Keywords: auditory brainstem response, auditory nerve fiber, cochlear synaptopathy, masking, noise
Abstract
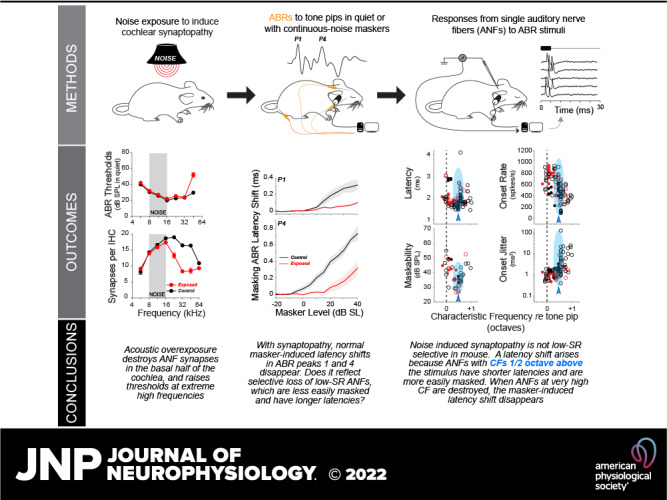
After acoustic overexposure, many auditory-nerve fiber (ANF) synapses permanently retract from surviving cochlear hair cells. This synaptopathy is hard to diagnose, since it does not elevate audiometric thresholds until almost no synapses remain, nevertheless it may degrade discrimination of complex stimuli especially in noisy environments. Here, we study an assay based on masking the auditory brainstem responses (ABRs) to a moderate-level probe tone with continuous noise of varied sound levels, and we investigate the underlying ANF responses at the single-fiber level. Synaptopathy was induced by overexposure to octave-band noise, resulting in a permanent synaptic loss of ∼50%, without permanent threshold elevation except at the highest frequencies. The normal progressive delay of ABR peaks with increasing masker level is diminished in synaptopathic ears; however, the single-fiber analysis suggests that this normal latency shift does not arise because contributing ANFs shift from low-threshold fibers (with high spontaneous rates) to high-threshold fibers (with low spontaneous rates). Rather, it may arise because of a shift in the cochlear region dominating the response. Surprisingly, the dynamic range of masking, i.e., the difference between the lowest masker level that attenuates the ABR to a fixed-level probe and the lowest masker level that eliminates the ABR, is enhanced in the synaptopathic ears. This ABR behavior mirrors the single-fiber data showing a paradoxical enhancement of onset-response synchrony and resistance to masking in responses of ANFs in the synaptopathic regions. An assay based on the dynamic range of masking could be useful in diagnosing synaptic damage in human populations.
NEW & NOTEWORTHY Using a masking paradigm, we demonstrate that noise-damaged animals exhibit “improved” performance, i.e., the dynamic range of masker levels over which an ABR remains detectable is increased in regions of damaged cochlear ribbon synapses. Furthermore, the normal masker-induced changes in ABR response latency, used as a biomarker of a healthy cochlear neural population, may reflect a shift in cochlear frequency regions dominating the response, rather than from high- to low spontaneous-rate groups as previously thought.
INTRODUCTION
Hearing impairment is typically quantified via the audiogram, which measures the lowest tone intensity required for reliable detection. However, audiometric thresholds are not always predictive of performance on more difficult auditory tasks (1). Hearing thresholds are largely determined by the health of outer hair cells (OHCs), which amplify cochlear sound-evoked vibrations, whereas inner hair cells (IHCs) transduce the vibrations into electrical signals that elicit action potentials in auditory-nerve fibers (ANFs) for transmission to the central auditory system (2, 3). Due to the large number of ANFs innervating each IHC (4), subtotal ANF loss, even approaching 90%, has minimal effect on threshold (5, 6). However, ANF survival is likely a key determinant of the ability to understand complex sounds such as speech, especially in a noisy environment (7, 8).
Cochlear histopathological studies in animals and humans, in both noise-damaged and normal-aging ears, have shown that the synapses between ANFs and their IHC targets often degenerate before any hair cell damage or associated threshold elevation (9, 10). In animals with permanent noise-induced cochlear synaptopathy, after exposures producing only temporary thresholds shifts (and, correspondingly, no permanent hair cell damage), the neuronal loss can be inferred from the decrement in suprathreshold amplitudes of the auditory brainstem responses (ABRs) (9). However, such inferences are complicated in an ear with hair cell damage, because the associated threshold elevation also decreases ABR suprathreshold amplitudes.
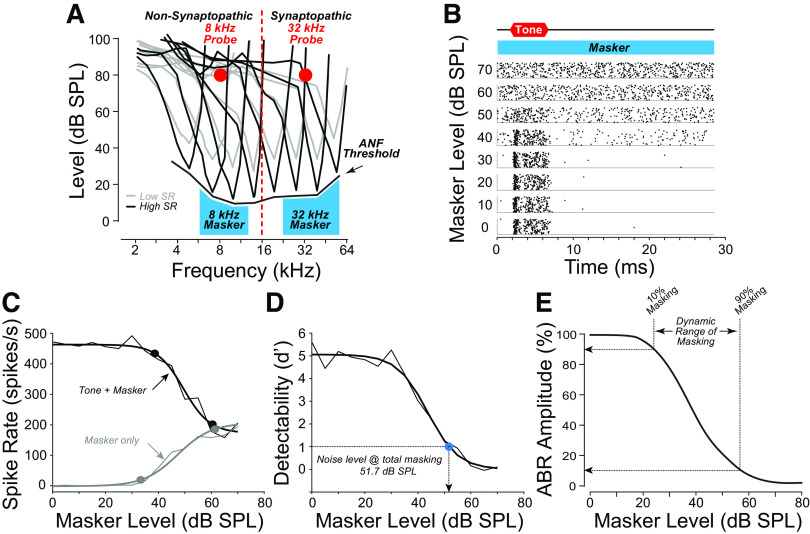
In an attempt to disambiguate the effects of hair cell and neural damage, and with an eye toward a minimally invasive metric of ANF performance in background noise, we designed an assay based on the masking of ABR responses to a fixed, suprathreshold-level probe tone in the presence of increasing levels of a continuous-noise masker (Fig. 1). Specifically, we wanted to measure the “dynamic range of masking,” i.e., the range of masker sound pressure levels (SPLs) between 1) the lowest level that reduces the ABR amplitude (i.e., 10% masking) and 2) the lowest level at which the ABR disappears (i.e., 90% masking). Importantly, we hoped to minimize the confound from hair cell damage and the threshold elevation it produces by measuring the range of masker levels over which the tone-pip response is detectable, rather than the absolute noise level at which some criterion level of masking is achieved, and, furthermore, by shaping the noise spectrum to the minimum threshold envelope.
Figure 1.
The auditory brainstem response (ABR) masking paradigm. A: schematic tuning curves of high- and low-spontaneous rate (SR) auditory-nerve fibers (ANFs) positioned with respect to the tone-burst probes for the nonsynaptopathic (8 kHz) or synaptopathic (32 kHz) regions, and their respective narrow-band maskers. B: spike-time dot-raster plots from an ANF [noise exposed animal; threshold at characteristic frequency (CF): 43.75 dB SPL at 29.41 kHz; SR: 9.83 spikes/s] responding to the 32-kHz probe at eight levels of the masker, showing disappearance of tone-evoked responses at levels ≥ 60 dB SPL. C: spike rates extracted from the raster show the decreasing response during the tone-on time (tone + masker) and increasing response during the tone-off time (masker only). D: complete masking is defined as the masker level required to reduce the detectability d′ to 1. E: the “dynamic range of masking” is defined as the dB difference between masker levels required to reduce P1 of the ABR by 10% and 90%.
Additional motivation for our study arose from the report that the ABR latency shift normally evoked by a broadband masker is smaller in people who perform more poorly on psychophysical tests involving fine temporal resolution or hearing in noise (11, 12). It has been suggested that the normal masker-induced latency shift with increasing masker level arises because the ANFs dominating the ABR response from short-latency fibers, with high spontaneous rates (SRs) to long-latency fibers with low SRs. Since, neurophysiological studies in guinea pig suggested that cochlear synaptopathy is initially selective for low-SR ANFs (13) and since low-SR fibers are, by virtue of their higher thresholds, more resistant to masking noise (14), it was suggested that the negative correlation between psychophysical performance and masker-induced ABR latency shift was due, at least in part, to selective low-SR neuropathy.
Here, we test both sets of ideas by applying an ABR masking assay in a mouse model of noise-induced synaptopathy and gain further insight into the underlying pathophysiology by recording the responses of single ANFs to the same stimulus paradigm. We conclude that the ABR-based paradigm is successful at revealing key aspects of the underlying ANF pathophysiology, which, in this now-popular mouse synaptopathy model, actually does not include a selective loss of low-SR fibers (15). The assay also reveals a paradoxically enhanced dynamic range of masking, which appears to reflect enhanced coherence of the onset response in the surviving ANFs and their enhanced resistance to continuous noise (15). The ABR assay also mirrors the differences in masker-induced ABR latency suggested by the human study, but the single-fiber data suggest that this may have more to do with differences in spread of excitation than a shift from high- to low-SR fibers dominating the response.
MATERIALS AND METHODS
Animals
A total of 105 male, CBA/CaJ mice were used in this study. Mice were obtained from Jackson Laboratories at 5 wk of age and acclimatized in our animal care facility for up to 2 wk before any experimental procedures. Animals were group-housed in cages of up to five animals, under constant light (12-h light/dark cycle) and temperature (22°C) control. Animals were randomly assigned into the normal or noise-exposed group. All procedures were approved by the Institutional Care and Use Committee of the Massachusetts Eye and Ear.
Noise Exposure
Experimental cohorts consisted of a single group of unexposed control animals and two synaptopathic groups, exposed to narrow-band noise (8–16 kHz) at 97.5 dB SPL for either 60- or 120 min, while awake and unrestrained. Noise exposure was always carried out at 7 wk of age on mice placed in mesh cages on an elevated turntable in a small reverberant booth, with an exponential horn directly overhead, through which a compression driver (JBL model 2446H) delivered the digitally generated (LABVIEW) and amplified (Crown Power Amplifier D75) noise waveform. The noise output level was continuously monitored via a ¼ in. condenser microphone (Bruel and Kjaer), and mice were visually monitored via an infrared camera.
ABRs and DPOAEs in Quiet
One week after exposure, cochlear function tests were performed under ketamine (0.1 mg/g) and xylazine (0.01 mg/g) anesthesia in an acoustically and electrically shielded chamber maintained at 32°C. Acoustic stimuli were generated digitally by I-O boards (National Instruments) controlled through custom software and delivered through a custom acoustic system consisting of two miniature dynamic drivers (CDMG15008-03A; CUI) and an electret condenser microphone (FG-23329-PO7; Knowles) coupled to a probe tube. Distortion product otoacoustic emissions (DPOAEs) and auditory brainstem responses (ABRs) in quiet were measured at seven frequency regions, in half-octave steps from 5.6 to 45.2 kHz. DPOAEs were measured using two primary tones separated in level by 10 dB and in frequency ratio of f2/f1 = 1.2. ABRs were recorded in response to 100 µs clicks or 5-ms tone-pips at 35 presentations/s with 0.5 ms rise-fall (cos2 shaping). Recordings were made through subdermal needle electrodes (Genuine Grass, Natus) inserted at the vertex and pinna, with a ground at the base of the tail. Responses to as many as 1,024 stimuli were amplified 10,000 times, passband filtered at 0.3–3 kHz, and averaged in 5 dB steps from 0 to 80 dB SPL. Both ABRs and DPOAEs were obtained as amplitude versus level series, in 5 dB level steps from 0 to 80 dB SPL. DPOAE thresholds were defined by interpolation as the f2 level required to produce a DPOAE of 10 dB SPL. ABR thresholds were defined by interpolation as the tone-pip level required to generate a criterion degree of cross-correlation between waveforms at adjacent stimulus levels (16).
ABR Masking Assay
In addition to routine cochlear function testing, ABR masker-level functions were recorded in response to a fixed-level (80 dB SPL) tone-pip “probe,” at either 8 kHz (“nonsynaptopathic”) or 32 kHz (“synaptopathic”), with a shaped continuous-noise masker randomly varied in 5 dB steps from 0 to 80 dB SPL. At each masker level, at least 512 tone-pip responses were averaged, with “Masker Off” runs interleaved every fourth presentation to control for potential long-term adaptation to the continuous noise. The noise was passband filtered to mask the cochlear region responding to the relevant probe tone: 5.6–11.3 kHz for the 8 kHz probe, and 22.6–64 kHz for the 32 kHz probe. The noise was also spectrally adjusted to account for threshold differences at different frequencies. In early experiments, the shaping spectrum was based on minimum ANF thresholds from a prior study of normal CBA/CaJ mice (17), as depicted in Fig. 1A. In later experiments, the spectrum was shaped according to mean ABR thresholds for the group (control vs. noise exposed) to account for the threshold differences at high frequencies as depicted in Fig. 5, A and B. The differing masking spectra did not affect the conclusions presented here.
Figure 5.
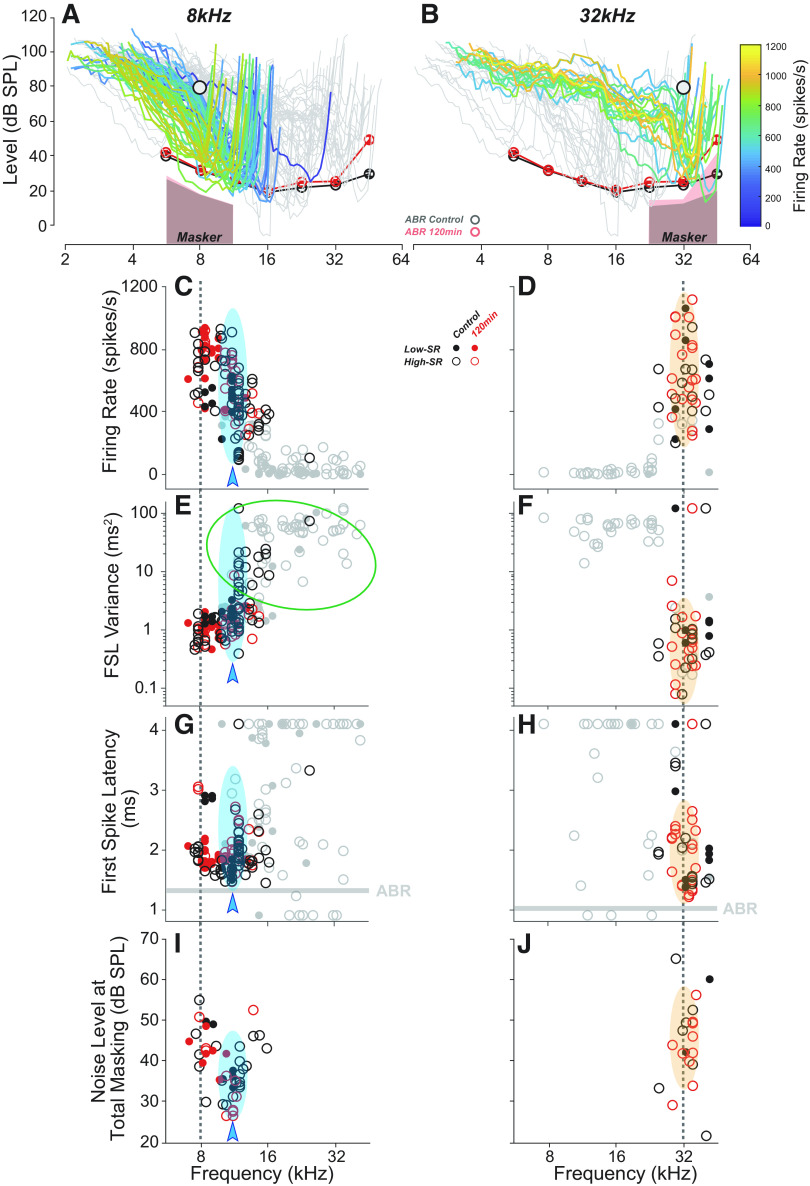
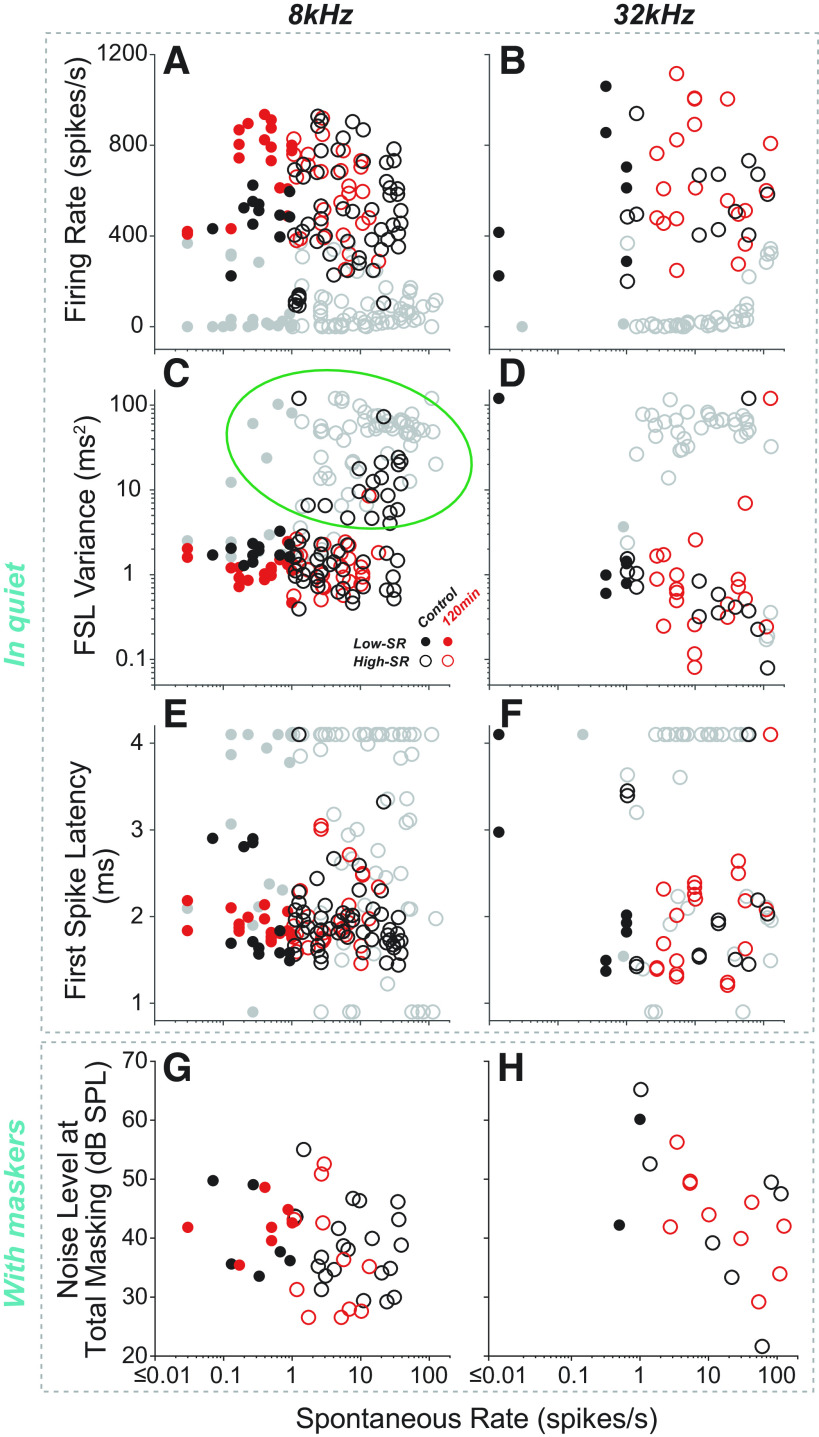
Auditory-nerve fiber (ANF) responses to the 8- or 32-kHz tone pips used to evoke auditory brainstem response (ABR) responses. A and B: all the ANF tuning curves from all groups are superimposed in each panel, coded by discharge rate in response to 8- or 32-kHz probe tones at 80 dB SPL in quiet, as indicated. Fibers not responding to the probe-tone pips (P > 0.05 for a paired t test comparing “tone-on” vs. “tone-off” spike counts) are in gray; others are color coded for onset rate as shown in the color bar to the right. Mean ABR thresholds (from Fig. 2A) and masker spectra are shown for reference. Key features of the ANF responses to either 8 kHz (C, E, G, and I) or 32 kHz (D, F, H, and J) tone pips at 80 dB SPL, as indicated on the vertical axis for each row. Symbol code in C applies to all panels. Firing rate (C and D) is averaged over the entire tone-pip duration (5 ms), and noise level at total masking (I and J) is as defined in Fig. 1D. Probe tone frequency is indicated by the vertical dashed lines. Relevant ABR P1 latencies are indicated by the horizontal bars in G and H, and the cyan arrowheads and bubbles in C, E, G, and I are positioned at the characteristic frequency (CF) region showing minimum response latency (G). The green oval in E indicates the same population of off-CF responses encircled in Fig. 6C. As for A and B, gray symbols denote fibers not responding to the tone pips. Symbols aligned at the max or min of the ordinate have off-scale values. FSL, first spike latency; SR, spontaneous rate.
Single-Fiber Recordings
Mice were allowed to recover for ∼1–2 wk (means ± SE = 1.9 ± 0.1) after the cochlear function test, then ANF recordings were made using beveled micropipettes (quartz glass filled with 0.45 M KCl in Tris buffer at pH 7.6; 200–600 MΩ initial resistance; 20–60 MΩ final resistance; P2000 & BV-10, Sutter Instrument) coupled to a low-noise preamplifier (Ithaco 1201 Low Noise Pre-Amplifier) or a bridge-balanced intracellular amplifier (NPI Electronic BA-03X) via a chloridated silver wire. Mice were anesthetized with urethane (1.32 mg/g) and xylazine (0.01 mg/g), tracheotomized and artificially respirated. The auditory nerve was accessed via a posterior fossa approach. After partial removal of the overlying occipital bone and cerebellar aspiration, the electrode was hydraulically advanced (Kopf, Model 607 W) through the cochlear nucleus using the crus commune of the semicircular canals as a landmark to guide the initial electrode placement superficially. Moderate-level broadband noise bursts were used as search stimuli, and ANFs were distinguished from cochlear nucleus neurons using established criteria (15, 17): i.e., electrode depth ≥ 1,000 µm re brainstem surface, first spike latency < 5 ms in response to tone bursts at 30 dB re threshold, coefficient of variance of interspike interval ≥ 0.5, and primary-like response type. To ensure stability of cochlear function, DPOAE thresholds were periodically remeasured and compared with baseline; if they rose by >10 dB, the experiment was terminated.
Tuning curves and peristimulus time histograms (PSTHs) used to identify ANFs were measured in response to 50 ms tone bursts at 10/s with 2.5-ms rise-fall time. The tuning curve algorithm tracks an iso-response contour corresponding to a 10 spike/s increase during stimulus on-time versus stimulus off-time (18–20). PSTHs were constructed from 150–300 presentations of a characteristic frequency (CF) tone burst presented 30 dB above threshold. Spontaneous discharge rate was measured during a 30-s quiet interval. All other sound-evoked responses reported here are for ABR-style tone pips, i.e., 5 ms in duration with cos2-shaped 0.5 ms rise-fall times. The ANF masking assay was identical to the ABR masking assay: i.e., 8 kHz or 32 kHz tone pips at 80 dB SPL, with continuous noise randomly varied from 0 to 80 dB SPL (in 5 dB increments) with a “masker off” condition interleaved between every 4th masker level. Spike times re stimulus onset were clocked with 0.1 µs accuracy and stored for offline analysis. All data analyses were performed using custom MATLAB scripts and inbuilt functions, with the exception of the following functions available from MATLAB Central File Exchange: ezyfit, num2clip, plotSpikeRaster, shadedErrorBar, and sigm_fit.
Experimental Design and Statistical Analysis
Significant interaction between main effects of group and frequency were expected for cochlear function testing and synaptopathy. Therefore, two-way ANOVA was followed by planned within-frequencies, between-cohort comparisons. Multiple comparisons were corrected using Sidak’s method. The d′ metric of detectability was used to define masking threshold for the ANF masking assay and was defined as per Eq. 1 of Kawase et al. (21). The transformation from spike times to a single d′ function is illustrated for an example fiber in Fig. 1, B–D. Masking threshold was always interpolated from a sigmoidal fit to the d′-level function for each fiber.
RESULTS
Cochlear Lesions
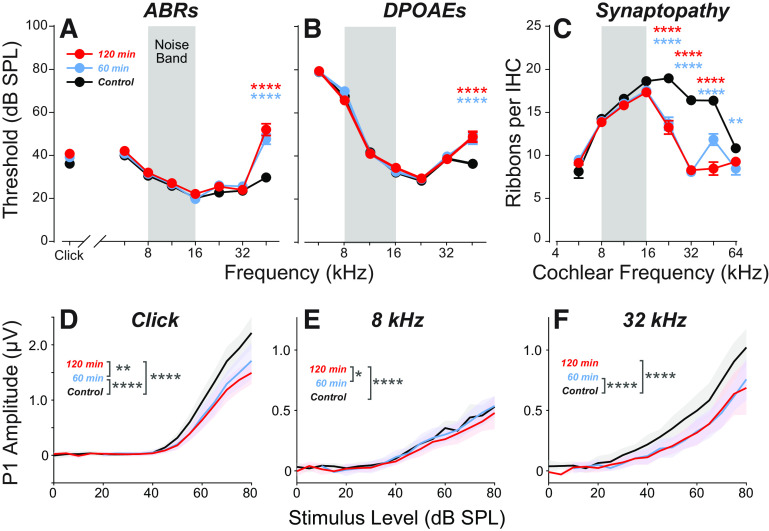
Exposing mice to an 8–16 kHz octave band of noise at 97.5 dB SPL for 2 h destroys a large number of the synapses between ANFs and IHCs in basal (high-frequency) cochlear regions (Fig. 2C), with permanent threshold shifts confined to the highest test frequency, 45.2 kHz for ABRs (Fig. 2A; P < 0.0001) and DPOAEs (Fig. 2B; P < 0.0001). As expected from prior studies (9), despite the recovery of thresholds at most frequencies, ABR suprathreshold amplitudes are significantly reduced in response to a click [Fig. 2D; FCOHORT(2,342) = 128.2, P < 0.0001; control vs. 120 min: P < 0.0001] and to tone pips in the synaptopathic region [32 kHz; Fig. 2F; FCOHORT(2,343) = 119.3, P < 0.0001; control vs. 120 min: P < 0.0001]. Although the amplitude-level functions in the nonsynaptopathic region are similar across groups, the main effect of cohort was also statistically significant at 8 kHz [Fig. 2E; FCOHORT(2,343) = 7.662, P = 0.0006].
Figure 2.
Exposure to narrow-band noise at 97.5 dB SPL for either 60 or 120 min causes minimal permanent threshold shift but significant synaptopathy throughout the basal turn of the cochlea. A and B: mean thresholds (means ± SE) for auditory brainstem responses (ABRs) and distortion product otoacoustic emissions (DPOAEs) in the three experimental groups (n = 14 in each), as measured 1 wk postexposure. C: mean synaptic ribbon counts (means ± SE) for inner hair cells in the three experimental groups (n = 8 in each). P1 amplitude-level functions (means ± SE) for the three experimental groups in response to clicks (D) or tone pips at either 8 kHz (E) or 32 kHz (F). Statistical significance of the group differences is indicated by asterisks: *P ≤ 0.05, **P ≤ 0.01, ****P ≤ 0.0001.
In an effort to reduce the threshold shift without reducing the synaptopathy, we decreased the exposure duration from 2 h to 1 h. This slightly reduced the synaptopathy at 45.2 Hz (Fig. 2C) without reducing the threshold shift (Fig. 2, A and B) or significantly affecting peak 1 (P1) amplitude-level functions for ABR in nonsynaptopathic regions (Fig. 2E; control vs. 60 min: P = 0.55). In response to suprathreshold clicks, P1 amplitudes were slightly higher than the 120 min group (P = 0.007), but still significantly attenuated compared with control (P < 0.0001). Suprathreshold amplitudes in synaptopathic regions were not significantly different from the 120 min group (P = 0.9357) but were significantly reduced compared with controls (Fig. 2F; control vs. 60 min: P < 0.0001). Interestingly, there was no significant difference in P1 amplitudes compared with controls at 8 kHz (P = 0.5510). The data from this 60-min exposure group are a useful control for the effects of threshold shift.
Masking ABR Responses
ABR masking results in the control group versus each of the two noise-exposure groups can be viewed as conventional waveforms or as heat maps of the same amplitude versus time data (Fig. 3). The mean ABR waveforms in response to the 8 kHz probe, which reflects activity in the nonsynaptopathic region, look similar across the three groups, i.e., amplitudes of all peaks decrease with increasing masker level. The amplitude of the P4 peak, dominated by responses in the inferior colliculus, is more robust to masking than P1, which is dominated by responses of ANFs (22). In all three groups, the latency of the P4 peak increases significantly with increasing masker level—much more so than the P1 latency, as is most clearly seen in the heat-map rendition of the data (boxed region). In contrast, the response to the 32 kHz probe, which reflects activity of ANFs in the synaptopathic region, behaves differently in the 120-min exposure group: the P4 latency shift is much smaller as masker levels increase, and, unexpectedly, the P1 amplitude is more robust to masking (masker levels of 10–40 dB SL).
Figure 3.
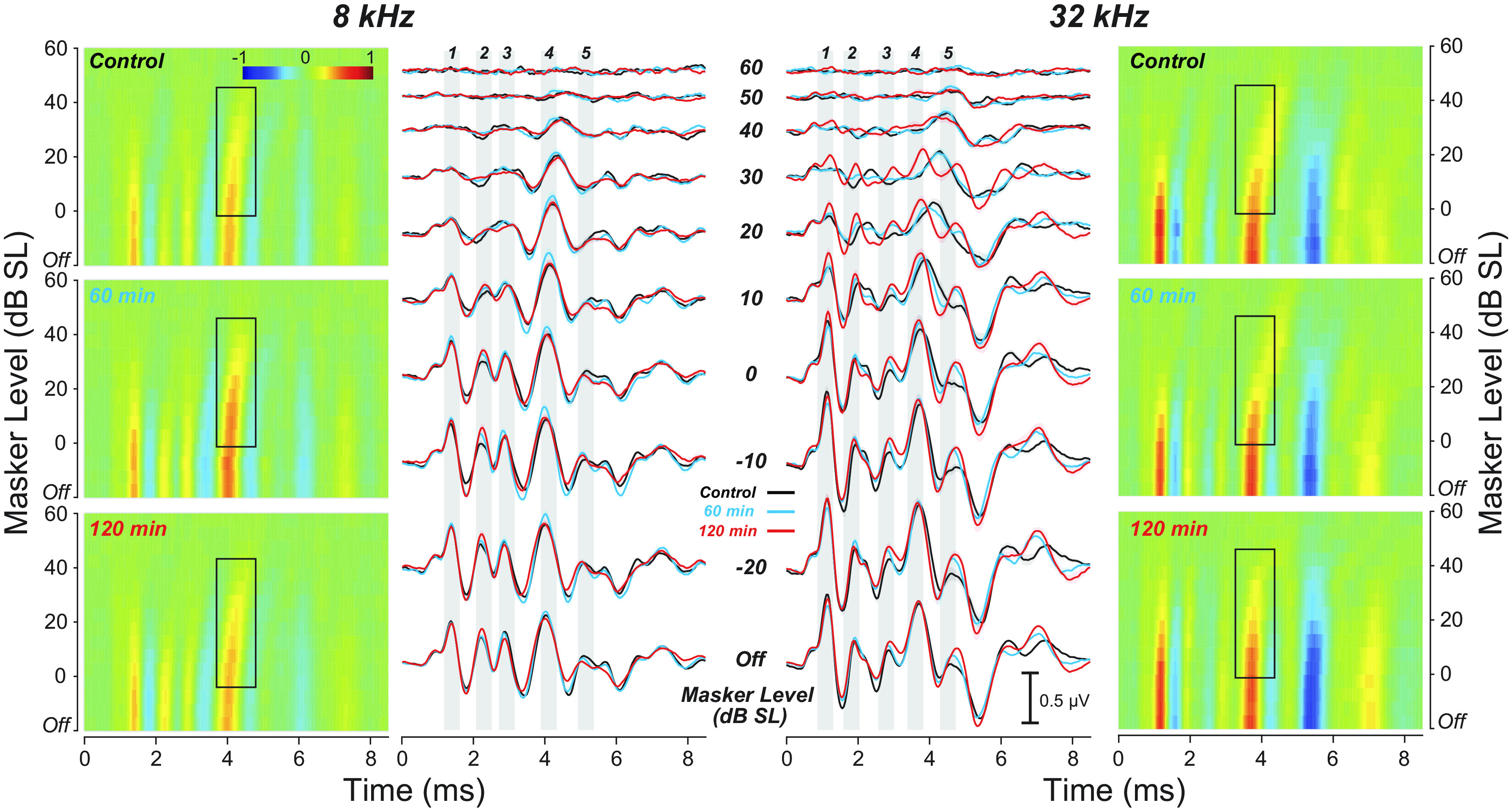
Mean auditory brainstem responses (ABRs) to tone pips at 8 or 32 kHz (80 dB SPL) with increasing levels of a continuous-noise masker for each experimental group. For each tone-pip frequency, the ABR data are shown two ways: 1) as heat maps (outer columns) and 2) as amplitude-vs.-time (waveform) plots (inner columns). Masker level is expressed in dB “Sensation Level” (SL), i.e. dB re the noise level required to decrease ABR P1 amplitude by 10% (Fig. 1E). Gray bars are centered on each of the five ABR peaks to facilitate comparisons of latency shifts across masker levels. The black boxes in the heatmaps highlight the masker-induced P4 latency shifts. Group sizes for the 8 kHz data were: control n = 12, 60 min n = 13, 120 min n = 14, and for 32 kHz: control n = 11, 60 min n = 12, 120 min n = 14. For each group, the masker noise was spectrally shaped according to the respective mean ABR thresholds (Fig. 5, A and B).
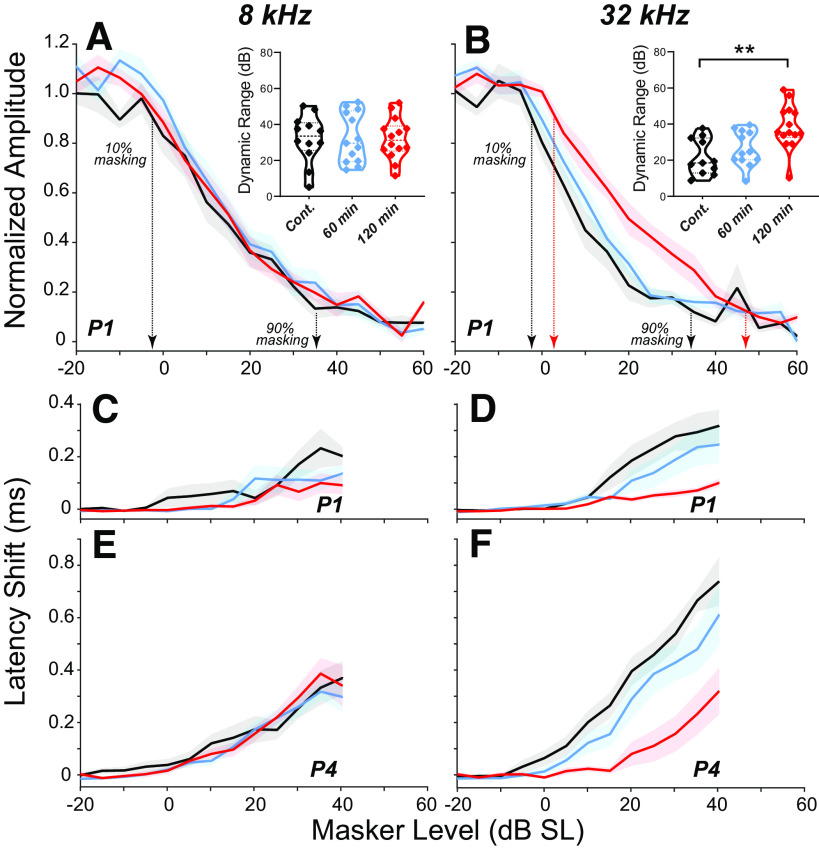
These differences in masking behavior across the three groups are most clearly seen by extracting amplitude- and latency-versus-masker-level functions (Fig. 4). Normalizing the P1 amplitudes to the masker-off condition shows the “dynamic range of masking,” i.e., the difference between the lowest masker level that reduces the probe response and the lowest masker level at which the probe response disappears. In the nonsynaptopathic region, where there are no differences in synaptic counts (Fig. 2C), the amplitude functions are comparable among cohorts (Fig. 4, A, C, and E); however, P1 latency shifts (Fig. 4C) were significantly smaller in both 60-min (P = 0.0049) and 120-min (P = 0.0023) groups compared with controls. At 32 kHz, the dynamic range of masking is extended in the synaptopathic ears from the 120-min group, but not in the 60-min group (Fig. 4B; control vs. 120 min: P = 0.0011; control vs. 60 min: P = 0.5184). The P1 amplitude function in the 120-min cohort was significantly different from both control (P < 0.001) and 60-min (P < 0.001) groups, however, there were no significant differences between control and 60-min groups (P = 0.1032). Similarly, the latency-level functions for both P1 and P4 show significant differences between the control and the 120-min group, with the 60-min group intermediate between the two but closer to control values (Fig. 4, D and F; P1 latency—control vs. 120 min: P < 0.001; control vs. 60 min: P < 0.001; 60 min vs. 120 min: P = 0.004; P4 latency—control vs. 120 min: P < 0.001; control vs. 60 min: P = 0.4175; 60 min vs. 120 min: P < 0.001). The differences between the two exposed groups suggest that the noise-induced extension of the dynamic range is not due to the threshold elevation at high CFs, which is observed in both exposure groups (Fig. 2, A and B).
Figure 4.
Auditory brainstem response (ABR) masking functions for control vs. exposed groups. A and B: mean amplitudes (means ± SE; baseline-to-peak) of P1 in response to 8- or 32-kHz probe tones at 80 dB SPL, as a function of masker level. P1 amplitude is normalized to the masker-off condition; masker level is normalized to the SPL producing a 10% reduction in P1 Amplitude (Fig. 1E). The calculated dynamic range of masking for each case is shown in the insets. Significance of the intergroup differences in shown by asterisks: **P ≤ 0.01. Mean latencies (means ± SE) of P1 (C and D) or P4 (E and F) as a function of masker level for the same assays shown in A and B. Latencies are normalized to the masker-off condition. For each group, the masker noise was spectrally shaped according to the respective mean ABR thresholds (Fig. 5, A and B).
Single-Unit Responses to ABR-Style Tone Pips
In a separate report on ANF responses from the same animals reported here, we showed that fibers from synaptopathic regions unexpectedly had enhanced onset responses to CF tone bursts in quiet, with significantly lower temporal jitter than normal fibers from control ears (15). This improved onset synchronization is likely related to the unexpected increase in dynamic range of masking observed here (Fig. 4B). However, the tone-burst stimuli used in that report were shaped as is customary for single-fiber ANF studies, i.e., with 2.5-ms rise-fall times, rather than the faster 0.5 ms rise times used to evoke ABR responses.
Here, to gain deeper insight into the ABR masking results, we measured single-ANF responses using the identical stimulus paradigm: fixed-level (80 dB SPL) “ABR-style” tone pips in quiet, and in the presence of varying levels of narrow-band continuous noise. Although the faster rise-fall times increase the spectral splatter of the stimuli, the ANF population responding to these ABR-style probe tones (in quiet) remained restricted to an octave-band range of CF (Fig. 5, A and B), even at 80 dB SPL, because the relatively high thresholds on the tuning curve “tails” in mice minimize the spread of excitation (17). Specifically, fibers responding to the 8 kHz probe were restricted to those with CFs between 7 and 16 kHz, whereas those responding to the 32 kHz probe all had CFs between 25 and 42 kHz. There was no obvious difference in this regard between fibers from exposed versus control ears.
There are several noteworthy features in the responses to tone pips in quiet (Fig. 5, A–H). The color coding in Fig. 5, A and B suggests that response rate falls as CF moves away from the probe frequency, thereby further restricting the CF region that likely dominates the ABR responses. Indeed, as further shown in the scatterplots of firing rate (Fig. 5C) and onset jitter [i.e., first-spike latency (FSL) variance, Fig. 5E], the strength and temporal coherence, respectively, of the ANF responses fall as the CF increases more than about ½ octave above CF, as marked by the cyan arrowheads. Curiously, the first-spike latency (Fig. 5G) and the noise level required to mask the probe-tone responses (Fig. 5I) are both minimal for CFs slightly higher than the probe frequency. This, in turn, suggests that the ABR latency shift with masker level (Fig. 4D) might arise because the CF region dominating the response is shifting, rather than because of a shift from high-SR to low-SR fibers dominating the tone-pip response. Again, there are no obvious differences in these trends between fibers from exposed versus control ears.
The effects of SR and exposure group on the responses to ABR-style tone pips are summarized in Fig. 6. The data show no significant relationship between SR and latency (Fig. 5, E and F; 8 kHz: ρ = 0.0166, P = 0.8169; 32 kHz: ρ = 0.0956, P = 0.4180). There is a positive correlation between SR and jitter at 8 kHz in control ears (8 kHz: ρ = 0.4336, P < 0.0001; 32 kHz: ρ = −0.0374, P = 0.7520), however, this arises from the off-CF responses of high-SR fibers (green oval in Fig. 5E and Fig. 6C). When this population is omitted, the correlation is no longer significant (as for the 32 kHz condition: ρ = −0.0478, P = 0.5938). In contrast, low-SR responses to tone pips with longer rise times (2.5 ms vs. the 0.5 ms used here) showed significantly more jitter than those of high-SR fibers (15). The maskability data for ABR-style tone pips (Fig. 6, G and H) are consistent with prior reports in cat that low-SR fibers are more resistant to masking than high SR fibers (14), however, this trend was only significant at 32 kHz (8 kHz: ρ = −0.2752, P = 0.0641; 32 kHz: ρ = −0.5489, P = 0.0135).
Figure 6.
Auditory-nerve fiber (ANF) responses to the 8 kHz (A, C, E, and G) or 32 kHz (B, D, F, and H) tone pips used to evoke auditory brainstem response (ABR) responses, viewed as a function of spontaneous discharge rate. These data are from the same as those plotted in Fig. 5, C–J as function of characteristic frequency. As in Fig. 5, gray symbols denote fibers not responding to the tone pips, according to a paired t test of “tone-on” vs. “tone-off” spike counts. The green oval in C encircles the same cloud of off-CF responses encircled in Fig. 5E. Symbols aligned at the max or min of the ordinate have off-scale values. FSL, first spike latency; SR, spontaneous rate.
To gain further insight into the reasons for the P1 latency shifts in ABRs with increasing masker level, we convolved single-fiber spike times with the hypothetical unitary contribution of each spike to a far-field extracellular potential (23, 24). To compensate for interfiber differences in absolute latency due to CF (Fig. 5, G and H), SR (Fig. 6, E and F), or precise recording position (re ANF somata), time was expressed re the latency of the most robust response for that fiber (i.e., tone pips at 80 dB SPL with masker off). The computed ensemble waveforms in response to tone pips in quiet (Fig. 7, A, B, E, and F) show a monotonic decrease in latency with increasing tone-pip level, similar in magnitude to that seen in the corresponding ABR responses in quiet (Fig. 7, I and K). In contrast, the computed ANF far-field responses to a constant-level tone pip shows virtually no latency shift with increasing levels of masking noise (Fig. 7, J and L). This suggests that 1) although both decrease the tone-evoked discharge rate, the effect of increasing masking noise on a tone-pip response is very different than the effect of turning down the sound pressure of a tone in quiet, and, 2) the masking-induced latency shift in ABR P1 must arise from shifting the population of responding ANFs, not from shifting the latency of individual fibers.
Figure 7.
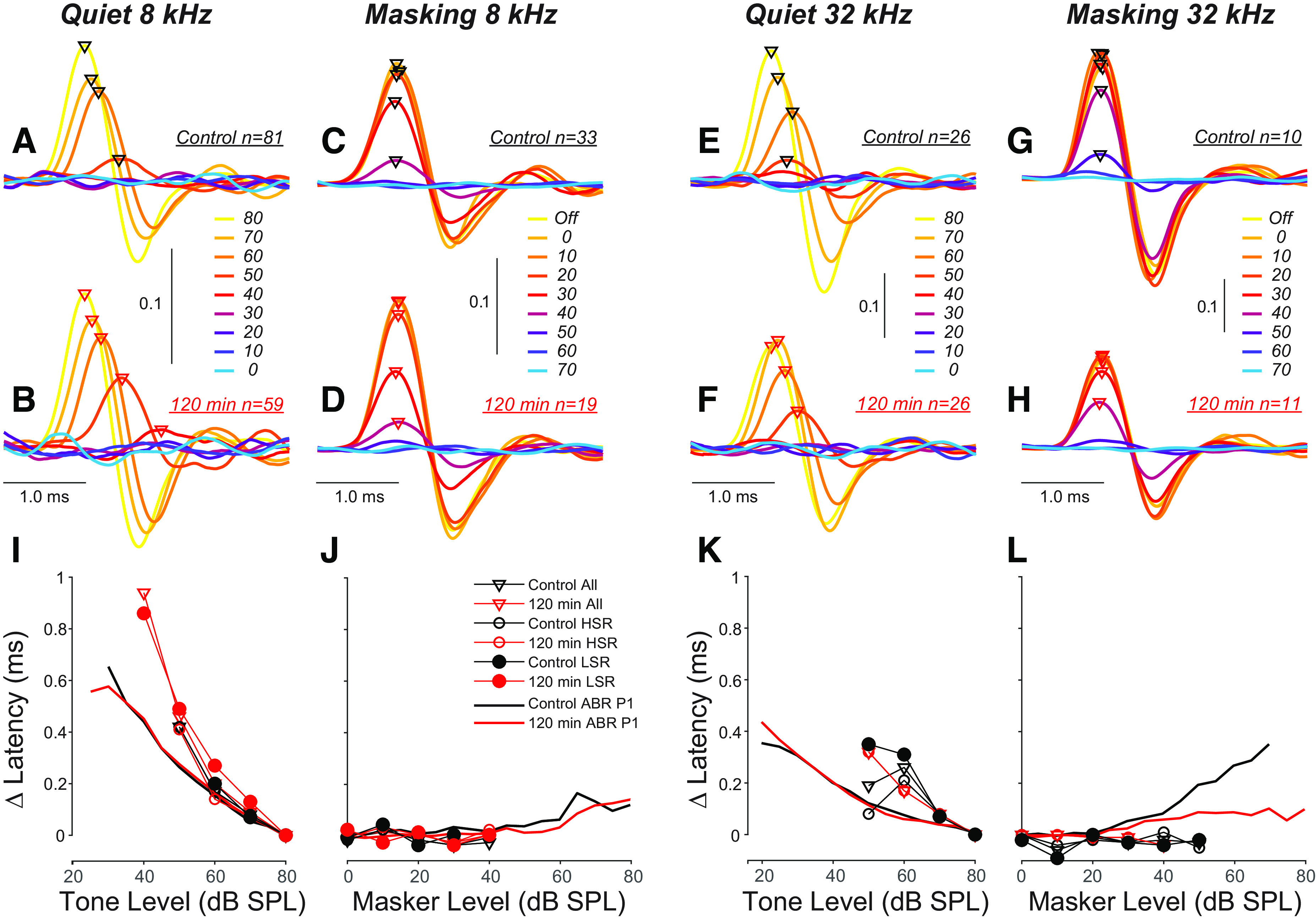
The latency of auditory-nerve fiber (ANF) contributions to auditory brainstem response (ABR) wave 1 increases with decreasing tone-pip level in quiet, but does not shift as the level of a noise masker is increased. A–H: these waveforms were created by convolving the spike-times underlying the summed peristimulus time histograms (PSTHs) in Fig. 8 with the idealized extracellular potential contributed by each spike to the ABR, i.e. a single cycle of an 800 Hz sinusoid (23, 25). Hot and cool colors represent highest and lowest signal to noise ratios, respectively. I–L: the thin lines with symbols show the mean latency shifts extracted from the waveforms in the upper panels; the thicker lines without symbols show P1 latency shifts calculated from ABRs. For each metric, latency is expressed re the relevant minimum value. The masker for these data was spectrally shaped to the threshold envelope of mouse ANFs from a prior study (17). HSR, high spontaneous rate; LSR, low spontaneous rate.
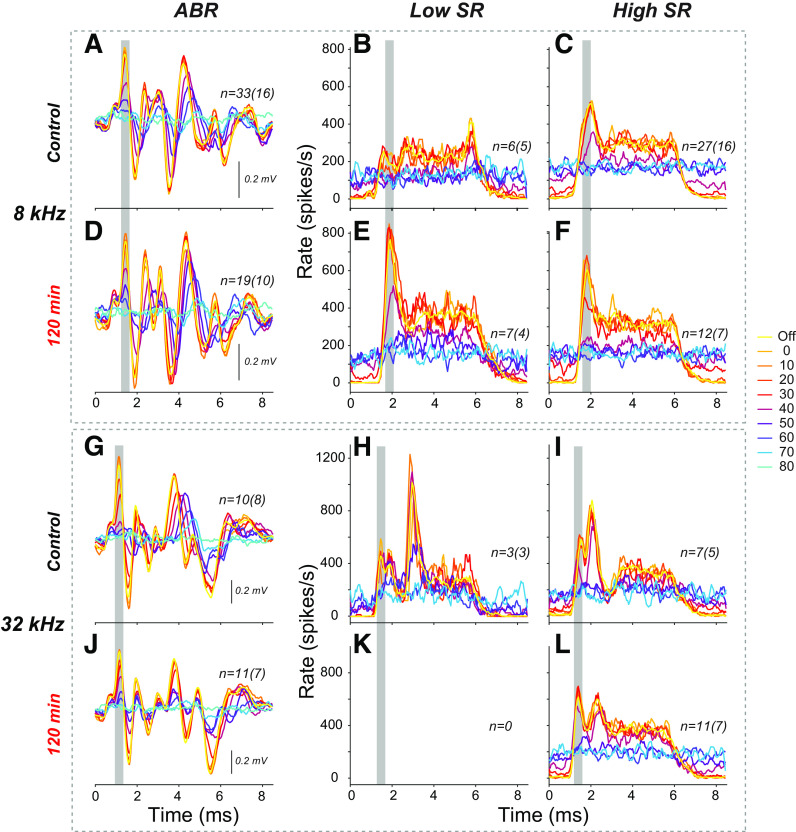
The ANF responses to these ABR-style tone pips can also be summarized by averaging the PSTHs (Fig. 8). With respect to the effects of masking noise, the summed PSTHs illustrate how, as masker level increases, the decrease in onset rates of ANFs (i.e., peaks highlighted with gray bars in Fig. 8, B, C, E, F, H, I, and L) is greater than the decrease in steady-state rates. Ultimately, the background firing rate is so elevated by the noise that the probe elicits no obvious change in rate (i.e., blue colors, see also Fig. 1B). First, for each of the probe frequencies, ANF latencies are slightly delayed re the ABR peak (gray bars). This delay is expected, as the ABR is dominated by current at their cell bodies, whereas single ANFs are recorded from their central axons near the cochlear nucleus. Second, in control ears, the low-SR fibers have smaller onset responses than high-SR fibers, especially at 8 kHz (Fig. 8B vs. Fig. 8C), as expected from prior studies of responses to classic tone-burst stimuli, i.e., with 2.5-ms rise-fall times (17, 26), and from the relatively higher variance of first-spike latencies for low-SR compared with most high-SR fibers (Fig. 6C). Third, fibers from the exposed ears show enhancements of the onset peak to both the 8 kHz and 32 kHz probes, and, at least at 8 kHz, among both low- and high-SR fibers. Finally, the ANF responses at 32 kHz are complex, with a double-peaked PSTH reminiscent of responses to clicks (18), although in response to classic ANF-style tone bursts, the same fibers displayed the typical monotonic decay from a single onset peak that defined them as “primary-like” responses (15). This complexity is not reflected in the ABR because 1) the biphasic nature of each spike’s contribution to the far-field potentials means that the repolarization phases of early spikes can cancel the depolarization phases of later spikes and 2) the ABR includes responses of cochlear nucleus neurons that will overlap in time with the later spikes from ANFs (22).
Figure 8.
Comparison of masker effects on mean auditory brainstem response (ABR) waveforms (A, D, G, and J) and the summed peristimulus time histograms (PSTHs) for low-spontaneous rate (SR) (B, E, K, and H) vs. high-SR (C, F, I, and L) auditory-nerve fibers (ANFs) in control and exposed ears, at 8 kHz or 32 kHz. Each panel superimposes data for the 80 dB probe in quiet (Off) or in the presence of eight levels of masking noise (dB SPL), as indicated in the line-color key at the right. ABRs were averaged from the same animals from which ANFs were recorded and are weighted by the number of fibers recorded from each case. The summed PSTHs were constructed by binning spikes for each unit-level combination in 0.05-ms bins, then averaging across units, within levels. Gray bars are positioned to aid comparison of response latencies. The number of fibers (animals) is given in each panel. The masker for these data was spectrally shaped to the threshold envelope of mouse ANFs from a prior study (17).
DISCUSSION
The Mouse Synaptopathy Model and an ABR-Based Masking Assay
Noise-induced cochlear synaptopathy has been studied in a number of species by exposing animals to band-limited noise at levels and durations that produce a severe, but ultimately reversible, threshold shift (9, 13, 27). In both animal and human studies, suprathreshold amplitudes of ABR responses have been used to infer neural health (9, 28). However, if there are permanent threshold shifts, abnormalities in ABR amplitude growth reflect a mix of contributions from neural and hair cell damage that are impossible to unambiguously disentangle. Other assays have been proposed to infer the magnitude of primary neural degeneration, including the evoked neural potentials to amplitude-modulated tones (12, 29, 30), the strength of the middle-ear muscle reflex (30), and the latency shift in ABR responses with increasing masker level (11). Here, we concentrate on the ABR masking assay, because hearing in noise is central to the putative functional consequences of cochlear synaptopathy and because measuring the dynamic range of masking theoretically offers a way to compensate for differences in absolute thresholds.
In a guinea pig model of noise-induced synaptopathy, ANF recordings at acute time points (∼2 wk postexposure) suggested that the neuronal loss was selective for low-SR fibers, as has also been reported in gerbils with age-related hearing loss or ouabain-induced cochlear neurodegeneration (13, 23, 31). In our mouse model of noise-induced synaptopathy, we saw no evidence for selective loss of low-SR fibers (15). However, ANF population statistics may be misleading if SRs are changed after noise, for example, transforming some high-SR fibers to a nominal low-SR classification, as occurs when acoustic overexposure damages stereocilia and raises thresholds (32).
We also saw increases in the amplitude of ANF onset responses, only from the synaptopathic region, and corresponding decreases in the variance of their first-spike latencies (15). These unexpected increases in the onset synchrony of the tone-burst responses were associated with an increased robustness to continuous-noise masking in the noise-exposed ears that mirrors the increase in the dynamic range of masking observed in the ABR-based test in the present study (Fig. 4B). Thus, a masking-based ABR assay of the type used here can be informative about key aspects of the underlying pathology, although it is not clear whether it can detect a selective loss of low-SR fibers.
Sound-evoked ANF responses are classically studied with 50-ms tone bursts at CF, presented at 10 repetitions/s with a 2.5-ms rise-fall time (18, 26, 33). The choice of repetition rate is a trade-off between the need for rapid data acquisition and the desire to avoid neuronal fatigue (adaptation) due to depletion of presynaptic vesicles (34, 35). Similarly, the rise-fall time is a trade-off between the desire to study rapid stimulus onsets that are important signal cues and the need to minimize spectral splatter, which increases with increasing steepness of stimulus onset. Far-field neural potentials like the ABR are usually evoked with faster repetition rates (i.e., 35/s in our mouse studies) to minimize averaging time, and with faster rise-fall times (i.e., 0.5 ms) to evoke better neural synchronization. Here, we concentrated on the ANF responses to ABR-style tone pips, at only one level (80 dB SPL) and two frequencies (8 and 32 kHz) to allow a more definitive analysis of the neural populations contributing to the phenomena observed in the ABR.
Interpreting ABR Amplitudes and Latencies Evoked by Tone Pips in Quiet
In addition to having higher thresholds, low-SR fibers also show smaller onset responses than high-SR fibers, when studied with classic “ANF-style” tone bursts (17, 26). Here, we show that onset responses among low-SR fibers are similarly attenuated in response to the ABR-style tone pips (Fig. 8B vs. Fig. 8C and Fig. 8H vs. Fig. 8I). The near-complete lack of onset responses in the low-SR responses to the 8 kHz tones (Fig. 6A; Fig. 8B vs. Fig. 8E) may also be influenced by the fast repetition rate of the ABR paradigm, as low-SR fibers are also more susceptible to rate adaptation (36). The double-peak responses to the high-frequency tones are surprising (Fig. 8, H, I, and L), since such complex PSTHs are not seen in the same fibers in response to ANF-style tone pips (data not shown but used to originally characterize fibers).
Given their attenuated onset responses, low-SR fiber loss should have a smaller fractional effect on suprathreshold ABR amplitude than high-SR fiber loss. Indeed in our synaptopathic mice, a 50% synapse loss at 32 kHz was associated with a 30% decrease in suprathreshold amplitudes (15). However, the novel finding of a noise-induced increase in onset responses, which was seen both for the slower rise-fall times (15) and the faster rise-fall times (Fig. 6A and Fig. 8), complicates the interpretation of the fractional changes in suprathreshold amplitudes.
As tone-pip level increases, the spread of excitation along the cochlear partition away from the region tuned to the tone-pip frequency will activate fibers with a broader range of CFs, which will also contribute to the growth of ABR suprathreshold amplitudes. That spread moves farther toward higher CF regions than to lower CF regions, due to the asymmetry of ANF tuning curves (Fig. 5). However, in mouse, the high-frequency spread is smaller than in other well-studied mammals, due to the relatively high thresholds of the low-frequency “tails” of the tuning curves (17). The spread of excitation is also restricted by the finding, novel to this study, that the jitter of the onset responses increases dramatically as the CF moves farther above the probe frequency (Fig. 5E). Responses that are not well synchronized either within or across fibers will not add effectively to generate a robust ABR peak.
Nevertheless, the basalwards spread becomes important in interpreting suprathreshold amplitudes in a noise-exposure model as seen here, where there is “tonotopically inappropriate” cochlear damage in the extreme cochlear base (Fig. 2, A and B). The loss of responsive neurons at CF regions ≥ 45 kHz, which we do not document because our acoustic system cannot be calibrated above ∼50 kHz, can also contribute to the suprathreshold amplitude differences seen at 32 kHz between normal and exposed ears. According to the cochlear frequency map for mouse (37), an estimated 20% of the cochlea is tuned to frequencies ≥ 45 kHz. Although the innervation density (ANFs/IHC) decreases at frequencies > 16 kHz (Fig. 2C), fibers with CFs ≥ 45 kHz likely comprise 10%–15% of the total ANF population.
Here, we saw a delay of roughly 0.2 ms between the latency of ABR wave 1 and the peak of the ensemble ANF PSTHs: compare, for example, gray bars in Fig. 8, A and C. This is to be expected if the ABR is dominated by current fluxes in the vicinity of the neuronal cell bodies in the spiral ganglion. Our single-fiber recordings, targeted near the Schwann-glial border where the nerve meets the cochlear nucleus (18), are roughly 1 mm from the ganglion in mouse (38). A computed conduction velocity of 5 m/s is a reasonable estimate (39–42) based on a central-axon diameter of 1.5 µm, thus a conduction delay of 0.2 ms is appropriate to our recording configuration.
ABR wave 1 latency decreases by ∼0.5 ms as tone-pip level increases from threshold levels to around 80 dB SPL in quiet (Fig. 7, I and K). Given the level-dependent spread of excitation toward higher CFs, and the decrease in response latency with increasing CFs (18), the level-dependent latency decrease in ABR could, at least in part, reflect the recruitment of fibers with progressively higher CFs. However, the present data show that response rates, latencies, and temporal jitter are also strongly dependent on the relation of tone-pip frequency to CF (Fig. 5, C, E, and G). Thus, as the CF moves farther from the probe, the response rate decreases, while jitter and latency increase. This limits the extent to which spread of excitation contributes to the ABR peak and suggests it adds little to the level-dependent decrease in ABR latency. As shown in Fig. 7, the level-dependent latency shift in single fibers is similar to the shift in ABR latencies suggesting that the latter is dominated by latency shifts among the fibers with CFs near the probe.
Interpreting the Normal Masker-Induced Latency Shift and Its Disappearance in Synaptopathy
The masking-induced latency shift in ABR responses was first invoked as a biomarker of a healthy low-SR population in a human study of psychophysical performance differences among “normal-hearing” young adults (11, 12). That study found that subjects with larger masker-induced latency shifts showed significantly better detection of interaural timing differences. The underlying hypotheses were 1) that the ABR peaks are normally delayed, as the masker level increases, because the ANFs dominating the probe response shift from high-SR to low-SR fibers, which are more robust to masking and have longer response latencies and 2) that a minimal masker-induced ABR latency shift suggests a weak or absent population of low-SR fibers.
Here, we replicated the phenotype, i.e., that ABR latency normally shifts with increasing masker level, and that a synaptopathic noise exposure reduces that shift (Fig. 3). However, we have recently shown that the pathophysiology in the mouse model studied here is not SR-selective (15); thus we sought an alternate explanation for the loss of masker-induced latency shift.
First we asked whether raising noise levels normally changes the response latency of individual ANFs to a constant-level tone pip (Fig. 7, C, D, and J). The lack of effect we observed suggests that the masking effect here is predominantly “excitatory,” i.e., due to synaptic fatigue, rather than “suppressive,” i.e., due to reduction in the mechanical response to the probe tone arising from cochlear nonlinearities. Suppressive effects might be more similar, with respect to response latencies, to turning down the probe-tone intensity (33, 43), which does cause prominent latency increases in single fibers (Fig. 7, A, B, and I).
If each fiber’s response latency is unaffected by masking noise, the masker-induced ABR latency shift must reflect a shift in the ANF population dominating the response. If not due to a shift in SR group, the phenomenon must arise from a shift in CF regions, and the present data support this hypothesis. Here, we showed for the first time that, in response to ABR-style tone pips, latency depends on the relation between stimulus frequency and CF. Furthermore, the CF region with the shortest latencies (cyan arrowheads in Fig. 5G) are most easily masked (cyan arrowheads in Fig. 5I) and will be the first to cease contributing to the ABR peak as noise level is increased. Conversely, the CF region most resistant to masking has longer latencies, thus latency should shift as noise level increases. At 8 kHz, this shift should be similar for control and exposed groups because there are no CF-related differences in threshold or synapse counts (Fig. 2). In contrast, for the 32-kHz probe, there is a loss of responsive fibers with CF above 45 kHz, due to synaptopathy and threshold elevation (Fig. 2), and to the hair cell loss documented in our prior reports on this synpatopathic model (44). This loss of responsive ANFs would minimize the basalwards spread of excitation in the exposed group and thus minimize the masker-induced latency shift, assuming that the same relations among CF, probe frequency, latency, and maskability are present in these high-frequency fibers as are seen in response to the 8-kHz probe. The fact that the 60-min exposure group, with slightly better 45 kHz thresholds, and clearly less synaptic loss (Fig. 2), has latency shifts intermediate between the control and 120-min exposure group (Fig. 4) is consistent with this view.
Similar confounds are at play in studies of “normal hearing” humans, given that the human cochlea extends to frequencies ∼20 kHz while standard audiometry fails to test above 8 kHz (45, 46). In human studies, as well as other mammals, the spread of excitation with increasing stimulus level is likely to be an even bigger confound than in mouse given that, as noted earlier, the below CF (i.e., “tail”) thresholds of mouse ANFs are exceptionally high compared with other mammals (17, 47). Such confounds also need to be considered when comparing suprathreshold amplitudes of ABRs in response to unmasked tones or clicks as a biomarker for putative low-SR loss, as a loss or threshold elevation at the extended high frequencies will also reduce ABRs even if thresholds near the probe frequency remain normal.
GRANTS
This research was supported by National Institute on Deafness and Other Communication Disorders (NIDCD) Grant R01 DC000188.
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.S. and M.C.L. conceived and designed research; K.S. performed experiments; K.S. and M.C.L. analyzed data; K.S. and M.C.L. interpreted results of experiments; K.S. and M.C.L. prepared figures; K.S. and M.C.L. drafted manuscript; K.S. and M.C.L. edited and revised manuscript; K.S. and M.C.L. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors acknowledge Dr. Ken Hancock for technical assistance with acquisition and analysis software, Evan Foss and Ishmael Stefanov-Wagner for engineering input, and Leslie Liberman for histological preparation.
Present address of K. Suthakar: Section on Neuronal Circuitry, National Institute on Deafness and Other Communication Disorders, NIH, Bethesda, MD, 20892, USA.
REFERENCES
- 1.Mepani AM, Verhulst S, Hancock KE, Garrett M, Vasilkov V, Bennett K, de Gruttola V, Liberman MC, Maison SF. Envelope following responses predict speech-in-noise performance in normal-hearing listeners. J Neurophysiol 125: 1213–1222, 2021. doi: 10.1152/jn.00620.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu PZ, O'Malley JT, de Gruttola V, Liberman MC. Age-related hearing loss is dominated by damage to inner ear sensory cells, not the cellular battery that powers them. J Neurosci 40: 6357–6366, 2020. doi: 10.1523/JNEUROSCI.0937-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nayagam BA, Muniak MA, Ryugo DK. The spiral ganglion: connecting the peripheral and central auditory systems. Hear Res 278: 2–20, 2011. doi: 10.1016/j.heares.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liberman MC. Morphological differences among radial afferent fibers in the cat cochlea: an electron-microscopic study of serial sections. Hear Res 3: 45–63, 1980. doi: 10.1016/0378-5955(80)90007-6. [DOI] [PubMed] [Google Scholar]
- 5.Chambers AR, Resnik J, Yuan Y, Whitton JP, Edge AS, Liberman MC, Polley DB. Central gain restores auditory processing following near-complete cochlear denervation. Neuron 89: 867–879, 2016. doi: 10.1016/j.neuron.2015.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schuknecht HF, Woellner RC. An experimental and clinical study of deafness from lesions of the cochlear nerve. J Laryngol Otol 69: 75–97, 1955. doi: 10.1017/s0022215100050465. [DOI] [PubMed] [Google Scholar]
- 7.Felder E, Schrott-Fischer A. Quantitative evaluation of myelinated nerve fibres and hair cells in cochleae of humans with age-related high-tone hearing loss. Hear Res 91: 19–32, 1995. doi: 10.1016/0378-5955(95)00158-1. [DOI] [PubMed] [Google Scholar]
- 8.Wu P-Z, O'Malley JT, de Gruttola V, Liberman MC. Primary neural degeneration in noise-exposed human cochleas: correlations with outer hair cell loss and word-discrimination scores. J Neurosci 41: 4439–4447, 2021. doi: 10.1523/JNEUROSCI.3238-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci 29: 14077–14085, 2009. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu PZ, Liberman LD, Bennett K, de Gruttola V, O'Malley JT, Liberman MC. Primary neural degeneration in the human cochlea: evidence for hidden hearing loss in the aging ear. Neuroscience 407: 8–20, 2019. doi: 10.1016/j.neuroscience.2018.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehraei G, Gallardo AP, Shinn-Cunningham BG, Dau T. Auditory brainstem response latency in forward masking, a marker of sensory deficits in listeners with normal hearing thresholds. Hear Res 346: 34–44, 2017. doi: 10.1016/j.heares.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehraei G, Hickox AE, Bharadwaj HM, Goldberg H, Verhulst S, Liberman MC, Shinn-Cunningham BG. Auditory brainstem response latency in noise as a marker of cochlear synaptopathy. J Neurosci 36: 3755–3764, 2016. doi: 10.1523/JNEUROSCI.4460-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furman AC, Kujawa SG, Liberman MC. Noise-induced cochlear neuropathy is selective for fibers with low spontaneous rates. J Neurophysiol 110: 577–586, 2013. doi: 10.1152/jn.00164.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costalupes JA, Young ED, Gibson DJ. Effects of continuous noise backgrounds on rate response of auditory nerve fibers in cat. J Neurophysiol 51: 1326–1344, 1984. doi: 10.1152/jn.1984.51.6.1326. [DOI] [PubMed] [Google Scholar]
- 15.Suthakar K, Liberman MC. Auditory-nerve responses in mice with noise-induced cochlear synaptopathy. J Neurophysiol 126: 2027–2038, 2021. doi: 10.1152/jn.00342.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suthakar K, Liberman MC. A simple algorithm for objective threshold determination of auditory brainstem responses. Hear Res 381: 107782, 2019. doi: 10.1016/j.heares.2019.107782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taberner AM, Liberman MC. Response properties of single auditory nerve fibers in the mouse. J Neurophysiol 93: 557–569, 2005. doi: 10.1152/jn.00574.2004. [DOI] [PubMed] [Google Scholar]
- 18.Kiang NY, Watanabe T, Thomas EC, Clark LF. Discharge Patterns of Single Fibers in the Cat's Auditory Nerve. Cambridge, MA: The MIT Press, 1966. [Google Scholar]
- 19.Kiang NYS, Moxon EC, Levine RA. Auditory nerve activity in cats with normal and abnormal cochleas. In: Sensorineural Hearing Loss, edited by Wolstenholme GEW, Knight J.. London: J & A Churchill, 1970, p. 241–273. [DOI] [PubMed] [Google Scholar]
- 20.Liberman MC. Auditory-nerve response from cats raised in a low-noise chamber. J Acoust Soc Am 63: 442–455, 1978. doi: 10.1121/1.381736. [DOI] [PubMed] [Google Scholar]
- 21.Kawase T, Delgutte B, Liberman MC. Antimasking effects of the olivocochlear reflex. II. Enhancement of auditory-nerve response to masked tones. J Neurophysiol 70: 2533–2549, 1993. doi: 10.1152/jn.1993.70.6.2533. [DOI] [PubMed] [Google Scholar]
- 22.Melcher JR, Knudson IM, Fullerton BC, Guinan JJ Jr, Norris BE, Kiang NYS. Generators of the brainstem auditory evoked potential in cat. I. An experimental approach to their identification. Hear Res 93: 1–27, 1996. doi: 10.1016/0378-5955(95)00178-6. [DOI] [PubMed] [Google Scholar]
- 23.Bourien J, Tang Y, Batrel C, Huet A, Lenoir M, Ladrech S, Desmadryl G, Nouvian R, Puel JL, Wang J. Contribution of auditory nerve fibers to compound action potential of the auditory nerve. J Neurophysiol 112: 1025–1039, 2014. doi: 10.1152/jn.00738.2013. [DOI] [PubMed] [Google Scholar]
- 24.Kiang NYS, Moxon EC, Kahn AR. The relationship of gross potentials recorded from the cochlea to single unit activity in the auditory nerve. In: Electrocochleography, edited by Ruben RJ, Eberling C, Solomon G.. Baltimore, MD: University Park Press, 1976. [Google Scholar]
- 25.Goldstein MH, Kiang NYS. Synchrony of neural activity in electrical responses evoked by transient acoustic stimuli. J Acoust Soc Am 30: 107–114, 1958. doi: 10.1121/1.1909497. [DOI] [Google Scholar]
- 26.Rhode WS, Smith PH. Characteristics of tone-pip response patterns in relationship to spontaneous rate in cat auditory nerve fibers. Hear Res 18: 159–168, 1985. doi: 10.1016/0378-5955(85)90008-5. [DOI] [PubMed] [Google Scholar]
- 27.Hickox AE, Larsen E, Heinz MG, Shinobu L, Whitton JP. Translational issues in cochlear synaptopathy. Hear Res 349: 164–171, 2017. doi: 10.1016/j.heares.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liberman MC, Epstein MJ, Cleveland SS, Wang H, Maison SF. Toward a differential diagnosis of hidden hearing loss in humans. PLoS One 11: e0162726, 2016. doi: 10.1371/journal.pone.0162726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaheen LA, Valero MD, Liberman MC. Towards a diagnosis of cochlear neuropathy with envelope following responses. J Assoc Res Otolaryngol 16: 727–745, 2015. doi: 10.1007/s10162-015-0539-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valero MD, Hancock KE, Liberman MC. The middle ear muscle reflex in the diagnosis of cochlear neuropathy. Hear Res 332: 29–38, 2016. doi: 10.1016/j.heares.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmiedt RA, Mills JH, Boettcher FA. Age-related loss of activity of auditory-nerve fibers. J Neurophysiol 76: 2799–2803, 1996. doi: 10.1152/jn.1996.76.4.2799. [DOI] [PubMed] [Google Scholar]
- 32.Liberman MC, Dodds LW. Single-neuron labeling and chronic cochlear pathology. II. Stereocilia damage and alterations of spontaneous discharge rates. Hear Res 16: 43–53, 1984. doi: 10.1016/0378-5955(84)90024-8. [DOI] [PubMed] [Google Scholar]
- 33.Sachs MB, Abbas PJ. Rate versus level functions for auditory-nerve fibers in the cat: tone-burst stimuli. J Acoust Soc Am 56: 1835–1847, 1974. doi: 10.1121/1.1903521. [DOI] [PubMed] [Google Scholar]
- 34.Kiang NY, Peake W. Components of electrical responses recorded from the cochlea. Ann Otol Rhinol Laryngol 69: 448–458, 1960. doi: 10.1177/000348946006900213. [DOI] [PubMed] [Google Scholar]
- 35.Smith RL, Brachman ML, Goodman DA. Adaptation in the auditory periphery. Ann NY Acad Sci 405: 79–93, 1983. doi: 10.1111/j.1749-6632.1983.tb31621.x. [DOI] [PubMed] [Google Scholar]
- 36.Relkin EM, Doucet JR. Recovery from prior stimulation. I. Relationship to spontaneous firing rates of primary auditory neurons. Hearing Res 55: 215–222, 1991. doi: 10.1016/0378-5955(91)90106-j. [DOI] [PubMed] [Google Scholar]
- 37.Muller M, von Hunerbein K, Hoidis S, Smolders JW. A physiological place-frequency map of the cochlea in the CBA/J mouse. Hear Res 202: 63–73, 2005. doi: 10.1016/j.heares.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 38.Berglund AM, Ryugo DK. Hair cell innervation by spiral ganglion neurons in the mouse. J Comp Neurol 255: 560–570, 1987. doi: 10.1002/cne.902550408. [DOI] [PubMed] [Google Scholar]
- 39.Brown AM, Hamann M. Computational modeling of the effects of auditory nerve dysmyelination. Front Neuroanat 8: 73, 2014. doi: 10.3389/fnana.2014.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nguyen BH, Javel E, Levine SC. Physiologic identification of eighth nerve subdivisions: direct recordings with bipolar and monopolar electrodes. Am J Otol 20: 522–534, 1999. [PubMed] [Google Scholar]
- 41.Woo J, Miller CA, Abbas PJ. The dependence of auditory nerve rate adaptation on electric stimulus parameters, electrode position, and fiber diameter: a computer model study. J Assoc Res Otolaryngol 11: 283–296, 2010. doi: 10.1007/s10162-009-0199-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller CA, Robinson BK, Hetke JF, Abbas PJ, Nourski KV. Feasibility of using silicon-substrate recording electrodes within the auditory nerve. Hear Res 198: 48–58, 2004. doi: 10.1016/j.heares.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 43.Delgutte B. Physiological mechanisms of psychophysical masking: observations from auditory-nerve fibers. J Acoust Soc Am 87: 791–809, 1990. doi: 10.1121/1.398891. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki J, Corfas G, Liberman MC. Round-window delivery of neurotrophin 3 regenerates cochlear synapses after acoustic overexposure. Sci Rep 6: 24907, 2016. doi: 10.1038/srep24907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schuknecht HF. Pathology of the Ear (2nd ed.). Baltimore, MD: Lea & Febiger, 1993, p. 1–503. [Google Scholar]
- 46.Greenwood DD. A cochlear frequency-position function for several species–29 years later. J Acoust Soc Am 87: 2592–2605, 1990. doi: 10.1121/1.399052. [DOI] [PubMed] [Google Scholar]
- 47.Kiang NY, Moxon EC. Tails of tuning curves of auditory-nerve fibers. J Acoust Soc Am 55: 620–630, 1974. doi: 10.1121/1.1914572. [DOI] [PubMed] [Google Scholar]