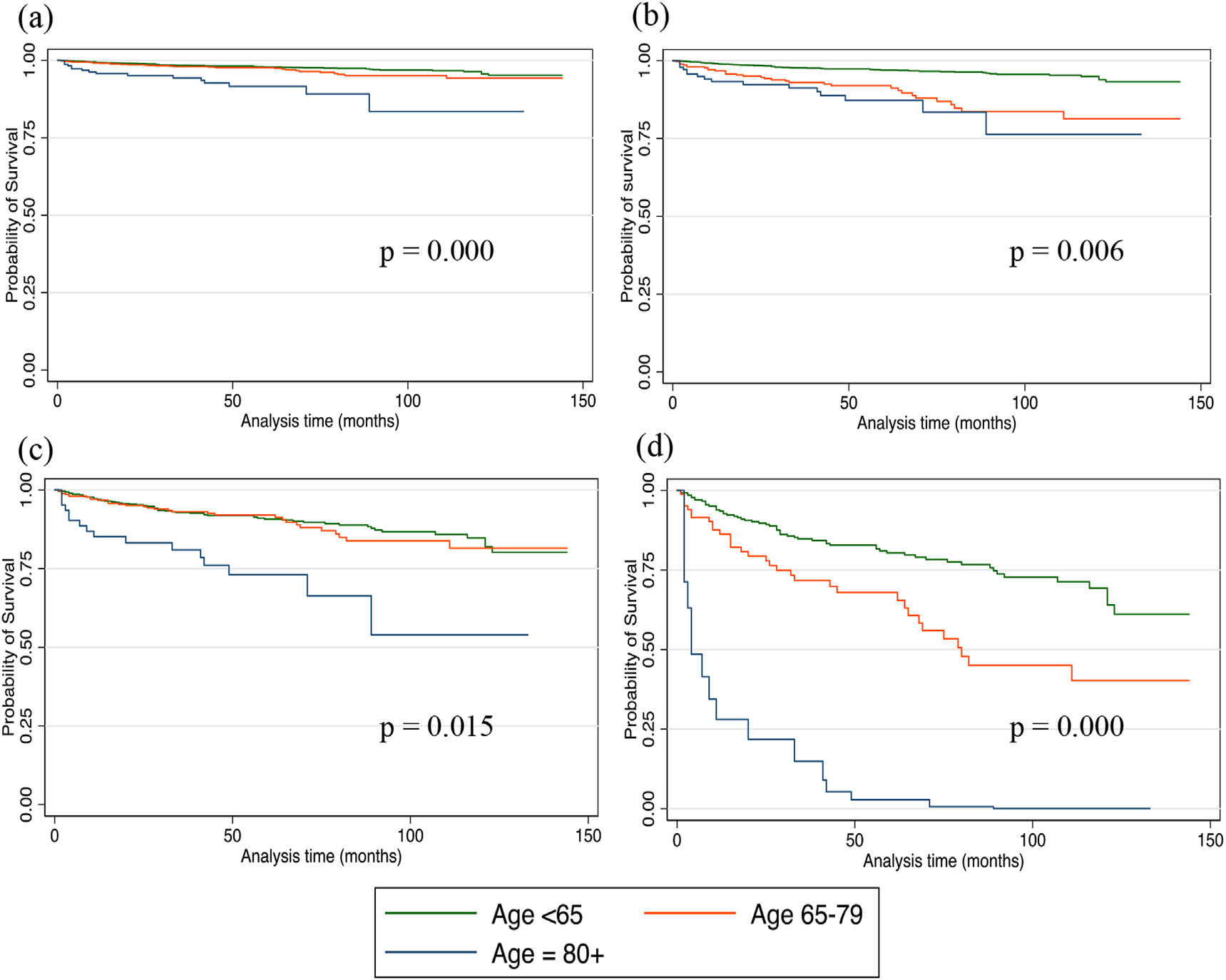
Kaplan Meier disease-specific survival among three age categories who presented with stage (a) T1 (b) T2 (c) T3 and (d) T4 disease.
On multivariable analysis (Table 4), older age (65–79 years HR = 2.908, 95%CI: 1.828–4.626, p = 0.000; ≥80 years HR = 6.709, 95%CI: 3.688–12.203, p = 0.000), African American race (HR = 2.182, 95%CI: 1.129–4.219, p = 0.020), T3 and T4 disease (T3 HR = 2.044, 95%CI: 1.047–3.992; p = 0.000 and T4 HR = 3.676, 95% CI: 1.798–7.516; p = 0.000), lymph node metastases (N1a HR = 3.138, 95%CI: 1.556–6.332, p = 0.001; N1b HR = 3.570, 95%CI: 1.914–6.659, p = 0.000), distant metastasis (HR = 19.716, 95%CI: 13.449–28.903, p = 0.000), and non-operative management (HR = 2.603, 95%CI: 1.131–5.995, p = 0.024) were all independently associated with worse DSM. Therefore, even after controlling for disease stage and management of MTC, older age was an independent prognostic factor for worse DSM. Gender, extent of lymph node dissection, extent of thyroidectomy, and Hispanic ethnicity were not significantly associated with DSM (Table 4).
Table 4.
Factors associated with disease-specific mortality on multivariable analysis.
| Hazard Ratio | 95% CI | P-value | ||
|---|---|---|---|---|
|
| ||||
| Age | ||||
| <65 | Ref | |||
| 65–79 | 2.908 | 1.828 | 4.626 | 0.000 |
| 80+ | 6.709 | 3.688 | 12.203 | 0.000 |
| Race | ||||
| White | Ref | |||
| Black | 2.182 | 1.129 | 4.219 | 0.020 |
| Other | 0.133 | 0.018 | 0.962 | 0.046 |
| Gender | ||||
| Female | Ref | |||
| Male | 1.028 | 0.677 | 1.562 | 0.896 |
| Ethnicity | ||||
| Non-Hispanic | Ref | |||
| Hispanic | 0.957 | 0.514 | 1.786 | 0.892 |
| Tumor Size | ||||
| T1 | Ref | |||
| T2 | 1.758 | 0.860 | 3.593 | 0.122 |
| T3 | 2.044 | 1.047 | 3.992 | 0.036 |
| T4 | 3.676 | 1.798 | 7.516 | 0.000 |
| Nodal Stage | ||||
| N0 | Ref | |||
| N1a | 3.138 | 1.556 | 6.332 | 0.001 |
| N1b | 3.570 | 1.914 | 6.659 | 0.000 |
| Metastasis | ||||
| M0 | Ref | |||
| M1 | 9.324 | 5.703 | 15.247 | 0.000 |
| Surgical Intervention | ||||
| Total Thyroidectomy | Ref | |||
| Lobectomy | 0.724 | 0.219 | 2.399 | 0.598 |
| No surgery | 2.603 | 1.131 | 5.995 | 0.024 |
| Lymph Nodes Removed | ||||
| None | Ref | |||
| 1–3 | 1.396 | 0.595 | 3.273 | 0.443 |
| 4 + | 0.864 | 0.380 | 1.966 | 0.727 |
| Other | 0.335 | 0.105 | 1.069 | 0.065 |
Discussion
In summary, our study represents one of the largest population-based studies over a 10-year period demonstrating an association between increased age (≥65 years) and increased DSM regardless of disease stage or surgical management of MTC. Among our cohort of 1457 patients, 109 (7.48%) patients died due to MTC. DSM was 2.9 and 6.7 times higher among patients 65–79 years and ≥80 years, respectively. Moreover, African American race was associated with increased DSM. Other factors such as non-operative management, T3 and T4 staging, and lymph node and distant metastasis were also associated with increased DSM.
Our results are in agreement with previously published studies showing an association between age and DSM among MTC patients.18–21 However, previously published nomograms for MTC that predict DSM did not take into account extent of surgical management.18,19 A 2015 retrospective single institution study by Ho et al. designed a predictive nomogram for MTC DSM using age, gender, postoperative calcitonin, perivascular invasion, pathologic T status, pathologic N status, and M status.18 After M status, age had the highest hazard ratio (1.71) for DSM. Moreover, Qu et al. proposed an age cutoff of 45 and 69 years to further classify MTC patients into three risk groups to be used with sex, race, and conventional AJCC TNM status to identify highrisk patients.19 Patient age 50–69 years and ≥70 years were significantly associated with increased DSM (HR: 2.853 and 5.804, respectively; p = 0.001). In contrast to our results, a 2006 retrospective single institutional study by De Groot et al. reported extrathyroidal extension and stage at diagnosis were the only independent predictors of MTC survival.22 However, a small patient cohort of 120 limited the study.
The alternate implication of our findings is that prognosis in older adults and super-elderly is worse because they present with more aggressive disease or that a significantly larger proportion of younger patients present with familial disease. Numerous studies have found more favorable outcomes among familial MTC patients when compared to those with sporadic MTC.23–25 Since sporadic MTC has a worse prognosis compared with hereditary MTCs, we would expect that those that are younger in age with MTC would be associated with a lower mortality due to the association familial MTCs. This is thought to be partly due to increased screening and, thus, earlier diagnosis among familial cases of MTC. Among our cohort, the T stage distribution varied with age group with older adults presenting more frequently with T1 and T4 disease. Younger adults had the highest proportion of T2 disease. Older and super-elderly groups presented more frequently with T3 disease. Despite presenting with more advanced stages (T3–4), older age was independently associated with increased DSM within each T stage.
We also observed a significant difference in extent of surgical management among the three age groups. Younger patients underwent more total thyroidectomy and lymphadenectomy than the super-elderly. This may be related to greater proportion of diagnostic lobectomies performed for patients with an indeterminate pre-operative biopsy (Bethesda III/IV) and on final pathology were discovered to have MTC. Therefore, the appropriate operation would not have been performed at the index operation as the MTC diagnosis was not available. This variation in surgical management did not affect DSM. However, we were unable to prove this association using the SEER data due to the lack of pre-operative biopsy results. It is also possible older patients may have been offered less than the recommended treatment due to age or co-morbidities, however, this dataset does not provide that level of granularity.
There are several limitations in this study in addition to those inherent in retrospective database studies including coding errors, missing values, confounding by indication, and selection bias into the database. The SEER database lacks information on relevant risk factors including family history of thyroid cancer, history of radiation exposure, and mutational or RET status. It also lacks information regarding receipt of chemotherapy post-operatively, follow up methods, development of recurrence, socioeconomic status, or recognized biochemical variables such as CEA or calcitonin levels. Elevated post-operative calcitonin levels have been associated with increased DSM after adjusting for age.18,25 However, a study by De Groot et al. found no association between persistent biochemical MTC and disease-specific survival.22 Calcitonin doubling time of less than one year was associated with a worse recurrence free survival independent of age. Our analysis was unable to adjust for calcitonin levels or doubling time. The description of the extent and type of lymph node dissection among patients with MTC was also not clearly delineated in this database. This information was inferred from nodal staging and reported number of lymph nodes removed, which was categorized into specific ranges in the SEER database. Furthermore, the database does not have information regarding patient comorbidities in order to determine frailty or comorbidity scores and whether MTC is familial or sporadic. However, most familial cases would be expected to present prior to age 65. We limited our analysis to a modern cohort of patients diagnosed between 2004 and 2015 because the presentation and treatment of MTC have changed over time and survival has improved.
Despite these limitations, the results presented in this paper illustrate that regardless of disease stage or surgical management of MTC, DSM is increased among older patients. Due to MTC’s low incidence and lack of prospective studies, there is a paucity of convincing evidence to demonstrate a significant correlation between prognostic indicators and DSM. Moreover, our results also show an increased DSM among African American patients. Additional prospective studies are needed to validate these findings and identify risk factors such as frailty, comorbidity scores, and calcitonin levels and doubling time to evaluate the association with DSM. This will allow clinicians to improve oncologic patient counselling among this patient population. Identifying this highrisk patient population can potentially help with determining the need for increased frequency of follow-up and imaging.
Conclusion
Increased age (≥65 years) is an independent predictor of increased DSM regardless of disease stage or surgical management of MTC. Additional prospective studies are needed to understand the true association between age and DSM among adults with MTC.
Funding source
NIH K23 AG053429 (Corresponding Author).
Footnotes
Declaration of competing interest
No potential or real conflicts to report.
Acute acalculous cholecystitis (AAC) constitutes 5–10% of all cases of cholecystitis in adults, and is even less common in children. The recent literature has described an association between primary Epstein-Barr virus (EBV) infection and AAC, however, it still remains an uncommon presentation of the infection. Most authors advise that the management of AAC in patients with primary EBV infection should be supportive, since the use of antibiotics does not seem to alter the severity or prognosis of the illness. Furthermore, surgical intervention has not been described as necessary or indicated in the management of uncomplicated AAC associated with EBV infection. We report a case of a 16-year-old Lebanese girl with AAC associated with primary EBV infection. She presented to the emergency department, with high-grade fever, fatigue, vomiting and abdominal pain. Liver enzymes were elevated with a cholestatic pattern, and imaging confirmed the diagnosis of AAC. She was admitted to the regular floor, and initial management was conservative. Owing to persistence of fever, antibiotics were initiated on day 3 of admission. She had a smooth clinical course and was discharged home after a total of 9 days, with no complications.Acute acalculous cholecystitis (AAC) constitutes 5–10% of all cases of cholecystitis in adults, and is even less common in children. The recent literature has described an association between primary Epstein-Barr virus (EBV) infection and AAC, however, it still remains an uncommon presentation of the infection. Most authors advise that the management of AAC in patients with primary EBV infection should be supportive, since the use of antibiotics does not seem to alter the severity or prognosis of the illness. Furthermore, surgical intervention has not been described as necessary or indicated in the management of uncomplicated AAC associated with EBV infection. We report a case of a 16-year-old Lebanese girl with AAC associated with primary EBV infection. She presented to the emergency department, with high-grade fever, fatigue, vomiting and abdominal pain. Liver enzymes were elevated with a cholestatic pattern, and imaging confirmed the diagnosis of AAC. She was admitted to the regular floor, and initial management was conservative. Owing to persistence of fever, antibiotics were initiated on day 3 of admission. She had a smooth clinical course and was discharged home after a total of 9 days, with no complications.
References
- 1.Cancer Stat Facts. Thyroid cancer. National cancer Institute; 2020. Available from: https://seer.cancer.gov/statfacts/html/thyro.html; 2020. Accessed March 18, 2020. [Google Scholar]
- 2.Randle RW, Balentine CJ, Leverson GE, et al. Trends in the presentation, treatment, and survival of patients with medullary thyroid cancer over the past 30 years. Surgery. 2017;161(1):137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roman S, Lin R, Sosa JA. Prognosis of medullary thyroid carcinoma: demographic, clinical, and pathologic predictors of survival in 1252 cases. Cancer: Interdisciplinary International Journal of the American Cancer Society. 2006;107(9):2134–2142. [DOI] [PubMed] [Google Scholar]
- 4.Wells SA Jr, Asa SL, Dralle H, et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma: the American Thyroid Association Guidelines Task Force on medullary thyroid carcinoma. Thyroid. 2015;25(6):567–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Panigrahi B, Roman SA, Sosa JA. Medullary thyroid cancer: are practice patterns in the United States discordant from American Thyroid Association guidelines? Ann Surg Oncol. 2010;17(6):1490–1498. [DOI] [PubMed] [Google Scholar]
- 6.Ortman JM, Velkoff VA, Hogan H. An Aging Nation: The Older Population in the United States. Washington, DC: US Census Bureau; 2014:25–1140. [Google Scholar]
- 7.Lang BH-H, Lo C-Y. Technological innovations in surgical approach for thyroid cancer. Journal of oncology. 2010:2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christmas C, Makary MA, Burton JR. Medical considerations in older surgical patients. J Am Coll Surg. 2006;203(5):746–751. [DOI] [PubMed] [Google Scholar]
- 9.Kowal P, Dowd JE. Definition of an Older Person. Proposed Working Definition of an Older Person in Africa for the MDS Project. Geneva: World Health Organization; 2001. 10.13140/2.1.5188.9286. [DOI] [Google Scholar]
- 10.Biliotti G, Martini F, Vezzosi V, et al. Specific features of differentiated thyroid carcinoma in patients over 70 years of age. J Surg Oncol. 2006;93(3):194–198. [DOI] [PubMed] [Google Scholar]
- 11.Park HS, Roman SA, Sosa JA. Treatment patterns of aging Americans with differentiated thyroid cancer. Cancer. 2010;116(1):20–30. [DOI] [PubMed] [Google Scholar]
- 12.Ganly I, Nixon IJ, Wang LY, et al. Survival from differentiated thyroid cancer: what has age got to do with it? Thyroid. 2015;25(10):1106–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan H, Winchester DJ, Prinz RA, et al. Differences in the impact of age on mortality in well-differentiated thyroid cancer. Ann Surg Oncol. 2018;25(11):3193–3199. [DOI] [PubMed] [Google Scholar]
- 14.Institute NC. [4/25/2018]. Available from: http://seer.cancer.gov/data. [Google Scholar]
- 15.Park HS, Roman SA, Sosa JA. Treatment patterns of aging Americans with differentiated thyroid cancer. Cancer: Interdisciplinary International Journal of the American Cancer Society. 2010;116(1):20–30. [DOI] [PubMed] [Google Scholar]
- 16.Sutton W, Canner JK, Segev DL, et al. Treatment variation in older adults with differentiated thyroid cancer. J Surg Res. 2020;254:154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grogan RH, Mitmaker EJ, Hwang J, et al. A population-based prospective cohort study of complications after thyroidectomy in the elderly. J Clin Endocrinol. 2012;97(5):1645–1653. [DOI] [PubMed] [Google Scholar]
- 18.Ho AS, Wang L, Palmer FL, et al. Postoperative nomogram for predicting cancer-specific mortality in medullary thyroid cancer. Ann Surg Oncol. 2015;22(8):2700–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qu N, Shi RL, Luo TX, et al. Prognostic significance and optimal cutoff of age in medullary thyroid cancer. Oncotarget. 2016;7(13):15937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esfandiari NH, Hughes DT, Yin H, et al. The effect of extent of surgery and number of lymph node metastases on overall survival in patients with medullary thyroid cancer. J Clin Endocrinol Metabol. 2014;99(2):448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hyer S, Vini L, Harmer C. Medullary thyroid cancer: multivariate analysis of prognostic factors influencing survival. Eur J Surg Oncol. 2000;26(7):686–690. [DOI] [PubMed] [Google Scholar]
- 22.De Groot JWB, Plukker JT, Wolffenbuttel BH, et al. Determinants of life expectancy in medullary thyroid cancer: age does not matter. Clin Endocrinol. 2006;65(6):729–736. [DOI] [PubMed] [Google Scholar]
- 23.Saltiki K, Simeakis G, Anagnostou E, et al. Different outcomes in sporadic versus familial medullary thyroid cancer. Head Neck. 2019;41(1):154–161. [DOI] [PubMed] [Google Scholar]
- 24.Elisei R, Cosci B, Romei C, et al. Prognostic significance of somatic RET oncogene mutations in sporadic medullary thyroid cancer: a 10-year follow-up study. J Clin Endocrinol Metabol. 2008;93(3):682–687. [DOI] [PubMed] [Google Scholar]
- 25.Machens A, Hofmann C, Hauptmann S, Dralle H. Locoregional recurrence and death from medullary thyroid carcinoma in a contemporaneous series: 5-year results. Eur J Endocrinol. 2007;157(1):85–93. [DOI] [PubMed] [Google Scholar]


