Abstract
The size of the satellite cell pool is reduced in estradiol (E2)-deficient female mice and humans. Here, we use a combination of in vivo and in vitro approaches to identify mechanisms, whereby E2 deficiency impairs satellite cell maintenance. By measuring satellite cell numbers in mice at several early time points postovariectomy (Ovx), we determine that satellite cell numbers decline by 33% between 10 and 14 days post-Ovx in tibialis anterior and gastrocnemius muscles. At 14 days post-Ovx, we demonstrate that satellite cells have a reduced propensity to transition from G0/G1 to S and G2/M phases, compared with cells from ovary-intact mice, associated with changes in two key satellite cell cycle regulators, ccna2 and p16INK4a. Further, freshly isolated satellite cells treated with E2 in vitro have 62% greater cell proliferation and require less time to complete the first division. Using clonal and differentiation assays, we measured 69% larger satellite cell colonies and enhanced satellite cell-derived myoblast differentiation with E2 treatment compared with vehicle-treated cells. Together, these results identify a novel mechanism for preservation of the satellite cell pool by E2 via promotion of satellite cell cycling.
Keywords: muscle stem cells, ovariectomy, satellite cell cycling, skeletal muscle
INTRODUCTION
Skeletal muscle growth and regeneration require a mitotically quiescent stem cell population known as satellite cells (1, 2). During homeostasis, satellite cells reside on the periphery of terminally differentiated muscle fibers and are marked by the expression of paired box transcription factor 7 (Pax7) and several cell surface markers, including α7-integrin (3–6). Following a stimulus (e.g., injury or stress), a subset of the satellite cells transition from a quiescent to activated state and enter the G1 phase of the cell cycle (7). Satellite cells can then undergo asymmetric division where one daughter cell commits to the myogenic lineage, differentiates and fuses to new or existing damaged fibers, and the other daughter cell returns to quiescence to maintain the satellite cell pool, a process known as self-renewal (8). Satellite cells can also undergo symmetric proliferation followed by stochastic recruitment of proliferated progeny back into the satellite cell pool (9, 10). In healthy adult muscle, the appropriate balance of intrinsic and extrinsic factors is maintained to coordinate satellite cell fate decisions (i.e., myogenic commitment vs. self-renewal) with the demands of regenerating or growing muscle (11, 12).
The effects of disrupting the complex balance of factors that affect satellite cell fate can be observed in aging skeletal muscle, resulting in markedly compromised muscle regeneration. Major advances toward understanding how changes in the intrinsic and extrinsic factors that influence the satellite cell pool have been made in the last 60 yr since the satellite cell was discovered (13). Numerous groups have observed a decline in satellite cell number in aged rodents (14–22) and human skeletal muscles (23–25) with the rate and extent varying with muscle fiber type and function (e.g., locomotion, respiration, or mastication; 18, 20, 21). In addition, several studies have demonstrated that changes in extrinsic factors in the satellite cell microenvironment contribute to impaired regeneration with age (reviewed in Refs. 24–30). The decline of circulating hormones including insulin-like growth factor-1 (IGF-1) and oxytocin have been identified as contributors to age-associated impairments of satellite cells (31–33). Over the last decade, the relationship between satellite cell function and sex hormones has gained attention to rationalize sex-related differences in skeletal muscle regeneration (34–43). In particular, evidence is mounting that the major sex hormone in females, estradiol (E2), influences satellite cell function and muscle regeneration.
It is important to understand that E2 levels can decline in females due to a variety of reasons, including 1) natural age-induced menopause (44), 2) menstrual dysfunction experienced with the female athlete triad (45), 3) side effects of hormone therapy to treat cancer (46), 4) congenital conditions (e.g., Turner syndrome; 47, 48), and 5) hysterectomy with or without oophorectomy (i.e., surgical removal of the ovaries; referred to as ovariectomy in animals; 49). Health issues associated with E2 deficiency traditionally prompted studies focused on osteoporosis (reviewed in Ref. 50) and heart disease (reviewed in Ref. 51) leaving the role of E2 on skeletal muscle and satellite cell biology less clear. Early studies have shown that E2 deficiency in females blunts satellite cell activation and proliferation induced by injury or exercise (51–56). Interestingly, E2 treatment has been shown to both impair and enhance satellite cell differentiation in mice (C2C12) and rat (L6) myoblast cells (57–59). Inconsistencies in the effects of E2 on satellite cells are presumably due to different experimental conditions including animal model and age, E2 dose and treatment duration, as well as methods of measuring progression of satellite cells through myogenesis. Although these studies suggest potential mechanisms of action of E2 on muscle regeneration, well-defined mechanisms, whereby E2 regulates satellite cell function are yet to be determined.
Our recent findings show that the size of the satellite cell pool is reduced in ovarian hormone-deficient female mice and humans under normal homeostatic conditions (60, 61). Using hormone replacement, we demonstrate that E2 is the ovarian hormone responsible for affecting satellite cells (60, 61). Here we investigate mechanisms whereby the loss of circulating E2 in females results in the reduced satellite cell number under normal homeostatic conditions, i.e., without any muscle injury. We posit that E2 influences satellite cell maintenance by regulating satellite cell cycle kinetics, progression, proliferation, and differentiation. To test this hypothesis, we use ovariectomized (Ovx) female mice to study satellite cell biology in vivo and thus examine the cell cycle progression of satellite cells with and without circulating E2. In addition, considering that satellite cells are heterogeneous regarding their cell cycle progression, we assess cell cycle kinetics, proliferation, and differentiation in vitro by treating freshly isolated satellite cells from female mice with E2. Our results show decrements in satellite cell cycle progression with E2 deficiency, suggesting that satellite cell number declines due to inability of the satellite cells to cycle and generate progeny without E2 in the environment. These findings have implications in the preservation of efficient muscle regeneration, including targeting p16-mediated pathways to prevent cell cycle arrest of satellite cells and subsequent exhaustion of the satellite cell pool. Ultimately, understanding how E2 regulates satellite cells will help to determine therapies for improving muscle regeneration and recovery of strength that affect the quality of life.
METHODS
Mice
All procedures were performed in accordance with protocols approved by the Institutional Animal Care and Use Committees at the University of Minnesota (No. A3456-01). All experiments were conducted on female mice when they were mature adults (3–6 mo of age, life phase equivalent of ∼20–30 yr for humans; 62). Female wild-type (C57Bl/6) mice were obtained from Jackson Laboratory (000664; Bar Harbor, ME). Female Pax7-ZsGreen, Pax7CreERT2/+; Esr1fl/fl;Pax7-ZsGreen (scERαKO), and Pax7+/+Esr1fl/fl;Pax7-ZsGreen (scERαWT) mice were generated in-house (4). Mice were housed in groups of 4–5 and had access to phytoestrogen-free rodent chow (Harlan-Tekland No. 2019; Indianapolis, IN) and water ad libitum. The housing room was maintained on a 14:10 light:dark cycle with controlled temperature and humidity.
For time-course experiments, female C57Bl/6 mice were assigned to one of two groups: Sham or Ovariectomized (Ovx) and were euthanized 6, 10, or 14 days postsurgery (n = 25 for each group). Female scERαWT and scERαKO mice were treated with tamoxifen (2 mg/kg) for 5 days consecutively (63, 64). At 14 days after tamoxifen treatment, mice were used for satellite cell harvests (n = 6/group). For the Pax7CreERT2/+ effect experiment, female scERαWT and scERαKO mice were used for satellite cell harvests (n = 6/group). For the tamoxifen effect experiment, female scERαWT were treated with a vehicle [15% ethanol (EtOH) in sunflower seed oil] or tamoxifen (n = 5/group). For in vivo proliferation experiments, female C57Bl/6 mice that were either Sham or Ovx received 21 days slow-release 5-ethynyl-2′-deoxyuridine (EdU; 25 mg) immediately following surgery (n = 7–9). For in vitro experiments, satellite cells were harvested from Pax7-ZsGreen female mice (n = 3–6/group). Mice were euthanized with an intraperitoneal injection of sodium pentobarbital (200 mg/kg) followed with cervical dislocation as secondary euthanasia.
Surgical Procedures
Sham and Ovx surgeries were performed as previously described (65). Briefly, mice were given a subcutaneous injection of slow-release buprenorphine (1 mg/kg), and 2–4 h later were anesthetized by inhalation of isoflurane (2%–3%, 125 mL O2/min). Bilateral Ovx was performed through two small dorsal incisions between the iliac crest and the lower ribs, and Sham operations consisted of the same procedure as Ovx except that the ovaries were not removed. In a subset of mice, immediately after Ovx, mice were implanted with pellets containing 25 mg EdU released over a 21 days period (Innovative Research of America, Sarasota, FL). The daily dose of EdU is equivalent to that given by intraperitoneal injection daily (50 mg/kg). Mice were monitored daily for 3 days following surgery, and incision wound clips were removed at 10 days postsurgery. The estrous cycle of Sham and Ovx mice was tracked for 3–5 days consecutively via vaginal cytology to confirm normal estrous cycles or persistent diestrus, respectively (66). At the completion of all experiments, uteri were dissected and weighed. Uterine mass <30 mg was used as an inclusion parameter to indicate successful Ovx surgery (67).
Satellite Cell Isolation
Isolation of satellite cells from individual muscles [e.g., tibialis anterior (TA) and gastrocnemius (GC)] and bulk muscles (hindlimb muscles) were performed as described in detail previously (18, 60). Briefly, muscles were dissected, minced in parallel with muscle fibers, and digested with collagenase type II and dispase (17101-015 and 17105-041, respectively; Gibco, Grand Island, NY). Mononuclear cells were stained using an antibody mixture of 1 µL PE-Cy7 rat anti-mouse CD31 (clone 390; 561410; BD Biosciences, San Diego, CA), 1 µL PE-Cy7 rat anti-mouse CD45 (clone 30-F11; 552848; BD Biosciences), 1 µL biotin rat anti-mouse CD106 [clone 429 (MVCAM.A); 553331; BD Biosciences], 1 µL PE streptavidin (554061; BD Biosciences), and 2 µL α7 integrin 647 (clone R2F2; AbLab; Vancouver, BC, Canada). Samples were incubated with an antibody cocktail, washed, and resuspended with fluorescence-activated cell sorting (FACS) staining medium [2% fetal bovine serum (FBS; 16000044; Gibco) in phosphate-buffered saline (PBS)] containing 0.5 µg/mL propidium iodide (PI) for analysis on a FACSAriaII SORP (BD Biosciences, San Diego, CA). Total satellite cells (lineage negative; VCAM, α7 double-positive cells) were analyzed from the entire muscle sample. For isolation of satellite cells from Pax7-ZsGreen mice, mononuclear cells were incubated in FACS staining medium containing PI and ZsGreen+ cells were examined as described previously (68). Absolute satellite cell counts by FACS were confirmed through gating ZsGreen+ cells and counting beads (CountBright absolute counting beads; C36950; Lot No. 2361079; Invitrogen, Waltham, MA) according to the manufacturer’s instructions. Cell concentration was calculated using the formula: (number of cell events ÷ number of bead events) × (assigned bead count of the lot ÷ volume of sample).
Pax7 Immunostaining
TA muscles were removed and placed in OCT compound, frozen in 2-methylbutane (Sigma-Aldrich), cooled by liquid nitrogen, and stored at −80°C until use. For visualization of satellite cells, Pax7 and laminin staining were performed on 7 µM cryosections (CM 1850, Leica Microsystems, Buffalo Grove, IL). Sections were fixed in 4% paraformaldehyde (PFA), washed with PBS, and boiled in heat-induced antigen retrieval buffer (1.8 mM citric acid and 8.2 mM sodium citrate in water) for 30 min using an Instant Pot pressure cooker (Instant Appliances). Sections were incubated for 10 min in H2O2 to block endogenous peroxidase activity and then blocked for nonspecific binding in 0.5% PerkinElmer TNB blocking reagent [0.1 M Tris-HCl, pH 7.5; 0.15 M NaCl; 0.5% tyramide signal amplification (TSA) blocking reagent, FP1020] for 1 h at room temperature. Following blocking, sections were incubated with anti-pax7 mouse IgG1 primary antibody (PAX7, Developmental Studies Hybridoma Bank, 1:10) and anti-laminin rabbit (L9393; Sigma-Aldrich, 1:250) in TNB blocking buffer overnight at 4°C. After washing with PBS, sections were incubated with goat anti-mouse biotin-conjugated secondary antibody (115-065-205; Jackson Immuno Research Laboratories Inc, West Grove, PA; 1:1,000) and Alexa Fluor 488 goat anti-rabbit (A11034; Invitrogen; 1:500) in TNB blocking buffer for 2 h at room temperature. Visualization of the Pax7 primary antibody was achieved by incubating the sections with the Vectastain ABC reagent (PK-6100; Vector Laboratories, Burlingame, CA) for 3 h and incubation in the dark with TSA cyanine 3 kit (NEL744; PerkinElmer, Waltham, MA; 1:50) in diluent buffer for 10 min. Finally, the sections were mounted with antifade Prolong gold with 4,6-diamidino-2-phenylindole (DAPI). All images were processed and analyzed in a blinded manner with samples being de-identified as to the group. Mouse muscle samples were examined and imaged using a Leica DM5500B microscope (Leica Microsystems) at ×5 to ×20 magnification. Images were stitched using the automated tile-scan tool to construct an image of the entire cross section of the TA muscle. Satellite cells were identified by DAPI+ and Pax7+ cells residing along the myofiber border and were quantified using the region of interest (ROI) manager in the ImageJ software package (NIH, Bethesda, MD). For determination of the cross-sectional area of the TA muscle, the freehand and wand selection tools of the ImageJ were used to measure maximum Feret’s diameter.
DNA Content Analysis
Pax7-ZsGreen cells were isolated by FACS and fixed by adding cooled 70% EtOH dropwise while vortexing cell suspension. Cells were then washed with PBS and incubated in a staining solution containing 0.1% (vol/vol) Triton-X100 in PBS, 2 mg DNase-free RNase (Sigma), and 1 mg/mL PI for 30 min at 37°C. Samples were analyzed on a FACSAriaII SORP (BD Biosciences, San Diego, CA). Cell cycle distributions for satellite cells in G1, S, and G2 phases were performed using FlowJo v.10 univariate modeling with the Watson pragmatic algorithm.
RT-qPCR
RNA from freshly FACS-isolated satellite cells was isolated using Qiagen RNeasy Plus Universal Mini kit (73404; Hilden, Germany) according to manufacturer’s instructions. cDNA was synthesized from 100 ng RNA according to directions in SUPERVILO cDNA Synthesis Kit (11756050; Thermo Fisher Scientific, Waltham, MA). Relative quantitation of cdkn1b/p27Kip1 (Mm00438168_m1), cdkn2a/p16INK4a (Mm00494449_m1), ccnd1 (Mm00432359_m1), ccna2 (Mm00438063_m1), mapk14/p38 (Mm01301009_m1), and house-keeping gene GAPDH (Mm99999915_g1) were determined using TaqMan fast advanced master mix (4444557; Thermo Fisher Scientific; Waltham, MA).
In Vivo EdU Proliferation Assay
Sham and Ovx mice received a pellet containing 25 mg EdU. Following 21 days of exposure, flow cytometry analysis was performed as described in the Click-iT EdU Alexa Fluor 488 Flow Cytometry kit (C104020; Invitrogen) combined with the mononuclear antibody mixture as described earlier in the satellite cell isolation section. A total of 50,000–100,000 events were recorded for the analysis. Proliferating satellite cells (i.e., S-phase satellite cells) were identified as lineage negative; VCAM, α7; FITC triple-positive cells (Supplemental Fig. S3B; see https://doi.org/10.6084/m9.figshare.17096918.v1). A positive control comprised of a 72 h post-barium chloride injured TA muscle was included to demonstrate robust EdU+ incorporation by satellite cells (Supplemental Fig. S3, C and D).
In Vitro EdU Proliferation Assay
Pax7-ZsGreen cells were isolated by FACS and plated into 0.1% gelatin-coated 96-well plates (1,000 cells/well) containing muscle growth medium (MGM) with 20% charcoal-stripped (CS) FBS (NB036790; Thermo Fisher Scientific). The cells received MGM with or without E2 daily (100 pM final concentration; E8875; Sigma-Aldrich). On day 6, Click-iT EdU cell proliferation kit for imaging, Alexa Fluor 594 dye (C10339; Invitrogen) was performed according to the manufacturer’s instructions. The cells were then incubated in DAPI (1:1,000 dilution) in PBS for 20 min at room temperature. EdU+ nuclei were identified and imaged at ×10 magnification, taken on a Zeiss Observer.Z1 inverted microscope equipped with an AxioCam MRm camera (Thornwood, NY).
MTT Proliferation Assay
Pax7-ZsGreen cells were isolated by FACS and plated into 0.1% gelatin-coated 96-well plate (2,000 cells/well) with Hams/F10 medium (SH30025.01; Hyclone, Logan, UT) supplemented with 20% CS-FBS (NB036790; Thermo Fisher Scientific), 10 ng/mL human basic fibroblast growth factor (bFGF; 100-18C; Peprotech), 1% Pen/Strep (15140122; Gibco), and 1% Glutamax (35050061; Gibco) and were incubated at 37°C and 5% CO2. Satellite cells were treated with E2 every 12 or 24 h to establish a final concentration of: 0 pM, 3.125 pM (0.85 pg/mL), 50 pM (13.62 pg/mL), or 100 pM (27.24 pg/mL) E2. The MTT assay was performed according to manufacturer’s instructions (11465007001; Roche). After 24 or 72 h, the MTT labeling reagent [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide in PBS] was added to each well (final concentration 0.5 mg/mL) and incubated for 4 h. The solubilization solution was then added to each well and incubated overnight. The formazan product was measured in a microplate reader at a 570-nm wavelength.
ATP Cell Proliferation Luciferase Assay
Pax7-ZsGreen cells were isolated by FACS and plated into 0.1% gelatin-coated 96-well plates (1,000 cells/well) containing MGM with 20% CS-FBS. The cells either received MGM with or without 100 pM E2. On days 4 and 6, CellTiter-Glo luminescent cell viability assay (G7570; Promega, Madison, WI) was performed. Medium was replaced with CellTitre-Glo reagent (1:3) in 100 µL of PBS. Plates were allowed to equilibrate for 3 min, then read on a Cytation3 plate reader (BioTek, Winooski, VT).
Time to First Division and Cell Size
Pax7-ZsGreen cells were isolated by FACS and plated for live-cell imaging into 0.1% gelatin-coated 24-well glass-bottom dishes (NC9988706; Mattek; Thermo Fisher Scientific; Waltham, MA; 12,000 cells/well) containing MGM with 20% CS-FBS. Cells were treated with MGM containing vehicle (0.03% ethanol in PBS) or E2 (final concentration 100 pM) at the time of plating and again 18 h after plating. Time-lapse imaging was performed from 18 h to 72 h after plating with a Nikon Eclipse Ti-inverted fluorescence microscope equipped with an automated stage (Prior), and a custom chamber to maintain a constant 37°C temperature, high humidity, and 5% CO2. Multiple positions were analyzed per group with images acquired every 10 min using phase contrast. Images were collected using a ×20 CFI Plan Apochromat Lambda (NA = 0.75) objective (Nikon). For each condition, at least 100 individual cells were tracked. Following imaging, data were exported as individual TIFFs for each position and time point. ImageJ software package was used to concatenate TIFF images from each location and manually measure time to first division of each cell. Cell size at 18 h after plating was measured following pixel-based classification and cell segmentation with ilastik (version 1.3.3) and CellProfiler (version 4.0.5), respectively.
Colony-Forming Assay
Pax7-ZsGreen cells were isolated by FACS and single cells were sorted into 0.1% gelatin-coated 96-well plates containing mouse myoblast medium (MMM): Dulbecco’s modified Eagle’s medium (DMEM; SH30284.01; Hyclone) without phenol red containing 4.00 mM l-glutamine, 4,500 mg/L glucose, and sodium pyruvate; 20% CS-FBS; 10% charcoal-stripped horse serum (CS-HS; NC9058780; Thermo Fisher Scientific); 10 ng/mL human basic fibroblast growth factor (bFGF; 100-18C; Peprotech), 1% Pen/Strep, and 1% Glutamax with or without E2 (final concentration 100 pM E2). Cells were allowed to adhere for 24 h and were then supplemented daily with MMM with or without 100 pM E2. After culturing plates for 8 days at 37°C and 5% CO2, cells were fixed with 4% PFA for 20 min at room temperature. For immunostaining of colonies, cells were permeabilized with 0.3% Triton-X100 for 20 min at room temperature, washed with PBS, and blocked with 3% BSA in PBS for 1 h at room temperature. Colonies were stained for MF-20 antibody supernatant (Developmental Studies Hybridoma Bank, University of Iowa; 1:20 dilution) in 3% BSA in PBS overnight at 4°C. After PBS washes, cells were incubated with Alexa Fluor 555 goat anti-mouse secondary antibody (Life Technologies; 1:500 dilution) in the dark for 45 min at room temperature. The cells were then incubated in DAPI (1:1,000 dilution) in PBS for 20 min at room temperature. Colonies were imaged at ×10 magnification, taken on a Zeiss Observer.Z1 inverted microscope equipped with an AxioCam MRm camera (Thornwood, NY). Intensity thresholding of ImageJ software package was used to measure the number of nuclei and number of colonies. The percentage of clonal efficiency was calculated by dividing the number of colonies in each plate by the number of wells in which a single cell was sorted then multiplying by 100. Colony size was measured using the freehand selection tool.
Satellite Cell-Derived Myoblast Differentiation
Pax7-ZsGreen cells were isolated by FACS and plated into 0.1% gelatin-coated or Matrigel matrix (353234; Corning; Bedford, MA) 48-well plates (20,000 cells/well) containing MGM with 20% FBS. Cells were incubated at 37°C and 5% CO2 with MGM medium changed every other day. Cells reached 80%–100% confluence on day 3 and were induced to differentiate in a low serum medium: DMEM supplemented with 2% normal or CS-HS, 1% Pen/Strep, and 1% Glutamax for 3.5 days with or without 100 pM E2. Immunofluorescent staining of cells for MF-20 and DAPI was performed as described in the clonal ability assay section. Fusion index was calculated as the percentage of nuclei in myotubes.
Statistical Analysis
Two-way analysis of variance (ANOVA) was utilized to determine differences among times and groups. Holm–Sidak post hoc tests were performed in the event of a significant interaction or main effect of time. All other data were analyzed with two-tailed unpaired Student t tests for determining significant differences between two groups or one-way ANOVA with Holm–Sidak post hoc for determining significant differences among three or more groups. An α level of <0.05 was used for all analyses. Data are presented as means ± SE unless otherwise indicated. Time to first division data are presented as histograms representing individual cells dividing within time points and as scatter plots with means ± SD. Satellite cell size and colony size data are presented as scatter plots with means ± SD. All statistical testing was performed using GraphPad Prism 8.0 (GraphPad Software Inc., San Diego, CA) or SigmaPlot version 12.5 (Systat Software, San Jose, CA). Sample sizes are reported as the number of independent mice from which the cells were analyzed or isolated. All immunofluorescent images were processed and analyzed in a blinded manner with samples being de-identified as to treatment or group.
RESULTS
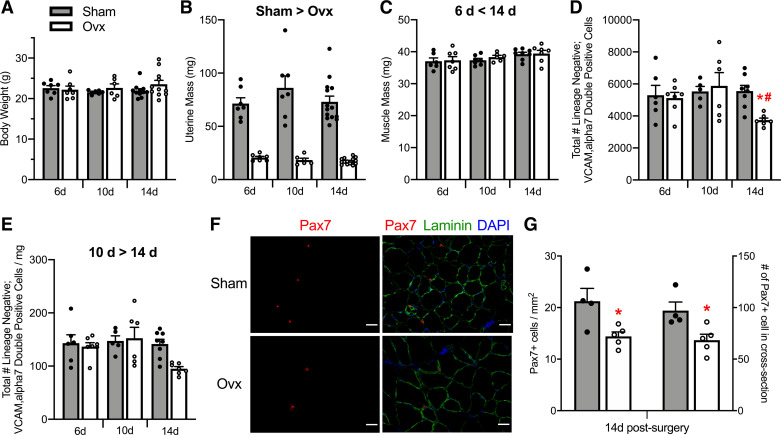
Body mass did not differ between Sham and Ovx mice or across 6, 10, and 14 days postsurgery (P ≥ 0.416; Fig. 1A). Vaginal cytology confirmed estrous cycling in Sham mice and persistent diestrus in Ovx mice. Further, Ovx surgery was considered successful with uterine mass approximately, about fourfold less in Ovx than in Sham mice (P < 0.001) and all uteri being <26 mg in Ovx mice (Fig. 1B). The duration of E2 deficiency did not affect the uterine mass (P = 0.393). Mass of TA muscles was 6% greater at 14 compared with 6 days post-Ovx (P = 0.040) but did not differ between Sham and Ovx (P = 0.471; Fig. 1C).
Figure 1.
Effects of ovarian hormone deficiency on organ masses and satellite cell number. Body masses (A), uterine masses (B), and TA muscle masses of Sham and Ovx female mice (n = 6–14/group; C). D: total number of satellite cells quantified by FACS as lineage negative; VCAM, α7 double-positive cells in TA muscles 6, 10, or 14 days following a Sham or Ovx surgery. E: density of satellite cells as calculated from the total number of satellite cells normalized to wet TA muscle masses. F: satellite cell number quantified by Pax7 immunohistochemistry in TA muscle cross sections from Sham (n = 4) and Ovx (n = 5) at 14 days postsurgery. Pax7+ cells are presented normalized to TA cross-section area and as absolute satellite cells per cross section. Scale bars = 50 µm. Values are presented as means ± SE. Significant main effects of two-way ANOVAs (P < 0.05) are indicated above the bars (B, C, and E) and when significant interactions occurred, Holm–Sidak post hoc results are indicated by *different from Sham at corresponding time (D) and #different from 10 days Ovx (D). *Different from Sham by student t tests (G). FACS, fluorescence-activated cell sorting; Ovx; ovariectomized; TA; tibialis anterior.
Effects of Ovarian Hormones and ERα Signaling on Satellite Cell Number
To identify the best time post-Ovx to study satellite cell cycling, FACS was used to quantify the total number of satellite cells (lineage negative; VCAM α7 double-positive cells) in TA and GC muscles (Supplemental Fig. S1, A and B; see https://doi.org/10.6084/m9.figshare.17096900.v1 respectively). An interaction between group and time was observed for satellite cell number in the TA (P = 0.049; Fig. 1D). Satellite cell number did not differ between Sham and Ovx at 6 or 10 days postsurgery (P ≥ 0.862); however, was 33% lower in Ovx than Sham mice at 14 days (3,738 ± 128 vs. 5,554 ± 354, respectively; P = 0.010; Fig. 1D). We evaluated the density of satellite cells, calculated by dividing the absolute cell number by the wet mass of each muscle. Satellite cell density in TA muscles was lower at 14 days compared with 10 days (time effect; P = 0.031; Fig. 1E). Similarly, satellite cell number and density were 33% lower in GC muscles from Ovx than Sham mice at 14 days postsurgery (group effect; P ≤ 0.001; Supplemental Fig. S1, C–E). Compilation of satellite cell number at these early time points of ovarian hormone deficiency with later time points previously reported (i.e., 56, 112, and 196 days post-Ovx; 58), identifies 14 days as the earliest time point analyzed where a decline in satellite cell number is measured (Supplemental Fig. S1, F and G). Pax7-immunostaining of TA muscle cross sections at 14 days post-Ovx showed 30% and 32% fewer satellite cells per cross section and per mm2, respectively (P ≤ 0.025; Fig. 1, F and G), recapitulating the decline in satellite cell number at 14 days post-Ovx observed with FACS quantification.
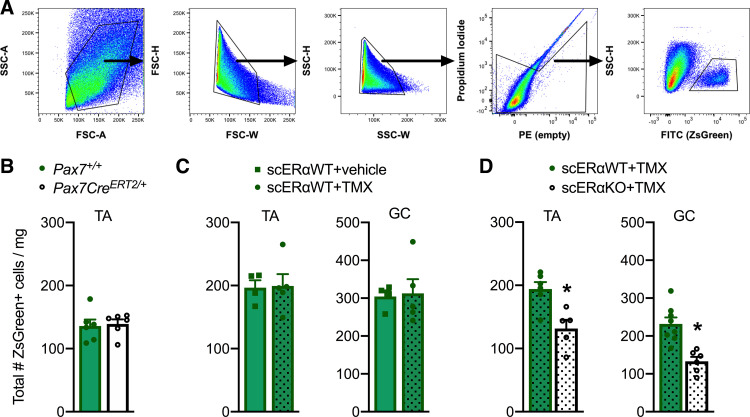
We recently determined that Esr1, the gene encoding estrogen receptor α (ERα), is more highly expressed in satellite cells than the two other estrogen receptors, Esr2 (ERβ) and Gper, and the progesterone receptor, Pgr (60). This led us to develop an inducible satellite cell-specific ERα knockout mouse (scERαKO) to specifically probe E2-ERα signaling in satellite cells (described in Ref. 60) by measuring ZsGreen+ cells (Fig. 2A). First, we completed control experiments to directly show that the presence of Pax7CreERT2/+ and tamoxifen treatment did not influence satellite cell numbers (Fig. 2, B and C). Next, we measured ZsGreen+ cells in TA and GC muscles from scERαKO and control littermates (scERαWT) 14 days after ablation of ERα. Similar to Ovx mice, scERαKO mice have 24%–62% fewer satellite cells (P ≤ 0.050; Fig. 2D) indicating that E2 deficiency drives the loss of satellite cells with Ovx as opposed to any other ovarian hormone. Accuracy of satellite cell counts by FACS was confirmed by the concurrent analysis of flow cytometry counting beads and ZsGreen+ satellite cells (Supplemental Fig. S2, A and B; see https://doi.org/10.6084/m9.figshare.17096915.v1). Together, these results indicate that deficiency of the hormone E2 drives the loss of satellite cells with Ovx as opposed to any other ovarian hormone and that the loss of E2 or its receptor for only 14 days causes a reduction in the number of satellite cells in skeletal muscles of female mice. Importantly, these data identify the most appropriate time point for conducting the following in vivo experiments to investigate impaired satellite cell cycling as a mechanism for the decline in satellite cell number with disruption of E2-ERα signaling.
Figure 2.
Satellite cell number with the loss of E2-ERα signaling. A: gating scheme for ZsGreen+ cells in tibialis anterior and gastrocnemius muscles. ZsGreen+ cells were gated based on forward/side scatter (plots 1–3) and live cells (propidium iodide negative – plot 4). These cells were then selected for ZsGreen-positive cells SSC-H X FITC (ZsGreen; absolute). Total number of ZsGreen+ satellite cells normalized to muscle masses from Pax7+/+ (scERαWT; n = 6) and Pax7CreERT2/+ (scERαKO; n = 6) female mice (B), scERαWT female mice treated with vehicle (n = 4) or TMX (n = 5) (C), and scERαWT and scERαKO female mice treated with TMX (n = 5–8/group; D). For C and D, TA and GC muscles were harvested and analyzed 14 days after treatment or the loss of E2-ERα signaling. Values are presented as means ±SE. *Different from scERαWT. ERα; estrogen receptor α; E2; estradiol; GC, gastrocnemius; TA; tibialis anterior; TMX; tamoxifen.
Effects of E2 on Satellite Cell Cycle Progression
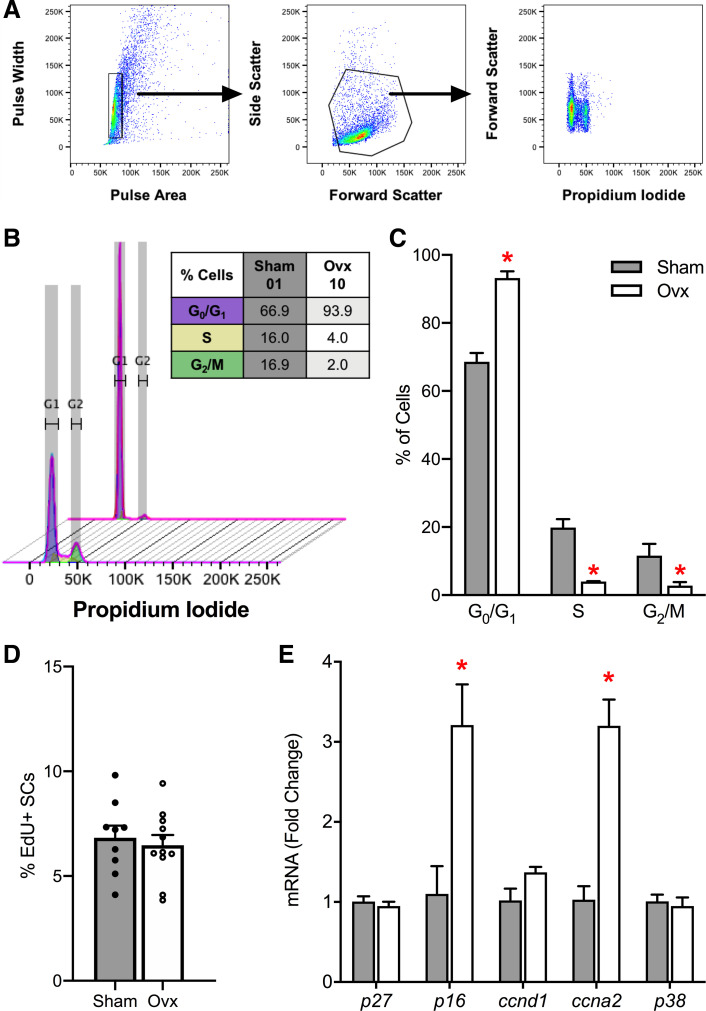
First, we investigated whether the decline in satellite cell number with E2-ERα disruption is due to changes in satellite cell cycle progression. The most common method for evaluating the cell cycle is DNA content; thus, we used isolated satellite cells from Sham and Ovx mice 14 days postsurgery and stained the DNA stoichiometrically with PI (Fig. 3A). This analysis revealed significant differences between Sham and Ovx mouse muscles in the distribution of satellite cells in each cell cycle phase (P ≤ 0.048; Fig. 3, B and C). To evaluate the percentage of S-phase satellite cells long-term, Sham and Ovx mice received EdU slow-release pellets, implanted on the day of Ovx surgery, for 21 days (Supplemental Fig. S3A). Flow cytometry analysis indicated that the percentage of EdU+ satellite cells accumulated over 21 days did not differ between TA muscles from Sham and Ovx mice (P = 0.646; Fig. 3D). qPCR analysis showed that gene expression of p16INK4a, a negative regulator of the cell cycle, and ccna2, a regulator of both DNA replication and mitotic entry, were upregulated threefold in satellite cells from Ovx mice (P ≤ 0.020; Fig. 3E).
Figure 3.
Role of E2 in satellite cell cycle progression. A: cell cycle analysis by quantitation of DNA content via flow cytometry. Sorted satellite cells were fixed and gated based on pulse area/width (plot 1), forward/side scatter (plot 2), and propidium iodide histogram plot (plot 3). B: overlay histogram of the cell cycle analysis and table of cell cycle distribution of satellite cells from representative Sham (sample 01) and Ovx (sample 10) TA muscles. C: cell cycle distributions for satellite cells in G1, S, and G2 phases (n = 4/group). D: in vivo percentage of EdU+ satellite cells 21 days post-Ovx (n = 9–11/group). E: RT-qPCR mRNA expression of cell cycle-related genes in ZsGreen+ satellite cells isolated from hindlimb muscles of Sham (n = 4) and Ovx mice (n = 4) at 14 days postsurgery. Values are presented as means ± SE. *Different from Sham. E2; estradiol; Ovx; ovariectomized; SC; satellite cell; TA; tibialis anterior.
Effects of E2 on Proliferation and Cell Cycle Kinetics of Satellite Cells In Vitro
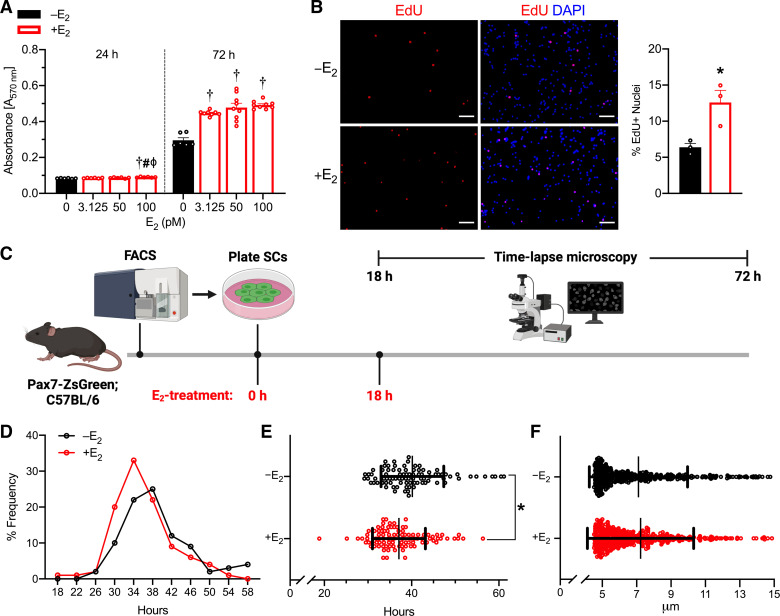
To further characterize the impaired cycling of the satellite cell pool with E2-ERα disruption, we isolated satellite cells from female Pax7-ZsGreen mice and assessed cell cycle kinetics in vitro. First, we evaluated satellite cell proliferation at 24 and 72 h postplating with E2 (final concentrations 0, 3.125, 50, and 100 pM). Satellite cell proliferation was 51%–67% greater with E2-treatment at 72 h postplating, regardless of the dose (P < 0.001; Fig. 4A). The 100 pM E2 concentration was used for all subsequent experiments, as it significantly affected satellite cell proliferation at both 24 and 72 h postplating and represents physiologically relevant E2 levels in mice (66). Furthermore, satellite cell proliferation measured by ATP luciferase assay was six- and sevenfold greater with 100 pM E2 treatment at 4 and 6 days after plating, respectively (P < 0.001; Supplemental Fig. S4; see https://doi.org/10.6084/m9.figshare.17096927.v1), and the percentage of EdU+ nuclei was twofold greater with 100 pM E2 treatment at 6 days after plating (P = 0.025; Fig. 4B).
Figure 4.
Proliferation and cell cycle kinetics of E2-treated satellite cells. A: Formazan absorbance expressed as a measure of cell viability from satellite cells treated with physiological doses of E2 (n = 6–9/group). B: representative images and quantification of the percentage of EdU+ nuclei 6 days postplating of satellite cells (n = 3/group). Scale bars = 50 µm. C: schematic of study design for time-lapse microscopy experiment to quantify satellite cell time to first division. D: frequency of satellite cell first division (n = 3/group). E: mean time of satellite cell first division. F: satellite cell size at 18 h after plating and initial E2-treatment. E2; estradiol. Values are presented as means ± SE in A and B and means ± SD for E and F. †Different from 0 (vehicle), #different from 3.125 pM, ϕdifferent from 50 pM at corresponding time points. *Different from –E2. E2; estradiol.
To measure the rate of satellite cell division, we treated satellite cells with E2 at 0 and 18 h after plating and observed cell division by time-lapse imaging from 18 to 72 h after plating (Fig. 4C). We found that E2-treated satellite cells require less time to complete the first division compared with vehicle-treated (37.1 ± 0.6 and 40.2 ± 0.8 h; P = 0.001; Fig. 4, D and E). Since cell size is proposed to be indicative of cell growth, we measured satellite cell size at 18 h postplating; E2 treatment did not affect satellite cell size (P = 0.547; Fig. 4F).
Effects of E2 on Satellite Cell Colony-Forming Ability and Differentiation
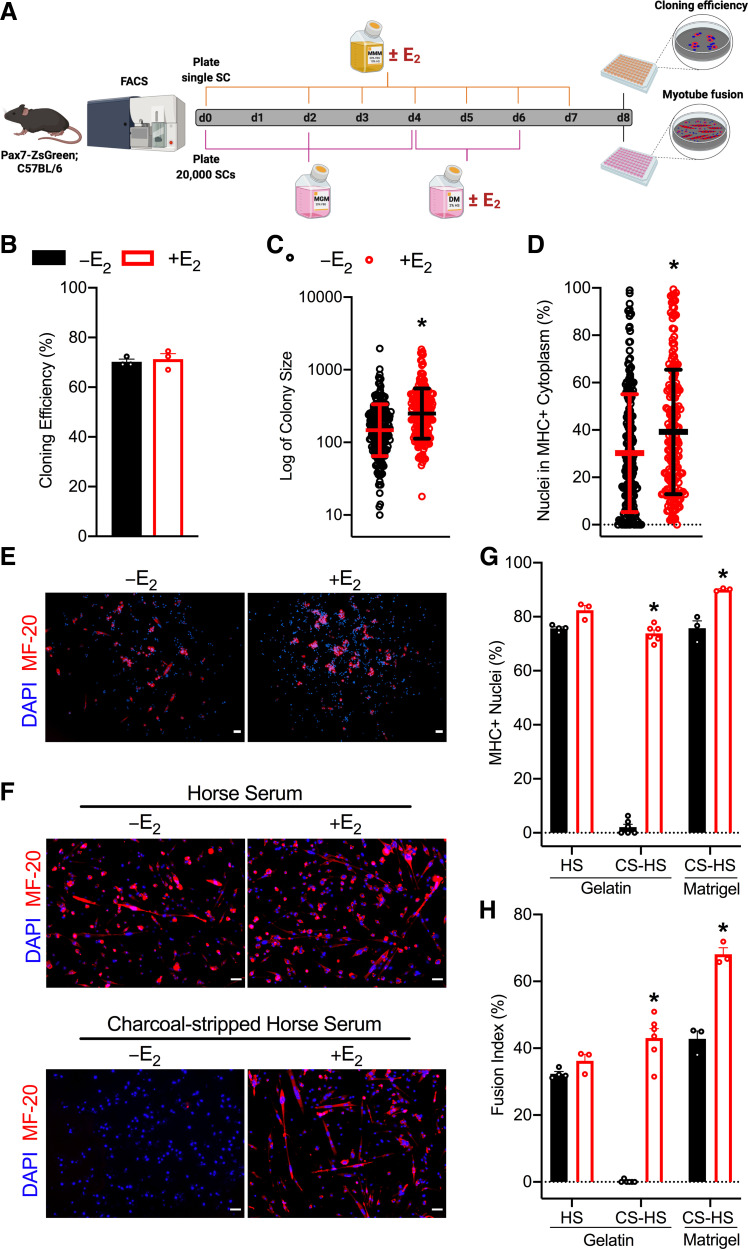
To assess clonogenicity of single satellite cells with and without E2, we treated single satellite cells with vehicle or E2 and allowed colonies to form for 8 days (Fig. 5A, top). The ability of the cells to survive and form colonies in vitro was not affected by E2 treatment (P = 0.687; Fig. 5B). However, mean colony size and spontaneous differentiation, quantified as nuclei in myosin heavy chain (MHC)+ cytoplasm, were ∼69% and 30% greater with E2 treatment (P < 0.001; Fig. 5, C and D, respectively). Because we observed greater satellite cell proliferation in vitro with E2 treatment under standard culture conditions (Fig. 4, A and B; Supplemental Fig. S4), to evaluate the effects of E2 on satellite cell differentiation alone, we cultured satellite cells under identical conditions until confluent and then began vehicle or E2 treatment after switching to low-serum differentiation medium (Fig. 5A, bottom). The number of nuclei in MHC+ myotubes and fusion index did not differ between –E2 and +E2 groups when normal HS was used (P = 0.053 and P = 0.180, respectively; Fig. 5, F–H). However, the number of nuclei in MHC+ myotubes and fusion index was greater with E2 treatment compared with no E2 when charcoal-stripped serum was used regardless of the matrix (P ≤ 0.033 and P ≤ 0.001, respectively; Fig. 5, F–H middle and right bars). In fact, myotubes were almost nonexistent in the –E2, vehicle-treated wells on a gelatin matrix, supporting the concept that the lack of E2 impairs terminal differentiation (Fig. 5F; −E2 on left).
Figure 5.
Effects of E2 on satellite cell colony-forming ability and differentiation. A: schematic for colony-forming assay (top) where single satellite cells were plated and treated with MMM with vehicle or E2 until day 8 when colony number, size, and spontaneous differentiation are measured (n = 3/group). Schematic for differentiation assay (bottom) where 20,000 satellite cells were plated and treated with vehicle or E2 only after switching to low-serum differentiation medium (n = 3–6/group). B: clonal efficiency quantified by counting the number of colonies in each plate and the number of wells in which a single cell was sorted. C: geometric mean of colony size. D: percentage of nuclei in MHC+ cytoplasm. Representative images of immunofluorescence of MF-20 (MHC) and DAPI on a gelatin matrix after 8 days of MMM with or without E2 (E) or after low-serum medium conditions with normal horse serum (HS; top) and charcoal-stripped horse serum (CS-HS; bottom) for 3.5 days with or without E2 (F). Scale bars = 50 µm. G: percentage of MHC+ nuclei. H: quantitative analysis of myotube fusion index. Values are presented as means ± SE for B, D, G, and H and means ± SD for C. *Different from –E2 within condition. CS-HS; charcoal stripped horse serum; DM; differentiation medium; E2; estradiol; FACS; fluorescence-activated cell sorting; HS; horse serum; MGM; muscle growth medium; MHC; myosin-heavy chain; MMM; mouse myoblast medium; SC; satellite cell.
DISCUSSION
Recent developments in satellite cell biology have highlighted the importance of circulating factors, such as sex hormones, in skeletal muscle growth and regeneration. Here, we expanded upon our previous work demonstrating that there is a substantial decline in the number of satellite cells in muscles from female mice with an ovarian hormone deficiency, which can be prevented with E2 treatment (60). Results of the present study show that deficiency of the hormone E2 drives the loss of satellite cells with Ovx as opposed to any other ovarian hormone and that the loss of E2 or its receptor for only 14 days impairs satellite cell maintenance. We show mechanistically that impaired satellite cell maintenance caused by E2 deficiency includes altered satellite cell cycle progression, kinetics, proliferation, and differentiation.
When satellite cells exit quiescence, the noncycling G0 phase, they can adopt different cell fates: differentiation, cell death (i.e., apoptosis, necrosis, and autophagy), or senescence. These satellite cell fate decisions are carefully regulated by intrinsic and extrinsic cues and significant alterations can lead to exhaustion of the satellite cell pool (14, 69, 70). In vivo FACS analysis of cell cycle distribution at 14 days post-Ovx indicated that E2-deficient satellite cells have impaired cell cycle progression at both G1 to S and S to G2 transitions. There were four- to fivefold fewer satellite cells from Ovx than Sham muscles in S-phase and G2-phase (Fig. 3C). In contrast, 93% of the satellite cell population was in G0 phase in Ovx muscles versus only 69% for Sham (Fig. 3C). Theoretically, there is no need for elevated cell cycling or proliferation in the ovary-intact mice because the satellite cell pool is maintained. However, given that there are ∼33% fewer satellite cells in muscles of Ovx mice compared with ovary-intact mice, we proposed that there would be an increase in proliferating satellite cells to counter the loss of satellite cells caused by E2 deficiency previously suggested to occur through apoptosis (60). We found that the cumulative proportion of proliferating satellite cells in vivo over 21 days is the same in muscles of Ovx and ovary-intact mice (Fig. 3D) and that the percentage of EdU-labeled satellite cells was similar to that previously reported. Specifically, studies have shown that 1%–2% of satellite cells are labeled per week (71) or 0.2 ± 0.1% of satellite cells are labeled per day (72), suggesting that after 21 days ∼6% of satellite cells will be EdU+, which is supported by our observations in this study. Interestingly, in vitro, we observed that E2-treatment of isolated satellite cells have 97% greater EdU incorporation compared with vehicle-treated satellite cells (Fig. 4B). These results are consistent with other reports showing that disruption of E2-ERα/β signaling impairs the proliferation of cultured satellite cells or exercise-induced satellite cell proliferation (53–56, 73, 74).
Consequently, we evaluated genes that are known to regulate satellite cell cycle progression. The progression of satellite cells from G1 to S phase is promoted by insulin-like growth factor-1 (IGF-1) via downregulation of p27Kip1 (75) and ccnd1 via downregulation of transforming growth factor β (TGF-β) signaling (76), whereas accumulation of cyclin-dependent kinase (CDK) inhibitors, such as p16INK4a, prevents satellite cell G1 to S phase progression resulting in cell cycle arrest and replicative senescence (14). Changes in satellite cell cycling in a short period of time are also a result of rapid molecular changes via protein-kinase cascades, such as phosphatidylinositol 3-kinase (PI3K; 52, 55). E2 deficiency did not affect p27Kip1 or ccnd1 mRNA expression (Fig. 3E), supporting the notion that p27Kip1, a negative regulator of cell cycling, is not associated with E2-dependent satellite cell proliferation, and potentially occurs through PI3K as previously demonstrated (52, 55). We instead show a threefold upregulation in mRNA expression of p16INK4a, a marker of permanent cell cycle arrest, in satellite cells from muscles of Ovx mice (Fig. 3E). Sousa-Victor et al. (14) recently showed for the first time that the loss of cell cycle protective mechanisms with age results in senescent p16INK4a-expressing satellite cells. That study revealed a two- and fourfold upregulation in p16INK4a mRNA expression and 15% and 40% SA-β gal+ satellite cells from 28 to 32 mo geriatric mice and 75 yr humans compared with young adults, respectively (14). With p16INK4a-induced replicative senescence playing a role in satellite cell maintenance in aged mice and humans, it will be interesting to further investigate whether the inability of satellite cells to efficiently cycle and generate progeny in an environment lacking E2 is due to p16INK4a-induced replicative senescence and whether the loss of E2 stimulates a switch to a senescent, nonproliferative state or impairs the maintenance of quiescence.
We also present data that mRNA expression of ccna2, an essential regulator of both the onset of the S-phase transition and during the G2-M transition, is upregulated (Fig. 3E). This result is in contrast to a previous study that shows a decline in ccna2 mRNA expression at 6 days postinactivation of ERβ signaling in satellite cells (73). The discrepancy between these studies is presumably due to different analysis time points, hormone versus receptor signaling, and that the present study did not culture satellite cells before qPCR analysis as was done previously (72).
It may seem paradoxical that both p16INK4a and ccna2 are upregulated in satellite cells from Ovx muscles, but it is important to note that our study involved a satellite cell population analysis and not an individual satellite cell-based analysis. As such, we do not know if the p16INK4a-expressing satellite cells were the same or different satellite cells expressing ccna2. Functionally heterogeneous subpopulations of satellite cells have been previously identified using techniques such as single-cell RNA-sequencing (77–79), single-cell mass cytometry (CyTOF; 80), lineage tracing (7, 8), and label retaining (81, 82). These cell cycle gene expression data along with our finding that E2 is necessary for transitions between cell cycle phases strongly suggest that reduced cell cycle progression is one of the mechanisms whereby satellite cell number declines with E2 deficiency.
Satellite cell subpopulations are distinguished by differential expression of genes or cell surface markers, or phenotypic changes (e.g., time to first division). For instance, noncycling satellite cells, in G0 phase, express high levels of Sprouty1 and p27Kip1 to maintain quiescence (77, 81, 83) and can reversibly transition from G0 to a primed GAlert phase permitting rapid cell cycle entry (7). We therefore cultured satellite cells in the presence or absence of E2 and observed time to first division. To note, normal serum can contain estrogens from female donors (84), so we utilized serum where the endogenous estrogens were removed by charcoal-stripping and then controlled estrogen exposure by adding specific amounts of exogenous E2. We observed that E2-treated satellite cells have reduced time to the first division, only taking 33–48 h to undergo the first division (Fig. 4E). The time to first division was not comparable to that of satellite cells from muscles 3 days postinjury, which is reported to be less than 20 h (7). Other time-lapse microscopy studies have shown that 16% of satellite cells do not divide and it takes the remaining satellite cells 36–48 h to undergo cell division (7, 85). It is worth mentioning that satellite cells grow more slowly in a medium containing charcoal-stripped serum than in normal serum, so the time to first division observed of vehicle-treated satellite cells in our study may not closely compare with previous satellite cell studies. Advancements in cell-labeling techniques have allowed analysis of satellite cell division history revealing that satellite cell division can be separated into slow- and fast-dividing subpopulations. Studies have shown that the slow-dividing subpopulation accounts for 10%–20% of satellite cells and are the long-term self-renewing population; whereas the fast-dividing subpopulation accounts for 80%–90% of satellite cells, which generate a great deal of differentiated cells but have limited replication (86–89). This study showed a faster rate of cell division with E2 treatment compared with vehicle treatment, suggesting that the satellite cells exhausted in an environment without E2 are from the fast-dividing subpopulation. These results identify impaired cell cycle kinetics as an additional mechanism, whereby the absence of E2 influences satellite cell maintenance.
Our study used a variety of methods to analyze the satellite cell proliferation in environments with and without E2, including colorimetric assays that measure metabolic activity (i.e., MTT, ATP luciferase), fluorescent dyes (i.e., PI), and incorporation of thymidine analogs (i.e., EdU). The collective data suggest that satellite cell viability and proliferation are enhanced when E2 is present (Fig. 4, A and B; Supplemental Fig. S4). Previous rodent studies investigating the effects of E2 on satellite cell proliferation did not observe changes in satellite cell proliferation with E2-treatment under normal conditions (i.e., without injury or exercise), when quantifying proliferation using the proliferation marker, proliferating cell nuclear antigen (PCNA; 54) or immunostaining for incorporated thymidine analog BrdU (53). Kamanga-Sollo et al. also used radioactive thymidine (3H-thymidine) in vitro and demonstrated greater proliferation in bovine satellite cells treated with E2 when medium is free of IGF binding protein (IGFBP)-3, a protein previously shown to antagonize IGF-1 actions on myogenic proliferation (90). The 1.5-fold increase in bovine satellite cell proliferation noted in their study was only observed when bovine satellite cells were cultured with 104 pM E2 and not 103 pM E2 (52, 74). Considering that circulating E2 concentrations in rodent models and premenopausal women range from ∼5 to 200 pM (91, 92), all of the currently published studies evaluating the effects of E2 on cultured satellite cells used supraphysiological E2 doses [ranging from 104 to 106 pM E2; (52, 58, 74, 93)]. Higher doses of E2 were possibly used due to the short half-life of E2 in culture (presumably 3 h), relatively high photodegradation half-life in solution (94), and adherence of E2 molecules to polypropylene (95). Here, we use physiologically relevant E2 doses to establish a final concentration of 100 pM E2 in culture and treated either every 24 or 48 h. Our data demonstrated greater proliferative capacity of satellite cells with E2 compared with vehicle at 3.125, 50, and 100 pM (final concentrations) at 72 h postplating (Fig. 3A). These findings suggest that previous studies using supraphysiological E2 doses may have observed opposite effects compared with those observed with physiological ranges, as has been shown in other cells/tissues (96, 97).
Assessment of the colony-forming ability of single satellite cells demonstrated that all satellite cells were able to generate clones, regardless of treatment, but the satellite cells supplemented with E2 had greater colony sizes, suggesting enhanced proliferative capacity (Fig. 5C). Satellite cell-derived myoblasts treated with E2 had augmented differentiation, indicated by increased MHC+ nuclei and myotube fusion index (Fig. 5, G and H). Other studies have yielded similar results using other methodologies. Kitajima et al. (98) cultured myofibers from Ovx in floating conditions for 3 days and observed significantly lower numbers of differentiating satellite cells compared with those from ovary-intact mice, and Galluzzo et al. (59) found that E2-treated rat myoblast cells (L6) had augmented expression of differentiation markers, MHC and myogenin. On the contrary, Ogawa et al. (58) previously demonstrated that satellite cells treated with E2 (104 pM) and cultured for 8 days in differentiation medium displayed inhibited myogenesis and reduced fusion index . We propose this negative regulation by E2 is due to supraphysiological dosing and the prolonged duration of culture in the differentiation medium.
The present study showed no noticeable difference in myotube fusion index when satellite cells were cultured in normal HS (Fig. 5H). This result emphasizes the importance of using charcoal-stripped serum to deplete E2 and other endogenous nonpolar lipid-bound materials (e.g., hormones, growth factors, and cytokines), which have demonstrated estrogenic activity and could potentially confound results (99, 100). Given that the pH indicator phenol-red has also been shown to have estrogenic effects (101–103), this study used phenol-red free DMEM with CS-HS. The absence of myotubes in vehicle-treated satellite cells cultured in phenol-red free DMEM with CS-HS on a gelatin matrix (Fig. 5F) demonstrates that E2, possibly as well as the other lipid-modified proteins, are crucial for myoblast differentiation. In addition, repeating these conditions with a Matrigel matrix indicates that E2 is largely beneficial at the terminal differentiation stage (i.e., fusion of myocytes to form myotubes; Fig. 5F). These results suggest that E2 regulates both cell division of undifferentiated satellite cells and fusion of differentiated myocytes, resulting in enhanced myogenesis.
In summary, we show that mechanisms underlying the E2 deficiency-induced decline in satellite cell number are multifactorial, involving impaired satellite cell cycle progression, kinetics, proliferation, and differentiation. These findings have implications in the preservation of efficient muscle regeneration, including targeting p16-mediated pathways to prevent cell cycle arrest of satellite cells and subsequent exhaustion of the satellite cell pool and are relevant to all women experiencing a decline in circulating E2 levels. Ultimately, understanding how E2 regulates satellite cells will help to determine therapies for improving muscle regeneration and recovery of strength that affect the quality of life.
DATA AVAILABILITY
The data that support the findings of this study are available from the corresponding author upon reasonable request.
SUPPLEMENTAL DATA
Supplemental Fig. S1: https://doi.org/10.6084/m9.figshare.17096900.v1.
Supplemental Fig. S2: https://doi.org/10.6084/m9.figshare.17096915.v1.
Supplemental Fig. S3: https://doi.org/10.6084/m9.figshare.17096918.v1.
Supplemental Fig. S4: https://doi.org/10.6084/m9.figshare.17096927.v1.
GRANTS
This study was funded by the National Institute of Health (NIH) Grants R01-AG062899 (D.A.L. and M.K.) and R01-AG031743 (D.A.L.). A.A.L. was supported on T32-AG029796 and S.L.M. was supported on T32-AR007612.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.A.L., S.L.M., E.B., M.K., and D.A.L. conceived and designed research; A.A.L., A.S.S., S.L.M., B.P.S., C.V., and E.B. performed experiments; A.A.L., A.S.S., B.P.S., and Z.A.R. analyzed data; A.A.L., S.L.M., M.K., and D.A.L. interpreted results of experiments; A.A.L., A.S.S., and B.P.S. prepared figures; A.A.L. drafted manuscript; A.A.L., S.L.M., E.B., M.K., and D.A.L. edited and revised manuscript; A.A.L., A.S.S., S.L.M., B.P.S., C.V., Z.A.R., E.B., M.K., and D.A.L. approved final version of manuscript.
ACKNOWLEDGMENTS
Graphical abstract and experimental design figures were created with Biorender.com.
REFERENCES
- 1.Relaix F, Zammit PS. Satellite cells are essential for skeletal muscle regeneration: the cell on the edge returns centre stage. Development 139: 2845–2856, 2012. doi: 10.1242/dev.069088. [DOI] [PubMed] [Google Scholar]
- 2.Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiol Rev 93: 23–67, 2013. doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA. Pax7 is required for the specification of myogenic satellite cells. Cell 102: 777–786, 2000. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- 4.Bosnakovski D, Xu Z, Li W, Thet S, Cleaver O, Perlingeiro RC, Kyba M. Prospective isolation of skeletal muscle stem cells with a pax7 reporter. Stem Cells 26: 3194–3204, 2008. doi: 10.1634/stemcells.2007-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lepper C, Partridge TA, Fan CM. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development 138: 3639–3646, 2011. doi: 10.1242/dev.067595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Maltzahn J, Jones AE, Parks RJ, Rudnicki MA. Pax7 is critical for the normal function of satellite cells in adult skeletal muscle. Proc Natl Acad Sci USA 110: 16474–16479, 2013. doi: 10.1073/pnas.1307680110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodgers JT, King KY, Brett JO, Cromie MJ, Charville GW, Maguire KK, Brunson C, Mastey N, Liu L, Tsai C-R, Goodell MA, Rando TA. mTORC1 controls the adaptive transition of quiescent stem cells from G0 to GAlert. Nature 510: 393–396, 2014. doi: 10.1038/nature13255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuang S, Kuroda K, Le Grand F, Rudnicki MA. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell 129: 999–1010, 2007. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pawlikowski B, Dalla Betta N, Antwine T, Olwin BB. Skeletal muscle stem cell self-renewal and differentiation kinetics revealed by EdU lineage tracing during regeneration. bioRxiv : 627851, 2019. doi: 10.1101/627851. [DOI]
- 10.Robinson DCL, Ritso M, Nelson GM, Mokhtari Z, Nakka K, Bandukwala H, Goldman SR, Park PJ, Mounier R, Chazaud B, Brand M, Rudnicki MA, Adelman K, Dilworth FJ. Negative elongation factor regulates muscle progenitor expansion for efficient myofiber repair and stem cell pool repopulation. Dev Cell 56: 1014–1029.e7, 2021. doi: 10.1016/j.devcel.2021.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brack AS, Rando TA. Intrinsic changes and extrinsic influences of myogenic stem cell function during aging. Stem Cell Rev 3: 226–237, 2007. doi: 10.1007/s12015-007-9000-2. [DOI] [PubMed] [Google Scholar]
- 12.Dumont NA, Wang YX, Rudnicki MA. Intrinsic and extrinsic mechanisms regulating satellite cell function. Development 142: 1572–1581, 2015. doi: 10.1242/dev.114223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engquist EN, Zammit PS. The satellite cell at 60: the foundation years. J Neuromuscul Dis 8: S183–S203, 2021. doi: 10.3233/JND-210705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sousa-Victor P, Gutarra S, García-Prat L, Rodriguez-Ubreva J, Ortet L, Ruiz-Bonilla V, Jardí M, Ballestar E, González S, Serrano AL, Perdiguero E, Muñoz-Cánove P. Geriatric muscle stem cells switch reversible quiescence into senescence. Nature 506: 316–321, 2014. doi: 10.1038/nature13013. [DOI] [PubMed] [Google Scholar]
- 15.Allbrook D, Han M, Hellmuth A. Population of muscle satellite cells in relation to age and mitotic activity. Pathology 3: 233–243, 1971. doi: 10.1080/00313027109073739. [DOI] [PubMed] [Google Scholar]
- 16.Snow MH. The effects of aging on satellite cells in skeletal muscles of mice and rats. Cell Tissue Res 185: 399–408, 1977. doi: 10.1007/BF00220299. [DOI] [PubMed] [Google Scholar]
- 17.Gibson MC, Schultz E. Age‐related differences in absolute numbers of skeletal muscle satellite cells. Muscle Nerve 6: 574–580, 1983. doi: 10.1002/mus.880060807. [DOI] [PubMed] [Google Scholar]
- 18.Arpke RW, Shams AS, Collins BC, Larson AA, Lu N, Lowe DA, Kyba M. Preservation of satellite cell number and regenerative potential with age reveals locomotory muscle bias. Skeletal Muscle 11: 22, 2021. doi: 10.1186/s13395-021-00277-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collins CA, Zammit PS, Ruiz AP, Morgan JE, Partridge TA. A population of myogenic stem cells that survives skeletal muscle aging. Stem cells 25: 885–894, 2007. doi: 10.1634/stemcells.2006-0372. [DOI] [PubMed] [Google Scholar]
- 20.Day K, Shefer G, Shearer A, Yablonka-Reuveni Z. The depletion of skeletal muscle satellite cells with age is concomitant with reduced capacity of single progenitors to produce reserve progeny. Dev Biol 340: 330–343, 2010. doi: 10.1016/j.ydbio.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shefer G, Van de Mark DP, Richardson JB, Yablonka-Reuveni Z. Satellite-cell pool size does matter: defining the myogenic potency of aging skeletal muscle. Dev Biol 294: 50–66, 2006. doi: 10.1016/j.ydbio.2006.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neal A, Boldrin L, Morgan JE. The satellite cell in male and female, developing and adult mouse muscle: distinct stem cells for growth and regeneration. PloS One 7: e37950, 2012. doi: 10.1371/journal.pone.0037950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suetta C, Frandsen U, Mackey AL, Jensen L, Hvid LG, Bayer ML, Petersson SJ, Schrøder HD, Andersen JL, Aagaard P, Schjerling P, Kjaer M. Ageing is associated with diminished muscle re‐growth and myogenic precursor cell expansion early after immobility‐induced atrophy in human skeletal muscle. J Physiology 591: 3789–3804, 2013. doi: 10.1113/jphysiol.2013.257121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verdijk LB, Koopman R, Schaart G, Meijer K, Savelberg HH, van Loon LJ. Satellite cell content is specifically reduced in type II skeletal muscle fibers in the elderly. Am J Physiol Endocrinol Physiol 292: E151–E157, 2007. doi: 10.1152/ajpendo.00278.2006. [DOI] [PubMed] [Google Scholar]
- 25.Brack AS, Bildsoe H, Hughes SM. Evidence that satellite cell decrement contributes to preferential decline in nuclear number from large fibres during murine age-related muscle atrophy. J Cell Sci 118: 4813–4821, 2005. doi: 10.1242/jcs.02602. [DOI] [PubMed] [Google Scholar]
- 26.Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433: 760–764, 2005. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 27.Price FD, von Maltzahn J, Bentzinger CF, Dumont NA, Yin H, Chang NC, Wilson DH, Frenette J, Rudnicki MA. Inhibition of JAK-STAT signaling stimulates adult satellite cell function. Nat Med 20: 1174–1181, 2014. doi: 10.1038/nm.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giordani L, Parisi A, Le Grand F. Satellite cell self-renewal. Curr Top Dev Biol 126: 177–203, 2018. doi: 10.1016/bs.ctdb.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 29.von Maltzahn J. Regulation of muscle stem cell function. Vitam Horm 116: 295–311, 2021. doi: 10.1016/bs.vh.2021.02.012. [DOI] [PubMed] [Google Scholar]
- 30.Thorley M, Malatras A, Duddy W, Le Gall L, Mouly V, Butler Browne G, Duguez S. Changes in communication between muscle stem cells and their environment with aging. J Neuromuscul Dis 2: 205–217, 2015. doi: 10.3233/JND-150097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allen RE, Boxhorn LK. Regulation of skeletal muscle satellite cell proliferation and differentiation by transforming growth factor‐beta, insulin‐like growth factor I, and fibroblast growth factor. J Cell Physiol 138: 311–315, 1989. doi: 10.1002/jcp.1041380213. [DOI] [PubMed] [Google Scholar]
- 32.Elabd C, Cousin W, Upadhyayula P, Chen RY, Chooljian MS, Li J, Kung S, Jiang KP, Conboy IM. Oxytocin is an age-specific circulating hormone that is necessary for muscle maintenance and regeneration. Nat Commun 5: 4082, 2014. doi: 10.1038/ncomms5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ascenzi F, Barberi L, Dobrowolny G, Villa Nova Bacurau A, Nicoletti C, Rizzuto E, Rosenthal N, Scicchitano BM, Musarò A. Effects of IGF‐1 isoforms on muscle growth and sarcopenia. Aging Cell 18: e12954, 2019. doi: 10.1111/acel.12954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bär PR, Amelink GJ, Oldenburg B, Blankenstein MA. Prevention of exercise-induced muscle membrane damage by oestradiol. Life Sci 42: 2677–2681, 1988. doi: 10.1016/0024-3205(88)90243-3. [DOI] [PubMed] [Google Scholar]
- 35.Amelink GJ, Kamp HH, Bär PR. Creatine kinase isoenzyme profiles after exercise in the rat: sex-linked differences in leakage of CK-MM. Pflugers Arch 412: 417–421, 1988. doi: 10.1007/BF01907561. [DOI] [PubMed] [Google Scholar]
- 36.Amelink GJ, Bär PR. Exercise-induced muscle protein leakage in the rat. Effects of hormonal manipulation. J Neurol Sci 76: 61–68, 1986. doi: 10.1016/0022-510x(86)90142-5. [DOI] [PubMed] [Google Scholar]
- 37.Deasy BM, Lu A, Tebbets JC, Feduska JM, Schugar RC, Pollett JB, Sun B, Urish KL, Gharaibeh BM, Cao B, Rubin RT, Huard J. A role for cell sex in stem cell-mediated skeletal muscle regeneration: female cells have higher muscle regeneration efficiency. J Cell Biol 177: 73–86, 2007. doi: 10.1083/jcb.200612094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kadi F, Charifi N, Denis C, Lexell J. Satellite cells and myonuclei in young and elderly women and men. Muscle Nerve 29: 120–127, 2004. doi: 10.1002/mus.10510. [DOI] [PubMed] [Google Scholar]
- 39.Petrella JK, Kim J-S, Cross JM, Kosek DJ, Bamman MM. Efficacy of myonuclear addition may explain differential myofiber growth among resistance-trained young and older men and women. Am J Physiol Endocrinol Physiol 291: E937–E946, 2006. doi: 10.1152/ajpendo.00190.2006. [DOI] [PubMed] [Google Scholar]
- 40.Velleman SG, Liu X, Nestor KE, McFarland DC. Heterogeneity in growth and differentiation characteristics in male and female satellite cells isolated from turkey lines with different growth rates. Comp Biochem Physiol A Mol Integr Physiol 125: 503–509, 2000. doi: 10.1016/S1095-6433(00)00178-1. [DOI] [PubMed] [Google Scholar]
- 41.Bonavaud S, Thibert P, Gherardi RK, Barlovatz-Meimon G. Primary human muscle satellite cell culture: variations of cell yield, proliferation and differentiation rates according to age and sex of donors, site of muscle biopsy, and delay before processing. Biol Cell 89: 233–240, 1997. [PubMed] [Google Scholar]
- 42.Salimena MC, Lagrota-Candido J, Quírico-Santos T. Gender dimorphism influences extracellular matrix expression and regeneration of muscular tissue in mdx dystrophic mice. Histochem Cell Biol 122: 435–444, 2004. doi: 10.1007/s00418-004-0707-8. [DOI] [PubMed] [Google Scholar]
- 43.Doumit ME, McFarland DC, Minshall RD. Satellite cells of growing turkeys: influence of donor age and sex on proliferation and differentiation in vitro. Exp Cell Res 189: 81–86, 1990. doi: 10.1016/0014-4827(90)90259-d. [DOI] [PubMed] [Google Scholar]
- 44.Hall JE. Endocrinology of the menopause. Endocrinol Metab Clin North Am 44: 485–496, 2015. doi: 10.1016/j.ecl.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nazem TG, Ackerman KE. The female athlete triad. Sports Health 4: 302–311, 2012. doi: 10.1177/1941738112439685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rugo HS, Rumble RB, Macrae E, Barton DL, Connolly HK, Dickler MN, Fallowfield L, Fowble B, Ingle JN, Jahanzeb M, Johnston SRD, Korde LA, Khatcheressian JL, Mehta RS, Muss HB, Burstein HJ. Endocrine therapy for hormone receptor–positive metastatic breast cancer: American Society of Clinical Oncology guideline. J Clin Oncol 34: 3069–3103, 2016. doi: 10.1200/JCO.2016.67.1487. [DOI] [PubMed] [Google Scholar]
- 47.Rosenfield RL, Devine N, Hunold JJ, Mauras N, Moshang T Jr, Root AW. Salutary effects of combining early very low-dose systemic estradiol with growth hormone therapy in girls with Turner syndrome. J Clin Endocrinol Metab 90: 6424–6430, 2005. doi: 10.1210/jc.2005-1081. [DOI] [PubMed] [Google Scholar]
- 48.Hagen CP, Main KM, Kjaergaard S, Juul A. FSH, LH, inhibin B and estradiol levels in Turner syndrome depend on age and karyotype: longitudinal study of 70 Turner girls with or without spontaneous puberty. Hum Reprod 25: 3134–3141, 2010. doi: 10.1093/humrep/deq291. [DOI] [PubMed] [Google Scholar]
- 49.Secoșan C, Balint O, Pirtea L, Grigoraș D, Bălulescu L, Ilina R. Surgically induced menopause—A practical review of literature. Medicina (Kaunas) 55: 482, 2019. doi: 10.3390/medicina55080482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gosset A, Pouillès J-M, Trémollieres F. Menopausal hormone therapy for the management of osteoporosis. Best Pract Res Clin Endocrinol Metab 35: 101551, 2021. doi: 10.1016/j.beem.2021.101551. [DOI] [PubMed] [Google Scholar]
- 51.Maas AHEM. Hormone therapy and cardiovascular disease: benefits and harms. Best Pract Res Clin Endocrinol Metab 35: 101576, 2021. doi: 10.1016/j.beem.2021.101576. [DOI] [PubMed] [Google Scholar]
- 52.Kamanga-Sollo E, White ME, Hathaway MR, Chung KY, Johnson BJ, Dayton WR. Roles of IGF-I and the estrogen, androgen and IGF-I receptors in estradiol-17β- and trenbolone acetate-stimulated proliferation of cultured bovine satellite cells. Domestic Anim Endocrinol 35: 88–97, 2008. doi: 10.1016/j.domaniend.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 53.Enns DL, Tiidus PM. Estrogen influences satellite cell activation and proliferation following downhill running in rats. J Appl Physiol (1985) 104: 347–353, 2008. doi: 10.1152/japplphysiol.00128.2007. [DOI] [PubMed] [Google Scholar]
- 54.Velders M, Schleipen B, Fritzemeier KH, Zierau O, Diel P. Selective estrogen receptor-β activation stimulates skeletal muscle growth and regeneration. FASEB J 26: 1909–1920, 2012. doi: 10.1096/fj.11-194779. [DOI] [PubMed] [Google Scholar]
- 55.Mangan G, Bombardier E, Mitchell AS, Quadrilatero J, Tiidus PM. Oestrogen‐dependent satellite cell activation and proliferation following a running exercise occurs via the PI 3K signalling pathway and not IGF‐1. Acta Physiol (Oxf) 212: 75–85, 2014. doi: 10.1111/apha.12317. [DOI] [PubMed] [Google Scholar]
- 56.Thomas A, Bunyan K, Tiidus PM. Oestrogen receptor-alpha activation augments post-exercise myoblast proliferation. Acta Physiol (Oxf) 198: 81–89, 2010. doi: 10.1111/j.1748-1716.2009.02033.x. [DOI] [PubMed] [Google Scholar]
- 57.Go G-Y, Lee S-J, Jo A, Lee J-R, Kang J-S, Yang M, Bae G-U. Bisphenol A and estradiol impede myoblast differentiation through down-regulating Akt signaling pathway. Toxicology Lett 292: 12–19, 2018. doi: 10.1016/j.toxlet.2018.04.019. [DOI] [PubMed] [Google Scholar]
- 58.Ogawa M, Yamaji R, Higashimura Y, Harada N, Ashida H, Nakano Y, Inui H. 17β-estradiol represses myogenic differentiation by increasing ubiquitin-specific peptidase 19 through estrogen receptor α. J Biol Chem 286: 41455–41465, 2011. doi: 10.1074/jbc.M111.276824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Galluzzo P, Rastelli C, Bulzomi P, Acconcia F, Pallottini V, Marino M. 17β-Estradiol regulates the first steps of skeletal muscle cell differentiation via ER-α-mediated signals. Am J Physiol Cell Physiol 297: C1249–C1262, 2009. doi: 10.1152/ajpcell.00188.2009. [DOI] [PubMed] [Google Scholar]
- 60.Collins BC, Arpke RW, Larson AA, Baumann CW, Xie N, Cabelka CA, Nash NL, Juppi HK, Laakkonen EK, Sipilä S, Kovanen V, Spangenburg EE, Kyba M, Lowe DA. Estrogen regulates the satellite cell compartment in females. Cell Rep 28: 368–381, 2019. doi: 10.1016/j.celrep.2019.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Larson AA, Baumann CW, Kyba M, Lowe DA. Oestradiol affects skeletal muscle mass, strength and satellite cells following repeated injuries. Exp Physiol 105: 1700–1707, 2020. doi: 10.1113/EP088827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Flurkey K, Joanne MC, Harrison D. Mouse models in aging research. In: The Mouse in Biomedical Research (2nd ed.), edited by Fox J, Barthold SW, Davisson MT, Newcomer CE, Quimby FW, Smith AL.. Burlington; Amsterdam: American College Laboratory Animal Medicine; Elsevier, 2007. [Google Scholar]
- 63.Keefe AC, Lawson JA, Flygare SD, Fox ZD, Colasanto MP, Mathew SJ, Yandell M, Kardon G. Muscle stem cells contribute to myofibres in sedentary adult mice. Nat Commun 6: 7087, 2015. doi: 10.1038/ncomms8087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development 138: 3625–3637, 2011. doi: 10.1242/dev.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moran AL, Nelson SA, Landisch RM, Warren GL, Lowe DA. Estradiol replacement reverses ovariectomy-induced muscle contractile and myosin dysfunction in mature female mice. J Appl Physiol (1985) 102: 1387–1393, 2007. doi: 10.1152/japplphysiol.01305.2006. [DOI] [PubMed] [Google Scholar]
- 66.Nelson JF, Felicio LS, Randall PK, Sims C, Finch CE. A longitudinal study of estrous cyclicity in aging C57BL/6J mice: I. Cycle frequency, length and vaginal cytology. Biol Reprod 27: 327–339, 1982. doi: 10.1095/biolreprod27.2.327. [DOI] [PubMed] [Google Scholar]
- 67.Wood GA, Fata JE, Watson KLM, Khokha R. Circulating hormones and estrous stage predict cellular and stromal remodeling in murine uterus. Reproduction 133: 1035–1044, 2007. doi: 10.1530/REP-06-0302. [DOI] [PubMed] [Google Scholar]
- 68.Arpke RW, Darabi R, Mader TL, Zhang Y, Toyama A, Lonetree CL, Nash N, Lowe DA, Perlingeiro RC, Kyba M. A new immuno-, dystrophin-deficient model, the NSG-mdx(4Cv) mouse, provides evidence for functional improvement following allogeneic satellite cell transplantation. Stem Cells 31: 1611–1620, 2013. doi: 10.1002/stem.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yue F, Bi P, Wang C, Li J, Liu X, Kuang S. Conditional loss of Pten in myogenic progenitors leads to postnatal skeletal muscle hypertrophy but age-dependent exhaustion of satellite cells. Cell Rep 17: 2340–2353, 2016. doi: 10.1016/j.celrep.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Contreras O, Córdova-Casanova A, Brandan E. PDGF-PDGFR network differentially regulates the fate, migration, proliferation, and cell cycle progression of myogenic cells. Cell Signal 84: 110036, 2021. doi: 10.1016/j.cellsig.2021.110036. [DOI] [PubMed] [Google Scholar]
- 71.Schmalbruch H, Lewis D. Dynamics of nuclei of muscle fibers and connective tissue cells in normal and denervated rat muscles. Muscle Nerve 23: 617–626, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 72.Hardy D, Besnard A, Latil M, Jouvion G, Briand D, Thépenier C, Pascal Q, Guguin A, Gayraud-Morel B, Cavaillon JM, Tajbakhsh S, Rocheteau P, Chrétien F. Comparative study of injury models for studying muscle regeneration in mice. PLoS One 11: e0147198, 2016. doi: 10.1371/journal.pone.0147198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Seko D, Fujita R, Kitajima Y, Nakamura K, Imai Y, Ono Y. Estrogen receptor β controls muscle growth and regeneration in young female mice. Stem Cell Reports 15: 577–586, 2020. doi: 10.1016/j.stemcr.2020.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kamanga-Sollo E, Pampusch M, Xi G, White ME, Hathaway MR, Dayton WR. IGF‐I mRNA levels in bovine satellite cell cultures: effects of fusion and anabolic steroid treatment. J Cell Physiol 201: 181–189, 2004. doi: 10.1002/jcp.20000. [DOI] [PubMed] [Google Scholar]
- 75.Chakravarthy MV, Abraha TW, Schwartz RJ, Fiorotto ML, Booth FW. Insulin-like growth factor-I extends in vitro replicative life span of skeletal muscle satellite cells by enhancing g1/s cell cycle progression via the activation of phosphatidylinositol 3′-kinase/akt signaling pathway. J Biol Chem 275: 35942–35952, 2000. doi: 10.1074/jbc.M005832200. [DOI] [PubMed] [Google Scholar]
- 76.Brett JO, Arjona M, Ikeda M, Quarta M, de Morrée A, Egner IM, Perandini LA, Ishak HD, Goshayeshi A, Benjamin DI, Both P, Rodríguez-Mateo C, Betley MJ, Wyss-Coray T, Rando TA. Exercise rejuvenates quiescent skeletal muscle stem cells in old mice through restoration of Cyclin D1. Nat Metab 2: 307–317, 2020. doi: 10.1038/s42255-020-0190-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barruet E, Garcia SM, Striedinger K, Wu J, Lee S, Byrnes L, Wong A, Xuefeng S, Tamaki S, Brack AS, Pomerantz JH. Functionally heterogeneous human satellite cells identified by single cell RNA sequencing. eLife 9: e51576, 2020. doi: 10.7554/eLife.51576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.De Micheli AJ, Laurilliard EJ, Heinke CL, Ravichandran H, Fraczek P, Soueid-Baumgarten S, De Vlaminck I, Elemento O, Cosgrove BD. Single-cell analysis of the muscle stem cell hierarchy identifies heterotypic communication signals involved in skeletal muscle regeneration. Cell Rep 30: 3583–3595.e5, 2020. doi: 10.1016/j.celrep.2020.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yartseva V, Goldstein LD, Rodman J, Kates L, Chen MZ, Chen Y-JJ, Foreman O, Siebel CW, Modrusan Z, Peterson AS, Jovičić A. Heterogeneity of satellite cells implicates DELTA1/NOTCH2 signaling in self-renewal. Cell Rep 30: 1491–1503.e6, 2020. doi: 10.1016/j.celrep.2019.12.100. [DOI] [PubMed] [Google Scholar]
- 80.Yucel N, Wang YX, Mai T, Porpiglia E, Lund PJ, Markov G, Garcia BA, Bendall SC, Angelo M, Blau HM. Glucose metabolism drives histone acetylation landscape transitions that dictate muscle stem cell function. Cell Rep 27: 3939–3955.e6, 2019. doi: 10.1016/j.celrep.2019.05.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chakkalakal JV, Jones KM, Basson MA, Brack AS. The aged niche disrupts muscle stem cell quiescence. Nature 490: 355–360, 2012. doi: 10.1038/nature11438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chakkalakal JV, Christensen J, Xiang W, Tierney MT, Boscolo FS, Sacco A, Brack AS. Early forming label-retaining muscle stem cells require p27kip1 for maintenance of the primitive state. Development 141: 1649–1659, 2014. doi: 10.1242/dev.100842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shea KL, Xiang W, LaPorta VS, Licht JD, Keller C, Basson MA, Brack AS. Sprouty1 regulates reversible quiescence of a self-renewing adult muscle stem cell pool during regeneration. Cell Stem Cell 6: 117–129, 2010. doi: 10.1016/j.stem.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sikora MJ, Johnson MD, Lee AV, Oesterreich S. Endocrine response phenotypes are altered by charcoal-stripped serum variability. Endocrinology 157: 3760–3766, 2016. doi: 10.1210/en.2016-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Siegel AL, Kuhlmann PK, Cornelison DD. Muscle satellite cell proliferation and association: new insights from myofiber time-lapse imaging. Skelet Muscle 1: 7–7, 2011. doi: 10.1186/2044-5040-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ono Y, Masuda S, Nam H-S, Benezra R, Miyagoe-Suzuki Y, Takeda S. Slow-dividing satellite cells retain long-term self-renewal ability in adult muscle. J Cell Sci 125: 1309–1317, 2012. doi: 10.1242/jcs.096198. [DOI] [PubMed] [Google Scholar]
- 87.Schultz E. Satellite cell proliferative compartments in growing skeletal muscles. Dev Biol 175: 84–94, 1996. doi: 10.1006/dbio.1996.0097. [DOI] [PubMed] [Google Scholar]
- 88.Chen W, Datzkiw D, Rudnicki MA. Satellite cells in ageing: use it or lose it. Open Biol 10: 200048, 2020. doi: 10.1098/rsob.200048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rocheteau P, Gayraud-Morel B, Siegl-Cachedenier I, Blasco MA, Tajbakhsh S. A subpopulation of adult skeletal muscle stem cells retains all template DNA strands after cell division. Cell 148: 112–125, 2012. doi: 10.1016/j.cell.2011.11.049. [DOI] [PubMed] [Google Scholar]
- 90.Pampusch MS, Kamanga-Sollo E, White ME, Hathaway M, Dayton WR. Effect of recombinant porcine IGF-binding protein-3 on proliferation of embryonic porcine myogenic cell cultures in the presence and absence of IGF-I. J Endocrinol 176: 227–236, 2003. doi: 10.1677/joe.0.1760227. [DOI] [PubMed] [Google Scholar]
- 91.Nilsson ME, Vandenput L, Tivesten Å, Norlén A-K, Lagerquist MK, Windahl SH, Börjesson AE, Farman HH, Poutanen M, Benrick A, Maliqueo M, Stener-Victorin E, Ryberg H, Ohlsson C. Measurement of a comprehensive sex steroid profile in rodent serum by high-sensitive gas chromatography-tandem mass spectrometry. Endocrinology 156: 2492–2502, 2015. doi: 10.1210/en.2014-1890. [DOI] [PubMed] [Google Scholar]
- 92.Nelson JF, Felicio LS, Osterburg HH, Finch CE. Altered profiles of estradiol and progesterone associated with prolonged estrous cycles and persistent vaginal cornification in aging C578L/6J mice. Biol Reprod 24: 784–794, 1981. doi: 10.1095/biolreprod24.4.784. [DOI] [PubMed] [Google Scholar]
- 93.La Colla A, Vasconsuelo A, Boland R. Estradiol exerts antiapoptotic effects in skeletal myoblasts via mitochondrial PTP and MnSOD. J Endocrinol 216: 331–341, 2013. doi: 10.1530/JOE-12-0486. [DOI] [PubMed] [Google Scholar]
- 94.Silva CP, Lima DL, Otero M, Esteves VI. Photosensitized degradation of 17β‐estradiol and 17α‐ethinylestradiol: role of humic substances fractions. J Environ Qual 45: 693–700, 2016. doi: 10.2134/jeq2015.07.0396. [DOI] [PubMed] [Google Scholar]
- 95.Walker CW, Watson JE. Adsorption of estrogens on laboratory materials and filters during sample preparation. J Environ Qual 39: 744–748, 2010. doi: 10.2134/jeq2009.0017. [DOI] [PubMed] [Google Scholar]
- 96.Bayer J, Gläscher J, Finsterbusch J, Schulte LH, Sommer T. Linear and inverted U-shaped dose-response functions describe estrogen effects on hippocampal activity in young women. Nat Commun 9: 1–12, 2018. doi: 10.1038/s41467-018-03679-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Garrido P, Salehzadeh F, Duque-Guimaraes DE, Al-Khalili L. Negative regulation of glucose metabolism in human myotubes by supraphysiological doses of 17β-estradiol or testosterone. Metabolism 63: 1178–1187, 2014. doi: 10.1016/j.metabol.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 98.Kitajima Y, Ono Y. Estrogens maintain skeletal muscle and satellite cell functions. J Endocrinol 229: 267–275, 2016. doi: 10.1530/JOE-15-0476. [DOI] [PubMed] [Google Scholar]
- 99.Liang Z-R, Qu L-H, Ma L-M. Differential impacts of charcoal-stripped fetal bovine serum on c-Myc among distinct subtypes of breast cancer cell lines. Biochem Biophys Res Commun 526: 267–272, 2020. doi: 10.1016/j.bbrc.2020.03.049. [DOI] [PubMed] [Google Scholar]
- 100.Lee EJ, Choi J, Hyun JH, Cho K-H, Hwang I, Lee H-J, Chang J, Choi I. Steroid effects on cell proliferation, differentiation and steroid receptor gene expression in adult bovine satellite cells. Asian Australas J Anim Sci 20: 501–510, 2007. doi: 10.5713/ajas.2007.501. [DOI] [Google Scholar]
- 101.Katzenellenbogen BS, Choi I, Delage-Mourroux R, Ediger TR, Martini PG, Montano M, Sun J, Weis K, Katzenellenbogen JA. Molecular mechanisms of estrogen action: selective ligands and receptor pharmacology. J Steroid Biochem Mol Biol 74: 279–285, 2000. doi: 10.1016/s0960-0760(00)00104-7. [DOI] [PubMed] [Google Scholar]
- 102.Moreno-Cuevas JE, Sirbasku DA. Estrogen mitogenic action. III. Is phenol red a “red herring”? In Vitro Cell Dev Biol Anim 36: 447–464, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 103.Welshons WV, Wolf MF, Murphy CS, Jordan VC. Estrogenic activity of phenol red. Mol Cell Endocrinol 57: 169–178, 1988. doi: 10.1016/0303-7207(88)90072-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. S1: https://doi.org/10.6084/m9.figshare.17096900.v1.
Supplemental Fig. S2: https://doi.org/10.6084/m9.figshare.17096915.v1.
Supplemental Fig. S3: https://doi.org/10.6084/m9.figshare.17096918.v1.
Supplemental Fig. S4: https://doi.org/10.6084/m9.figshare.17096927.v1.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.