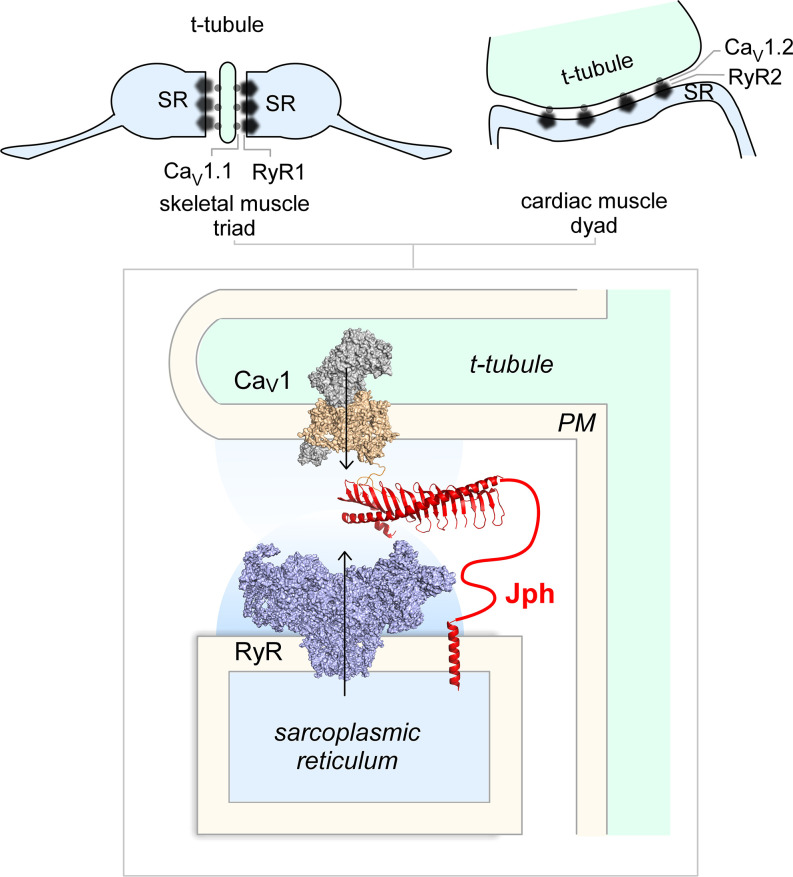
Every breath of life and every beat of the heart relies on the faithful translation of fast voltage signals into a dynamic influx of Ca2+ ions that initiates the contraction of skeletal and cardiac muscle. This process, known as excitation–contraction coupling, requires the precise geometric arrangement and functional coordination of two large ion channel macromolecular complexes that reside in two opposing membrane compartments which are separated by a narrow cleft―the L-type voltage-gated Ca2+ channels (CaV1) in the traverse-tubular (t-tubule) membrane and the ryanodine receptor (RyR) in the sarcoplasmic reticulum (SR). In the heart, this compartment is known as a dyad based on its bipartite organization (Fig. 1). The propagation of the cardiac action potential into the t-tubule results in the activation of dyadic L-type channels (CaV1.2) that convey a local influx of Ca2+ into the cleft. The freely diffusing Ca2+, in turn, binds RyR2 across the dyadic cleft to elicit a much larger release of Ca2+ from intracellular stores (1). In addition to the two ion channel behemoths, a wide range of Ca2+-effector proteins such as calmodulin-dependent kinases and calcineurin also reside in this space and couple changes in Ca2+ signals to various cellular functions, rendering the dyadic junction a privileged Ca2+-signaling domain (1). In the skeletal muscle, the analogous subcellular compartment is known as a triad owing to its tripartite arrangement (Fig. 1). In the triad, voltage-dependent activation of the CaV1.1 L-type channel is conformationally coupled to RyR1, thus obviating the need for Ca2+ ions in evoking Ca2+ release from the intracellular store (2). Beyond striated muscle, close apposition of the endoplasmic reticulum and the plasma membrane (ER–PM junctions) is found in many cell types including neurons, where they play a vital role in Ca2+ homeostasis and signaling and lipid exchange (3, 4). The function and formation of ER–PM junctions can be static or dynamic, involving a rich repertoire of proteins. For example, depletion of intracellular Ca2+ stores causes the coaccumulation of stromal interaction molecule and Orai channels in ER–PM junctions (5). Ensuing localized Ca2+ entry through Orai replenishes the intracellular stores (5). By comparison, dyadic and triadic junctions are preassembled. In this broader context, the junctophilin family of proteins (Jph1 to Jph4) have been shown to play an essential role in both the formation of ER–PM junctions in muscle and neurons as well as to serve as a scaffold that recruits relevant ion channels to this specialized Ca2+-signaling compartment (6). In PNAS, the paper by Yang et al. (7) presents high-resolution atomic structures of a key Jph domain alone and associated with an isolated cytosolic segment of the CaV1.1 channel. This study opens new frontiers in understanding the structure and mechanisms underlying the formation of ER–PM junctions and the recruitment of ion channels to this subcellular domain.
Fig. 1.
Cartoon illustrates the excitation–contraction machinery in skeletal and cardiac muscle. The CaV1 channels (PDB: 5GJV) are localized to the t-tubular membrane, while the RyR (PDB: 6W1N) is in the SR membrane. Jph plays a key role in formation of the junction and is proposed to recruit both CaV1 and RyR to this exquisite Ca2+-signaling compartment.
The junctophilin family of proteins were identified in 2000 as an important component of junctional membrane complexes by Takeshima et al. (8). Four different subtypes of Jph have been identified, with Jph1 primarily expressed in skeletal muscle, Jph2 in cardiac and skeletal muscle, and Jph3 and Jph4 in neurons (see reviews in refs. 6 and 9). In terms of molecular architecture, the Jphs are composed of eight membrane occupation and recognition nexus (MORN) domains, a long α-helical region, thought to be a spacer between the ER and PM, a long divergent region, and a transmembrane domain. The first six MORN domains are linked to the remaining two via a joining region that is largely disordered. The MORN domains are thought to associate with the PM through interactions with phosphoinsoitol-containing phospholipids, palmitoylation, or association with adapter proteins, while the transmembrane domain spans the ER (6, 9). The α-helical domain has been proposed to function as a molecular spacer that determines the size of the cleft. Beyond its structural role, Jph1 to Jph4 have emerged as versatile modulators of various ion channels, recruiting and fine-tuning the function of RyR1 to RyR3 (10–12), CaV1/2 (11, 13), and KCNQ channels (10). In skeletal muscle, Jph1 recruits CaV1.1 to triads through a physical interaction with the channel carboxy terminus (13) and serves as one of the minimal requisite components necessary for reconstituting voltage-dependent excitation–contraction coupling (the others being the CaV1.1 β1a subunit and the adaptor protein stac3) (14). In the heart, the interaction of Jph2 with RyR2 stabilizes channel function and prevents diastolic Ca2+ leak (12). In the brain, Jph3/4 recruits CaV1 and CaV2 channels to ER–PM junctions and can selectively modify the inactivation kinetics of CaV2 channels (11). Not surprisingly, constitutive knockout of Jph1 and Jph2 is lethal, while knockout of Jph3 and Jph4 results in deficits in motor coordination, learning, and memory (6). In the heart, acute loss of Jph2 has been shown to result in heart failure and increased mortality, presumably due to deficits in t-tubule maturation and altered Ca2+ homeostasis (12). By contrast, Jph2 overexpression promotes t-tubule formation and is protective against heart failure in mice (15). Human mutations in Jph have been linked to various diseases. Specifically, Jph2 mutations have been linked to hypertrophic cardiomyopathies (HCM), dilated cardiomyopathies, and atrial fibrillation. Jph1 mutations have been identified in Charcot–Marie–Tooth disease, while trinucleotide expansion mutations in Jph3 have been linked to Huntington disease-like 2 syndrome (6). The pathophysiological mechanisms of these human mutations are likely complex, involving changes in ER–PM structure and regulation of multiple ion channels—a seemingly intractable problem.
In this context, the study by Yang et al. (7) represents a major leap forward by furnishing a molecular understanding of Jph and a structural blueprint to dissect the complex effects of human Jph mutations. In this work, the authors have solved the structures of the tandem MORN motifs and α-helix rich domain (ARD) of Jph1 and Jph2 in isolation as well as that of Jph2 bound to a cytosolic fragment of the CaV1.1 channel (7). These structures provide an in-depth view at the bridge linking the plasma membrane and SR and reveal that the β-sheet–rich MORN motifs of Jph1 and Jph2 adopt an exquisite “rib cage”–like repetitive structure, the convex side of which cradles the long contiguous α-helix of the ARD, while the concave side forms a narrow basic groove. In the structure of the Jph2–CaV1.1 complex, the CaV1.1 peptide was found to interact with this groove, suggesting that it may serve as a site of interaction for other plasma membrane-localized binding partners. Interestingly, although the structures of the MORN and ARD regions of the two Jph isoforms were nearly identical in isolation, or when bound to the CaV1.1 peptide, the region that joins the sixth and seventh MORN repeats was found to adopt different conformations in the three structures. In all four Jph isoforms, the joining region between the sixth and seventh MORN motifs is considerably longer than that found between the other MORN motifs and has low sequence complexity, which has led to the prediction that it is intrinsically disordered. While this joining region was truncated in the Jph constructs used by Yang et al. for crystallization, the conformational differences observed in the three structures may provide a glimpse into the ensemble of states sampled by this region. Complementing their structural studies, the authors also undertake functional analysis to demonstrate that disruption of the interaction between Jph1 and the CaV1.1 carboxy terminus through structure-informed mutagenesis diminished CaV1.1 clustering and weakened excitation–contraction coupling, consistent with previous studies (13). As noted, the structures also shed light on how human mutations in Jph1 to Jph3 may ultimately contribute to human disease by disrupting Jph folding and interactions with target proteins. Notably, a number of variants have been found at the interface between the MORN motifs and ARD. As these mutations either remove stabilizing interactions or introduce steric hinderance between these regions, it is expected that they would weaken or prevent this interaction, greatly perturbing the tertiary structure of Jph2. More concretely, biochemical studies revealed that an HCM-linked mutation in Jph2 may weaken its binding to CaV1 channels, potentially informing on pathophysiological mechanisms.
The structures by Yang et al. (7) also raise many new questions. First, the Jph MORN domains have been classically thought to anchor Jph to the PM by binding phosphatidylserine (PS)- and phosphatidylinositol (PI)-containing phospholipids. Surprisingly, the authors found no density corresponding to PS or PI in crystals grown in the presence of these phospholipids. This suggests that the Jph MORN domains may only weakly bind lipids. While it is possible that the high ionic strength utilized for crystallization weakened the Jph–phospholipid interaction, it is also possible that the MORN domain may require additional partners for robust association with the membrane. Second, the authors clearly demonstrate that the association of Jph with the CaV1 channel carboxy terminus is important for determining channel localization. Yet this domain is also a hub for many regulatory proteins that tune channel gating. If so, are there as-yet-unknown effects of Jph2 on channel activity? Superresolution scanning patch-clamp experiments in rat ventricular myocytes hint at spatially altered activity profile of CaV1.2 upon overexpression of Jph2 (16). Third, the present structures provide an unparalleled view of the Jph interfaces involved in binding CaV1 channels. However, this is only one part of the full picture. Jph is also capable of recruiting other ion channels, including the RyR (6). Are there conserved motifs that support binding to the Jph MORN domain? It is also possible that Jph may use multiple interfaces to glue together various channel complexes. Indeed, both the joining region and the carboxyl-terminal divergent regions of Jph have been reported to bind RyR (11, 12).
Excitation–contraction coupling is one of the most fundamental phenomena in physiology, and disruption of this process is at the epicenter of many life-threatening human diseases. Since the early electron micrographs that revealed the ultrastructure of the dyad and the triad (17), identifying the molecular structures and events that translate membrane depolarization to Ca2+ release has been a high priority. Pieces of this elaborate puzzle are now falling into place, one domain at a time.
Footnotes
The authors declare no competing interest.
See companion article, “Structures of the junctophilin/voltage-gated calcium channel interface reveal hot spot for cardiomyopathy mutations,” 10.1073/pnas.2120416119.
References
- 1.Bers D. M., Cardiac excitation-contraction coupling. Nature 415, 198–205 (2002). [DOI] [PubMed] [Google Scholar]
- 2.Bannister R. A., Bridging the myoplasmic gap: Recent developments in skeletal muscle excitation-contraction coupling. J. Muscle Res. Cell Motil. 28, 275–283 (2007). [DOI] [PubMed] [Google Scholar]
- 3.Dickson E. J., Endoplasmic reticulum-plasma membrane contacts regulate cellular excitability. Adv. Exp. Med. Biol. 997, 95–109 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Sahu G., et al. , Junctophilin proteins tether a Cav1-RyR2-KCa3.1 tripartite complex to regulate neuronal excitability. Cell Rep. 28, 2427–2442.e6 (2019). [DOI] [PubMed] [Google Scholar]
- 5.Prakriya M., Lewis R. S., Store-operated calcium channels. Physiol. Rev. 95, 1383–1436 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lehnart S. E., Wehrens X. H. T., The role of junctophilin proteins in cellular function. Physiol. Rev., 10.1152/physrev.00024.2021 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang Z. F., et al. , Structures of the junctophilin/voltage-gated calcium channel interface reveal hot spot for cardiomyopathy mutations. Proc. Natl. Acad. Sci. U.S.A. 119, 10.1073/pnas.2120416119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takeshima H., Komazaki S., Nishi M., Iino M., Kangawa K., Junctophilins: A novel family of junctional membrane complex proteins. Mol. Cell 6, 11–22 (2000). [DOI] [PubMed] [Google Scholar]
- 9.Perni S., The builders of the junction: Roles of Junctophilin1 and Junctophilin2 in the assembly of the sarcoplasmic reticulum-plasma membrane junctions in striated muscle. Biomolecules 12, 109 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang M., et al. , JPH-2 interacts with Cai-handling proteins and ion channels in dyads: Contribution to premature ventricular contraction-induced cardiomyopathy. Heart Rhythm 13, 743–752 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perni S., Beam K., Neuronal junctophilins recruit specific CaV and RyR isoforms to ER-PM junctions and functionally alter CaV2.1 and CaV2.2. eLife 10, e64249 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Oort R. J., et al. , Disrupted junctional membrane complexes and hyperactive ryanodine receptors after acute junctophilin knockdown in mice. Circulation 123, 979–988 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakada T., et al. , Physical interaction of junctophilin and the CaV1.1 C terminus is crucial for skeletal muscle contraction. Proc. Natl. Acad. Sci. U.S.A. 115, 4507–4512 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perni S., Lavorato M., Beam K. G., De novo reconstitution reveals the proteins required for skeletal muscle voltage-induced Ca2+ release. Proc. Natl. Acad. Sci. U.S.A. 114, 13822–13827 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reynolds J. O., et al. , Junctophilin-2 gene therapy rescues heart failure by normalizing RyR2-mediated Ca2+ release. Int. J. Cardiol. 225, 371–380 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poulet C., et al. , Junctophilin-2 tethers T-tubules and recruits functional L-type calcium channels to lipid rafts in adult cardiomyocytes. Cardiovasc. Res. 117, 149–161 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franzini-Armstrong C., Studies of the Triad: I. Structure of the junction in frog twitch fibers. J. Cell Biol. 47, 488–499 (1970). [DOI] [PMC free article] [PubMed] [Google Scholar]