Abstract
Sarcolemmal/plasmalemmal ATP-sensitive K+ (KATP) channels have key roles in many cell types and tissues. Hundreds of studies have described how the KATP channel activity and ATP sensitivity can be regulated by changes in the cellular metabolic state, by receptor signaling pathways and by pharmacological interventions. These alterations in channel activity directly translate to alterations in cell or tissue function, that can range from modulating secretory responses, such as insulin release from pancreatic β-cells or neurotransmitters from neurons, to modulating contractile behavior of smooth muscle or cardiac cells to elicit alterations in blood flow or cardiac contractility. It is increasingly becoming apparent, however, that KATP channels are regulated beyond changes in their activity. Recent studies have highlighted that KATP channel surface expression is a tightly regulated process with similar implications in health and disease. The surface expression of KATP channels is finely balanced by several trafficking steps including synthesis, assembly, anterograde trafficking, membrane anchoring, endocytosis, endocytic recycling, and degradation. This review aims to summarize the physiological and pathophysiological implications of KATP channel trafficking and mechanisms that regulate KATP channel trafficking. A better understanding of this topic has potential to identify new approaches to develop therapeutically useful drugs to treat KATP channel-related diseases.
Keywords: inward rectifier potassium channel, K+channel, KATP channel, trafficking
ATP-SENSITIVE K+ CHANNELS
Sarcolemmal/plasmalemmal ATP-sensitive K+ (KATP) channel opening is regulated by alterations in cytosolic ATP and ADP concentrations, which couples cellular energy metabolism to membrane excitability. This property of “metabolo-electrical” coupling is essential in several biological processes, for example in pancreatic β-cells where KATP channels close in response to increased plasma glucose and intracellular ATP production to trigger insulin secretion (1), in glucose sensitive neurons in the hypothalamus where KATP channels link low glucose levels to feeding behavior (2), in the heart where KATP channel activation during rapid heart rates promote adaptive shortening of the cardiac action potential (3), and during metabolic impairment where KATP channels in the vascular smooth muscle and the endothelium contribute to vasodilatation and increased blood flow (3). In the heart, the actions of KATP channels are most pronounced during pathological states that involve deficits in energy metabolism. During hypoxia, for example, KATP channel opening rapidly shortens the cardiac action potential (3). Sarcolemmal KATP channels are also instrumental in mediating the cardioprotective response of ischemic preconditioning (4).
A KATP channel is composed of four pore-forming inward rectifier potassium channel (Kir6) subunits and four regulatory sulfonylurea receptor (SUR) subunits, which belong to the ATP-binding cassette (ABC) superfamily (Fig. 1A). The Kir6 subfamily has two members, Kir6.1 and Kir6.2, whereas the SUR subfamily has two; namely SUR1 and SUR2, with SUR2 existing as one of at least two splice variants, SUR2A and SUR2B. Pancreatic β-cells predominantly express Kir6.2 and SUR1, smooth muscle cells highly express Kir6.1 and SUR2B, and cardiomyocytes express all subunit isoforms but are relatively abundant in Kir6.2 and SUR2A (3).
Figure 1.
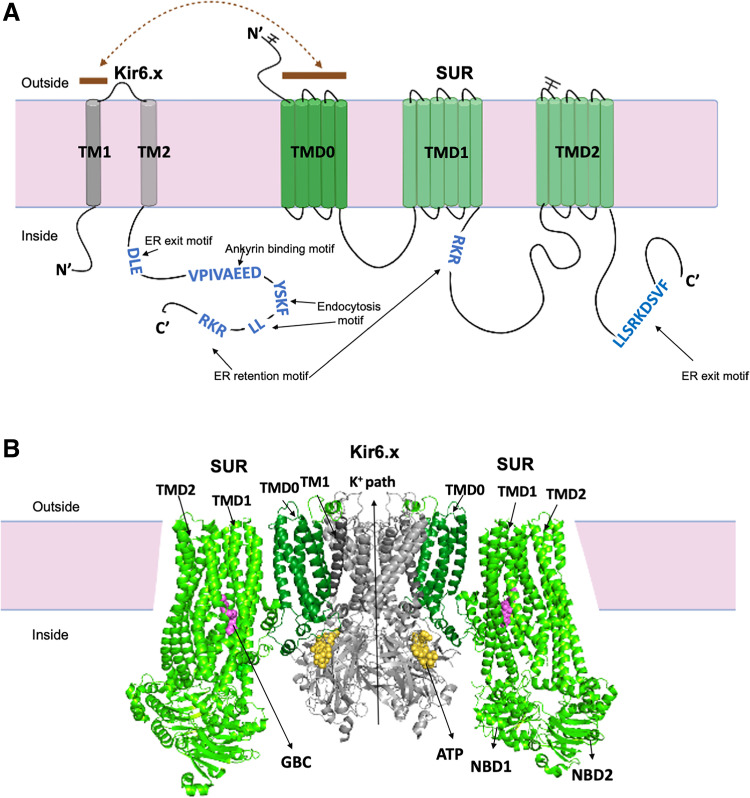
Topology and structural information of Kir6.x and SURx channels. A: topology of the SURx/Kir6.x channel with domains labeled and colored to match the three-dimensional (3-D) structures shown below. The branched sticks represent the location of the two N-linked glycosylation sites in SUR. The sky-blue letters represent the signaling motifs involved in ATP-sensitive K+ (KATP) channel trafficking. The brown dashed line represents the interacting domains between SUR and Kir6 in the assembled KATP hetero-octameric complex. Also shown are some of the trafficking motifs identified in the KATP channel subunits that regulate KATP channel anterograde trafficking, membrane anchoring, and endocytosis. The motif sequences shown are referred to human Kir6.2 and SUR1. B: cryoEM structure of the KATP channel based on a structural model of the pancreatic KATP channel generated using PDB ID 6BAA, with the core Kir6.2 tetramer forming the potassium ion pore (gray). The outer regulatory proteins SUR1 has three transmembrane domains, TMD0 (forest green), TMD1 and TMD2 (pale green). Each Kir6.2 subunit has two transmembrane helices, the inner helix (M2) and outer helix (M1), an intermembrane pore helix. ATP is bound to Kir6.2 (yellow spheres), and the K+ ion path is closed. Glibenclamide (GBC) is captured bound to the channel (purple spheres). ER, endoplasmic reticulum.
SUBCELLULAR TRAFFICKING OF MEMBRANE PROTEINS
The total amount of current through a particular channel type is determined by
| (1) |
where I represents the amount of ionic current in a cell, n the number of available channels, i the unitary channel current, and Po the average open probability of each channel.
Many studies aim to understand how KATP channels are regulated by alterations in Po (e.g., during disease states, with pharmacological interventions, with receptor signaling, etc.). It is becoming increasingly clear that the number of channels (n) is not fixed and can be dynamically altered by de novo protein synthesis, degradation of existing channel subunits, and by endocytic recycling of channels from intracellular compartments to and from the surface. Cells internalize the equivalent of their entire surface area up to five times per hour and most of the endocytosed proteins are recycled back to the membrane (5, 6). Not only is endocytic recycling a rapid process occurring at a time scale of minutes, but the rates of endocytosis and exocytosis are tightly controlled by specialized trafficking proteins and signaling pathways (6). Thus, endocytic recycling is a potentially powerful mechanism to regulate the surface density and availability of ion channels (7). In the case of the KATP channel, the major regulators of function were long thought to directly affect Po, for example, block by intracellular ATP, activation by intracellular ADP, activation by PIP2, opening by KATP channel openers such as diazoxide or pinacidil, and block of the channels by sulfonylureas such as tolbutamide or glibenclamide (3). It is becoming increasingly apparent, however, that some of these factors also affect endocytic recycling and surface expression. Moreover, sulfonylureas not only decrease Po but also enhance channel biogenesis and assembly efficiency thus increasing surface expression, whereas channel openers tend to decrease channel surface expression. This review extends previous publications (3, 8) by highlighting studies demonstrating that modulating the number of available channels (n) is a major regulator of the KATP channel current under both physiological and pathophysiological conditions.
PHYSIOLOGICAL AND PATHOPHYSIOLOGICAL RELEVANCE OF KATP CHANNEL TRAFFICKING
There are several studies demonstrating that KATP channel surface density is dynamically regulated during health, and can be negatively impacted by disease.
Glucose-Induced Trafficking in the Pancreatic β-Cell
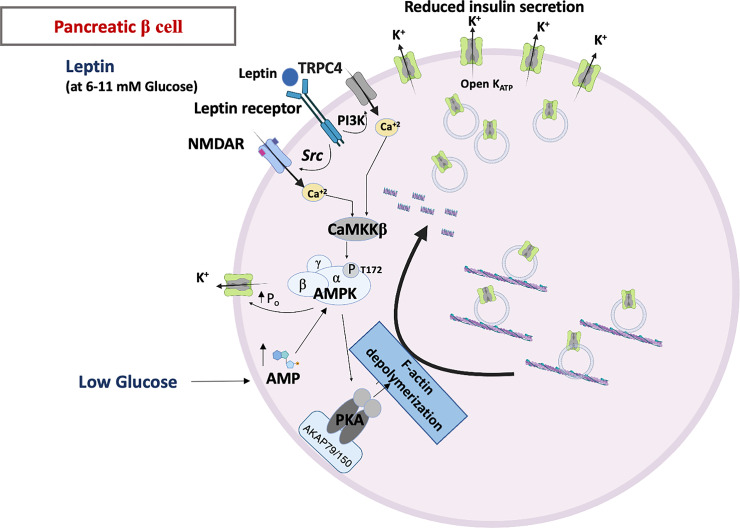
The role that KATP channels have as a regulator of insulin secretion from pancreatic β-cells in the islet of Langerhans is well established. During fasting states, when intracellular ATP levels are low, the KATP channel is open, which clamps the membrane potential to negative values, and in turn mitigates electrical activity of the β-cell. After a meal, glucose uptake and alterations in the ATP and ADP levels inside the cell causes the KATP channel open probability to decrease, which depolarizes the β-cell and sets in motion a cascade of events that includes activation of other ion channels and transporters, action potential firing, increases in intracellular Ca2+, and vesicular release of insulin from intracellular granules (9). Hormones released from the gut after a meal, such as glucagon-like peptide 1 (GLP-1), sensitize this process, in part by further decreasing the KATP channel open probability through a noncanonical cAMP/EPAC-mediated pathway (10). What is not commonly appreciated, however, is that changes in KATP channel open probability are mirrored by corresponding trafficking alterations and that the change in the number of surface channels (n) may be as important, if not more important, than the changes in activity (Fig. 2). In one of the earliest studies demonstrating a link between glucose and trafficking, glucose stimulation was found to increase KATP surface expression (11). A more recent study, however, suggests the opposite namely that the surface expression of Kir6.2 is profoundly increased by glucose deprivation, an intervention that also decreases the open probability of the channel, without changes in the total Kir6.2 protein level (12). This process is reversible and occurs with a relatively rapid time scale (in the minute range), strongly indicative that trafficking mechanisms are involved. Intracellular energy homeostatic changes caused by glucose deprivation lead to an increase in intracellular AMP levels, which activates AMP-activated protein kinase A (AMPK) (13). This pathway turns out to be key: apart from the fact that AMPK strongly enhances the open probability of KATP channels (14), AMPK activation also plays an integral role in increasing the surface expression of KATP channels upon glucose deprivation in pancreatic β-cells (12). This action of AMPK goes beyond glucose deprivation. Leptin, a hormone released by fat cells and is known to regulate insulin secretion, also increases the KATP channel activity (15). Consistent with a trafficking mechanism, an early study has demonstrated that leptin activation of KATP channels involves disruption of the actin cytoskeleton (16). Indeed, subsequent studies have shown that leptin transiently increases the KATP channel surface expression in a manner that depends on AMPK activation (17, 18) and Rac-mediated remodeling of the actin cytoskeleton (19). This increase results primarily from increased channel trafficking to the plasma membrane rather than reduced endocytosis of surface channels. Thus, surface trafficking is a necessary element to regulate KATP channels in pancreatic β-cells.
Figure 2.
Signaling pathways involved in ATP-sensitive K+ (KATP) channel trafficking in the pancreatic β-cell. In the pancreatic β-cell, AMP-activated protein kinase A (AMPK) is activated by phosphorylation at T172 site either in low glucose conditions where AMP levels are elevated, or in the presence of adipocyte hormone, leptin (at 6–11 mM glucose). Leptin binding to its biospecific receptor elicits a downstream signaling cascade that involves an increase in Ca2+ influx into the cells through either activation of surface TRPC4 channels or potentiation of NMDA receptors (NMDAR) activity, which activates CaMKKβ leading to phosphorylation and activation of AMPK. Active AMPK elicits activation of AKAP79/150-plasma membrane-anchored PKA, which then triggers depolymerization of the actin cytoskeleton to allow vesicles containing KATP channels to traffic to the plasma membrane.
Subcellular Trafficking in Vascular Smooth Muscle
Vascular smooth muscle expresses KATP channels that consist of a combination of Kir6.1 and SUR2B subunits (20). There is ample evidence that KATP channels regulate smooth muscle tone and blood flow, particularly in small vessels. During hypoxia, for example, increased KATP open probability causes smooth muscle relaxation (21). PKA signaling, which causes coronary vasodilation, also stimulates vascular KATP channel open probability (22). By contrast, PKC signaling, associated with vasoconstriction, inhibits vascular KATP channel opening. In parallel with the decreased open probability of vascular KATP channels, PKC also decreases the number of surface KATP channels (23). In the latter study, PKC activation with phorbol esters led to a decrease in KATP channel current, which was prevented by a dominant-negative dynamin mutant. Internalization was also observed in response to the vasoconstrictor, angiotensin II, demonstrating a role for internalization by endocytosis as a key regulator of vascular KATP channel function. Apart from this single study, little is known about regulated surface expression of vascular KATP channels.
Subcellular Trafficking in Cardiomyocytes
Spatially defined subcellular expression patterns.
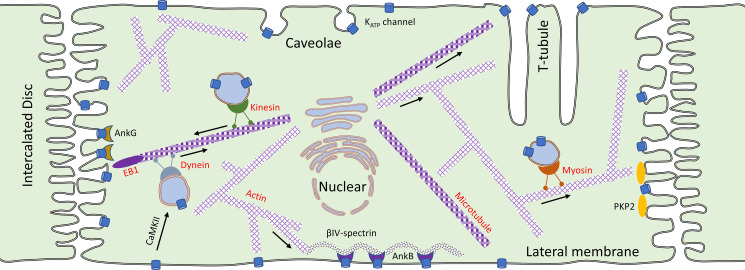
Cellular functions often depend on the distribution of ion channels, transporters, and pumps to specialized areas of the cell membrane. For example, voltage-gated Na+ and K+ channels are concentrated at the nodes of Ranvier, which are essential for saltatory conduction in myelinated nerves, whereas nicotinic acetylcholine receptor channels are mainly at the postsynaptic membrane of the neuromuscular junction, to initiate excitation-contraction (EC) coupling of skeletal muscle. Targeted trafficking of several membrane proteins occurs in cardiac myocytes and the currently proposed mechanisms are summarized in Fig. 3. For example, there is a distinct subpopulation of L-type Ca2+ channels (LTCCs) present at the transverse (T)-tubule, where their close proximity with ryanodine receptors (RYR2) of the sarcoplasmic reticulum (SR) allows for Ca2+-induced Ca2+ release from the SR and cardiac contractions (24). Some LTCCs, however, are targeted to another physically and functionally distinct location, namely to lipid rafts and caveolae (25), where they are subject to local regulation by signaling pathways (26, 27). Separate pools of Na+ channels have also been identified in cardiac myocytes (28). One pool is targeted to the lateral membrane by the syntrophin/dystrophin complex (29, 30). Another subpopulation of Na+ channels is organized in a highly specialized macromolecular complex at the intercalated disk (ICD) (31), where targeting and anchoring is coordinated by AnkG, SAP97, EB1, and PKP2 (28, 32). The concept of regional specification of channels has recently been extended to K+ channels. For example, some inward rectifier K+ channels (Kir2.1) localize to the perinexus (33) and may co-traffic with Nav1.5 (34). A subpopulation of another class of K+ channels (the Kv2 delayed rectifier) clusters in specific membrane subcompartments in cardiomyocytes (35) and neurons (36), whereas other Kv2 channels locate to lipid rafts (37). There is now evidence that the clustered Kv2 channels are in fact nonfunctional (38) and serve an important scaffolding role, interacting with VAP proteins (VAMP-associated proteins) to initiate membrane/endoplasmic reticulum (ER) contact sites (39, 40), which have essential roles in a variety of biological processes (41). Thus, understanding the channel composition and their presence at specific membrane compartments within a cardiac myocyte provides new insights into the mechanism of electrical excitation and may pave the way for novel therapies against cardiac rhythm disturbances. There may also be distinct KATP channel subpopulations within a cardiac myocyte. Similar to the cardiac Na+ channel, KATP channels localize to at least two subcellular membrane domains in a ventricular myocyte (Fig. 3).
Figure 3.
Targeted trafficking of channels in cardiac myocytes. Depicted is a cardiac myocyte that connect to its immediate neighbors in an end-to-end manner at the intercalated disk (ICD). De novo synthesized membrane proteins can be targeted to specific subcellular regions by anterograde trafficking mechanisms and by anchoring to specific subcellular domains. Shown are targeted delivery via myosin or microtubule based mechanisms and targeted delivery to lateral membranes, the specialized t-tubule structures or the ICD. Channels can be stabilized at specific regions through anchoring mechanisms [e.g., binding to ankyrin-B (Ank-B) or ankyrin-G (Ank-G)] or by localization to specific subcellular domains, such as caveolae. Our understanding of ATP-sensitive K+ (KATP) channel subcellular trafficking in a cardiac myocyte is still in its infancy.
The presence of KATP channels in lateral membranes was established by their initial identification with patch clamp methods (42). Early scanning ion conductance microscopy, combined with whole cell voltage clamping, suggested that lateral KATP channels are present as submicrometer clusters in Z-grooves of the sarcolemma (43). Moreover, the Kir6.2 subunit of KATP channels was shown to physically interact with ankyrin-B (AnkB) (44, 45), which is expressed at lateral membranes in cardiac myocytes and localizes to Z- and M-lines in an isoform-dependent manner (46). Finally, some KATP channels localize to caveolae (47, 48), which are present in t-tubules and lateral membranes (49).
KATP channels are also present at the ICD region (Fig. 3), which links cardiac myocytes end-to-end, ensures rapid propagation of electrical impulses between cells, and allows mechanical coupling sufficiently strong to withstand the mechanical forces during cardiac contraction. While searching for novel KATP channel interactors with mass spectrometry approaches, we previously identified desmosomal proteins, including plakoglobin (PG) and plakophilin-2 (PKP2) (50, 51). We verified that they physically interact with KATP channels with coimmunoprecipitation assays (50, 51). With differential centrifugation assays under detergent-free conditions, we found that rat ventricular KATP channel subunits cosegregate with PKP2, but not with markers of other ICD substructures including Cx43 (gap junctions). Kir6.2 colocalizes with PG and PKP2 in confocal immunofluorescence microscopy. Using super-resolution microscopy techniques, we demonstrated that KATP channels cluster within nanometer distances from these junctional proteins at the ICD region of cardiomyocytes (51). From recordings made in the inside-out or in the cell-attached patch configuration, we found that the local KATP channel density in membrane patches at the ICD region of isolated mouse or rat cardiomyocytes is substantially higher than at lateral membranes, and that this difference is mitigated in PKP2+/− mice (51).
Functional interaction between KATP channels and Na+ channels at the ICD.
A theme in channel biology that has received much recent activity is that ion channels, exchangers, and pumps present in the same subdomain have the potential to physically and functionally interact with each other. For example, there are numerous studies demonstrating functional interaction between KATP channels and the Na+/K+ pump in a variety of cell type, including cardiac myocytes (52–58). Although physical interaction between these proteins is suggested by mass spectrometry data showing that the Na+/K+ ATPase α-subunit was present in KATP channel immunoprecipitates (50), the structural basis and mechanisms of physical interaction between these two proteins remain to be elucidated. The enrichment of both Na+ and KATP channels at the cardiac ICD and the fact that both interact with desmosomal proteins and are similarly affected by PKP2 deficiency (51, 59, 60) suggested the possibility that these two channels may functionally interact. Indeed, these two channels were found to reciprocally regulate each other in heterologous expression systems. Overexpressing the KATP channel subunits led to a decrease of Nav1.5 Na+ channel subunit, and overexpression of Nav1.5 led to a decrease of Kir6.2/SUR2A channel current. The relevance of this finding to cardiomyocytes was demonstrated by the finding that overexpression of Kir6.2 by adenoviral delivery significantly reduced the whole cell Na+ current density in cardiomyocytes (61). Using super-resolution microscopy techniques, it was found that native Na+ and KATP channels co-cluster at the ICD of cardiac myocytes. Mechanisms of anchoring and co-clustering will be discussed in Anchoring Mechanisms. Although the functional relevance of co-clustering of these two channels is still under investigation, this study provides a paradigm in which to consider the pharmacological and genetic aspects of arrhythmogenesis.
Dynamic changes in surface expression during physiological conditions.
Changes in surface expression of KATP channels in cardiac myocytes under physiological conditions are suggested from the literature, but not well studied. For example, exercise training of mice for only 5 days leads to an approximately two times increase in the number of KATP channels per patch in isolated cardiac myocytes, with relatively small changes in mRNA expression (62). Given that AMPK is activated with exercise training, and that the positive regulation of KATP channel surface expression can occur via this pathway (see the previous section), the untested possibility is raised that elevated surface expression occurred. Regardless of the mechanism, the physiological consequence of the increased surface expression of KATP channels with exercise is clear. With an elevated heart rate, such as during strenuous exercise, action potential duration adaption occurs over the time course of a few minutes (63). This progressive action potential shortening allows for adequate filling time for the ventricle, and is responsible for a decreased refractoriness and prevention of arrhythmias. Loss of KATP channel activity with glibenclamide, or cardiac-specific overexpression of dominant-negative Kir6 subunits strongly mitigates action potential frequency adaptation (62). There is a strong possibility that AMPK-mediated changes in KATP surface expression underlies this response and future studies are needed to investigate this possibility and the potential role(s) of altered KATP channel surface expression in other physiological processes.
Dynamic changes in surface expression during pathophysiological conditions.
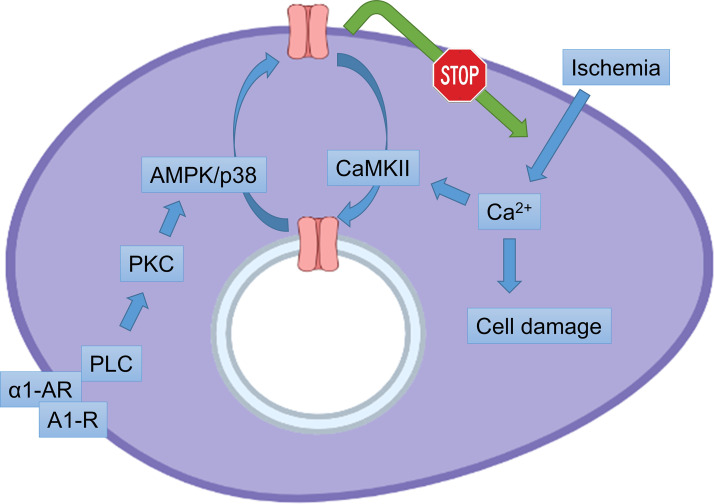
Dynamic alterations in KATP channel surface expression in cardiomyocytes under pathophysiological conditions is an emerging topic in the literature (Fig. 4). The role of KATP channels in the cardioprotective effects of ischemic preconditioning (IPC) has been well established—both using pharmacological and genetic approaches (3). To our knowledge, one of the first studies demonstrating that an increase in surface KATP channels underlies the protective effects of IPC came from a study using a mouse model with transgenic overexpression of a specific CaMKII inhibitory peptide AC3-I (64). Cardiomyocytes from the transgenic mice exhibited significantly increased IK,ATP current density and greater sarcolemmal expression of Kir6.2, without significant changes in total Kir6.2 expression or alterations in open probability or other channel properties. The AC3-I transgenic hearts were protected against infarct development after ischemia/reperfusion to the same extent as with a KATP channel opener. Although direct evidence was not provided, this study strongly suggested that myocardial CaMKII inhibition confers resistance to ischemia-reperfusion injury (similar to IPC) by enhancing the surface expression of KATP channels (64). Acute upregulation of IK,ATP density was directly demonstrated in a cellular model of pharmacological preconditioning using isolated rat cardiac myocytes (65). In the latter study, pretreatment of myocytes with an α1 adrenergic receptor agonist phenylephrine afforded improved protection against cell death after oil-overlay ischemic pelleting, which was prevented by a KATP channel blocker, strongly suggesting that the enhanced KATP channel surface expression underlies protective effects of preconditioning. Interestingly, a role for AMPK, PKC-δ, and p38 was suggested to underlie the increase in IK,ATP current induced by phenylephrine preconditioning (65).
Figure 4.
Mechanisms that regulate ATP-sensitive K+ (KATP) channel subcellular trafficking in a cardiac myocyte during ischemia and ischemic preconditioning. Prevailing data show that sarcolemmal KATP channels are protective against metabolic stress, possibly by reducing Ca2+ overload (e.g., during ischemia) and/or preserving mitochondrial function. Ischemia appears to mitigate the protective effects of KATP channels by causing internalization through endocytosis, mediated by a CaMKII-dependent mechanism. One of the cardioprotective mechanisms of ischemic preconditioning may reside in the activation of a PKC/AMPK/p38 signaling axis, which promotes KATP channel surface expression, which counteracts the detrimental ischemia-induced internalization of KATP channels from the sarcolemma. Further research into this proposed mechanism is needed, which could lead to new therapeutic approaches to develop cardioprotective strategies.
An interesting twist to the tale came about when the mechanism by which CaMKII regulates KATP channel surface expression was elucidated. The Zingman’s laboratories demonstrated that the β1 adrenergic receptor agonist isoproterenol led to a reversible inhibition of whole cell IK,ATP in isolated mouse ventricular cardiomyocytes over a time course of minutes and that this inhibition did not occur in myocytes overexpressing the CaMKII inhibitory peptide AC3-I (66). An acute trafficking mechanism was suggested by the finding that surface density of Kir6.2 and SUR2A (measured with surface biotinylation assays) was decreased by CaMKII activation. Indeed, a role for the Ca2+/CaMKII pathway in stimulating endocytosis was demonstrated by the finding that internalization was prevented by a dominant negative dynamin peptide or the endocytosis inhibitor, dynasore. A tyrosine-based motif was identified as mediating endocytic internalization (66) (see Sorting and Anchoring Motifs in KATP Channel Subunits).
A study with cardiac-specific Kir6.2 knockout mice clarified the role of KATP channels in the perspective of cardiac ischemia and IPC (4). As expected, hearts from wild-type mice were protected from infarct development after ischemia by IPC perfusion protocols or by adenosine (67). Cardiomyocyte-specific Kir6.2 knockout did not mitigate infarct development after ischemia, but reversed the protective effects of IPC or adenosine, demonstrating a key role for KATP channels in the protective mechanisms. In parallel, KATP channel subunit expression was measured in membrane fractions obtained with density gradient centrifugation techniques. KATP channel subunits were present both in fractions enriched with sarcolemmal proteins and in membrane fractions that contain endosomal proteins. In an early study, surface KATP channel expression was found to be increased during ischemia (68). Experimental conditions were largely uncontrolled in the latter study, however, since the ischemia model used was to excise hearts from rats 20–30 min postmortem. When performing experiments under laboratory controlled conditions (temperature, ionic compositions, etc.), it was found that ischemia actually reduces the KATP channel content in sarcolemmal fractions, which was prevented by a cocktail of trafficking inhibitors (brefeldin A, colchicine, and nocodazole) or by the inhibitor of endocytosis, dynasore. Moreover, consistent with the findings discussed in the previous paragraph, CaMKII inhibition also prevented ischemia-induced KATP channel internalization. Thus, unlike the paradigm in pancreatic β-cells and smooth muscle cells, where KATP channel activity parallels surface expression changes (i.e., reduced channel activity is associated with internalization), the reverse seems to occur in cardiomyocytes, where the expected activation of KATP channel opening during ischemia (69) is associated with internalization. This is a counterintuitive phenomenon since KATP channels are cardioprotective (70) and internalization would mitigate this protective mechanism. The same study highlighted a mechanism by which KATP channels contribute to the protective effects of ischemic preconditioning. Building on the idea that endosomal KATP channels may serve as a reservoir for recycling of channels to the surface (68), a key finding was made that the internalized KATP channels are restored to the cell surface with ischemic preconditioning or by adenosine pretreatment (4). This process was PKC dependent, highlighting at least one of the mechanisms by which PKC activation participate in the protective effects of preconditioning. Overall, these studies make a strong case for a role of subcellular trafficking and endocytic recycling in the well-described protective effects of KATP channels.
The time course of dynamic regulation of KATP channel turnover in cardiac myocytes has not been studied formally, mainly due to the challenges of studying native channels with currently available antibodies. Experiments performed with heterologous expression systems suggest a rapid turnover rate in the order of minutes (see mechanisms of subcellular trafficking and endocytic recycling of kATP channels). Experiments with an electrophysiological approach in cardiomyocytes showed that CaMKII-dependent internalization (and reversal) similarly occurs in a time scale of minutes (66), suggesting that dynamic regulation of KATP channel surface expression is rapid in the heart. Experiments with antibody uptake and recycling or similar approaches are needed to verify this expectation. Such experiments are expected to be challenging given the compact nature of the cardiomyocyte microarchitecture and the limitations of the available antibodies against native KATP channels.
Trafficking Defects Caused by Genetic Variants in KATP Channel Genes
Variants in KATP channel genes, KCNJ8, KCNJ11, ABCC8, and ABCC9 are associated with several disease states and syndromes in humans (3, 71). The most extensively studied are those in KCNJ11 and ABCC8 encoding Kir6.2 and SUR1, respectively, which form the predominant Kir6.2/SUR1 KATP channel subtype in pancreatic endocrine islets and neurons. Variants that cause loss of channel function (LOF) underlie congenital hyperinsulinism, which is characterized by persistent and unregulated insulin secretion and life-threatening hypoglycemia (72). By contrast, variants resulting in gain of channel function (GOF) are the most common cause of neonatal diabetes mellitus (73), found in ∼40% of patients diagnosed with permanent diabetes at <6 mo of age (74). Some GOF mutations also lead to developmental delay, epilepsy in additional to neonatal diabetes, known as the DEND syndrome (74). Although many of the loss- or gain-of-function variants affect KATP channel gating, many are found to prevent or reduce channel trafficking to the plasma membrane (75) especially those associated with congenital hyperinsulinism. Counterintuitively, several neonatal diabetes/DEND syndrome associated mutations also reduce channel expression at the plasma membrane. With these mutations, the severe gating defects override reduced surface expression to yield an overall gain of function disease phenotype (76–78). For most trafficking mutations reported to date, misfolding or misassembly of channel subunits leading to ER retention and proteasome degradation of mutant proteins account for the trafficking defect. However, a Kir6.2 mutation E282K at a di-acidic ER exit signal, 280DLE282 has been reported to disrupts Sar1-GTPase-dependent ER exit (79). Of note, trafficking defects of a subgroup of ER-retained mutants can be corrected by treatment with antidiabetic sulfonylureas or other pharmacological chaperone molecules (75). These mutations are seen clustered at the SUR1-TMD0 and Kir6.2 TM1 helix where the two subunits make contact in recent cryoEM structures of the channel (Fig. 1B) (80, 81). Mutations at this interface likely interfere with channel assembly. In cryoEM structures of channels bound to pharmacological chaperones, the distal N-terminus of Kir6.2 lies in a cleft formed by the two transmembrane domains of the ABC core of SUR1 and adjacent to the chaperone bound in the transmembrane helix bundle above the first nucleotide binding domain. The distal N-terminus of Kir6.2 has been shown to be involved in KATP channel assembly (82, 83) and high affinity binding of sulfonylureas (84). Thus, sulfonylureas and other KATP pharmacological chaperones likely facilitate assembly of mutant channels by stabilizing interactions between Kir6.2-N terminus and the SUR1 ABC core to correct mutant channel trafficking defects (81).
Additional diseases, including Brugada syndrome, ventricular fibrillation with early repolarization, early infantile epileptic encephalopathy, sudden infant death syndrome, heart failure, dilated cardiomyopathy, familial atrial fibrillation, coronary spasm, acute myocardial infarction (MI), Cantú syndrome, and AIMS (ABCC9-related Intellectual disability Myopathy) syndrome have been linked to variations in KATP channel genes (3, 71). Among these, Cantú syndrome has been most definitively linked to KCNJ8 and ABCC9 mutations causing gain of function of vascular KATP channels comprising Kir6.1 and SUR2B (85). Whether any of the genetic variants associated with the aforementioned diseases affect trafficking and surface expression of the resulting channels remain largely unexplored.
MECHANISMS OF SUBCELLULAR TRAFFICKING AND ENDOCYTIC RECYCLING OF KATP CHANNELS
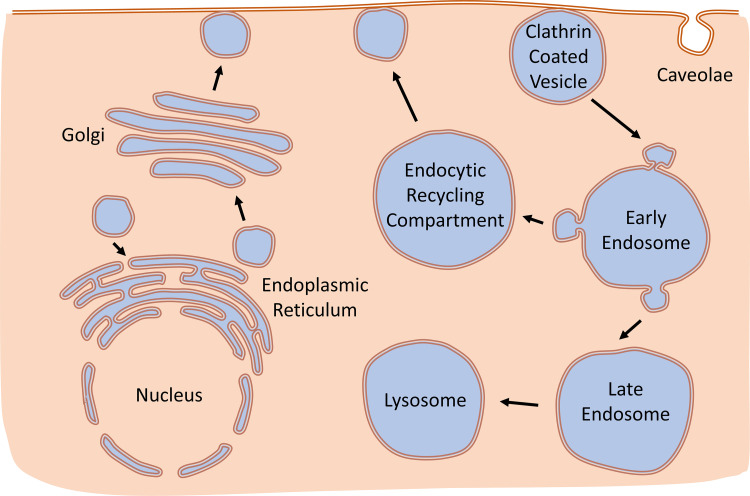
The surface expression of KATP channels is regulated by a fine balance between synthesis, assembly, anterograde trafficking, membrane anchoring, endocytosis, endocytic recycling, and degradation (Fig. 5). There are multiple trafficking steps and each of these is tightly controlled by enzymatic activity, subunit interaction, specific amino acid targeting motifs present in the transported cargo, posttranslational modifications of trafficking proteins, and amino acid mutations causing misfolding defects of the cargo. In the case of the KATP channel, an additional element to consider is that there are multiple possible subunit combinations of Kir6.1, Kir6.2, SUR1, SUR2A, and SUR2B and that isoform-specific sequence differences and assembly may result in shared and cell-specific trafficking mechanisms. Factors that determine surface expression density of membrane proteins include the rate of protein synthesis, modifications taking place within the endoplasmic reticulum (ER), and the activity of trafficking proteins that interact with specific membrane-bound cargos. These determine the rate of the anterograde trafficking pathway (when a protein travels from the ER to the plasma membrane through the Golgi apparatus). Protein stability at the plasma membrane is determined in part by anchoring mechanisms and/or localization in specific subcellular domains (e.g., t-tubules, the ICD, or caveolae). The presence of channels or transporters at the membrane is a transient phenomenon since all membrane proteins are endocytosed, after which they can be recycled to the plasmalemma or trafficked to the cell’s protein degradation machinery.
Figure 5.
A simplified depiction of some of the subcellular processes responsible for ATP-sensitive K+ (KATP) channel endocytic recycling. De novo synthesized KATP channel complexes are assembled in the endoplasmic reticulum and are directed to the plasma membrane via the Golgi apparatus, where specific posttranslational modifications (e.g., glycosylation) take place. Surface channels can be stabilized by several anchoring mechanisms (not shown here). Multiple lines of evidence suggest that plasmalemmal KATP channels are internalized through a dynamin- and clathrin-dependent endocytic process. Endocytosed vesicles are likely directed to early endosomes, from where trafficking can be directed to late endosomes (which may serve as a subcellular buffer) or to the endocytic recycling compartment, from where channels may be redirected for surface expression. There are multiple trafficking steps, proteins controlling these steps, and their regulation by receptor signaling pathways that remain to be elucidated.
Transcriptional Regulation, Protein Stability, and Degradation
Regulation of KATP channels by transcriptional alterations are well documented, but not well studied mechanistically. Prevailing data show that transcription of Kir6.2 mRNA can be upregulated by mechanical stress (86). The subunit expression of KATP channels is also altered following hypoxia or ischemia. In H9c2 rat heart cells, hypoxia upregulates mRNA expression of Kir6.1 and SUR2A (87, 88). These changes occur with a relatively slow time course. For example, in a model of left coronary occlusion of rat hearts upregulation of Kir6.1, SUR1, and SUR2A mRNA was reported to occur after 8–20 wk postinfarction (89). Similarly, a strong increase in Kir6.1 mRNA expression was observed 2–6 h after renal ischemia in the rat (90), and in rat heart, Kir6.1 mRNA expression was upregulated after 60 min of myocardial regional ischemia followed by 24–72 h (but not 3–6 h) of reperfusion (91). The latter study reported that Kir6.2 and SUR2 mRNA expression levels were unchanged. The local renin-angiotensin system was described to have a role in this process (92). Transcription factors that have been implicated in KATP channel mRNA expression include forkhead box (FOX) O1 and hypoxia-inducible factor (HIF)-1α (93, 94).
Protein stability may also influence KATP channel availability. This is not a rate-limiting factor, however, since the half-life of Kir6.2/SUR1 and Kir6.2/SUR2A channels is ∼15–20 h (68, 95–97). When expressed by itself, the half-life of Kir6.2 is ∼2 h, suggesting that association with SURx stabilizes the protein complex (98). Kir6.1 protein levels are upregulated in hearts and brains of rats subjected to chronic hypoxia, whereas Kir6.2 protein expression levels are downregulated by hypoxia (89). It has been suggested that tissue hypoxia may operate through posttranscriptional mechanisms by inhibiting Kir6.2 protein degradation (86). The mechanisms of KATP channel expression can be twofold by involving both lysosomal pathway as has been shown upon PKC activation (99) and the ubiquitin-proteasome degradation pathway since degradation of both Kir6.2 and SUR1 is mitigated by the proteasome inhibitor MG132 (96, 98).
The expression and function of KATP channels in the hours to days following ischemia/reperfusion is an important topic for future studies. Importantly, patients experiencing a myocardial infarction are at an increased risk for a second cardiac event in the post-MI period (e.g., re-infarction, heart failure, and sudden death). This occurs despite the availability and use of effective methods for limiting infarct size with thrombolytic agents and primary angioplasty. Given the protective role of KATP channels in stress events, it is essential to understand how their expression and function is altered following an ischemic episode. It is clear, however, that these transcriptional and posttranscriptional changes are slow relative to the dynamic alterations in endosomal recycling, which represents another important therapeutic target.
Folding and Assembly in the Endoplasmic Reticulum
Proper folding, essential posttranslational modifications, and correct assembly of multisubunit complexes are necessary steps for membrane proteins to exit the endoplasmic reticulum and to be forwarded along the anterograde trafficking pathway to the plasmalemma. Soon after the molecular identification of KATP channel subunits it was realized that Kir6.x and SURx subunits need to be co-expressed to form functional channels (100). Mechanistic insight was provided by the finding that co-assembly of Kir6/SUR subunits is needed for effective membrane trafficking (101). The latter study has identified a specific ER retention motif (RKR) in both Kir6.x and SURx subunits (RXR in SUR2). These motifs were proposed to hide in the co-assembled protein, thus allowing the protein complex to escape the ER and enter the anterograde pathway. Unfortunately, these motifs are not well resovled in the cryoEM structures reported to date to confirm this notion. Aside from this ER retention motif, a di-acidic motif (DXE) in Kir6.2 was found to function as an ER exit signal, which concentrates KATP channels into COPII-enriched ER exit sites before ER export via a process that requires Sar1-GTPase (79). Some reports suggested that SUR subunits may also have an ER exit motif (LLXXXXXXF) that functions as an anterograde signal, required for KATP channels to exit the ER/Golgi compartments (102). This result is controversial, however, since the distal C-terminus of SUR1 was reported not to be required for efficient surface expression (82). A pair of conserved cysteine residues at the N-terminus of SUR1 (C6 and C26), initially identified in ABCB6, forms an intramolecular disulfide bond critical for ER processing (103). Indeed, the C6G mutation in patients shows greatly reduced surface KATP channel expression (8). The interesting possibility was raised that these and other trafficking motifs might serve to create a reservoir of intracellular channels to regulate and buffer the number of available surface channels (101). Even though studies reviewed here are making headway to understanding this phenomenon, there remains many open questions and there is much work to be done to gain a full understanding.
Amino acid substitutions due to missense variants in KATP channel genes may cause trafficking defects (see Trafficking Defects Caused by Genetic Variants in KATP Channel Genes). These mutations may cause misfolding defects leading to slow trafficking or targeting for degradation. Misfolded KATP channels may form complexes with Derlin-1 and an associated p97 protein, which promotes KATP channel degradation (104). Consistent with the role of p97 in protein ubiquitination, ubiquitin proteasome inhibitors or Derlin-1 knockdown slows the degradation rate and increases surface expression of KATP channels in pancreatic β-cells or heterologous expression system (96, 104).
Anterograde Trafficking
Little is known about the anterograde trafficking of KATP channels, but there is indirect evidence from the studies with SUR1 glycosylation. SUR1 is glycosylated at two N-linked glycosylation sites (105). The mature, complex glycosylated form of SUR1 can be distinguished from the core-glycosylated form with SDS-PAGE (101). The N-glycosylation is sensitive to Endo H digestion when occurs in the ER, but is insensitive to Endo H after modification in the Golgi. Since the mature form of SUR1 resist Endo H digestion (102, 106), one can assume that KATP channels traffic through the Golgi. A few regulators of anterograde trafficking have been identified. The ER-retention signal (RKR) can be recognized by both COPI vesicle coat and 14-3-3 proteins (107, 108). This competitive binding results in promoted KATP channel anterograde trafficking by 14-3-3 while inhibited by COPI (109). Syntaxin-1A, a SNARE protein, binds to the intracellular nucleotide binding domains of SUR1 and SUR2 (110, 111), impairs maturation and anterograde trafficking of KATP channels by retaining KATP channels in the endoplasmic reticulum-Golgi intermediate compartment (112). Finally, Rab8a was shown to be involved in membrane trafficking of Kir6.2 in the MIN6 insulinoma cell line, but the subcellular mechanism is unresolved (113). Exploring anterograde trafficking mechanisms must be a focus for future research since there are many unresolved questions. For example, we know that targeted delivery of certain proteins (e.g., Cx43 and Nav1.5) to the cardiomyocyte intercalated disk is by microtubule-based vesicle transport pathways and a microtubule plus-end binding protein EB1 (31, 114), but how KATP channel trafficking to this subcellular structure is determined is not yet known (Fig. 3).
Internalization
Even though KATP channels are anchored at the surface membrane, they are continuously being internalized and recycled to the membrane. In an antibody uptake experiment with transfected cells, ∼50% of SUR1/Kir6.2 channels was found to be internalized within 5 min, reaching a maximum by 15 min. SUR2-containing channels are internalized even more rapidly than SUR1-containing channels (68, 115). Although it was initially suggested that KATP channels are internalized via a caveolar-based mechanism (23), nystatin, which blocks caveolae-mediated endocytosis, does not reduce internalization (116). The reality is that caveolae are highly immobile membrane microdomains and are generally not thought to be involved in constitutive endocytic trafficking (49). The current consensus is that KATP channels are internalized through a dynamin- and clathrin-dependent endocytosis pathway. Readers are referred to an excellent review on this topic (117). In brief, the adaptor complex AP-2 is recruited to the inner membrane face, which serves to link clathrin to the membrane and to coordinate the structural assembly of the clathrin coat with the selection of specific cargo proteins and lipids. The membrane develops curvature until a deeply invaginated clathrin-coated pit is formed. Dynamin GTPase mediates fission of the coated pit to form the newly budded endocytotic vesicle that can be further transported in the endocytic recycling process. Evidence that KATP channels are internalized through this pathway include observations that 1) internalized KATP channels colocalize with clathrin, 2) the Kir6.2 C-terminus directly interacts with the μ2 subunit of the AP2 adaptor complex, and 3) a dominant-negative form of μ2 subunit of the AP2 adaptor complex (D176A/W421A), but not the wild-type μ2, inhibits KATP channel endocytosis (115). A role for dynamin is demonstrated by the findings that endocytosis can be prevented by dominant negative dynamin-1 and dynamin-2 (K44A), but not their WT versions (115), by a dominant negative dynamin peptide (66) and by the dynamin inhibitor dynasore (4, 116, 118). Other indirect evidence that KATP channel endocytosis occurs through a clathrin-dependent pathway includes the finding that it is blocked by chlorpromazine or potassium depletion (116). It is not clear whether SUR contains endocytosis motifs. Some studies suggested that SUR1 expressed by itself (with a mutated ER retention signal) does not internalize by itself, but can be internalized when coexpressed with Kir6.2 (115). Another study, however, suggested that SUR1 by itself with the ER retention signal mutated (RKR to AAA mutant) can undergo endocytosis independent of Kir6.2 (116). There appears to be better consensus with Kir6.2 since endocytosis of an HA-tagged Kir6.2 truncation mutant (Kir6.2Δ36) expresses better at the cell surface in the absence of SURx subunits (66). Moreover, a chimeric protein of CD4 with the Kir6.2 C-terminus (178–364; lacks RKR) can be endocytosed, whereas CD4 by itself is not (115). Internalization of Kir6.1 channels remains to be studied.
Endocytic Recycling
The majority of endocytosed membrane proteins recycle back to the surface membrane (6). Endocytosed proteins first enter early endosomes (Fig. 5). Some proteins are directly and efficiently recycled to the plasma membrane by Rab4 from the early endosomes, including internalized or “desensitized” β2-adrenergic receptors (119, 120). Most other cargo is recycled back to the plasma membrane via a somewhat slower pathway through the endocytic recycling compartment (ERC). An antibody capture assay showed that KATP channels are recycled within ∼10 min (99), suggesting that KATP channels recycle through the ERC. Indeed, KATP channel subunits colocalize with Rab11 (48), which is often used as an ERC marker (121) but can also influence trans-Golgi network-to-plasma membrane transport (122). Moreover, the GFP-Rab11-S25N dominant interfering mutant, known to prevent exit of cargo from the ERC (123), led to accumulation of KATP channels in the ERC, strongly implicating this pathway in KATP channel recycling, similar to the well-described Rab11B-mediated pathway for recycling of transferrin (124). Other channels that follow a Rab11-dependent recycling pathway include Kv1.5 (125–127), KCNQ1/KCNE1 (128), hERG (129), HCN4 (130), TRPC6 (131), and CaV2.2 (132), whereas some others such as Kv4.2 appear to traffic independently of Rab11 (133). Further studies are needed to assess the role(s) of these and other trafficking proteins in mediating the surface expression of KATP channels from the ERC.
Aside from endocytic recycling of KATP channels to the plasma membrane, there is also evidence that intracellular KATP channels traffic to late endosomal/lysosomal compartments marked by LAMP2 (68). The authors suggested that the late endosomes may be a reservoir for recycling KATP channels to the membrane. The role of this recycling pathway and trafficking proteins that regulate late endosomal recycling, such as LRRK2 (134), Rab7 that regulates transport from early to late endocytic compartments (135) or Rab9 that regulates transport from late endosomes back to the trans Golgi network (136), remain to be elucidated.
Role of the Cytoskeleton
The cytoskeleton is a key component of ion channel expression and endocytic recycling. Newly synthesized channels, transporters, and pumps exit the endoplasmic reticulum and, with the assistance of molecular motors, travel along tracks formed by actin or microtubules to the plasma membrane and between trafficking compartments inside the cell. The reciprocal regulation of ion channels and the cytoskeleton has been reviewed (137). The actin cytoskeleton is often studied using compounds such as the fungal metabolite cytochalasin that inhibits growth and depolymerizes actin by binding to the actin barbed ends, or by the mushroom poison phalloidin that stabilizes filamentous (f) actin (138). Actin-destabilizing compounds lead to the activation of Na+ channels but they frequently suppress the activity of K+ channels (139). A well-described example of a K+ channel protein that interacts with the actin cytoskeleton by association with α-actinin is Kv1.5, which forms a voltage-activated delayed rectifier K+ channel. In mammalian cell lines, Kv1.5 coimmunoprecipitates and colocalize with α-actinin, whereas cytochalasin D inhibits Kv1.5 currents. Treating KATP channels in inside-out patches from ventricular myocytes with deoxyribonuclease (DNase) I (which forms complexes with G actin and prevents actin filament formation) or with cytochalasin D, antagonizes sulfonylurea-induced inhibition of KATP channels, causes a loss of ATP sensitivity, and induces a progressive loss of channel activity that can be reversed with f-actin (140, 141). Disruption of sulfonylurea binding by actin-disrupting compounds was observed in rat glomeruli and aortic rings (142, 143). A direct role for actin was demonstrated in the process by which leptin activates KATP channels in pancreatic β-cells (16, 17). Intracellular dialysis with the actin filament stabilizer phalloidin or treating cells with the actin-stabilizing agent jasplakinolide prevented KATP channel activation or trafficking by leptin. Moreover, the actin filament destabilizing agents DNase I, cytochalasin B, or latrunculin B increased KATP channel activity by increasing channel surface expression, whereas anti-microtubule agents nocodazole and colchicine had no effect on KATP channel activity. Since leptin modulated actin dynamics, the conclusion was reached that leptin activates KATP channels by disrupting the actin cytoskeleton (16, 17). A role for the AMPK/Rac-dependent cytoskeletal remodeling and the myosin II motor protein was demonstrated to be key in promoting KATP channel translocation to the plasma membrane in pancreatic β-cells (19). Overall, these data demonstrate that the actin cytoskeleton can have effects on channel opening, ATP-sensitivity, and surface trafficking, while microtubules appear to have less of a role.
Anchoring Mechanisms
The persistence of proteins at the plasmalemma can be enhanced by being localized in stable subdomains, such as caveolae, or their binding to perimembrane stable structures, such as the cortical cytoskeleton (Fig. 5).
Caveolae are small (50–100 nm) raft-like invaginations of the plasma membrane that are enriched in cholesterol and sphingolipids and are inherently stable membrane structures (49). Many ion channels, transporters, pumps, and G-protein coupled receptors localize to caveolae (47, 144). KATP channels can also be found in caveolae. Evidence includes the finding that KATP channel subunits co-fractionate with caveolar proteins (such as caveolins) after density gradient centrifugation of membrane proteins under detergent-free conditions (48, 145–147). Disrupting caveolar structure by cholesterol depletion (e.g., with methyl-β-cyclodextrin) reduces the ability of receptor signaling to affect KATP channel function (145–147) (see Receptor Signaling Pathways). KATP channels have been proposed to colocalize with, and directly interact with caveolins (147–149), which is expected to contribute to stabilizing KATP channels in these membrane microdomains. Caveolae are known to be stabilized by some of the C-terminal Eps15 homology domain-containing proteins, such as EHD2 (150). Indeed, EHD2 participates in KATP channel surface stability since a dominant negative EHD2 protein increases surface motility of KATP channels, leads to rapid internalization and a decrease in KATP channel surface density (48). In cardiomyocytes, caveolae are generally thought to be present at the mouth of t-tubules and/or the crests between t-tubules (24) and they are likely to be partially responsible for targeting/anchoring KATP channels to these subcellular regions.
Ankyrins are intracellular perimembrane scaffolding proteins that anchor many membrane proteins, including channels, transporters, and pumps, to the actin/spectrin cytoskeleton. The result is that the membrane proteins are targeted to specific subcellular microdomains as required for cell function (151). Ankyrin B (Ank-B), for example coordinates the expression of the Na+/K+ pump, the Na+/Ca2+ exchanger, and the inositol (1,4,5)-trisphosphate (IP3) receptor to T-tubules in cardiac myocytes (152). KATP channels may be targeted to the similar domains due to their interaction with Ank-B. Evidence for a role of Ank-B in KATP channel targeting comes from the findings that Kir6.2 interacts with Ank-B binding domain in GST pulldown experiments and that Ank-B knockout mice or Ank-B disease-causing mutations leads to altered KATP channel expression (153). Ankyrin G also coordinate membrane expression of Na+ channels, the Na+/K+ pump, and KCNQ channels (154–157). A key role for Ank-G was demonstrated for protein targeting and interactions of Cx43, PKP2, and Nav1.5 at the cardiac intercalated disk (158–160). Evidence that KATP channels are targeted to the ICD, in part by being anchored to Ank-G, includes the findings that peptides against an ankyrin binding domain of Kir6.2 decreases the amplitude of a subpopulation of KATP channels at the ICD but not at lateral membranes, and that both Nav1.5 and Kir6.2 colocalize with Ank-G at the ICD and co-clusters, as determined by super-resolution techniques (61). Thus, Ank-B and Ank-G may have distinct roles to anchor KATP channels to specific regions in cardiac myocytes. These anchoring mechanisms of KATP channels in other tissues remain to be explored, such as the Ank-G-mediated anchoring of Na+ channels to the initial segment of the axon (155).
Clustering
Clustering of ion channels modulate physiological processes such as excitation-contraction coupling, regulation of vascular tone, and modulating conduction velocity. For example, clustering of ORAI channels, L-type Ca2+ channels, ryanodine receptors, or IP3 receptors is responsible for producing transient, localized Ca2+ events (named sparks, puffs, sparklets, or microdomains), that summate to underlie global Ca2+ changes in cells (161, 162). Na+ channels also cluster in specialized subcellular regions of neurons, such as the axon initial segment, node of Ranvier, axonal terminals, postsynaptic membranes of dendrites and muscle fibers, and premyelinated axons (163) and at the ICD of cardiac myocytes (164). KATP channels also cluster in cardiomyocytes, particularly at the ICD, as evidenced by the large variability in currents measured in cell-attached or excised patches (51) and by direct observation of KATP clusters at the ICD with super-resolution methods (51, 61). One of the functional consequences of clustering is that it results in cooperative gating of some types of channels, such as L-type Ca2+ channels, Na+ channels, several types of K+ channels, ryanodine receptors, and IP3 receptors (165). Another is that close juxtapositioning of different types of channels leads to functional interaction, which is best illustrated by the well-described interaction of L-type Ca2+ channels and RYR2 to mediate Ca2+-induced Ca2+ release in cardiac myocytes. The functional relevance of KATP channel clustering remains to be elucidated, but their close association with glycolytic enzymes, Na+ channels, and the Na+/K+ pump suggests a role in local ionic control mechanisms, possibly linked via changes in local ATP/ADP concentrations (166).
Receptor Signaling Pathways
Regulation of KATP channel activity by receptor signaling pathways is well described and will not be reviewed here (20). Receptor signaling pathways may also regulate KATP channel trafficking and endocytic recycling, but data are sparse and in some cases contradictory. CaMKII signaling has already been discussed briefly as mediating internalization in cardiac myocytes (see Dynamic changes in surface expression during pathophysiological conditions). CaMKII activation, for example during cardiac ischemia or heart failure (4, 64), appears to lead to phosphorylation of Kir6.2 at T180 and T224, which in turn enhances the binding of Kir6.2 to the μ2 subunit of the AP2 adaptor complex, thereby promoting clathrin-dependent endocytosis (66).
Glucose-induced alterations in KATP channel surface expression have been discussed (see Glucose-Induced Trafficking in the Pancreatic β-Cell). In pancreatic β-cells, leptin similarly increases KATP surface expression, by activating AMPK (Fig. 2). One study suggests that AMPK activation by leptin is mediated by Ca2+ influx through TRPC4 channels, which stimulates CaMKKβ to phosphorylate AMPK (18). However, other recent studies have implicated NMDA receptors as mediators of AMPK activation by leptin (167–169). In this case, leptin was shown to activate Src, which phosphorylates NMDA receptors to potentiate their activity, resulting in increased Ca2+ influx, activation of CaMKKβ, and phosphorylation of AMPK. Downstream of AMPK, F-actin depolymerization underlies the surface trafficking of KATP channels, which has been reported to be caused by AMPK-induced activation of Rac GTPase and the phosphorylation of myosin regulatory light chain (MRLC) (19). F-actin depolymerization following the leptin-AMPK signaling axis is dependent on PKA whose activity increases in response to leptin or the AMPK activator AICAR. Interestingly, knockdown of the PKA-anchoring protein AKAP79/150 prevents leptin-induced KATP channel trafficking, suggesting leptin signaling may lead to localized PKA activation to control actin remodeling and channel trafficking.
In cardiomyocytes, AMPK signaling has also been proposed to increase KATP channel surface expression (Fig. 4) particularly during ischemic preconditioning (65, 170). A key role for PKC activation has also been identified in heart cells. Ischemia, for example, causes a CaMKII-dependent internalization of KATP channels. Re-expression of surface KATP channels takes place during ischemic preconditioning, which is mimicked by adenosine and mitigated by PKC inhibitors (4). Thus, PKC signaling during preconditioning promotes KATP channel surface expression. PKC activation during preconditioning may well be upstream of AMPK activation, as has been demonstrated for GLUT4 upregulation in the late phase of cardioprotection (171). PKC was also shown to be involved in the upregulation of KATP channel currents by isoflurane-induced preconditioning (172). The signaling cascade during IPC is complex and may consist of a PKC/AMPK/p38 MAPK cascade (65). Activation by PKC with phorbol esters in heart cells also led to upregulation of surface KATP channel density, but curiously only in female rats (173). In heterologous expression systems, however, phorbol esters limit KATP channel surface expression and may divert the KATP channel to lysosomal degradation (68, 99, 174, 175). The underlying reasons for this difference are not clear at present and may involve participation of different PKC isoforms and/or the ability of PKC activation to signal via the AMPK/p38 MAPK axis in the non-native environment of a heterologous expression system.
Sorting and Anchoring Motifs in KATP Channel Subunits
Sorting of transmembrane proteins to various intracellular organelles and compartments is governed by a multitude of trafficking steps and is highly cargo specific. This specificity is imparted primarily by signals present within the cytosolic domains of the cargo proteins. These signals generally consist of short, linear sequences of amino acid residues, called motifs. For an excellent treatise of these sorting signals, the reader is referred to a comprehensive review on this topic (176). Some sorting motifs on KATP channel subunits have already been discussed and will not be repeated here. These include ER retention and exit motifs in Kir6.x and SURx subunits (see Folding and Assembly in the Endoplasmic Reticulum); see also Fig. 1A. Internalization signals include tyrosine-based motifs [YXXθ, with θ denoting any hydrophobic amino acid (ILMVF)] (176).
Although tyrosine-based motifs have been demonstrated to be involved in endocytosis, it should be noted that these motifs can also have roles in sorting at endosomal-lysosomal organelles and cytotoxic granules (176). Kir6.2 contains two C-terminal tyrosine-based motifs (258YhvI261 and 330YskF333). One study demonstrated that internalization of channels can occur with the Y330C or F333I mutations, that these mutations led to an overall reduction surface expression and may be involved in neonatal diabetes by reducing ATP sensitivity (116). Another study showed that mutagenesis of the first of these (Y258A) did not affect endocytosis of Kir6.2, whereas mutagenesis of the second motif (Y330A) prevented internalization and doubled KATP channel surface expression (115). Internalization of KATP channels following CaMKII activation was also found to require an intact 330YskF333 motif (66). A key role for this tyrosine-based internalization motif is further suggested in patients with a genetic form of neonatal diabetes that harbor a missense variants causing amino acid mutations Y330C and F333I in Kir6.2 that inhibit spontaneous KATP channel endocytosis and result in a doubling of surface channels (115). The tetrapeptide tyrosine-based motif in the membrane protein coordinates its binding to the µ2 subunit of the AP2 complex, which is an important selection step during the early phases of the endocytic vesicle development. Molecular modeling of the Kir6.2 subunit has predicted that the 258YhvI261 motif is hidden due to protein folding and that 330YskF333 is exposed (115). Molecular modeling methods have further predicted that the Kir6.2 330YskF333 motif directly interacts with the AP2 complex µ2 subunit (66). Currently, there is no cryoEM structures of the KATP channel in complex with µ2 subunit. In recent cryoEM structures, the YSKF motif is involved in ATP binding (80). The µ2 docking needs to be revisited using available KATP channel cryoEM structures. The Kir6.2 330YskF333 motif is fully conserved across species as well as in Kir6.1 subunits, but its role in Kir6.1-containing smooth muscle and pericyte KATP channels has not been explored. SUR2A contains 13 tyrosine-based motifs, but these have also not been studied.
Dileucine internalization motifs can consist minimally of adjacent Leu residues (LL). Kir6.2 contains no fewer than 28 such LL motifs and SUR2A has even more. It is extremely unlikely that each of these govern internalization. More commonly, dileucine motifs take the form of acidic cluster-dileucine motifs [DE]XXXL[LI] or DXXLL. Kir6.2 has a single C-terminal consensus sequence that corresponds to both of these forms, namely 351EdhsLL356 or 352DhsLL356. Both Kir6.1 and SUR2A lack these dileucine motifs. Whether this Kir6.2 dileucine motif contributes to internalization is questionable. An initial study demonstrated that mutagenesis of the dileucine motif (356LL358) prevented decreased Kir6.2 surface density following PKC activation, either when co-expressed with SUR1 or SUR2, which was interpreted to mean that PKC-induced endocytosis of Kir6.2 was dependent on the dileucine motif (174). Another study confirmed that mutagenesis of 356LL358 led to a doubling of KATP channel surface expression, but failed to observe a decrease in the rate of endocytosis (115). The possibility was raised that this dileucine motif affects the rate of recycling to the plasma membrane, but this issue remains to be addressed experimentally. Dileucine motifs have been implicated in subcellular trafficking events other than internalization, including sorting from the trans-Golgi to the endosomal/lysosomal compartments, and mutagenesis of these dileucine motifs led to increased plasmalemmal expression of other membrane proteins as well (177–179). Mutagenesis of a dileucine pair in the distal SUR1 C-terminus (1566LL1567) impaired surface expression (102). Curiously, however, the effect was restricted to only the first of these Leu residues since the L1567A mutation did not have the same impact on surface expression. One interpretation of this result is that L1566 in SUR1 is a part of an anterograde sorting motif that remains to be identified.
There are other sorting motifs to consider as well. For example, NPXY or NXXY motifs regulate endocytic processing of several membrane proteins, including the LDL receptor, integrin β-1, insulin receptors and the epidermal growth factor (EGF) receptor. These motifs may coordinate binding to the membrane protein to the AP2 complex, or the Phox-homology (PX) domain-containing proteins sorting nexins, SNX17, SNX27, and SNX31 (180). Kir6 subunits lack these motifs. SUR2x contains a single NXXY motif (1206NiaY1209), which is not present in SUR1. Since SUR2-containing channels are endocytosed more rapidly than SUR1 channels (68), it may be worth investigating whether this sorting motif has a role.
Aside from sorting motifs, one also has to consider anchoring motifs, which can stabilize expression of membrane proteins and thereby affect surface expression and/or subcellular localization/targeting. For example, an ankyrin-binding motif (VPIALGESD) in the intracellular loop between domains II and III of Nav1.2 was found to be responsible for binding to ankyrin G (Ank-G), leading to clustering of the channel at the axonal initial segment (181). This sequence is relatively well conserved in other Na+ channels, including Nav1.5, where the VPIAVAESD sequence was shown to mediate binding of Nav1.5 to ankyrin G (156). KATP channels also have an ankyrin binding motif. Pulldown assays have demonstrated that Kir6.2 interacts with a GST-fusion protein that contains the ankyrin B (AnkB) membrane-binding domain (MBD), but not with AnkG-MBD (45). A motif in the C-terminus of Kir6.2 (316VPI-VAEED323), almost identical in sequence to the Nav1.5 AnkG binding motif, mediated this interaction (153). In ventricular myocytes, AnkB is localized at lateral membranes (182) and predominantly colocalizes with the Z-line marker protein α-actinin, whereas a minor AnkB-212 isoform co-localizes only with the M-line resident protein, myomesin (46). The finding that Kir6.2 can be anchored by AnkB can therefore partly explain anchoring of KATP channels in lateral membranes and T-tubules of cardiac ventricular myocytes.
Kir6.2 also interacts with AnkG, apparently via the same 316VPIVAEED323 domain. Overexpression of Kir6.2/SUR2A and Nav1.5 reciprocally inhibit each other electrophysiologically and reduce each other’s surface expression (without changing the total protein content) (61). Mutating the putative AnkG binding domain prevents this reciprocal functional interaction. A functional KATP channel is not needed since a pore-mutant nonconducting Kir6.2 subunit behaved similar to wild-type. Kir6.1, which differs from Kir6.2 by three amino acids in this region (325VSIVTEEE332) failed to affect Nav1.5 currents. Peptides corresponding to the Nav1.5 and Kir6.2 domains both affected Na+ and KATP channels at the ICD region, but not lateral regions, of cardiomyocytes (61). These observations, coupled with the fact that AnkG is predominantly, but not exclusively, expressed at the ICD (156, 183) support the concept that the ankyrin binding domain anchors both Na+ channels and KATP channels at the ICD (51, 61, 183).
PERSPECTIVES
Maintaining the normal level of surface KATP channels has been demonstrated to be of great importance for controlled insulin secretion and cardioprotection. The surface expression of KATP channels is regulated by a fine balance between synthesis, assembly, anterograde trafficking, membrane anchoring, endocytosis, endocytic recycling, and degradation. The mechanisms of these trafficking steps are still poorly understood. KATP channels express widely across different cells and tissues. KATP channel trafficking is mainly investigated in pancreatic β-cells, followed by cardiomyocytes and smooth muscle cells. More studies are needed to explore the trafficking of the KATP channels in neurons and immune cells. This knowledge is critically important for future efforts to design more effective drugs targeting the trafficking of KATP channels.
GRANTS
This study was supported by National Institutes of Health Grants HL146514 (to W.A.C.), HL148609 (to W.A.C. and M.D.), DA051876 (to W.A.C.), and DK066485 and DK057699 (to S.L.S.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
This article is part of the special collection “Inward Rectifying K+ Channels.” Jerod Denton, PhD, and Eric Delpire, PhD, served as Guest Editors of this collection.
AUTHOR CONTRIBUTIONS
H.-Q.Y., A.E., S.-L.S., and W.A.C. prepared figures; H.-Q.Y., F.A.E., A.E., and W.A.C. drafted manuscript; H.-Q.Y., F.A.E., A.E., S.A.A., N.S., T.C., M.D., S.-L.S., and W.A.C. edited and revised manuscript; H.-Q.Y., F.A.E., A.E., I.G., S.A.A., N.S., T.C., M.D. and S.-L.S., and W.A.C. approved final version of manuscript.
REFERENCES
- 1.Tarasov AI, Girard CA, Ashcroft FM. ATP sensitivity of the ATP-sensitive K+ channel in intact and permeabilized pancreatic β-cells. Diabetes 55: 2446–2454, 2006. doi: 10.2337/db06-0360. [DOI] [PubMed] [Google Scholar]
- 2.Miki T, Liss B, Minami K, Shiuchi T, Saraya A, Kashima Y, Horiuchi M, Ashcroft F, Minokoshi Y, Roeper J, Seino S. ATP-sensitive K+ channels in the hypothalamus are essential for the maintenance of glucose homeostasis. Nat Neurosci 4: 507–512, 2001. doi: 10.1038/87455. [DOI] [PubMed] [Google Scholar]
- 3.Foster MN, Coetzee WA. KATP channels in the cardiovascular system. Physiol Rev 96: 177–252, 2016. doi: 10.1152/physrev.00003.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang HQ, Foster MN, Jana K, Ho J, Rindler MJ, Coetzee WA. Plasticity of sarcolemmal KATP channel surface expression: relevance during ischemia and ischemic preconditioning. Am J Physiol Heart Circ Physiol 310: H1558–H1566, 2016. doi: 10.1152/ajpheart.00158.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mukherjee S, Ghosh RN, Maxfield FR. Endocytosis. Physiol Rev 77: 759–803, 1997. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- 6.Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol 5: 121–132, 2004. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 7.Robertson GA. Endocytic control of ion channel density as a target for cardiovascular disease. J Clin Invest 119: 2531–2534, 2009. doi: 10.1172/JCI40427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin GM, Rex EA, Devaraneni P, Denton JS, Boodhansingh KE, DeLeon DD, Stanley CA, Shyng SL. Pharmacological correction of trafficking defects in ATP-sensitive potassium channels caused by sulfonylurea receptor 1 mutations. J Biol Chem 291: 21971–21983, 2016. doi: 10.1074/jbc.M116.749366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ashcroft FM, Rorsman P. K channels and islet hormone secretion: new insights and controversies. Nat Rev Endocrinol 9: 660–669, 2013. doi: 10.1038/nrendo.2013.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang G, Leech CA, Chepurny OG, Coetzee WA, Holz GG. Role of the cAMP sensor EPAC as a determinant of KATP channel ATP sensitivity in human pancreatic β-cells and rat INS-1 cells. J Physiol 586: 1307–1319, 2008. doi: 10.1113/jphysiol.2007.143818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang SN, Wenna ND, Yu J, Yang G, Qiu H, Yu L, Juntti-Berggren L, Kohler M, Berggren PO. Glucose recruits K(ATP) channels via non-insulin-containing dense-core granules. Cell Metab 6: 217–228, 2007. doi: 10.1016/j.cmet.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Lim A, Park SH, Sohn JW, Jeon JH, Park JH, Song DK, Lee SH, Ho WK. Glucose deprivation regulates KATP channel trafficking via AMP-activated protein kinase in pancreatic β-cells. Diabetes 58: 2813–2819, 2009. doi: 10.2337/db09-0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hardie DG, Carling D. The AMP-activated protein kinase–fuel gauge of the mammalian cell? Eur J Biochem 246: 259–273, 1997. doi: 10.1111/j.1432-1033.1997.00259.x. [DOI] [PubMed] [Google Scholar]
- 14.Yoshida H, Bao L, Kefaloyianni E, Taskin E, Okorie U, Hong M, Dhar-Chowdhury P, Kaneko M, Coetzee WA. AMP-activated protein kinase connects cellular energy metabolism to KATP channel function. J Mol Cell Cardiol 52: 410–418, 2012. doi: 10.1016/j.yjmcc.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harvey J, McKenna F, Herson PS, Spanswick D, Ashford ML. Leptin activates ATP-sensitive potassium channels in the rat insulin-secreting cell line, CRI-G1. J Physiol 504: 527–535, 1997. doi: 10.1111/j.1469-7793.1997.527bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harvey J, Hardy SC, Irving AJ, Ashford ML. Leptin activation of ATP-sensitive K+ (KATP) channels in rat CRI-G1 insulinoma cells involves disruption of the actin cytoskeleton. J Physiol 527: 95–107, 2000. doi: 10.1111/j.1469-7793.2000.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen PC, Kryukova YN, Shyng SL. Leptin regulates KATP channel trafficking in pancreatic β-cells by a signaling mechanism involving AMPK and PKA. J Biol Chem 288: 34098–34109, 2013. doi: 10.1074/jbc.M113.516880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park SH, Ryu SY, Yu WJ, Han YE, Ji YS, Oh K, Sohn JW, Lim A, Jeon JP, Lee H, Lee KH, Lee SH, Berggren PO, Jeon JH, Ho WK. Leptin promotes KATP channel trafficking by AMPK signaling in pancreatic β-cells. Proc Natl Acad Sci USA 110: 12673–12678, 2013. doi: 10.1073/pnas.1216351110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han YE, Lim A, Park SH, Chang S, Lee SH, Ho WK. Rac-mediated actin remodeling and myosin II are involved in KATP channel trafficking in pancreatic β-cells. Exp Mol Med 47: e190, 2015. doi: 10.1038/emm.2015.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tinker A, Aziz Q, Li Y, Specterman M. ATP-sensitive potassium channels and their physiological and pathophysiological roles. Compr Physiol 8: 1463–1511, 2018. doi: 10.1002/cphy.c170048. [DOI] [PubMed] [Google Scholar]
- 21.Von Beckerath N, Cyrys S, Dischner A, Daut J. Hypoxic vasodilatation in isolated, perfused guinea-pig heart: an analysis of the underlying mechanisms. J Physiol 442: 297–319, 1991. doi: 10.1113/jphysiol.1991.sp018794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quinn KV, Giblin JP, Tinker A. Multisite phosphorylation mechanism for protein kinase A activation of the smooth muscle ATP-sensitive K+ channel. Circ Res 94: 1359–1366, 2004. doi: 10.1161/01.RES.0000128513.34817.c4. [DOI] [PubMed] [Google Scholar]
- 23.Jiao J, Garg V, Yang B, Elton TS, Hu K. Protein kinase C-epsilon induces caveolin-dependent internalization of vascular adenosine 5'-triphosphate-sensitive K+ channels. Hypertension 52: 499–506, 2008. [Erratum in Hypertension 58: e29, 2011]. doi: 10.1161/HYPERTENSIONAHA.108.110817. [DOI] [PubMed] [Google Scholar]
- 24.Hong T, Shaw RM. Cardiac T-tubule microanatomy and function. Physiol Rev 97: 227–252, 2017. doi: 10.1152/physrev.00037.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Best JM, Kamp TJ. Different subcellular populations of L-type Ca2+ channels exhibit unique regulation and functional roles in cardiomyocytes. J Mol Cell Cardiol 52: 376–387, 2012. doi: 10.1016/j.yjmcc.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balijepalli RC, Foell JD, Hall DD, Hell JW, Kamp TJ. Localization of cardiac L-type Ca(2+) channels to a caveolar macromolecular signaling complex is required for β2-adrenergic regulation. Proc Natl Acad Sci USA 103: 7500–7505, 2006. doi: 10.1073/pnas.0503465103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calaghan S, White E. Caveolae modulate excitation-contraction coupling and β2-adrenergic signalling in adult rat ventricular myocytes. Cardiovasc Res 69: 816–824, 2006. doi: 10.1016/j.cardiores.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Shy D, Gillet L, Abriel H. Cardiac sodium channel NaV1.5 distribution in myocytes via interacting proteins: the multiple pool model. Biochim Biophys Acta 1833: 886–894, 2013. doi: 10.1016/j.bbamcr.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 29.Gavillet B, Rougier JS, Domenighetti AA, Behar R, Boixel C, Ruchat P, Lehr HA, Pedrazzini T, Abriel H. Cardiac sodium channel Nav1.5 is regulated by a multiprotein complex composed of syntrophins and dystrophin. Circ Res 99: 407–414, 2006. doi: 10.1161/01.RES.0000237466.13252.5e. [DOI] [PubMed] [Google Scholar]
- 30.Hund TJ, Koval OM, Li J, Wright PJ, Qian L, Snyder JS, Gudmundsson H, Kline CF, Davidson NP, Cardona N, Rasband MN, Anderson ME, Mohler PJ. A β(IV)-spectrin/CaMKII signaling complex is essential for membrane excitability in mice. J Clin Invest 120: 3508–3519, 2010. doi: 10.1172/JCI43621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agullo-Pascual E, Lin X, Leo-Macias A, Zhang M, Liang FX, Li Z, Pfenniger A, Lubkemeier I, Keegan S, Fenyo D, Willecke K, Rothenberg E, Delmar M. Super-resolution imaging reveals that loss of the C-terminus of connexin43 limits microtubule plus-end capture and NaV1.5 localization at the intercalated disc. Cardiovasc Res 104: 371–381, 2014. doi: 10.1093/cvr/cvu195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gillet L, Shy D, Abriel H. Elucidating sodium channel NaV1.5 clustering in cardiac myocytes using super-resolution techniques. Cardiovasc Res 104: 231–233, 2014. doi: 10.1093/cvr/cvu221. [DOI] [PubMed] [Google Scholar]
- 33.Veeraraghavan R, Lin J, Keener JP, Gourdie R, Poelzing S. Potassium channels in the Cx43 gap junction perinexus modulate ephaptic coupling: an experimental and modeling study. Pflugers Arch 468: 1651–1661, 2016. doi: 10.1007/s00424-016-1861-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ponce-Balbuena D, Guerrero-Serna G, Valdivia CR, Caballero R, Diez-Guerra FJ, Jimenez-Vazquez EN, Ramirez RJ, Monteiro da Rocha A, Herron TJ, Campbell KF, Willis BC, Alvarado FJ, Zarzoso M, Kaur K, Perez-Hernandez M, Matamoros M, Valdivia HH, Delpon E, Jalife J. Cardiac Kir2.1 and NaV1.5 channels traffic together to the sarcolemma to control excitability. Circ Res 122: 1501–1516, 2018. doi: 10.1161/CIRCRESAHA.117.311872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Connell KM, Whitesell JD, Tamkun MM. Localization and mobility of the delayed-rectifer K+ channel Kv2.1 in adult cardiomyocytes. Am J Physiol Heart Circ Physiol 294: H229–H237, 2008. doi: 10.1152/ajpheart.01038.2007. [DOI] [PubMed] [Google Scholar]
- 36.Scannevin RH, Murakoshi H, Rhodes KJ, Trimmer JS. Identification of a cytoplasmic domain important in the polarized expression and clustering of the Kv2.1 K+ channel. J Cell Biol 135: 1619–1632, 1996. doi: 10.1083/jcb.135.6.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martens JR, Navarro-Polanco R, Coppock EA, Nishiyama A, Parshley L, Grobaski TD, Tamkun MM. Differential targeting of Shaker-like potassium channels to lipid rafts. J Biol Chem 275: 7443–7446, 2000. doi: 10.1074/jbc.275.11.7443. [DOI] [PubMed] [Google Scholar]
- 38.Fox PD, Loftus RJ, Tamkun MM. Regulation of Kv2.1 K+ conductance by cell surface channel density. J Neurosci 33: 1259–1270, 2013. doi: 10.1523/JNEUROSCI.3008-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fox PD, Haberkorn CJ, Akin EJ, Seel PJ, Krapf D, Tamkun MM. Induction of stable ER-plasma-membrane junctions by Kv2.1 potassium channels. J Cell Sci 128: 2096–2105, 2015. doi: 10.1242/jcs.166009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson B, Leek AN, Sole L, Maverick EE, Levine TP, Tamkun MM. Kv2 potassium channels form endoplasmic reticulum/plasma membrane junctions via interaction with VAPA and VAPB. Proc Natl Acad Sci USA 115: E7331–E7340, 2018. doi: 10.1073/pnas.1805757115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu H, Carvalho P, Voeltz GK. Here, there, and everywhere: the importance of ER membrane contact sites. Science 361: eaan5835, 2018. doi: 10.1126/science.aan5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noma A. ATP-regulated K+ channels in cardiac muscle. Nature 305: 147–148, 1983. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- 43.Korchev YE, Negulyaev YA, Edwards CR, Vodyanoy I, Lab MJ. Functional localization of single active ion channels on the surface of a living cell. Nat Cell Biol 2: 616–619, 2000. doi: 10.1038/35023563. [DOI] [PubMed] [Google Scholar]
- 44.Kline CF, Hund TJ, Mohler PJ. Ankyrin regulates KATP channel membrane trafficking and gating in excitable cells. Channels (Austin) 4: 55–57, 2010. doi: 10.4161/chan.4.1.10362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li J, Kline CF, Hund TJ, Anderson ME, Mohler PJ. Ankyrin-B regulates Kir6.2 membrane expression and function in heart. J Biol Chem 285: 28723–28730, 2010. doi: 10.1074/jbc.M110.147868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu HC, Yamankurt G, Luo J, Subramaniam J, Hashmi SS, Hu H, Cunha SR. Identification and characterization of two ankyrin-B isoforms in mammalian heart. Cardiovasc Res 107: 466–477, 2015. doi: 10.1093/cvr/cvv184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maguy A, Hebert TE, Nattel S. Involvement of lipid rafts and caveolae in cardiac ion channel function. Cardiovasc Res 69: 798–807, 2006. doi: 10.1016/j.cardiores.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 48.Yang HQ, Jana K, Rindler MJ, Coetzee WA. The trafficking protein, EHD2, positively regulates cardiac sarcolemmal KATP channel surface expression: role in cardioprotection. FASEB J 32: 1613–1625, 2018. doi: 10.1096/fj.201700027R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thomsen P, Roepstorff K, Stahlhut M, van Deurs B. Caveolae are highly immobile plasma membrane microdomains, which are not involved in constitutive endocytic trafficking. Mol Biol Cell 13: 238–250, 2002. doi: 10.1091/mbc.01-06-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hong M, Kefaloyianni E, Bao L, Malester B, Delaroche D, Neubert TA, Coetzee WA. Cardiac ATP-sensitive K+ channel associates with the glycolytic enzyme complex. FASEB J 25: 2456–2467, 2011. doi: 10.1096/fj.10-176669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hong M, Bao L, Kefaloyianni E, Agullo-Pascual E, Chkourko H, Foster M, Taskin E, Zhandre M, Reid DA, Rothenberg E, Delmar M, Coetzee WA. Heterogeneity of ATP-sensitive K+ channels in cardiac myocytes: enrichment at the intercalated disk. J Biol Chem 287: 41258–41267, 2012. doi: 10.1074/jbc.M112.412122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mayorga-Wark O, Dubinsky WP, Schultz SG. Reconstitution of a KATP channel from basolateral membranes of Necturus enterocytes. Am J Physiol Cell Physiol 269: C464–C471, 1995. doi: 10.1152/ajpcell.1995.269.2.C464. [DOI] [PubMed] [Google Scholar]
- 53.Welling PA. Cross-talk and the role of KATP channels in the proximal tubule. Kidney Int 48: 1017–1023, 1995. doi: 10.1038/ki.1995.384. [DOI] [PubMed] [Google Scholar]
- 54.Priebe L, Friedrich M, Benndorf K. Functional interaction between KATP channels and the Na+-K+ pump in metabolically inhibited heart cells of the guinea-pig. J Physiol 492: 405–417, 1996. doi: 10.1113/jphysiol.1996.sp021317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Urbach V, Van KE, Maguire D, Harvey BJ. Cross-talk between ATP-regulated K+ channels and Na+ transport via cellular metabolism in frog skin principal cells. J Physiol 491: 99–109, 1996. doi: 10.1113/jphysiol.1996.sp021199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Akaike N, Jin YH, Koyama S. Functional interaction between Na+-K+ pump and KATP channels in the CNS neurons under experimental brain ischemia. Jpn J Physiol 47: S50–S51, 1997. [PubMed] [Google Scholar]
- 57.Kabakov AY. Activation of KATP channels by Na/K pump in isolated cardiac myocytes and giant membrane patches. Biophys J 75: 2858–2867, 1998. doi: 10.1016/S0006-3495(98)77728-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Elmi A, Idahl LA, Sehlin J. Modulation of β-cell ouabain-sensitive 86Rb+ influx (Na+/K+ pump) by d-glucose, glibenclamide or diazoxide. Int J Exp Diabetes Res 1: 265–274, 2001. doi: 10.1155/edr.2000.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cerrone M, Noorman M, Lin X, Chkourko H, Liang FX, van der Nagel R, Hund T, Birchmeier W, Mohler P, van Veen TA, van Rijen HV, Delmar M. Sodium current deficit and arrhythmogenesis in a murine model of plakophilin-2 haploinsufficiency. Cardiovasc Res 95: 460–468, 2012. doi: 10.1093/cvr/cvs218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin X, Liu N, Lu J, Zhang J, Anumonwo JM, Isom LL, Fishman GI, Delmar M. Subcellular heterogeneity of sodium current properties in adult cardiac ventricular myocytes. Heart Rhythm 8: 1923–1930, 2011. doi: 10.1016/j.hrthm.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang HQ, Perez-Hernandez M, Sanchez-Alonso J, Shevchuk A, Gorelik J, Rothenberg E, Delmar M, Coetzee WA. Ankyrin-G mediates targeting of both Na+ and KATP channels to the rat cardiac intercalated disc. eLife 9: e52373, 2020. doi: 10.7554/eLife.52373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zingman LV, Zhu Z, Sierra A, Stepniak E, Burnett CM, Maksymov G, Anderson ME, Coetzee WA, Hodgson-Zingman DM. Exercise-induced expression of cardiac ATP-sensitive potassium channels promotes action potential shortening and energy conservation. J Mol Cell Cardiol 51: 72–81, 2011. doi: 10.1016/j.yjmcc.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eisner DA, Dibb KM, Trafford AW. The mechanism and significance of the slow changes of ventricular action potential duration following a change of heart rate. Exp Physiol 94: 520–528, 2009. doi: 10.1113/expphysiol.2008.044008. [DOI] [PubMed] [Google Scholar]
- 64.Li J, Marionneau C, Koval O, Zingman L, Mohler PJ, Nerbonne JM, Anderson ME. Calmodulin kinase II inhibition enhances ischemic preconditioning by augmenting ATP-sensitive K+ current. Channels (Austin) 1: 387–394, 2007. doi: 10.4161/chan.5449. [DOI] [PubMed] [Google Scholar]
- 65.Turrell HE, Rodrigo GC, Norman RI, Dickens M, Standen NB. Phenylephrine preconditioning involves modulation of cardiac sarcolemmal KATP current by PKC delta, AMPK and p38 MAPK. J Mol Cell Cardiol 51: 370–380, 2011. doi: 10.1016/j.yjmcc.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 66.Sierra A, Zhu Z, Sapay N, Sharotri V, Kline CF, Luczak ED, Subbotina E, Sivaprasadarao A, Snyder PM, Mohler PJ, Anderson ME, Vivaudou M, Zingman LV, Hodgson-Zingman DM. Regulation of cardiac ATP-sensitive potassium channel surface expression by calcium/calmodulin-dependent protein kinase II. J Biol Chem 288: 1568–1581, 2013. doi: 10.1074/jbc.M112.429548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Downey JM, Davis AM, Cohen MV. Signaling pathways in ischemic preconditioning. Heart Fail Rev 12: 181–188, 2007. doi: 10.1007/s10741-007-9025-2. [DOI] [PubMed] [Google Scholar]
- 68.Bao L, Hadjiolova K, Coetzee WA, Rindler MJ. Endosomal KATP channels as a reservoir after myocardial ischemia: a role for SUR2 subunits. Am J Physiol Heart Circ Physiol 300: H262–H270, 2011. doi: 10.1152/ajpheart.00857.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vleugels A, Vereecke J, Carmeliet E. Ionic currents during hypoxia in voltage-clamped cat ventricular muscle. Circ Res 47: 501–508, 1980. doi: 10.1161/01.res.47.4.501. [DOI] [PubMed] [Google Scholar]
- 70.Zingman LV, Hodgson DM, Bast PH, Kane GC, Perez-Terzic C, Gumina RJ, Pucar D, Bienengraeber M, Dzeja PP, Miki T, Seino S, Alekseev AE, Terzic A. Kir6.2 is required for adaptation to stress. Proc Natl Acad Sci USA 99: 13278–13283, 2002. doi: 10.1073/pnas.212315199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang HQ, Gando I, Coetzee WA. Functional characterization of ion channel gene variants in sudden unexplained natural death. In: Zipes and Jalife's Cardiac Electrophysiology: From Cell to Bedside, edited by Jalife J, Stevenson WG.. Philadelphia, PA: Elsevier, 2022, p. 143–154. [Google Scholar]
- 72.De Leon DD, Stanley CA. Mechanisms of disease: advances in diagnosis and treatment of hyperinsulinism in neonates. Nat Clin Pract Endocrinol Metab 3: 57–68, 2007. doi: 10.1038/ncpendmet0368. [DOI] [PubMed] [Google Scholar]
- 73.Hattersley AT, Ashcroft FM. Activating mutations in Kir6.2 and neonatal diabetes: new clinical syndromes, new scientific insights, and new therapy. Diabetes 54: 2503–2513, 2005. doi: 10.2337/diabetes.54.9.2503. [DOI] [PubMed] [Google Scholar]
- 74.Pipatpolkai T, Usher S, Stansfeld PJ, Ashcroft FM. New insights into KATP channel gene mutations and neonatal diabetes mellitus. Nat Rev Endocrinol 16: 378–393, 2020. doi: 10.1038/s41574-020-0351-y. [DOI] [PubMed] [Google Scholar]
- 75.Martin GM, Chen PC, Devaraneni P, Shyng SL. Pharmacological rescue of trafficking-impaired ATP-sensitive potassium channels. Front Physiol 4: 386, 2013. doi: 10.3389/fphys.2013.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lin CW, Lin YW, Yan FF, Casey J, Kochhar M, Pratt EB, Shyng SL. Kir6.2 mutations associated with neonatal diabetes reduce expression of ATP-sensitive K+ channels: implications in disease mechanism and sulfonylurea therapy. Diabetes 55: 1738–1746, 2006. doi: 10.2337/db05-1571. [DOI] [PubMed] [Google Scholar]
- 77.Zhou Q, Garin I, Castano L, Argente J, Munoz-Calvo MT, Perez de Nanclares G, Shyng SL. Neonatal diabetes caused by mutations in sulfonylurea receptor 1: interplay between expression and Mg-nucleotide gating defects of ATP-sensitive potassium channels. J Clin Endocrinol Metab 95: E473–E478, 2010. doi: 10.1210/jc.2010-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lin YW, Li A, Grasso V, Battaglia D, Crino A, Colombo C, Barbetti F, Nichols CG. Functional characterization of a novel KCNJ11 in frame mutation-deletion associated with infancy-onset diabetes and a mild form of intermediate DEND: a battle between K(ATP) gain of channel activity and loss of channel expression. PLoS One 8: e63758, 2013. doi: 10.1371/journal.pone.0063758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Taneja TK, Mankouri J, Karnik R, Kannan S, Smith AJ, Munsey T, Christesen HB, Beech DJ, Sivaprasadarao A. Sar1-GTPase-dependent ER exit of KATP channels revealed by a mutation causing congenital hyperinsulinism. Hum Mol Genet 18: 2400–2413, 2009. doi: 10.1093/hmg/ddp179. [DOI] [PubMed] [Google Scholar]
- 80.Martin GM, Yoshioka C, Rex EA, Fay JF, Xie Q, Whorton MR, Chen JZ, Shyng SL. Cryo-EM structure of the ATP-sensitive potassium channel illuminates mechanisms of assembly and gating. eLife 6: e24149, 2017. doi: 10.7554/eLife.24149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Martin GM, Sung MW, Yang Z, Innes LM, Kandasamy B, David LL, Yoshioka C, Shyng SL. Mechanism of pharmacochaperoning in a mammalian KATP channel revealed by cryo-EM. eLife 8: e46417, 2019. doi: 10.7554/eLife.46417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Giblin JP, Quinn K, Tinker A. The cytoplasmic C-terminus of the sulfonylurea receptor is important for KATP channel function but is not key for complex assembly or trafficking. Eur J Biochem 269: 5303–5313, 2002. doi: 10.1046/j.1432-1033.2002.03249.x. [DOI] [PubMed] [Google Scholar]
- 83.Devaraneni PK, Martin GM, Olson EM, Zhou Q, Shyng SL. Structurally distinct ligands rescue biogenesis defects of the KATP channel complex via a converging mechanism. J Biol Chem 290: 7980–7991, 2015. doi: 10.1074/jbc.M114.634576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kuhner P, Prager R, Stephan D, Russ U, Winkler M, Ortiz D, Bryan J, Quast U. Importance of the Kir6.2 N-terminus for the interaction of glibenclamide and repaglinide with the pancreatic KATP channel. Naunyn Schmiedebergs Arch Pharmacol 385: 299–311, 2012. doi: 10.1007/s00210-011-0709-8. [DOI] [PubMed] [Google Scholar]
- 85.Huang Y, Hu D, Huang C, Nichols CG. Genetic discovery of ATP-sensitive K(+) channels in cardiovascular diseases. Circ Arrhythm Electrophysiol 12: e007322, 2019. doi: 10.1161/CIRCEP.119.007322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Raeis-Dauve V, Philip-Couderc P, Faggian G, Tessari M, Roatti A, Milano AD, Bochaton-Piallat ML, Baertschi AJ. Increased expression of adenosine triphosphate-sensitive K+ channels in mitral dysfunction: mechanically stimulated transcription and hypoxia-induced protein stability? J Am Coll Cardiol 59: 390–396, 2012. doi: 10.1016/j.jacc.2011.08.077. [DOI] [PubMed] [Google Scholar]
- 87.Melamed-Frank M, Terzic A, Carrasco AJ, Nevo E, Avivi A, Levy AP. Reciprocal regulation of expression of pore-forming KATP channel genes by hypoxia. Mol Cell Biochem 225: 145–150, 2001. doi: 10.1023/a:1012286624993. [DOI] [PubMed] [Google Scholar]
- 88.Crawford RM, Jovanovic S, Budas GR, Davies AM, Lad H, Wenger RH, Robertson KA, Roy DJ, Ranki HJ, Jovanovic A. Chronic mild hypoxia protects heart-derived H9c2 cells against acute hypoxia/reoxygenation by regulating expression of the SUR2A subunit of the ATP-sensitive K+ channel. J Biol Chem 278: 31444–31455, 2003. doi: 10.1074/jbc.M303051200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Isidoro Tavares N, Philip-Couderc P, Papageorgiou I, Baertschi AJ, Lerch R, Montessuit C. Expression and function of ATP-dependent potassium channels in late post-infarction remodeling. J Mol Cell Cardiol 42: 1016–1025, 2007. doi: 10.1016/j.yjmcc.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 90.Sgard F, Faure C, Drieu lR, Graham D, O'Connor SE, Janiak P, Besnard F. Regulation of ATP-sensitive potassium channel mRNA expression in rat kidney following ischemic injury. Biochem Biophys Res Commun 269: 618–622, 2000. doi: 10.1006/bbrc.2000.2342. [DOI] [PubMed] [Google Scholar]
- 91.Akao M, Otani H, Horie M, Takano M, Kuniyasu A, Nakayama H, Kouchi I, Murakami T, Sasayama S. Myocardial ischemia induces differential regulation of KATP channel gene expression in rat hearts. J Clin Invest 100: 3053–3059, 1997. [Erratum in J Clin Invest 101: 915, 1998]. doi: 10.1172/JCI119860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Akao M, Sakurai T, Horie M, Otani H, Takano M, Kouchi I, Murakami T, Sasayama S. Angiotensin II type 1 receptor blockade abolishes specific K(ATP) channel gene expression in rats with myocardial ischemia. J Mol Cell Cardiol 32: 2239–2247, 2000. doi: 10.1006/jmcc.2000.1251. [DOI] [PubMed] [Google Scholar]
- 93.Philip-Couderc P, Tavares NI, Roatti A, Lerch R, Montessuit C, Baertschi AJ. Forkhead transcription factors coordinate expression of myocardial KATP channel subunits and energy metabolism. Circ Res 102: e20–e35, 2008. doi: 10.1161/CIRCRESAHA.107.166744. [DOI] [PubMed] [Google Scholar]
- 94.Raeis V, Philip-Couderc P, Roatti A, Habre W, Sierra J, Kalangos A, Beghetti M, Baertschi AJ. Central venous hypoxemia is a determinant of human atrial ATP-sensitive potassium channel expression: evidence for a novel hypoxia-inducible factor 1α-Forkhead box class O signaling pathway. Hypertension 55: 1186–1192, 2010. doi: 10.1161/HYPERTENSIONAHA.109.148767. [DOI] [PubMed] [Google Scholar]
- 95.Yan F, Lin CW, Weisiger E, Cartier EA, Taschenberger G, Shyng SL. Sulfonylureas correct trafficking defects of ATP-sensitive potassium channels caused by mutations in the sulfonylurea receptor. J Biol Chem 279: 11096–11105, 2004. doi: 10.1074/jbc.M312810200. [DOI] [PubMed] [Google Scholar]
- 96.Yan FF, Lin CW, Cartier EA, Shyng SL. Role of ubiquitin-proteasome degradation pathway in biogenesis efficiency of β-cell ATP-sensitive potassium channels. Am J Physiol Cell Physiol 289: C1351–C1359, 2005. doi: 10.1152/ajpcell.00240.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Crane A, Aguilar-Bryan L. Assembly, maturation, and turnover of KATP channel subunits. J Biol Chem 279: 9080–9090, 2004. doi: 10.1074/jbc.M311079200. [DOI] [PubMed] [Google Scholar]
- 98.Tanaka H, Miake J, Notsu T, Sonyama K, Sasaki N, Iitsuka K, Kato M, Taniguchi S, Igawa O, Yoshida A, Shigemasa C, Hoshikawa Y, Kurata Y, Kuniyasu A, Nakayama H, Inagaki N, Nanba E, Shiota G, Morisaki T, Ninomiya H, Kitakaze M, Hisatome I. Proteasomal degradation of Kir6.2 channel protein and its inhibition by a Na+ channel blocker aprindine. Biochem Biophys Res Commun 331: 1001–1006, 2005. doi: 10.1016/j.bbrc.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 99.Manna PT, Smith AJ, Taneja TK, Howell GJ, Lippiat JD, Sivaprasadarao A. Constitutive endocytic recycling and protein kinase C-mediated lysosomal degradation control KATP channel surface density. J Biol Chem 285: 5963–5973, 2010. doi: 10.1074/jbc.M109.066902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Inagaki N, Gonoi T, Clement JPt, Namba N, Inazawa J, Gonzalez G, Aguilar-Bryan L, Seino S, Bryan J. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science 270: 1166–1170, 1995. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- 101.Zerangue N, Schwappach B, Jan YN, Jan LY. A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane KATP channels. Neuron 22: 537–548, 1999. doi: 10.1016/s0896-6273(00)80708-4. [DOI] [PubMed] [Google Scholar]
- 102.Sharma N, Crane A, Clement JPT, Gonzalez G, Babenko AP, Bryan J, Aguilar-Bryan L. The C terminus of SUR1 is required for trafficking of KATP channels. J Biol Chem 274: 20628–20632, 1999. doi: 10.1074/jbc.274.29.20628. [DOI] [PubMed] [Google Scholar]
- 103.Fukuda Y, Aguilar-Bryan L, Vaxillaire M, Dechaume A, Wang Y, Dean M, Moitra K, Bryan J, Schuetz JD. Conserved intramolecular disulfide bond is critical to trafficking and fate of ATP-binding cassette (ABC) transporters ABCB6 and sulfonylurea receptor 1 (SUR1)/ABCC8. J Biol Chem 286: 8481–8492, 2011. doi: 10.1074/jbc.M110.174516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang F, Olson EM, Shyng SL. Role of Derlin-1 protein in proteostasis regulation of ATP-sensitive potassium channels. J Biol Chem 287: 10482–10493, 2012. doi: 10.1074/jbc.M111.312223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Raab-Graham KF, Cirilo LJ, Boettcher AA, Radeke CM, Vandenberg CA. Membrane topology of the amino-terminal region of the sulfonylurea receptor. J Biol Chem 274: 29122–29129, 1999. doi: 10.1074/jbc.274.41.29122. [DOI] [PubMed] [Google Scholar]
- 106.Arakel EC, Brandenburg S, Uchida K, Zhang H, Lin YW, Kohl T, Schrul B, Sulkin MS, Efimov IR, Nichols CG, Lehnart SE, Schwappach B. Tuning the electrical properties of the heart by differential trafficking of KATP ion channel complexes. J Cell Sci 127: 2106–2119, 2014. doi: 10.1242/jcs.141440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Michelsen K, Schmid V, Metz J, Heusser K, Liebel U, Schwede T, Spang A, Schwappach B. Novel cargo-binding site in the β and δ subunits of coatomer. J Cell Biol 179: 209–217, 2007. doi: 10.1083/jcb.200704142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Heusser K, Yuan H, Neagoe I, Tarasov AI, Ashcroft FM, Schwappach B. Scavenging of 14-3-3 proteins reveals their involvement in the cell-surface transport of ATP-sensitive K+ channels. J Cell Sci 119: 4353–4363, 2006. doi: 10.1242/jcs.03196. [DOI] [PubMed] [Google Scholar]
- 109.Yuan H, Michelsen K, Schwappach B. 14-3-3 dimers probe the assembly status of multimeric membrane proteins. Curr Biol 13: 638–646, 2003. doi: 10.1016/S0960-9822(03)00208-2. [DOI] [PubMed] [Google Scholar]
- 110.Pasyk EA, Kang Y, Huang X, Cui N, Sheu L, Gaisano HY. Syntaxin-1A binds the nucleotide-binding folds of sulphonylurea receptor 1 to regulate the KATP channel. J Biol Chem 279: 4234–4240, 2004. doi: 10.1074/jbc.M309667200. [DOI] [PubMed] [Google Scholar]
- 111.Kang Y, Leung YM, Manning-Fox JE, Xia F, Xie H, Sheu L, Tsushima RG, Light PE, Gaisano HY. Syntaxin-1A inhibits cardiac KATP channels by its actions on nucleotide binding folds 1 and 2 of sulfonylurea receptor 2A. J Biol Chem 279: 47125–47131, 2004. doi: 10.1074/jbc.M404954200. [DOI] [PubMed] [Google Scholar]
- 112.Chen PC, Bruederle CE, Gaisano HY, Shyng SL. Syntaxin 1A regulates surface expression of β-cell ATP-sensitive potassium channels. Am J Physiol Cell Physiol 300: C506–C516, 2011. doi: 10.1152/ajpcell.00429.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Uchida K, Nomura M, Yamamoto T, Ogawa Y, Teramoto N. Rab8a is involved in membrane trafficking of Kir6.2 in the MIN6 insulinoma cell line. Pflugers Arch 471: 877–887, 2019. doi: 10.1007/s00424-018-02252-1. [DOI] [PubMed] [Google Scholar]
- 114.Epifantseva I, Shaw RM. Intracellular trafficking pathways of Cx43 gap junction channels. Biochim Biophys Acta Biomembr 1860: 40–47, 2018. doi: 10.1016/j.bbamem.2017.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mankouri J, Taneja TK, Smith AJ, Ponnambalam S, Sivaprasadarao A. Kir6.2 mutations causing neonatal diabetes prevent endocytosis of ATP-sensitive potassium channels. EMBO J 25: 4142–4151, 2006. doi: 10.1038/sj.emboj.7601275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bruederle CE, Gay J, Shyng SL. A role of the sulfonylurea receptor 1 in endocytic trafficking of ATP-sensitive potassium channels. Traffic 12: 1242–1256, 2011. doi: 10.1111/j.1600-0854.2011.01227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Takei K, Haucke V. Clathrin-mediated endocytosis: membrane factors pull the trigger. Trends Cell Biol 11: 385–391, 2001. doi: 10.1016/s0962-8924(01)02082-7. [DOI] [PubMed] [Google Scholar]
- 118.Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T. Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell 10: 839–850, 2006. doi: 10.1016/j.devcel.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 119.von Zastrow M, Kobilka BK. Ligand-regulated internalization and recycling of human β 2-adrenergic receptors between the plasma membrane and endosomes containing transferrin receptors. J Biol Chem 267: 3530–3538, 1992. [PubMed] [Google Scholar]
- 120.Seachrist JL, Anborgh PH, Ferguson SS. β2-adrenergic receptor internalization, endosomal sorting, and plasma membrane recycling are regulated by rab GTPases*. J Biol Chem 275: 27221–27228, 2000. doi: 10.1016/S0021-9258(19)61500-0. [DOI] [PubMed] [Google Scholar]
- 121.Holleran JP, Zeng J, Frizzell RA, Watkins SC. Regulated recycling of mutant CFTR is partially restored by pharmacological treatment. J Cell Sci 126: 2692–2703, 2013. doi: 10.1242/jcs.120196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen W, Feng Y, Chen D, Wandinger-Ness A. Rab11 is required for trans-golgi network-to-plasma membrane transport and a preferential target for GDP dissociation inhibitor. Mol Biol Cell 9: 3241–3257, 1998. doi: 10.1091/mbc.9.11.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ullrich O, Reinsch S, Urbe S, Zerial M, Parton RG. Rab11 regulates recycling through the pericentriolar recycling endosome. J Cell Biol 135: 913–924, 1996. doi: 10.1083/jcb.135.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schlierf B, Fey GH, Hauber J, Hocke GM, Rosorius O. Rab11b is essential for recycling of transferrin to the plasma membrane. Exp Cell Res 259: 257–265, 2000. doi: 10.1006/excr.2000.4947. [DOI] [PubMed] [Google Scholar]
- 125.McEwen DP, Schumacher SM, Li Q, Benson MD, Iniguez-Lluhi JA, Van Genderen KM, Martens JR. Rab-GTPase-dependent endocytic recycling of Kv1.5 in atrial myocytes. J Biol Chem 282: 29612–29620, 2007. doi: 10.1074/jbc.M704402200. [DOI] [PubMed] [Google Scholar]
- 126.Zadeh AD, Xu H, Loewen ME, Noble GP, Steele DF, Fedida D. Internalized Kv1.5 traffics via Rab-dependent pathways. J Physiol 586: 4793–4813, 2008. [Erratum in J Physiol 587: 505, 2009]. doi: 10.1113/jphysiol.2008.161570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Balse E, El-Haou S, Dillanian G, Dauphin A, Eldstrom J, Fedida D, Coulombe A, Hatem SN. Cholesterol modulates the recruitment of Kv1.5 channels from Rab11-associated recycling endosome in native atrial myocytes. Proc Natl Acad Sci USA 106: 14681–14686, 2009. doi: 10.1073/pnas.0902809106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Seebohm G, Strutz-Seebohm N, Birkin R, Dell G, Bucci C, Spinosa MR, Baltaev R, Mack AF, Korniychuk G, Choudhury A, Marks D, Pagano RE, Attali B, Pfeufer A, Kass RS, Sanguinetti MC, Tavare JM, Lang F. Regulation of endocytic recycling of KCNQ1/KCNE1 potassium channels. Circ Res 100: 686–692, 2007. doi: 10.1161/01.RES.0000260250.83824.8f. [DOI] [PubMed] [Google Scholar]
- 129.Chen J, Guo J, Yang T, Li W, Lamothe SM, Kang Y, Szendrey JA, Zhang S. Rab11-dependent recycling of the human ether-a-go-go-related Gene (hERG) channel. J Biol Chem 290: 21101–21113, 2015. doi: 10.1074/jbc.M115.636324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hardel N, Harmel N, Zolles G, Fakler B, Klocker N. Recycling endosomes supply cardiac pacemaker channels for regulated surface expression. Cardiovasc Res 79: 52–60, 2008. doi: 10.1093/cvr/cvn062. [DOI] [PubMed] [Google Scholar]
- 131.Cayouette S, Bousquet SM, Francoeur N, Dupre E, Monet M, Gagnon H, Guedri YB, Lavoie C, Boulay G. Involvement of Rab9 and Rab11 in the intracellular trafficking of TRPC6. Biochim Biophys Acta 1803: 805–812, 2010. doi: 10.1016/j.bbamcr.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 132.Meyer JO, Dolphin AC. Rab11-dependent recycling of calcium channels is mediated by auxiliary subunit α2δ-1 but not α2δ-3. Sci Rep 11: 10256, 2021. doi: 10.1038/s41598-021-89820-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang T, Cheng Y, Dou Y, Goonesekara C, David JP, Steele DF, Huang C, Fedida D. Trafficking of an endogenous potassium channel in adult ventricular myocytes. Am J Physiol Cell Physiol 303: C963–C976, 2012. doi: 10.1152/ajpcell.00217.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rivero-Rios P, Gomez-Suaga P, Fernandez B, Madero-Perez J, Schwab AJ, Ebert AD, Hilfiker S. Alterations in late endocytic trafficking related to the pathobiology of LRRK2-linked Parkinson’s disease. Biochem Soc Trans 43: 390–395, 2015. doi: 10.1042/BST20140301. [DOI] [PubMed] [Google Scholar]
- 135.Mukhopadhyay A, Funato K, Stahl PD. Rab7 regulates transport from early to late endocytic compartments in Xenopus oocytes. J Biol Chem 272: 13055–13059, 1997. doi: 10.1074/jbc.272.20.13055. [DOI] [PubMed] [Google Scholar]
- 136.Lombardi D, Soldati T, Riederer MA, Goda Y, Zerial M, Pfeffer SR. Rab9 functions in transport between late endosomes and the trans Golgi network. EMBO J 12: 677–682, 1993. doi: 10.1002/j.1460-2075.1993.tb05701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Steele DF, Fedida D. Cytoskeletal roles in cardiac ion channel expression. Biochim Biophys Acta 1838: 665–673, 2014. doi: 10.1016/j.bbamem.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 138.Cooper JA. Effects of cytochalasin and phalloidin on actin. J Cell Biol 105: 1473–1478, 1987. doi: 10.1083/jcb.105.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Janmey PA. The cytoskeleton and cell signaling: component localization and mechanical coupling. Physiol Rev 78: 763–781, 1998. doi: 10.1152/physrev.1998.78.3.763. [DOI] [PubMed] [Google Scholar]
- 140.Brady PA, Alekseev AE, Aleksandrova LA, Gomez LA, Terzic A. A disrupter of actin microfilaments impairs sulfonylurea-inhibitory gating of cardiac KATP channels. Am J Physiol Heart Circ Physiol 271: H2710–H2716, 1996. doi: 10.1152/ajpheart.1996.271.6.H2710. [DOI] [PubMed] [Google Scholar]
- 141.Furukawa T, Yamane T, Terai T, Katayama Y, Hiraoka M. Functional linkage of the cardiac ATP-sensitive K+ channel to the actin cytoskeleton. Pflugers Arch 431: 504–512, 1996. doi: 10.1007/BF02191896. [DOI] [PubMed] [Google Scholar]
- 142.Metzger F, Loffler C, Quast U. Sulphonylurea binding in rat isolated glomeruli: pharmacological characterization and dependence on cell metabolism and cytoskeleton. Naunyn Schmiedebergs Arch Pharmacol 355: 141–149, 1997. doi: 10.1007/pl00004925. [DOI] [PubMed] [Google Scholar]
- 143.Loffler-Walz C, Quast U. Disruption of the actin cytoskeleton abolishes high affinity 3 H-glibenclamide binding in rat aortic rings. Naunyn Schmiedebergs Arch Pharmacol 357: 183–185, 1998. doi: 10.1007/pl00005153. [DOI] [PubMed] [Google Scholar]
- 144.Balijepalli RC, Kamp TJ. Caveolae, ion channels and cardiac arrhythmias. Prog Biophys Mol Biol 98: 149–160, 2008. doi: 10.1016/j.pbiomolbio.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sampson LJ, Hayabuchi Y, Standen NB, Dart C. Caveolae localize protein kinase A signaling to arterial ATP-sensitive potassium channels. Circ Res 95: 1012–1018, 2004. doi: 10.1161/01.RES.0000148634.47095.ab. [DOI] [PubMed] [Google Scholar]
- 146.Sampson LJ, Davies LM, Barrett-Jolley R, Standen NB, Dart C. Angiotensin II-activated protein kinase C targets caveolae to inhibit aortic ATP-sensitive potassium channels. Cardiovasc Res 76: 61–70, 2007. doi: 10.1016/j.cardiores.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 147.Garg V, Jiao J, Hu K. Regulation of ATP-sensitive K+ channels by caveolin-enriched microdomains in cardiac myocytes. Cardiovasc Res 82: 51–58, 2009. doi: 10.1093/cvr/cvp039. [DOI] [PubMed] [Google Scholar]
- 148.Davies LM, Purves GI, Barrett-Jolley R, Dart C. Interaction with caveolin-1 modulates vascular ATP-sensitive potassium (KATP) channel activity. J Physiol 588: 3255–3266, 2010. doi: 10.1113/jphysiol.2010.194779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sun W, Hu K. Role for SUR2A in coupling cardiac KATP channels to caveolin-3. Cell Physiol Biochem 25: 409–418, 2010. doi: 10.1159/000303045. [DOI] [PubMed] [Google Scholar]
- 150.Shah C, Hegde BG, Moren B, Behrmann E, Mielke T, Moenke G, Spahn CM, Lundmark R, Daumke O, Langen R. Structural insights into membrane interaction and caveolar targeting of dynamin-like EHD2. Structure 22: 409–420, 2014. doi: 10.1016/j.str.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cunha SR, Mohler PJ. Cardiac ankyrins: essential components for development and maintenance of excitable membrane domains in heart. Cardiovasc Res 71: 22–29, 2006. doi: 10.1016/j.cardiores.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 152.Mohler PJ, Davis JQ, Bennett V. Ankyrin-B coordinates the Na/K ATPase, Na/Ca exchanger, and InsP3 receptor in a cardiac T-tubule/SR microdomain. PLoS Biol 3: e423, 2005. doi: 10.1371/journal.pbio.0030423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kline CF, Kurata HT, Hund TJ, Cunha SR, Koval OM, Wright PJ, Christensen M, Anderson ME, Nichols CG, Mohler PJ. Dual role of KATP channel C-terminal motif in membrane targeting and metabolic regulation. Proc Natl Acad Sci USA 106: 16669–16674, 2009. doi: 10.1073/pnas.0907138106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Thevananther S, Kolli AH, Devarajan P. Identification of a novel ankyrin isoform (AnkG190) in kidney and lung that associates with the plasma membrane and binds α-Na, K-ATPase. J Biol Chem 273: 23952–23958, 1998. doi: 10.1074/jbc.273.37.23952. [DOI] [PubMed] [Google Scholar]
- 155.Zhou D, Lambert S, Malen PL, Carpenter S, Boland LM, Bennett V. AnkyrinG is required for clustering of voltage-gated Na channels at axon initial segments and for normal action potential firing. J Cell Biol 143: 1295–1304, 1998. doi: 10.1083/jcb.143.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mohler PJ, Rivolta I, Napolitano C, LeMaillet G, Lambert S, Priori SG, Bennett V. Nav1.5 E1053K mutation causing Brugada syndrome blocks binding to ankyrin-G and expression of Nav1.5 on the surface of cardiomyocytes. Proc Natl Acad Sci USA 101: 17533–17538, 2004. doi: 10.1073/pnas.0403711101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hill AS, Nishino A, Nakajo K, Zhang G, Fineman JR, Selzer ME, Okamura Y, Cooper EC. Ion channel clustering at the axon initial segment and node of Ranvier evolved sequentially in early chordates. PLoS Genet 4: e1000317, 2008. doi: 10.1371/journal.pgen.1000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sato PY, Coombs W, Lin X, Nekrasova O, Green KJ, Isom LL, Taffet SM, Delmar M. Interactions between ankyrin-G, Plakophilin-2, and Connexin43 at the cardiac intercalated disc. Circ Res 109: 193–201, 2011. doi: 10.1161/CIRCRESAHA.111.247023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Agullo-Pascual E, Reid DA, Keegan S, Sidhu M, Fenyo D, Rothenberg E, Delmar M. Super-resolution fluorescence microscopy of the cardiac connexome reveals plakophilin-2 inside the connexin43 plaque. Cardiovasc Res 100: 231–240, 2013. doi: 10.1093/cvr/cvt191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Makara MA, Curran J, Little SC, Musa H, Polina I, Smith SA, Wright PJ, Unudurthi SD, Snyder J, Bennett V, Hund TJ, Mohler PJ. Ankyrin-G coordinates intercalated disc signaling platform to regulate cardiac excitability in vivo. Circ Res 115: 929–938, 2014. doi: 10.1161/CIRCRESAHA.115.305154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Llinas R, Sugimori M, Silver RB. Time resolved calcium microdomains and synaptic transmission. J Physiol Paris 89: 77–81, 1995. doi: 10.1016/0928-4257(96)80554-7. [DOI] [PubMed] [Google Scholar]
- 162.Guse AH, Gil Montoya DC, Diercks BP. Mechanisms and functions of calcium microdomains produced by ORAI channels, d-myo-inositol 1,4,5-trisphosphate receptors, or ryanodine receptors. Pharmacol Ther 223: 107804, 2021. doi: 10.1016/j.pharmthera.2021.107804. [DOI] [PubMed] [Google Scholar]
- 163.Eshed-Eisenbach Y, Peles E. The clustering of voltage-gated sodium channels in various excitable membranes. Develop Neurobiol 81: 427–437, 2021. doi: 10.1002/dneu.22728. [DOI] [PubMed] [Google Scholar]
- 164.Leo-Macias A, Agullo-Pascual E, Sanchez-Alonso JL, Keegan S, Lin X, Arcos T, Feng Xia L, Korchev YE, Gorelik J, Fenyo D, Rothenberg E, Rothenberg E, Delmar M. Nanoscale visualization of functional adhesion/excitability nodes at the intercalated disc. Nat Commun 7: 10342, 2016. doi: 10.1038/ncomms10342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Dixon RE, Navedo MF, Binder MD, Santana LF. Mechanisms and physiological implications of cooperative gating of ion channels clusters. Physiol Rev 102: 1159–1210, 2022. doi: 10.1152/physrev.00022.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Dhar-Chowdhury P, Malester B, Rajacic P, Coetzee WA. The regulation of ion channels and transporters by glycolytically derived ATP. Cell Mol Life Sci 64: 3069–3083, 2007. doi: 10.1007/s00018-007-7332-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wu Y, Fortin DA, Cochrane VA, Chen PC, Shyng SL. NMDA receptors mediate leptin signaling and regulate potassium channel trafficking in pancreatic β-cells. J Biol Chem 292: 15512–15524, 2017. doi: 10.1074/jbc.M117.802249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cochrane VA, Wu Y, Yang Z, ElSheikh A, Dunford J, Kievit P, Fortin DA, Shyng SL. Leptin modulates pancreatic β-cell membrane potential through Src kinase-mediated phosphorylation of NMDA receptors. J Biol Chem 295: 17281–17297, 2020. doi: 10.1074/jbc.RA120.015489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cochrane VA, Yang Z, Dell'Acqua ML, Shyng SL. AKAP79/150 coordinates leptin-induced PKA signaling to regulate KATP channel trafficking in pancreatic β-cells. J Biol Chem 296: 100442, 2021. doi: 10.1016/j.jbc.2021.100442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sukhodub A, Jovanovic S, Du Q, Budas G, Clelland AK, Shen M, Sakamoto K, Tian R, Jovanovic A. AMP-activated protein kinase mediates preconditioning in cardiomyocytes by regulating activity and trafficking of sarcolemmal ATP-sensitive K+ channels. J Cell Physiol 210: 224–236, 2007. doi: 10.1002/jcp.20862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Nishino Y, Miura T, Miki T, Sakamoto J, Nakamura Y, Ikeda Y, Kobayashi H, Shimamoto K. Ischemic preconditioning activates AMPK in a PKC-dependent manner and induces GLUT4 up-regulation in the late phase of cardioprotection. Cardiovasc Res 61: 610–619, 2004. doi: 10.1016/j.cardiores.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 172.Marinovic J, Bosnjak ZJ, Stadnicka A. Preconditioning by isoflurane induces lasting sensitization of the cardiac sarcolemmal adenosine triphosphate-sensitive potassium channel by a protein kinase C-δ-mediated mechanism. Anesthesiology 103: 540–547, 2005. doi: 10.1097/00000542-200509000-00017. [DOI] [PubMed] [Google Scholar]
- 173.Edwards AG, Rees ML, Gioscia RA, Zachman DK, Lynch JM, Browder JC, Chicco AJ, Moore RL. PKC-permitted elevation of sarcolemmal KATP concentration may explain female-specific resistance to myocardial infarction. J Physiol 587: 5723–5737, 2009. doi: 10.1113/jphysiol.2009.181040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hu K, Huang CS, Jan YN, Jan LY. ATP-sensitive potassium channel traffic regulation by adenosine and protein kinase C. Neuron 38: 417–432, 2003. doi: 10.1016/s0896-6273(03)00256-3. [DOI] [PubMed] [Google Scholar]
- 175.Aziz Q, Thomas AM, Khambra T, Tinker A. Regulation of the ATP-sensitive potassium channel subunit, Kir6.2, by a Ca2+-dependent protein kinase C. J Biol Chem 287: 6196–6207, 2012. doi: 10.1074/jbc.M111.243923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem 72: 395–447, 2003. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- 177.Letourneur F, Klausner RD. A novel di-leucine motif and a tyrosine-based motif independently mediate lysosomal targeting and endocytosis of CD3 chains. Cell 69: 1143–1157, 1992. doi: 10.1016/0092-8674(92)90636-Q. [DOI] [PubMed] [Google Scholar]
- 178.Tikkanen R, Obermuller S, Denzer K, Pungitore R, Geuze HJ, von Figura K, Honing S. The dileucine motif within the tail of MPR46 is required for sorting of the receptor in endosomes. Traffic 1: 631–640, 2000. doi: 10.1034/j.1600-0854.2000.010807.x. [DOI] [PubMed] [Google Scholar]
- 179.Takatsu H, Katoh Y, Shiba Y, Nakayama K. Golgi-localizing, gamma-adaptin ear homology domain, ADP-ribosylation factor-binding (GGA) proteins interact with acidic dileucine sequences within the cytoplasmic domains of sorting receptors through their Vps27p/Hrs/STAM (VHS) domains. J Biol Chem 276: 28541–28545, 2001. doi: 10.1074/jbc.C100218200. [DOI] [PubMed] [Google Scholar]
- 180.Ghai R, Bugarcic A, Liu H, Norwood SJ, Skeldal S, Coulson EJ, Li SS, Teasdale RD, Collins BM. Structural basis for endosomal trafficking of diverse transmembrane cargos by PX-FERM proteins. Proc Natl Acad Sci USA 110: E643–E652, 2013. doi: 10.1073/pnas.1216229110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Garrido JJ, Giraud P, Carlier E, Fernandes F, Moussif A, Fache MP, Debanne D, Dargent B. A targeting motif involved in sodium channel clustering at the axonal initial segment. Science 300: 2091–2094, 2003. doi: 10.1126/science.1085167. [DOI] [PubMed] [Google Scholar]
- 182.Mohler PJ, Hoffman JA, Davis JQ, Abdi KM, Kim CR, Jones SK, Davis LH, Roberts KF, Bennett V. Isoform specificity among ankyrins. An amphipathic α-helix in the divergent regulatory domain of ankyrin-b interacts with the molecular co-chaperone Hdj1/Hsp40. J Biol Chem 279: 25798–25804, 2004. doi: 10.1074/jbc.M401296200. [DOI] [PubMed] [Google Scholar]
- 183.Lowe JS, Palygin O, Bhasin N, Hund TJ, Boyden PA, Shibata E, Anderson ME, Mohler PJ. Voltage-gated Nav channel targeting in the heart requires an ankyrin-G dependent cellular pathway. J Cell Biol 180: 173–186, 2008. doi: 10.1083/jcb.200710107. [DOI] [PMC free article] [PubMed] [Google Scholar]