Abstract
Background and Objectives
Evaluating and understanding the heterogeneity in dementia course has important implications for clinical practice, health care decision-making, and research. However, inconsistent findings have been reported with regard to the disease courses of the 2 most common dementias: Alzheimer disease (AD) and dementia with Lewy bodies (DLB). Using autopsy-confirmed diagnoses, we aimed to examine the disease trajectories in the years before death among patients with dementia with pure AD, pure DLB, or mixed (AD and DLB) pathologies.
Methods
The current retrospective longitudinal study included 62 participants with autopsy-confirmed diagnoses of pure AD (n = 34), mixed AD and DLB (AD + DLB; n = 17), or pure DLB (n = 11) from the Predictors 2 Cohort Study, a prospective, clinic-based, cohort of patients with dementia. Generalized estimating equation models, with time zero at death, were used to examine the trajectory of cognition (Folstein Mini-Mental State Examination [MMSE]), function (activities of daily living [ADL]), and Dependence Scale among patients with different autopsy-confirmed diagnosis (pure AD, AD + DLB, and pure DLB). The models were adjusted for age, sex, education, and baseline features including extrapyramidal signs, MMSE, ADL, and Dependence Scale.
Results
The participants on average received 9.4 ± 4.6 assessments at 6-month intervals during a mean 5.4 ± 2.9 years of follow-up. The 3 groups were similar in both cognition and function status at baseline. Cognition and function were highly correlated among patients with AD + DLB but not in pure AD or pure DLB at baseline. Patients of the 3 groups all declined in both cognition and function but had different trajectories of decline. More specifically, the patients with pure DLB experienced approximately double the rate of both cognitive decline and functional decline than the patients with pure AD, and the mixed pathology group showed double the rate of functional decline as compared to pure AD.
Discussion
In this longitudinal study, we found that among patients with dementia, those with Lewy body pathology experienced faster cognitive and functional decline than those with pure AD pathology.
Alzheimer disease (AD), the leading cause of dementia, is clinically characterized by progressive memory and functional decline and pathologically characterized by neurofibrillary tau tangles beginning in the medial temporal lobes and amyloid plaques starting in the neocortex.1 Dementia with Lewy bodies (DLB), the second most common type of dementia, has core clinical features including cognitive fluctuations, extrapyramidal motor features, REM sleep behavior disorder, and visual hallucinations and is pathologically characterized by the accumulation of aggregated α-synuclein into Lewy bodies and Lewy neurites in neurons and neuronal processes.2 Understanding differences in how these diseases progress has important implications for clinical management, health care utilization, and decision-making.
The current literature regarding the trajectory of clinical symptoms in AD compared with DLB shows inconsistent results. Some studies suggest that DLB has a faster decline relative to AD,3-8 but not others.9-11 Part of this inconsistency may be due to changes over time in clinical diagnosis guidelines.12 Moreover, past studies have relied mainly on clinical diagnoses, which likely represent a mixture of underlying pathologies.13-17 For example, approximately half of patients with AD also have α-synuclein pathology of the Lewy body type,2,13,18,19 and similarly, up to half of patients with LBD have pathology characteristic of AD.16,20
In contrast to clinical diagnoses, studies based on pathologically confirmed diagnoses provide an objective gold standard of disease and thus are needed to fully understand disease trajectories across these illnesses. Whereas cross-sectional neuropathologic studies provide a snapshot of clinical symptoms at specific disease stages and inform the pathologic substrates of specific clinical symptoms,19,21-24 longitudinal studies with pathologic dementia diagnosis are necessary to understand the overall trajectory of disease course. Such studies are scarce and often have few repeated antemortem clinical measurements, have short follow-up periods, begin at later disease stages, or do not follow patients to the end of life.5,11,14,15,24-31
In addition to cognitive impairment, loss of function, especially the ability to perform self-care tasks,32 is a defining feature of these degenerative diseases33 and is inevitably linked to dependence on family members or formal caregivers. However, few studies have examined the trajectories of noncognitive features, such as functioning and dependence. In the current study, we aimed to compare the trajectories of key clinical features, including cognition, function, and dependence, in 3 autopsy-confirmed groups—AD, AD + DLB, and DLB—based on the Predictors 2 study,34 a longitudinal, multicenter, clinic-based study with detailed biannual clinical assessments designed to predict major disease outcomes in AD and DLB.
Methods
Participants
The participants of the current study were from the Predictors 2 study, a cohort of patients with dementia clinically diagnosed with predominantly AD but also DLB.34 Recruitment of this cohort was initiated in 1997 following the same methods as the Predictors 1 cohort.34 Patients who were diagnosed with mild to moderate probable dementia in the clinic were referred by their physicians to be recruited into this study. Participants were then followed up every 6 months with repeated clinical measurements including medical, neuropsychological, functional, and dependence measures. AD was clinically diagnosed according to National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer’s Disease and Related Disorders Association criteria33 and DLB was diagnosed according to the 1996 Consensus Guidelines36 for probable DLB. A total of 211 participants with probable AD and 28 with DLB were recruited into the cohort at 3 sites: Columbia University, Johns Hopkins University, and Massachusetts General Hospital. We limited our analyses to participants who had pathologic data available and had longitudinal measures of clinical symptoms. Among 78 participants who donated brains, we excluded 8 participants who did not have α-synuclein immunohistochemistry staining to confirm the presence of Lewy body disease/synucleinopathy, 7 participants who did not have pathologic features required for AD or DLB diagnosis, and 1 participant who had no follow-up visits to assess clinical trajectory. Thus, the current study included 62 participants who had autopsy-confirmed diagnosis of AD (n = 34), mixed AD and DLB (n = 17), or DLB (n = 11), and had longitudinal measure of clinical symptoms. The 16 excluded participants were not significantly different from the included participants in age, sex, education, or baseline clinical symptoms.
Standard Protocol Approvals, Registrations, and Patient Consents
The project was approved by the institutional review board at each of the 3 study sites. All patients and their proxy decision makers provided written informed consent.
Cognitive Measures
Participants underwent detailed cognitive and clinical assessment at baseline and follow-up visits. Global cognitive status was assessed with the Folstein Mini-Mental State Examination (MMSE) (0–30, with higher score indicating better cognitive performance).
Functional Assessment
Functional capacity of the patients was reported by the patient's reliable informant using the Blessed Dementia Rating Scale activities of daily living (ADL) subscore,32 including 7 instrumental ADL items (difficulty performing chores around the house [e.g., cleaning], handling money, remembering short lists [e.g., shopping], walking across a room, walking several blocks, recognizing one's whereabouts, and remembering things that happened recently) and 3 basic ADL items (eating, dressing, and bladder and bowel control). The response options for instrumental ADL items were none (0), some (0.5), and a lot of difficulty (1), and for basic ADL items, ranged from 0 to 3, with higher score indicating more difficulty. The total ADL score was the sum of scores on all 10 items (range 0–16), with higher scores indicating worse functional capacity. The ADL scale has good reliability and validity, with reliability coefficients reported to be between 0.60 and 0.80.32
Dependence Scale
The Dependence Scale (DS) was developed to measure the amount of assistance patients with AD require to fulfill daily functions.35 The DS was administered to the patient's reliable informant who lived with the patient or one who was well informed about the patient's daily activities and needs. The DS consists of 13 items representing different levels of care required by a patient, from mild (e.g., “Does the patient need frequent help finding misplaced objects?”) to severe (e.g., “Does the patient need to be tube fed?”). Two items (“Needs reminders to manage chores”; “Needs help to remember important things such as appointments”) are coded as 0 (no), 1 (occasionally, at least once a month), or 2 (frequently, at least once a week), while responses to the rest of the items are coded dichotomously, indicating whether the patient requires assistance in a particular item (0 = no, 1 = yes). The total DS score ranges from 0 to 15, with higher score indicating greater dependence on others. The DS has strong psychometric properties and is reliable and valid, with reliability coefficients ranging between 0.66 and 0.93.35,36 It is related to, but distinct from, existing cognitive, functional, and behavioral measures of disease severity, and predicts disease progression independent of other measures of functional and cognitive status.35,36
Other Demographic and Clinical Measurements
Patient age at baseline, sex, and highest level of education were recorded. Sex was used as a dichotomous variable with male as the reference group. Age and years of education were used as continuous variables. The Columbia University Scale for Psychopathology in Alzheimer's Disease was used to measure patients' psychotic, behavioral, and depressive symptoms.37 The Unified Parkinson's Disease Rating Scale38 was used to measure extrapyramidal signs (EPS) and treated as a binary variable, with 1 indicating severity rating of mild to moderate or greater on any item.
Pathologic Diagnoses
The pathologic categorization for each case into AD, DLB, or AD + DLB was based on review of neuropathologic reports, and slides if necessary, by a coauthor (J.B.L.), and staging of AD and Lewy body pathology as outlined in the National Institute on Aging–Alzheimer's Association pathologic assessment of AD and Lewy body disease.39 For this study, an AD pathologic diagnosis required both a staging of moderate or frequent neuritic plaques (Consortium to Establish a Registry for Alzheimer’s Disease criteria)40 and Braak stage IV, V, or VI neurofibrillary tangle stage.41 Braak stages IV, V, and VI have been consistently associated with clinical dementia.42,43 For a pathologic DLB classification, Lewy body pathology required a staging of either limbic or neocortical Lewy body disease.44 Participants were diagnosed with pure AD if they had above-mentioned AD neuropathologic changes but no Lewy body neuropathologic changes or with insufficient Lewy body pathology density or spread to meet criteria for DLB, pure DLB if they had limbic or neocortical Lewy body neuropathologic changes but insufficient neuropathologic changes for AD, AD + DLB if they met the above-defined neuropathologic changes for both DLB and AD, or negative pathology if they did not meet the pathologic criteria for either AD or DLB as defined above.
Statistical Analysis
The demographic and clinical characteristics were summarized by mean and SD for continuous measures and by frequency and proportions for categorical measures. The measures were compared among the 3 autopsy-confirmed groups using χ2 test for categorical variables and one-way analysis of variance for continuous variables. Pearson correlation coefficient was used to examine the relationship among baseline MMSE, ADL, and DS.
We used generalized estimating equation (GEE) models, with linear identity link function and independent working correlation matrix structure, to examine the trajectory of the outcomes. The time variable was the main predictor in the model, which was calculated as years before death, with time zeroed on confirmed death date and a visit occurring 1 year before death coded as −1, 2 years before death coded as −2, et cetera.3 The model was adjusted for age, sex, education, the autopsy-confirmed diagnosis group (pure AD, AD + DLB, pure DLB), and baseline features including EPS, MMSE, ADL, and DS.
To compare the trajectories among the diagnosis groups (as the predictor, treated as a categorical variable with pure AD being the reference group) separately for the 3 outcomes (i.e., MMSE, ADL, DS), an interaction term between diagnosis group × time was added to the GEE model, with a significant interaction indicating different trajectory rate between AD + DLB and pure AD and between pure DLB and pure AD. The models were adjusted for age, sex, education, and EPS at baseline (model 1), and in addition adjusted for the outcome status at baseline, individually (model 2) and simultaneously (model 3). Nonlinear trajectories of decline including quadratic and piecewise regression were assessed for suitability but did not improve model fit over the linear alternative, consistent with the literature.15
Because of the unbalanced duration from baseline to death among diagnostic groups, we performed sensitivity analyses by limiting the analysis to the last 5 years of life and repeated the analyses. Because APOE was missing in a quarter of the participants (n = 15) and cholinesterase inhibitor use was missing in 11 participants, we did not include these 2 variables in the main analyses but performed sensitivity analyses adjusting for APOE and cholinesterase inhibitor use in addition to model 3 covariates.
All analyses were conducted using SPSS 26. Due to the exploratory nature of this analysis, the significance level was defined as p < 0.05 without corrections for multiple comparisons.
Data Availability
Deidentified participant data will be made available to qualified investigators with appropriate data transfer agreements and institutional board approval.
Results
Characteristics of the Patients With Dementia With Postmortem Diagnoses of Pure AD, AD + DLB, and Pure DLB
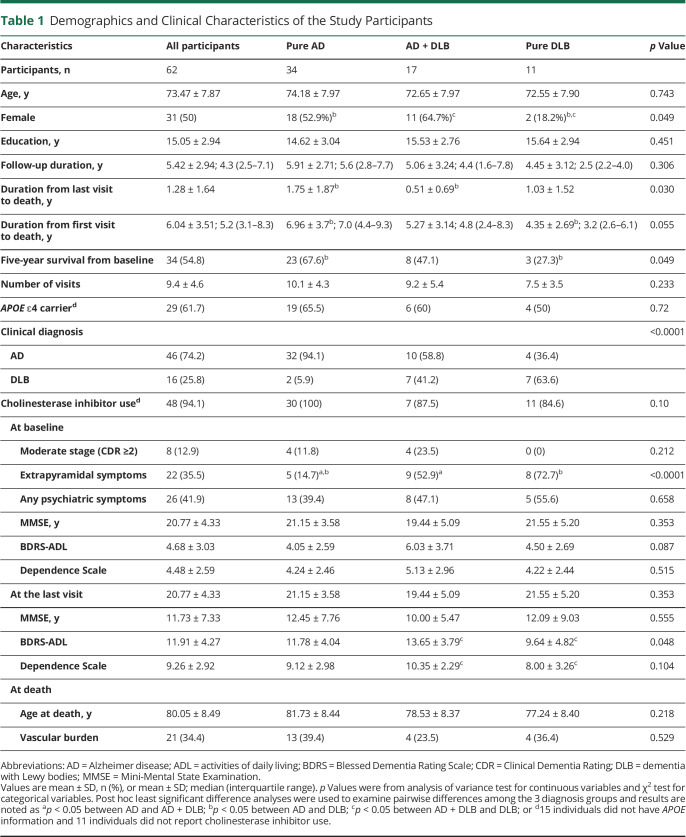
At time of recruitment, the participants were 73.5 ± 7.9 (mean ± SD) years old, had 15 ± 2.9 years of education, and scored 20.8 ± 4.3 on MMSE, 4.68 ± 3.0 on ADL, and 4.48 ± 2.6 on DS (Table 1). The majority (87%) of the participants had mild dementia, with the other 13% having moderate dementia (Clinical Dementia Rating [CDR] ≥2). Half of the participants were female, 36% had EPS, and 42% had psychotic symptoms (Table 1). At baseline, MMSE was negatively correlated with ADL (r = −0.51; p < 0.0001) and DS (r = −0.43; p < 0.0001), whereas the latter 2 were positively correlated (r = 0.79; p < 0.0001). The participants on average received 9.4 ± 4.6 assessments at 6-month intervals during a mean 5.4 ± 2.9 years of follow-up time, and survived 6.0 ± 3.5 years, with 54.8% surviving at least 5 years (Table 1).
Table 1.
Demographics and Clinical Characteristics of the Study Participants
About two-thirds of the study participants entered the study with a clinical diagnosis of AD (cAD), 91% (42 of 46) of whom were confirmed to have AD pathology; among patients with a clinical diagnosis of DLB (cDLB), 88% (14 of 16) were found to have Lewy body neuropathologic changes on autopsy. However, 30% (14/46) of the patients with cAD also had Lewy body neuropathologic changes, and 56% (9/16) of the patients with cDLB had AD pathology.
Patients with pure DLB were more likely to be men compared with the pure AD (p = 0.044) or AD + DLB (p = 0.016) groups, whereas the latter 2 groups did not differ (p = 0.424). Patients with pure AD were less likely to have EPS compared with the AD + DLB (p = 0.004) or pure DLB (p < 0.0001) groups, whereas the latter 2 did not differ (p = 0.295). The patients with pure AD on average survived longer than patients with pure DLB from baseline (7.0 ± 3.7 vs 4.4 ± 2.7 years; p = 0.031). Five-year survival rate was higher (p = 0.018) among the patients with pure AD (68%) than in patients with pure DLB (27%).
At the last visit, patients with AD + DLB had worse ADL (p = 0.015) and DS (p = 0.038) scores than the patients with pure DLB. The patients with AD + DLB were followed up to a later stage of life compared with patients with pure AD (p = 0.01).
The pairwise correlations among MMSE, ADL, and DS were all statistically significant in patients with AD + DLB (r = −0.85 for MMSE–ADL, r = −0.83 for MMSE–DS, and r = 0.93 ADL–DS correlations; p < 0.0001 for all). ADL was highly correlated with DS in both pure AD (r = 0.76; p < 0.0001) and pure DLB (r = 0.71; p = 0.014). However, MMSE was not correlated with ADL in pure AD (r = −0.25; p = 0.152) or pure DLB (r = −0.39; p = 0.237), nor with DS in pure AD (r = −0.20; p = 0.277) or pure DLB (r = −0.55; p = 0.077).
Change of Cognition and Function in Patients With Dementia at the End of Life
Among all participants, there was a significant change over time for all outcomes. Specifically, MMSE declined 0.74 (SE 0.17; p < 0.0001), ADL increased 0.66 (SE 0.14; p < 0.0001), and DS increased 0.47 (SE 0.09; p < 0.0001) points per year.
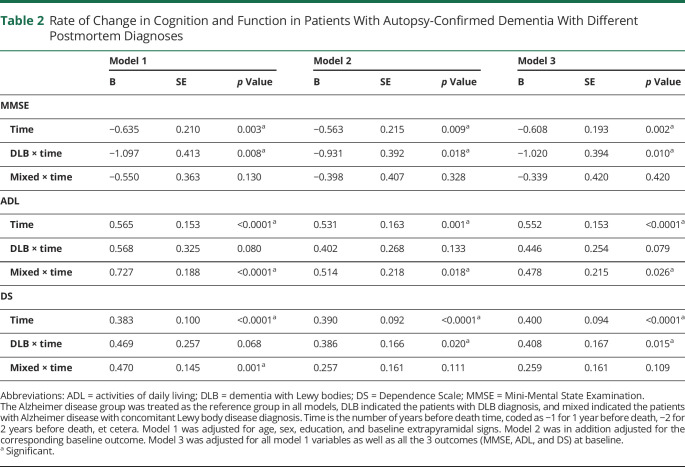
Differential Trajectory of Cognition and Function Among Patients With Dementia With Postmortem Diagnoses of Pure AD, AD + DLB, or Pure DLB
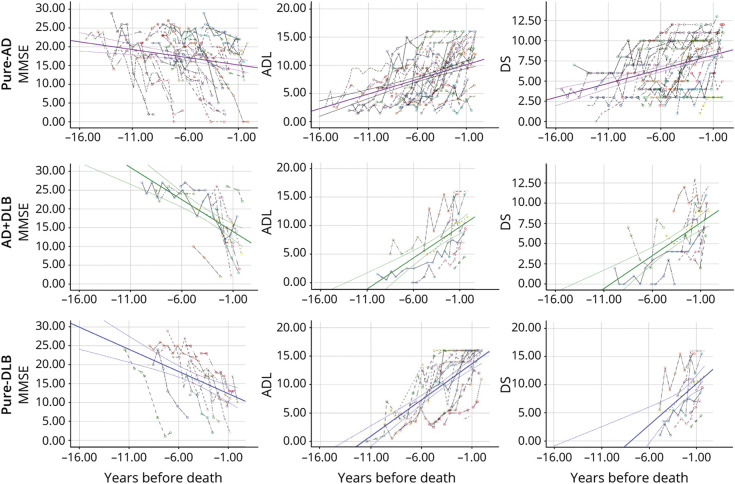
For patients with pure AD, MMSE declined 0.61 (SE 0.19; p = 0.002), ADL increased 0.55 (SE 0.15; p < 0.0001), and DS increased 0.40 (SE 0.09; p < 0.0001) points per year (Table 2, model 3; Figure). Compared with the pure AD group, the pure DLB group experienced a faster decline in MMSE (b = −1.02 [SE 0.39]; p = 0.010) and faster deterioration in DS (b = 0.41 [SE 0.17]; p = 0.015) in the fully adjusted model (Table 2, model 3; Figure). That is, compared with a patient with pure AD who experienced a 0.61-point decline in MMSE in 1 year, a patient with pure DLB of similar characteristics would decline an additional 1.02 points, or a total of 1.63 points per year (approximately 2.7 times of the rate in patients with pure AD). In other words, the changes a patient with pure DLB experienced in 1 year were similar to the changes typically seen in a patient with pure AD of similar characteristics in approximately 2.7, 1.8, and 2.0 years for MMSE, ADL, and DS, respectively.
Table 2.
Rate of Change in Cognition and Function in Patients With Autopsy-Confirmed Dementia With Different Postmortem Diagnoses
Figure. Cognition and Functional Change in the Years Preceding Death in Patients With Autopsy-Confirmed Dementia.
Cognition (Mini-Mental State Examination [MMSE]) and functional (activities of daily living [ADL] and Dependence Scale [DS]) changes in the years preceding death in patients with autopsy-confirmed dementia for patients with dementia with pure AD (purple), AD + DLB (green), or pure DLB (blue). Each colored dashed line shows the observed MMSE (first column), ADL (second column), and DS (third column) scores for each individual.
Compared with the pure AD group, the AD + DLB group experienced a faster deterioration in ADL (b = 0.48 [SE 0.21]; p = 0.026) (Table 2, model 3; Figure). The annual change in ADL a patient with AD + DLB experienced was similar to the change typically seen in a patient with pure AD of similar characteristics in approximately 1.9 years.
There was no difference in the trajectory of MMSE, ADL, or DS between AD + DLB and pure DLB groups (data not shown).
Supplementary Analyses
Limiting the analyses to the assessments within 5 years preceding death, the results in the fully adjusted model were similar to the main results, even with larger effect sizes, although the results were no longer significant. Specifically, compared with the AD group, the DLB group experienced a nonsignificant faster deterioration in MMSE (b = −1.50 [SE 0.96]; p = 0.120), DS (b = 0.53 [SE 0.32]; p = 0.096), and ADL (b = 0.81 [SE 0.50]; p = 0.106). There was no difference between the pure AD and AD + DLB groups. Compared with the AD + DLB group, the pure DLB group experienced a significantly faster deterioration in DS (b = 0.77 [SE 0.31]; p = 0.01) and in ADL (b = 0.94 [SE 0.48]; p = 0.053), but not in MMSE (b = −1.10 [SE 0.93]; p = 0.24).
When we included APOE status (ɛ4 carriers, noncarriers, and unknown) into model 3, we found the results were attenuated but remained similar to the main findings. The results remained similar to main results when cholinesterase inhibitor use was further adjusted in the models (data not shown).
Discussion
In this clinic-based, longitudinal study of an autopsy-confirmed cohort of patients with dementia, we described and compared the trajectories among pure AD, pure DLB, and AD + DLB. We found the 3 groups were similar in both cognitive and functional status at baseline but had different trajectories of decline. In comparison with the pure AD group, the patients with pure DLB experienced approximately doubled rates of cognitive and functional decline, and the AD + DLB group experienced approximately doubled rates of functional decline only.
The clinical diagnoses of the study participants were confirmed in most patients by presence of the corresponding pathologic features in autopsy. However, consistent with the literature, a large proportion of the patients also had other neuropathologic changes.2,13,16,18,20 The current study showed that patients with pure AD were less likely to have EPS19,22 at study enrollment than those with Lewy body neuropathology.
We found that baseline cognition and function were highly correlated in AD + DLB but not in pure AD or pure DLB, probably due to the mixed pathologies affecting a wider range of brain areas responsible for the phenotypes. The correlation between cognition and function has rarely been evaluated in the literature and the only autopsy-based study evaluated both cognition and function and found that cognition predicted ADL in both autopsy-confirmed AD and DLB.45 In clinically diagnosed AD and DLB, the additional functional impairments in DLB compared with AD were found to be mainly attributable to extrapyramidal motor symptoms.46 Thus, the stronger cognition–function correlation in AD + DLB than in pure AD in the current study might at least partially be due to the higher prevalence of extrapyramidal symptoms in AD + DLB. Meanwhile, cognition fluctuation is one of the core clinical features in DLB,2 whereas the patients with AD + DLB might have progressive cognitive deterioration and more consistent cognitive impairment due to the underlying AD pathology, thus overriding the cognitive fluctuation feature and leading to a stronger cognition–function correlation compared with patients with pure DLB.2,11 More studies are needed to confirm our findings and explore the reasons for differences in cognition–function correlation in patients with AD + DLB than in patients with pure AD or pure DLB. Such studies may also help confirm whether EPS and a parallel impairment in cognition and function seen in a patient with clinically diagnosed AD indicates underlying mixed AD and DLB pathologies.
We found that individual key cognitive and functional features were similar across the 3 groups at enrollment and at the last visit, except that the patients with AD + DLB were functionally worse compared with the pure DLB group at the last visit, which does not seem to be explained by the starting level, assessments’ proximity to the death time, or duration of follow-up. In addition, although there was no significant difference in symptoms at the last assessment between pure AD and pure DLB groups, it is unclear whether patients with pure AD would have been worse than those with pure DLB at the last visit if the patients with pure AD continued to be assessed until a later date. Cross-sectional neuropathologic studies have suggested that concomitant Lewy body pathology makes little difference on the clinical phenotype of AD.19,21-24 With the potential limitations of such snapshot analyses mentioned above, however, it may be important to examine the longitudinal trajectory to capture the full disease course.
Many longitudinal studies have focused on comparing the duration of disease or survival. Consistent with the reported 1.6 years shorter survival in cDLB than in cAD in 11 previous studies,3,4 we found that patients with pure AD had longer disease duration than patients with pure DLB, suggesting that patients with pure AD have better survival than patients with pure DLB. In one study9 using CSF biomarkers to assess pathology, it was found that patients with AD + DLB had a higher risk of nursing home admittance and death, whereas no differences in the rate of cognitive decline were found between groups. The study had a mean 1.65 years of follow-up and neuropsychological scores were derived from multiple imputation due to missing data. Another study found different survival rates but similar cognitive trajectories between autopsy-diagnosed AD and DLB, but the study was limited by having few repeated cognitive measures.26 In contrast, some studies did not find a significant difference in disease duration or survival between patients with different pathologies.21 In another study, disease duration was not different across the 3 groups, but the AD + DLB and DLB groups had a faster cognitive decline than the AD group.25 One potential limitation of studies examining survival, however, is that the disease duration or survival can be influenced by many factors, such as comorbidities, especially cardiovascular diseases and pneumonia, hospice use, and end-of-life treatment intensity. Thus, disease course is best described not by the survival time itself, but rather by direct, repeated measurements of the key clinical features of the disease, ideally through a reasonably long follow-up time with multiple measures.
With an average of 9 repeated measurements over 5 years, we found the 3 groups had different rates of decline, with the patients with pure DLB or AD + DLB experiencing approximately doubled rates of decline as compared with the patients with pure AD. Only a few longitudinal autopsy studies have examined cognitive trajectories or overall disease stage trajectories. Some studies have suggested that patients with AD + DLB exhibit faster cognitive decline or disease progression compared with pure AD.11,15,25 However, other studies report no difference in rate of cognitive decline.14,24,26,27 In a large national autopsy sample from the National Alzheimer's Coordinating Center, patients with AD + DLB were found to have faster decline on the CDR–sum of boxes than did patients with pure AD.15 In the Arizona Study of Aging and Neurodegenerative Disorders study, the AD with Lewy bodies group (those with Lewy body pathology restricted to a limbic-predominant stage but not yet in the neocortical regions), but not the pure DLB group, had a significantly greater MMSE decline compared with the pure AD group.5 There are few autopsy-based studies comparing disease progression of pure DLB with pure AD, or pure DLB with AD + DLB.28 We found faster cognitive decline in pure DLB than in pure AD in our study, consistent with previous findings that showed a faster MMSE decline25,31 or shorter survival26,31 in pure DLB than in pure AD. However, in several pathologic studies, patients with pure DLB did not seem to decline significantly faster than patients with pure AD.5,15,24,26,29,30 Several previous studies have found that there was no difference in survival between pure DLB and AD + DLB groups,14,26 consistent with our findings.
Therefore, existing evidence has been inconsistent, but rarely have studies found patients with pure AD to decline faster than those with pure DLB or AD + DLB. Given the emerging evidence suggesting that patients with AD + DLB and patients with pure DLB may have faster decline than patients with pure AD, it is possible that Lewy body pathology might play a key role in aggressive disease progression. The faster decline in AD + DLB than in pure AD can be due to increased neurodegeneration as a result of the multiple pathologies.47 More research is needed to fully understand the potential synergistic interactions of AD and DLB pathology at molecular levels. In addition, as the neocortical type (diffuse) and limbic type (transitional) of Lewy body pathology may have different patterns of cognitive decline for certain cognitive domains14 and survival rates,48,49 future studies may investigate the different types of Lewy body pathology and use more specific cognitive measures.
Functional trajectory has rarely been compared among autopsy-confirmed patients with dementia. We previously reported that compared with patients with AD, patients with DLB were significantly more impaired in ADLs and showed greater dependence on caregivers at first evaluation, but there were no significant differences in the rate of decline between the 2 groups.29 In a separate cross-sectional study, no difference in functional impairment was observed between AD and DLB.24 Findings from the current study that both AD + DLB and pure DLB groups had faster functional decline than the pure AD group may point to a role of Lewy body pathology in functional changes of patients with dementia.
This study has some limitations. Incident dementia cases were not examined, so we may have missed the observation of the earliest period of the disease. However, most of the participants were at the mild stage of dementia when enrolled into the study, and the age at baseline was similar to the age at onset of dementia symptoms reported by other studies.3 Similar to other neuropathologic studies that have an overrepresentation of APOE ɛ4 carriers,9,22 the current study also found a high percentage of participants carrying an APOE ɛ4 allele, which might indicate a potential selection bias. The MMSE is an overall measure of cognition and more specific neuropsychological tests tapping into individual cognitive domains might have larger or smaller difference among the 3 pathologic groups.19 We only examined a few cognitive and functional measures; however, other clinical symptoms, such as urinary incontinence, that show higher prevalence in DLB than in AD50 are worth exploration in future studies. Although the clinical symptoms are assumed to be driven by the underlying neuropathologic changes, the postmortem neuropathologic assessments may not reflect neuropathologic burden when clinical progression was measured as neuropathologic changes might continue to accumulate over time. However, limiting the analyses to the last 5 years before death found similar results to the main findings. We did not adjust for APOE in our main analysis because a large number of participants did not have APOE ɛ4 information. However, sensitivity analyses taking APOE into consideration did not change the main findings, similar to previous reports.17 Previous studies showed that individuals meeting neuropathologic criteria for AD and having insufficient Lewy body pathology to meet distribution and density thresholds for DLB may have a faster clinical course than those with pure AD.5 We did not examine this group separately due to small number of participants (n = 6). However, including these participants in the pure AD group may have biased our results toward null and would not change our main findings that the pure DLB and AD + DLB groups had faster decline than participants with pure AD. The relatively small sample size also limited our ability to perform additional subgroup analyses according to severity of AD neuropathology, types of Lewy body pathology,15,48,49 or sex.26 Finally, the study participants were predominantly White and well-educated, limiting the generalizability of the findings.
Our study adds innovative information to the literature by providing an almost complete disease history for patients with pure AD, AD + DLB, or pure DLB. The clinical symptoms were measured directly and frequently (biannually, on average 9 antemortem visits) throughout the disease course until close to death, ensuring more accurate and reliable information compared with less frequent measures. We performed comprehensive clinical assessments in a standardized and consistent manner. To our knowledge, the current study is the first to examine the functional and dependence trajectory among patients with dementia. The pathologic data were carefully reviewed and diagnosed by an experienced neuropathologist according to the most recent guidelines. We included 3 diagnosis groups to provide a more comprehensive comparison of these conditions involving AD and DLB pathologies.
In this longitudinal study, we that found patients with dementia with Lewy body pathology experienced faster cognitive and functional decline than patients with pure AD. The findings of this autopsy-based study have implications for clinical management, future clinical study design, and research on pathology-specific biomarkers.
Glossary
- AD
Alzheimer disease
- ADL
activities of daily living
- cAD
clinical diagnosis of Alzheimer disease
- cDLB
clinical diagnosis of dementia with Lewy bodies
- CDR
Clinical Dementia Rating
- DLB
dementia with Lewy bodies
- DS
Dependence Scale
- EPS
extrapyramidal signs
- GEE
generalized estimating equation
- MMSE
Mini-Mental State Examination
Appendix. Authors

Footnotes
CME Course: NPub.org/cmelist
Study Funding
This study was funded by the National Institute on Aging (AG007370).
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's disease. Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. Neurobiol Aging. 1997;18:S1-S2. [PubMed] [Google Scholar]
- 2.McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology. 2017;89(1):88-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mueller C, Soysal P, Rongve A, et al. Survival time and differences between dementia with Lewy bodies and Alzheimer's disease following diagnosis: a meta-analysis of longitudinal studies. Ageing Res Rev. 2019;50:72-80. [DOI] [PubMed] [Google Scholar]
- 4.Price A, Farooq R, Yuan JM, Menon VB, Cardinal RN, O'Brien JT. Mortality in dementia with Lewy bodies compared with Alzheimer's dementia: a retrospective naturalistic cohort study. BMJ Open. 2017;7(11):e017504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malek-Ahmadi M, Beach TG, Zamrini E, et al. Faster cognitive decline in dementia due to Alzheimer disease with clinically undiagnosed Lewy body disease. PLoS One. 2019;14:e0217566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kramberger MG, Auestad B, Garcia-Ptacek S, et al. Long-term cognitive decline in dementia with Lewy bodies in a large multicenter, international cohort. J Alzheimers Dis. 2017;57(3):787-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson RS, Capuano AW, Bennett DA, Schneider JA, Boyle PA. Temporal course of neurodegenerative effects on cognition in old age. Neuropsychology. 2016;30(5):591-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galasko DR, Gould RL, Abramson IS, Salmon DP. Measuring cognitive change in a cohort of patients with Alzheimer's disease. Stat Med. 2000;19:1421-1432. [DOI] [PubMed] [Google Scholar]
- 9.Lemstra AW, de Beer MH, Teunissen CE, et al. Concomitant AD pathology affects clinical manifestation and survival in dementia with Lewy bodies. J Neurol Neurosurg Psychiatry. 2017;88(2):113-118. [DOI] [PubMed] [Google Scholar]
- 10.Breitve MH, Chwiszczuk LJ, Hynninen MJ, et al. A systematic review of cognitive decline in dementia with Lewy bodies versus Alzheimer's disease. Alzheimers Res Ther. 2014;6(5-8):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kraybill ML, Larson EB, Tsuang DW, et al. Cognitive differences in dementia patients with autopsy-verified AD, Lewy body pathology, or both. Neurology. 2005;64(12):2069-2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamada M, Komatsu J, Nakamura K, et al. Diagnostic criteria for dementia with Lewy bodies: updates and future directions. J Mov Disord. 2020;13(1):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamilton RL. Lewy bodies in Alzheimer's disease: a neuropathological review of 145 cases using alpha-synuclein immunohistochemistry. Brain Pathol. 2000;10(3):378-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schneider JA, Arvanitakis Z, Yu L, Boyle PA, Leurgans SE, Bennett DA. Cognitive impairment, decline and fluctuations in older community-dwelling subjects with Lewy bodies. Brain. 2012;135(pt 10):3005-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brenowitz WD, Hubbard RA, Keene CD, et al. Mixed neuropathologies and estimated rates of clinical progression in a large autopsy sample. Alzheimers Dement. 2017;13(6):654-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irwin DJ, Hurtig HI. The contribution of tau, amyloid-beta and alpha-synuclein pathology to dementia in Lewy body disorders. J Alzheimers Dis Parkinsonism. 2018;8(4):444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brenowitz WD, Keene CD, Hawes SE, et al. Alzheimer's disease neuropathologic change, Lewy body disease, and vascular brain injury in clinic- and community-based samples. Neurobiol Aging. 2017;53:83-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nelson PT, Abner EL, Schmitt FA, et al. Modeling the association between 43 different clinical and pathological variables and the severity of cognitive impairment in a large autopsy cohort of elderly persons. Brain Pathol. 2010;20(1):66-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshizawa H, Vonsattel JP, Honig LS. Early neuropsychological discriminants for Lewy body disease: an autopsy series. J Neurol Neurosurg Psychiatry. 2013;84(12):1326-1330. [DOI] [PubMed] [Google Scholar]
- 20.Barker WW, Luis CA, Kashuba A, et al. Relative frequencies of Alzheimer disease, Lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the State of Florida Brain Bank. Alzheimer Dis Assoc Disord. 2002;16:203-212. [DOI] [PubMed] [Google Scholar]
- 21.Roudil J, Deramecourt V, Dufournet B, et al. Influence of Lewy pathology on Alzheimer's disease phenotype: a retrospective clinico-pathological study. J Alzheimers Dis. 2018;63(4):1317-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Savica R, Beach TG, Hentz JG, et al. Lewy body pathology in Alzheimer's disease: a clinicopathological prospective study. Acta Neurol Scand. 2019;139(1):76-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stern Y, Jacobs D, Goldman J, et al. An investigation of clinical correlates of Lewy bodies in autopsy-proven Alzheimer disease. Arch Neurol. 2001;58(3):460-465. [DOI] [PubMed] [Google Scholar]
- 24.Lopez OL, Hamilton RL, Becker JT, Wisniewski S, Kaufer DI, DeKosky ST. Severity of cognitive impairment and the clinical diagnosis of AD with Lewy bodies. Neurology. 2000;54(9):1780-1787. [DOI] [PubMed] [Google Scholar]
- 25.Nelson PT, Kryscio RJ, Jicha GA, et al. Relative preservation of MMSE scores in autopsy-proven dementia with Lewy bodies. Neurology. 2009;73(14):1127-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams MM, Xiong C, Morris JC, Galvin JE. Survival and mortality differences between dementia with Lewy bodies vs Alzheimer disease. Neurology. 2006;67(11):1935-1941. [DOI] [PubMed] [Google Scholar]
- 27.Hamilton JM, Salmon DP, Galasko D, et al. Visuospatial deficits predict rate of cognitive decline in autopsy-verified dementia with Lewy bodies. Neuropsychology. 2008;22(6):729-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Breitve MH, Chwiszczuk LJ, Brønnick K, et al. A longitudinal study of neurocognition in dementia with Lewy bodies compared to Alzheimer's disease. Front Neurol. 2018;9:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stavitsky K, Brickman AM, Scarmeas N, et al. The progression of cognition, psychiatric symptoms, and functional abilities in dementia with Lewy bodies and Alzheimer disease. Arch Neurol. 2006;63(10):1450-1456. [DOI] [PubMed] [Google Scholar]
- 30.Ballard CG, O'Brien J, Lowery K, et al. A prospective study of dementia with Lewy bodies. Age Ageing. 1998;27(5):631-636. [DOI] [PubMed] [Google Scholar]
- 31.Olichney JM, Galasko D, Salmon DP, et al. Cognitive decline is faster in Lewy body variant than in Alzheimer's disease. Neurology. 1998;51(2):351-357. [DOI] [PubMed] [Google Scholar]
- 32.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114(512):797-811. [DOI] [PubMed] [Google Scholar]
- 33.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939-944. [DOI] [PubMed] [Google Scholar]
- 34.Stern Y, Folstein M, Albert M, et al. Multicenter study of predictors of disease course in Alzheimer disease (the “predictors study”): I: study design, cohort description, and intersite comparisons. Alzheimer Dis Assoc Disord. 1993;7:3-21. [DOI] [PubMed] [Google Scholar]
- 35.Stern Y, Albert SM, Sano M, et al. Assessing patient dependence in Alzheimer's disease. J Gerontol. 1994;49(5):M216-M222. [DOI] [PubMed] [Google Scholar]
- 36.Lenderking WR, Wyrwich KW, Stolar M, et al. Reliability, validity, and interpretation of the dependence scale in mild to moderately severe Alzheimer's disease. Am J Alzheimers Dis Other Dement. 2013;28(8):738-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Devanand DP. Use of the Columbia University Scale to assess psychopathology in Alzheimer's disease. Int Psychogeriatr. 1997;9(suppl 1):137-142; discussion 143-150. [DOI] [PubMed] [Google Scholar]
- 38.Louis ED, Lynch T, Marder K, Fahn S. Reliability of patient completion of the historical section of the Unified Parkinson's Disease Rating Scale. Mov Disord. 1996;11(2):185-192. [DOI] [PubMed] [Google Scholar]
- 39.Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol. 2012;123(1):1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD): part II: standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41(4):479-486. [DOI] [PubMed] [Google Scholar]
- 41.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239-259. [DOI] [PubMed] [Google Scholar]
- 42.Knopman DS, Parisi JE, Salviati A, et al. Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol. 2003;62(11):1087-1095. [DOI] [PubMed] [Google Scholar]
- 43.Gold G, Bouras C, Kövari E, et al. Clinical validity of Braak neuropathological staging in the oldest-old. Acta Neuropathol. 2000;99(5):579-582; discussion 583-584. [DOI] [PubMed] [Google Scholar]
- 44.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863-1872. [DOI] [PubMed] [Google Scholar]
- 45.Hamilton JM, Salmon DP, Raman R, et al. Accounting for functional loss in Alzheimer's disease and dementia with Lewy bodies: beyond cognition. Alzheimers Dement. 2014;10(2):171-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McKeith IG, Rowan E, Askew K, et al. More severe functional impairment in dementia with Lewy bodies than Alzheimer disease is related to extrapyramidal motor dysfunction. Am J Geriatr Psychiatry. 2006;14(7):582-588. [DOI] [PubMed] [Google Scholar]
- 47.Nedelska Z, Ferman TJ, Boeve BF, et al. Pattern of brain atrophy rates in autopsy-confirmed dementia with Lewy bodies. Neurobiol Aging. 2015;36(1):452-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferman TJ, Aoki N, Crook JE, et al. The limbic and neocortical contribution of α-synuclein, tau, and amyloid β to disease duration in dementia with Lewy bodies. Alzheimers Dement. 2018;14(3):330-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Graff-Radford J, Aakre J, Savica R, et al. Duration and pathologic correlates of Lewy body disease. JAMA Neurol. 2017;74:310-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ransmayr GN, Holliger S, Schletterer K, et al. Lower urinary tract symptoms in dementia with Lewy bodies, Parkinson disease, and Alzheimer disease. Neurology. 2008;70(4):299-303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Deidentified participant data will be made available to qualified investigators with appropriate data transfer agreements and institutional board approval.