Significance
A variety of mechanisms safeguard the body from autoimmune reactions, yet how these processes cooperate is largely unclear. Using a range of mouse genetic models, we uncover a critical tolerogenic axis between the autoimmune regulator, AIRE, and the immune checkpoint molecule, PD-1. Their combined loss induced an early-onset lethal autoimmune syndrome driven by autoreactive CD4+ T cells that could not be restrained by regulatory T cells. These data shed light on how central and peripheral tolerance mechanisms work together, and highlight thymic function as a potentially key modifier of responses to anti–PD-1 therapies.
Keywords: autoimmunity, immunological tolerance, thymus, anergy, checkpoint
Abstract
Immunological tolerance is established and maintained by a diverse array of safeguards that work together to protect against autoimmunity. Despite the identification of numerous tolerogenic processes, the basis for cooperation among them remains poorly understood. We sought to identify synergy among several well-defined tolerance mediators that alone provide protection only from mild autoimmune symptoms in C57BL/6 mice: BIM, AIRE, CBL-B, and PD-1. Survey of a range of compound mutant mice revealed that the combined loss of the autoimmune regulator, AIRE, with PD-1 unleashed a spontaneous, lethal autoimmune disease. Pdcd1−/−Aire−/− mice succumbed to cachexia before adulthood, with near-complete destruction of the exocrine pancreas. Such fatal autoimmunity was not observed in Pdcd1−/−Bim−/−, Bim−/−Aire−/−, or Cblb−/−Bim−/− mice, suggesting that the cooperation between AIRE-mediated and PD-1–mediated tolerance was particularly potent. Immune profiling revealed largely normal development of FOXP3+ regulatory T (Treg) cells in Pdcd1−/−Aire−/− mice, yet excessive, early activation of effector T cells. Adoptive transfer experiments demonstrated that autoimmune exocrine pancreatitis was driven by conventional CD4+ T cells and could not be prevented by the cotransfer of Treg cells from wild-type mice. The development of autoimmunity in mixed bone marrow chimeras supported these observations, indicating that failure of recessive tolerance was responsible for disease. These findings reveal a potent tolerogenic axis between AIRE and PD-1 that has implications for our understanding of how immune checkpoint blockade might synergize with subclinical defects in central tolerance to elicit autoimmune disease.
Autoimmune disease develops in ∼3% of the US population, typically after a long period of latency (1). A variety of immunological tolerance mechanisms orchestrate the removal or silencing of autoreactive lymphocytes to prevent tissue damage (2, 3). The long latency for development of autoimmune pathology has led to suggestions that serial breakdown of multiple immune tolerance mechanisms (or checkpoints) must occur for overt disease to become apparent (3).
T cell tolerance is imposed in two main locations. The first location is the thymus, where a variety of mechanisms delete or censor highly autoreactive clones during the differentiation of T cells. These processes are collectively referred to as thymic negative selection (4, 5). For example, the proapoptotic BH3-only protein, BIM, is required for the deletion of self-reactive thymocytes receiving high-avidity T cell receptor (TCR) signals; yet Bim−/− mice on the C57BL/6 background only develop mild autoimmune symptoms (6, 7). Similarly, C57BL/6.Aire−/− mice have impaired negative selection of thymocytes reactive to peripheral tissue antigens (PTA) normally produced by the thymic epithelium; yet they also only develop relatively mild autoimmune symptoms (8, 9). These findings reinforce the notion that additional tolerance mechanisms act as fail-safes to control autoreactive T cells when thymic deletion is impaired.
Mature T cells that are exported from the thymus to peripheral lymphoid and nonlymphoid organs are subject to peripheral tolerance mechanisms, such as deletion, anergy, inhibitory receptor engagement, or suppression by regulatory cells (10). The ubiquitin ligase, CBL-B, can enforce anergy and restrain the activation of autoreactive T cells by imposing the requirement for CD28 costimulation (11, 12). By contrast, ligation of the inhibitory receptor, PD-1, antagonizes activated T cell responses and curtails spontaneous autoimmunity in certain contexts (13, 14). However, in these and many other examples, the loss of individual tolerance genes does not elicit catastrophic autoimmune disease in C57BL/6 mice. This observation supports the hypothesis that there must exist a substantial degree of cooperation among the various tolerance processes preventing autoimmune disease. Yet the most critical components of this cooperation remain unclear.
Although the concept that peripheral tolerance provides an essential backup to when thymic negative selection fails is well established, examples of the mechanisms underpinning this interaction are surprisingly few. One example is provided by Aire−/−;Cblb−/− mice on the B10.Br background, which develop fatal autoimmune pancreatitis and sialitis that was not observed with deficiency of either gene alone (15). In this case, CBL-B was required to antagonize peripheral activation of autoreactive T cells escaping AIRE-dependent thymic deletion. By contrast, compound mutant Aire−/− mice also lacking FASL, Roquin, or CARD11 revealed no cooperation between these tolerance mechanisms (15). Due to the paucity of information on further cooperativity among the distinct tolerance mechanisms, we generated mice with genetic deletion of key central and peripheral tolerance mediators to uncover critical interactions between different tolerance-inducing processes.
PD-1 is notable in this context because it is an important target in cancer immunotherapies that overcome immune inhibition to promote tumor cell killing. However, these responses are often attended by autoimmune side effects. Initial evidence that PD-1 plays a role in immunological tolerance came from mice lacking Pdcd1 (the gene encoding PD-1) which, on a C57BL/6 genetic background, exhibit spontaneous development of a late-onset, lupus-like disease characterized by autoantibodies and mild glomerulonephritis (13). This tolerance defect was exacerbated on autoimmune-prone backgrounds, such BALB/c, where Pdcd1 deficiency induced fatal myocarditis and gastritis (13). These studies indicate that the PD-1–mediated immune suppressive pathway plays a critical role in maintaining immunological tolerance. PD-1 appears to control peripheral tolerance by limiting the initial phase of activation and expansion of self-reactive T cells, and restricts self-reactive T cell effector functions in the nonlymphoid tissues of perinatal mice (16). Thus, the adverse immune events observed in patients receiving PD-1 or PD-L1 blocking agents could reflect manifestations that have their origin in defects in central tolerance mechanisms. We sought to investigate the impact of interfering simultaneously with central and peripheral tolerance mechanisms to uncover critical synergies that may inform on the best combinations for new treatment strategies and on the mechanisms of toxicity from currently used immunotherapies.
Results
Pd1−/−Aire−/− Compound-Deficient Mice Succumb to Fatal Exocrine Pancreatitis.
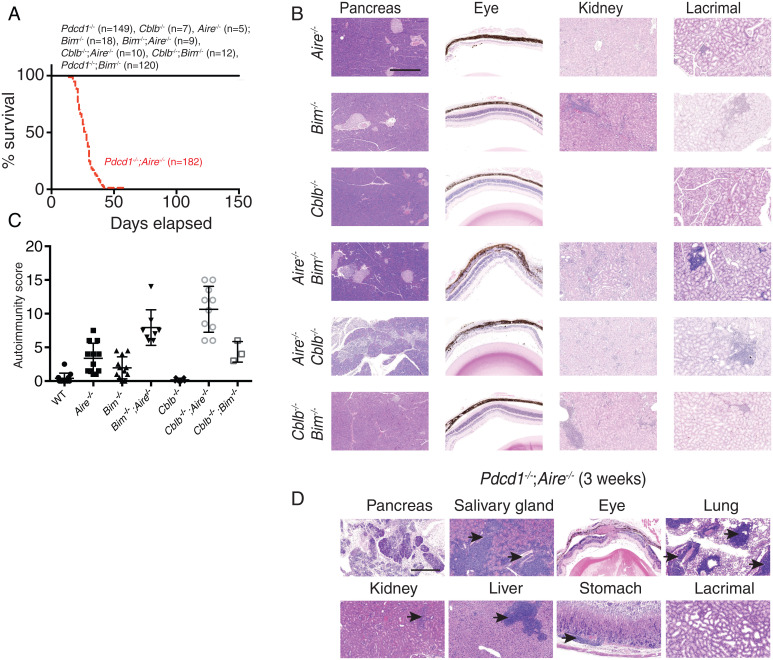
To investigate the cooperation between central and peripheral tolerance mechanisms, we took a genetic loss-of-function approach. BIM and AIRE are critical mediators of thymic negative selection that act via distinct mechanisms. BIM is a proapoptotic protein required for the killing of thymocytes that receive high-avidity TCR signals (6). On a C57BL/6 background, Bim−/− mice develop features of systemic lupus erythematosus but do not succumb to organ-specific autoimmunity (7). AIRE is required for the expression of PTAs by thymic epithelial cells, and defects in this process lead to autoimmunity in a range of organs (8, 9). The presentation of AIRE-regulated PTAs on thymic epithelial cells or dendritic cells is required to mediate deletion of thymocytes with autoreactive TCRs or Treg cell generation (5). To test whether AIRE and BIM activities overlap in the induction of thymic tolerance or are distinct, we aged cohorts of Aire−/−, Bim−/−, and Bim−/−;Aire−/− mice and examined the impact on organ-specific autoimmunity. All mice in these groups survived to the 150-d end point of the experiment (Fig. 1A), whereupon histological analysis was undertaken in organs normally targeted by autoimmune manifestations in Aire−/− or Bim−/− mice. Bim−/−;Aire−/− mice exhibited the autoimmune features frequently observed in AIRE-deficient mice (i.e., severe retinopathy, sialitis, mild pneumonitis, lacrimal gland infiltration), combined with the mild renal pathology that is associated with BIM deficiency (Fig. 1B and SI Appendix, Fig. S1A). For further comparison, we performed a semiquantitative assessment of lymphocytic infiltration and tissue destruction in all organs and summed these values into an overall “autoimmunity score” (7, 9) (an example is shown for pancreas in SI Appendix, Fig. S1B). We found that the average overall score in Bim−/−;Aire−/− mice was effectively the sum of those observed in the groups of Aire−/− or Bim−/− mice (Fig. 1C), suggesting that the tolerogenic defects induced by the compound loss of BIM and AIRE were additive (Fig. 1C). The relatively mild impact of these combined defects in negative selection suggests that peripheral tolerance mechanisms are sufficient to prevent the activation of autoreactive T cells and the development of severe autoimmune disease.
Fig. 1.
Autoimmune pathology resulting from combined defects in peripheral and central tolerance mechanisms. (A) Survival of Bim−/−, Aire−/−, Cblb−/−, Pdcd1−/−, Bim−/−;Aire−/−, Cblb−/−;Aire−/−, Cblb−/−;Bim−/−, Pdcd1−/−;Bim−/−, and Pdcd1−/−;Aire−/− mice (all on a C57BL/6 background). (B) Representative images of H&E-stained sections of the pancreas, eye, kidney, and lacrimal gland from mice of the indicated genotypes taken at 150 d. (Scale bar, 500 μm.) (C) Combined “autoimmunity score” for the various mouse strains, summing infiltration/destruction scores from the major organs of the mice indicated (n = 3 to 12). (D) Representative images of H&E-stained sections of the indicated organs taken from Pdcd1−/−;Aire−/− mice upon euthanasia. Black arrows indicate examples of lymphocytic infiltration. (Scale bar, 500 μm.)
To explore peripheral tolerance mechanisms that may be important for preserving tolerance when thymic tolerance is perturbed, we genetically ablated Pdcd1 or Cblb in conjunction with either Bim or Aire. All single- or double-knockout mice survived until the end of the experiment (150 d), except those with a combined deficiency of Pdcd1 and Aire (Fig. 1A). These Pdcd1−/−;Aire−/− mice became runted and died at weaning. Comparative histological analysis of organs from those mice surviving to 150 d showed that Cblb−/−;Aire−/− mice had much more severe infiltration and tissue destruction than the single-knockout controls (Fig. 1 B and C). It was previously reported that Cblb−/−;Aire−/− mice on a B10.BR background succumbed to fatal autoimmune disease with a median survival of 25 d (15). However, on the C57BL/6 background we tested here, we did not observe lethal autoimmune disease within 150 d of age (Fig. 1A). Nevertheless, there was substantial leukocyte infiltration in the salivary gland, liver, retina, and pancreas; the latter is an organ that is not normally targeted in either of the single-mutant mice (Fig. 1 B and C). These data highlight the potent synergy between AIRE-mediated thymic tolerance and CBL-B–mediated peripheral tolerance.
In contrast to all other strains examined, Pdcd1-/;−Aire−/− mice succumbed to a wasting disease at a young age (Fig. 1A). Analysis of organs from 3-wk-old Pdcd1−/−;Aire−/− mice revealed substantial lymphocytic infiltration in the lung, salivary gland, liver, and retina and near-complete destruction of the pancreas (Fig. 1D and SI Appendix, Fig. S1C). Extensive destruction of the exocrine acinar cells was evident, yet the islets of Langerhans were typically spared, and these mice did not develop diabetes (Fig. 1D and SI Appendix, Fig. S1C). These results show that AIRE-dependent mechanisms synergize with CBL-B– or PD-1–dependent processes to safeguard immunological tolerance and prevent severe autoimmune disease.
Fatal Exocrine Pancreatitis in Pdcd1−/−;Aire−/− Mice Is Mediated by CD4+ T Cells.
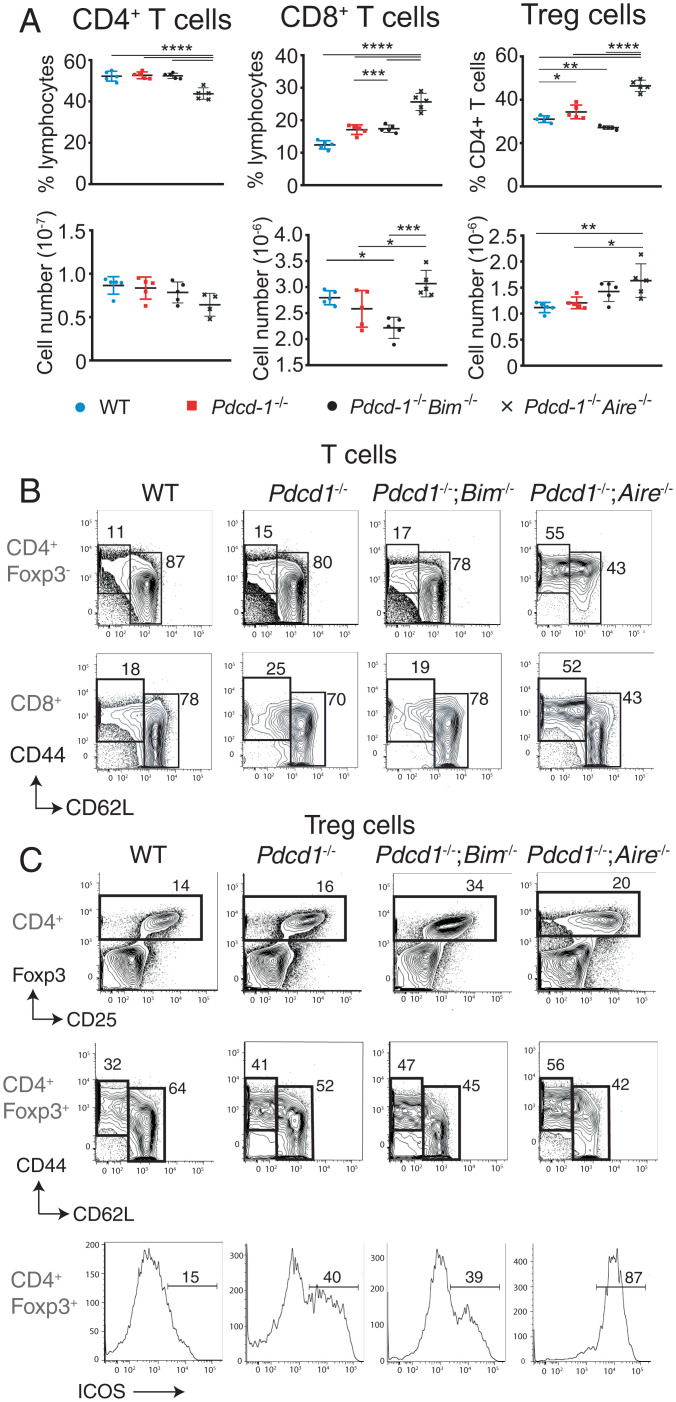
We next sought to determine the basis of the fatal autoimmune disease observed in mice lacking both AIRE and PD-1. Flow cytometric analysis of splenocytes from Pdcd1−/−;Aire−/− compound mutant mice at 2.5 wk of age (just prior to the onset of lethal wasting) revealed an increased frequency and number of CD8+ T cells and FOXP3+ Treg cells compared to wild-type (WT), Pdcd1−/−, and Pdcd1−/−;Bim−/− mice of the same age (Fig. 2A). CD4+ and CD8+ conventional T cells from mice lacking PD-1 and AIRE had markedly heightened activation compared to controls and Pdcd1−/−;Bim−/− mice (Fig. 2B). FOXP3+ Treg cells from young Pdcd1−/−;Aire−/− mice also showed signs of heightened activation, with an increase in cells with a CD44highCD62LlowICOS+FOXP3+ Treg effector phenotype (Fig. 2C). These phenotypes are consistent with the autoimmune features observed being driven by loss of T cell tolerance.
Fig. 2.
Abnormally increased T cell activation in Pdcd-1−/−;Aire−/− mice. (A) Graphs of the mean percentages and total numbers (±SEM) of splenic CD4+ and CD8+ T cells and CD4+Foxp3+ Treg cells from 2.5-wk-old mice of the indicated genotypes. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 Student’s t test. (B) CD44 and CD62L expression gated on CD4+ or CD8+ T cells from 19-d-old mice of the indicated genotypes. Percentages of naïve T cells and effector/memory T cells based on the indicated regions are shown. Data are representative of three individual experiments (n > 4 mice per genotype). (C) FOXP3 and CD25 expression in splenic CD4+ T cells (Top), CD44 and CD62L expression on splenic Foxp3+ CD4+ T cells (Middle), and ICOS expression on splenic Foxp3+ CD4+ T cells (Bottom) from mice of the indicated genotypes at 2.5 wk for Pdcd-1−/−;Aire−/− mice and at 8 wk for mice of the other genotypes. The percentages of the indicated regions are shown. Data in A–C are representative of three or four experiments performed with n = 3 to 6 per genotype.
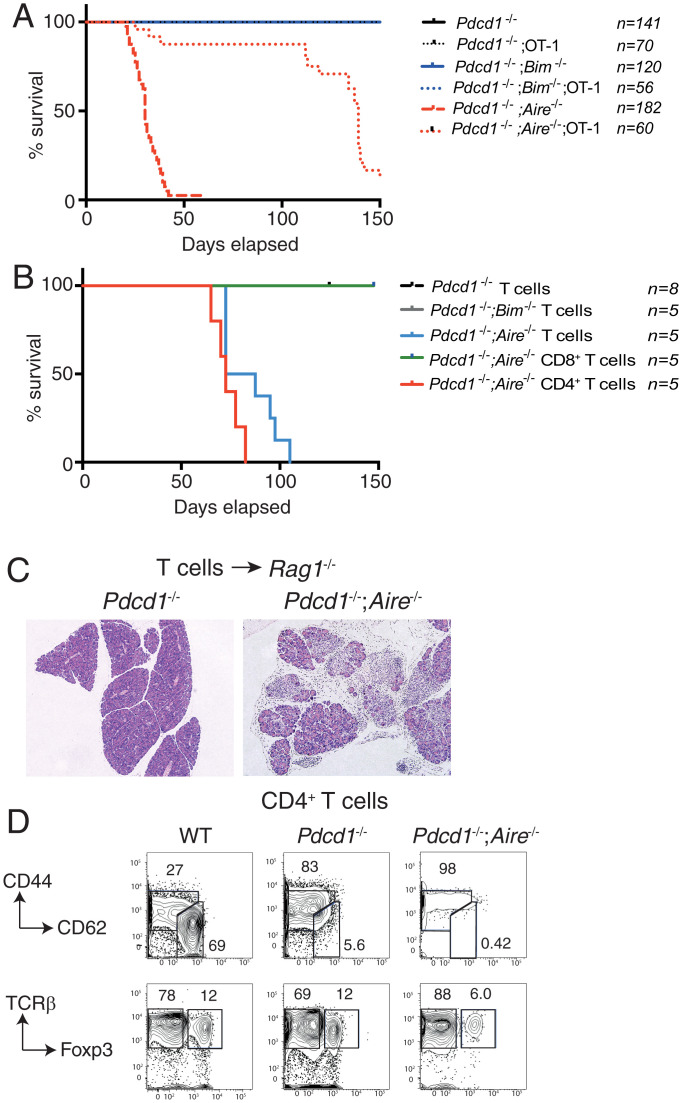
To establish whether the diversity of the TCR repertoire was a driver of the fatal autoimmune disease of the Pdcd1−/−;Aire−/− mice, we introduced the OT-I TCR transgene onto this background. OT-1 TCR tg mice have a restricted TCR repertoire, with most T cells bearing antigen receptors specific for an ovalbumin-derived peptide presented by H2-Kb. Therefore, we compared the survival of Pdcd1−/−;Aire−/− mice with that of OT-I tg;Pdcd1−/−;Aire−/− mice to determine whether reducing TCR repertoire diversity would impact the development of disease. Pdcd1−/−;Aire−/− mice bearing the OT-I TCR transgene had substantially delayed disease development, with most animals surviving beyond 110 d (Fig. 3A). These older OT-I tg;Pdcd1−/−;Aire−/− mice succumbed to a wasting condition similar to the younger, nontransgenic Pdcd1−/−;Aire−/− controls. These findings demonstrate that restricting the diversity of the TCR repertoire in Pdcd1−/−;Aire−/− mice substantially delays the development of severe autoimmunity, further implicating autoreactive T cells as drivers of the disease.
Fig. 3.
Fatal exocrine pancreatitis in Pdcd-1−/−;Aire−/− mice is driven by CD4+ T cells. (A) Survival of Pdcd1−/−, Pdcd1−/−;Bim−/−, and Pdcd1−/−;Aire−/− mice with or without the OT-1 TCR transgene. (B) Survival of Rag1−/− mice adoptively transferred with T cells from Pdcd1−/−;Aire−/−, Pdcd1−/−;Bim−/−, or Pdcd-1−/− mice or with purified CD4+ or CD8+ cells from Pdcd1−/−;Aire−/− mice. (C) Histological analysis of sections of the pancreas from Rag1−/− mice that had been injected i.v. with total T cells from Pdcd1−/− or Pdcd1−/−;Aire−/− mice. (Scale bar, 500 μm.) (D) Representative flow cytometric analysis of splenocytes from Rag1−/− mice that had been injected i.v. with purified CD4+ T cells from WT, Pdcd1−/−, or Pdcd1−/−;Aire−/− mice. The percentage of naive/activated T cells (Top) or Treg cells (Bottom) bounded by region gating are shown. Data are representative of two experiments performed with three to five mice per group.
We next performed adoptive transfers of T cells from PD-1–deficient and AIRE-deficient mice into Rag1−/− recipients to test whether this population was sufficient to induce disease. Previous studies have established that mature lymphocytes from Aire−/− mice can transfer mild autoimmunity in Rag1−/− recipients, but do not cause early lethality (8). By contrast, all Rag1−/− mice that we had inoculated with T cells from Pdcd1−/−;Aire−/− animals died within 5 wk, presenting with severe weight loss and extensive destruction of pancreatic acinar cells (Fig. 3 B and C). Flow cytometric analysis of these animals revealed greatly increased CD44high CD62Llow activated/memory T cells (Fig. 3D), similar to the phenotype observed in the intact Pdcd1−/−;Aire−/− mice. Rag1−/− mice receiving T cells from mice lacking only PD-1 also had increased activated/memory T cells, but they remained healthy for 150 d and did not display any signs of autoimmune pancreatitis (Fig. 3 B and C). These outcomes indicate that autoreactive T cells are sufficient to drive the fatal exocrine pancreatitis observed in Pdcd1−/−;Aire−/− mice. Furthermore, although PD-1–deficient T cells can become activated in lymphopenic recipients, AIRE-mediated thymic tolerance likely curtails the autoreactive repertoire and prevents the lethal autoimmunity.
To determine whether CD4+ or CD8+ T cells from Pdcd1−/−;Aire−/− mice were the key autoimmune effectors, these populations were enriched and injected separately into Rag1−/− mice. Residual contaminating CD8+ or CD4+ cells were depleted with anti-CD4 or anti-CD8 antibodies in the recipients. Only recipients of CD4+ T cells from Pdcd1−/−;Aire−/− mice succumbed to autoimmune pancreatitis within 4 wk, whereas the mice that had been injected with CD8+ T cells remained healthy to at least 150 d following transfer (Fig. 3B). These findings demonstrate that the fatal autoimmune destruction of the exocrine pancreas in Pdcd1−/−;Aire−/− mice is driven by a breakdown of tolerance in CD4+ T cells.
Cell-Intrinsic Tolerance Defects in Pdcd1−/−;Aire−/− Mice Drive Fatal Autoimmunity.
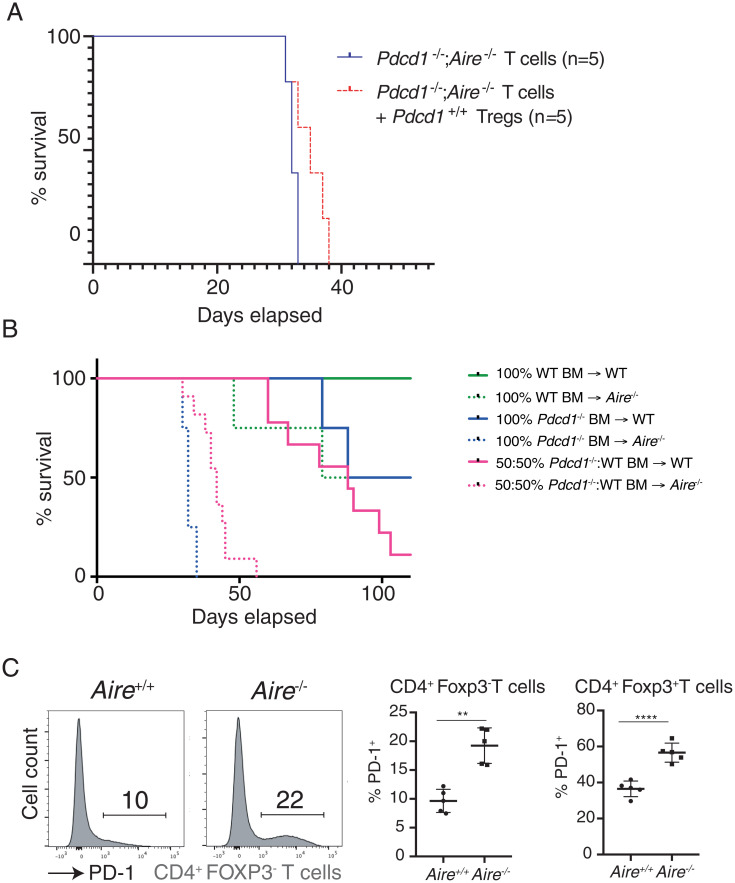
The CD4+ population includes immune-suppressive FOXP3+ Treg cells. Treg cells express high levels of PD-1 which functions to restrain their differentiation and suppressive function (17). Nevertheless, it remained possible that the additional loss of AIRE modified the capacity of PD-1–deficient Treg cells to suppress autoimmune responses. Therefore, we sought to determine whether the impaired tolerance observed in Pdcd1−/−;Aire−/− mice stemmed from defects in recessive (cell intrinsic) or dominant (cell extrinsic) mechanisms of tolerance. The suppressive capacity of Treg cells from Pdcd1−/−;Aire−/− was comparable to that of WT Treg cells (SI Appendix, Fig. S2A), indicating that there was not a gross functional defect in PD-1–deficient Treg cells. Cotransfer of Treg cells from WT mice with CD4+ T cells taken from Pdcd1−/−;Aire−/− mice into Rag1−/− mice (SI Appendix, Fig. S2B) did not rescue these recipients from lethal autoimmune disease (Fig. 4A). This finding suggests that the breakdown in tolerance observed in PD-1/AIRE compound mutant mice was not due to defects in Treg cell function.
Fig. 4.
A cell-intrinsic requirement for PD-1 to prevent autoimmunity in AIRE-deficient mice. (A) Survival of Rag1−/− mice adoptively transferred with T cells from Pdcd-1−/−Aire−/− mice with or without coadministration of CD4+Foxp3+ Treg cells from WT mice. (B) Survival of WT or Aire−/− hematopoietic chimeras reconstituted with bone marrow cells from WT, Pdcd-1−/−, or a 50:50 mixture of both. (C) Representative histograms of PD-1 expression on splenic CD4+ FOXP3− T cells from Aire+/+ (WT) or Aire−/− mice. Right quantify the mean percentage (±SEM) of PD-1+ Tconv or Treg cells from Aire+/+ (WT) or Aire−/− mice. **P < 0.01, ****P < 0.0001 Student’s t test. Data in C are representative of two experiments, each with n = 5 mice per group.
To investigate the possibility that defects in other immunoregulatory hematopoietic cell types were the basis of autoimmune disease in Pdcd1−/−;Aire−/− mice, we established mixed hematopoietic chimeras in lethally irradiated WT or Aire−/− recipient mice with donor cells from WT and PD-1–deficient mice. Mice were then analyzed for the onset of lethal autoimmunity, to test whether the presence of WT T cells could impose tolerance on PD-1–deficient T cells “educated” in an AIRE-deficient thymus. Controls included irradiated WT or Aire−/− mice receiving only WT or only PD-1–deficient bone marrow cells, respectively. As expected, Ly5.2 WT mice reconstituted with 100% Ly5.1 WT bone marrow cells remained healthy, whereas Aire−/− mice reconstituted with 100% bone marrow cells from Pdcd1−/− mice swiftly succumbed to a wasting disease with a similar latency as mice with combined germline deletion of both AIRE and PD-1 (Fig. 4B). A fraction of control WT mice that had been reconstituted with either 100% bone marrow cells from Pdcd1−/− mice or a 1:1 mixture of WT and PD-1–deficient bone marrow cells developed wasting disease with a delayed onset (Fig. 4B). This finding suggested a degree of autoimmunity in this setting, consistent with previous findings in Pdcd1−/−→Rag1−/− chimeras (18), and that this disease could not be inhibited by WT hematopoietic cells. Aire−/− mice reconstituted with a 1:1 mixture of WT and PD-1–deficient bone marrow cells died within 6 wk of transplantation, with histological analysis revealing severe autoimmune destruction of the pancreas to the same extent as that observed in the intact Pdcd1−/−;Aire−/− mice (SI Appendix, Fig. S2C). These findings establish that PD-1 deficiency in the hematopoietic compartment and AIRE deficiency in the stromal compartment is sufficient to induce lethal autoimmunity in mice. Furthermore, the breakdown in immunological tolerance observed in Pdcd1−/−;Aire−/− mice could not be corrected by complementation with WT cells, indicating that defects in recessive tolerance drive autoimmunity in this setting.
Previous studies using transgenic systems have found roles for PD-1 in thymic beta and positive selection events (19–21). To determine whether PD-1 may also play a role in thymic deletion in the polyclonal setting, we used an approach developed by Breed et al. (22) to quantify thymocytes undergoing deletion using cleaved (i.e., activated) caspase-3 (SI Appendix, Fig. S3A). As expected, genetic loss of the apoptotic effector proteins BAX and BAK reduced clonal deletion in thymocytes, and BIM accounted for much of this activity (SI Appendix, Fig. S3 B and C). The loss of AIRE did not change the proportion of deleted cells, suggesting this assay is not sensitive enough to detect the deletion of those thymocytes reactive to PTA (SI Appendix, Fig. S3 B and C). Similar levels of apoptotic thymocytes were detected in Pdcd1−/− mice and WT controls (SI Appendix, Fig. S3 B and C), suggesting that PD-1 does not grossly affect thymic deletion, at least within the dynamic range of this assay.
Collectively, these data are consistent with a scenario where increased numbers of autoreactive T cells emerge from the thymus of AIRE-deficient mice which are then restrained by PD-1–mediated signals. In accord with this notion, we found an increased proportion of splenic PD-1+ Tconv and Treg cells in Aire−/− mice (Fig. 4C).
Discussion
It has been proposed that multiple mechanisms of immune tolerance must fail for autoimmune pathology to ensue (3). However, there are few examples highlighting cooperation among the various mechanisms imposing tolerance. The current study adds to our understanding by identifying cooperation between key central and peripheral tolerance mechanisms that prevent severe autoimmune pathology. We find that the loss of AIRE alone does not result in severe autoimmunity on the C57BL/6 background, because peripheral tolerance mechanisms restrain the activation of the autoreactive cells that have escaped the thymus. We confirm a key role for the ubiquitin ligase, CBL-B, in maintaining T cell tolerance in Aire−/− mice (15), with Cblb−/−;Aire−/− mice developing multiple signs of organ-specific autoimmunity. Although autoimmunity in Cblb−/−;Aire−/− mice on the B10.Br background was fatal, we found that, on the relatively autoimmune-resistant C57BL/6 background, they can survive well into adulthood. By contrast, PD-1 expression in Aire−/− mice was essential for preventing an early onset, fulminant, and lethal autoimmune disease. Together, these findings reveal potent synergy between AIRE-mediated thymic tolerance and certain peripheral mechanisms that restrain T cell activation.
The rapid, multiorgan, fatal autoimmune disease we found in Pdcd1−/−;Aire−/− mice was caused by defective recessive tolerance in CD4+ T cells. Treg cells from WT mice could not restrain autoreactive T cells from PD-1 and AIRE double-deficient mice, nor were mixed bone marrow chimeras able to maintain tolerance. The primary role for pathogenic CD4+ T cells echoes findings with PD-1–deficient cell reconstitution and T cell transfer studies in lymphopenic Rag1−/− mice (18, 23) and the effector cells responsible for autoimmunity in Aire−/− mice (24). The PD-1 ligands, PD-L1 and PD-L2, are expressed on thymic stromal cells (25), and roles for PD-1 in thymocyte selection have been reported in some systems (19–21). We did not find evidence of gross impacts on thymic deletion in nontransgenic Pdcd1−/− mice; however, we cannot exclude that there may be a subtle role for PD-1 in thymic selection that contributes to the autoimmune phenotype in Pdcd1−/−Aire−/− mice. Nevertheless, given the preponderance of evidence that PD-1 plays critical roles in preserving peripheral tolerance, we favor the hypothesis that autoreactive T cells specific for AIRE-regulated self-antigens that escape from the thymus are normally held in check by PD-1–mediated anergy. Consistent with this notion, we found elevated PD-1 expression in T cells from Aire−/− mice.
It was also recently found that high PD-1 expression was a characteristic of T cells infiltrating the nonlymphoid tissues of perinatal AIRE-deficient mice (16). It is well established that the perinatal stage is the critical time window for the establishment of AIRE-mediated tolerance (26). PD-1 antibody blockade in NOD.Aire−/− mice triggered early multiorgan autoimmunity, including the exocrine pancreatitis that is characteristic of this background (9, 16). These findings demonstrate an important role for PD-1 in establishing and maintaining an anergic phenotype in tissue-infiltrating T cells specific for AIRE-regulated antigens that have egressed from the perinatal thymus and are consistent with the early onset of the lethal autoimmune disease we describe here.
Therefore, the picture that emerges is that AIRE-dependent thymic tolerance acts to reduce the number of autoreactive cells emerging into the periphery, while PD-1 antagonizes the activation/effector function of any autoreactive clones that somehow escape from the thymus into the periphery. The efficiency of thymic selection is likely to vary in the population due to a range of factors, such as variation in AIRE expression, polymorphisms at HLA or specific PTA loci that affect thymic presentation, and differences in the efficiency of apoptotic deletion of autoreactive cells (27). An implication of the concept that PD-1–mediated peripheral tolerance is essential for supporting central tolerance is that individuals at the lower range of effective thymic tolerance may be at greater risk of adverse immune events from therapies blocking PDL1/PD-1 interactions. Indeed, pancreatitis has been observed as an immune-mediated adverse effect during cancer immunotherapy (28–30). Conversely, patients with lower thymic selection thresholds might also initiate better anticancer responses during immunotherapy. A study of a small number of melanoma patients receiving the CTLA-4 antagonist, ipilimumab, found associations between AIRE polymorphisms and treatment outcomes (31). Certainly, in animal models, there is evidence that constitutive or transient AIRE deficiency can improve antimelanoma responses (32, 33), particularly in concert with CTLA-4 blockade (31). The outcome of a clinical trial of the combination of PD-1 blockade (with or without CTLA-4 blockade) and RANK-L inhibition for the treatment of metastatic melanoma will be an interesting test of this idea (NCT03161756).
Compound deficiency in two key mediators of thymic negative selection, BIM and AIRE, did not elicit fatal autoimmune disease in C57BL/6 mice. This finding suggests that peripheral tolerance mechanisms remain capable of controlling the increased burden of autoreactive cells emerging from severe, compound defects in thymic negative selection. To put it another way, thymocyte deletion may be dispensable for immunological tolerance, since PD-1– and/or CBL-B–mediated mechanisms can control pathological autoreactive T cell responses, at least on the C57BL/6 genetic background. One caveat is that other proapoptotic proteins can contribute to thymic deletion processes in addition to BIM [e.g., PUMA (7)]; therefore, further defects on thymic negative selection might induce autoimmunity. Nevertheless, our data suggest that synergy between central and peripheral tolerance mechanisms are more potent than cooperation between different central tolerance mechanisms.
In this context, it was somewhat surprising that mice deficient in both BIM and PD-1 did not display overt signs of autoimmune disease, unlike Pdcd1−/−;Aire−/− animals. BIM is responsible for the apoptotic deletion of many autoreactive thymocytes (6, 22). This role extends to many thymocytes expressing TCRs recognizing self-antigens that are AIRE dependent (although not all; the related BH3-only protein, PUMA, is also required for efficient deletion of PTA-reactive thymocytes) (7). However, one important distinction between the T cells emerging from the thymus of Aire−/− and Bim−/− mice is that only in the latter are thymocytes likely to have encountered their cognate self-antigen during selection (e.g., ref. 34). Therefore, antigen experience in the thymus may induce other central tolerance mechanisms, such as anergy or diversion into the Treg cell lineage. Indeed, Bim−/− mice exhibit a large expansion of the FOXP3+ Treg cell population (35, 36) that is not observed in Aire−/− mice, perhaps further buttressing peripheral tolerance mechanisms, even in the absence of PD-1.
In conclusion, this study identifies a potent tolerogenic axis between AIRE-mediated thymic tolerance and PD-1–mediated control of autoreactive T cell responses. Conversely, defective BIM-mediated apoptosis did not synergize with AIRE-mediated or CBL-B– or PD-1–mediated tolerance mechanisms. These findings highlight the importance of AIRE function as a factor influencing responses to immunotherapy.
Materials and Methods
Mice.
All mice were housed at Walter and Eliza Hall Institute (WEHI) under specific pathogen-free housing conditions, and experiments were carried out in accordance with the guidelines and requirements of the Walter and Eliza Hall Research Animal Ethics Committee. Bim−/− mice (Bcl2l11−/−) were generated on a mixed C57BL/6 × 129SV background (37) and were then backcrossed to the C57BL/6 background >20 times prior to their use in intercrosses for this study (7). OT-1 TCR transgenic mice generated on a C57BL/6 genetic background have been described (38), and Rag1J−/− and Ly5.1 on a C57BL/6 background mice were obtained from WEHI Bioservices. Pdcd1−/− mice were obtained from Arlene Sharpe, Harvard Medical School, Cambridge, MA, and backcrossed to the C57BL/6 background. Cblb−/− mice on a C57BL/6 background were obtained from Chris Goodnow, Garvan Institute for Medical Research, Darlinghurst, NSW, Australia. Aire−/− (8) and Bak−/− BaxΔCd4 mice (39) were generated as described and maintained on a C57BL/6 background.
Bone Marrow Reconstitution Experiments.
Recipient mice were irradiated with two doses of 5.5 Gy 3 h apart prior to injection of donor cells. The hind legs of donor mice were dissected, and the bone marrow was flushed into cold fluorescence-activated cell sorter buffer (phosphate-buffered saline [PBS] containing 2% fetal calf serum). Cell suspensions were filtered and centrifuged at 1,500 rpm for 5 min at 4 °C. Cells were counted and resuspended in sterile PBS, and 200 μL (5 million cells) was injected intravenously (i.v.) into the tail vein of irradiated recipients. The following day, 100 μg of anti-Thy1 mAb (clone T24) was administered intraperitoneally to deplete residual host T cells. Chimeras were assessed for signs of disease and euthanized if they lost more than 10% of their peak body weight, according to the animal ethics guidelines.
Adoptive Transfer.
Spleens and lymph nodes were dissected, and cell suspensions were enriched for CD4+ or CD8+ T cells using AutoMACS column enrichment (Miltenyi Biotec). Cells (1 × 106) were then i.v. administered into Rag1−/− recipients, that were subsequently treated with CD4-depleting antibodies after injection with CD8+ T cells or with CD8-depleting Ab after injection with CD4+ T cells on days 0, 1, 7, and 14 to ensure depletion of residual contaminating cells of the unwanted T cell subset.
Histology.
Tissues were dissected and fixed in formalin, then embedded, sectioned, and stained with hematoxylin and eosin (H&E). Lymphocytic infiltration was scored as described (9, 40) with 0, 0.5, 1, 2, or 3 indicating none, trace, mild, moderate, or severe lymphocytic infiltration and tissue destruction, respectively. The scores from the analysis of the retina, salivary glands, lacrimal glands, thyroid glands, lung, liver, stomach, kidney, and pancreas were summed to derive the overall infiltration score.
Statistical Analysis.
Statistical comparisons were made using one-way ANOVA with multiple comparisons with Prism v.6.0 (GraphPad). P values of <0.05 were considered to indicate a statistically significant difference.
Further methods are supplied in SI Appendix.
Supplementary Material
Acknowledgments
We thank Drs. A. Sharpe, D. Mathis, J. Villadangos, P. Bouillet, and C. Goodnow for the provision of mice. We are grateful to WEHI Bioservices staff for expert mouse husbandry; the WEHI Flow Cytometry Facility; Histology Core; and B. Helbert, R. Chan, and K. Mackwell for genotyping. This work was supported by the National Health and Medical Research Council (NHMRC) Australia (Grant1078763, Fellowship 1090236, Grant 145888, Fellowship 1158024, and Grant 1187367 to D.H.D.G.; Fellowship 1089072 and Grant 2002618 to C.E.T.; and Grant 1116937 and Grant 1113133 to A.S.), the Cancer Council of Victoria (Grant-in-Aid 1120104 to A.S. and D.H.D.G.; Postgraduate Research Scholarship and Postdoctoral Cancer Research Fellowship for A.P.), and the Victorian Cancer Agency (MCRF20026 to C.E.T.). This work was made possible through Victorian State Government Operational Infrastructure Support and Australian Government NHMRC Independent Research Institutes Infrastructure Support.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2120149119/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Jacobson D. L., Gange S. J., Rose N. R., Graham N. M., Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin. Immunol. Immunopathol. 84, 223–243 (1997). [DOI] [PubMed] [Google Scholar]
- 2.Xing Y., Hogquist K. A., T-cell tolerance: Central and peripheral. Cold Spring Harb. Perspect. Biol. 4, a006957 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodnow C. C., Multistep pathogenesis of autoimmune disease. Cell 130, 25–35 (2007). [DOI] [PubMed] [Google Scholar]
- 4.Daley S. R., Teh C., Hu D. Y., Strasser A., Gray D. H. D., Cell death and thymic tolerance. Immunol. Rev. 277, 9–20 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Klein L., Kyewski B., Allen P. M., Hogquist K. A., Positive and negative selection of the T cell repertoire: What thymocytes see (and don’t see). Nat. Rev. Immunol. 14, 377–391 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouillet P., et al. , BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature 415, 922–926 (2002). [DOI] [PubMed] [Google Scholar]
- 7.Gray D. H., et al. , The BH3-only proteins Bim and Puma cooperate to impose deletional tolerance of organ-specific antigens. Immunity 37, 451–462 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson M. S., et al. , Projection of an immunological self shadow within the thymus by the aire protein. Science 298, 1395–1401 (2002). [DOI] [PubMed] [Google Scholar]
- 9.Jiang W., Anderson M. S., Bronson R., Mathis D., Benoist C., Modifier loci condition autoimmunity provoked by Aire deficiency. J. Exp. Med. 202, 805–815 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ElTanbouly M. A., Noelle R. J., Rethinking peripheral T cell tolerance: Checkpoints across a T cell’s journey. Nat. Rev. Immunol. 21, 257–267 (2021). [DOI] [PubMed] [Google Scholar]
- 11.Bachmaier K., et al. , Negative regulation of lymphocyte activation and autoimmunity by the molecular adaptor Cbl-b. Nature 403, 211–216 (2000). [DOI] [PubMed] [Google Scholar]
- 12.Chiang Y. J., et al. , Cbl-b regulates the CD28 dependence of T-cell activation. Nature 403, 216–220 (2000). [DOI] [PubMed] [Google Scholar]
- 13.Nishimura H., Nose M., Hiai H., Minato N., Honjo T., Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 11, 141–151 (1999). [DOI] [PubMed] [Google Scholar]
- 14.Nishimura H., et al. , Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 291, 319–322 (2001). [DOI] [PubMed] [Google Scholar]
- 15.Teh C. E., Daley S. R., Enders A., Goodnow C. C., T-cell regulation by casitas B-lineage lymphoma (Cblb) is a critical failsafe against autoimmune disease due to autoimmune regulator (Aire) deficiency. Proc. Natl. Acad. Sci. U.S.A. 107, 14709–14714 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tuncel J., Benoist C., Mathis D., T cell anergy in perinatal mice is promoted by T reg cells and prevented by IL-33. J. Exp. Med. 216, 1328–1344 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan C. L., et al. , PD-1 restraint of regulatory T cell suppressive activity is critical for immune tolerance. J. Exp. Med. 218, e20182232 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thangavelu G., et al. , Programmed death-1 is required for systemic self-tolerance in newly generated T cells during the establishment of immune homeostasis. J. Autoimmun. 36, 301–312 (2011). [DOI] [PubMed] [Google Scholar]
- 19.Blank C., et al. , Absence of programmed death receptor 1 alters thymic development and enhances generation of CD4/CD8 double-negative TCR-transgenic T cells. J. Immunol. 171, 4574–4581 (2003). [DOI] [PubMed] [Google Scholar]
- 20.Nishimura H., Honjo T., Minato N., Facilitation of beta selection and modification of positive selection in the thymus of PD-1-deficient mice. J. Exp. Med. 191, 891–898 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keir M. E., Latchman Y. E., Freeman G. J., Sharpe A. H., Programmed death-1 (PD-1):PD-ligand 1 interactions inhibit TCR-mediated positive selection of thymocytes. J. Immunol. 175, 7372–7379 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Breed E. R., Watanabe M., Hogquist K. A., Measuring thymic clonal deletion at the population level. J. Immunol. 202, 3226–3233 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ellestad K. K., et al. , Prior to peripheral tolerance, newly generated CD4 T cells maintain dangerous autoimmune potential: Fas- and Perforin-independent autoimmunity controlled by Programmed Death-1. Front. Immunol. 9, 12 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Devoss J. J., et al. , Effector mechanisms of the autoimmune syndrome in the murine model of autoimmune polyglandular syndrome type 1. J. Immunol. 181, 4072–4079 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liang S. C., et al. , Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses. Eur. J. Immunol. 33, 2706–2716 (2003). [DOI] [PubMed] [Google Scholar]
- 26.Guerau-de-Arellano M., Martinic M., Benoist C., Mathis D., Neonatal tolerance revisited: A perinatal window for Aire control of autoimmunity. J. Exp. Med. 206, 1245–1252 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liston A., Lesage S., Gray D. H., Boyd R. L., Goodnow C. C., Genetic lesions in T-cell tolerance and thresholds for autoimmunity. Immunol. Rev. 204, 87–101 (2005). [DOI] [PubMed] [Google Scholar]
- 28.Weber J. S., et al. , Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): A randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 16, 375–384 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Hofmann L., et al. , Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur. J. Cancer 60, 190–209 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Naidoo J., et al. , Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann. Oncol. 26, 2375–2391 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bakhru P., et al. , Combination central tolerance and peripheral checkpoint blockade unleashes antimelanoma immunity. JCI Insight 2, e93265 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khan I. S., et al. , Enhancement of an anti-tumor immune response by transient blockade of central T cell tolerance. J. Exp. Med. 211, 761–768 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu M. L., Nagavalli A., Su M. A., Aire deficiency promotes TRP-1-specific immune rejection of melanoma. Cancer Res. 73, 2104–2116 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moran A. E., et al. , T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J. Exp. Med. 208, 1279–1289 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhan Y., et al. , Defects in the Bcl-2-regulated apoptotic pathway lead to preferential increase of CD25 low Foxp3+ anergic CD4+ T cells. J. Immunol. 187, 1566–1577 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pierson W., et al. , Antiapoptotic Mcl-1 is critical for the survival and niche-filling capacity of Foxp3+ regulatory T cells. Nat. Immunol. 14, 959–965 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bouillet P., et al. , Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science 286, 1735–1738 (1999). [DOI] [PubMed] [Google Scholar]
- 38.Hogquist K. A., et al. , T cell receptor antagonist peptides induce positive selection. Cell 76, 17–27 (1994). [DOI] [PubMed] [Google Scholar]
- 39.Teh C. E., et al. , Linear ubiquitin chain assembly complex coordinates late thymic T-cell differentiation and regulatory T-cell homeostasis. Nat. Commun. 7, 13353 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gray D. H., Gavanescu I., Benoist C., Mathis D., Danger-free autoimmune disease in Aire-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 104, 18193–18198 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.