Abstract
The oxidation of elemental sulfur by Thiobacillus thiooxidans was studied at pH 2.3, 4.5, and 7.0 in the presence of different concentrations of various anions (sulfate, phosphate, chloride, nitrate, and fluoride) and cations (potassium, sodium, lithium, rubidium, and cesium). The results agree with the expected response of this acidophilic bacterium to charge neutralization of colloids by ions, pH-dependent membrane permeability of ions, and osmotic pressure.
Thiobacillus ferrooxidans and Thiobacillus thiooxidans are involved in bacterial leaching of metals from sulfide ores and as such are considered to be extremely tolerant to high concentrations of certain metals (11, 24, 25). The growth of these bacteria and the oxidation of ferrous iron or sulfur are nevertheless inhibited at high concentrations of these metals. The inhibition of Fe2+ oxidation by Fe3+ is competitive (10, 12). The inhibition by Cu2+ or Zn2+ of Fe2+ oxidation is also competitive (20, 21), suggesting a simple mechanism for the effect of these metals on Fe2+ oxidation. The oxidation of sulfur is also inhibited by high concentrations of metals (20, 21), but the mechanism seems to be more complex. A preliminary study indicated that Na2SO4 or K2SO4 was even more inhibitory at high concentrations than was CuSO4 or ZnSO4, and KCl or NaCl was more inhibitory than was K2SO4 or Na2SO4 at the same cation concentrations (18). It was therefore necessary to carry out a systematic study to obtain general rules governing the effects of various anions and cations before trying to understand any specific effect. It was also essential to study these effects at different pH values to analyze their causes in these acidophilic bacteria.
Since T. thiooxidans is specialized in the oxidation of sulfur and is unable to oxidize ferrous iron, this organism was used for the present study. The oxidation of elemental sulfur by thiobacilli is a complex process involving the contact of cells with sulfur particles (23), the oxidation of sulfur to sulfite (19), and the oxidation of sulfite to sulfate (22). All of these processes are influenced by pH. T. thiooxidans can oxidize sulfur at a wide range of pHs from pH 1 to 9 but can grow only under acidic conditions of pH 1 to 5. The determination of sulfur-oxidizing activity of T. thiooxidans is complicated by the solid nature of the substrate. The plot of activity versus pH shows two peaks at pH 2.5 to 3.0 and pH 6.5 to 7.0 in 0.05 to 0.1 M potassium phosphate buffer but only one peak at pH 4 to 5 in 0.5 M potassium phosphate buffer (23).
We have studied the effects of increasing concentrations of different anions and cations on the oxidation of sulfur by T. thiooxidans at three different pH values: pH 2.3 (pH for normal growth), pH 4.5 (near upper limit of pH for growth), and pH 7.0 (pH where the organism cannot grow but oxidizes sulfur). The results are in general agreement with the expected behavior of various anions in acidophilic bacteria, where the higher internal pH of 6 to 7 is supposed to be maintained against the lower external pH by the inside-positive membrane potential, Δψ, to prevent H+ entry (1, 3, 7, 13), although the mechanism seems to be complex (4, 5). Permeant anions under acidic conditions inhibited sulfur oxidation presumably by destroying the Δψ, leading to the lowering of internal pH, the effect being counteracted by some cations. General activation of sulfur oxidation by low concentrations of salts, as expected from the charge neutralization on colloidal surfaces, causing a reduction in the repulsive force for contact based on the Derjaguin-Landau-Verwey-Overbeek theory (9, 14, 28), and the inhibition at high concentrations in addition to the extended lag periods due to osmotic stress were also observed but were irrespective of the pH of the experiments.
Microorganism.
T. thiooxidans ATCC 8085 was grown statically for 4 days on elemental sulfur at 28°C in Starkey’s medium 1 adjusted to pH 2.3 with H2SO4 as described previously (22). Cultures were first filtered through Whatman no. 1 filter paper under suction to remove sulfur. Cells were collected by centrifugation at 8,000 × g for 10 min, washed once in glass-distilled water adjusted to pH 2.3 with H2SO4, and suspended in the same pH 2.3 water at a concentration of 50 mg (wet weight) of cells per ml. The cell suspension was kept at 4°C and used immediately (within 24 h).
Determination of sulfur oxidation activities.
Oxidation of sulfur by fresh cells was determined by measuring the rate of O2 consumption polarographically in a Gilson Oxygraph with a Clark electrode and a magnetic stirrer at 25°C. The reaction mixture in a total volume of 1.2 ml contained 1 mg (wet weight) of cells (20 μl of the cell suspension) and 32 mg of powdered sulfur or 5 μg of sulfur dissolved in dimethyl sulfoxide (DMSO) in reaction media with a variety of salts at different concentrations and three different pHs (pH 2.3, 4.5, and 7.0). Powdered sulfur suspension as substrate was prepared by stirring 32 g of BDH precipitated sulfur, low in Fe, in 100 ml of glass-distilled water containing 500 ppm of Tween 80 for 1 h. Sulfur in DMSO was prepared by dissolving 5 mg of the above sulfur in 10 ml of DMSO by stirring. Addition of 0.1 ml of sulfur suspended in Tween 80 (S0/Tween) or injection of 10 μl of sulfur dissolved in DMSO (S0/DMSO) into the cell suspension in various conditions started the reaction, and O2 consumption was monitored normally until either substrate S0 or O2 was fully consumed (S + 1 1/2O2 + H2O → H2SO4). A linear rate of O2 consumption (nanomoles of O2 per minute) following a lag period (normally less than 5 min) was recorded for each experiment. Since the cell activities of suspensions were not stable, one set of experiments was carried out with one batch of cells within 24 h, and the results were duplicated with another batch of cells. Although the absolute activity values of each batch of cells were not identical, the patterns presented in the figures were reproducible. Two types of sulfur were used as substrates, because powdered sulfur added in large excess made cells sometimes less responsive to various effects than were those cells with sulfur dissolved in DMSO, which was limiting in concentration and was consumed by cells during the experiments.
Effect of monovalent and divalent cations.
Divalent metals such as Cu2+ and Zn2+ are often released in high concentrations during bacterial leaching of sulfide ores. The divalent cations Mg2+, Zn2+, and Cu2+, however, were less inhibitory than the monovalent cation K+ for the rate of sulfur oxidation by T. thiooxidans cells when tested at the same concentration of sulfate, the natural anion produced by the organism. Concentrations required for 50% inhibition of the oxidation (S0/Tween) at pH 2.3 were 150 mM K2SO4 and 300 mM MgSO4, ZnSO4, or CuSO4. Since the organism failed to grow in the growth medium with 100 mM CuSO4 or 200 mM ZnSO4 without adaptation (20), these metals may inhibit other reactions essential for growth of the organism different from the sulfur oxidation. These sulfur oxidation results show that the inhibitory effect is likely due to the high osmotic pressure rather than to the ionic strength, since the colligative molarity of K2SO4 is one-and-a-half times those of salts of divalent metals.
Effect of potassium and sodium salts of sulfate, phosphate, chloride, and nitrate.
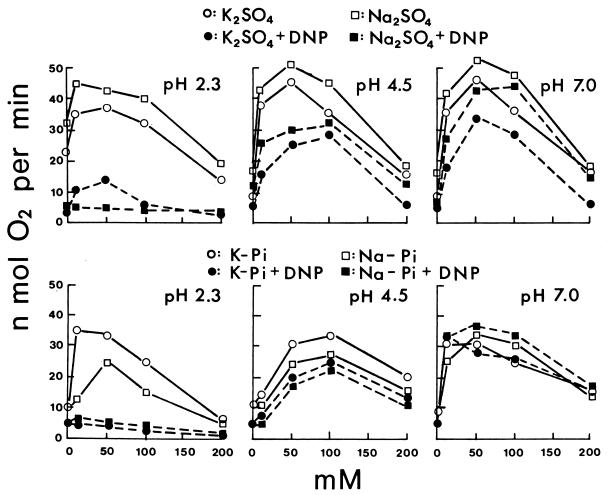
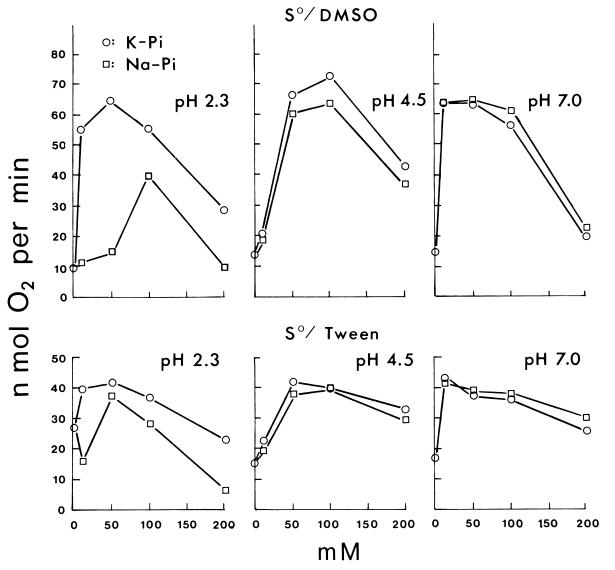
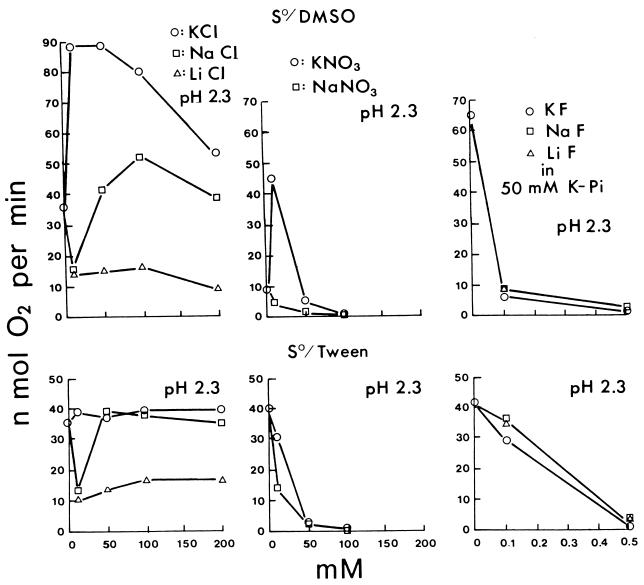
Potassium sulfate and sodium sulfate as standard salts of a nonpermeant anion both increased the sulfur oxidation rate of T. thiooxidans at 10 to 50 mM and decreased it at higher concentrations either with sulfur dissolved in DMSO (Fig. 1) or with powdered sulfur suspended in Tween 80 (data not shown) at pH 2.3, 4.5, or 7.0. Results with phosphate were more complicated (Fig. 1 and Fig. 2). At pH 2.3, potassium phosphate increased the rate at 10 to 50 mM as potassium or sodium sulfate did, but sodium phosphate either decreased the rate or did not increase it as much. At pH 4.5, both potassium and sodium phosphate required 50 to 100 mM for increased activity, 10 mM being ineffective. At pH 7.0, both potassium and sodium phosphate increased the activity at 10 to 50 mM, similarly to sulfates. The results are in agreement with the pH activity profile (23) at low potassium phosphate concentrations (minimum activity at pH 4.5) and at high potassium phosphate concentrations (maximum activity at pH 4.5). The effect of potassium and sodium chloride (Fig. 3) at pH 2.3 was similar to that of phosphates. Sodium chloride at 10 mM was definitely inhibitory, requiring 50 to 100 mM to reach the activity in potassium chloride fully (S0/Tween 80) or only partially (S0/DMSO). Lithium chloride was even more inhibitory than sodium chloride at pH 2.3 (Fig. 3). The decreased activity at 10 mM LiCl did not increase appreciably at higher LiCl concentrations. Potassium chloride increased the oxidation rate at 10 to 50 mM at pH 2.3, 4.5, or 7.0 as potassium sulfate. At pH 4.5 and 7.0 (data not shown), sodium chloride and lithium chloride were no longer inhibitory, increasing the activity with increasing concentrations to 50 mM, only slightly less stimulatory than KCl. Rubidium chloride (data not shown) had the same effect as did potassium chloride, and cesium chloride (data not shown) was similar to sodium chloride as shown in Fig. 3. The effect of potassium nitrate and sodium nitrate was even more dramatic (Fig. 3). At pH 2.3, both nitrates were strongly inhibitory and decreased the activity to near zero in 100 mM. Potassium nitrate but not sodium nitrate at 10 mM, however, increased the activity (S0/DMSO). At pH 4.5 and 7.0, the inhibition disappeared.
FIG. 1.
Effect of potassium or sodium sulfate and phosphate concentrations on the oxidation of sulfur at pH 2.3, 4.5, and 7.0 and effect of DNP. The rate of O2 consumption was determined with sulfur dissolved in DMSO. The DNP concentration, when DNP was present, was 6.25 μM. Different batches of cells were used for the potassium and sodium sulfate experiments, while the phosphate experiments were carried out with the same batch of cells.
FIG. 2.
Effect of potassium or sodium phosphate concentrations on the oxidation of sulfur at pH 2.3, 4.5, and 7.0.
FIG. 3.
Effect of potassium, sodium, or lithium chloride (left); potassium or sodium nitrate (middle); and potassium, sodium, or lithium fluoride (right) concentrations on the oxidation of sulfur at pH 2.3.
These results agree with the following interpretation. Salts at low concentrations activate the cells by neutralizing surface charges when the anions are membrane impermeable (K2SO4 and Na2SO4). Permeable anions will enter the cells at pH 2.3 in response to Δψ (positive inside), leading to proton entry and decrease in pH and activity (phosphate, chloride, and nitrate). Potassium (or rubidium) can enter as a counterion with permeant anions, preventing the loss of Δψ, but sodium (or cesium) is less effective, and lithium is ineffective. The permeability of nitrate is much higher than that of chloride or phosphate, resulting in stronger inhibition; thus, the salt activation is observed only with 10 mM KNO3. At a higher pH, the salt activation is more pronounced because Δψ will be smaller and proton penetration will be negligible. Phosphate is unique in that at pH 4.5 the potassium salt required 50 mM for significant activation and 10 mM was consistently ineffective, unlike the potassium salts of other anions. The reason remains unclear, but it could be related to the dissociation properties of phosphoric acid: H3PO4 → H+ + H2PO4− → H+ + HPO42− with pKa values of 2.12 and 7.21.
Effect of fluoride.
Potassium, sodium, or lithium fluoride was strongly inhibitory at pH 2.3 (Fig. 3), less inhibitory at pH 4.5, and not inhibitory at pH 7.0 (data not shown). The cation had no effect at these low concentrations. The degree of inhibition by fluoride was even more pH dependent than that by nitrate. Hydrofluoric acid is a weak acid with a pKa of 3.45: HF ↔ H+ + F−. So at pH 2.3, fluoride exists largely as HF, the undissociated free acid which can penetrate membranes. Fluoride is therefore taken up by cells at pH 2.3 as HF and dissociates inside at a neutral pH as H+ and F−, thus destroying ΔpH and activity. At pH 4.5, only 10% of fluoride existed as HF and the inhibition required a 10-times-higher concentration of fluoride. At pH 7.0, NaF even at a concentration as high as 200 mM did not appreciably inhibit the sulfur oxidation. Thus, although both chloride and fluoride are taken up by cells, the former responds to Δψ and the latter responds to ΔpH. Thiocyanate (SCN−), a very permeable anion which responds to Δψ (positive inside), inhibited the activity by over 90% at 0.1 mM NaSCN in 10 mM potassium phosphate (pH 2.3) but had no effect even at 1 mM NaSCN in 10 mM potassium phosphate (pH 7.0).
Effect of osmotic pressure.
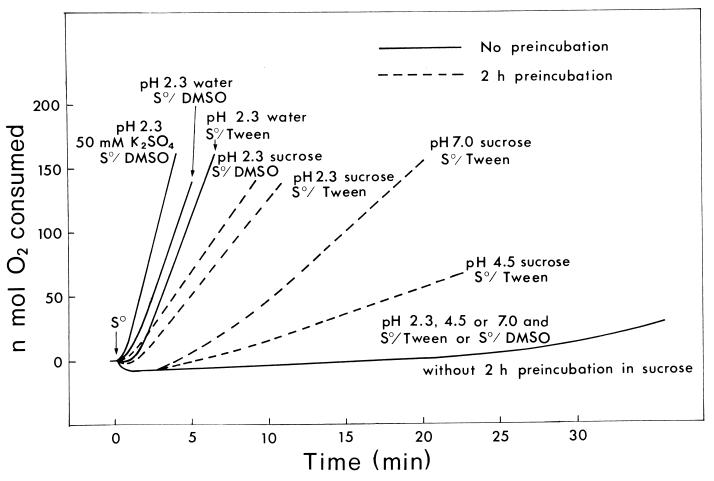
A high concentration (200 mM) of any salt decreased the sulfur oxidation rate at all the pHs tested (Fig. 1 to 3). The effect of osmotic pressure was suspected, since an extended lag period of 5 to 10 min was observed before the oxidation at the inhibited rate. Sucrose at 0 to 200 mM (data not shown) did not affect the activity as much as did potassium sulfate (Fig. 1), i.e., little activation or inhibition was observed. In the presence of 8.3 mM K2SO4, sucrose up to 200 mM had no effect at all on the activity at the three different pH values (data not shown). Sucrose did have a drastic effect, however, at 500 mM, stopping the O2 consumption nearly completely for over half an hour. Potassium sulfate at 200 mM produced an extended lag period of around 5 min before a linear rate of O2 consumption, but preincubation of cells for 5 min in 200 mM K2SO4 at pH 2.3, 4.5, or 7.0 before the addition of sulfur eliminated the extended lag period, although the salt-inhibited activity remained the same (data not shown). Obviously, the cells had to adjust to the high salt concentration before the initiation of sulfur oxidation. Interestingly, potassium sulfate at 200 mM did not affect the growth in the sulfur medium over 4 days. The extended lag period in 500 mM sucrose was much longer (30 to 45 min), although the osmotic pressure at 25°C of 1.23 MPa is slightly lower than the 1.47 MPa calculated for 200 mM K2SO4. When cells were preincubated for 2 h in 500 mM sucrose before the addition of sulfur, the extended lag period was largely eliminated. At pH 2.3, a considerable rate of oxidation was restored either with S0/DMSO or with S0/Tween (Fig. 4). At pH 7.0, only the S0/Tween activity was partially restored, and not the S0/DMSO activity (data not shown). At pH 4.5, the restored activity with S0/Tween was even lower than that at pH 7.0. Thus, T. thiooxidans cells can recover from the osmotic shock faster in K2SO4 than in sucrose. In sucrose, the recovery was better at pH 2.3, i.e., in a sucrose solution adjusted to pH 2.3 with H2SO4.
FIG. 4.
Effect of 500 mM sucrose with and without preincubation. Conditions were as described in the text, except that the activity in 500 mM sucrose was determined with (dashed line) and without (solid line) 2 h of preincubation of cells in sucrose before the addition of sulfur to start the reaction. O2 consumption tracings in the absence of 500 mM sucrose (in water and in 50 mM K2SO4) are also shown for the oxidation of sulfur at pH 2.3.
Effect of valinomycin.
Since potassium was more effective than other cations in counteracting the deleterious effect of permeable anions, the effect of valinomycin (4.2 μM) was studied with sulfate or phosphate as an anion and potassium or sodium as a cation. Valinomycin generally increased the rate of sulfur oxidation in K2SO4 and lowered the rate in Na2SO4 by as much as 15 to 30% at pH 2.3, 4.5, or 7.0 with S0/DMSO or S0/Tween as substrate (data not shown). Valinomycin also shortened the lag period in 200 mM K2SO4 and extended it in 200 mM Na2SO4. The effect of valinomycin in potassium or sodium phosphate (data not shown) was similar to the effect in potassium or sodium sulfate only at pH 4.5, where valinomycin clearly increased the activity in the potassium phosphate and decreased the activity in the sodium phosphate as expected. At pH 2.3 and 7.0, valinomycin inhibited sulfur oxidation generally either in potassium or in sodium phosphate. The reason for these results in phosphate is unclear. The general activation effect of valinomycin in potassium salts and the inhibition in sodium salts agree with the concept of K+ being the natural cation for T. thiooxidans showing the highest activity of sulfur oxidation (Fig. 1 to 3).
Effect of DNP.
The protonophore 2,4-dinitrophenol (DNP) is expected to collapse the proton gradient, ΔpH, and inhibit the oxidation of sulfur under acidic conditions. Sulfur oxidation was inhibited very strongly at pH 2.3, only moderately at pH 4.5, and still less at pH 7.0 by DNP (6.25 μM) with S0/DMSO (Fig. 1). The results were similar in potassium or sodium sulfate and in potassium or sodium phosphate. At pH 7.0, however, DNP inhibited the sulfur oxidation slightly in potassium or sodium sulfate but very little or not at all in potassium phosphate and even slightly activated the oxidation in sodium phosphate. Although not shown in Fig. 1, the effects of pH on the DNP inhibition were similar in potassium chloride and in sodium chloride, i.e., there was stronger inhibition at lower pH, but the extent of overall inhibition was larger. The results with S0/Tween 80 were essentially similar to results in Fig. 1, DNP inhibiting sulfur oxidation more strongly at a lower pH. DNP, however, increased the rate of oxidation by 10 to 20% in sodium phosphate or chloride at pH 7.0 with some batches of cells. Thus, at pH 7.0 where ΔpH was expected to be small and Δψ was negative inside, DNP inhibition was also small and sometimes DNP even increased the activity, depending on the complex response of cations and anions and the physiological state of cells.
The results reported in this paper for the effect of salts and pH on sulfur oxidation by T. thiooxidans are best explained by a combination of the following five events: (i) activation of sulfur oxidation in T. thiooxidans by increasing concentrations of salts at low concentrations (below 0.1 M) according to the Derjaguin-Landau-Verwey-Overbeek theory of charge neutralization on the surface of colloid particles (2, 9, 14, 28); (ii) based on the expected response of acidophilic bacteria (1, 7, 13), inhibition by some permeant anions at low-pH conditions due to destruction of Δψ (positive inside), allowing the H+ to leak in from outside (order of increasing inhibition: HSO4− ≪ H2PO4−, Cl− ≪ NO3−); (iii) counteraction of anionic inhibition by cations when they move inside, restoring the positive charge (order of increasing restoration: Li+ < Cs+, Na+ < Rb, K+); (iv) inhibition by HF as a weak acid permeable at low pH moving inside in response to ΔpH, acidifying the cellular contents; and (v) inhibition of sulfur oxidation at high salt concentrations (0.2 M) accompanied by extended lag periods caused probably by high osmotic pressures, similar to the effect produced by sucrose (0.5 M). The extended lag periods can be largely eliminated by preincubation of cells in the high concentrations of salts.
The degree of inhibition by anions under acidic conditions followed the order SCN− > NO3− > Cl− > H2PO4− > HSO4−, the same order as that of the Hofmeister series. Fluoride was a strong inhibitor only as HF and not as F−, similar to sulfurous acid, H2SO3, with a pKa of 1.81 (H2SO3 ↔ HSO3− + H+), which inhibits the oxidation of sulfur (19) and sulfite (22) under acidic conditions. Collins (6, 26) studied the behavior of various ions on Sephadex G-10 and showed chaotropes such as SCN− adsorbing to the gel more strongly than polar kosmotropes such as sulfate because of the weakly held water molecules of SCN−, which are easily lost, making the ion “sticky”. Collins (6) states that K+ channels are passable by chaotropic K+ (radius, 1.38 Å) by dehydration, while not by a smaller Na+ ion (radius, 1.02 Å), which cannot be dehydrated easily. Rb+ (radius, 1.49 Å) is permeable, but not Cs+ (radius, 1.7 Å), because of the large size. The results in this paper agree with the possibility of T. thiooxidans having a similar channel. Li+ is highly hydrated (8) and not expected to pass through the channel. Recently, the significance of water and water activities affected by salts and osmotic pressure in biological systems has been emphasized (8, 15, 17). Detailed analyses of the behavior of water, describing different states of water, high-density water (reactive) and low-density water (less reactive; ice or glass), and the distribution of various ions between these two states, which follows the Hofmeister series, have appeared (16, 27). In sulfur oxidation, cells must make contact with hydrophobic sulfur across water and somehow oxidize it to hydrophilic sulfate. Thus, the water activities are expected to have significant influence.
Acknowledgments
This work was supported by a grant from the Natural Sciences and Engineering Research Council of Canada.
REFERENCES
- 1.Alexander B, Leach S, Ingledew W J. The relationship between chemiosmotic parameters and sensitivity to anions and organic acids in the acidophile Thiobacillus ferrooxidans. J Gen Microbiol. 1987;133:1171–1179. [Google Scholar]
- 2.Blake R C, II, Shute E A, Howard G T. Solubilization of minerals by bacteria: electrophoretic mobility of Thiobacillus ferrooxidans in the presence of iron, pyrite, and sulfur. Appl Environ Microbiol. 1994;60:3349–3357. doi: 10.1128/aem.60.9.3349-3357.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Booth I R. Regulation of cytoplasmic pH in bacteria. Microbiol Rev. 1985;49:359–378. doi: 10.1128/mr.49.4.359-378.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cobley J G. The maintenance of pH gradients in acidophilic and alkalophilic bacteria: Gibbs-Donnan equilibrium calculations. In: Strohl W R, Tuovinen O H, editors. Microbial chemoautotrophy. Columbus, Ohio: The Ohio State University Press; 1984. pp. 121–132. [Google Scholar]
- 5.Cobley J G, Cox J C. Energy conservation in acidophilic bacteria. Microbiol Rev. 1983;47:579–595. doi: 10.1128/mr.47.4.579-595.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins K M. Sticky ions in biological systems. Proc Natl Acad Sci USA. 1995;92:5553–5557. doi: 10.1073/pnas.92.12.5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cox J C, Nicholls D J, Ingledew W J. Transmembrane electrical potential and transmembrane pH gradient in the acidophile Thiobacillus ferro-oxidans. Biochem J. 1979;178:195–200. doi: 10.1042/bj1780195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Douzou P. Osmotic regulation of gene action. Proc Natl Acad Sci USA. 1994;91:1657–1661. doi: 10.1073/pnas.91.5.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordon A S, Millero F J. Electrolyte effects on attachment of an estuarine bacterium. Appl Environ Microbiol. 1984;47:495–499. doi: 10.1128/aem.47.3.495-499.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelly D P, Jones C A. Factors affecting metabolism and ferrous iron oxidation in suspensions and batch cultures of Thiobacillus ferrooxidans: relevance to ferric iron leach solution regeneration. In: Murr L E, Torma A E, Brierley J A, editors. Metallurgical applications of bacterial leaching and related microbiological phenomena. New York, N.Y: Academic Press, Inc.; 1978. pp. 19–44. [Google Scholar]
- 11.Leduc L G, Feroni G D. The chemotrophic bacterium Thiobacillus ferrooxidans. FEMS Microbiol Rev. 1994;14:103–120. [Google Scholar]
- 12.Lizama H M, Suzuki I. Synergistic competitive inhibition of ferrous iron oxidation by Thiobacillus ferrooxidans by increasing concentrations of ferric iron and cells. Appl Environ Microbiol. 1989;55:2588–2591. doi: 10.1128/aem.55.10.2588-2591.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matin A. Bioenergetics parameters and transport in obligate acidophiles. Biochim Biophys Acta. 1990;1018:267–270. [Google Scholar]
- 14.Mills A L, Herman J S, Hornberger G M, DeJesús T H. Effect of solution ionic strength and iron coatings on mineral grains on the sorption of bacterial cells to quartz sand. Appl Environ Microbiol. 1994;60:3300–3306. doi: 10.1128/aem.60.9.3300-3306.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parsegian A D. Hopes for Hofmeister. Nature. 1995;378:335–336. [Google Scholar]
- 16.Potts M. Desiccation tolerance of prokaryotes. Microbiol Rev. 1994;58:755–805. doi: 10.1128/mr.58.4.755-805.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rand R P. Raising water to new heights. Science. 1992;256:618. doi: 10.1126/science.256.5057.618. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki, I. 1987. Unpublished data.
- 19.Suzuki I, Chan C W, Takeuchi T L. Oxidation of elemental sulfur to sulfite by Thiobacillus thiooxidans cells. Appl Environ Microbiol. 1992;58:3767–3769. doi: 10.1128/aem.58.11.3767-3769.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki I, Oh J K, Tackaberry P D, Lizama H. Determination of activity parameters in Thiobacillus ferrooxidans strains as criteria for mineral leaching efficiency. I. Growth characteristics and effect of metals on growth and on sulfur or ferrous iron oxidation. In: McCready R G L, editor. Biominet proceedings SP87-10. Ottawa, Ontario, Canada: CANMET; 1988. pp. 179–209. [Google Scholar]
- 21.Suzuki I, Oh J K, Tackaberry P D, Lizama H, Takeuchi T L. Determination of activity parameters in Thiobacillus ferrooxidans strains as criteria for mineral leaching efficiency. Final report to CANMET, Ottawa. UP-M-589, DSS File no. 24 ST. 23440-6-9126. Ottawa, Ontario, Canada: CANMET; 1989. [Google Scholar]
- 22.Takeuchi T L, Suzuki I. Effect of pH on sulfite oxidation by Thiobacillus thiooxidans cells with sulfurous acid or sulfur dioxide as a possible substrate. J Bacteriol. 1994;176:913–916. doi: 10.1128/jb.176.3.913-916.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takeuchi T L, Suzuki I. Cell hydrophobicity and sulfur adhesion of Thiobacillus thiooxidans. Appl Environ Microbiol. 1997;63:2058–2061. doi: 10.1128/aem.63.5.2058-2061.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trevors J T, Oddie K M, Belliveau B H. Metal resistance in bacteria. FEMS Microbiol Rev. 1985;32:39–54. [Google Scholar]
- 25.Tuovinen O H, Niemelä S I, Gyllenberg H G. Tolerance of Thiobacillus ferrooxidans to some metals. Antonie Leeuwenhoek. 1971;37:489–496. doi: 10.1007/BF02218519. [DOI] [PubMed] [Google Scholar]
- 26.Washabaugh M W, Collins K M. The systematic characterization by aqueous column chromatography of solutes which affect protein stability. J Biol Chem. 1986;261:12477–12485. [PubMed] [Google Scholar]
- 27.Wiggins P M. Role of water in some biological processes. Microbiol Rev. 1990;54:432–449. doi: 10.1128/mr.54.4.432-449.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zita A, Hermansson M. Effects of ionic strength on bacterial adhesion and stability of flocs in a wastewater activated sludge system. Appl Environ Microbiol. 1994;60:3041–3048. doi: 10.1128/aem.60.9.3041-3048.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]