Abstract
Background and Objectives
Serum antioxidant vitamins and carotenoids may protect against neurodegeneration with age. We examined associations of these nutritional biomarkers with incident all-cause and Alzheimer disease (AD) dementia among US middle-aged and older adults.
Methods
Using data from the third National Health and Nutrition Examination Surveys (1988–1994), linked with Centers for Medicare & Medicaid follow-up data, we tested associations and interactions of serum vitamins A, C, and E and total and individual serum carotenoids and interactions with incident AD and all-cause dementia. Cox proportional hazards regression models were conducted.
Results
After ≤26 years follow-up (mean 16–17 years, 7,283 participants aged 45–90 years at baseline), serum lutein+zeaxanthin was associated with reduced risk of all-cause dementia (65+ age group), even in the lifestyle-adjusted model (per SD: hazard ratio [HR] 0.93, 95% CI 0.87–0.99; p = 0.037), but attenuated in comparison with a socioeconomic status (SES)–adjusted model (HR 0.92, 95% CI 0.86–0.93; p = 0.013). An inverse relationship was detected between serum β-cryptoxanthin (per SD increase) and all-cause dementia (45+ and 65+) for age- and sex-adjusted models (HR 0.86, 95% CI 0.80–0.93; p < 0.001 for 45+; HR 0.86, 95% CI 0.80–0.93; p = 0.001 for 65+), a relationship remaining strong in SES-adjusted models (HR 0.89, 95% CI 0.82–0.96; p = 0.006 for 45+; HR 0.88, 95% CI 0.81–0.96; p = 0.007 for 65+), but attenuated in subsequent models. Antagonistic interactions indicate putative protective effects of 1 carotenoid may be observed at lower levels other carotenoids or antioxidant vitamin.
Discussion
Incident all-cause dementia was inversely associated with serum lutein+zeaxanthin and β-cryptoxanthin levels. Further studies with time-dependent exposures and randomized trials are needed to test neuroprotective effects of supplementing the diet with select carotenoids.
Classification of Evidence
This study provides Class II evidence that incident all-cause dementia was inversely associated with serum lutein+zeaxanthin and β-cryptoxanthin levels.
Dementia of all causes and subtypes, including Alzheimer disease (AD), is a key determinant for disability and long-term institutionalization among older adults.1 Extending intact cognitive functioning into old age is an increasingly important public health challenge. Such an endeavor would have a sizeable effect on quality of life and costs of care in late life. Thus, a greater focus on and understanding of factors that alter the risk of dementia in general and AD dementia in particular is needed.2
Oxidative stress has received considerable attention over the past several decades given its possible role in neurodegenerative processes, such as AD, as well as other age-related conditions that affect cardiovascular health and some cancers.3 Oxidative stress is a form of metabolic stress that emerges due to an imbalance between the production of reactive oxygen species (ROS) and the antioxidant mechanisms that counteract it.2 The brain comprises a high concentration of lipid and iron content, potentially making neurons especially susceptible to these processes.4 For example, exposure to ROS can increase brain oxidative processes, which may become chronic due to the impaired DNA repair mechanisms that decline with age.5
Epidemiologic studies show that dietary intake of antioxidants (e.g., β-carotene, vitamins A, C, and E) may help mitigate oxidative DNA damage through the reduction of ROS.6 Such antioxidants, consumed via diet or supplements, may protect against neurodegenerative processes including cognitive decline.7 Studies have also revealed the potential for synergistic effects between some carotenoids and antioxidants.8 To date, however, no studies have investigated whether carotenoids in general may interact with each other and with vitamins A, C, or E in relation to incidence rates of AD or all-cause dementia.
In this report, we use longitudinal data from a large nationally representative sample of middle-aged and older adults to examine adjusted associations among several serum antioxidants and incidence of AD and all-cause dementia using a retrospective cohort design. Specifically, we examined relationships of serum vitamins A, C, and E with both incident outcomes across levels of serum total carotenoid intake and tested interactions of serum vitamin A, C, and E with serum total and individual carotenoids, namely α-carotene, β-carotene, lutein+zeaxanthin, β-cryptoxanthin, and lycopene, in relation to the 2 incident outcomes. Interactions between individual carotenoids were also tested in relation to the 2 incident outcomes of interest.
Methods
Database
Participants from the Third National Health and Nutrition Examination Survey (NHANES III) comprised a cross-sectional sample representative of the US civilian noninstitutionalized population obtained through a complex multistage probability sample design. Between 1988 and 1994, participants received a household interview and physical examination, including phlebotomy.9 The NHANES has been linked to several administrative databases, including the Centers for Medicare & Medicaid Services (CMS) and the National Death Index (NDI). Details on these linkage procedures are provided in eMethods 1 (links.lww.com/WNL/B921).
Standard Protocol Approvals, Registrations, and Patient Consents
The procedures followed were in accordance with the ethical standards of the institution or regional committee on human experimentation and approval was obtained from the relevant committee on human subjects at the Centers for Disease Control and Prevention National Center for Health Statistics. Institutional review board (IRB) approval for the current retrospective analysis of the parent IRB-approved study (i.e., NHANES III linked to CMS-Medicare) was obtained from the NIH Intramural Research Program and the ethics board determined that participant consent was not required or waived.
Study Sample
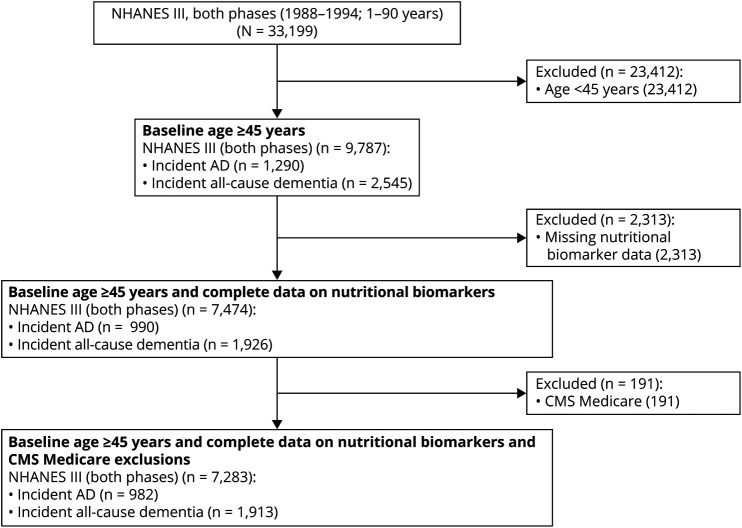
Details on sample selection criteria are shown in the Figure. We defined our eligible analytic sample to include respondents to the NHANES III who were 45–90 years of age (≥45 years) at baseline (1988–1994) for whom nutritional biomarkers and linkage to outcome were available, accounting for Health Maintenance Organization (HMO) exclusions. Of 33,199 respondents to the NHANES III aged 1–90 years who had complete sociodemographic information, 9,787 were ≥45 year old in their baseline interview. Among these respondents, we further excluded 2,313 respondents for whom nutritional biomarker data were missing or extreme (≥100 μg/dL for α-carotene, n = 3 for the 45+ group; ≥300 μg/dL for β-carotene, n = 6 for the 45+ group), producing an analytic sample of 7,474. Respondents for whom CMS linkage information was missing were assumed to have no event of interest until end of 2013 or censored upon death. Upon further exclusion due to missing covariates of interest and lack of CMS linkage, the sample was reduced to up to 7,283. We included observations that were missing information on some potential confounders and used multiple imputation on these cases. The average rate of missingness on key imputed confounders was <10%. We conducted the same procedure in a further restricted sample to respondents aged ≥65 years at baseline for sensitivity analyses (final sample n = 3,618 out of an initial sample n = 5,252).
Figure. CONSORT Flow Diagram.
AD = Alzheimer disease; CMS = Centers for Medicare & Medicaid Services; CONSORT = Consolidated Standards of Reporting Trials; NHANES III = Third National Health and Nutrition Examination Survey.
Incident All-Cause and AD Dementia
We used detailed information obtained from the CMS Chronic Condition Data Warehouse to identify cases of AD and all-cause dementia as well as onset time. Diagnostic categories contained 21 chronic conditions with varying reference time periods, numbers and types of claims to qualify, exclusions, and a set of ICD-9/CPT4/Healthcare Common Procedural Coding System codes. Details are provided in eMethods 1 (links.lww.com/WNL/B921). We used age at study (in years to the nearest month) as the underlying time scale, with baseline age defined as the earliest examination date obtained from the Medical Examination Center (MEC). The follow-up period was 1999–2013 for the pre-estimated earliest occurrence date. Follow-up time was truncated to January 1, 2014. We used the same algorithm to estimate AD/dementia earliest diagnosis date during 1991–1998.10 Thus, for most participants, the follow-up time could go up to 26 years, with a mean of ∼16–17 years, depending on the outcome.
Serum Carotenoid and Antioxidant Exposures
Serum levels of vitamin A (retinol), vitamin E (α-tocopherol), retinyl esters, and carotenoids were measured by isocratic high-performance liquid chromatography with detection at wavelengths of 300, 325, and 450 nm. Quantitation was accomplished by comparison of peak heights with a standard solution.11 Serum concentrations of vitamin C were measured using a total vitamin C, fully reduced method using high-performance liquid chromatography with electrochemical detection analysis.
Covariates
Sociodemographic and Socioeconomic Status Covariates
Covariates added in multivariable models were previously shown to be related to the outcomes or the exposures, or both. Those included age at baseline (in years), sex, race (non-Hispanic [NH] White [ref], NH Black, Mexican American, other), urban–rural residence, household size, marital status (never married, married, divorced, widowed, other), poverty income ratio (PIR), and completed years of education.
Lifestyle and Health-Related Covariates
We accounted for lifestyle and health-related covariates, which included smoking, alcohol use, diet, physical activity, and social support. Smoking was defined by the number of cigarettes smoked per day as well as the person-years of smoking (i.e., number of years that a respondent smoked cigarettes). A single 24-hour dietary recall was elicited from NHANES III participants by trained interviewers in a private room in the MEC. Data were collected on personal computers using the Dietary Data Collection system, an automated, interactive data collection and coding system. Interviewers were fluent in Spanish and English and had a set of measuring guides to help respondents estimate portion sizes. Data were collected for all days of the week. NHANES III data were coded with the 7-digit food codes from the US Department of Agriculture survey nutrient database.12 Nutrient intakes were calculated with a database provided for NHANES III.13 Alcohol was assessed as part of a single 24-hour dietary recall from which nutrient and food group intakes were derived. Alcohol use in this study was measured in g/d. Diet quality was assessed using the 1995 Healthy Eating Index (1995-HEI) and the mean adequacy ratio (MAR) score (eMethods 2, links.lww.com/WNL/B921). We classified physical activity using 3 survey items that assessed (1) the respondent's relative change in activity over the past month to the past year (0 = less, 1 = same, 2 = more), (2) self-reported activity levels among respondents relative to men/women their age (0 = less, 1 = same, 2 = more), and (3) self-reported activity levels among respondents relative to their levels of activity 10 years ago (0 = less, 1 = same, 2 = more). Five survey items were used to define social support, which included the following: (1) “In a typical week, how many times do you talk on the telephone with family, friends, or neighbors?” (2) “How often do you get together with friends or relatives—things like going out together or visiting in each other's homes? (per year)” (3) “About how often do you visit with any of your other neighbors, either in their homes or in your own? (per year)” (4) “How often do you attend church or religious services? (per year)” (5)“Altogether, how often do you attend meetings of clubs or organizations? (per year)”
We defined a health construct using measures on 4 health assessments, including self-related health (excellent, very good, good, fair, poor), comorbidity index (arthritis, congestive heart failure, stroke, asthma, chronic bronchitis, emphysema, hay fever, cataracts, goiter, thyroid disease, lupus, gout, skin cancer, other cancer), body mass index (BMI), and allostatic load, which was defined using 9 biochemical and anthropometric indices detailed in eMethods 2 (links.lww.com/WNL/B921). Allostatic load was defined such that higher scores reflected poorer health.14
Other Nutritional Biomarkers
The INCSTAR 25(OH)D assay consists of a 2-step procedure. The first step involves rapid extraction of 25(OH)D and other hydroxylated metabolites from serum or plasma with acetonitrile. The second step involves assaying the treated sample using an equilibrium radioimmunoassay procedure.11 In the NHANES III, serum folate, which is required in cellular metabolism and hematopoiesis, is measured by using the Bio-Rad Laboratories Quantaphase Folate Radioassay Kit.11
Statistical Analysis
Analyses were completed with Stata release 16.15 All covariates aside from carotenoids, antioxidants, and other nutritional biomarkers were multiple imputed (5 imputations, 10 iterations), assuming missingness at random. Descriptions of key variable distributions were presented for the total sample and stratified by tertiles (T) of total carotenoids for the total eligible sample (45+ years at baseline). Means of continuous variables across tertiles were compared using linear regression models, first to examine trends across tertiles, and then to contrast T2 vs T1 and T3 vs T1. Multiple linear, logistic, and multinomial logit models were used to test those differences across carotenoid tertiles, while adjusting for age, sex, race, and PIR. The analyses testing the main hypotheses consisted of several Cox proportional hazards regression models that were stratified by total carotenoid intake tertiles.16 In each model, and for each stratum, outcomes included 1 of 2 incident outcomes (all-cause or AD dementia) with up to 26 years of follow-up, and predictors were each of 5 individual carotenoids, total carotenoids, and vitamins A, C, and E measured at baseline. All models accounted for number of years elapsed between age at entry ≥45 years (delayed entry) and age at outcome of interest or censoring by end of follow-up or age of death. All participants were dementia-free at baseline, by design, and models included potentially confounding baseline covariates. These covariates (listed in the Covariates section) included other antioxidants and total carotenoids, sociodemographic, lifestyle, and health-related factors. Modeling was done in 6 steps. In model 1, minimal adjustment was made on the other 2 antioxidants, total carotenoids, and age. Model 2 further adjusted for sex, race, marital status, urban–rural area of residence, and household size. Model 3 further adjusted for PIR and years of education. In model 4, further adjustment was made for lifestyle and social support variables. Model 5 was model 4 further adjusted for health-related factors as well as additional nutritional biomarkers (i.e., serum folate and 25-hydroxyvitamin D). The model was conducted overall and stratified by serum total carotenoid tertiles. Two-way interaction terms were added to test heterogeneity of antioxidant effects on outcomes across tertiles of total serum carotenoids in the overall unstratified model. Most of the main analyses were also conducted in the 65+ age group, as a subanalysis. Dose–response relationships were tested by including tertiles of total carotenoids as an ordinal variable. Individual carotenoids and antioxidants were examined as standardized z scored exposures, with a per 1 SD increase interpretation. Finally, to test synergism and antagonism, 2-way interaction terms were added alternately between each individual carotenoid and each antioxidant vitamin (45+ years), while adjusting for the remaining factors and nutritional biomarkers (i.e., the full model), and including their main effects. The 2-way interaction was interpreted as synergism if negative, and antagonism if positive, given the expected protective effects on each outcome. A similar approach was applied to interactions among individual carotenoids, presenting only the final model among those aged 45+ years, and adjusting for the remaining carotenoids in all models.
Type I errors for each main effect and interaction term were set at 0.05 and 0.10, respectively,17 prior to multiple testing correction. A familywise Bonferroni approach was applied for this adjustment, accounting only for outcome multiplicity. We thus assumed that each outcome was a distinctive substantive hypothesis.18 Thus, significance levels for main effects were adjusted to p < 0.025 (0.05/2); 0.10/2 = 0.05 for the 2-way interaction terms.19
Results
Characteristics of Study Participants by Total Carotenoid Tertiles
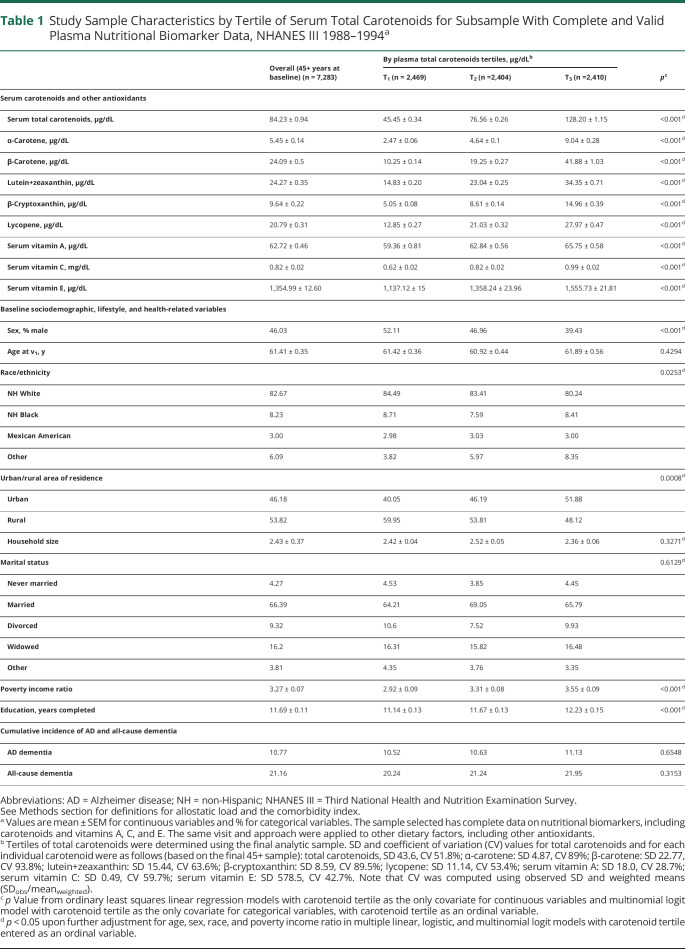
Study sample characteristics are presented in Table 1 and eTable 1 (links.lww.com/WNL/B921) across baseline serum total carotenoid tertiles. There was a linear increase in all nutritional biomarkers between the lowest and the uppermost tertiles of serum total carotenoids (p < 0.001) that was independent of age, sex, race, and PIR. Similarly, the proportion of male participants (39.4% vs 52.1%) was significantly lower in the uppermost tertile vs the lowest tertile of total carotenoids, as was the proportion of NH White participants (80.2% vs 84.5%), the percentage living in rural areas (48.1% vs 60.0%), the number of cigarettes and years smoked (p < 0.001), alcohol consumption (p = 0.003), percentage with fair/poor self-rated health (p < 0.001), mean comorbidity index (p = 0.045), mean allostatic load (p < 0.001), and mean BMI (p < 0.001). In contrast, those in the uppermost tertile of total carotenoids vs the lowest were more likely to report being more active than age peers or self 10 years ago (p < 0.05), and more frequently attended church or meetings in clubs, independently of age, sex, race, or PIR. Other results are summarized in eResults 1.
Table 1.
Study Sample Characteristics by Tertile of Serum Total Carotenoids for Subsample With Complete and Valid Plasma Nutritional Biomarker Data, NHANES III 1988–1994a
All-Cause and AD Dementia vs Individual/Total Carotenoids and Other Antioxidants: Cox Proportional Hazards Models
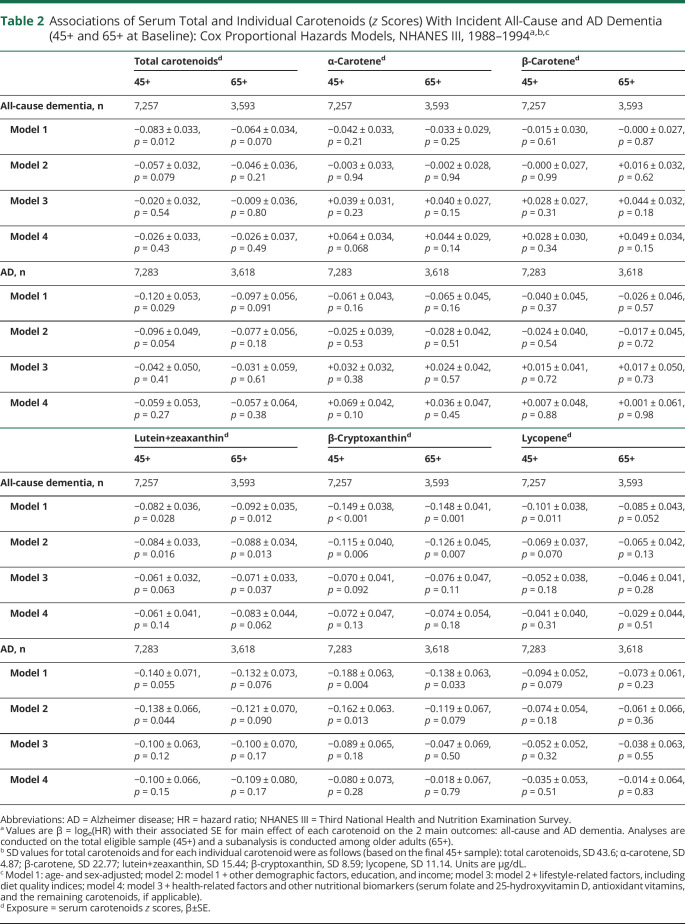
Table 2 shows results from Cox proportional hazards models examining associations of total and individual carotenoids with incidence of AD and all-cause dementia. In the 45+ baseline age group, age- and sex-adjusted models indicated an inverse relationship between total carotenoids and both outcomes of interest (per SD of total carotenoids, hazard ratio [HR] 0.92, 95% CI 0.86–0.98, p = 0.012 for all-cause dementia; HR 0.89, 95% CI 0.80–0.98, p = 0.029 for AD). However, these associations were attenuated upon adjustment for other sociodemographic and socioeconomic status (SES) factors, including education and PIR (p < 0.10), and became null upon further adjustment for diet quality and other lifestyle factors (model 3). Nevertheless, when examining individual carotenoids, lutein+zeaxanthin plasma concentration was associated with reduced risk of all-cause dementia in the 65+ baseline age group, even upon adjustment for lifestyle factors such as diet quality (HR 0.93, 95% CI 0.87–0.99, p = 0.037), although with a marked attenuation compared to model 2 (HR 0.92, 95% CI 0.86–0.93, p = 0.013). The relationship became nonsignificant when health-related factors such as allostatic load were introduced into the model (HR 0.92, 95% CI 0.84–1.00, p = 0.062). A strong inverse relationship was also detected between serum β-cryptoxanthin and all-cause dementia in both age groups for the age- and sex-adjusted models (HR 0.86, 95% CI 0.80–0.93, p < 0.001 for 45+; HR 0.86, 95% CI 0.80–0.93, p = 0.001 for 65+). This relationship remained strong in models adjusted for other sociodemographic and SES factors (HR 0.89, 95% CI 0.82–0.96, p = 0.006 for 45+; HR 0.88, 95% CI 0.81–0.96, p = 0.007 for 65+). Nevertheless, it was attenuated upon further adjustment for diet quality and other lifestyle factors, suggesting mediation through healthy dietary patterns. The inverse relationship between β-cryptoxanthin and incident AD was detected in the 45+ group, retaining statistical significance in model 2. Unlike lutein+zeaxanthin and β-cryptoxanthin, the initial inverse relationship between lycopene and all-cause dementia was highly confounded by SES factors (model 2 vs model 1). No association was found between α-carotene or β-carotene and any of the outcomes within both age groups of interest. Upon correction for multiple testing, only inverse associations in models 1 and 2 of lutein+zeaxanthin (45+ and 65+) and β-cryptoxanthin (45+) with all-cause dementia (and AD for β-cryptoxanthin, 45+) remained statistically significant (p < 0.025).
Table 2.
Associations of Serum Total and Individual Carotenoids (z Scores) With Incident All-Cause and AD Dementia (45+ and 65+ at Baseline): Cox Proportional Hazards Models, NHANES III, 1988–1994a,b,c
All-Cause and AD Dementia vs Vitamin Antioxidants, Overall and Across Total Carotenoid Tertiles: Cox Proportional Hazards Models
eTable 2 (links.lww.com/WNL/B921) displays findings from a series of Cox proportional hazards models in the 45+ and 65+ age groups and show findings of associations for antioxidant vitamins A, C, and E with all-cause and AD dementia at increasing level of covariate adjustment, in the total population and across tertiles of serum total carotenoids. Overall, serum vitamin C was inversely associated with incident all-cause dementia only in the age- and sex-adjusted model (i.e., model 1), with a stronger effect shown in the 45+ age group. The association remained statistically significant in the model adjusting for other sociodemographic and SES factors, although it was attenuated in both age groups. In model 3, which added diet quality and other lifestyle factors among adjusted covariates, the association between vitamin C and all-cause dementia was no longer detected, as was the case for model 4. When examining interaction with total carotenoids, only 1 passed the threshold of statistical significance, in model 3 (which adjusted for diet and other lifestyle factors), indicating that at higher levels of carotenoids, vitamin A may potentially increase the risk for all-cause dementia in the older group (65+ at baseline), an association that differed significantly between the top and bottom tertiles of serum carotenoids.
All-Cause and AD Dementia vs Interaction Between Individual Carotenoids and Other Antioxidants: Cox Proportional Hazards Models
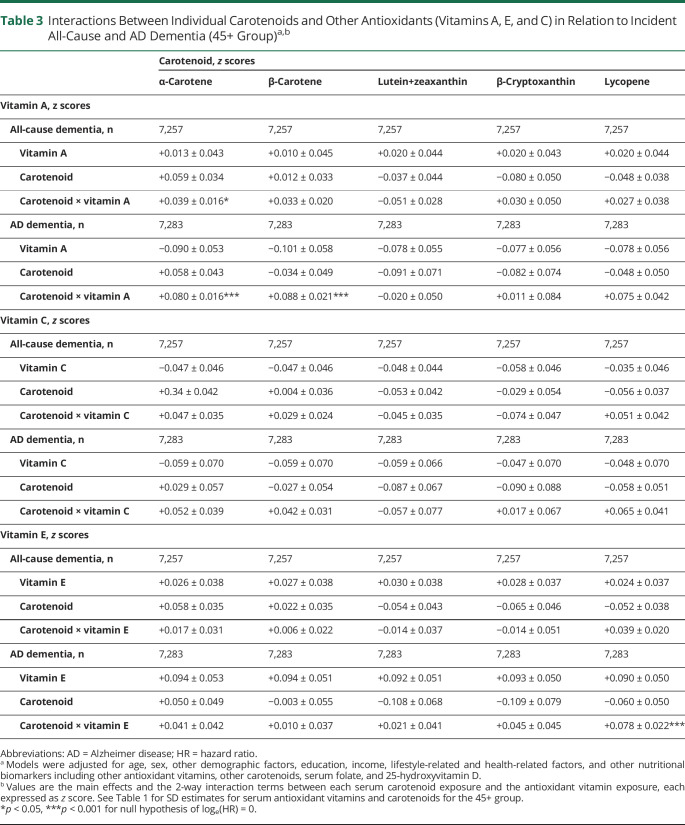
Table 3 presents key findings from Cox proportional hazards models for all-cause and AD dementia incidence among participants aged 45+ at baseline, in the full models, with 2-way interactions added between each individual carotenoid and each antioxidant vitamin. In those fully adjusted models, antagonistic interactions were observed between serum vitamin A and α-carotene vs all-cause dementia (β±standard error of estimate [SEE] +0.039 ± 0.016, p = 0.017); vitamin A and α-carotene vs AD dementia (β±SEE +0.080 ± 0.016, p < 0.001); vitamin A and β-carotene vs AD incidence (β±SEE +0.088 ± 0.021, p < 0.001); and vitamin E and lycopene vs AD incidence (β±SEE +0.078 ± 0.022, p = 0.001).
Table 3.
Interactions Between Individual Carotenoids and Other Antioxidants (Vitamins A, E, and C) in Relation to Incident All-Cause and AD Dementia (45+ Group)a,b
All-Cause and AD Dementia vs Interactions Among Individual Carotenoids: Cox Proportional Hazards Models
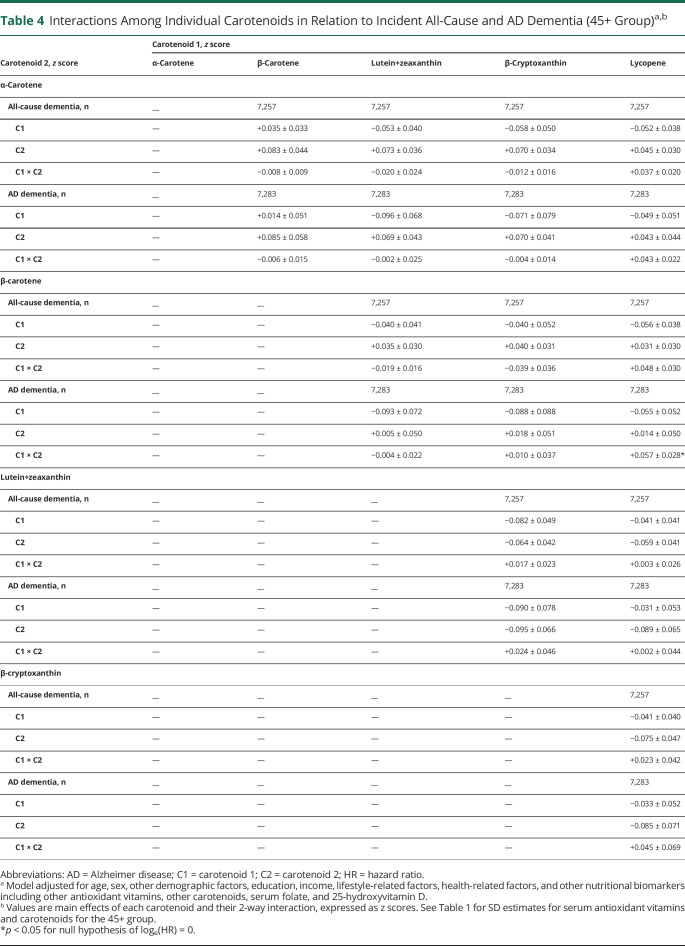
Table 4 shows findings from full models with interactions added between individual carotenoids in relation to incidence of all-cause and AD dementia within the 45+ baseline age group. Only 1 interaction was deemed statistically significant, namely a potential antagonistic interaction between lycopene and β-carotene vs incident AD (C1×C2: β±SE +0.057 ± 0.028, p = 0.046), indicating that putative protective effects on incident AD of lycopene are reduced at higher levels of β-carotene. Other relevant results showing findings from Table 2, model 4 for covariates included in the model are presented in eResults 1 (links.lww.com/WNL/B921). This study provides Class II evidence that incident all-cause dementia was inversely associated with serum lutein+zeaxanthin and β-cryptoxanthin levels.
Table 4.
Interactions Among Individual Carotenoids in Relation to Incident All-Cause and AD Dementia (45+ Group)a,b
Discussion
In this study, we evaluated whether carotenoids and other antioxidants act synergistically in their association with AD and all-cause dementia using a nationally representative prospective cohort of US adults with administrative linkage. Inverse associations of total and individual carotenoid plasma concentrations with both outcomes were detected, with lutein+zeaxanthin and β-cryptoxanthin meeting statistical significance upon multiple testing adjustment. Specifically, lutein+zeaxanthin was associated with reduced risk of all-cause dementia (65+ age group), even in the lifestyle-adjusted model (per SD, HR 0.93, 95% CI 0.87–0.99, p = 0.037), although attenuated in comparison with a sociodemographic and SES factors–adjusted model (HR 0.92, 95% CI 0.86–0.93, p = 0.013). A strong inverse relationship was detected between serum β-cryptoxanthin (per SD increase) and all-cause dementia (45+ and 65+) for age- and sex-adjusted models (HR 0.86, 95% CI 0.80–0.93, p < 0.001 for 45+; HR 0.86, 95% CI 0.80–0.93, p = 0.001 for 65+), a relationship remaining strong in sociodemographic and SES factor–adjusted models (HR 0.89, 95% CI 0.82–0.96, p = 0.006 for 45+; HR 0.88, 95% CI 0.81–0.96, p = 0.007 for 65+), but attenuated in subsequent models. In fully adjusted models, antagonistic interactions were observed between serum vitamin A and α-carotene vs all-cause dementia and vitamin A and α-carotene, vitamin E and lycopene, vitamin A and β-carotene, and lycopene and β-carotene vs AD incidence.
Studies examining the link between dietary antioxidant intake and the risk of dementia have produced mixed findings. For example, one study20 reported no association between midlife dietary intake of vitamins E and C and incident dementia, a finding that was consistent with 5 other cohort studies with respect to these 2 dietary antioxidants.21-24 Another study,22 however, found that carotenoids, particularly β-carotene intake, may have beneficial effects on various cognitive outcomes, whereas associations between cognitive outcomes and other carotenoids were not detected in other studies.23-25
Among carotenoids, lutein or lutein+zeaxanthin were found to have beneficial cognitive effects in older men and women as indicated by a recent randomized controlled trial26 and a large cohort study27; 2 recent meta-analyses of randomized controlled trials and cohort studies came to the conclusion that carotenoids in general, and lutein in particular, may have cognitive benefits.28,29 In the first study,26 the cognitive benefit of docosahexaenoic acid (DHA), an essential omega-3 fatty acid, and lutein in unimpaired older women were explored in a 4-month, double-blind, intervention trial supplementing DHA and lutein for eye health. Most notably, the study's results indicated that memory scores and rate of learning improved significantly in the combined treatment group vs placebo (p < 0.03).26 The second study indicated that higher total carotenoid intake was indeed linked to substantially lower hazard of AD after controlling for age, sex, education, participation in cognitively stimulating activities, APOE4 status, and physical activity level.27 Comparing the uppermost with the lowest quintile (median intake: 24.8 compared with 6.7 mg/d) of total carotenoids, the multivariate HR (95% CI) was 0.52 (0.33, 0.81), ptrend < 0.01. A similar association was observed for lutein+zeaxanthin, with a weaker inverse relationship observed for β-carotene, and a marginally significant inverse association found for β-cryptoxanthin.27 In the deceased group, decedents with higher total carotenoids consumption (uppermost vs lowest tertile, 18.2 compared with 8.2 mg/d) had less global AD pathology (b −0.10; SE 0.04; ptrend = 0.01).27 For individual carotenoids, lutein+zeaxanthin and lycopene were inversely related to brain global pathology, whereas lutein+zeaxanthin exhibited an additional inverse association with AD diagnostic score, neuritic plaque severity, and neurofibrillary tangle density and severity.27 Our study had comparable findings for lutein+zeaxanthin in serum in relation to AD and all-cause dementia in the older group (65+ years of age at baseline), with some additional evidence for a protective effect of β-cryptoxanthin. Nevertheless, a recent randomized controlled trial of >3,000 participants with age-related macular degeneration (AREDS2 study) showed that supplementation with omega-3 fatty acids and lutein/zeaxanthin had no significant effect on cognitive function.30
Serum concentrations of antioxidant vitamins may be a better biomarker for oxidative stress status whether derived from dietary intake or supplementation. Several recent cohort studies31-33 reported an inverse relationship between serum vitamin E levels and cognitive impairment and disorders. In one of these studies, researchers observed a U-shaped association between blood tocopherol subtypes and cognitive impairment.32 Moreover, numerous other studies reported protective associations between serum carotenoids and cognitive impairment,34-42 including a recent study conducted in our same cohort that detected similar potentially protective associations between plasma lutein+zeaxanthin, lycopene, and AD mortality.42 Taken together, the previous literature indicates that both carotenoids and serum antioxidant vitamins tended to be protective against various adverse cognitive outcomes, including incident AD and all-cause dementia. However, only one recent study has examined interactions between those bioactive micronutrients and cognitive performance or decline in midlife.43 The findings indicated that among others, there was a synergistic interaction between vitamin E and total carotenoids, particularly lycopene, whereby vitamin E was directly associated with baseline performance on a test of verbal memory at higher carotenoid levels, with antagonistic interactions detected between vitamin A and some carotenoids in relation to visual memory decline.43 Our current study did not detect any synergistic interactions or a potential protective effect of vitamin E against incidence of all-cause or AD dementia. In contrast, vitamin E and lycopene exhibited an antagonistic interaction in our study in relation to AD incidence, suggesting that interactions between carotenoids and antioxidant vitamins are patterned differently across time. A study conducted on brain tissues acquired from frontal and temporal cortices of 47 centenarians from the Georgia Centenarian Study indicated that brain nutrient pattern explained mainly by carotenoid concentrations is correlated with cognitive function among participants who had no dementia, re-enforcing the biological plausibility of our detected associations.44 Other related biological mechanisms are summarized in eDiscussion 1 (links.lww.com/WNL/B921).
Our study has notable strengths. First, we used a nationally representative study that sufficiently powered our analyses to detect interactions between various nutritional biomarkers of antioxidant status in relation to 2 key cognitive impairment outcomes, namely all-cause and AD dementia. We used a nationally representative sample together with administrative linkages that allowed us to combine detailed demographic and behavioral health information with medical records. In prior work, studies have typically relied solely on medical claims information, which do not necessarily contain demographic and behavioral health information.45 Second, advanced statistical techniques such as multiple Cox proportional hazards models were used with multiple imputed covariates, thus reducing selection bias and preserving statistical power within the eligible sample with complete exposure and outcome data. Third, this study is among few studies to examine serum nutritional biomarkers of antioxidant status, rather than dietary intakes, the latter being known for reflecting only short-term exposure and having considerable measurement error. Fourth, our analyses were carried out among middle-aged and older adults, with a subanalysis carried out among older adults (aged 65+) to determine the influence of age at exposure on the outcome.
Our study also has limitations. First, in terms of outcome, those diagnosed earlier may be at worse overall health or have better access to health care than those who were diagnosed later. In addition, baseline exclusion of dementia or cognitive impairment cases was based on a household screener46 rather than a formal set of cognitive performance tests. Nevertheless, the large majority of incident dementia cases were diagnosed after at least 10 years of follow-up, thus reducing the possibility of reverse causality. Second, although nutritional biomarkers are an improvement over dietary intakes, their association with the key outcomes may be confounded by other biomarkers. In addition, despite some genetic effect, dietary influence on these nutritional biomarkers is often predominant. In addition, the serum antioxidant levels reflect current intakes and may not accurately reflect the person's lifetime habitual intakes. Another class of antioxidants, the flavonoids, have been shown to be protective against oxidative DNA damage47 but were not accounted for in this study because of the lack of a flavonoid database. Whereas some drugs like aspirin and l-dopa preparations can affect antioxidant systems,48 this study did not control for these drugs. Moreover, the levels of serum antioxidants needed to beneficially modify the aging of the brain are unknown, resulting in the need for further exploration of the association between serum antioxidant levels and dementia. Two other limitations of the study are the unavailability of vitamin E isoforms in the data and the possibility of regression dilution due to elongated follow-up periods.
Incident all-cause dementia was inversely associated with serum lutein+zeaxanthin and β-cryptoxanthin levels. Antagonistic interactions indicate that putative protective effects of one carotenoid may be observed at a lower level of another carotenoid or antioxidant vitamin. Further studies with time-dependent exposures and randomized trials are needed to test neuroprotective effects of supplementing the diet with select carotenoids.
Disclaimer
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of CDC/NCHS or Fort Belvoir Community Hospital, the Defense Health Agency, the Department of Defense, or the US Government. Reference to any commercial products within this publication does not create or imply any endorsement by Fort Belvoir Community Hospital, the Defense Health Agency, the Department of Defense, or the US Government.
Acknowledgment
The authors thank the NHANES staff, investigators, and participants and the NIA/NIH/IRP internal reviewers of this article; Negasi Beyene from the Centers for Disease Control and Prevention National Center for Health Statistics, Hyattsville, MD, for assistance with the statistical analysis process at the Research Data Center in Rockville, MD; and Ray Kuntz, AHRQ, for supervising the data analysis process at the Research Data Center.
Glossary
- 1995 HEI
Healthy Eating Index, 1995 version
- AD
Alzheimer disease
- BMI
body mass index
- CMS
Centers for Medicare & Medicaid Services
- CPT4
Common Procedural Terminology
- DHA
docosahexaenoic acid
- HMO
Health Maintenance Organization
- HR
hazard ratio
- ICD-9
International Classification of Diseases, 9th revision
- ICD-10
International Classification of Diseases, 10th revision
- IRB
institutional review board
- MAR
mean adequacy ratio
- MEC
mobile examination center
- NDI
National Death Index
- NH
Non-Hispanic
- NHANES III
Third National Health and Nutrition Examination Survey
- PIR
poverty income ratio
- ROS
reactive oxygen species
- SES
socioeconomic status
Appendix. Authors
Footnotes
Editorial, page 871
Class of Evidence: NPub.org/coe
Study Funding
This work was supported in part by the Intramural Research Program of the NIH, National Institute on Aging, NIH project number AG000513.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Waldstein SR, Elias MF. Introduction to the special section on health and cognitive function. Health Psychol. 2003;22(6):555-558. [DOI] [PubMed] [Google Scholar]
- 2.Rafnsson SB, Dilis V, Trichopoulou A. Antioxidant nutrients and age-related cognitive decline: a systematic review of population-based cohort studies. Eur J Nutr. 2013;52(6):1553-1567. [DOI] [PubMed] [Google Scholar]
- 3.Chikara S, Nagaprashantha LD, Singhal J, Horne D, Awasthi S, Singhal SS. Oxidative stress and dietary phytochemicals: role in cancer chemoprevention and treatment. Cancer Lett. 2018;413:122-134. [DOI] [PubMed] [Google Scholar]
- 4.Carney JM, Starke-Reed PE, Oliver CN, et al. Reversal of age-related increase in brain protein oxidation, decrease in enzyme activity, and loss in temporal and spatial memory by chronic administration of the spin-trapping compound N-tert-butyl-alpha-phenylnitrone. Proc Natl Acad Sci USA. 1991;88(9):3633-3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacob KD, Noren Hooten N, Tadokoro T, Lohani A, Barnes J, Evans MK. Alzheimer's disease-associated polymorphisms in human OGG1 alter catalytic activity and sensitize cells to DNA damage. Free Radic Biol Med. 2013;63:115-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ekinci FJ, Linsley MD, Shea TB. Beta-amyloid-induced calcium influx induces apoptosis in culture by oxidative stress rather than tau phosphorylation. Brain Res Mol Brain Res. 2000;76(2):389-395. [DOI] [PubMed] [Google Scholar]
- 7.Beydoun MA, Beydoun HA, Gamaldo AA, Teel A, Zonderman AB, Wang Y. Epidemiologic studies of modifiable factors associated with cognition and dementia: systematic review and meta-analysis. BMC Public Health. 2014;14:643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gropper SS, Smith JL. Advanced Nutrition and Human Metabolism. Cengage Learning; 2017. [Google Scholar]
- 9.Center for Disease Control and Prevention. The Third National Health and Nutrition Examination Survey (NHANES III 1988-94) Reference Manuals and Reports (CD-ROM). Centers for Disease Control and Prevention; 1996. [Google Scholar]
- 10.Center for Disease Control and Prevention. NHANES and CMS linked data overview [online]. cdc.gov/nchs/tutorials/NHANES-CMS/Orientation/Overview/index.htm
- 11.Gunter EW, Lewis BG, Koncikowski SM. Laboratory Procedures Used for the Third National Health and Nutrition Examination Survey (NHANES III), 1988-1994. US DHHS, Public Health Service, Centers for Disease Control and Prevention, National Center for Environmental Health and National Center for Health Statistics; 2010. cdc.gov/nchs/data/nhanes/nhanes3/cdrom/nchs/manuals/labman.pdf [Google Scholar]
- 12.Westat I. NHANES III Dietary Interviewer's Manual. Prepared for the National Center for Health Statistics, US Department of Health and Human Services; 1992. [Google Scholar]
- 13.Human Nutrition Information Service. Survey Nutrient Database for NHANES III, Phase I. Human Nutrition Information Service; 1993. [Google Scholar]
- 14.Seeman T, Merkin SS, Crimmins E, Koretz B, Charette S, Karlamangla A. Education, income and ethnic differences in cumulative biological risk profiles in a national sample of US adults: NHANES III (1988-1994). Soc Sci Med. 2008;66:72-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Statistics/Data Analysis: Release 16.0 [Computer Program]. Stata Corporation; 2019. [Google Scholar]
- 16.Blackwell E, de Leon CF, Miller GE. Applying mixed regression models to the analysis of repeated-measures data in psychosomatic medicine. Psychosom Med. 2006;68:870-878. [DOI] [PubMed] [Google Scholar]
- 17.Selvin S. Statistical Analysis of Epidemiologic Data, 3rd ed. Oxford University Press; 2004. [Google Scholar]
- 18.Hochberg Y, Tamhane AC. Multiple Comparison Procedures. Wiley; 1987. [Google Scholar]
- 19.Beydoun MA, Canas JA, Dore GA, et al. Serum uric acid and its association with longitudinal cognitive change among urban adults. J Alzheimers Dis. 2016;52(4):1415-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laurin D, Masaki KH, Foley DJ, White LR, Launer LJ. Midlife dietary intake of antioxidants and risk of late-life incident dementia: the Honolulu-Asia Aging Study. Am J Epidemiol. 2004;159(10):959-967. [DOI] [PubMed] [Google Scholar]
- 21.Peacock JM, Folsom AR, Knopman DS, Mosley TH, Goff DC Jr., Szklo M. Dietary antioxidant intake and cognitive performance in middle-aged adults: The Atherosclerosis Risk in Communities (ARIC) Study investigators. Public Health Nutr. 2000;3(3):337-343. [DOI] [PubMed] [Google Scholar]
- 22.Jama JW, Launer LJ, Witteman JC, et al. Dietary antioxidants and cognitive function in a population-based sample of older persons: The Rotterdam Study. Am J Epidemiol. 1996;144(3):275-280. [DOI] [PubMed] [Google Scholar]
- 23.McNeill G, Jia X, Whalley LJ, et al. Antioxidant and B vitamin intake in relation to cognitive function in later life in the Lothian Birth Cohort 1936. Eur J Clin Nutr. 2011;65(5):619-626. [DOI] [PubMed] [Google Scholar]
- 24.Devore EE, Kang JH, Stampfer MJ, Grodstein F. Total antioxidant capacity of diet in relation to cognitive function and decline. Am J Clin Nutr. 2010;92(5):1157-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morris MC, Evans DA, Bienias JL, Tangney CC, Wilson RS. Vitamin E and cognitive decline in older persons. Arch Neurol. 2002;59(7):1125-1132. [DOI] [PubMed] [Google Scholar]
- 26.Johnson EJ, McDonald K, Caldarella SM, Chung HY, Troen AM, Snodderly DM. Cognitive findings of an exploratory trial of docosahexaenoic acid and lutein supplementation in older women. Nutr Neurosci. 2008;11(2):75-83. [DOI] [PubMed] [Google Scholar]
- 27.Yuan C, Chen H, Wang Y, Schneider JA, Willett WC, Morris MC. Dietary carotenoids related to risk of incident Alzheimer dementia (AD) and brain AD neuropathology: a community-based cohort of older adults. Am J Clin Nutr. 2020;113(1):200-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davinelli S, Ali S, Solfrizzi V, Scapagnini G, Corbi G. Carotenoids and cognitive outcomes: a meta-analysis of randomized intervention trials. Antioxidants. 2021;10(2):223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yagi A, Nouchi R, Butler L, Kawashima R. Lutein has a positive impact on brain health in healthy older adults: a systematic review of randomized controlled trials and cohort studies. Nutrients. 2021;13(6):1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chew EY, Clemons TE, Agrón E, Launer LJ, Grodstein F, Bernstein PS. Effect of omega-3 fatty acids, lutein/zeaxanthin, or other nutrient supplementation on cognitive function: the AREDS2 randomized clinical trial. JAMA. 2015;314(8):791-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dunn JE, Weintraub S, Stoddard AM, Banks S. Serum alpha-tocopherol, concurrent and past vitamin E intake, and mild cognitive impairment. Neurology. 2007;68(9):670-676. [DOI] [PubMed] [Google Scholar]
- 32.Ravaglia G, Forti P, Lucicesare A, et al. Plasma tocopherols and risk of cognitive impairment in an elderly Italian cohort. Am J Clin Nutr. 2008;87(5):1306-1313. [DOI] [PubMed] [Google Scholar]
- 33.Mangialasche F, Solomon A, Kåreholt I, et al. Serum levels of vitamin E forms and risk of cognitive impairment in a Finnish cohort of older adults. Exp Gerontol. 2013;48(12):1428-1435. [DOI] [PubMed] [Google Scholar]
- 34.Akbaraly NT, Faure H, Gourlet V, Favier A, Berr C. Plasma carotenoid levels and cognitive performance in an elderly population: results of the EVA Study. J Gerontol A Biol Sci Med Sci. 2007;62(3):308-316. [DOI] [PubMed] [Google Scholar]
- 35.Johnson EJ, Vishwanathan R, Johnson MA, et al. . Relationship between serum and brain carotenoids, alpha-tocopherol, and retinol concentrations and cognitive performance in the oldest old from the Georgia Centenarian Study. J Aging Res. 2013;2013:951786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kesse-Guyot E, Andreeva VA, Ducros V, et al. . Carotenoid-rich dietary patterns during midlife and subsequent cognitive function. Br J Nutr. 2014;111:915-923. [DOI] [PubMed] [Google Scholar]
- 37.Feeney J, O'Leary N, Moran R, et al. Plasma lutein and zeaxanthin are associated with better cognitive function across multiple domains in a large population-based sample of older adults: findings from the Irish longitudinal study on aging. J Gerontol A Biol Sci Med Sci. 2017;72(10):1431-1436. [DOI] [PubMed] [Google Scholar]
- 38.Zuniga KE, Moran NE. Low serum carotenoids are associated with self-reported cognitive dysfunction and inflammatory markers in breast cancer survivors. Nutrients. 2018;10(8):1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zwilling CE, Talukdar T, Zamroziewicz MK, Barbey AK. Nutrient biomarker patterns, cognitive function, and fMRI measures of network efficiency in the aging brain. Neuroimage. 2019;188:239-251. [DOI] [PubMed] [Google Scholar]
- 40.von Arnim CA, Herbolsheimer F, Nikolaus T, et al. . Dietary antioxidants and dementia in a population-based case-control study among older people in South Germany. J Alzheimers Dis. 2012;31:717-724. [DOI] [PubMed] [Google Scholar]
- 41.Mewborn CM, Lindbergh CA, Robinson TL, et al. Lutein and zeaxanthin are positively associated with visual-spatial functioning in older adults: an fMRI study. Nutrients. 2018;10(4):458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Min JY, Min KB. Serum lycopene, lutein and zeaxanthin, and the risk of Alzheimer's disease mortality in older adults. Dement Geriatr Cogn Disord. 2014;37(3-4):246-256. [DOI] [PubMed] [Google Scholar]
- 43.Beydoun MA, Canas JA, Fanelli-Kuczmarski MT, et al. Association of antioxidant vitamins A, C, E and carotenoids with cognitive performance over time: a cohort study of middle-aged adults. Nutrients. 2020;12(11):3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanprasertsuk J, Scott TM, Barbey AK, et al. Carotenoid-rich brain nutrient pattern is positively correlated with higher cognition and lower depression in the oldest old with no dementia. Front Nutr. 2021;8:704691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Douglas MD, Dawes DE, Holden KB, Mack D. Missed policy opportunities to advance health equity by recording demographic data in electronic health records. Am J Public Health. 2015;105(suppl 3):S380-S388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Westat I. National Health and Nutrition Examination Survey III, Home Examiner's Manual [online]. Accessed December 2, 2021. https://wwwn.cdc.gov/nchs/data/nhanes3/manuals/homeexam.pdf
- 47.Mecocci P, Boccardi V, Cecchetti R, et al. A long journey into aging, brain aging, and Alzheimer's disease following the oxidative stress tracks. J Alzheimers Dis. 2018;62(3):1319-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Foy CJ, Passmore AP, Vahidassr MD, Young IS, Lawson JT. Plasma chain-breaking antioxidants in Alzheimer's disease, vascular dementia and Parkinson's disease. QJM. 1999;92(1):39-45. [DOI] [PubMed] [Google Scholar]