Abstract
Background:
The gut microbiota potentially plays an important role in the immunologic education of the host during early infancy.
Objective:
We sought to determine how the infant gut microbiota evolve during infancy, particularly in relation to hygiene-related environmental factors, atopic disorders, and a randomized introduction of allergenic solids.
Methods:
A total of 1303 exclusively breast-fed infants were enrolled in a dietary randomized controlled trial (Enquiring About Tolerance study) from 3 months of age. In this nested longitudinal study, fecal samples were collected at baseline, with additional sampling of selected cases and controls at 6 and 12 months to study the evolution of their gut microbiota, using 16S ribosomal RNA gene-targeted amplicon sequencing.
Results:
In the 288 baseline samples from exclusively breast-fed infant at 3 months, the gut microbiota was highly heterogeneous, forming 3 distinct clusters: Bifidobacterium-rich, Bacteroides-rich, and Escherichia/Shigella-rich. Mode of delivery was the major discriminating factor. Increased Clostridium sensu stricto relative abundance at 3 months was associated with presence of atopic dermatitis on examination at age 3 and 12 months. From the selected cases and controls with longitudinal samples (n = 70), transition to Bacteroides-rich communities and influx of adult-specific microbes were observed during the first year of life. The introduction of allergenic solids promoted a significant increase in Shannon diversity and representation of specific microbes, such as genera belonging to Prevotellaceae and Proteobacteria (eg, Escherichia/Shigella), as compared with infants recommended to exclusively breast-feed.
Conclusions:
Specific gut microbiota characteristics of samples from 3-month-old breast-fed infants were associated with cesarean birth, and greater Clostridium sensu stricto abundance was associated with atopic dermatitis. The randomized introduction of allergenic solids from age 3 months alongside breast-feeding was associated with differential dynamics of maturation of the gut microbial communities.
Keywords: Atopic dermatitis, bacteria, diet, environment, food, microbiome, colonization, tolerance
Graphical Abstract
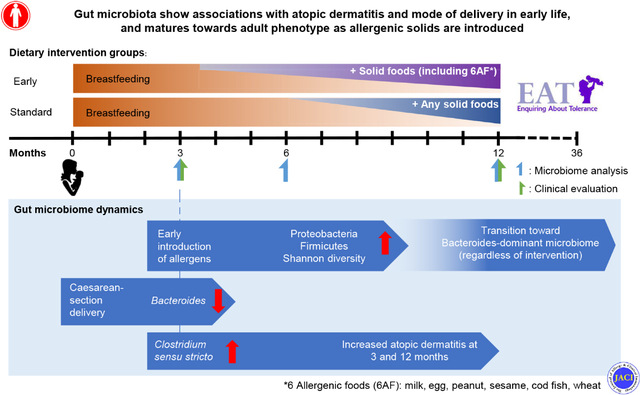
Patterns of gut microbiota acquisition during infancy, especially during the first months, are highly dynamic and associated with the development of various conditions in later life, such as atopic diseases.1–4 Putative mechanisms for how early colonization patterns may influence health and disease are currently subjects of intense research. Studies have shown that microbial colonization is an indispensable process for immune maturation and organ development. Major events—such as birth and mode of delivery, feeding patterns, and antibiotic usage—have been shown to affect the early microbiota acquisition.5–11 Although many studies have been conducted to explore the effects of environmental factors on gut microbiome development, most of them are retrospective and observational, therefore, challenged by mixed confounders.
Mode of delivery has been identified as a key exposure affecting successional microbiota development during the neonatal period, because it determines colonizing microorganisms that are shared from mother to newborn and may also influence gut microbiota characteristics.5–7,12 It has been commonly reported that cesarean-delivered infants harbor less Bacteroides and more hospital-associated pathobionts, such as Enterococcus spp. and Klebsiella spp. An infant’s diet may also influence its gut microbiota. Several reports have highlighted gut microbiota differences between breast-fed and formula-fed infants.8,13,14 Two small uncontrolled studies have described an increase in infants’ gut microbiota complexity when dietary solids are introduced,15,16 and a nonrandomized study of 98 maternal-infant dyads observed that cessation of breast-feeding was associated with maturation into an adult-like microbiota.8
Here, we investigated a nested cohort of infants undergoing randomized introduction of allergenic solids as part of a randomized controlled trial to prevent food allergy. This study provided an opportunity to independently evaluate the impact of dietary solid food introduction alongside ascertaining associations between other factors including mode of delivery and environmental exposures on the stability and maturation of the gut microbiota, and in turn the risk of host infants developing atopic disorders.17,18
METHODS
The Enquiring About Tolerance cohort
In the Enquiring About Tolerance (EAT) study, 1303 exclusively breast-fed, healthy infants from England and Wales, aged between 12 and 17 weeks, were enrolled into a randomized controlled trial to examine whether the regular consumption of allergenic solids reduced the prevalence of food allergy when compared with continued exclusive breast-feeding up to 6 months of age (ISRCTN: 14254740).17 Participants were randomly assigned to either the regular consumption of 6 allergenic foods (boiled hen’s egg, peanut, cow’s milk [yogurt], wheat, white fish, and sesame) from 3 months twice weekly alongside continued breast-feeding (early introduction group) or exclusive breast-feeding until 6 months (standard introduction group). Beyond 6 months, food consumption was at parental discretion. Food specific IgE level to each of the 6 foods was measured at enrollment and at age 1 and 3 years in both groups using ImmunoCap (Phadia) assays (cutoff ≥0.35 kU/L to determine food sensitization). The primary outcome of the original EAT study was the prevalence of challenge-proven food allergy to 1 or more of the 6 study foods between age 1 and 3 years. Parents completed online questionnaires eliciting exposure data, such as mode of delivery, sibship size, pet ownership and antibiotic usage.
All infants were examined for atopic dermatitis (AD) at their enrollment visit at 3 months and 12 months of age, using the UK diagnostic criteria–based photographic protocol of the International Study of Asthma and Allergies in Childhood Phase Two.19 AD severity was determined by the SCORing Atopic Dermatitis index.20 Skin barrier function was assessed by measuring transepidermal water loss using the Biox Aquaflux AF200 (Biox, London, UK) closed condenser chamber device on the unaffected skin of the left volar forearm. Venous blood samples were screened for the 6 commonest FLG mutations (TaqMan allelic discrimination assays for mutations R501X, 2282del4, R2447X, and S3247X; ABI 7900 HT; Applied Biosystems, Foster City, Calif) or by sizing of fluorescent PCR products on an Applied Biosystems 3130 DNA sequencer (mutations 3673delC and 3702delG). These 6 mutations detect 99% of FLG mutation carriers in the UK population.
Ethical approval for the EAT study was provided by the St Thomas’ Hospital Research Ethics Committee (Reference 08/H0802/93), and informed consent was obtained from the parents of all children enrolled in the study. All sequencing data were deposited and are available at the National Center for Biotechnology Information (NCBI) Sequence Read Archive under BioProject number PRJNA597342.
Gut microbiota sample collection, DNA extraction and sequencing
Beginning October 1, 2011, consecutively enrolling families (n = 359) were asked to provide stool samples (see Fig E1 in this article’s Online Repository at www.jacionline.org). Stool samples were collected from 288 of enrolled participants, with follow-up samples requested at age 6 and 12 months. Stool samples were retrieved from diapers and transferred to the King’s College London Molecular Microbiology Research Laboratory for storage at −80°C until DNA extraction. The cell lysis and DNA extraction protocol used both phenol-chloroform liquid-liquid phase separation and bead-beating, followed by −8°C storage (see this article’s Methods section in the Online Repository at www.jacionline.org). To evaluate the assembly of gut microbial communities during infancy, a total of 428 samples were sequenced (of the total 288 subjects providing baseline samples, 218 participants provided baseline samples only, and a subset of 70 individuals provided samples from multiple time points of 3, 6, and 12 months). The subsets of cases and controls (42 in the standard introduction group and 28 in the early introduction group) were selected on the basis of intervention assignment and clinical atopic diagnoses (either challenge-proven food allergy or AD on examination). DNA extracts were amplified using primers targeting the hypervariable V4 (515F-806R) region of the 16S ribosomal RNA gene and performed on the MiSeq instrument.21
Sequence analysis
The open-source software mothur22 and DADA223 were used to process the raw sequencing data (see this article’s Online Repository at www.jacionline.org). Reads were aligned and classified down to the genus level with ribosomal database project naive Bayesian classifier24 or species level with Greengenes database v13.8.25 Operational taxonomic units were defined at 97% similarity using the opticlust algorithm.26 We also undertook comparative analyses with 977 samples from the TwinsUK (adults from the same UK region),27,28 which were sequenced using the same primers (accession no. ERP006339 and ERP006342; 9 samples were removed during preprocessing), and TEDDY (large, multicenter infant cohort)11 studies. The Environmental Determinants of Diabetes in the Young (TEDDY) data set samples were selected on the basis of (1) exclusive breast-feeding before solid food introduction and (2) longitudinal data (≥ 2 samples before age 6 months) to permit comparable analyses.
Statistical analysis
The beta-diversity and linear discriminant statistical analyses were performed using R software, with libraries including vegan, ape, and bios2mds. Thetayc distance (theta) metric was used to measure the distance between communities (beta diversity),29 and principal-coordinate analysis (PCoA) was performed on the basis of theta distance, unless otherwise indicated. Significance of differences between groups was tested by various methods, including Wilcoxon rank-sum, Kruskal-Wallis, and the analysis of molecular variance.30 Covariates of community variation were evaluated by calculating correlation between indicated PCoA ordination and metadata or genus abundance (envfit function in vegan R package; 10,000 permutations with Bonferroni correction).
RESULTS
Of enrolled families, 80.2% (288 of 359) contributed baseline samples, a subset of which (n = 70) also contributed follow-up samples. Within the subset, cases were composed of 12 participants with allergies to egg (1 additionally allergic to peanut), 1 milk allergy, and 1 codfish allergy, and 13 with AD (SCORing Atopic Dermatitis index more than 15 at either 3 or 12 months).
Exclusively breast-fed infants’ gut microbial communities form 3 distinct clusters
To understand the landscape of the gut microbiome during breast-feeding, we analyzed baseline (3-month) samples (n = 288, and a mean age of 105 days and interquartile range 100–112 days). The most abundant genera established in the microbial communities at 3 months were Bifidobacterium (median abundance [MA], 38.75%), Bacteroides (MA, 5.43%), and multiple genera belonging to Firmicutes and Proteobacteria (Fig 1, A and B). The gut microbial communities of exclusively breast-fed 3-month-old infants were diverse, in examining the PCoA with theta distance29 and Bray-Curtis distance based on genus-level classification (Fig 1, A; see Fig E2, A, in this article’s Online Repository at www.jacionline.org). k-means clustering (see Fig E2, A and B) revealed that the microbial communities of exclusively breast-fed infants clustered into 3 groups, in which cluster number 1 was the Bifidobacterium-rich cluster, cluster number 2 was the Bacteroides-rich cluster, and cluster number 3 was predominated by Escherichia/Shigella and genera belonging to Firmicutes phylum (Fig 1, A and B). Although minor genera, such as Streptococcus, Dorea, and Rothia, also contributed to the community variation, the 3 major genera were sufficient to reproduce clustering, underscoring their importance in community variation (Fig 1, C). Community diversity was lower in cluster 1 than in clusters 2 and 3 (alpha diversity; Shannon index, P < .005; Wilcoxon rank-sum test) due to the predominance of Bifidobacterium (Fig 1, D). Of note, the age of participants did not have a significant effect on clustering (see Fig E2, C; P >.05). PCoA and k-means clustering based on theta distance calculated from species-level (see Fig E2, D) and de novo operational taxonomic unit (97% similarity) abundance (see Fig E2, E) and on Bray-Curtis distance (see Fig E2, A) were highly comparable to the genus-based clustering (Fig 1, B).
FIG 1.
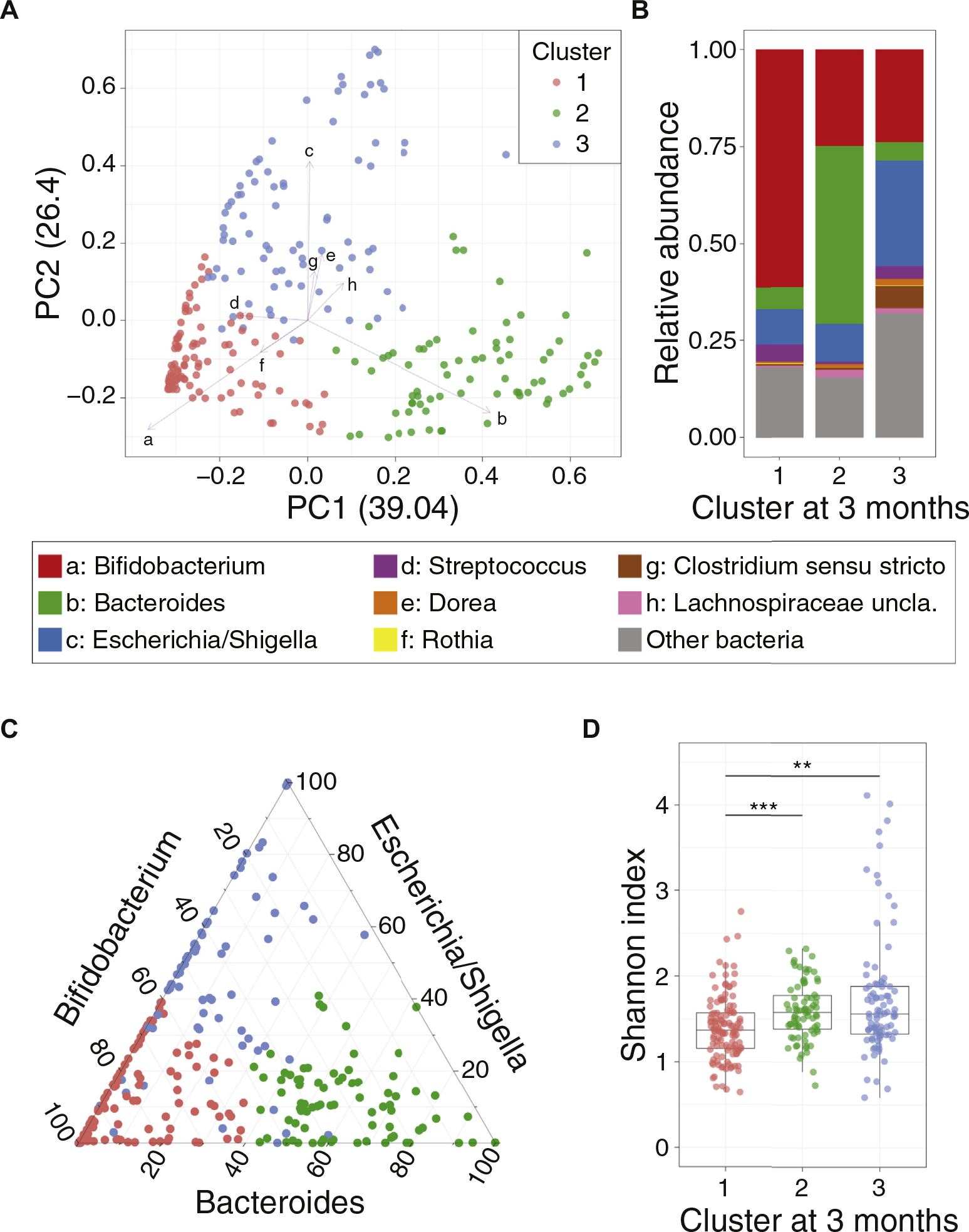
Gut microbiota characteristics at age 3 months. A, PCoA of the gut microbiome at 3 months. Pair-wise distances (theta distance) among all samples were calculated and 2 major axes (PC1 and PC2) from the multidimensional distance space were calculated and depicted on a scatter plot. Colors indicate different clusters, according to k-means clustering. Arrows and letters indicate specific genera significantly correlated with PCoA ordination (P < .05, lengths of arrows are proportion to R2, calculated from EnvFit function from R). Corresponding genera for letters are showing on the right panel of B. B, Averaged stacked bar chart of infants’ microbiome within each cluster. Genera that are significantly correlated with PCoA ordination are depicted as colored bar (legend on right) and others are merged as “Other bacteria.” C, Triangle plot showing relative abundances of the 3 key genera (Bifidobacterium, Bacteroides, and Escherichia/Shigella) in the gut microbiota of baseline samples. The colors indicate clusters (same as Fig 1, A). D, Boxplot of bacterial community diversity (Shannon index) differences according to microbiome community clusters. Shannon diversity of cluster 1 community is significantly lower than those of clusters 2 and 3. (**P < .01, ***P < .001; Wilcoxon rank-sum test, after Kruskal-wallis test)
Infants’ gut microbial communities mature in first year
Data from 6- and 12-month samples (n = 70 samples per each time point; see Methods) were superimposed onto the existing 3-month PCoA coordinates (see Fig E3, A, in this article’s Online Repository at www.jacionline.org). Microbiota from 6-month-old infants was similarly dispersed as 3-month communities, whereas 12-month samples were exclusively overlaid on cluster 2, indicating that gut microbial communities mature toward Bacteroides-rich communities at age 1 year, in keeping with the previous literature.7,10 Interpersonal similarity increased during aging (see Fig E3, B) due to the convergence to Bacteroides-rich communities. We then investigated whether the infant gut microbiomes matured toward an adult-like pattern. Because gut microbiomes can vary in adult populations from distinct geographic regions,31 we compared our data set to a representative data set of fecal microbiota from adults in a geographic region analogous to our infant cohort, revealing that infants begin to transition toward adult gut microbiomes during age 6 to 12 months (see Fig E4 in this article’s Online Repository at www.jacionline.org). Collectively, these results demonstrated the extensive transition and maturation of gut microbiota during the first year of life.
Early environmental exposures are associated with the infant gut microbiota
Among the environmental exposures that were evaluated, mode of delivery was the most significant factor contributing to microbiome community variation (P < .01) at 3 months of age (Fig 2, A), with underrepresentation of Bacteroides in individuals born by cesarean section (Fig 2, B and C) as shown in previous reports.5,32,33 Because the mode of delivery profoundly contributed to microbiome variation, PCoA and clustering were re-analyzed after stratification by delivery methods. Gut microbial communities of vaginally delivered infants also formed 3 clusters, with the same 3 key genera—Bifidobacterium, Bacteriodes, and Escherichia/Shigella—as the major contributing taxa (see Fig E5, A, in this article’s Online Repository at www.jacionline.org), indicating contribution of other exposures in defining microbiome communities. Therefore, pair-wise associations between major bacterial genera (MA, >1%) and environmental factors were investigated (see Fig E5, B). Results highlighted additional bacterial genera associated with delivery mode (eg, Bifidobacterium, Clostridium sensu stricto, Enterobacteriaceae, and Lachnospiraceae were enriched in cesarean-delivered infants), underscoring the importance of delivery mode in shaping infant gut microbiomes.
FIG 2.
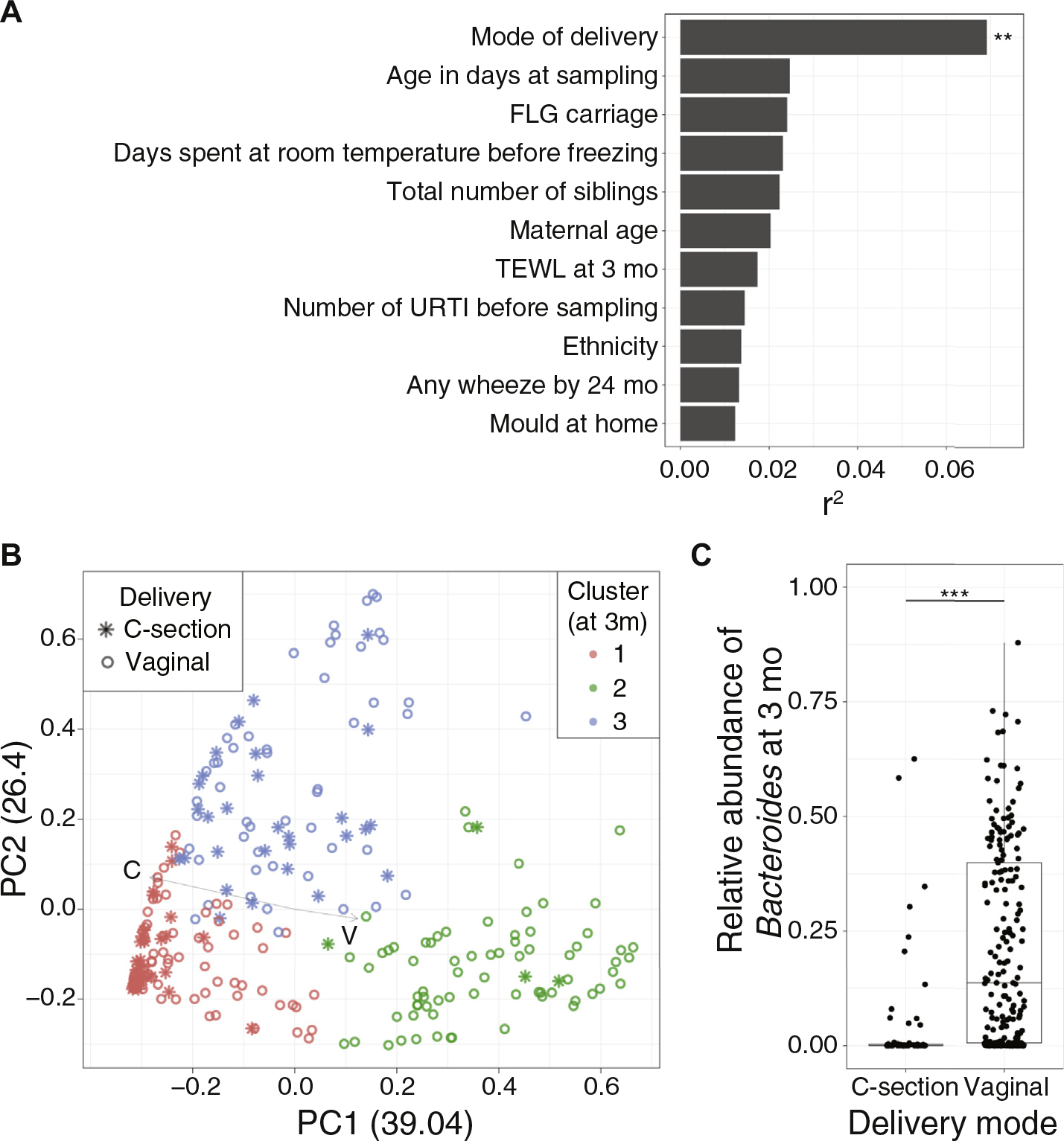
Evaluation of associations between gut microbiota characteristics and cesarean delivery. A, Association analysis of clinical, hygiene, and lifestyle factors (“covariates”) with microbiome variations. Among a total of 48 covariates tested, 11 covariates with r2 greater than 0.01 are plotted here. Each bar indicates the amount of variance explained by each covariate, calculated by EnvFit function from R (**P < .01; Bonferroni corrected). After correction for multiple comparison, mode of delivery remained as a significant factor that associated with microbiome variation. B, PCoA of microbiomes at 3 months with delivery mode delineated. Color indicates cluster (same as Fig 1, A), and the shape indicates delivery mode. Arrows demonstrate the direction of each covariate in the ordination space. Infants born by cesarean section tend to cluster on the left portion of the scatter plot. C, Boxplot showing the difference in relative abundances of Bacteroides by mode of delivery. Bacteriodes are largely missing in infants born by cesarean section (***P < .001; Wilcoxon rank-sum test). TEWL, Transepidermal water loss; URTI, Upper respiratory tract infection.
Clinical outcomes are linked with specific infant gut microbiota
We next examined potential links between clinical outcomes and the 3-month gut microbiome clusters; however no statistically significant associations were identified. Therefore, associations between major bacterial genera (MA, >1%) and clinical manifestations were also investigated (see Fig E6, A, in this article’s Online Repository at www.jacionline.org). Interestingly, SCORing Atopic Dermatitis index at age 12 months was significantly associated with the abundance of Clostridium sensu stricto and Haemophilus at enrollment. Equally, raised transepidermal water loss at 3 months showed a positive association with Haemophilus. Raised transepidermal water loss at 12 months was also linked to an increased abundance in Veillonella (Fig E6, A). Linear discriminant analysis confirmed that higher relative abundances of Clostridium sensu stricto at age 3 months were observed in infants who had AD at age 3 months and 12 months (see Fig E6, B and C). Family history of allergic disease and food allergy/sensitization did not significantly differ according to the microbiome variation or clustering.
The introduction of allergenic foods alters infants’ gut microbiota
The EAT study randomized exclusively breast-fed infants to dietary introduction of 6 allergenic foods (“early introduction,” n = 28) versus continued exclusive breast-feeding (“standard introduction,” n = 42) until age 6 months.17 Therefore, the maturation in the gut microbiota was investigated relative to the timing of introduction of allergenic solids. Participants in the intervention group had a significantly increased Shannon microbial diversity index at 6 months as compared with their enrollment samples (Fig 3, A; see Fig E7, A, in this article’s Online Repository at www.jacionline.org). PCoA revealed that the gut microbial communities of the early and standard introduction groups were largely indistinguishable (Fig 3, B, P nonsignificant; analysis of molecular variance) at 3 and 12 months. In contrast, these microbial communities significantly differed at age 6 months (Fig 3, B, middle panel, P < .05; analysis of molecular variance), demonstrating differing maturation trajectories of early versus standard introduction groups at age 6 months. Interestingly, early introduction intervention group infants predominantly transitioned along the second principal coordinate (y-axis) from age 3 to 6 months (Fig 3, C). Abundances of Prevotella and Escherichia/Shigella were positively correlated with the second principal coordinate (P < .05, and r2 = 0.52 and 0.44, respectively). Similarly, linear discriminant analysis revealed that Paraprevotella and various genera belonging to Proteobacteria and Firmicutes phyla were enriched in the early intervention group (see Fig E7, B and D) at 6 months, but not evident at age 12 months (see Fig E7, C and E). Infants in both groups moved along with the first principal coordinate (x-axis) and converged toward Bacteroides-rich communities during 6 to 12 months (Fig 3, D). To validate that the introduction of solids altered the gut microbiota in exclusively breast-fed infants, we analyzed gut microbiome changes in exclusively breast-fed infants after dietary solids introduction within the TEDDY cohort (see Methods for detailed criteria)11 and superimposed their data onto the same PCoA dimensions (see E8, A, in this article’s Online Repository at www.jacionline.org). TEDDY study infants with early introduction of solids (n = 48, no formula and started solid foods before 150 days of life) also transitioned along with the second principal coordinate from 3 months to 6 months, whereas TEDDY study infants mimicking the standard introduction group (n = 45, no formula and started solid foods after 150 days of life) did not follow the same gut microbiome transition (Fig 3, E).
FIG 3.
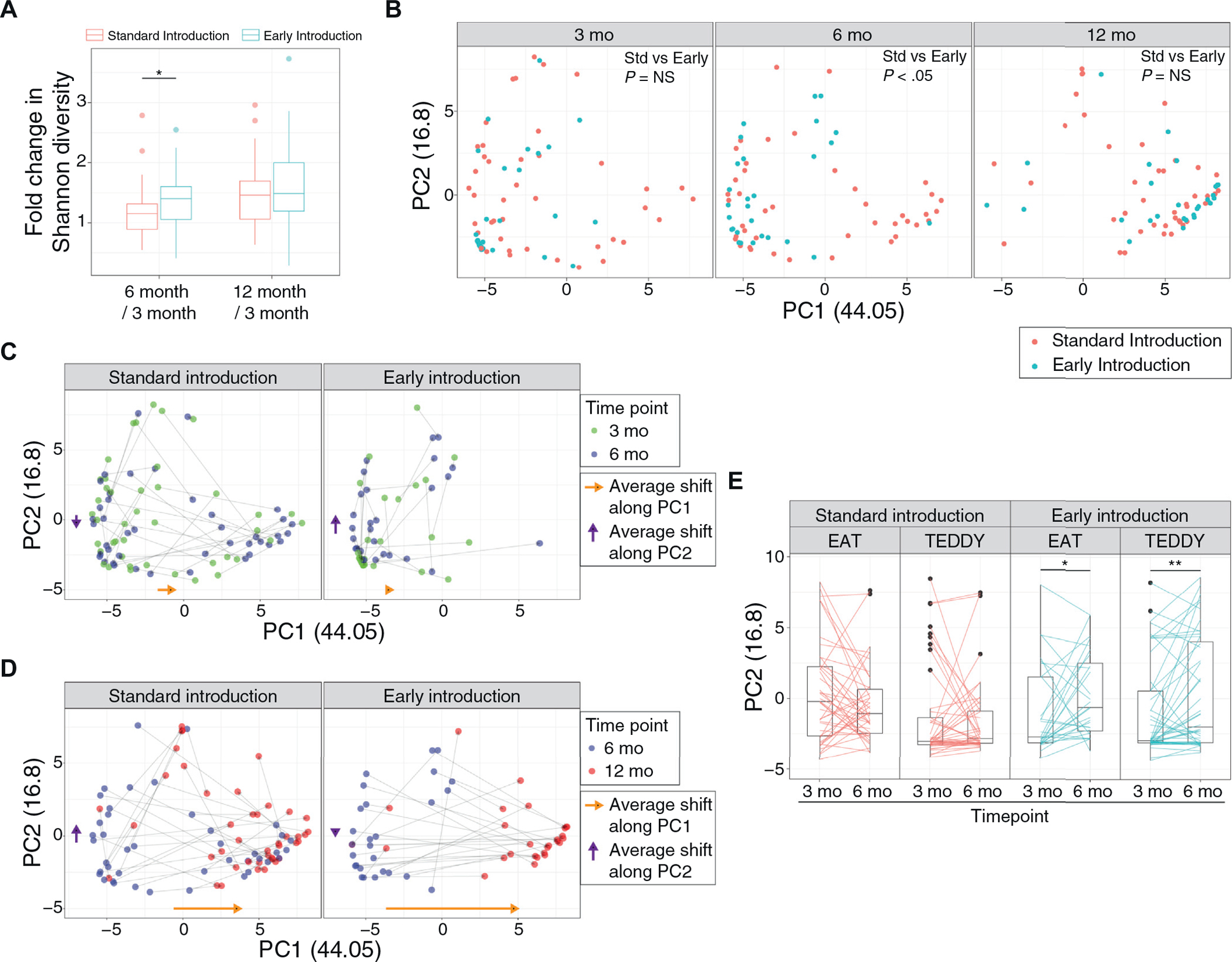
Impact of allergenic solid introduction on infants’ gut microbiota. A, Boxplot comparing Shannon diversity changes among participants’ longitudinal samples according to randomized allocation to continued exclusive breast-feeding (standard introduction group) or the introduction of allergenic solids (early introduction group) (*P < .05; Wilcoxon rank-sum test). B, PCoA plot showing longitudinal transition of the gut microbiome from age 3 to 12 months in the different dietary intervention groups. At 6 months, the microbiota of early introduction is significantly different from standard introduction (P < .05 by analysis of molecular variance). C, PCoA scatter plot demonstrating longitudinal transition from age 3 to 6 months. Gray arrows connect samples from the same individuals. Yellow and purple arrows alongside the axes indicate the average shift of the microbiota in each PCoA axis. D, PCoA scatter plot demonstrating longitudinal transition from age 6 to 12 months. Gray arrows connect samples from the same individual. Yellow and purple arrows alongside the axes indicate average shift of the microbiota in each PCoA axis. E, Boxplot showing changes in microbiome from 3 to 6 months, in different dietary intervention groups and cohorts (EAT and TEDDY). Early introduction in both studies led to an increase along the second principal-coordinate axis (*P < .05 and **P < .01; paired Wilcoxon rank-sum test). NS, Nonsignificant; Std, standard.
DISCUSSION
This cohort provided a unique opportunity to examine the relationship between the gut microbiome and many factors including dietary intervention, clinical outcomes and hygiene-related factors. Gut microbiota is widely heterogeneous among exclusively breast-fed 3-month-old infants, and there is lesser abundance of Bacteroides species if infants are born by cesarean section. After 3 months, the gut microbiota underwent major transformation in diversity and complexity, maturing toward a predominance of “adult-like” bacteria. Babies’ gut microbiota diversified when allergenic foods were introduced and matured toward Bacteroides-rich communities at age 12 months, with significant changes observed at a younger age in infants with earlier introduction of allergenic solids beginning at 3 months. Although there were significant microbiota-related associations with AD and AD severity at 3 and 12 months, none of the observed gut microbiota changes were associated with the development of food allergy or sensitization.
Study strengths and limitations
This study allowed strict characterization of fecal samples according to exclusivity of breastfeeding at 3 months by virtue of the EAT Study trial design, which only recruited exclusively breastfed infants. It also supported population-representative sampling from the general population across England and Wales, and a randomized intervention with allergenic solid introduction between age 3 and 6 months, rather than an observational study design with no control of food intake.17 The nested observational study within the EAT cohort allowed comparisons of the effects of the dietary intervention on the gut microbiota over the first year of life. We validated these findings in exclusively breast-fed infants with comparable introductions of solid foods in the TEDDY cohort, recapitulating these observations in other populations with Westernized diets. Detailed phenotyping of this cohort enabled assessment of alterations in infant gut microbiota in association with exposures, such as mode of delivery, antibiotic prescribing, communal child care, sibship size, rural versus urban residence, and pet ownership. Validated scores were used to examine participants for AD, and diagnosis of food allergy was challenge-based, rather than relying on parental report. A limitation of our study is that we were unable to evaluate the gut microbiota before 3 months of life, which would have allowed an even earlier characterization of microbiota development.
Neonatal environmental exposures may influence gut microbiota at age 3 months
Delivery by cesarean had the most profound effects on the shaping of the gut microbiota during early infancy, with reduction in Bacteroides, similar to other cohorts.5,11,32,33 Colonization with Bacteroides in the neonatal period may have considerable health benefits, especially immune maturation.34 Bacteroides fragilis has polysaccharide A molecular moieties that stimulate the development of regulatory T cells in the lamina propria,35 and samples from human fecal microbiota have found that at least 16 Bacteroides taxa contribute to this phenomenon in vivo.36 Therefore, the abundance of Bacteroides spp. may relate to the nature of early-life immunologic priming and warrants further investigation. In addition, if these mechanistic studies are fruitful, intervention trials may consider targeting infants born by cesarean to try and alter Bacteroides relative abundances and/or Bacteroides diversity.7
Although we did not find other exposures contributing to the heterogeneity of microbiome at 3 months of infancy, there may be mixed effects of multiple factors and/or hidden factors that were not included in our metadata; therefore, an optimized model for discovering associated factors might be necessary. With the current data set, we trained a random forest classifier with metadata to predict 3 clusters at 3 months. Although the classifier did not perform well (out-of-box error rate, >40%, data not shown), it calculated maternal age at birth as a second important factor for classifying clusters, suggesting the importance of maternal/household factors in microbiome development at infancy. Similarly, studies have reported the long-term colonization of vertically transmitted microbes.12
Diet contributes to evolution of the infant gut microbiota
Infants’ diet also had significant effects on the shaping of the gut microbiota during early infancy. Several reports have demonstrated differential microbiome colonization in breast-fed versus formula-fed infants, with a higher prevalence of Bifidobacterium spp (B longum and B breve) and Lactobacillus in breast-fed infants.8,9,11,14 In addition, the introduction of solid foods has been proposed as a key contributor to the maturation of the gut microbiota. For example, a Scandinavian observational study concluded that the gut microbiota matured after the cessation of breast-feeding.8
Because studies randomizing dietary interventions in infants are very unusual, this study offered a unique opportunity to study the effects of introducing allergenic solids on the gut microbiome. In the EAT study, early peanut and egg introduction, if consumed in sufficient quantity, was shown to protect against the development of peanut and egg allergies between age 1 and 3 years.17,18 We have demonstrated that the early introduction of allergenic foods alongside ongoing breast-feeding between age 3 and 6 months led to an increase in overall gut microbiota Shannon diversity, in particular promoting an influx of various microbes including Prevotellaceae and Escherichia/Shigella. Interestingly, the presence of Prevotella has been shown to be associated with high-fiber diet including remote villages with less frequent chronic inflammatory disorders.37–40 In addition, preceding studies have noted that the cessation of breast-feeding may promote further diversification of infants’ gut microbiota,8 whereas our results showed that the introduction of various allergenic foods in the setting of exclusive breast-feeding increased diversity of the gut microbiota. Of note, 6-month gut microbiota Shannon diversities were not statistically significantly different between the early and standard introduction groups, indicating that the increased microbial community diversity between 3- to 6-months by early introduction of allergenic foods was still comparable to exclusively breast-fed infants (Fig E7, A). However, the combined findings from this study demonstrate that early introduction of solid foods elicits considerable and consistent changes in gut microbial composition and diversity from 3 to 6 months of infancy. Therefore, controlled dietary interventions in infancy may promote selective colonization of desired microbes and potentially exerts health and developmental benefit to the host. However, we found no evidence that the observed changes in the gut microbiota driven by the early introduction of allergenic foods have an impact on the development of AD.
Conclusions
We confirmed that cesarean delivery is associated with reduced gut microbiota diversity at 3 months. The early introduction of solids into infants’ diets accelerates maturation of microbiota diversity as well as increases the relative abundances of Prevotella and Escherichia/Shigella. This manipulation of the gut microbiota in early life toward diversity and maturity, in particular in those delivered by cesarean section or at risk of allergy and atopic diseases, should be examined in future studies to examine potential wider health benefits.
Supplementary Material
Key messages.
Cesarean delivery is associated with reduced gut microbiota diversity at 3 months.
The infant gut microbiomes mature toward Bacteroides-rich communities that begin to transition during the latter half of the first year of life.
Dietary introduction of solids into babies’ diets accelerates microbiota diversity and promotes different trajectory of maturation.
Clostridium sensu stricto abundance at 3 months was associated with AD and AD severity.
Acknowledgments
We thank the parents and children for taking part in this trial; the members of the Trial Steering Committee (Graham Roberts [chair], David Strachan [vice chair], Mary Fewtrell, Christine Edwards, David Reading, Ian Kimber, Anne Greenough, Andy Grieve, Mary Feeney, Kate Grimshaw, Judy More, Debbie Palmer, Carina Venter, and Rebecca Knibb) for contributions to the study design; Monica Basting and Gemma Deutsch for project management; Helen Fisher, Una O’Dwyer-Leeson, Amy Nixon, Louise Coverdale, and Muhsinah Adam for nursing support; Alicia Parr for dietetic support; George Du Toit and Susan Chan for assistance with medical supervision; Jenna Heath and Kathryn Hersee for play-specialist support; and Joelle Buck, Sarah Hardy, Elizabeth Kendall, and Shuhana Begum, of the Food Standards Agency, for their commitment to the trial.
The main components of the Enquiring About Tolerance study were jointly funded by the UK Food Standards Agency (grant code T07051) and the Medical Research Council (MRC, grant no.MC_G1001205). The skin-related aspects of the study were supported by a Clinician Scientist Award from the UK National Institute for Health Research (NIHR) held by C.F. (NIHRCS/01/2008/009). The British Skin Foundation (BSF) funded the fecal sample sequencing work (BSF large grant 5047i, principle investigator C.F.). T.M., C.F., and G.L. are supported by the NIHR Biomedical Research Centre at Guy’s & St Thomas’ NHS Foundation Trust and King’s College London. C.F. is also a Principle Investigator of the EU-funded IMI Consortium BIOMAP, and the work described in this project was performed within the frame of the BIOMAP project which has received funding from the Innovative Medicines Initiative 2 Joint Undertaking (JU) under grant agreement No 821511 (BIOMAP). The JU receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA. The McLean lab is funded by Wellcome Trust Programme (092530/Z/10/Z to W.H.I.M.) and Bioresources grants (090066/B/09/Z to W.H.I.M.). The Centre for Dermatology and Genetic Medicine, University of Dundee, is supported by a Wellcome Trust Strategic Award (098439/Z/12/Z to W.H.I.M.). This work was supported by the Intramural Research Program of the US National Institutes of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases (J.-H.J., H.H.K.). J.-H.J. was also supported by a grant of the Korean Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (grant no. HI15C1095). The views expressed in this publication are those of the authors and not necessarily those of the Food Standards Agency, the MRC, the NHS, the UK NIHR, the UK Department of Health, the US National Institutes of Health, or the Wellcome Trust.
Disclosure of potential conflict of interest: G. Lack has received research funding from ALK Abello, the Immune Tolerance Network, US National Peanut Board, Food Standards Agency, Medical Research Council, Food Allergy Initiative, Action Medical Research, and Guys and St Thomas’ Charity; sponsorship from Novartis, Sodilac, Spanish Society of Allergy and Clinical Immunology, Danone Nutricia, and Nestle; has been a scientific advisor for DBV Technologies; and gives voluntary advice to the Anaphylaxis Campaign and US National Peanut Board. The rest of the authors declare that they have no relevant conflicts of interest.
Abbreviations used
- AD
Atopic dermatitis
- EAT
Enquiring About Tolerance
- MA
Median abundance
- PCoA
Principal-coordinate analysis
- TEDDY
The Environmental Determinants of Diabetes in the Young
REFERENCES
- 1.Zeissig S, Blumberg RS. Life at the beginning: perturbation of the microbiota by antibiotics in early life and its role in health and disease. Nat Immunol 2014;15: 307–10. [DOI] [PubMed] [Google Scholar]
- 2.Arrieta MC, Stiemsma LT, Dimitriu PA, Thorson L, Russell S, Yurist-Doutsch S, et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med 2015;7:307ra152. [DOI] [PubMed] [Google Scholar]
- 3.Fujimura KE, Sitarik AR, Havstad S, Lin DL, Levan S, Fadrosh D, et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat Med 2016;22:1187–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stokholm J, Blaser MJ, Thorsen J, Rasmussen MA, Waage J, Vinding RK, et al. Maturation of the gut microbiome and risk of asthma in childhood. Nat Commun 2018;9:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A 2010;107: 11971–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stokholm J, Thorsen J, Chawes BL, Schjorring S, Krogfelt KA, Bonnelykke K, et al. Cesarean section changes neonatal gut colonization. J Allergy Clin Immunol 2016;138:881–9.e2. [DOI] [PubMed] [Google Scholar]
- 7.Bokulich NA, Chung J, Battaglia T, Henderson N, Jay M, Li H, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med 2016;8:343ra82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Backhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 2015;17:852. [DOI] [PubMed] [Google Scholar]
- 9.Baumann-Dudenhoeffer AM, D’Souza AW, Tarr PI, Warner BB, Dantas G. Infant diet and maternal gestational weight gain predict early metabolic maturation of gut microbiomes. Nat Med 2018;24:1822–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yassour M, Vatanen T, Siljander H, Hamalainen AM, Harkonen T, Ryhanen SJ, et al. Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci Transl Med 2016;8:343ra81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stewart CJ, Ajami NJ, O’Brien JL, Hutchinson DS, Smith DP, Wong MC, et al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature 2018;562:583–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shao Y, Forster SC, Tsaliki E, Vervier K, Strang A, Simpson N, et al. Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature 2019;574:117–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 2006;118:511–21. [DOI] [PubMed] [Google Scholar]
- 14.Bezirtzoglou E, Tsiotsias A, Welling GW. Microbiota profile in feces of breast- and formula-fed newborns by using fluorescence in situ hybridization (FISH). Anaerobe 2011;17:478–82. [DOI] [PubMed] [Google Scholar]
- 15.Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol 2007;5:e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koenig JE, Spor A, Scalfone N, Fricker AD, Stombaugh J, Knight R, et al. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci U S A 2011;108:4578–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perkin MR, Logan K, Marrs T, Radulovic S, Craven J, Flohr C, et al. Enquiring About Tolerance (EAT) study: feasibility of an early allergenic food introduction regimen. J Allergy Clin Immunol 2016;137:1477–86.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perkin MR, Logan K, Tseng A, Raji B, Ayis S, Peacock J, et al. Randomized trial of introduction of allergenic foods in breast-fed infants. N Engl J Med 2016;374: 1733–43. [DOI] [PubMed] [Google Scholar]
- 19.Weiland SK, Bjorksten B, Brunekreef B, Cookson WO, von Mutius E, Strachan DP, et al. Phase II of the International Study of Asthma and Allergies in Childhood (ISAAC II): rationale and methods. Eur Respir J 2004;24:406–12. [DOI] [PubMed] [Google Scholar]
- 20.Kunz B, Oranje AP, Labreze L, Stalder JF, Ring J, Taieb A. Clinical validation and guidelines for the SCORAD index: consensus report of the European Task Force on Atopic Dermatitis. Dermatology 1997;195:10–9. [DOI] [PubMed] [Google Scholar]
- 21.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci U S A 2011;108:4516–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 2009;75:7537–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 2016; 13:581–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 2007;73:5261–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol 2006;72:5069–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Westcott SL, Schloss PD. OptiClust, an improved method for assigning amplicon-based sequence data to operational taxonomic units. mSphere 2017;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodrich JK, Waters JL, Poole AC, Sutter JL, Koren O, Blekhman R, et al. Human genetics shape the gut microbiome. Cell 2014;159:789–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jackson MA, Verdi S, Maxan ME, Shin CM, Zierer J, Bowyer RCE, et al. Gut microbiota associations with common diseases and prescription medications in a population-based cohort. Nat Commun 2018;9:2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yue JC, Clayton MK. A similarity measure based on species proportions. Commun Stat Theory Meth 2005;34:2123–31. [Google Scholar]
- 30.Excoffier L, Smouse PE, Quattro JM. Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics 1992;131:479–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clemente JC, Pehrsson EC, Blaser MJ, Sandhu K, Gao Z, Wang B, et al. The microbiome of uncontacted Amerindians. Sci Adv 2015;1:e1500183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rutayisire E, Huang K, Liu Y, Tao F. The mode of delivery affects the diversity and colonization pattern of the gut microbiota during the first year of infants’ life: a systematic review. BMC Gastroenterol 2016;16:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jakobsson HE, Abrahamsson TR, Jenmalm MC, Harris K, Quince C, Jernberg C, et al. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by caesarean section. Gut 2014;63:559–66. [DOI] [PubMed] [Google Scholar]
- 34.Kelly D, Campbell JI, King TP, Grant G, Jansson EA, Coutts AG, et al. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-gamma and RelA. Nat Immunol 2004;5:104–12. [DOI] [PubMed] [Google Scholar]
- 35.Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci U S A 2010; 107:12204–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 2011; 331:337–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, et al. Human gut microbiome viewed across age and geography. Nature 2012;486: 222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A 2010;107: 14691–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schnorr SL, Candela M, Rampelli S, Centanni M, Consolandi C, Basaglia G, et al. Gut microbiome of the Hadza hunter-gatherers. Nat Commun 2014;5:3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marungruang N, Tovar J, Bjorck I, Hallenius FF. Improvement in cardiometabolic risk markers following a multifunctional diet is associated with gut microbial taxa in healthy overweight and obese subjects. Eur J Nutr 2018;57: 2927–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


