Abstract
Hyaluronan is a versatile macromolecule capable of an exceptional range of functions from cushioning and hydration to dynamic signaling in development and disease. Because of its critical roles, hyaluronan production is regulated at multiple levels including epigenetic, transcriptional, and posttranslational control of the three hyaluronan synthase (HAS) enzymes. Precursor availability can dictate the rate and amount of hyaluronan synthesized and shed by the cells producing it. However, the nucleotide-activated sugar substrates for hyaluronan synthesis by HAS also participate in exquisitely fine-tuned cross-talking pathways that intersect with glycosaminoglycan production and central carbohydrate metabolism. Multiple UDP-sugars have alternative metabolic fates and exhibit coordinated and reciprocal allosteric control of enzymes within their biosynthetic pathways to preserve appropriate precursor ratios for accurate partitioning among downstream products, while also sensing and maintaining energy homeostasis. Since the dysregulation of nucleotide sugar and hyaluronan synthesis is associated with multiple pathologies, these pathways offer opportunities for therapeutic intervention. Recent structures of several key rate-limiting enzymes in the UDP-sugar synthesis pathways have offered new insights to the overall regulation of hyaluronan production by precursor fate decisions. The details of UDP-sugar control and the structural basis for underlying mechanisms are discussed in this review.
Keywords: hexosamine biosynthesis pathway, hyaluronan, nucleotide sugars, UDP-glucuronate, UDP-N-acetylglucosamine
INTRODUCTION
Hyaluronan (HA) is a linear anionic glycosaminoglycan (GAG) polymer consisting of a simple repeating disaccharide unit of N-acetylglucosamine and glucuronate, produced abundantly by many diverse species. Unlike other sulfated GAGs, HA is produced at the plasma membrane by one of three HA synthase isozymes (HAS1, HAS2, and HAS3) and is shed to the extracellular space without additional modifications or covalent attachment to a protein core. In mammals, HA is essential for development, wound healing, immune function, and tissue homeostasis. Its dysregulated production and turnover are widely implicated in multiple pathologies and disease states from atherosclerosis to cancer (1–3).
The complexity of HA impacts is due in part to its viscoelastic properties and highly hygroscopic nature, but also to its differential association with diverse receptors and binding proteins, based on its molecular mass. Processing and turnover involving hyaluronidases and receptor-mediated endocytosis, coupled with its recently demonstrated presence in extracellular vesicles, further enriches the spectrum of mechanisms by which this versatile polymer achieves its functional complexity (4, 5).
Production of HA requires the continuous cytosolic availability of two nucleotide sugar precursors, UDP-glucuronate (UDP-GlcA) and UDP-N-acetylglucosamine (UDP-GlcNAc). The synthesis of these multifunctional precursors is highly energy-dependent and the pathways through which they are produced yield high-energy intermediates that serve as branch points and feedback regulators for cross talk to coordinate metabolic outcomes (6). For example, HA and proteoglycan production are biosynthetic processes. Opposing the generation of these polymers is the use of UDP-GlcA for cellular detoxification through the glucuronidation pathway. The cellular mechanisms that resolve such competing interests are multifold (7). Besides metabolite binding and direct allosteric regulation of pathway proteins, individual processes are compartmentalized: HA synthesis occurs at the plasma membrane whereas proteoglycans are synthesized within the Golgi and cellular detoxification is largely confined to the endoplasmic reticulum (ER) lumen. A specific subfamily of solute carrier proteins, known as nucleotide sugar transporters (NST), is differentially distributed among organelle membranes to regulate ER and Golgi concentrations of nucleotide sugars by gradient-driven antiport of the hydrolyzed nucleotide leaving groups (8).
Further regulation can occur at the level of expression of specific essential enzymes that serve as “gatekeepers” to the production of rate-limiting metabolites. In this review, we focus on the role of key allosteric gatekeeper enzymes in regulating flux through critical pathways via metabolite control. In particular, we discuss insights provided by examination of structures, posttranslational modifications, enzyme kinetics, and pharmacological inhibition of sensory nodes in HA biosynthesis, that reveal the complex intracellular cross talk and intimate link to overall carbohydrate and nucleotide metabolic homeostasis.
ORIGIN AND COMPETING FATES OF UDP-GLUCURONATE AND UDP-N-ACETYLGLUCOSAMINE
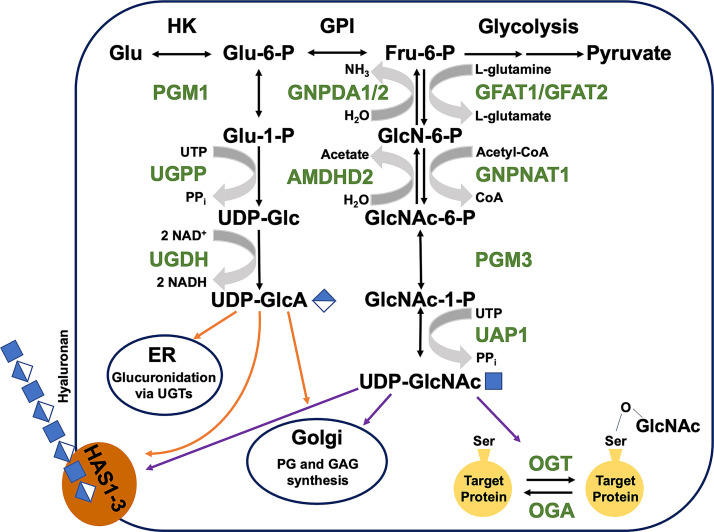
Nucleotide sugars are chemically activated precursors that fundamentally derive from cellular glucose and glutamine intake by well-established pathways that integrate with central metabolism (9) (Fig. 1). The nucleotide sugars important for HA production have several key roles: 1) as substrates of HA synthases, 2) in regulation of HA synthesis as allosteric feedback modulators of gatekeeper enzymes in their own synthetic pathways, and 3) as precursors for posttranslational modifications that increase or decrease HA accumulation. HAS enzymes use UDP-GlcA and UDP-GlcNAc as substrates and both of these essential HA building blocks are also used for other metabolic processes.
Figure 1.
HA precursor biosynthesis pathways. UDP-GlcA and UDP-GlcNAc are produced through a series of enzymatic reactions downstream of glucose metabolism. UDP-GlcA synthesis begins with Glu-6-P and ends with oxidation of UDP-Glc catalyzed by UDP-glucose dehydrogenase (UGDH). UDP-GlcNAc is produced from Fru-6-P and the first reaction of this pathway, the production of d-glucosamine-6-phosphate, is considered the rate-limiting step. Both nucleotide sugars have three downstream competing fates, but both are utilized at the Golgi for proteoglycan (PG) and glycosaminoglycan (GAG) production and at the plasma membrane for HA synthesis via HA synthase (HAS1-3) enzymes. In addition, UDP-GlcA is utilized in the ER for glucuronidation via UDP-glucuronosyltransferase (UGT) enzymes and UDP-GlcNAc is used in the cytosol for serine O-linked GlcNAcylation. ER, endoplasmic reticulum; HA, hyaluronan; UDP-GlcA, UDP-glucuronate; UDP-GlcNAc, UDP-N-acetylglucosamine.
Glucose and glutamine enter the cell through specific regulated or gradient-driven transporters. Glucose (Glc) is phosphorylated in the cytoplasm to Glc-6-P and is available for glycolysis if energy demand is high, or for biosynthesis via the multifunctional metabolite UDP-Glc if energy is abundant. UDP-Glc is a precursor for glycogen, and for enzymes such as UDP-Glc:ceramide glucosyltransferase needed for control of sphingolipid distribution (10), or UDP-Glc:glycoprotein glucosyltransferase, which is essential for quality control of protein glycosylation and protein folding (11). UDP-Glc also has a demonstrated role in intracellular and extracellular signaling processes, via direct interaction with RNA binding proteins thereby controlling stability of multiple target transcripts (12), or ligation of cell surface P2Y14 receptors that trigger gene expression cascades (13), respectively. UDP-Glc is one of two substrates for UDP-galactose-4-epimerase (GALE), which produces UDP-galactose (UDP-Gal) to support GAG chain elongation on proteoglycans (14).
UDP-Glc is also a substrate for the unique enzyme UDP-Glc dehydrogenase (UGDH). UGDH catalyzes oxidation of UDP-Glc to UDP-GlcA, which is a multifunctional precursor that is partitioned among the Golgi- and ER-localized processes of protein glycosylation, proteoglycan formation, and glucuronidation, in addition to being used by plasma membrane-embedded HAS isozymes HAS1, HAS2, and HAS3 for the synthesis and extracellular secretion of polymeric HA (7). Besides supporting HA synthesis and GAG chain elongation directly, UDP-GlcA is also the essential substrate for UDP-Xylose synthase to produce UDP-Xylose for N-glycan initiation on all proteoglycan core proteins in the Golgi.
In the other arm of the HA precursor synthesis process, the hexosamine biosynthesis pathway (HBP, Fig. 1), Glc-6-P that is isomerized to Fru-6-P in an energetically neutral reaction can be used to synthesize activated amino sugars required for production of HA and other GAGs, as well as protein glycosylation, and the posttranslational modification of target serine residues, termed O-GlcNAcylation. Fru-6-P is rate-limiting in the synthesis of amino sugars, catalyzed by glutamine-fructose-6-phosphate amidotransferases (GFAT1/2). The initial conversion to glucosamine-phosphate (GlcN-6-P) is an amidotransferase reaction dependent on cytosolic glutamine. The subsequent acetylation by glucosamine-phosphate N-acetyltransferase 1 (GNPNAT1) utilizes an acetylCoA cofactor. Phosphoacetylglucosamine mutase 3 (PGM3) converts GlcNAc-6-P to GlcNAc-1-P before the activation by UDP-N-acetylglucosamine pyrophosphorylase 1 (UAP1) to yield UDP-GlcNAc. UDP-GlcNAc is a potent feedback inhibitor of the HBP, through its ability to bind and allosterically inhibit GFAT1 (15), much as UDP-xylose can bind and feedback inhibit UGDH (16). UDP-GlcNAc is also the second substrate of GALE, which catalyzes its epimerization to yield UDP-GalNAc for glycan elongation. Overall, it has been estimated that 2%–5% of all cellular glucose use results from HBP flux (17).
Cytosolic UDP-GlcA and UDP-GlcNAc can also be generated from other sugar precursors, which can enter central metabolism and be subsequently channeled into the respective pathways following activation by UDP esterification. In addition, the turnover of cell surface proteoglycans produces glucuronate, N-acetylglucosamine, and glucosamine that can be phosphorylated, acetylated, and ultimately again UDP-esterified and thereby salvaged for HA synthesis. Internalization of HA from exogenous sources can also produce sugar precursors for cellular recycling. Specific sugar and amino sugar transporters present in late endosomes and lysosomes permit the release of these metabolites back to the cytosol for modification and reactivation. In general, the availability of these precursors in the compartments that house their synthetic enzymes is regulated by the presence of membrane-embedded transporters (NSTs) that have differential specificity for monosaccharides, amino sugars, and nucleotide sugars (8). Since exogenous HA has frequently been found to impact cellular processes differently than endogenously produced HA, this source of precursors does not constitute a futile cycle and may be critical to cell survival, proliferation, and cell fate plasticity in development and disease.
REGULATION OF PRECURSOR BIOSYNTHETIC ENZYMES
The biosynthesis of HA maintains a 1:1 stoichiometry of UDP-GlcA and UDP-GlcNAc. Therefore, HA production requires a balance of competing metabolic needs with regulated flux of sugar moieties through the UDP-GlcA and UDP-GlcNAc pathways. Both branches feature interconversion of sugar isomers via phosphoglucomutases PGM1 or PGM3 and their activation by UTP:hexose uridylyl-transferases (UGPP/UAP1). The latter enzymes interconvert UTP and the appropriate hexose-1-phosphate to UDP-hexose and inorganic pyrophosphate (PPi). Posttranslational regulatory control of metabolite flow through these two pathways appears to reside primarily in UGDH and GFAT1/2. Recent kinetic, structural, and functional characterizations of these two enzymes have revealed intricate allosteric regulatory mechanisms that support metabolite cross talk between the two pathways.
UDP-GlcA Synthesis
Glc-6-P is diverted to UDP-GlcA by the successive action of PGM1, UDP-Glc pyrophosphorylase (UGPP), and UGDH (Fig. 1). PGM1 is located at a critical metabolic node and catalyzes the reversible, Mg2+-dependent interconversion of Glc-6-P and Glc-1-P via a glucose-1,6-bisphosphate intermediate, mechanistic details of which were revealed by characterization of clinically observed mutations (18, 19). The resulting Glc-1-P and UTP are then converted by UGPP to UDP-Glc with the concomitant production of PPi. Similar to PGM1, the core catalytic domains of UGPP are conserved from bacteria to humans (20, 21). However, some eukaryotes may employ an unusual regulatory mechanism that is dependent on the formation of higher-order oligomers. Although these octomeric species have lower catalytic activity, they appear to have greater stability and may allow for allosteric regulation through intersubunit communication (22, 23).
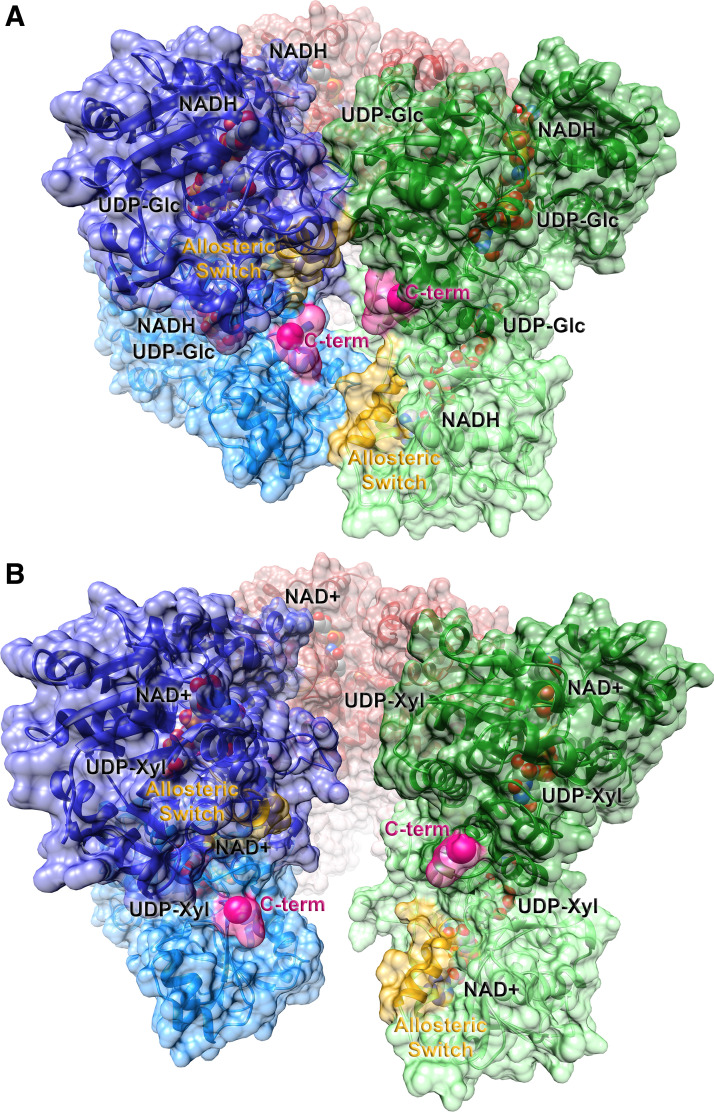
Human UGDH catalyzes two successive NAD+-dependent oxidations of the C6′ hydroxyl group of UDP-Glc to generate UDP-GlcA. The enzymatic mechanism of UGDH has been studied comprehensively and core catalytic features are conserved across species (24, 25). Whereas bacterial UGDH is homodimeric, eukaryotic UGDH exists primarily in homohexamers (Fig. 2) that can sample dimeric and tetrameric states (27, 28). Under physiological conditions, this “trimer of dimers” appears to be the relevant species, as introduction of point mutations that disrupt the hexameric state results in significant reductions in catalytic efficiency (28–30). However, the enzyme must also retain the ability to transiently disrupt subunit-subunit interactions (27, 31), highlighting that conformational dynamics at subunit interfaces clearly contribute to efficient catalysis (32).
Figure 2.
Ribbon representations of the active (E) and inhibited (EΩ) substate conformations of hexameric human UGDH. The abortive ternary complex of the substrate, UDP-glucose, and the reduced cofactor, NADH [2Q3E (26); A] and the feedback inhibited complex of UDP-xylose and NAD+ [3PTZ (27); B] are shown in ribbon representation. The solvent accessible surface representation is superimposed. Individual dimers are illustrated in dark/light pairs of red, green, and blue. The allosteric switch, which includes the Thr 131 loop and the adjacent α6 helix, is labeled and highlighted in yellow; the start of the intrinsically disordered C-terminal tail is labeled and colored in pink. Ligands are shown in space-filling representation with oxygen colored in red, nitrogen in blue, carbon in gray, and phosphorus in orange. UGDH, UDP-glucose dehydrogenase.
Detailed mechanistic studies have suggested that feedback inhibitors and allosteric regulators influence the relative distribution of three distinct substates of UGDH: an active hexamer (E), an inactive hexamer (E*), and a UDP-Xylose inhibited hexamer (EΩ) (27, 29, 30, 33–38). Stabilization of a given substate likely modulates metabolic flux through this critical pathway. Like many key metabolic branch point enzymes, UGDH is feedback inhibited by one of its downstream products. UDP-Xylose binds in the UDP-Glc binding site and leads to significant changes in the position of a proposed allosteric switch. Figure 2 illustrates the comparison of the holoenzyme interface (Fig. 2A) and the “open” UDP-Xylose-inhibited interface (Fig. 2B). Relative to glucose, the inhibitor lacks a hydroxymethyl substituent at the C5 position of xylose, allowing Thr 131 within the allosteric switch loop to move deeper into the active site pocket, displacing the nicotinamide ring of the cofactor and reducing activity (27). Movement of this allosteric switch (labeled in each subunit in yellow) is propagated along the adjacent α6 helix, leading to disruption of subunit-subunit interfaces.
An intriguing observation is that the removal of the intrinsically disordered C-terminal tail (residues 465–494, colored and labeled in pink within each subunit) of UGDH results in a 10-fold increase in the Ki for UDP-Xylose (36), suggesting that entropic movements can impact the relative distribution of the three postulated substates. Subsequent studies established that the length of the C-terminal tail, but not its sequence or charge distribution, is the major determinant in shifting the conformational equilibrium toward the inhibited EΩ substate (36). Recently, Tyr 473 in the endogenous C-terminal tail has been shown to be phosphorylated (39), suggesting that posttranslational modifications may also contribute to UGDH regulation by shifting the relative distribution of substates.
Both the disordered C-terminal tail and α6 helix of the allosteric switch are located near the dimer-dimer interface (compare Fig. 2A and Fig. 2B). As additional allosteric effectors and posttranslational modifications are identified, it is likely that they will also influence the dynamic equilibrium in this region of the hexameric species and regulate UDP-GlcA production. These observations suggest that pharmacological compounds can be developed to modulate conformational sampling that contributes to quaternary assembly, and thus tune UGDH activity.
UDP-GlcNAc Synthesis
GFAT catalyzes the initial and rate-limiting step in the synthesis of UDP-GlcNAc (40, 41). The closely related mammalian isoforms of the enzyme, GFAT1 and GFAT2 (sometimes denoted GFPT1/2) share ∼75% sequence identity but differ in their tissue distribution (42, 43). Many of the mechanistic details of the conversion of Fru-6-P and l-glutamine to d-glucosamine 6-phosphate (GlcN-6P) have been established in the bacterial homolog, glucosamine-6-phosphate synthase (GlmS) (44, 45). GFAT1 and GlmS show significant sequence conservation (∼35%–40% identity), suggesting that the two enzymes likely share a common mechanism.
Both GlmS and GFAT are homodimeric proteins. Each subunit contains an N-terminal glutaminase domain and a C-terminal synthase/isomerase domain (41, 44–46). The C-terminal domain is structurally conserved and catalyzes the interconversion of Fru-6-P to Glc-6-P with the subsequent addition of ammonia to generate d-glucosamine 6-phosphate. The free ammonia is supplied by the coordinated hydrolysis of l-glutamine by the N-terminal glutaminase domain, which is a member of the N-terminal nucleophile hydrolase (Ntn-hydrolase) family (47). Following translation, the glutaminase domain undergoes an autocatalytic processing event in which the thiol nucleophile of Cys 2 attacks the peptide bond between it and the initiator methionine. Hydrolysis of this peptide bond results in a new N-terminus and the revealed α-amino group of Cys 2 further activates its own side chain in the glutaminase reaction (45).
To ensure both the sugar acceptor and the nitrogen donor are present before the initiation of catalysis, a series of conformational changes must occur. Before sugar binding, the C-terminal tail of GlmS and the entire glutaminase domain exhibit considerable flexibility (44), and key active site residues in both the glutaminase and synthase/isomerase domains are not properly oriented for efficient catalysis. Binding of the Fru-6-P substrate leads to the ordering of the C-terminal tail over the substrate and the proper alignment of catalytic residues. This promotes the formation of an ammonia channel that links the glutaminase active site to that of the synthase/isomerase domain, and overall ordering of the glutaminase domain. These coordinated movements are strongly supported by mechanistic and structural studies. Notably, glutaminase activity is stimulated >100-fold in the presence of Fru-6-P and this stimulation can be attenuated by amino acid substitutions that disrupt interdomain communication (45).
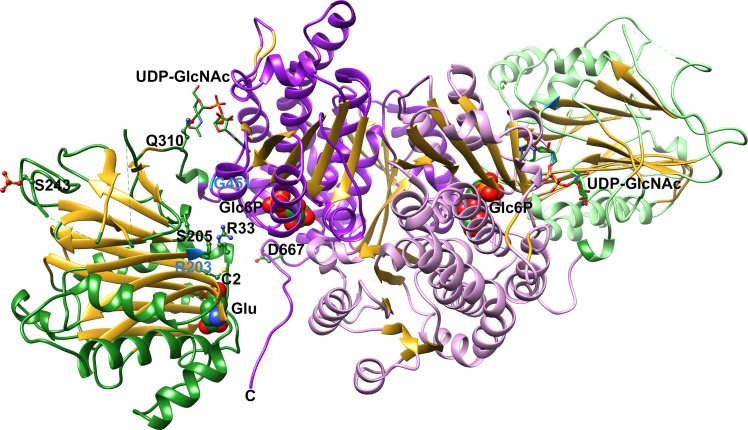
Recent work on human GFAT1 has focused on the exquisite posttranslational regulation of this critical enzyme. As illustrated by the mechanistic studies of GlmS, conformational dynamics are critical for efficient catalysis and disruption or facilitation of these coordinated movements could impact flux through this pathway. GFAT is feedback inhibited by the end product of the pathway, UDP-GlcNAc (15), and the molecular details of inhibition were recently revealed (41). UDP-GlcNAc binding disrupts communication between the N-terminal glutaminase and C-terminal synthase/isomerase domains by interacting with the interdomain linker (Fig. 3). Subsequent cellular studies show that GFAT1 is significantly inhibited at physiological UDP-GlcNAc concentrations, suggesting that additional factors are needed to stimulate activity (41).
Figure 3.
Ribbon representation of the structure of human GFAT1. The structure of human GFAT1 in complex with glutamate and Glc6P was determined by x-ray crystallography (6R4E). Subunit A (darker shade) and subunit B (lighter shade) each contain a glutaminase domain (green) and a synthase/isomerase domain (purple), with β-strands colored in gold. Glutamate and Glc6P are rendered in a space filling representation and relevant side chains in ball and stick format. The superimposed position of UDP-GlcNAc from the feedback inhibited structure (6R4G) is presented in stick format. Atoms are colored as follows: oxygen—red, nitrogen—blue, phosphate—orange, and sulfur—yellow. Sites of gain-of-function mutants are colored pale blue in the ribbon representation (41). UDP-GlcNAc, UDP-N-acetylglucosamine.
GFAT1 has been shown to be regulated by several protein kinases. Ser 205 and Ser 235 are phosphorylated by cAMP-dependent protein kinase (PKA) (48), whereas phosphorylation of Ser 243 occurs via AMP-activated protein kinase (AMPK) and calcium/calmodulin-dependent kinase II (49, 50). Recent structural and biochemical studies have resolved conflicting reports about phosphorylation of Ser 205, which reduces the in vitro catalytic efficiency of GFAT1 (41, 46). However, Ser 205 phosphorylation also interferes with binding of UDP-GlcNAc at the allosteric site, leading to greater production of UDP-GlcNAc by limiting feedback inhibition despite the compromised kinetic parameters (41, 46). A definitive role for Ser 235 has not yet been identified.
In vitro characterizations using a phosphomimetic substitution at position 243 suggests that a posttranslational serine modification would lead to increased production of GlcN-6P (50). A recent study in mouse embryonic fibroblasts supports this assertion and further provides evidence that mTORC2-mediated phosphorylation of Ser-243 stabilizes GFAT1 to promote flux through the HBP under nutrient-limiting conditions (49). Examination of the available GFAT1 structures does not provide an obvious explanation, as Ser 243 is remote from the glutaminase active site. However, an adjacent arginine residue, Arg 288, may form favorable interactions with the added phosphate group and influence conformational dynamics that mediate stability and activity.
To complete UDP-GlcNAc synthesis, the resulting GlcN-6P is acetylated by GNPNAT1 to generate GlcNAc-6-phosphate (GlcNAc-6P). A recent study also identified GlcNAc Deacetylase (AMDHD2) as an enzyme that can oppose UDP-GlcNAc synthesis by hydrolysis of GlcNAc-6P (51). The structure determination of human GNPNAT1 (52) revealed that it is a member of the well-characterized GNAT superfamily of acetyltransferases (53). The final two steps in UDP-GlcNAc synthesis closely resemble the first two steps in the production of UDP-GlcA. PGM3 (also known as AGM1) catalyzes the conversion of GlcNAc-6-P into GlcNAc-1-P, in analogous catalytic fashion to PGM1 (54, 55). UAP1 then catalyzes the nucleophilic attack of a phosphate oxygen of GlcNAc-1-P on the UTP α-phosphate to generate UDP-GlcNAc and PPi. The mechanistic features of this final enzyme closely resemble those of UGPP (56, 57). Currently, the extent of posttranslational regulation of these final steps appears to be minimal relative to that of GFAT1.
IMPACT OF UDP-GlcA AND UDP-GlcNAc ON HYALURONAN LEVELS
Developmental events and neoplastic pathologies such as cancer share the common feature of dependence on carbohydrate metabolism to enable morphogenesis and processes that require cellular plasticity. Programs of metabolic energetics that harvest energy while continuing to provide reduced carbon skeleton precursors for macromolecular synthesis are capable of both maintaining stemness and multipotency, and executing dedifferentiation transformations. Because a key metabolic feature of cancer involves the increased uptake of glucose to facilitate aerobic glycolysis, most cancers have elevated pools of nucleotide sugars, including UDP-GlcA and UDP-GlcNAc. Excesses in the relative abundance of UDP-sugar precursors can drive HA accumulation that promotes or exacerbates disease states, whereas HA deficiency is associated with multiple congenital developmental disorders.
UDP-Sugar Precursors Can Drive HA Production
Availability of UDP-GlcA and UDP-GlcNAc directly impacts HA levels, though cellular content of UDP-sugars varies among cell types and does not correlate with HAS1-3 transcript levels (58). Of the nucleotide sugars, UDP-GlcNAc is the most abundant found in mammalian cells (59), with concentrations reported in the range of 25 nmol/g of tissue in muscle (60) and 400 nmol/g of tissue in the liver (61). The apparent Km of purified HAS for UDP-GlcNAc is relatively high, reflecting the greater abundance of this nucleotide sugar (62). UDP-GlcA is found in ∼10%–30% of the levels of UDP-GlcNAc. However, the HAS Km for UDP-GlcA is an order of magnitude lower than that for UDP-GlcNAc (62), so the different intrinsic affinities likely ensure the equimolar incorporation of both substrates in HA synthesis.
Factors that contribute to changes in cellular availability of these nucleotide sugars include alterations in upstream precursor supply and dysregulation of precursor biosynthetic enzymes. UDP-GlcNAc, though present at high levels in the cytosol of many cells, has an important role in supporting posttranslational modification of a variety of target proteins for the enzyme O-GlcNAc Transferase (OGT) (63, 64). OGT regulates the activity of dozens of metabolic enzymes and transcription factors using UDP-GlcNAc as a substrate for O-GlcNAcylation of serine and threonine residues on its targets. HAS2 is O-GlcNAcylated at serine 221, which significantly enhances the output of HA by extending the half-life of HAS2 protein from a few minutes to a few hours (65). Interestingly, this serine is not conserved in HAS1 but the analogous residue is a threonine in HAS3, and the manipulation of OGT or OGA (the enzyme that hydrolyzes the reversible O-GlcNAc modification) was shown to enhance or reduce HA production by HAS3-expressing cells as well (66). In the case of HAS3, protein levels were not significantly affected but its residence on the plasma membrane versus within endocytic vesicles was dramatically altered by OGT or OGA manipulation. The putative O-GlcNAcylated residue occurs in a stretch of cytosolic residues flanked by extended conserved sequences and may contribute to the reported need for increased concentration of UDP-GlcNAc to support HAS1-catalyzed HA production, relative to that of HAS2 or HAS3 (58). The Km for UDP-GlcNAc reported for purified OGT is 0.5 µM (67) whereas liposome-reconstituted HAS2 and HAS3 exhibit a Km for UDP-GlcNAc ∼200- to 500-fold higher (62), so the effect of O-GlcNAc on HAS2 or HAS3 at UDP-GlcNAc levels sufficient to drive HA synthesis is clearly dependent on respective activities of OGT and OGA. In turn, the ongoing production of HA is required to relieve inhibition of GFAT1 by UDP-GlcNAc and continue flux through the HBP (68).
HA metabolism is a dynamic event that involves interactions with signaling and endocytic receptors at the plasma membrane (69). Since UDP-sugar availability directly impacts HA production it is important to consider also whether UDP-sugars can modulate HA receptor expression levels or their ability to interact with HA. Transcript levels of the HA receptor CD44 were not found to be affected by cellular depletion of UDP-GlcA (70). Both extracellular HA (internalized via binding to hyaluronidases and HA receptors) and internal UDP-sugar precursors accelerate endocytic vesicle trafficking and recycling. Enhanced endocytosis also translates to receptor modulation, since multiple cell surface receptors are concurrently internalized and differentially recycled as part of this process (66, 71). HA-dependent effects on vesicle trafficking have been shown to lead to sustained changes in distribution of cell-cell and cell-matrix adhesion receptors and increased cell motility and metastasis (71, 72).
UDP-Glc is released to the extracellular space through the constitutive Golgi secretory pathway. Many cell types express purinergic receptors such as P2Y14, which is a G-protein-coupled receptor that specifically recognizes UDP and UDP-Glc as ligands, acting via inhibitory G proteins to suppress cAMP production (13). Extracellular UDP-Glc was found to signal through P2Y14 via Stat3 phosphorylation, which increased HAS2 transcription and led to increased HA production (73). Extracellular UTP and UDP were also able to signal through a homologous receptor to increase HAS2 and HA (74). These mechanisms constitute a potential means for nucleotide sugar precursors to activate HA production in trans through cellular cross talk.
UDP-GlcA elevation.
The oxidation of UDP-Glc to UDP-GlcA is a rate-limiting step in HA production. Differential expression of UGDH at the transcript and protein level impacts extracellular HA accumulation, which has functional disease outcomes. Basal UGDH expression requires the transcription factor Sp1 (75). Numerous factors known to impact HA synthesis have been shown do so via Sp1-mediated control of UGDH levels. For example, increased expression of UGDH by factors such as TGFβ1 that stimulate Sp1-mediated transcriptional activity also lead to the accumulation of HA in orbital fibroblasts of patients with Graves disease (76). UGDH is also elevated following epithelial to mesenchymal transition (EMT) in metastatic breast cancer models, in a manner dependent on PDGF receptor β and NFκB (77).
Transcriptional regulation of UGDH can also occur through androgen and estrogen receptor-mediated activation via the respective consensus response elements in the distal UGDH promoter. In hormone-responsive tissues such as the prostate, UGDH expression is increased by the presence of hormone receptor ligands, and its upregulation stimulates UDP-GlcA production (78–80). In prostate cancer, UDP-GlcA is elevated in castration-resistant cells relative to hormone-dependent cells. Elevated availability of UDP-GlcA in castration-resistant cells corresponds to a modest increase in HA production and increased proteoglycan biosynthesis (79) and leads to therapeutic resistance (80). UGDH and HA are both found elevated in advanced prostate (81, 82), breast (83), and ovarian (84) cancers. The functional consequence of the increased HA production in breast (85) and prostate (86) tumor cells is enhanced metastasis.
UDP-GlcNAc elevation.
In breast cancer biopsies, both UDP-GlcA and UDP-GlcNAc are elevated, concurrently with strong HA accumulation, relative to normal glandular tissue (87). Increased UDP-GlcNAc availability correlates positively with expression of GFAT2 mRNA in these tissues, illustrating the potential for increases in upstream HBP precursors to drive HA production and thereby override endogenous UDP-GlcNAc suppression of HBP.
In human keratinocytes, augmenting UDP-GlcNAc production by glucosamine supplementation of the cultures, which bypasses the GFAT1-catalyzed reaction, led to a decrease in HAS2 mRNA levels by O-GlcNAcylation of Sp1, required for UGDH transcription, and YY1, both required for HAS2 transcription (88). In this case, elevated UDP-sugar pools may have activated HAS2 by O-GlcNAc modification at the plasma membrane while also triggering the downregulation of new HAS2 transcription as a compensatory mechanism to preserve HA homeostasis and limit glucose demand.
Multiple HBP enzymes are found overexpressed in castration-resistant prostate cancer patient-derived specimens and cell lines (89). Although HA was not measured specifically in these tumors, HA accumulation is predictive of prostate cancer that is likely to progress and to recur following prostatectomy, so there is a probable causative association. Importantly, in this study, the addition of UDP-GlcNAc to cells in which HBP had been knocked down promoted tumor growth, metastasis, and resistance to anti-androgen therapy. These effects were found to be mediated through changes in Sp1 binding to its target promoters, which is negatively regulated by glycosylation, and alterations in signaling through PI-3-kinase and Akt. Thus, cellular changes in global gene expression in cancer clearly involve UDP-sugars and their effects may be HA-associated.
Deficiencies in Precursor Synthesis Limit HA Production
The consequences of UDP-GlcA insufficiency for HA production in vivo have been demonstrated in model organisms and human congenital disorders. In organisms that produce HA, UGDH deletions or mutants with reduced activity reveal the unique roles of HA in development. UGDH deletion halts gastrulation in Xenopus (90) and cardiac valve formation in zebrafish, in both cases due to the loss of HA production for organ formation and cellular transformation (91). UGDH also fuels production of HA that is mechanically essential for ear development and for initiation of mammalian left-right asymmetry (92).
In general, the negative regulation of UGDH corresponds with reduced accumulation of HA and sulfated GAGs. Examples of negative regulators of UGDH include hypoxia, where a complete mechanism has not been defined but reduced Sp1 expression is observed, leading to transcriptional downregulation (75); some xenobiotics that may act as ligands for the PPARα/RXR transcriptional heterodimer through a functional suppressive element in the UGDH promoter (93, 94); and IL-1β, a proinflammatory cytokine that alters UGDH transcription through SAP/JNK signaling, leading to impaired chondrogenesis and contributing to osteoarthritis (95).
Additional mechanisms that reduce UGDH expression have been revealed by investigating congenital developmental defects linked to UGDH deficiency. Missense mutations in UGDH that interfere with quaternary assembly of the enzyme result in impaired catalytic activity and reduced stability of UGDH expression, ultimately manifesting in conditions such as cardiac valve malformation (96), global developmental delay (97), and epileptic encephalopathy (98). Loss-of-function variants of UGDH in these studies impair the binding of substrate and cofactor and/or disrupt hexameric assembly and stability of the enzyme as discussed in UDP-GlcA Synthesis. Patients show a general deficiency in HA and sulfated GAGs, particularly in specific areas of the brain, that underscore the essential demand for the UGDH product in tissue morphogenesis.
In further support of metabolite-regulated HA homeostasis, the depletion of either UDP-GlcA or UDP-GlcNAc was shown to induce endocytosis of HAS3 from the plasma membrane, which inhibited HA synthesis (66). Because of the critical nature of UDP-GlcNAc in multiple nonredundant processes, as seen in the case of UGDH, most of the enzymes of the HBP branch do not have homozygous deleterious mutations identified with disease states. Interestingly, although essential as a precursor, feedback regulation by UDP-GlcNAc is a two-edged sword. The recently published structure of GFAT1 demonstrates the location of the UDP-GlcNAc suppression site and provides insight to the underlying cause of a gain of function mutation found in Caenorhabditis elegans (41). The mutation eliminates UDP-GlcNAc inhibition and elevates UDP-GlcNAc production, which actually improves protein homeostasis, extends lifespan, and elevates tolerance of tunicamycin-induced ER stress.
GENETIC AND PHARMACOLOGICAL CONTROL OF HA PRODUCTION THROUGH PRECURSOR MANIPULATION
The manipulation of gatekeeper enzymes in the pathways controlling UDP-sugar precursors of HA has provided insights and validation for the critical roles of the enzymes and the HA product in health and disease. Here we will focus on the studies that have defined the gatekeepers and elucidated the roles of HA.
UDP-GlcA Manipulation
Elevation of UDP-GlcA.
Several studies have used ectopic overexpression of UGDH to formally demonstrate the link between UGDH expression, UDP-GlcA production, and HA output, showing that UGDH activity correlates strongly with capacity for HA production in cells that express one or more of the HAS isozymes. Eukaryotic UGDH is well conserved among multiple species and in fact, Xenopus UGDH was found to functionally augment contribution of its human ortholog in vascular smooth muscle cells, where its expression fueled the excess production of HA (90). In primary articular surface cells from joint tissue, the overexpression of UGDH supported large increases in the production of both HA and sulfated GAGs in a manner that was dependent on MEK-ERK signaling, augmented pericellular HA matrices retained by the cells, and promoted chondrogenesis (99). The coexpression of UGDH with HAS3 in HEK293 cells produced multifold increases in HA production, and the ability of UGDH to promote HA production was found to depend on its dynamic hexameric association, which allowed the favorable cooperative interactions conferred by the binding of substrate and cofactor (28). Neither obligate dimeric nor obligate hexameric point mutants of UGDH were capable of supporting HA production in this study. Importantly, although UGDH elevation can promote HA production, the robust activity of HAS is also necessary, as in the above study. This was also shown using prostate tumor cells, where HAS3 expression dramatically elevated HA production and pericellular retention of HA in cells (100) whereas hormone-stimulated elevation of UGDH expression did not affect HAS levels and had only modest effects on HA output (78). It is also noteworthy that reduced precursor levels correspond to reduced overall HA polymer output, and are not associated with production of smaller sizes of HA (e.g., <105 Da), suggesting that HA polymers are not released from the HAS enzymes until a critical threshold size is reached. This is probably due to endocytosis of stalled complexes.
Depletion of UDP-GlcA.
In addition to studies of elevated HA biosynthesis, there are many examples of attempts at targeted disruption of UGDH, GFAT1, and other HBP enzymes that illustrate lethality of homozygous deletion of the pathways for UDP sugar production. Numerous pathologies and studies in model organisms revealed the interrelatedness of control mechanisms affecting HA production, which is critical for development. Use of transient, inducible or knockdown approaches, and/or pharmacological manipulation of UDP-GlcA has been successful in revealing the rapid and dynamic impact of UDP-GlcA depletion on cellular processes.
Genetic knockdown of UGDH in aortic smooth muscle cells clearly reveals the link between precursor availability and HA synthesis, where HA quantity is selectively reduced whereas GAG synthesis remains largely unaffected (90). In prostate tumor cells, UGDH knockdown diminishes cellular availability of UDP-GlcA, which leads to significant diminution of both HA production and proteoglycan biosynthesis, and results in reduced intrinsic growth as well as resensitization of therapeutically resistant cells to growth suppression by the anti-androgen enzalutamide (80). In triple-negative breast cancer cells, knockdown of UGDH decreased cellular UDP-GlcA, lowering HA production and resulting in loss of 3-dimensional proliferation and cell invasion potential (85). In this study, the authors suggested that metabolic reprogramming to support EMT may require a shift to increased glucose metabolism to facilitate remodeling of the extracellular matrix via elevated HA production. In contrast, another study found that knocking down UGDH also reduced the migration of lung adenocarcinoma cells, but feeding back UDP-GlcA and HA was insufficient to recover this effect (12), suggesting it is not dependent on the UGDH product. Such apparently contrasting results may be partially reconciled by the vesicular trafficking of HAS, as discussed above in UDP-Sugar Precursors Can Drive HA Production.
Inhibition of HA synthesis can be achieved through pharmacological manipulation of either UDP-GlcA or UDP-GlcNAc. The most widely used pharmacological agent for inhibition of HA synthesis is 4-methylumbelliferone (4MU, also called hymecromone), which scavenges UDP-GlcA through glucuronidation, a critical Phase II detoxification process. The use of this chemical scavenger results in severe depletion of cellular UDP-GlcA through the action of endogenous UDP-glucuronosyltransferase enzymes, which use 4MU and UDP-GlcA as substrates to produce 4MU-glucuronide and UDP. The 4MU-glucuronide is excreted from the cell, driving the continued loss of UDP-GlcA through this pathway and leaving significantly less available for HA production.
More than 100 studies published in the past decade using multiple systems have empirically demonstrated the relatively selective effect of this agent on inhibition of HA synthesis. As it has demonstrated low toxicity, use of 4MU to reduce HA accumulation has clinical promise, and its efficacy in relieving complications of vascular pathologies, autoimmune disease, and aggressive progression or therapeutic resistance of solid tumors, has been discussed in recent reviews (101, 102). In vascular pathologies, HA plays a critical role in the initiation of vessel wall thickening. In aortic smooth muscle cells, 4MU treatment was shown to reduce HA synthesis through depletion of UDP-GlcA and by downregulation of transcripts encoding HAS 1–3 and UGDH, which further indicates that nucleotide sugar abundance can impact HA not only through substrate availability but also by affecting gene expression (70). Although 4MU specifically depletes cellular pools of UDP-GlcA, 4MU treatment modestly decreases the availability of UDP-GlcNAc indicating that nucleotide sugars may act as cellular sensors in the transcriptional regulation of precursor and biosynthetic enzyme genes (70, 103). The metabolized form of 4MU, 4-MU glucuronide, has also recently been shown to inhibit HA synthesis in mice demonstrating both a direct and indirect inhibition of HA synthesis (104).
UDP-GlcNAc and HBP Manipulation
Expression of HBP enzymes impacting HA production through provision of UDP-GlcNAc has been found to be reciprocally regulated by changes in the relative abundance of UDP-GlcA or UDP-GlcNAc. Knockdown of the rate-limiting enzyme of the HBP, GFAT1, depletes cellular pools of UDP-GlcNAc, which results in loss of pericellular HA coat in cultured cells and an upregulation of HAS2 mRNA (105). The significant elevation in HAS2 transcript was attributed to cellular compensation to preserve HA homeostasis during precursor flux (105). Conversely, siRNA targeting of glucosamine-6-phosphate deaminase-1 (GNPDA1), which catalyzes the reverse of the rate-limiting step, induces GFAT2 mRNA expression and increases the size of the pericellular HA coat.
UDP-GlcNAc can be modulated by treatment with d-mannose. In cultured epidermal keratinocytes, this resulted in a dose-dependent decrease in UDP-GlcNAc and inhibition of HA synthesis (103). Interestingly, d-mannose treatment did not downregulate HAS1-3 mRNA as has been reported with 4-MU treatment (70, 103). Pharmacological targeting of either UDP-GlcNAc or UDP-GlcA results in HA synthesis inhibition, but cotreatment with mannose and 4-MU does not have an additive effect indicating that either precursor can be rate-limiting and modulate HA synthesis (103). Treatment with mannose coupled with selective targeting of genes involved in the HBP does synergize to inhibit HA synthesis, likely due to additional significant reduction in UDP-GlcNAc availability (105).
Excess UDP-GlcNAc availability as a result of HBP enzyme overexpression has been implicated in recent studies of heart disease and cancer, in which both its impact on cellular carbohydrate metabolism and on HA production are important. Chronically high expression of GFAT1 was reported in heart disease patients with cardiac dysfunction, hypertrophy, and fibrosis (106). Using cultured cardiomyocytes, the authors found that use of an α1-adrenergic receptor agonist to stimulate proliferation of the cells led to significant elevation of the enzymes GFAT1, GNPNAT1, PGM3 and GALE, all of which contribute to UDP-GlcNAc synthesis when elevated. This metabolic reprogramming resulted from activation of the unfolded protein response, which triggered HBP gene transcription by the XBP1 transcription factor. Cardiac-specific GFAT1 knockout, or treatment with diazo-norleucine (DON, an amidotransferase inhibitor) to block GFAT1 activity, could relieve pressure-induced cardiac dysfunction in mice (106). The effect of HBP elevation was dependent on the master energy sensor mTORC1 and although HA was not measured in this study, the critical contributions of HA to cardiac function may be partially explained by these results.
The combined overexpression of GFAT1 and HAS2 was examined in breast cancer patients and indicated poor prognosis (107). Proteomic analysis of cancerous tissue revealed modest but significant increases in multiple HBP genes including GFAT1, GNPNAT1, PGM3, and UAP1, which correlated with reduced survival. The analysis further revealed a global elevation in O-GlcNAc modification of proteins. The authors found HBP gene expression comparably elevated in a mouse model of breast cancer, in conjunction with excess HA accumulation. Targeting GFAT1 with DON in cells cultured from this model resulted in decreased production of HA that could be rescued with glucosamine to bypass inhibition of GFAT. In the rescued cells, HAS2 was elevated, produced more HA, and led to expansion of cancer stem cells (characterized by biomarkers as CD44hi/CD24lo).
Three additional studies validate and expand on these profound disease-promoting impacts of elevated HBP and HA synthesis. In patient-derived xenograft tissue from pancreatic cancer patients, HBP enzymes were elevated, leading the authors to test the effect of targeting PGM3 (108). The overexpression of PGM3 led pancreatic tumors to become resistant to gemcitabine, a first-line therapy for pancreatic cancer, of which efficacy is known to be reduced by tumor HA content. Either genetic or pharmacological inhibition of PGM3, using a novel inhibitor (FR054), could reduce aggressive phenotype and tumorigenic potential of the cells and partially restore sensitivity to gemcitabine, suggesting a novel method to reduce tumor HA and improve treatment outcomes. A similar approach was taken using prostate cancer models, in which UAP1 was targeted (109). In this case, the increase in UAP1 was associated with resistance to anti-androgen therapy (termed castration resistance) and resulted in 10-fold higher UDP-GlcNAc. HA was not measured, but targeting UAP1 resulted in sensitizing cells to ER stress inducers, consistent with the heightened activity of the unfolded protein response. Lastly, a recent study found multiple HBP enzymes elevated in castration-resistant prostate cancer and tested the role of GNPNAT1 (89). Knockdown of GNPNAT1 expression also reduced GFAT1 expression, and as expected, UDP-GlcNAc was reduced. Interestingly, the net effect was an increase in the proliferation and tumorigenic potential of these cells, which was reversed by adding back UDP-GlcNAc. This study found that the impact of HBP reduction in castration-resistant cells resulted from diminished glycosyl-inhibition of Sp1 and expression of its transcriptional target genes and that targeting GNPNAT1 restored sensitivity to enzalutamide.
SUMMARY
The intricacies of HA regulation have become increasingly well characterized with the emergence of structures for key branch point enzymes determining flux through precursor synthesis bottlenecks and the mechanistic characterization of their endogenous regulators. Coupled with elegant integrative reports that examine global data sets resulting from aberrancies in HA metabolism or nucleotide sugar precursor synthesis pathways, in conjunction with mechanistic whole organism models, numerous advances in understanding how HA homeostasis is supported by energy status at the molecular level have been possible. The direct implication of altered HBP and UGDH activities in HA accumulation highlights the components of these pathways as obvious targets for innovative therapeutic options in the treatment of HA-mediated pathologies. The molecular visualization of structural details of the enzymes and conformational shifts that accompany their allosteric response to endogenous and pharmacological modulators provide an extensive array of new targets that have functional validation. These tools and insights to the role of UDP sugars as liaisons between metabolic energy status and extracellular HA production highlight novel ways that these pathways can be targeted to increase specificity of therapies directed against such universally critical processes.
GRANTS
This work was supported by National Cancer Institute Grant R21 CA185993 (to J. J. Barycki and M. A. Simpson).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
This article is part of the special collection “Deciphering the Role of Proteoglycans and Glycosaminoglycans in Health and Disease.” Liliana Schaefer, MD, served as Guest Editor of this collection.
AUTHOR CONTRIBUTIONS
B.M.Z., J.J.B., and M.A.S. prepared figures; B.M.Z., J.J.B., and M.A.S. drafted manuscript; B.M.Z., J.J.B., and M.A.S. edited and revised manuscript; B.M.Z., J.J.B., and M.A.S. approved final version of manuscript.
REFERENCES
- 1.Fischer JW. Role of hyaluronan in atherosclerosis: current knowledge and open questions. Matrix Biol 78–79: 324–336, 2019. doi: 10.1016/j.matbio.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Grandoch M, Bollyky PL, Fischer JW. Hyaluronan: a master switch between vascular homeostasis and inflammation. Circ Res 122: 1341–1343, 2018. doi: 10.1161/CIRCRESAHA.118.312522. [DOI] [PubMed] [Google Scholar]
- 3.Tammi MI, Oikari S, Pasonen-Seppänen S, Rilla K, Auvinen P, Tammi RH. Activated hyaluronan metabolism in the tumor matrix—causes and consequences. Matrix Biol 78-79: 147–164, 2019. doi: 10.1016/j.matbio.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 4.Rilla K, Siiskonen H, Tammi M, Tammi R. Hyaluronan-coated extracellular vesicles–a novel link between hyaluronan and cancer. Adv Cancer Res 123: 121–148, 2014. doi: 10.1016/B978-0-12-800092-2.00005-8. [DOI] [PubMed] [Google Scholar]
- 5.Tavianatou AG, Caon I, Franchi M, Piperigkou Z, Galesso D, Karamanos NK. Hyaluronan: molecular size-dependent signaling and biological functions in inflammation and cancer. FEBS J 286: 2883–2908, 2019. doi: 10.1111/febs.14777. [DOI] [PubMed] [Google Scholar]
- 6.Caon I, Parnigoni A, Viola M, Karousou E, Passi A, Vigetti D. Cell energy metabolism and hyaluronan synthesis. J Histochem Cytochem 69: 35–47, 2020. doi: 10.1369/0022155420929772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zimmer BM, Barycki JJ, Simpson MA. Integration of sugar metabolism and proteoglycan synthesis by UDP-glucose dehydrogenase. J Histochem Cytochem 69: 13–23, 2021. doi: 10.1369/0022155420947500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu L, Hirschberg CB. Developmental diseases caused by impaired nucleotide sugar transporters. Glycoconj J 30: 5–10, 2013. doi: 10.1007/s10719-012-9375-4. [DOI] [PubMed] [Google Scholar]
- 9.Freeze HH, Hart GW, Schnaar RL. Glycosylation precursors. In: Essentials of Glycobiology, edited by Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Darvill AG, Kinoshita T, Packer NH, Prestegard JH, Schnaar RL, Seeberger PH.. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 2015, p. 51–63. [Google Scholar]
- 10.Ishibashi Y, Kohyama-Koganeya A, Hirabayashi Y. New insights on glucosylated lipids: metabolism and functions. Biochim Biophys Acta 1831: 1475–1485, 2013. doi: 10.1016/j.bbalip.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Tannous A, Pisoni GB, Hebert DN, Molinari M. N-linked sugar-regulated protein folding and quality control in the ER. Semin Cell Dev Biol 41: 79–89, 2015. doi: 10.1016/j.semcdb.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Liu R, Zhu W, Chu H, Yu H, Wei P, Wu X, Zhu H, Gao H, Liang J, Li G, Yang W. UDP-glucose accelerates SNAI1 mRNA decay and impairs lung cancer metastasis. Nature 571: 127–131, 2019. doi: 10.1038/s41586-019-1340-y. [DOI] [PubMed] [Google Scholar]
- 13.Lazarowski ER, Harden TK. UDP-sugars as extracellular signaling molecules: cellular and physiologic consequences of P2Y14 receptor activation. Mol Pharmacol 88: 151–160, 2015. doi: 10.1124/mol.115.098756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindahl U, Couchman J, Kimata K, Esko JD. Proteoglycans and sulfated glycosaminoglycans. In: Essentials of Glycobiology, edited by Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Darvill AG, Kinoshita T, Packer NH, Prestegard JH, Schnaar RL, Seeberger PH.. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 2015, p. 207–221. [Google Scholar]
- 15.Kornfeld R. Studies on L-glutamine D-fructose 6-phosphate amidotransferase. I. Feedback inhibition by uridine diphosphate-N-acetylglucosamine. J Biol Chem 242: 3135–3141, 1967. [PubMed] [Google Scholar]
- 16.Neufeld EF, Hall CW. Inhibition of Udp-D-glucose dehydrogenase by Udp-d-xylose: a possible regulatory mechanism. Biochem Biophys Res Commun 19: 456–461, 1965. doi: 10.1016/0006-291x(65)90146-4. [DOI] [PubMed] [Google Scholar]
- 17.Marshall S, Bacote V, Traxinger RR. Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system. Role of hexosamine biosynthesis in the induction of insulin resistance. J Biol Chem 266: 4706–4712, 1991. [PubMed] [Google Scholar]
- 18.Beamer LJ. Enzyme dysfunction at atomic resolution: disease-associated variants of human phosphoglucomutase-1. Biochimie 183: 44–48, 2021. doi: 10.1016/j.biochi.2020.08.017. [DOI] [PubMed] [Google Scholar]
- 19.Lee Y, Stiers KM, Kain BN, Beamer LJ. Compromised catalysis and potential folding defects in in vitro studies of missense mutants associated with hereditary phosphoglucomutase 1 deficiency. J Biol Chem 289: 32010–32019, 2014. doi: 10.1074/jbc.M114.597914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thoden JB, Holden HM. The molecular architecture of glucose-1-phosphate uridylyltransferase. Protein Sci 16: 432–440, 2007. doi: 10.1110/ps.062626007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu Q, Zheng X. The crystal structure of human UDP-glucose pyrophosphorylase reveals a latch effect that influences enzymatic activity. Biochem J 442: 283–291, 2012. doi: 10.1042/BJ20111598. [DOI] [PubMed] [Google Scholar]
- 22.Führing J, Damerow S, Fedorov R, Schneider J, Münster-Kühnel AK, Gerardy-Schahn R. Octamerization is essential for enzymatic function of human UDP-glucose pyrophosphorylase. Glycobiology 23: 426–437, 2013. doi: 10.1093/glycob/cws217. [DOI] [PubMed] [Google Scholar]
- 23.Führing JI, Cramer JT, Schneider J, Baruch P, Gerardy-Schahn R, Fedorov R. A quaternary mechanism enables the complex biological functions of octameric human UDP-glucose pyrophosphorylase, a key enzyme in cell metabolism. Sci Rep 5: 9618, 2015. doi: 10.1038/srep09618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen J, Yang S. Catalytic mechanism of UDP-glucose dehydrogenase. Biochem Soc Trans 47: 945–955, 2019. doi: 10.1042/BST20190257. [DOI] [PubMed] [Google Scholar]
- 25.Egger S, Chaikuad A, Kavanagh KL, Oppermann U, Nidetzky B. UDP-glucose dehydrogenase: structure and function of a potential drug target. Biochem Soc Trans 38: 1378–1385, 2010. doi: 10.1042/BST0381378. [DOI] [PubMed] [Google Scholar]
- 26.Egger S, Chaikuad A, Kavanagh KL, Oppermann U, Nidetzky B. Structure and mechanism of human UDP-glucose 6-dehydrogenase. J Biol Chem 286: 23877–23887, 2011. doi: 10.1074/jbc.M111.234682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kadirvelraj R, Sennett NC, Polizzi SJ, Weitzel S, Wood ZA. Role of packing defects in the evolution of allostery and induced fit in human UDP-glucose dehydrogenase. Biochemistry 50: 5780–5789, 2011. doi: 10.1021/bi2005637. [DOI] [PubMed] [Google Scholar]
- 28.Hyde AS, Thelen AM, Barycki JJ, Simpson MA. UDP-glucose dehydrogenase activity and optimal downstream cellular function require dynamic reorganization at the dimer-dimer subunit interfaces. J Biol Chem 288: 35049–35057, 2013. doi: 10.1074/jbc.M113.519090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sennett NC, Kadirvelraj R, Wood ZA. Cofactor binding triggers a molecular switch to allosterically activate human UDP-α-D-glucose 6-dehydrogenase. Biochemistry 51: 9364–9374, 2012. doi: 10.1021/bi301067w. [DOI] [PubMed] [Google Scholar]
- 30.Kadirvelraj R, Custer GS, Keul ND, Sennett NC, Sidlo AM, Walsh RM Jr, Wood ZA. Hysteresis in human UDP-glucose dehydrogenase is due to a restrained hexameric structure that favors feedback inhibition. Biochemistry 53: 8043–8051, 2014. doi: 10.1021/bi500594x. [DOI] [PubMed] [Google Scholar]
- 31.Grady G, Thelen A, Albers J, Ju T, Guo J, Barycki JJ, Simpson MA. Inhibiting hexamer disassembly of human UDP-glucose dehydrogenase by photoactivated amino acid cross-linking. Biochemistry 55: 3157–3164, 2016. doi: 10.1021/acs.biochem.6b00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zimmer BM, Barycki JJ, Simpson MA. Integration of sugar metabolism and proteoglycan synthesis by UDP-glucose dehydrogenase. J Histochem Cytochem 69: 13–23, 2020. doi: 10.1369/0022155420947500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Easley KE, Sommer BJ, Boanca G, Barycki JJ, Simpson MA. Characterization of human UDP-glucose dehydrogenase reveals critical catalytic roles for lysine 220 and aspartate 280. Biochemistry 46: 369–378, 2007. doi: 10.1021/bi061537d. [DOI] [PubMed] [Google Scholar]
- 34.Egger S, Chaikuad A, Klimacek M, Kavanagh KL, Oppermann U, Nidetzky B. Structural and kinetic evidence that catalytic reaction of human UDP-glucose 6-dehydrogenase involves covalent thiohemiacetal and thioester enzyme intermediates. J Biol Chem 287: 2119–2129, 2012. doi: 10.1074/jbc.M111.313015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kadirvelraj R, Sennett NC, Custer GS, Phillips RS, Wood ZA. Hysteresis and negative cooperativity in human UDP-glucose dehydrogenase. Biochemistry 52: 1456–1465, 2013. [Erratum in Biochemistry 54: 629, 2015]. doi: 10.1021/bi301593c. [DOI] [PubMed] [Google Scholar]
- 36.Keul ND, Oruganty K, Schaper Bergman ET, Beattie NR, McDonald WE, Kadirvelraj R, Gross ML, Phillips RS, Harvey SC, Wood ZA. The entropic force generated by intrinsically disordered segments tunes protein function. Nature 563: 584–588, 2018. doi: 10.1038/s41586-018-0699-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sennett NC, Kadirvelraj R, Wood ZA. Conformational flexibility in the allosteric regulation of human UDP-α-d-glucose 6-dehydrogenase. Biochemistry 50: 9651–9663, 2011. doi: 10.1021/bi201381e. [DOI] [PubMed] [Google Scholar]
- 38.Sommer BJ, Barycki JJ, Simpson MA. Characterization of human UDP-glucose dehydrogenase. CYS-276 is required for the second of two successive oxidations. J Biol Chem 279: 23590–23596, 2004. doi: 10.1074/jbc.M401928200. [DOI] [PubMed] [Google Scholar]
- 39.Hagiuda D, Nagashio R, Ichinoe M, Tsuchiya B, Igawa S, Naoki K, Satoh Y, Murakumo Y, Saegusa M, Sato Y. Clinicopathological and prognostic significance of nuclear UGDH localization in lung adenocarcinoma. Biomed Res 40: 17–27, 2019. doi: 10.2220/biomedres.40.17. [DOI] [PubMed] [Google Scholar]
- 40.Broschat KO, Gorka C, Page JD, Martin-Berger CL, Davies MS, Huang Hc HC, Gulve EA, Salsgiver WJ, Kasten TP. Kinetic characterization of human glutamine-fructose-6-phosphate amidotransferase I: potent feedback inhibition by glucosamine 6-phosphate. J Biol Chem 277: 14764–14770, 2002. doi: 10.1074/jbc.M201056200. [DOI] [PubMed] [Google Scholar]
- 41.Ruegenberg S, Horn M, Pichlo C, Allmeroth K, Baumann U, Denzel MS. Loss of GFAT-1 feedback regulation activates the hexosamine pathway that modulates protein homeostasis. Nat Commun 11: 687, 2020. doi: 10.1038/s41467-020-14524-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oki T, Yamazaki K, Kuromitsu J, Okada M, Tanaka I. cDNA cloning and mapping of a novel subtype of glutamine:fructose-6-phosphate amidotransferase (GFAT2) in human and mouse. Genomics 57: 227–234, 1999. doi: 10.1006/geno.1999.5785. [DOI] [PubMed] [Google Scholar]
- 43.Zhou J, Neidigh JL, Espinosa R, LeBeau MM, McClain DA. Human glutamine: fructose-6-phosphate amidotransferase: characterization of mRNA and chromosomal assignment to 2p13. Hum Genet 96: 99–101, 1995. doi: 10.1007/BF00214194. [DOI] [PubMed] [Google Scholar]
- 44.Mouilleron S, Badet-Denisot MA, Golinelli-Pimpaneau B. Ordering of C-terminal loop and glutaminase domains of glucosamine-6-phosphate synthase promotes sugar ring opening and formation of the ammonia channel. J Mol Biol 377: 1174–1185, 2008. doi: 10.1016/j.jmb.2008.01.077. [DOI] [PubMed] [Google Scholar]
- 45.Isupov MN, Obmolova G, Butterworth S, Badet-Denisot MA, Badet B, Polikarpov I, Littlechild JA, Teplyakov A. Substrate binding is required for assembly of the active conformation of the catalytic site in Ntn amidotransferases: evidence from the 1.8 A crystal structure of the glutaminase domain of glucosamine 6-phosphate synthase. Structure 4: 801–810, 1996. [Erratum in Structure 5: 723, 1997]. doi: 10.1016/s0969-2126(96)00087-1. [DOI] [PubMed] [Google Scholar]
- 46.Ruegenberg S, Mayr F, Atanassov I, Baumann U, Denzel MS. Protein kinase A controls the hexosamine pathway by tuning the feedback inhibition of GFAT-1. Nat Commun 12: 2176, 2021. doi: 10.1038/s41467-021-22320-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brannigan JA, Dodson G, Duggleby HJ, Moody PC, Smith JL, Tomchick DR, Murzin AG. A protein catalytic framework with an N-terminal nucleophile is capable of self-activation. Nature 378: 416–419, 1995. [Erratum in Nature 378: 644, 1995]. doi: 10.1038/378416a0. [DOI] [PubMed] [Google Scholar]
- 48.Chang Q, Su K, Baker JR, Yang X, Paterson AJ, Kudlow JE. Phosphorylation of human glutamine:fructose-6-phosphate amidotransferase by cAMP-dependent protein kinase at serine 205 blocks the enzyme activity. J Biol Chem 275: 21981–21987, 2000. doi: 10.1074/jbc.M001049200. [DOI] [PubMed] [Google Scholar]
- 49.Moloughney JG, Vega-Cotto NM, Liu S, Patel C, Kim PK, Wu CC, Albaciete D, Magaway C, Chang A, Rajput S, Su X, Werlen G, Jacinto E. mTORC2 modulates the amplitude and duration of GFAT1 Ser-243 phosphorylation to maintain flux through the hexosamine pathway during starvation. J Biol Chem 293: 16464–16478, 2018. doi: 10.1074/jbc.RA118.003991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Y, Roux C, Lazereg S, LeCaer JP, Laprévote O, Badet B, Badet-Denisot MA. Identification of a novel serine phosphorylation site in human glutamine:fructose-6-phosphate amidotransferase isoform 1. Biochemistry 46: 13163–13169, 2007. doi: 10.1021/bi700694c. [DOI] [PubMed] [Google Scholar]
- 51.Kroef V, Ruegenberg S, Horn M, Allmeroth K, Ebert L, Bozkus S, Miethe S, Elling U, Schermer B, Baumann U, Denzel MS. GFPT2/GFAT2 and AMDHD2 act in tandem to control the hexosamine pathway. eLife 11: e69223, 2022. doi: 10.7554/eLife.69223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang J, Liu X, Liang YH, Li LF, Su XD. Acceptor substrate binding revealed by crystal structure of human glucosamine-6-phosphate N-acetyltransferase 1. FEBS Lett 582: 2973–2978, 2008. doi: 10.1016/j.febslet.2008.07.040. [DOI] [PubMed] [Google Scholar]
- 53.Vetting MW, S de Carvalho LP, Yu M, Hegde SS, Magnet S, Roderick SL, Blanchard JS. Structure and functions of the GNAT superfamily of acetyltransferases. Arch Biochem Biophys 433: 212–226, 2005. doi: 10.1016/j.abb.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 54.Nishitani Y, Maruyama D, Nonaka T, Kita A, Fukami TA, Mio T, Yamada-Okabe H, Yamada-Okabe T, Miki K. Crystal structures of N-acetylglucosamine-phosphate mutase, a member of the α-D-phosphohexomutase superfamily, and its substrate and product complexes. J Biol Chem 281: 19740–19747, 2006. doi: 10.1074/jbc.M600801200. [DOI] [PubMed] [Google Scholar]
- 55.Raimi OG, Hurtado-Guerrero R, van Aalten DMF. Evidence for substrate-assisted catalysis in N-acetylphosphoglucosamine mutase. Biochem J 475: 2547–2557, 2018. doi: 10.1042/BCJ20180172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peneff C, Ferrari P, Charrier V, Taburet Y, Monnier C, Zamboni V, Winter J, Harnois M, Fassy F, Bourne Y. Crystal structures of two human pyrophosphorylase isoforms in complexes with UDPGlc(Gal)NAc: role of the alternatively spliced insert in the enzyme oligomeric assembly and active site architecture. EMBO J 20: 6191–6202, 2001. doi: 10.1093/emboj/20.22.6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raimi OG, Hurtado-Guerrero R, Borodkin V, Ferenbach A, Urbaniak MD, Ferguson MAJ, van Aalten DMF. A mechanism-inspired UDP-N-acetylglucosamine pyrophosphorylase inhibitor. RSC Chem Biol 1: 13–25, 2020. doi: 10.1039/c9cb00017h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rilla K, Oikari S, Jokela TA, Hyttinen JM, Karna R, Tammi RH, Tammi MI. Hyaluronan synthase 1 (HAS1) requires higher cellular UDP-GlcNAc concentration than HAS2 and HAS3. J Biol Chem 288: 5973–5983, 2013. doi: 10.1074/jbc.M112.443879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oikari S, Venäläinen T, Tammi M. Borate-aided anion exchange high-performance liquid chromatography of uridine diphosphate-sugars in brain, heart, adipose and liver tissues. J Chromatogr A 1323: 82–86, 2014. doi: 10.1016/j.chroma.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 60.Hawkins M, Barzilai N, Liu R, Hu M, Chen W, Rossetti L. Role of the glucosamine pathway in fat-induced insulin resistance. J Clin Invest 99: 2173–2182, 1997. doi: 10.1172/JCI119390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Renner AB, Rieger K, Grunow D, Zimmermann-Kordmann M, Gohlke M, Reutter W. Liver-specific increase of UTP and UDP-sugar concentrations in rats induced by dietary vitamin B6-deficiency and its relation to complex N-glycan structures of liver membrane-proteins. Glycoconj J 24: 531–541, 2007. doi: 10.1007/s10719-007-9048-x. [DOI] [PubMed] [Google Scholar]
- 62.Itano N, Sawai T, Yoshida M, Lenas P, Yamada Y, Imagawa M, Shinomura T, Hamaguchi M, Yoshida Y, Ohnuki Y, Miyauchi S, Spicer AP, McDonald JA, Kimata K. Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J Biol Chem 274: 25085–25092, 1999. doi: 10.1074/jbc.274.35.25085. [DOI] [PubMed] [Google Scholar]
- 63.Hart GW. Nutrient regulation of signaling and transcription. J Biol Chem 294: 2211–2231, 2019. doi: 10.1074/jbc.AW119.003226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zachara N, Akimoto Y, Hart GW. The O-GlcNAc Modification. In: Essentials of Glycobiology, edited by Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Darvill AG, Kinoshita T, Packer NH, Prestegard JH, Schnaar RL, Seeberger PH.. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 2015, p. 239–251. [Google Scholar]
- 65.Vigetti D, Deleonibus S, Moretto P, Karousou E, Viola M, Bartolini B, Hascall VC, Tammi M, De Luca G, Passi A. Role of UDP-N-acetylglucosamine (GlcNAc) and O-GlcNAcylation of hyaluronan synthase 2 in the control of chondroitin sulfate and hyaluronan synthesis. J Biol Chem 287: 35544–35555, 2012. doi: 10.1074/jbc.M112.402347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Deen AJ, Arasu UT, Pasonen-Seppänen S, Hassinen A, Takabe P, Wojciechowski S, Kärnä R, Rilla K, Kellokumpu S, Tammi R, Tammi M, Oikari S. UDP-sugar substrates of HAS3 regulate its O-GlcNAcylation, intracellular traffic, extracellular shedding and correlate with melanoma progression. Cell Mol Life Sci 73: 3183–3204, 2016. doi: 10.1007/s00018-016-2158-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lubas WA, Hanover JA. Functional expression of O-linked GlcNAc transferase. Domain structure and substrate specificity. J Biol Chem 275: 10983–10988, 2000. doi: 10.1074/jbc.275.15.10983. [DOI] [PubMed] [Google Scholar]
- 68.Hascall VC, Wang A, Tammi M, Oikari S, Tammi R, Passi A, Vigetti D, Hanson RW, Hart GW. The dynamic metabolism of hyaluronan regulates the cytosolic concentration of UDP-GlcNAc. Matrix Biol 35: 14–17, 2014. doi: 10.1016/j.matbio.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Skandalis SS, Karalis TT, Chatzopoulos A, Karamanos NK. Hyaluronan-CD44 axis orchestrates cancer stem cell functions. Cell Signal 63: 109377, 2019. doi: 10.1016/j.cellsig.2019.109377. [DOI] [PubMed] [Google Scholar]
- 70.Vigetti D, Rizzi M, Viola M, Karousou E, Genasetti A, Clerici M, Bartolini B, Hascall VC, De Luca G, Passi A. The effects of 4-methylumbelliferone on hyaluronan synthesis, MMP2 activity, proliferation, and motility of human aortic smooth muscle cells. Glycobiology 19: 537–546, 2009. doi: 10.1093/glycob/cwp022. [DOI] [PubMed] [Google Scholar]
- 71.McAtee CO, Berkebile AR, Elowsky CG, Fangman T, Barycki JJ, Wahl JK 3rd, Khalimonchuk O, Naslavsky N, Caplan S, Simpson MA. Hyaluronidase Hyal1 increases tumor cell proliferation and motility through accelerated vesicle trafficking. J Biol Chem 290: 13144–13156, 2015. doi: 10.1074/jbc.M115.647446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McAtee CO, Booth C, Elowsky C, Zhao L, Payne J, Fangman T, Caplan S, Henry MD, Simpson MA. Prostate tumor cell exosomes containing hyaluronidase Hyal1 stimulate prostate stromal cell motility by engagement of FAK-mediated integrin signaling. Matrix Biol 78-79: 165–179, 2019. doi: 10.1016/j.matbio.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jokela TA, Kärnä R, Makkonen KM, Laitinen JT, Tammi RH, Tammi MI. Extracellular UDP-glucose activates P2Y14 receptor and induces signal transducer and activator of transcription 3 (STAT3) Tyr705 phosphorylation and binding to hyaluronan synthase 2 (HAS2) promoter, stimulating hyaluronan synthesis of keratinocytes. J Biol Chem 289: 18569–18581, 2014. doi: 10.1074/jbc.M114.551804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jokela T, Kärnä R, Rauhala L, Bart G, Pasonen-Seppänen S, Oikari S, Tammi MI, Tammi RH. Human keratinocytes respond to extracellular UTP by induction of hyaluronan synthase 2 expression and increased hyaluronan synthesis. J Biol Chem 292: 4861–4872, 2017. doi: 10.1074/jbc.M116.760322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bontemps Y, Vuillermoz B, Antonicelli F, Perreau C, Danan JL, Maquart FX, Wegrowski Y. Specific protein-1 is a universal regulator of UDP-glucose dehydrogenase expression: its positive involvement in transforming growth factor-beta signaling and inhibition in hypoxia. J Biol Chem 278: 21566–21575, 2003. doi: 10.1074/jbc.M209366200. [DOI] [PubMed] [Google Scholar]
- 76.Tsui S, Fernando R, Chen B, Smith TJ. Divergent Sp1 protein levels may underlie differential expression of UDP-glucose dehydrogenase by fibroblasts: role in susceptibility to orbital Graves disease. J Biol Chem 286: 24487–24499, 2011. doi: 10.1074/jbc.M111.241166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang Q, Karvelsson ST, Johannsson F, Vilhjalmsson AI, Hagen L, de Miranda Fonseca D, Sharma A, Slupphaug G, Rolfsson O. UDP-glucose dehydrogenase expression is upregulated following EMT and differentially affects intracellular glycerophosphocholine and acetylaspartate levels in breast mesenchymal cell lines. Mol Oncol 16: 1816–1840, 2021. doi: 10.1002/1878-0261.13172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wei Q, Galbenus R, Raza A, Cerny RL, Simpson MA. Androgen-stimulated UDP-glucose dehydrogenase expression limits prostate androgen availability without impacting hyaluronan levels. Cancer Res 69: 2332–2339, 2009. doi: 10.1158/0008-5472.CAN-08-3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zimmer BM, Howell ME, Wei Q, Ma L, Romsdahl T, Loughman EG, Markham JE, Seravalli J, Barycki JJ, Simpson MA. Loss of exogenous androgen dependence by prostate tumor cells is associated with elevated glucuronidation potential. Horm Cancer 7: 260–271, 2016. doi: 10.1007/s12672-016-0268-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zimmer BM, Howell ME, Ma L, Enders JR, Lehman D, Corey E, Barycki JJ, Simpson MA. Altered glucuronidation deregulates androgen dependent response profiles and signifies castration resistance in prostate cancer. Oncotarget 12: 1886–1902, 2021. doi: 10.18632/oncotarget.28059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Aaltomaa S, Lipponen P, Tammi R, Tammi M, Viitanen J, Kankkunen JP, Kosma VM. Strong stromal hyaluronan expression is associated with PSA recurrence in local prostate cancer. Urol Int 69: 266–272, 2002. doi: 10.1159/000066123. [DOI] [PubMed] [Google Scholar]
- 82.Huang D, Casale GP, Tian J, Lele SM, Pisarev VM, Simpson MA, Hemstreet GP 3rd.. Udp-glucose dehydrogenase as a novel field-specific candidate biomarker of prostate cancer. Int J Cancer 126: 315–327, 2010. doi: 10.1002/ijc.24820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Teoh ST, Ogrodzinski MP, Lunt SY. UDP-glucose 6-dehydrogenase knockout impairs migration and decreases in vivo metastatic ability of breast cancer cells. Cancer Lett 492: 21–30, 2020. doi: 10.1016/j.canlet.2020.07.031. [DOI] [PubMed] [Google Scholar]
- 84.Lin LH, Chou HC, Chang SJ, Liao EC, Tsai YT, Wei YS, Chen HY, Lin MW, Wang YS, Chien YA, Yu XR, Chan HL. Targeting UDP-glucose dehydrogenase inhibits ovarian cancer growth and metastasis. J Cell Mol Med 24: 11883–11902, 2020. doi: 10.1111/jcmm.15808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Arnold JM, Gu F, Ambati CR, Rasaily U, Ramirez-Pena E, Joseph R, Manikkam M, San Martin R, Charles C, Pan Y, Chatterjee SS, Den Hollander P, Zhang W, Nagi C, Sikora AG, Rowley D, Putluri N, Zhang XH, Karanam B, Mani SA, Sreekumar A. UDP-glucose 6-dehydrogenase regulates hyaluronic acid production and promotes breast cancer progression. Oncogene 39: 3089–3101, 2020. [Erratum in Oncogene 39: 3226–3228, 2020]. doi: 10.1038/s41388-019-0885-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Simpson MA, Weigel JA, Weigel PH. Systemic blockade of the hyaluronan receptor for endocytosis prevents lymph node metastasis of prostate cancer. Int J Cancer 131: E836–E840, 2012. doi: 10.1002/ijc.27427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Oikari S, Kettunen T, Tiainen S, Häyrinen J, Masarwah A, Sudah M, Sutela A, Vanninen R, Tammi M, Auvinen P. UDP-sugar accumulation drives hyaluronan synthesis in breast cancer. Matrix Biol 67: 63–74, 2018. doi: 10.1016/j.matbio.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 88.Jokela TA, Makkonen KM, Oikari S, Kärnä R, Koli E, Hart GW, Tammi RH, Carlberg C, Tammi MI. Cellular content of UDP-N-acetylhexosamines controls hyaluronan synthase 2 expression and correlates with O-linked N-acetylglucosamine modification of transcription factors YY1 and SP1. J Biol Chem 286: 33632–33640, 2011. doi: 10.1074/jbc.M111.265637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kaushik AK, Shojaie A, Panzitt K, Sonavane R, Venghatakrishnan H, Manikkam M, et al. Inhibition of the hexosamine biosynthetic pathway promotes castration-resistant prostate cancer. Nat Commun 7: 11612, 2016. doi: 10.1038/ncomms11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vigetti D, Ori M, Viola M, Genasetti A, Karousou E, Rizzi M, Pallotti F, Nardi I, Hascall VC, De Luca G, Passi A. Molecular cloning and characterization of UDP-glucose dehydrogenase from the amphibian Xenopus laevis and its involvement in hyaluronan synthesis. J Biol Chem 281: 8254–8263, 2006. doi: 10.1074/jbc.M508516200. [DOI] [PubMed] [Google Scholar]
- 91.Walsh EC, Stainier DY. UDP-glucose dehydrogenase required for cardiac valve formation in zebrafish. Science 293: 1670–1673, 2001. doi: 10.1126/science.293.5535.1670. [DOI] [PubMed] [Google Scholar]
- 92.Superina S, Borovina A, Ciruna B. Analysis of maternal-zygotic ugdh mutants reveals divergent roles for HSPGs in vertebrate embryogenesis and provides new insight into the initiation of left-right asymmetry. Dev Biol 387: 154–166, 2014. doi: 10.1016/j.ydbio.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 93.Vatsyayan J, Lee SJ, Chang HY. Effects of xenobiotics and peroxisome proliferator-activated receptor-alpha on the human UDPglucose dehydrogenase gene expression. J Biochem Mol Toxicol 19: 279–288, 2005. doi: 10.1002/jbt.20099. [DOI] [PubMed] [Google Scholar]
- 94.Vatsyayan J, Lin CT, Peng HL, Chang HY. Identification of a cis-acting element responsible for negative regulation of the human UDP-glucose dehydrogenase gene expression. Biosci Biotechnol Biochem 70: 401–410, 2006. doi: 10.1271/bbb.70.401. [DOI] [PubMed] [Google Scholar]
- 95.Wen Y, Li J, Wang L, Tie K, Magdalou J, Chen L, Wang H. UDP-glucose dehydrogenase modulates proteoglycan synthesis in articular chondrocytes: its possible involvement and regulation in osteoarthritis. Arthritis Res Ther 16: 484, 2014. doi: 10.1186/s13075-014-0484-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hyde AS, Farmer EL, Easley KE, van Lammeren K, Christoffels VM, Barycki JJ, Bakkers J, Simpson MA. UDP-glucose dehydrogenase polymorphisms from patients with congenital heart valve defects disrupt enzyme stability and quaternary assembly. J Biol Chem 287: 32708–32716, 2012. doi: 10.1074/jbc.M112.395202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Alhamoudi KM, Bhat J, Nashabat M, Alharbi M, Alyafee Y, Asiri A, Umair M, Alfadhel M. A missense mutation in the UGDH gene is associated with developmental delay and axial hypotonia. Front Pediatr 8: 71, 2020. doi: 10.3389/fped.2020.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hengel H, Bosso-Lefèvre C, Grady G, Szenker-Ravi E, Li H, Pierce S, et al. Loss-of-function mutations in UDP-Glucose 6-Dehydrogenase cause recessive developmental epileptic encephalopathy. Nat Commun 11: 595, 2020. doi: 10.1038/s41467-020-14360-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Clarkin CE, Allen S, Kuiper NJ, Wheeler BT, Wheeler-Jones CP, Pitsillides AA. Regulation of UDP-glucose dehydrogenase is sufficient to modulate hyaluronan production and release, control sulfated GAG synthesis, and promote chondrogenesis. J Cell Physiol 226: 749–761, 2011. doi: 10.1002/jcp.22393. [DOI] [PubMed] [Google Scholar]
- 100.Simpson MA, Wilson CM, Furcht LT, Spicer AP, Oegema TR Jr, McCarthy JB. Manipulation of hyaluronan synthase expression in prostate adenocarcinoma cells alters pericellular matrix retention and adhesion to bone marrow endothelial cells. J Biol Chem 277: 10050–10057, 2002. doi: 10.1074/jbc.M110069200. [DOI] [PubMed] [Google Scholar]
- 101.Liang J, Jiang D, Noble PW. Hyaluronan as a therapeutic target in human diseases. Adv Drug Deliv Rev 97: 186–203, 2016. doi: 10.1016/j.addr.2015.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nagy N, Kuipers HF, Frymoyer AR, Ishak HD, Bollyky JB, Wight TN, Bollyky PL. 4-methylumbelliferone treatment and hyaluronan inhibition as a therapeutic strategy in inflammation, autoimmunity, and cancer. Front Immunol 6: 123, 2015. doi: 10.3389/fimmu.2015.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jokela TA, Jauhiainen M, Auriola S, Kauhanen M, Tiihonen R, Tammi MI, Tammi RH. Mannose inhibits hyaluronan synthesis by down-regulation of the cellular pool of UDP-N-acetylhexosamines. J Biol Chem 283: 7666–7673, 2008. doi: 10.1074/jbc.M706001200. [DOI] [PubMed] [Google Scholar]
- 104.Nagy N, Gurevich I, Kuipers HF, Ruppert SM, Marshall PL, Xie BJ, Sun W, Malkovskiy AV, Rajadas J, Grandoch M, Fischer JW, Frymoyer AR, Kaber G, Bollyky PL. 4-Methylumbelliferyl glucuronide contributes to hyaluronan synthesis inhibition. J Biol Chem 294: 7864–7877, 2019. doi: 10.1074/jbc.RA118.006166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Oikari S, Makkonen K, Deen AJ, Tyni I, Karna R, Tammi RH, Tammi MI. Hexosamine biosynthesis in keratinocytes: roles of GFAT and GNPDA enzymes in the maintenance of UDP-GlcNAc content and hyaluronan synthesis. Glycobiology 26: 710–722, 2016. doi: 10.1093/glycob/cww019. [DOI] [PubMed] [Google Scholar]
- 106.Tran DH, May HI, Li Q, Luo X, Huang J, Zhang G, Niewold E, Wang X, Gillette TG, Deng Y, Wang ZV. Chronic activation of hexosamine biosynthesis in the heart triggers pathological cardiac remodeling. Nat Commun 11: 1771, 2020. doi: 10.1038/s41467-020-15640-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chokchaitaweesuk C, Kobayashi T, Izumikawa T, Itano N. Enhanced hexosamine metabolism drives metabolic and signaling networks involving hyaluronan production and O-GlcNAcylation to exacerbate breast cancer. Cell Death Dis 10: 803, 2019. doi: 10.1038/s41419-019-2034-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ricciardiello F, Votta G, Palorini R, Raccagni I, Brunelli L, Paiotta A, Tinelli F, D'Orazio G, Valtorta S, De Gioia L, Pastorelli R, Moresco RM, La Ferla B, Chiaradonna F. Inhibition of the hexosamine biosynthetic pathway by targeting PGM3 causes breast cancer growth arrest and apoptosis. Cell Death Dis 9: 377, 2018. doi: 10.1038/s41419-018-0405-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Itkonen HM, Engedal N, Babaie E, Luhr M, Guldvik IJ, Minner S, Hohloch J, Tsourlakis MC, Schlomm T, Mills IG. UAP1 is overexpressed in prostate cancer and is protective against inhibitors of N-linked glycosylation. Oncogene 34: 3744–3750, 2015. doi: 10.1038/onc.2014.307. [DOI] [PubMed] [Google Scholar]