Abstract
Background:
Calcium sulphate is a recent alternative for delayed antibiotic elution in infected bones and joints. The purpose of this study is to evaluate the use of antibiotic impregnated calcium sulphate (AICS) beads in the management of infected tibia and femur, with regards to patient outcomes and complication rates (including reinfection rate, remission rate and union rate).
Methods:
Searches of AMED, CINAHL, EMBASE, EMCARE, Medline, PubMed and Google Scholar were conducted in June 2020, with the mesh terms: “Calcium sulphate beads” or “Calcium sulfate beads” or “antibiotic beads” or “Stimulan” AND “Bone infection” or “Osteomyelitis” or “Debridement” AND “Tibia” or “Femur”. Risk of bias was assessed using the Risk of Bias in Non-randomised Studies of interventions (ROBINS-i) tool, and quality assessed via the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) criteria.
Results:
Out of 104 relevant papers, 10 met the inclusion criteria for data extraction. Total infection remission was 6.8%, which was greater than that of polymethylmethacrylate (PMMA, 21.2%). Complication rates varied. The main issue regarding AICS use was wound drainage, which was considerably higher in studies involving treatment of tibia alone. Studies using PMMA did not experience this issue, but there were a few incidences of superficial pin tract infection following surgery.
Conclusion:
Where AICS was used, it was consistently effective at infection eradication, despite variation in causative organism and location of bead placement. Wound drainage varied and was higher in papers regarding tibial cases alone.
Key Words: Antibiotic beads, Bone infection, Calcium sulphate beads, Long-bone osteomyelitis, Stimulan
Introduction
Osteomyelitis of the femur and tibia describes infection and inflammation of the bones. Multiple aetiologies exist while it mainly occurs after an open traumatic fracture or postoperatively, which exposes the bone to infectious agents(1,2). While acute cases may be treated with courses of antibiotics alone, with chronic cases, as the antibiotics have limited ability to penetrate into poorly vascularised and devitalised bone, they require a combination of local and systemic antibiotics, surgical resection and soft tissue coverage(2–6). This has led to a multi-disciplinary team approach while complete eradication of infection is still a challenge to the orthopaedic surgeon(7).
Bone defects resulting from osteomyelitis have been managed by various techniques. Many surgeons use implant antibiotic-loaded materials, in order to produce high concentrations of antibiotics at the site of infection(8). Initially, surgeons used antibiotic loaded Poly-methyl methacrylate (PMMA) beads as a carrier and third space management, but even in bead form, this has known to have many unfavourable properties(9–11). The use of alternatives have therefore been increasing in the treatment of osteomyelitis in long bones in addition to arthroplasty, periprosthetic joint infection (PJI) and open fractures(12). However, antibiotic impregnated calcium sulphate (AICS) bead implantation has not been practised without difficulties and this still remains an ongoing debate within orthopaedic surgeons(9,10).
The aim of this systematic review is to assess the outcomes of AICS use in management of infected femur and tibia, particularly with regards to recurrence and complication rates. The evidence for AICS will be evaluated against that of some other PMMA alternatives, such as bioactive glass (BAG), focusing on the same outcome measures.
Materials and Methods
The Preferred Reporting Items for Systematic reviews and Meta-analysis (PRISMA) methodology guidelines were followed(13,14). A protocol for this systematic review was prospectively registered in the International Prospective register of systematic reviews (PROSPERO; 2020: CRD42020192818).
We used the Healthcare Databases Advanced Search (HDAS) tool to search AMED, CINAHL, EMBASE, EMCARE, Medline and PubMed databases with mesh terms: “Calcium sulphate beads” or “Calcium sulfate beads” or “antibiotic beads” or “Stimulan” AND “Bone infection” or “Osteomyelitis” or “Debridement” AND “Tibia” or “Femur”. These keywords were also used to search Google Scholar.
The initial screening of the studies was done by two authors independently, for inclusion, with any discrepancies resolved by a third reviewer. The two initial reviewers were blinded to one another’s decisions. References from search results were scanned for other relevant papers, not identified in the initial search.
Searches were re-run prior to final analysis and only published studies in the English language were reviewed. Studies were included if they concerned the use of AICS beads or other antibiotic-eluting biomaterials (for comparison) in treatment of tibial or femoral osteomyelitis for both primary and post traumatic osteomyelitis. We excluded non-human studies and those of other bones. Comparators were included: PMMA beads and BAG. Case series’, case controls, cohort studies and Randomised Controlled Trials (RCTs) were included while case reports were excluded. Once relevant studies had been identified, data were extracted for each of the following: Author, year published, type of study, treatment protocol, antibiotics used, gender, side, age, indications for treatment, number of cases, type of bone, remission rate, complication(s’) rate (including wound drainage), patients’ other pathologies, organisms, numbers of different type of procedure performed (flaps, internal fixation and external fixation) and average bead volume used. The type of osteomyelitis was analysed using Cierny-Mader (CM) classification which has four types; where Type I is Medullary Osteomyelitis, Type II is Superficial Osteomyelitis, Type III is Localised osteomyelitis and Type IV is Diffuse osteomyelitis(7).
A meta-analysis could not be conducted due to heterogeneity of participant and comparator data (which was additionally only present in 1 study). The Risk of Bias in Non-randomised Studies of interventions (ROBINS-i) tool was used to assess bias and the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) criteria to assess quality of the study.
Supplementary data
Infection recurrence rates were collected where possible from each study.
Results
Search results
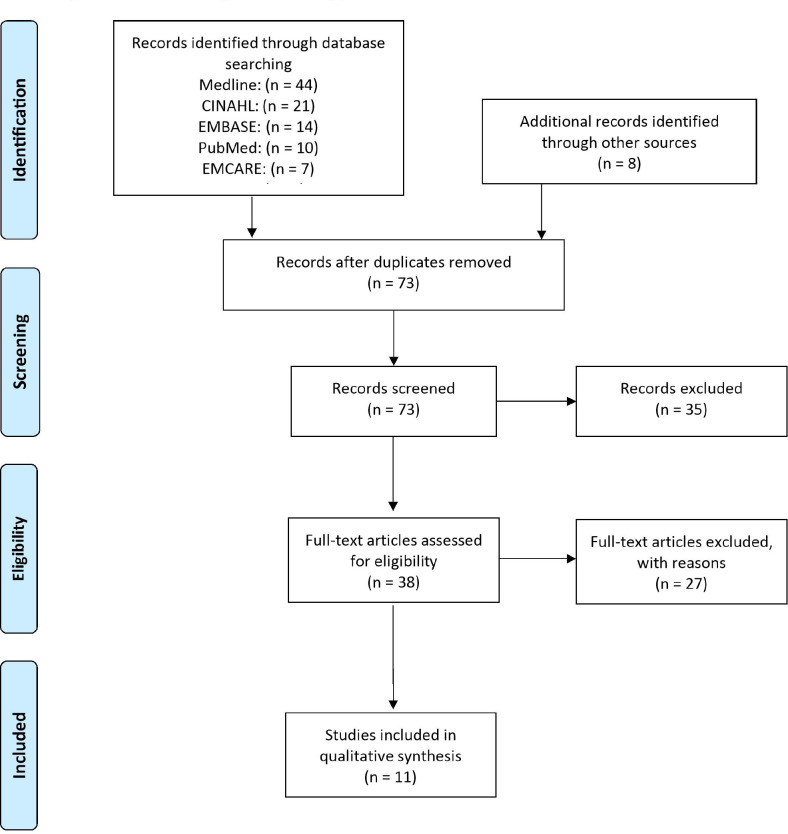
The HDAS search returned 97 results. A further 8 relevant articles from Google Scholar and references were identified, allowing us to screen 105 studies. After duplicates were removed, there were 73 articles. Eventually, 38 articles were assessed for eligibility and 11 successfully met the inclusion criteria [Figure 1]. CM classification in osteomyelitis of the femur and tibia was retrievable in 4 studies. A total of 53 cases were reported as CM type IIIa, 49 as type IIIb, 40 as type IVa and 20 as type IVb. There were no reported cases of type I or II.
Figure 1.
PRISM flow diagram of screening process
Summary of studies
Of the 11 articles that met the inclusion criteria, 4 were concerning the use of AICS only, 1 with the use of AICS compared to BAG and 5 with the use of PMMA. All studies were published between 1998 and 2020, but the 5 studies concerning AICS use were published from 2014 to 2020. These studies reported on 467 patients with 469 cases, all treated for femoral (124) or tibial (347) osteomyelitis; 295 were treated with AICS, 163 by PMMA and 11 by BAG. All patients were treated with debridement and had courses of systemic antibiotics postoperatively. Patients’ ages were reported and able to be separated for tibia or femur cases in 8 studies, with a mean of 37.9 years (range 12-88). The male to female ratio (100:49) was extractable in 7 studies. A summary of the patient demographics can be seen in [Table 1].
Table 1.
Patient Demographics
| Demographic Variable | Total In Included Studies | Missing Data in Included Studies (n =) |
|---|---|---|
| Age | Mean 37.9 (n = 200) Range: 12-88 |
267 |
| Sex | Male (n = 100) Female (n = 49) |
320 |
| Type of Bone | Tibia (n = 347) Femur (n = 124) |
0 |
| Osteomyelitis Classification | CM Type IIIa (n = 53) CM Type IIIb (n = 49) CM Type IVa (n = 40) CM Type IVb (n = 20) |
309 |
CM = Cierny-Mader
Outcome measures
In all studies, recurrence of infection was the primary outcome measure and was confirmed by radiological and histological evidence. The most common secondary outcome measure was complication rate (15–21). Other secondary outcome measures reported include mean length of hospital stay, bone healing time and extent of bone defect void filling(17,19,21,22). The most common side effect with AICS use was aseptic serous drainage or discharge. A summary of the results can be seen on [Table 2].
Table 2.
Results from Studies
| Author | Article | Type of Treatment (Antibiotic) |
Bone(s) Affected
(n =) |
Organisms Isolated (n =) | Complications |
|---|---|---|---|---|---|
| Zhou et al. | Single-stage treatment of chronic localized tibial osteomyelitis with local debridement and antibiotic-loaded calcium sulfate implantation: a retrospective study of 42 patients | Calcium Sulfate (Vancomycin and Gentamycin) |
Tibia (n = 42) CM Type IIIa (n = 35) CM Type IIIb (n = 7) |
S. aureus (n = 11)P. aeruginosa (n= 3)E. faecalis (n= 2)A. baumannii (n = 1)K. pneumonia (n = 1)E. cloacae (n = 1) S. haemolyticus (n = 1)E. coli (n = 1)A. hydrophila (n = 1) |
Infection reoccurrence: 5/42 (11.9%) Pain: 4/38 (10.5%)Discomfort: 4/38 (10.5%) Joint stiffness: 1/38 (2.6%)Slight claudication: 1/38 (2.6%)Prolonged aseptic drainage: 13/42 (31.0%) |
| McNally et al. | Single-stage treatment of chronic osteomyelitis with a new absorbable, gentamicin-loaded, calcium sulphate/ hydroxyapatite biocomposite A PROSPECTIVE SERIES OF 100 CASES | Calcium Sulfate (Gentamycin) |
Tibia (n = 38) CM Type IIIa (n = 7) CM Type IIIb (n = 27)CM Type IVa (n = 1) CM Type IVb (n = 3)Femur (n = 24) CM Type IIIa (n = 5) CM Type IIIb (n = 14) CM Type IVb (n = 5)Femur and tibia (n = 1) CM Type IVb (n = 1) |
- | Infection reoccurrence: 2 (3.2%)Sterile wound leakage: 1 (1.6%)Sterile wound drainage: 4 (6.3%)Amputation:1 (1.6%)Perisistent-nonunion:1 (1.6%) |
| Ferguson et al. | The use of a biodegradable antibiotic-loaded calcium sulphate carrier containing tobramycin for the treatment of chronic osteomyelitis | Calcium Sulfate (Tobramycin) |
Tibia (n = 88) Femur (n = 73) |
- | Infection reoccurrence: 11 (6.8%) |
| Humm et al. | Adjuvant treatment of chronic osteomyelitis of the tibia following exogenous trauma using OSTEOSET(®)-T: a review of 21 patients in a regional trauma centre. | Calcium Sulfate (Tobramycin) |
Tibia (n = 21) CM Type IIIa (n = 6) CM Type IIIb (n = 1) CM Type IVa (n = 13) CM Type IVb (n = 1) |
Polymicrobial (n= 4)Coagulase -ve Staph (n= 4)S. aureus (n= 4)-ve cultures (n= 3)Serratia sp. (n = 1)Corynebacterium sp. (n = 1) Enterococci (n = 1)Mixed anaerobes (n = 1) Propionibacteria (n = 1) |
Infection reoccurrence: 1 (4.8%) Serious drainage: 7 (33.3%) |
| Ferrando et al. | Treatment of Cavitary Bone Defects in Chronic Osteomyelitis: Biogactive glass S53P4 vs. Calcium Sulphate Antibiotic Beads. | Calcium Sulfate (Vancomycin and Gentamycin) Bioactive glass |
Calcium Sulfate Tibia (n = 6) Femur (n = 2) Bioactive glass Tibia (n = 7) Femur (n = 4) |
- | Calcium Sulfate Infection reoccurrence: 1 (12.5%) Bioactive Glass Infection reoccurrence: 1 (9.1%) |
| Sun et al. | Application of antibiotic impregnated beads on the patients with tibial chronic osteomyelitis | PMMA (Gentamicin) |
Tibia (n = 36) | K. pneumonia (n = 13)S. aureus (n = 13) E. coli (n = 12)S. pneumonia (n = 6) |
Infection reoccurrence: 19 (52.8%) |
| Chan et al. | Management of small infected tibial defects with antibiotic-impregnated autogenic cancellous bone grafting. | PMMA (Vancomycin, Piperacillin, Ticarcillin and Cephalothrin) |
Tibia (n = 36) CM Type IVa (n = 26) CM Type IVb (n = 10) |
Enterococcus (n = 6)K. pneumonia (n = 3)S. epidermis (n = 6) S. aureus (n = 14)P. aerugoinosa (n = 15)P. maltophia (n = 1)P. vulgaris (n = 1)Group B streptococcus (n = 1) M. Morganii (n = 1)E. cloacae (n = 4) S. marcescens (n = 3) Acinetobacter (n = 1)S. viridans (n = 1)E. coli (n = 1)P. putrefaciens (n = 1) |
Infection reoccurrence: 2 (5.6%) Skin rash to antibiotic: 2 (5.6%) Superficial pin tract infection: 2 (5.6%) |
| McHale et al. | Treatment of infected tibial non-union with debridement, antibiotic beads, and the Ilizarov method | PMMA (Aminoglycosides) |
Tibia (n = 10) | S. aureus (n =4) MRSA (n = 2) B. subtilis (n = 1) C.perfringens (n = 1) |
Infection reoccurrence: 2 (20%) Non-union: 1 (10%) |
| Qiu et al. | Outcomes of cement beads and cement spacers in the treatment of bone defects associated with post-traumatic osteomyelitis. | PMMA (Vancomycin and Gentamycin) |
Tibia (n = 18) | - | Infection reoccurrence: 2 (11.1%) Superficial pin tract infection: 4 (22.2%) |
| Sancineto et al. | Treatment of long bone osteomyelitis with a mechanically stable intramedullary antibiotic dispenser: nineteen consecutive cases with a minimum of 12 months follow-up. | PMMA (Vancomycin, Gentamycin, Tobramycin and Imipenem) |
Tibia (n = 15) Femur (n = 4) |
MRSA (n = 13)Pseudomonas (n = 1)E. cloacae (n = 2)Serratia sp. (n = 1)MSSA (n = 1) | Infection reoccurrence: 0 (0.0%) Vancomycin hypersensitivity: 1 (5.6%) |
| Walenkamp et al. |
Osteomyelitis treated with gentamicin-PMMA beads: 100 patients followed for 1–12 years |
PMMA (Gentamycin) |
Tibia (n = 29) Femur (n = 16) |
- | Infection reoccurrence: 7 (15.5%) Amputation due to treatment intolerance: 1 (2.2%) |
Types of Calcium Sulphate beads
At present, different types of beads exist with differing compositions. Zhou et al. and Ferrando et al. used Stimulan from Biocomposite Ltd., Staffordshire, UK(15,19). Both studies used vancomycin and gentamycin. The manufacturer recommends mixing 1g of vancomycin and 240 mg of liquid tobramycin per 10 cc of calcium sulphate, but these studies used gentamycin instead of tobramycin. Ferguson et al. and Humm et al. used Osteoset-T from Wright Medical Technology Inc., Arlington, Tennessee, USA(17,18). These beads are prepared as 96% pure calcium sulphate and 4% tobramycin, which is the only antibiotic used in these studies. McNally et al. used a calcium sulphate/ hydroxyapatite composite, produced by CERAMENT G from Bonesupport, Lund, Sweden(16). This preparation involves 175 mg gentamycin mixed in per 10 cc of bead composite. It is suggested that the hydroxyapatite allows the dissolving beads to provide a scaffold, as well as carrying the osteoconductive properties of calcium sulphate.
The contents of all calcium sulphate-based bead products must be mixed until doughy and then left to set at room temperature. This provides a number of advantages over PMMA beads, as these must set at high temperatures.
Recurrences
With use of AICS, mean infection recurrence rate was 6.8% (range 3.2% - 11.9%, n = 295). Of the patients who underwent treatment with PMMA, 32 patients (19.6%, n = 163) suffered reinfection [Tables 3; 4]. Ferrando et al. reported 1/11 (9.1%) recurrences with BAG(19).
Table 3.
Infection recurrence in calcium sulphate, PMMA and Bioactive Glass groups
|
Calcium Sulphate
(n = 295) |
PMMA
(n = 163) |
Bioactive Glass
(n = 11) |
|
|---|---|---|---|
| Infection Recurrence | 20 (6.8%) | 32 (19.6%) | 1 (9.1%) |
Table 4.
Comparison of Infection recurrence between calcium sulphate and bioactive glass
|
Calcium Sulphate
(n = 295) |
Bioactive Glass
(n = 11) |
p | |
|---|---|---|---|
| Infection Recurrence | 20 (6.8%) | 1 (9.1%) | 0.549 |
In the AICS studies, the lowest reinfection rate was in McNally et al. (3.2%, n = 63). Humm et al. only reported 1 patient (4.8%, n = 21) requiring further debridement for complete infection eradication and all cases in this series were treated for chronic osteomyelitis following trauma(16, 18). The Ferguson et al. series had 11 recurrences (6.8%, n = 161)(17). A higher rate was seen by Zhou et al. (11.9%, n = 42)(15). However, there was more heterogeneity in infection implication in this series, with 31 limbs (72.1%) needing treatment for trauma/ inappropriate treatment, 10 (23.3%) for haematogenous infection and 2 (4.7%) for continuous penetration from soft tissue infection. Only 11 (36.7%) of the trauma cases in this series were open fractures. The highest rate was in the Ferrando et al. series (12.5%), but this was a small sample (n = 8)(19).
In the PMMA studies, the lowest reinfection rate was in Sancineto et al. (0%, n = 19), but 1 patient did not finish treatment due to antibiotic intolerance(23). This study used an intramedullary dispenser different to bead-like structures used in the others. Chan et al. had 2 recurrences (5.6%, n = 36), but only 15 (41.7%, n = 36) cases in this series were open fractures(20). Qiu et al. also had 2 recurrences (11.1%, n = 18)(21). Walenkamp et al. reported 7 recurrences (15.5%, n = 45)(10). McHale et al. had 2 (20%, n = 10) patients suffer reinfection. In this military case series, one of these recurrences was in a patient with a severely damaged posterior tibial artery(24). Eight patients were implicated for trauma, the other two for postoperative complications. The largest recurrence rate of all studies was in Sun et al. (52.8%, n = 36)(22). All of the cases in this study had chronic osteomyelitis. Despite this, this study was deemed to be of low quality, so the reported outcomes may not be representative.
Wound drainage
The 3 AICS studies for which these data with regards to wound drainage were separable for tibia and femur cases were Zhou et al., McNally et al. and Humm et al.(15,16,18). These reported rates of 31.0%, 7.9%, and 33.3%, respectively. Across the 126 patients included in these studies, the total wound drainage/ discharge rate was 19.8%, which is similar to that reported in the literature for use in other conditions(12). In the Zhou et al. series, 10 (23.3%) of the 13 (31.0%) of the patients with prolonged aseptic wound drainage were managed by appropriate non-surgical wound caring(15). Three (7.0%) patients required follow up surgery months later to resolve this. Humm et al. reports 7 (33.3%) incidences of wound leakage, causing problems with wound healing in 6/7 of these, compared to 5/14 for those with no leakage (p = 0.06)(18). No follow-up surgery was reported for resolving drainage. This paper also found, with p = 1.0, that there was no significant association between site of bead implantation (intramedullary or at wound site) and incidence of leakage. McNally et al. reported a much smaller rate of 5/63 cases (7.9)%, all of which resolved without further surgery(16). The authors noticed that most wound drainage (4/5 of these cases) occurred in the distal tibia, possibly owing to its relatively poor cutaneous coverage. This may explain the difference in magnitude in drainage rates between this study and the others. Humm et al. and Zhou et al. focused on tibia only, but of the data we extracted from the McNally et al. series, 61.9% were femur cases(15,16,18). McNally et al. also determined no significant association between wound drainage (or patient CM grade) and outcome (recurrence)(16). Overall, across these 3 studies’ 126 patients, only 3 (2.4%) required follow up surgery as a result of wound drainage.
Out of the 6 papers we used concerning the use of PMMA cement beads, there were no reported incidences of wounds complicated by drainage. Ferrando et al. did not include any data on this, so we have no data for occurrence with BAG use(19).
Other complications
Zhou et al. listed a variety of problems some patients had following AICS treatment(15). Four patients (10.5%) experienced pain, and the same number experienced discomfort. McNally et al. described 1 non-union and 1 case requiring amputation(16).
While there was no reported wound drainage from any of the PMMA cases, there were 2 studies that reported superficial pin tract infections. Qiu et al. had 4 incidences (22.2%) and Chan et al. had 2 (5.6%)(20,21). There were no other commonly reported PMMA-related complications.
Risk of bias and Quality
The 6 studies regarding use of PMMA beads were all deemed to be of moderate risk of bias(10,20–24). Of the 4 concerning AICS, we evaluated 2 studies (Zhou et al. and Ferguson et al.) to be at low risk of bias and 2 to be at moderate risk (McNally et al. and Humm et al.)(15–18). The study comparing AICS and BAG (Ferguson et al.) was rated as moderate(17). Using this and other GRADE criteria, we concluded that 10 of our studies were of moderate quality. Sun et al. was deemed to be low quality(22). This may present issues, as 19/32 of the PMMA recurrences described in this review were extracted from this study.
Discussion
This review found variable results with different authors. In cases of AICS use in tibial or femoral osteomyelitis, the most commonly reported postoperative complication is aseptic wound drainage, occurring in 7.9-33% of cases. It has also been noted that close attention must be paid to patients with renal insufficiency (9). Other complications have commonly been reported for usage in other conditions (notably PJI), such as transient hypercalcaemia and heterotopic ossification (HO) (25–28). However, unlike in PJI, no incidences of hypercalcaemia or HO have been described in tibial or femoral osteomyelitis cases. This suggests that the side effect profiles for use in such situations are more favourable than previously noted for PJI cases. Additionally, only 3 cases of wound drainage did not resolve spontaneously, requiring further surgery. Menon et al. presented a case series, in which many incidences of wound discharge were found to spontaneously resolve within 4-6 weeks, concluding that careful wound status interpretation is needed to avoid unnecessary re-exploration (29). This paper was not used in this study as data couldn’t be extracted for tibia or femur osteomyelitis cases.
As evident from the current study, cases which utilized AICS beads reported significantly lower recurrence rates than those using PMMA. Currently, one cannot conclude how this compares to bioactive glass, due to the incredibly limited availability of data regarding the use of this material. Aside from recurrence rates, we noted various quantitative and qualitative discussion of the superior properties of AICS compared to PMMA. PMMA beads do not readily dissolve, so require a second operation for removal; they may also be colonised if left in situ for long periods of time, potentially leading to biofilm formation. Additionally, some studies have shown poor pharmacokinetic characteristics of these beads(9). On the contrary, AICS beads have been shown to fully dissolve (marked by absence on X-rays) within 3-12 weeks, removing the need for a follow-up procedure and the possibility of biofilm formation(25–27). Pharmacokinetic studies also show its superiority. As AICS dissolves, 100% of its loaded antibiotic is released, compared to around 10% in PMMA, leading to much higher local concentrations for more prolonged periods(9). Further to local antibiotic concentrations with AICS being much higher than the minimum inhibitory concentration (MIC) for the implicated pathogen, systemic levels remain far from those potentiating toxicity. Moreover, preparation of AICS beads involves setting at a cool room temperature, permitting the use of heat-sensitive antibiotics that could not be used in PMMA beads.
The use of calcium sulphate beads in the management of osteomyelitis of femur and tibia is a useful adjunct with a variety of advantages including pharmacokinetics and the ability to use a larger range of antibiotics with higher doses. Is it possible to accommodate non-heat stable antibiotics unlike in PMMA. The rates of remission are significantly greater than with the use of PMMA in tibial and femoral osteomyelitis. There were only 3 cases of discharge requiring surgical intervention. With AICS, high rates if asymptomatic wound drainage may be observed, especially in tibial cases or where there is little cutaneous coverage. Unlike in PJI, in tibial and femoral infection, there were no incidences of hypercalcemia or HO with the use of AICS.
Disclosure:
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper
Acknowledgements
None
References
- 1.Dellinger EP, Miller SD, Wertz MJ, Grypma M, Droppert B, Anderson PA. Risk of infection after open fracture of the arm or leg. Archives of surgery. 1988;123(11):1320–7. doi: 10.1001/archsurg.1988.01400350034004. [DOI] [PubMed] [Google Scholar]
- 2.Lew DP, Waldvogel FA. Osteomyelitis. The Lancet. 2004;364(9431):369–79. doi: 10.1016/S0140-6736(04)16727-5. [DOI] [PubMed] [Google Scholar]
- 3.Buchman J, Blair JE. Penicillin in the treatment of chronic osteomyelitis: A preliminary report. Archives of Surgery. 1945;51(2):81–92. doi: 10.1001/archsurg.1945.01230040086003. [DOI] [PubMed] [Google Scholar]
- 4.Orr HW. The treatment of acute osteomyelitis by drainage and rest. JBJS. 1927;9(4):733–9. doi: 10.1097/01.blo.0000238778.34939.66. [DOI] [PubMed] [Google Scholar]
- 5.Trueta J. Treatment of war wounds and fractures. British medical journal. 1942;1(4245) doi: 10.1136/bmj.1.4245.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stark WJ. The use of pedicled muscle flaps in the surgical treatment of chronic osteomyelitis resulting from compound fractures. JBJS. 1946;28(2):343–50. [PubMed] [Google Scholar]
- 7.Cierny Iii G, Mader JT, Penninck JJ. The classic: a clinical staging system for adult osteomyelitis. Clinical Orthopaedics and Related Research®. 2003;414:7–24. doi: 10.1097/01.blo.0000088564.81746.62. [DOI] [PubMed] [Google Scholar]
- 8.Pincher B, Fenton C, Jeyapalan R, Barlow G, Sharma HK. A systematic review of the single-stage treatment of chronic osteomyelitis. Journal of orthopaedic surgery and research. 2019;14(1):1–8. doi: 10.1186/s13018-019-1388-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wahl P, Guidi M, Benninger E, Rönn K, Gautier E, Buclin T, et al. The levels of vancomycin in the blood and the wound after the local treatment of bone and soft-tissue infection with antibiotic-loaded calcium sulphate as carrier material. The bone & joint journal. 2017;99(11):1537–44. doi: 10.1302/0301-620X.99B11.BJJ-2016-0298.R3. [DOI] [PubMed] [Google Scholar]
- 10.Walenkamp GH, Kleijn LL, de Leeuw M. Osteomyelitis treated with gentamicin-PMMA beads: 100 patients followed for 1–12 years. Acta Orthopaedica Scandinavica. 1998;69(5):518–22. doi: 10.3109/17453679808997790. [DOI] [PubMed] [Google Scholar]
- 11.Boyd D, Towler MR. The processing, mechanical properties and bioactivity of zinc based glass ionomer cements. Journal of Materials Science: Materials in Medicine. 2005;16(9):843–50. doi: 10.1007/s10856-005-3578-1. [DOI] [PubMed] [Google Scholar]
- 12.Abosala A, Ali M. The use of calcium sulphate beads in periprosthetic joint infection, a systematic review. Journal of bone and joint infection. 2020;5(1):43–9. doi: 10.7150/jbji.41743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG, Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS medicine. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of clinical epidemiology. 2009;62(10):e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Zhou CH, Ren Y, Ali A, Meng XQ, Zhang HA, Fang J, et al. Single-stage treatment of chronic localized tibial osteomyelitis with local debridement and antibiotic-loaded calcium sulfate implantation: a retrospective study of 42 patients. Journal of Orthopaedic Surgery and Research. 2020;15:1–0. doi: 10.1186/s13018-020-01721-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McNally MA, Ferguson JY, Lau AC, Diefenbeck M, Scarborough M, Ramsden AJ, et al. Single-stage treatment of chronic osteomyelitis with a new absorbable, gentamicin-loaded, calcium sulphate/hydroxyapatite biocomposite: a prospective series of 100 cases. The bone & joint journal. 2016;98(9):1289–96. doi: 10.1302/0301-620X.98B9.38057. [DOI] [PubMed] [Google Scholar]
- 17.Ferguson JY, Dudareva M, Riley ND, Stubbs D, Atkins BL, McNally MA. The use of a biodegradable antibiotic-loaded calcium sulphate carrier containing tobramycin for the treatment of chronic osteomyelitis: a series of 195 cases. The bone & joint journal. 2014;96(6):829–36. doi: 10.1302/0301-620X.96B6.32756. [DOI] [PubMed] [Google Scholar]
- 18.Humm G, Noor S, Bridgeman P, David M, Bose D. Adjuvant treatment of chronic osteomyelitis of the tibia following exogenous trauma using OSTEOSET®-T: a review of 21 patients in a regional trauma centre. Strategies in Trauma and Limb Reconstruction. 2014;9(3):157–61. doi: 10.1007/s11751-014-0206-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferrando A, Part J, Baeza J. Treatment of cavitary bone defects in chronic osteomyelitis: bioactive glass S53P4 vs calcium sulphate antibiotic beads. Journal of bone and joint infection. 2017;2(4):194–201. doi: 10.7150/jbji.20404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan YS, Ueng SW, Wang CJ, Lee SS, Chao EK, Shin CH. Management of small infected tibial defects with antibiotic-impregnated autogenic cancellous bone grafting. Journal of Trauma and Acute Care Surgery. 1998;45(4):758–64. doi: 10.1097/00005373-199810000-00023. [DOI] [PubMed] [Google Scholar]
- 21.Qiu XS, Chen YX, Qi XY, Shi HF, Wang JF, Xiong J. Outcomes of cement beads and cement spacers in the treatment of bone defects associated with post-traumatic osteomyelitis. BMC musculoskeletal disorders. 2017;18(1):1–6. doi: 10.1186/s12891-017-1614-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun PQ, Ma Y, Zhang YC, Cheng MG. Application of antibiotic impregnated beads on the patients with tibial chronic osteomyelitis. Pak J Pharm Sci. 2018;31(6):2783–6. [PubMed] [Google Scholar]
- 23.Sancineto CF, Barla JD. Treatment of long bone osteomyelitis with a mechanically stable intramedullar antibiotic dispenser: nineteen consecutive cases with a minimum of 12 months follow-up. Journal of Trauma and Acute Care Surgery. 2008;65(6):1416–20. doi: 10.1097/TA.0b013e31818c6a09. [DOI] [PubMed] [Google Scholar]
- 24.McHale KA, Ross AE. Treatment of infected tibial nonunions with debridement, antibiotic beads, and the Ilizarov method. Military medicine. 2004;169(9):728–34. doi: 10.7205/milmed.169.9.728. [DOI] [PubMed] [Google Scholar]
- 25.Kallala R, Haddad FS. Hypercalcaemia following the use of antibiotic-eluting absorbable calcium sulphate beads in revision arthroplasty for infection. The bone & joint journal. 2015;97(9):1237–41. doi: 10.1302/0301-620X.97B9.34532. [DOI] [PubMed] [Google Scholar]
- 26.McPherson E, Dipane M, Sherif S. Dissolvable antibiotic beads in treatment of periprosthetic joint infection and revision arthroplasty-the use of synthetic pure calcium sulfate (Stimulan®) impregnated with vancomycin & tobramycin. Reconstructive Review. 2013;3:1. [Google Scholar]
- 27.Lum ZC, Pereira GC. Local bio-absorbable antibiotic delivery in calcium sulfate beads in hip and knee arthroplasty. Journal of orthopaedics. 2018;15(2):676–8. doi: 10.1016/j.jor.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kallala R, Harris WE, Ibrahim M, Dipane M, McPherson E. Use of Stimulan absorbable calcium sulphate beads in revision lower limb arthroplasty: safety profile and complication rates. Bone & joint research. 2018;7(10):570–9. doi: 10.1302/2046-3758.710.BJR-2017-0319.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menon A, Soman R, Rodrigues C, Phadke S, Agashe VM. Careful interpretation of the wound status is needed with use of antibiotic impregnated biodegradable synthetic pure calcium sulfate beads: Series of 39 cases. Journal of bone and joint infection. 2018;3(2):87–93. doi: 10.7150/jbji.22684. [DOI] [PMC free article] [PubMed] [Google Scholar]