Abstract
Background
Germline inactivating variants in the CDH1 tumor suppressor gene impart an elevated lifetime risk of diffuse gastric cancer. The current endoscopic surveillance method depends upon random gastric biopsies for early cancer detection.
Methods
Asymptomatic adults with pathogenic or likely pathogenic CDH1 variants referred for endoscopic gastric cancer surveillance were included in this retrospective cohort. Upper gastrointestinal endoscopy was performed according to the consensus Cambridge method, in the early period, or a systematic (Bethesda) protocol as part of an ongoing natural history study. The primary outcome measure was cancer detection.
Results
Collectively, 135 endoscopic surveillance procedures were performed in 120 patients. Twenty-six (19%, 26/135) procedures were performed using Cambridge method and 109 (81%) using the Bethesda protocol. Gastric signet ring cell carcinomas were detected in 15% (4/26) using the Cambridge method and 36% (40/109) using the Bethesda protocol (p<0.05). Almost half (44.2%, 53/120) of patients later elected for prophylactic total gastrectomy, of whom 51 (96%, 51/53) had a signet ring cell carcinoma (T1a) discovered by histopathology. On a per endoscopy basis, the false-negative rates of detection using Cambridge method and Bethesda protocol were 80% (12/15) and 37.7% (17/45), respectively (p<0.01).
Conclusions
Gastric cancer detection was more frequent with implementation of a systematic surveillance protocol in CDH1 variant carriers. Given the decision for prophylactic surgery is often made by patients in the context of family history and pathologic result of surveillance biopsies, we propose the Bethesda protocol offers patients an opportunity to make more informed decisions.
Keywords: Hereditary cancer syndromes, Endoscopy, Cancer early detection, Gastric cancer
Introduction
Gastric cancer is highly prevalent worldwide and the third leading cause of cancer deaths. Approximately 1–3% of incident cases are considered heritable [1, 2]. The hereditary diffuse gastric cancer (HDGC) syndrome is most often attributed to inactivating germline pathogenic variants in the CDH1 tumor suppressor gene [3]. CDH1 encodes the transmembrane glycoprotein E-cadherin located at adherens junctions and functions in cell–cell adhesion and signal transduction [4, 5]. Carriers of a CDH1 pathogenic or likely pathogenic (PLP) variant have a 33–70% lifetime risk of developing diffuse-type gastric cancer thus prompting the recommendation for prophylactic total gastrectomy as early as age 20 years [6–9]. Total gastrectomy performed in asymptomatic patients for cancer prophylaxis is associated with life-long morbidity. Currently, endoscopic gastric surveillance is the only alternative to this major operation.
Annual endoscopic surveillance is recommended to carriers of CDH1 PLP variants who decline or choose to delay prophylactic total gastrectomy. Consensus management guidelines recommend standard, white-light upper endoscopy with a total minimum of thirty, non-targeted, gastric mucosal biopsies obtained from five discrete anatomic areas of the stomach [8]. Reported detection rates of early-stage cancer are mixed and suggest limited efficacy with this strategy [10–13]. Although occult gastric cancer detection rates have been reported up to 63.5% in specialty centers, these findings are not widely reproducible, with more common detection rates of pathognomonic intramucosal signet ring cell (SRC) foci of 9–16% [11, 14–16]. Advanced endoscopic methods such as ultrasound and chromoendoscopy have not demonstrated improved sensitivity [17, 18]. Therefore, reliable and reproducible surveillance methods are necessary not only to improve detection of occult gastric cancer, but also properly counsel patients about their gastric cancer risk.
Early-stage occult gastric cancer detection is necessary if endoscopic surveillance is to be considered a viable alternative for patients with CDH1 PLP variants who decline prophylactic total gastrectomy. Given the lack of sensitivity of the current consensus method of endoscopic surveillance and associated high false-negative detection rates, we sought to evaluate a systematic method of gastric mucosal surveillance and compare its detection rate to the consensus method. In addition, we aimed to convey rates of endoscopic cancer detection with reference to gastrectomy explant pathologic analysis and identify factors associated with gastric cancer detection using a systematic method of mucosal biopsy.
Methods
Study design and setting
Patients were enrolled in an Institutional Review Board-approved, prospective natural history study of hereditary gastric cancers (clinicaltrials.gov, NCT03030404) from 2017 through 2019. As part of this study patients were offered clinical care by an integrated multidisciplinary team composed of surgical oncologists, gastroenterologists, genetic counselors, dietitians, and pathologists. Demographic data, CDH1 genotype, medical history, family history, gross endoscopic findings, and pathologic details were collected prospectively. Patients over the age of 18 years with a CDH1 variant classified as pathogenic or likely pathogenic (PLP) and who underwent surveillance endoscopy during the study period were included in this analysis. Genotype was confirmed by genetic test reports from clinical diagnostic laboratories that adhere to the guidelines for interpretation of sequence variants put forth by the American College of Medical Genetics and Genomics and the Association for Molecular Pathology [19]. Consistent with international guidelines, all patients with a CDH1 PLP variant, and in the absence of concurrent cancer diagnosis or major medical comorbidity, were recommended total gastrectomy following initial gastroscopic screening. Patients who declined gastrectomy were offered annual endoscopic surveillance.
Definitions and outcomes
Prior to endoscopy, all patients were counseled regarding the method and number of gastric biopsies. All endoscopies were performed with general endotracheal tube anesthesia or monitored anesthesia care with intravenous propofol. Esophagogastroduodenoscopy (EGD) was performed with white-light, high-definition visualization (GIF Q180-2603574, Olympus). Gastric mucosal biopsies were obtained according to consensus guidelines with a minimum of thirty non-targeted biopsies from five discrete anatomical areas of the stomach, generally referred to as the Cambridge method. As our clinical practice evolved, we elected to expand gastric mucosal sampling according to a systematic method, hereby referred to as the Bethesda protocol. The Bethesda protocol is a systematic visualization and biopsy approach of the gastric mucosa adapted from a method previously described by Yao [20]. Briefly, a standard endoscopic examination was performed with visualization of the esophagus, stomach, and duodenum with appropriate landmarks. Next, twenty-two separate anatomic sites were examined and photographed: ante-grade 4-section view (anterior wall, lesser curve, posterior wall, and greater curve) of the antrum, lower body, and middle upper body, followed by retroflex 3-section view (anterior wall, lesser curve, and posterior wall) of incisura and middle upper body, and retroflex 4-section view of the cardia/fundus (Fig. 1a). Four, non-targeted biopsies were obtained from each of the 22 sites. Abnormal findings such as pale mucosal areas, nodularity, polyps, and ulceration were noted and/or biopsied in addition to the systematic biopsies. Cold biopsy forceps with radial jaw and needle were used (Boston Scientific, 2.8 mm). All specimens were processed and evaluated by experienced gastrointestinal pathologists. Tissue was processed through cycles of formalin, alcohol, xylene, and paraffin blocks cut 4-microns thick which were then stained with hematoxylin and eosin. All patients were notified of biopsy findings and received counseling regarding gastric cancer risk according to pathologic results.
Fig. 1.
Gastric anatomic zones that comprise the Bethesda protocol (a) and heat map of positive biopsy location and frequency (b)
Statistical methods
Summary statistics of baseline data are presented as either frequencies for categorical data or median and interquartile range (IQR) for continuous data, unless otherwise specified. Student t tests and Chi-squared tests were performed to analyze differences between the groups. In patients who elected for total gastrectomy SRC detection by endoscopy was compared to that on final surgical pathology, and sensitivity of SRC detection was calculated. All the authors had access to the study data and reviewed and approved the final manuscript.
Results
Patient and procedure characteristics
One hundred and thirty-five esophagogastroduodenoscopies were performed between January 2016 and September 2019 in 120 asymptomatic patients with CDH1 PLP variants. Median age was 47 years (range 19–78) and majority of patients were female (N = 81, 67.5%) and Caucasian (N = 118, 98.4%). The study group represents 63 families with 38 distinct CDH1 variants, and most patients (N = 100, 83.3%) reported a family history of gastric cancer (Table 1). The majority of procedures were performed using monitored anesthesia care (MAC; 68.1%) with less frequent use of general anesthesia and moderate sedation. There were no complications following endoscopy that required intervention during the follow-up period. All patients had a minimum 30 non-targeted biopsies with additional biopsies of any focal abnormalities.
Table 1.
Characteristics of patients and CDH1 variants
| Characteristic | Overall | Cambridge method | Bethesda protocol |
|---|---|---|---|
|
| |||
| Patients | 120 | 26 | 104 |
| Age, median (range) | 47 (19–78) | 46 (25–71) | 47 (19–78) |
| Sex | |||
| Female (%) | 81 (67.5) | 19 (73.0) | 70 (67.3) |
| Male (%) | 39 (32.5) | 7 (26.9) | 34 (32.7) |
| Race | |||
| Caucasian (%) | 118 (98.4) | 25 (96.1) | 102 (98.0) |
| African American (%) | 1 (0.8) | 1 (3.8) | 1 (1.0) |
| Other (%) | 1 (0.8) | 0 (0.0) | 1 (1.0) |
| Families | 63 | 19 | 60 |
| CDH1 gene variants | 38 | 16 | 37 |
| Nonsense | 36 | 3 | 33 |
| Splice site (canonical) | 29 | 5 | 24 |
| Splice site (cryptic)a | 24 | 9 | 15 |
| Frameshift | 24 | 7 | 17 |
| Deletion (complete) | 2 | 0 | 2 |
| Deletion (large) | 5 | 2 | 3 |
| Family history of gastric cancer (%) | 100 (83.3) | 24 (92.3%) | 85 (81.7) |
Includes missense variants
Occult gastric cancer is detected with non-targeted biopsies
Overall, 37 procedures (27.4%, n = 37/135) resulted in the discovery of occult SRC foci in at least one biopsy. There were 14 (10.4%, n = 14/135) procedures in which SRC were detected on more than one biopsy. Twenty-six procedures (19.2%, n = 26/135) were performed via the Cambridge method of 30 non-targeted biopsies. This biopsy method resulted in SRC detection in 4 procedures (15.3%, n = 4/26), in which all positive biopsies were obtained in the gastric fundus (Table 2). Twenty-two patients (84.6%, n = 22/26) demonstrated gross mucosal abnormality at endoscopy: one with a pale area, four with nodular mucosa, seven with gastric polyps, and four demonstrated mucosal inflammation (Fig. 2). Targeted biopsies did not reveal any SRC foci on pathologic evaluation. Two patients harbored Helicobacter pylori organisms on pathologic analysis, both of whom had notable mucosal inflammation. One of those patients were found to have SRC foci on a non-targeted biopsy.
Table 2.
Endoscopy findings according to surveillance method
| Category | Cambridge method | Bethesda protocol |
|---|---|---|
|
| ||
| Endoscopy procedures, number (%) | 26 (19) | 109 (81) |
| Median endoscopy time, min (range) | 57 (41–79) | 41 (27–101) |
| Mucosal findings, per procedure Any focal abnormality (%) |
22 (85) | 84 (77) |
| Nodular appearance | 4 | 13 |
| Polyps | 7 | 27 |
| Inflammation | 4 | 48 |
| Pale area | 1 | 0 |
| Ulceration | 0 | 6 |
| Biopsy positive for signet ring cells, no. (%) | 4 (15) | 40 (37) |
| Signet ring cell location Fundus |
4 | 44 |
| Body | 0 | 10 |
| Incisura | 0 | 5 |
Fig. 2.
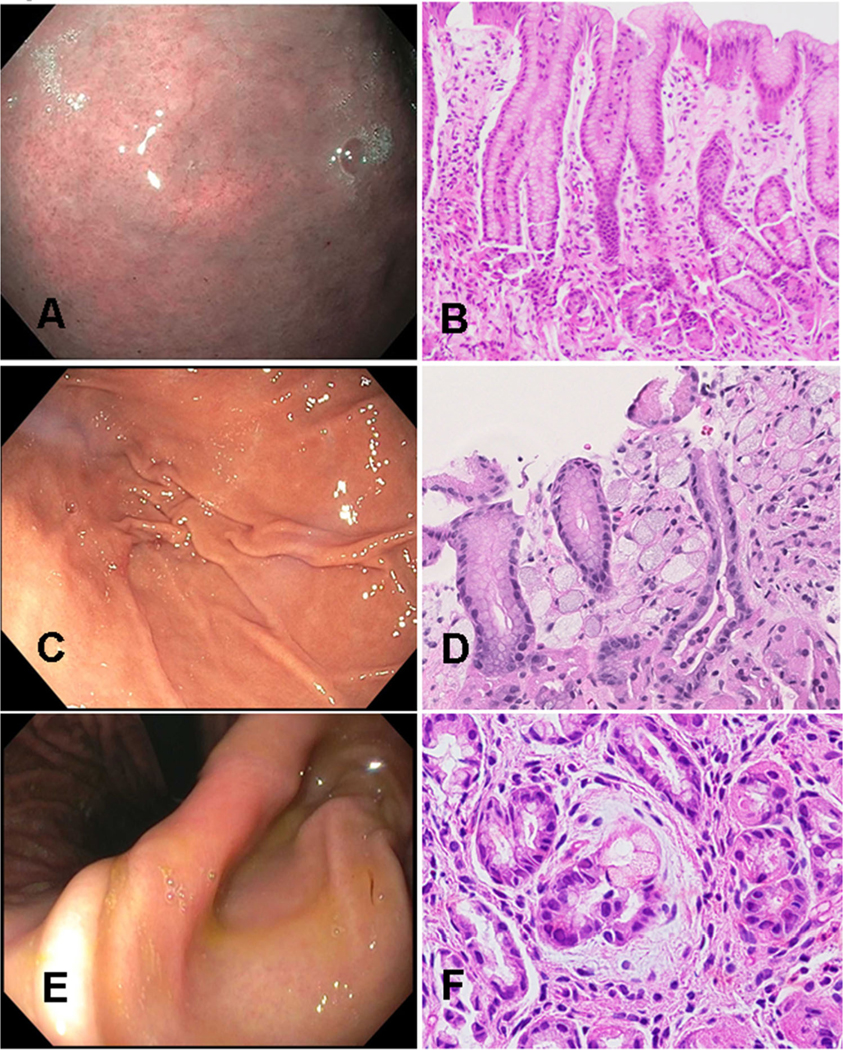
Endoscopic surveillance images and corresponding photomicrographs
One hundred and nine patients (80.7%) underwent gastric surveillance using the Bethesda protocol. Median endoscopy time was 41 min (range 27–101). The systematic method of gastric mucosal biopsy detected 60 SRC foci among 40 distinct endoscopic procedures (36%, n = 40/109). Eighty-four patients (77%, n = 84/109) had a gross mucosal abnormality that was biopsied and did not demonstrate SRC: 13 with nodular mucosa, 27 with gastric polyps, and 6 with mucosal ulceration (Table 2). Genotypes for the 120 asymptomatic patients with CDH1 PLP variants were evaluated for correlation with SRC prevalence. Canonical and cryptic splice site variants (including missense) were the most common genotypes for the entire study group (both Cambridge and Bethesda groups). Cryptic splice site variants were represented more in the Cambridge method group (p<0.05) and nonsense variants more in the Bethesda protocol group (p<0.05). Overall, there were no significant differences found in SRC detection rate based on genotype. Positive pathologic findings from the Bethesda protocol were further evaluated according to anatomic location (Fig. 1b). Signet ring cells were most frequently identified in the gastric fundus (n = 44/60, 73.3%), while the body (n = 10/60, 16.6%) and incisura (n = 5/60, 8.3%) were less frequent. Only one focus of SRC was discovered in the antrum of a patient; this patient was also found to have SRC foci in the body and fundus. Within the fundus, more SRC appeared in biopsies obtained from the posterior wall (n = 16/44, 36.3%) and lesser curvature (n = 13/44, 29.5%) compared to greater curvature (n = 9/44, 20.4%) and anterior wall (n = 6/44, 13.6%).
CDH1 variant carriers frequently harbor early-stage gastric cancer
Nearly half of patients (n = 53/120, 44.2%) proceeded to prophylactic total gastrectomy. Ultimately, 51 patients (n = 51/53, 96%) had at least one focus of SRC carcinoma (T1a) detected at final pathologic analysis. On a per patient level, 7 of 7 patients in the Cambridge group, 8 of 8 patients who had both Cambridge and Bethesda protocol endoscopies, and 36 patients in the Bethesda cohort had SRC foci in gastrectomy explants. The 53 patients who underwent total gastrectomy had a combined 55 endoscopic surveillance procedures. One patient had two Bethesda protocol procedures prior to gastrectomy; the first of which was negative for SRC and the second, a year later, was positive for SRC. Determination of endoscopic biopsy sensitivity and false-negative rate calculations were based on a per endoscopy basis. Of the 15 total gastrectomy explants from patients who underwent Cambridge method of surveillance, only three had pre-operative surveillance endoscopic biopsies positive for SRC, resulting in a false-negative rate of 80% (12/15). Out of the 45 total gastrectomy explants from patients who had a Bethesda protocol surveillance procedure, 28 had pre-operative surveillance biopsies positive for SRC, resulting in a false-negative rate of 37.7% (17/45) (p<0.001).
Discussion
Endoscopic detection of occult gastric cancer in patients with CDH1 PLP variants was augmented with the application of a systematic method of gastric biopsy. This reproducible endoscopic biopsy protocol was more sensitive for detection of occult SRC when compared to the recommended Cambridge method. Moreover, the Bethesda protocol demonstrated a substantially lower false-negative rate of early cancer detection when evaluated with respect to total gastrectomy explants. The findings from this large prospective cohort of CDH1 PLP variant carriers reinforces the need for a more reliable surveillance method for detection of occult gastric cancer. Identification of the specific cancer foci that are destined to progress to advanced cancer would also be desirable, but this first depends on their accurate and reproducible detection.
Effective endoscopic surveillance strategies for patients with CDH1 PLP variants are currently lacking [21, 22]. Computational models estimate nearly 1800 biopsies would be required to achieve a 90% SRC detection rate, which is clinically not feasible [23]. Strategies to maximize occult SRC detection via high-definition endoscopy have been proposed, such as attention to pale white mucosal areas as potential harbingers of malignancy [24, 25]. In our cohort, there was no correlation between gross endoscopic findings and pathologic diagnosis of cancer. Moreover, a modest increase in the number of gastric biopsies, from 30 to 88, appeared to substantially increase likelihood of SRC detection. The substantial proportion of patients who elected not to pursue total gastrectomy in this study underscores the need for effective endoscopic surveillance strategies as many patients desire non-surgical management.
The stochastic nature of gastric SRC development underlies our inability to provide accurate and reliable cancer surveillance in high-risk anatomic areas of the stomach. Contradictory evidence exists in the literature as to the geographical predilection for SRC foci, as some studies suggest the body-antral transitional zone is high yield while others demonstrate the proximal third of the stomach has the highest yield [26–28]. Our findings may provide additional insight into the location of SRC in patients with inactivating CDH1 PLP gene variants. Our data demonstrate that most SRC foci detected at endoscopy were located in the fundus, which is consistent with the originators of the Cambridge method who also found 9/10 patients had SRC foci in the proximal stomach (cardia/fundus) [10]. As our understanding of SRC development in this high-risk patient population increases, gastric biopsy of higher risk mucosa may improve cancer detection rates. Investigational strategies with advanced endoscopic techniques should also consider this anatomic phenomenon.
Hereditary diffuse gastric cancer due to CDH1 variants is a low incidence disease, thus small cohort studies are common and limit reliable attribution of small differences in cancer detection. Furthermore, and potentially more challenging, patient or physician bias in favor of more rigorous gastric surveillance could negatively influence comparison of novel techniques to anything considered less effective outside of a randomized trial. Patient-specific or genotypic factors that could contribute to differences in cancer surveillance outcomes would likely require a larger study with repeat endoscopic procedures. However, many patients will inevitably proceed with prophylactic total gastrectomy, as in our cohort, and in accordance with international consensus management guidelines. It follows that the natural history of gastric cancer development, or progression in those undergoing serial gastroscopic surveillance, also is unknown and likely would result only with longitudinal follow-up. Nevertheless, this single-institution cohort adds to our knowledge of this rare cancer syndrome as it represents the largest cohort of CDH1 variants carriers to undergo surveillance endoscopy to date. Even so, the need for more robust clinical evidence to improve early and accurate cancer detection in these patients is unmistakable.
In conclusion, the Bethesda protocol for surveillance of CDH1 variant carriers was associated with a low false-negative cancer detection rate when compared to the consensus endoscopy method. Furthermore, the potential relevance of an anatomic predilection of signet ring cancers observed in this study warrants attention. The current data reinforce the challenges of cancer surveillance in this patient population and serves to focus our attention on understanding the fundamental biology of CDH1-related gastric carcinogenesis. Patients who elect for endoscopic surveillance over total gastrectomy should be counseled regarding the limitations of current endoscopic methods.
Supplementary Material
Acknowledgments
Funding This study was supported in part by the Intramural Research Program, National Institutes of Health, National Cancer Institute.
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare no conflict of interest. The authors have no relevant personal, professional or financial disclosures.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s00535-020-01749-w) contains supplementary material, which is available to authorized users.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2.Palli D, Galli M, Caporaso NE, et al. Family history and risk of stomach cancer in Italy. Cancer Epidemiol Biomarkers Prev. 1994;3:15–8. [PubMed] [Google Scholar]
- 3.Guilford P, Hopkins J, Harraway J, et al. E-cadherin germline mutations in familial gastric cancer. Nature. 1998;392:402–5. [DOI] [PubMed] [Google Scholar]
- 4.Berx G, Becker KF, Hofler H, van Roy F. Mutations of the human E-cadherin (CDH1) gene. Hum Mutat. 1998;12:226–37. [DOI] [PubMed] [Google Scholar]
- 5.Oliveira C, Pinheiro H, Figueiredo J, et al. Familial gastric cancer: genetic susceptibility, pathology, and implications for management. Lancet Oncol. 2015;16:e60–70. [DOI] [PubMed] [Google Scholar]
- 6.Hansford S, Kaurah P, Li-Chang H, et al. Hereditary diffuse gastric cancer syndrome: CDH1 mutations and beyond. JAMA Oncol. 2015;1:23–32. [DOI] [PubMed] [Google Scholar]
- 7.Roberts ME, Ranola JMO, Marshall ML, et al. Comparison of CDH1 penetrance estimates in clinically ascertained families vs families ascertained for multiple gastric cancers. JAMA Oncol. 2019. 10.1001/jamaoncol.2019.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Post RS, Vogelaar IP, Carneiro F, et al. Hereditary diffuse gastric cancer: updated clinical guidelines with an emphasis on germline CDH1 mutation carriers. J Med Genet. 2015;52:361–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xicola RM, Li S, Rodriguez N, et al. Clinical features and cancer risk in families with pathogenic CDH1 variants irrespective of clinical criteria. J Med Genet. 2019;56:838–43. [DOI] [PubMed] [Google Scholar]
- 10.Lim YC, di Pietro M, O’Donovan M, et al. Prospective cohort study assessing outcomes of patients from families fulfilling criteria for hereditary diffuse gastric cancer undergoing endoscopic surveillance. Gastrointest Endosc. 2014;80:78–87. [DOI] [PubMed] [Google Scholar]
- 11.Mi EZ, Mi EZ, di Pietro M, et al. Comparative study of endoscopic surveillance in hereditary diffuse gastric cancer according to CDH1 mutation status. Gastrointest Endosc. 2018;87:408–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moreira L, Castells A. Surveillance of patients with hereditary gastrointestinal cancer syndromes. Best Pract Res Clin Gastroenterol. 2016;30:923–35. [DOI] [PubMed] [Google Scholar]
- 13.Moslim MA, Heald B, Tu C, et al. Early genetic counseling and detection of CDH1 mutation in asymptomatic carriers improves survival in hereditary diffuse gastric cancer. Surgery. 2018;164:754–9. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, Kingham K, Ford JM, et al. A prospective study of total gastrectomy for CDH1-positive hereditary diffuse gastric cancer. Ann Surg Oncol. 2011;18:2594–8. [DOI] [PubMed] [Google Scholar]
- 15.Hebbard PC, Macmillan A, Huntsman D, et al. Prophylactic total gastrectomy (PTG) for hereditary diffuse gastric cancer (HDGC): the Newfoundland experience with 23 patients. Ann Surg Oncol. 2009;16:1890–5. [DOI] [PubMed] [Google Scholar]
- 16.Pandalai PK, Lauwers GY, Chung DC, et al. Prophylactic total gastrectomy for individuals with germline CDH1 mutation. Surgery. 2011;149:347–55. [DOI] [PubMed] [Google Scholar]
- 17.Kumar S, Katona BW, Long JM, et al. Endoscopic ultrasound has limited utility in diagnosis of gastric cancer in carriers of CDH1 mutations. Clin Gastroenterol Hepatol. 2020;18(505–508):e501. [DOI] [PubMed] [Google Scholar]
- 18.Shaw D, Blair V, Framp A, et al. Chromoendoscopic surveillance in hereditary diffuse gastric cancer: an alternative to prophylactic gastrectomy? Gut. 2005;54:461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yao K The endoscopic diagnosis of early gastric cancer. Ann Gastroenterol. 2013;26:11–22. [PMC free article] [PubMed] [Google Scholar]
- 21.Garland SN, Lounsberry J, Pelletier G, Bathe OF. How do you live without a stomach? A multiple case study examination of total gastrectomy for palliation or prophylaxis. Palliat Support Care. 2011;9:305–13. [DOI] [PubMed] [Google Scholar]
- 22.Hamilton JG, Long JM, Brandt AC, et al. Patients’ medical and psychosocial experiences after detection of a CDH1 variant with multigene panel testing. JCO Precis Oncol. 2019;3:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujita H, Lennerz JK, Chung DC, et al. Endoscopic surveillance of patients with hereditary diffuse gastric cancer: biopsy recommendations after topographic distribution of cancer foci in a series of 10 CDH1-mutated gastrectomies. Am J Surg Pathol. 2012;36:1709–17. [DOI] [PubMed] [Google Scholar]
- 24.de Almeida Artifon EL, Marinho FRT. Endoscopic screening for hereditary diffuse gastric cancer: one size does not fit all. Gastrointest Endosc. 2018;87:405–7. [DOI] [PubMed] [Google Scholar]
- 25.Leung WK, Wu MS, Kakugawa Y, et al. Screening for gastric cancer in Asia: current evidence and practice. Lancet Oncol. 2008;9:279–87. [DOI] [PubMed] [Google Scholar]
- 26.Barber ME, Save V, Carneiro F, et al. Histopathological and molecular analysis of gastrectomy specimens from hereditary diffuse gastric cancer patients has implications for endoscopic surveillance of individuals at risk. J Pathol. 2008;216:286–94. [DOI] [PubMed] [Google Scholar]
- 27.Charlton A, Blair V, Shaw D, et al. Hereditary diffuse gastric cancer: predominance of multiple foci of signet ring cell carcinoma in distal stomach and transitional zone. Gut. 2004;53:814–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruff S, Curtin B, Quezado M, et al. Evaluation of confocal endoscopic microscopy for detection of early-stage gastric cancer in hereditary diffuse gastric cancer (HDGC) syndrome. J Gastrointest Oncol. 2019;10:407–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



