Abstract
Background and Objectives
To assess the accuracy of baseline CT perfusion (CTP) ischemic core estimates.
Methods
From SELECT (Optimizing Patient Selection for Endovascular Treatment in Acute Ischemic Stroke), a prospective multicenter cohort study of imaging selection, patients undergoing endovascular thrombectomy who achieved complete reperfusion (modified Thrombolysis In Cerebral Ischemia score 3) and had follow-up diffusion-weighted imaging (DWI) available were evaluated. Follow-up DWI lesions were coregistered to baseline CTP. The difference between baseline CTP core (relative cerebral blood flow [rCBF] <30%) volume and follow-up infarct volume was classified as overestimation (core ≥10 mL larger than infarct), adequate, or underestimation (core ≥25 mL smaller than infarct) and spatial overlap was evaluated.
Results
Of 101 included patients, median time from last known well (LKW) to imaging acquisition was 138 (82–244) minutes. The median baseline ischemic core estimate was 9 (0–31.9) mL and median follow-up infarct volume was 18.4 (5.3–68.7) mL. All 6/101 (6%) patients with overestimation of the subsequent infarct volume were imaged within 90 minutes of LKW and achieved rapid reperfusion (within 120 minutes of CTP). Using rCBF <20% threshold to estimate ischemic core in patients presenting within 90 minutes eliminated overestimation. Volumetric correlation between the ischemic core estimate and follow-up imaging improved as LKW time to imaging acquisition increased: Spearman ρ <90 minutes 0.33 (p = 0.049), 90–270 minutes 0.63 (p < 0.0001), >270 minutes 0.86 (p < 0.0001). Assessment of the spatial overlap between baseline CTP ischemic core lesion and follow-up infarct demonstrated that a median of 3.2 (0.0–9.0) mL of estimated core fell outside the subsequent infarct. These regions were predominantly in white matter.
Discussion
Significant overestimation of irreversibly injured ischemic core volume was rare, was only observed in patients who presented within 90 minutes of LKW and achieved reperfusion within 120 minutes of CTP acquisition, and occurred primarily in white matter. Use of a more conservative (rCBF <20%) threshold for estimating ischemic core in patients presenting within 90 minutes eliminated all significant overestimation cases.
Trial Registration Information
ClinicalTrials.gov: NCT03876457.
The randomized clinical trials (RCTs) that established endovascular thrombectomy (EVT) efficacy and safety for patients with ischemic stroke presenting with large vessel occlusions used different modalities to assess imaging eligibility.1-7 Most of the early window RCTs utilized noncontrast CT (NCCT) to identify patients with minimal ischemic changes (Alberta Stroke Program Early CT Score [ASPECTS] ≥6).1-3 EXTEND-IA (Extending the Time for Thrombolysis in Emergency Neurological Deficits–Intra-Arterial) and the initial phase of SWIFT-PRIME (Solitaire With the Intention For Thrombectomy as Primary Endovascular Treatment) used perfusion imaging to identify eligible candidates based on a mismatch between the estimated ischemic core and the region of hypoperfusion.4,5 The current American Heart Association/American Stroke Association and European guidelines indicate that perfusion imaging is not required for EVT patient selection within 6 hours of last known well (LKW).8,9 Perfusion imaging or MRI is recommended by the guidelines for potential EVT cases between 6 and 24 hours.8,9
Both NCCT and CT perfusion (CTP) imaging are often utilized for patient selection for EVT, irrespective of the time from LKW. NCCT detects hypodense irreversibly injured tissue and can be semiquantitatively assessed with the ASPECTS; perfusion images measure blood flow and provide a quantitative estimate of the ischemic core volume and ischemic penumbra. Although CTP and NCCT often have concordant findings, discordance may occur, especially in patients presenting in the early time window.10,11
Despite reasonable agreement in the ischemic core volume as assessed by CTP and follow-up infarct volume on magnetic resonance diffusion-weighted imaging (MR-DWI),12,13 the reliability of CTP for assessing ischemic core has been questioned, with reports of overestimation (“ghost core”), especially for early window patients.14 Other reports have suggested that for patients who present early after symptom onset (within 60–90 minutes of LKW), a stricter relative cerebral blood flow (rCBF) threshold (such as <20%) provides a better estimate of ischemic core.15 Final infarct volume in early reperfusers have been previously used to validate ischemic core thresholds.16
Several other variables may affect the correlation between preprocedural CTP core estimates and follow-up MR-DWI volumes, including time from baseline imaging to reperfusion, the robustness of collateral flow, and the degree of reperfusion achieved.
We aimed to assess volumetric and spatial agreement between CTP estimated ischemic core lesions and the follow-up infarct on DWI in patients who achieved complete reperfusion after EVT. We hypothesized that if ischemic core estimates are accurate, patients who achieve complete reperfusion should have a strong correlation between the baseline ischemic core estimate and the subsequent infarct volume. In addition, we investigated the relationship between time to imaging acquisition and time to successful reperfusion and the accuracy of CTP core estimates. We further evaluated whether the rCBF <20% threshold provided more accurate predictions of infarct volume for patients scanned within 90 minutes of LKW.
Methods
Patient Cohort
This is a post hoc analysis of SELECT (Optimizing Patient Selection for Endovascular Treatment in Acute Ischemic Stroke), a prospective, multicenter cohort study. The study population, methodology, and primary results have been published.17,18 Further details regarding patient cohort, informed consent, CTP acquisition, imaging review, and data availability are provided in the eMethods (links.lww.com/WNL/B926).
Imaging Analysis
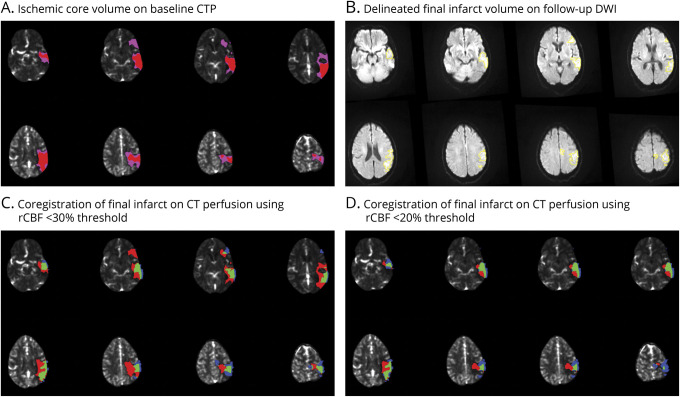
All source perfusion images were reprocessed using RAPID (research/commercial) v5.1. Ischemic core volume was estimated using the rCBF threshold of <30%. rCBF <20% maps were also generated to estimate ischemic core volumes (Figure 1A). Infarct volumes on DWI were manually delineated by the core laboratory using OsirixMD v12.0. Hemorrhagic transformation within the infarct lesion was included in the manual region of interest (ROI) delineations, whereas parenchymal hemorrhages outside the infarct were excluded. Infarct volumes from the ROIs were calculated using VoxelVolume plugin for Osirix MD (Figure 1B). After processing with RAPID, maps delineating rCBF volumes were exported. A rigid body transformation (SimpleElastix v1.2) was used to coregister both follow-up MR-DWI (mutual information cost function) and the base precontrast CTP (cross correlation cost function) image to the baseline NCCT image (Figure 1, C and D). Using the baseline NCCT image as the reference image results in more reliable registrations in dual slab than coregistration of DWI to the individual slabs. The analysis was constrained to the volume covered by the CTP scan. Significant underestimation of infarct volume was defined as the follow-up infarct volume being at least 25 mL larger than estimated ischemic core volume on baseline CTP,19,20 whereas core overestimation was defined as ischemic core volume at baseline being ≥10 mL larger than infarct volume on follow-up DWI.14,21 The rest were defined as adequate estimation (core corresponding to DWI infarct).
Figure 1. Illustration of Coregistration Process Employed During the Imaging Evaluation.
(A) Ischemic core on baseline CT perfusion (CTP) measured using relative cerebral blood flow (rCBF) thresholds of <30% (pink) and <20% (red). (B) Delineated infarct volume (yellow) on follow-up diffusion-weighted imaging (DWI). (C) Coregistration of baseline CTP and follow-up DWI using rCBF <30% threshold to measure ischemic core, representing overcall (red), undercall (blue), and adequate estimation (green). (D) Coregistration of baseline CTP and follow-up DWI, using rCBF <20% threshold to measure ischemic core, representing overcall (red), undercall (blue), and adequate estimation (green).
For spatial analysis, coregistered RAPID processed images and delineated ROIs on follow-up DWI were superimposed for visualization and volumetric quantification of 3 distinct regions:
Regions of the estimated ischemic core that progressed to infarction (green area in Figure 1, C and D)
Regions of the estimated ischemic core that did not progress to infarction (red area in Figure 1, C and D)
Regions that progressed to infarction but were not identified as ischemic core at baseline (blue area in Figure 1, C and D)
Statistical Analysis
Values were described using proportions with percentages for categorical variables and using median (interquartile range) for continuous variables. A nonparametric test for trend was used to assess trends across multiple categories. Scatterplot and boxplot illustrations were used to depict various volume distributions across various time measures. Volumetric agreement between ischemic core on baseline CTP and infarct volume on follow-up DWI was assessed using Bland-Altman plots.
To evaluate spatial agreement between ischemic core on baseline CTP and infarct volume on follow-up DWI, the positive and negative predictive values for the CTP rCBF <30% threshold were reported, using DWI infarct as the reference standard. Furthermore, Dice coefficient was calculated using the following equation from the coregistered set of images:
Average Hausdorff distance (the average of all minimum distances between the 2 segmentations) was also calculated to quantify spatial agreement.
A comparison of baseline characteristics and clinical outcomes was also completed between patients with limited (<25 mL) and significant infarct growth (≥25 mL). Factors independently associated with significant infarct growth (≥25 mL) were evaluated using a multivariable logistic regression model, with backwards stepwise variable selection to ensure model parsimony.
Statistical analysis was completed using STATA 15 (StataCorp 2017). All hypothesis testing was conducted using 2-sided statistical tests and p < 0.05 was considered statistically significant.
Standard Protocol Approvals, Registrations, and Patient Consents
The study protocol for SELECT was approved by local institutional review boards and registered at clinicaltrials.gov (NCT02446587). All participants or their legally authorized representatives provided informed consent prior to enrollment in the study.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Results
Study Cohort
Of the 361 patients enrolled in SELECT, 101 were included in the analysis. eFigure 1 (links.lww.com/WNL/B926) describes the study flowchart. Tables 1 and 2 provide the baseline characteristics and outcomes for patients included in the analysis. Further details regarding the study cohort are provided in the eResults.
Table 1.
Baseline Characteristics of SELECT EVT Patients Who had Complete Endovascular Reperfusion (mTICI 3) and Follow-up Imaging on DWI
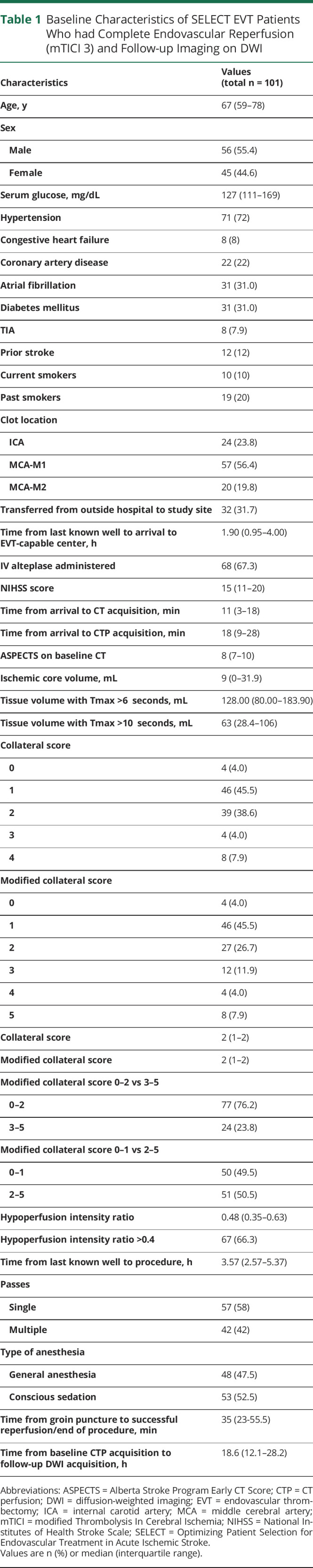
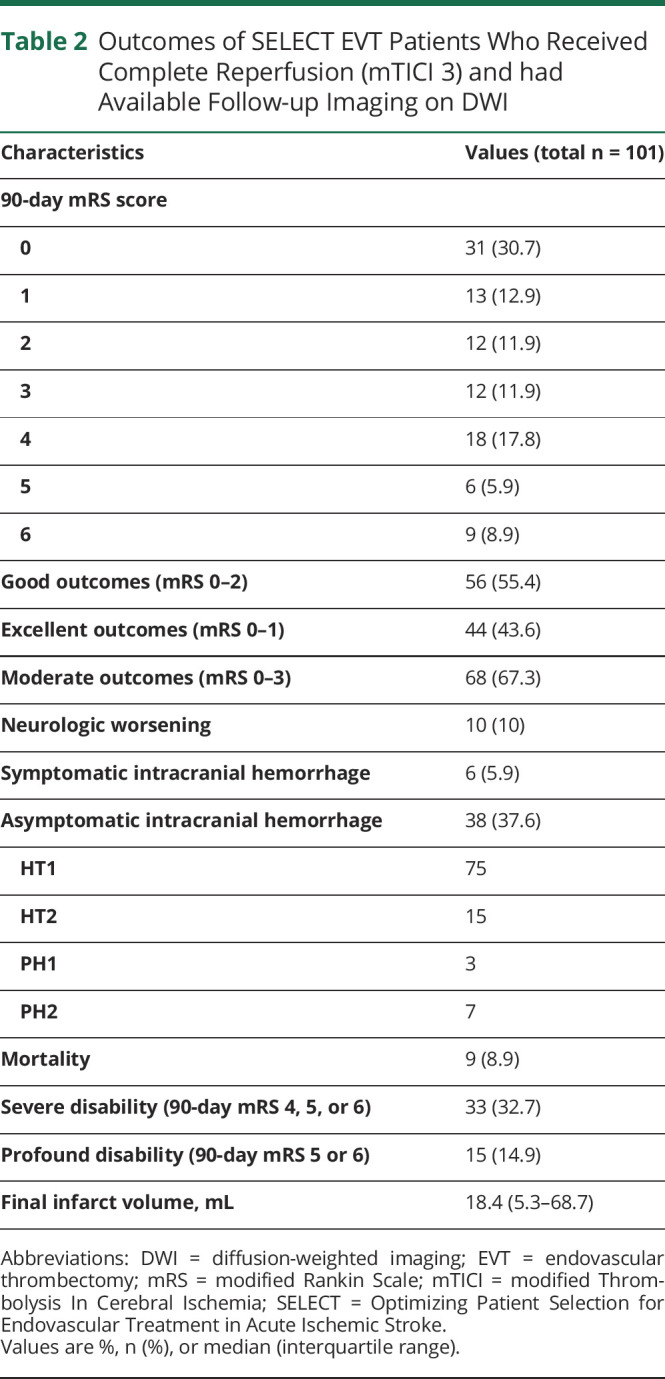
Table 2.
Outcomes of SELECT EVT Patients Who Received Complete Reperfusion (mTICI 3) and had Available Follow-up Imaging on DWI
Volumetric Analysis of CTP Core Estimation and the Correlation With Follow-up DWI
On EVT-capable center baseline images, the median volume of estimated ischemic core (rCBF <30% volume) was 9 (interquartile range [IQR], 0–31.9) mL, and median volume of critically hypoperfused tissue (Tmax >6 seconds volume) was 128 (IQR 80–183.9) mL. Median ASPECTS on arrival was 8 (IQR 7–10). On follow-up DWI, median (IQR) infarct volume was 18.4 (IQR 5.3–68.7) mL.
Median growth between the baseline ischemic core estimate on CTP and the infarct volume on follow-up DWI was 13.2 (IQR 2.9–35.4) mL. Figure 2 illustrates the agreement between ischemic core volume at presentation and infarct volume on follow-up DWI using Bland-Altman plot, the positive bias indicating that DWI infarct volumes were generally larger than estimated ischemic core on baseline CTP. The plot also demonstrated a tendency towards larger bias with increasing average lesion size.
Figure 2. Bland-Altman Plot Demonstrating Agreement in Ischemic Core Volume Using rCBF <30% Threshold Measured at Baseline and Infarct Volume Measured on Follow-up DWI.
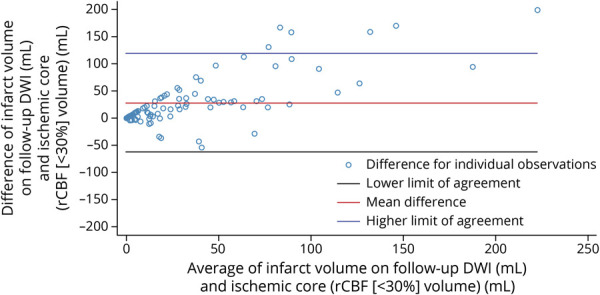
As demonstrated, the assessment revealed a positive bias (mean infarct volume on follow-up diffusion-weighted imaging [DWI] being larger than mean ischemic core on baseline CT perfusion) that increased as the average lesion size increased. rCBF = relative cerebral blood flow.
For the 40 of 101 (40%) patients without detectable ischemic core within the CTP coverage area, the median follow-up infarct volume (and thus median volumetric difference between baseline CTP ischemic core and follow-up infarct volume) was 9.8 mL (IQR 3.0–26.8 mL). In the remaining 61 (60%) patients, the baseline median ischemic core volume of 19.5 (IQR 10.8–42.7) mL increased to 46.2 (IQR 16.1–86.3) mL on follow-up DWI with a median difference of 21.5 (IQR 2.9–37.2) mL.
In a sensitivity analysis excluding the 39 patients with HT, the median volume difference between the CTP core estimate and the DWI follow-up study was 9.8 (IQR 0.2–31) mL. Median volume difference in the 39 patients with HT was 26.5 mL (IQR 8.4–55.4 mL). Increased absolute volumetric difference was associated with increased estimated baseline ischemic core volume (overall: ρ = 0.44, p < 0.0001; without hemorrhage: 0.34, p = 0.0076; with hemorrhage: 0.56, p = 0.0002).
Quantification of Volumetric Differences and Effect of Time From LKW to Imaging and Imaging to Reperfusion
Thirty-seven (37%) patients demonstrated significant growth of ≥25 mL (median 44.6 [IQR 34.6–95.6] mL) between baseline ischemic core and follow-up infarct volumes, whereas 58 (57%) had adequate estimation with a median volumetric difference of 5.4 (IQR 2–12.5) mL. Six (6%) patients had significant core overestimation, with a median overestimation of 34.8 (IQR 28.1–43) mL (eTables 1 and 2, links.lww.com/WNL/B926). Whereas only 25/101 patients had a moderate to large ischemic core (≥30 mL) on rCBF < 30% threshold in our cohort, 5/6 patients with significant overestimation had moderate to large ischemic core ≥30 mL on CTP.
As time from LKW to imaging acquisition increased, the volumetric accuracy of the ischemic core prediction of follow-up infarct volume increased: 6/36 (17%) patients with CTP acquired within 90 minutes of LKW demonstrated significant overestimation (≥10 mL) using rCBF <30% threshold, whereas none of the 65 patients receiving CTP beyond 90 minutes (43 within 90–270 minutes, 22 beyond 270 minutes of LKW) demonstrated significant overestimation (ptrend = 0.004).
Similarly, the frequency of adequate estimation increased from 14/36 (39%) in the first 90 minutes to 29/43 (67%) in 90–270 minutes and 15/22 (68%) in >270 minutes groups (ptrend = 0.015). Volumetric correlation between preprocedure estimated core and follow-up infarct volume also improved as LKW time to imaging acquisition increased: Spearman ρ <90 minutes, 0.33 (p = 0.049); 90–270 minutes, 0.63 (p < 0.0001); >270 minutes, 0.86 (p < 0.0001).
Similarly, significant overestimation of ischemic core was limited to patients who had faster reperfusion from imaging acquisition (<120 minutes 6/53 [11.3%] vs ≥120 minutes 0/47 [0%]; p = 0.028) and significant infarct growth (≥25 mL) increased as time from imaging to reperfusion increased (<120 minutes 15/53 [28.3%] vs ≥120 minutes 21/47 [44.7%]; p = 0.089). The median volumetric difference between estimated core and subsequent infarct volume stratified based on time from imaging acquisition to reperfusion was 3.7 mL (IQR 0.1–31.3 mL) if reperfusion occurred within 120 minutes and 21.5 mL (IQR 9.8–41.5 mL) if reperfusion occurred >120 minutes (p = 0.001). Volumetric correlation between preprocedure and follow-up volumes did not differ with time from imaging to reperfusion: Spearman ρ <120 minutes, 0.59 (p < 0.0001) vs ≥120 minutes, 0.59 (p < 0.0001).
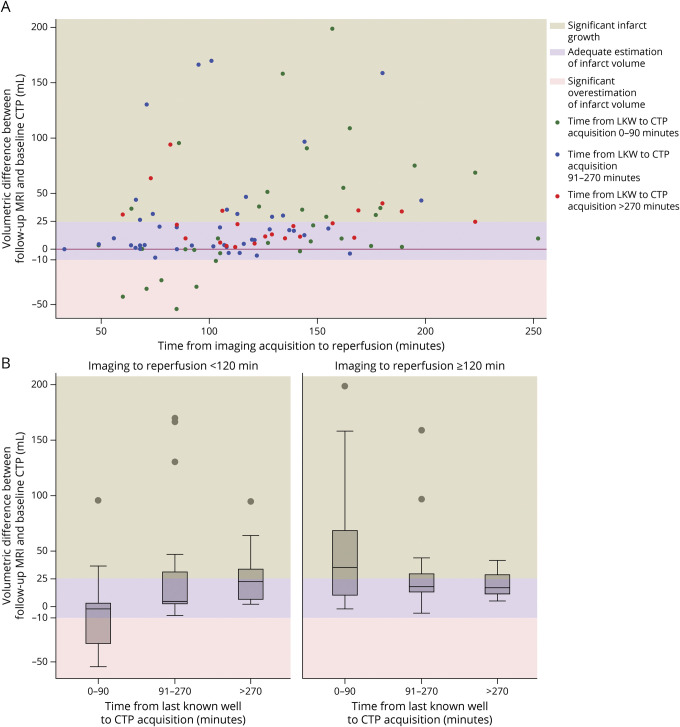
Figure 3A demonstrates the distribution of growth in infarct volume from baseline to follow-up imaging by time from imaging to reperfusion, stratified based on the time from LKW to imaging acquisition, with all 6 cases of overestimation demonstrating CTP imaging acquired within 90 minutes LKW and reperfusion achieved within 120 minutes of imaging acquisition. Figure 3B presents a box plot demonstrating the distribution of growth in infarct volume across categories for time from LKW to imaging and time from imaging to reperfusion.
Figure 3. Illustration of Volumetric Difference Between Ischemic Core on CTP and Infarct on Follow-up MRI From Baseline as Associated With Time From Last Known Well to CTP Acquisition and Time From CTP Acquisition to Successful Reperfusion.
(A) Scatterplot diagram. (B) Boxplot diagram. Overall, ischemic core volume on CT perfusion (CTP) and subsequent infarct volume on magnetic resonance diffusion-weighted imaging (MR-DWI) demonstrated significant correlation (Spearman ρ 0.58, p < 0.0001). Pearson correlation coefficient also demonstrated high correlation between ischemic core on CT perfusion and infarct volume on follow-up diffusion-weighted imaging (DWI) (r = 0.69, p < 0.0001). Significant core overestimation (≥10 mL) was shown to be limited to patients who received perfusion imaging within 90 minutes of last known well (LKW) and complete reperfusion within 120 minutes of imaging acquisition. Longer imaging to reperfusion intervals were associated with larger infarct growth in patients presenting very early (within 90 minutes of LKW) and early (90–270 minutes of LKW). Patients receiving perfusion imaging beyond 270 minutes of LKW demonstrated limited infarct growth, whether reperfusion was achieved within or beyond 120 minutes of imaging acquisition.
Furthermore, patients who had longer times from imaging acquisition to reperfusion demonstrated larger infarct growth in both very early (imaging <90 minutes from LKW) and early (imaging 90–270 minutes from LKW) presentations. In patients receiving imaging relatively late (>270 minutes from LKW), the growth in infarct volume was not significantly different, whether complete reperfusion was achieved within or beyond 120 minutes of imaging acquisition.
Effect of Collaterals Status on Accuracy of CTP Ischemic Core
The proportion of patients with good collaterals (collaterals score 2–4) demonstrating underestimation, adequate estimation, and overestimation of infarct volume were 29%, 67%, and 4%, whereas in those with poor collaterals (0–1), these proportions were 44%, 48%, and 8%.
rCBF <20% Threshold in Patients Receiving Imaging Within 90 Minutes of LKW
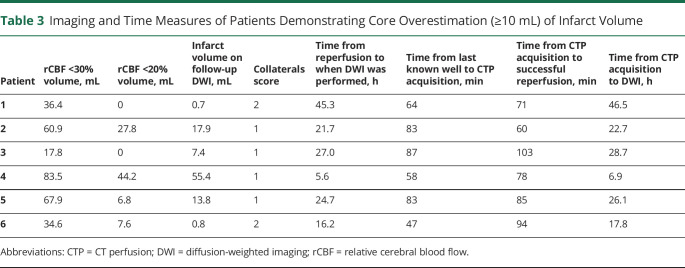
All 6 cases of significant ischemic core overestimation were observed when CTP was acquired within 90 minutes of LKW and successful reperfusion was achieved within 120 minutes of imaging acquisition. When these cases were assessed using the rCBF <20% threshold to estimate ischemic core, none of the cases demonstrated significant overestimation. Volumes of ischemic core using rCBF <30%, rCBF <20%, and infarct volume on follow-up DWI in these cases are shown in Table 3 and Figure 4 illustrates one of these cases. Of note, 2 patients demonstrated ≥5 mL overestimation using 20% threshold, including 1 case that approached significant (9.9 mL) infarct volume overestimation. All 6 patients with significant overestimation using the rCBF <30% threshold demonstrated poor (n = 4) or moderate (n = 2) collaterals (Table 3).
Table 3.
Imaging and Time Measures of Patients Demonstrating Core Overestimation (≥10 mL) of Infarct Volume
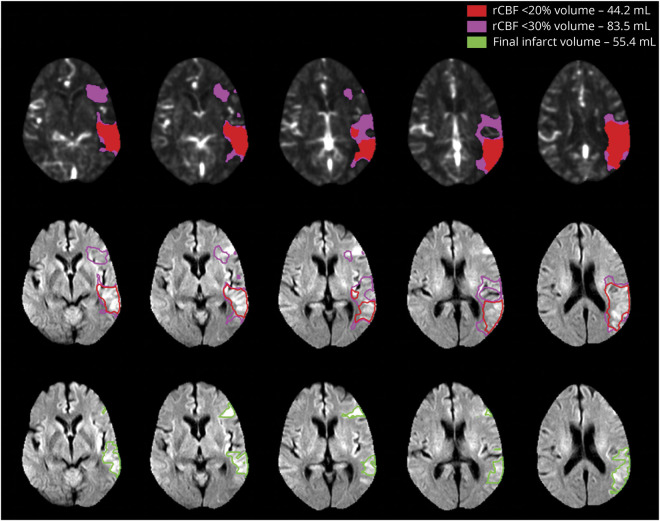
Figure 4. Illustration of a Case in the Hyperacute Period (<90 Minutes from Symptom Onset) With Significant Overestimation Ischemic Core Volumes Estimated Using the rCBF Threshold of <30% and How Using rCBF < 20% Threshold Resulted in Adequate Estimation of Infarct Volume.
The top panel demonstrates the relative cerebral blood flow (rCBF) ischemic core estimate using the <30% threshold in pink (83.5 mL) and with the <20% threshold overlaid red (44.2 mL). The bottom panel demonstrates coregistration of these volumes onto the follow-up diffusion-weighted imaging (DWI), which demonstrated an infarct volume of 55.4 mL. The pink outline of the <30% rCBF volume overestimates the DWI lesion; the red outline of the <20% rCBF volume provides a better prediction of the DWI lesion in this patient, whose baseline scan was performed 58 minutes after symptom onset.
Effect of Altering rCBF Threshold From 30% to 20% in Overall Population
In the overall population, rCBF threshold <30% was more accurate, with a median (IQR) of 13.2 (2.9–35.4) mL of infarct volume underestimation, whereas rCBF threshold <20% demonstrated a median (IQR) of 20.1 (5.9–55.4) mL of underestimation. Similarly, overall correlation of ischemic core and follow-up infarct volume also appears to be better with 30% threshold (Spearman ρ 0.58, p < 0.0001) as compared with 20% (Spearman ρ 0.48, p < 0.0001).
Effect of Time From LKW to Follow-up DWI Acquisition
Fifty-two (53.1%) patients had follow-up DWI within 24 hours of LKW, whereas 40 (40.8%) and 6 (6.1%) had DWI within 24–72 hours and beyond 72 hours from LKW, respectively. Three patients did not have information available regarding time from LKW to follow-up MRI acquisition. Volumetric difference between baseline CTP ischemic core volume and follow-up DWI infarct volume and its correlation with time from LKW to follow-up DWI is illustrated in eFigure 2 (links.lww.com/WNL/B926), demonstrating no clear relationship. We also did not observe any specific trends in infarct growth or proportion of overestimation, underestimation, or adequate estimation of infarct volume with time from LKW to follow-up DWI (eTable 3).
Factors Independently Associated With Infarct Growth
A comparison between baseline characteristics and clinical outcomes in patients exhibiting minimal infarct growth (<25 mL) and patients exhibiting significant infarct growth (≥25 mL) are provided in the eTables 4 and 5 (links.lww.com/WNL/B926). In a multivariable analysis incorporating perfusion imaging measures, procedural measures, and time metrics, only ischemic core volume (0.51 [0.21–0.81] mL of infarct growth for each 1 mL increase in baseline ischemic core volume; p = 0.001) and time from CTP acquisition to complete reperfusion (0.23 [0.03–0.43] mL of infarct growth for each 1-minute increase in time from CTP acquisition to reperfusion; p = 0.025) were independent predictors of infarct growth.
Spatial Analysis
After coregistration of baseline CTP imaging with follow-up MRI, infarct areas on MRI were classified based on whether they were superimposed on ischemic core regions on CTP. Volumes were determined for (1) tissue classified as ischemic core on CTP but not infarct on the subsequent DWI and (2) tissue classified as infarct on DWI but not ischemic core on CTP. Overall, the median volume of tissue classified as ischemic core on CTP but not infarcted on the subsequent DWI was 3.2 (0–9) mL. Tissue classified as infarct on DWI but not ischemic core on CTP had a median volume of 17.9 (5.6–43.9) mL.
Upon visual inspection of cases with tissue classified as ischemic core on CTP but not infarct on the subsequent DWI, the involved tissue was primarily in white matter in 46/60 cases. In 12 cases, these regions of “core overcall” were primarily in cortical/subcortical gray matter, whereas 2 cases involved both white and gray matter. Regions of infarct growth (tissue classified as infarct on DWI but not ischemic core on CTP) were observed primarily in cortical/subcortical gray matter in 82/97 cases, whereas 13 cases demonstrated infarct growth in both white and gray matter and 2 in primarily white matter.
Median (IQR) positive and negative predictive values for rCBF <30% threshold were 0.57 (0.26–0.81) and 0.98 (0.95–0.99), respectively. Median Dice coefficient for spatial agreement between the CTP core and DWI infarct regions was 0.1 (0–0.4). Ischemic core size was 0 mL in 40 cases and infarct volume on follow-up DWI was 0 mL in 1 case, thus calculation of the Hausdorff distance was not feasible. For the rest of the cases (n = 60), median average Hausdorff distance was 34.3 (28.1–46.1) mm. Time from imaging to reperfusion time did not correlate with calculated Dice coefficient (ρ = −0.005, p = 0.95) but correlated significantly with average Hausdorff distance (ρ = 0.31, p = 0.017).
Discussion
This substudy of the SELECT trial provides the largest prospectively collected dataset evaluating the relationship between CTP ischemic core estimates and subsequent infarct volumes in patients who achieved complete reperfusion after EVT. We found that baseline CTP ischemic core volume predicted subsequent infarct volume with a median error of approximately 13 mL, which is similar to the error reported in prior studies.19,20 In our study, none of the patients who received CTP beyond 90 minutes of LKW demonstrated significant (≥10 mL) overestimation of infarct, which occurred in 6 patients and was restricted to those who received CTP within 90 minutes of LKW and achieved rapid reperfusion (<120 minutes) subsequently. Using the <20% threshold for patients imaged within 90 minutes, we found that none demonstrated significant overestimation. These data support a recommendation to adjust the rCBF threshold to <20% for patients who are scanned within 90 minutes of LKW. Our results are compatible with prior studies that suggested that ischemic core thresholds may be time-dependent22-24 and stricter core thresholds should be used for patients who received perfusion imaging very early after symptom onset.25
Ischemic core overestimation is a potential concern that has been controversial in the stroke community, with recent articles14,21,26 suggesting that patients may be excluded from receiving EVT based on CTP overestimation of irreversible injury. The purpose of this study was to evaluate whether infarct core overestimation is frequent with the currently established rCBF <30% threshold, the effect of time from LKW and time from imaging to successful reperfusion on the incidence of potential overestimation, and whether the core overestimation can be eliminated with a more stringent rCBF <20% threshold.
There are a number of potential explanations for why a stricter CBF threshold provides a more accurate ischemic core estimate in patients who receive CTP very early after symptom onset. CTP assesses the hemodynamic status of the brain and not tissue viability. Ischemic core is estimated from CTP based on the severity of CBF reduction. Tissue death results when CBF is reduced for an adequate duration and severity to cause irreversible injury. Therefore, shorter duration of ischemia is less likely to cause irreversible injury unless the ischemia is very severe27; this concept is supported by our findings that a more strict CBF threshold was more accurate for patients receiving CTP within 90 minutes of LKW. Furthermore, CTP provides an assessment of CBF at a single point in time; if CBF values differed in the minutes or hours prior to the scan, the predictive accuracy will be reduced.
A substantial increase in subsequent infarct volume, compared with the baseline core estimate, occurred in about one third of the patients, more frequently in patients with hemorrhagic transformation, longer imaging to reperfusion times, and poor collaterals. These factors suggest that substantial infarct growth may have occurred prior to reperfusion and that reperfusion-related hemorrhage and edema are also key contributors.
Whereas MR-DWI can demonstrate reversibility of ischemic changes in cases of reperfusion, these images are considered gold standard for evaluating brain ischemia in early phases after stroke.28,29 In one study, CBF <30% threshold was shown to undercall core by a median of 12 mL when compared with a DWI scan obtained shortly after the CTP.30 These data are compatible with our findings. Less strict rCBF thresholds, such as <38%, can produce less undercall, but at the expense of more core overcall. In general, a more conservative estimate of ischemic core has been favored to avoid the potential for undertreatment.
Previous single-center, retrospective studies that reported higher incidence of CTP ischemic core overestimation typically used software that has been associated with high rates of core overcall31 and only evaluated the correlation between baseline CTP and final infarct volume volumetrically; no spatial review or coregistration of images was attempted. In addition, the final infarct volume was delineated on follow-up NCCT, which is less sensitive to detect ischemic changes.14,32 Furthermore, one study included only patients with ASPECTS ≥6.32
We used the definitions of 25 mL to define significant underestimation and 10 mL to define significant overestimation based on prior published studies that assessed accuracy of perfusion imaging.14,19-21 The threshold of 90 minutes was prespecified based on prior studies that suggested stricter thresholds were needed in the ultraearly time window,25 whereas the threshold of 270 minutes was assumed to allow for EVT procedure to occur within 6 hours of LKW. Infarct growth between ischemic core on baseline CTP and follow-up DWI infarct volume increased as time from imaging acquisition to complete reperfusion increased. Therefore, estimating infarct volume from the baseline core is more reliable in patients who are reperfused rapidly.
Theoretically, longer intervals between imaging and reperfusion may lead to loss of established collateral flow and thus progression of infarct. However, varying effect of time from imaging acquisition to reperfusion on lesion growth is reported, with differences being attributed to differing imaging profiles.24,33,34 In our study, we found significant infarct growth of 0.23 mL for each minute of time from CTP acquisition to reperfusion, which varied based on the time to imaging acquisition. In patients imaged beyond 270 minutes, the effect of time from imaging to reperfusion on core growth was limited. These later treated patients may have more favorable collaterals and therefore experienced slower core growth.35 This also may be due to the limited number of patients (22 patients) in this group. A pooled analysis from EXTEND-IA TNK and HERMES collaboration found that overestimation was uncommon and not related to imaging to reperfusion time.36 However, this pooled analysis included patients who achieved substantial reperfusion (modified Thrombolysis In Cerebral Ischemia [mTICI] = 2b/3). Our analysis only included patients who achieved complete reperfusion (mTICI = 3), thus minimizing the potential for infarct growth following EVT.
Utilization of a more stringent rCBF threshold of 20% resulted in resolution of significant overestimation in all 6 cases who presented within 90 minutes of LKW and received rapid reperfusion in the cohort. Use of more stringent threshold has been proposed in prior studies for patients presenting very early after symptom onset.25,37 However, these thresholds were evaluated from retrospectively collected limited data and had inclusion of patients with incomplete reperfusion and a combination of follow-up CT and MRI to define follow-up infarct volumes. Our study evaluated rCBF <20% threshold in prospectively collected data of patients achieving complete reperfusion and used MRI to define follow-up infarct volume. This observation, based on a limited number of cases, needs further validation. In addition, rCBF threshold of 30% performed better overall in volumetric assessment. It is possible that both time and individual patient factors may affect optimal rCBF thresholds; we did not evaluate additional predictors in this study.
We examined not only the volumetric but also spatial accuracy of CTP. We also evaluated how time from LKW to imaging acquisition and time from imaging to reperfusion affects the ischemic core estimation with the most commonly used rCBF threshold of <30%. Volumetric assessment demonstrated a greater correlation between baseline core and follow-up infarct than the spatial analysis. Lower spatial agreement is expected, in part because coregistration cannot provide a perfect alignment between baseline CT images and the follow-up MRI.
Various factors can contribute to overestimation of ischemic core measured on perfusion imaging. CBF values in the early period after stroke onset may vary based on the adequacy of the early response of collaterals to vessel occlusion.38,39 As perfusion imaging provides only a snapshot of the tissue hemodynamics at the acquisition time, changes occurring over time such as clot migration and recruitment of collaterals will not be reflected by the baseline perfusion assessment. Existing brain lesions including encephalomalacia from prior ischemic events and chronic microvascular changes can also confound the assessment. Furthermore, gray and white matter have differential ischemic thresholds and may demonstrate varying accuracy at a given CBF threshold.40-42 Prior efforts have suggested the use of an anatomic atlas during image processing and using differential ischemic thresholds to improve spatial and overall accuracy of perfusion imaging assessment.36 We observed a similar trend in our analysis with overcalls predominantly observed in white matter and infarct growth observed predominantly in gray matter on visual inspection.
Despite high rates of reperfusion with low rates of early complications including neurologic worsening and symptomatic ICH, 45% of our cohort with complete reperfusion failed to achieve 90-day functional independence. This is largely comparable to prior reports describing outcomes in patients with complete reperfusion.43 Furthermore, prevalence of undetectable baseline ischemic core was higher in our cohort (40%) compared to prior reports using similar software in a patient-level analysis of EXTEND-IA and HERMES in early window (19%)36 and in the DEFUSE 3 trial (24%; unpublished data).6 This could be related to the larger number of M2 occlusions included and larger number of very early presenters. Our dataset reflects routine clinical practice at 9 high-volume EVT centers across the United States.
Our study has several limitations. The study is a post hoc analysis of the SELECT trial, with a moderate number of patients included. Follow-up MRIs were obtained between 5 hours from enrollment up to 10 days. Transient reversal can occur with MR-DWIs obtained within 24 hours of reperfusion, which may result in underestimation of final infarct volume.44 Furthermore, studies have shown that a modest degree of lesion growth often occurs between 24 and 72 hours, even in patients who achieved complete reperfusion.45 Unlike previous studies, we did not demonstrate a difference in infarct growth between patients who had their infarct volume assessed within the first 24 hours vs at 5 days. This may be due to the fact that all patients in our cohort achieved complete reperfusion and infarct growth beyond 24 hours occurs primarily in nonreperfusers.44,45 Delayed acquisition of follow-up DWI can also result in inclusion of tissue edema in the infarct volume. Being a pragmatic study, SELECT allowed for follow-up imaging acquisition up to 7 days, which may have contributed to increased frequency of volumetric and spatial disagreements. RCTs usually have stricter time intervals for follow-up imaging acquisition. Time from CTP to reperfusion ranged widely from 33 to 252 minutes, which could have affected the accuracy of CTP estimation due to infarct growth in patients who had significant infarct growth due to delay prior to reperfusion. We did not collect information on whether stroke onset was witnessed; therefore, in patients who did not have an observed onset, the time between stroke onset and imaging may be overestimated.
Our results suggest that baseline ischemic core estimates predict subsequent infarct volumes in patients who achieve complete reperfusion during EVT with a median error of 13 mL. Underestimation of infarct volume is often related to hemorrhagic transformation or an extended time between imaging and reperfusion. Overestimation of the ischemic core on CT perfusion occurred infrequently and was limited to patients presenting very early, within 90 minutes, after the stroke onset who achieved rapid reperfusion and was predominantly limited to white matter. In these early presenting patients, overestimation may be avoided by using a more conservative rCBF <20% threshold to identify the ischemic core.
Glossary
- ASPECTS
Alberta Stroke Program Early CT Score
- CTP
CT perfusion
- DWI
diffusion-weighted imaging
- EVT
endovascular thrombectomy
- IQR
interquartile range
- LKW
last known well
- MR-DWI
magnetic resonance diffusion-weighted imaging
- mTICI
modified Thrombolysis In Cerebral Ischemia
- NCCT
noncontrast CT
- rCBF
relative cerebral blood flow
- RCT
randomized clinical trial
- ROI
region of interest
- SELECT
Optimizing Patient Selection for Endovascular Treatment in Acute Ischemic Stroke
- SWIFT-PRIME
Solitaire With the Intention For Thrombectomy as Primary Endovascular Treatment
Appendix 1. Authors
Appendix 2. Coinvestigators

Footnotes
Editorial, page 867
Infographic: NPub.org/ig9821
Study Funding
The SELECT trial was funded by Stryker Neurovascular through a grant to UT McGovern Medical School. Stryker Neurovascular did not participate in the design or conduct of the study; collection, management, analysis, or interpretation of data; preparation of the manuscript; or decision to submit the manuscript for publication.
Disclosure
A. Sarraj received research grants for SELECT and SELECT2 from Stryker and receives consultant fees from Stryker, which manufactures one of the devices that was used in SELECT. G.W. Albers has ownership interest in and is a consultant for iSchemaView, which is the software used to process the neuroimages for this research. S. Christensen received consultant fees from iSchemaView. The other authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Berkhemer OA, Fransen PSS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372(1):11-20. [DOI] [PubMed] [Google Scholar]
- 2.Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372(24):2296-2306. [DOI] [PubMed] [Google Scholar]
- 3.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372(11):1019-1030. [DOI] [PubMed] [Google Scholar]
- 4.Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372(24):2285-2295. [DOI] [PubMed] [Google Scholar]
- 5.Campbell BCV, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372(11):1009-1018. [DOI] [PubMed] [Google Scholar]
- 6.Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378(8):708-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378(1):11-21. [DOI] [PubMed] [Google Scholar]
- 8.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. [DOI] [PubMed] [Google Scholar]
- 9.Turc G, Bhogal P, Fischer U, et al. European Stroke Organisation (ESO)–European Society for Minimally Invasive Neurological therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischaemic stroke endorsed by Stroke Alliance for Europe (SAFE). Eur Stroke J. 2019;4(1):6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarraj A, Hassan AE, Grotta J, et al. Optimizing Patient Selection For Endovascular Treatment in Acute Ischemic Stroke (SELECT): a prospective, multicenter cohort study of imaging selection. Ann Neurol. 2020;87(3):419-433. [DOI] [PubMed] [Google Scholar]
- 11.Nannoni S, Ricciardi F, Strambo D, et al. Correlation between ASPECTS and core volume on CT perfusion: impact of time since stroke onset and presence of large-vessel occlusion. AJNR Am J Neuroradiol. 2021;42(3):422-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wintermark M, Reichhart M, Thiran JP, et al. Prognostic accuracy of cerebral blood flow measurement by perfusion computed tomography, at the time of emergency room admission, in acute stroke patients. Ann Neurol. 2002;51(4):417-432. [DOI] [PubMed] [Google Scholar]
- 13.Campbell BCV, Christensen S, Levi CR, et al. Comparison of computed tomography perfusion and magnetic resonance imaging perfusion-diffusion mismatch in ischemic stroke. Stroke. 2012;43(10):2648-2653. [DOI] [PubMed] [Google Scholar]
- 14.García-Tornel Á, Campos D, Rubiera M, et al. Ischemic core overestimation on computed tomography perfusion. Stroke. 2021;52(5):1751-1760. [DOI] [PubMed] [Google Scholar]
- 15.Legault C, Lansberg M, Heit J, Albers G. Optimizing CT perfusion thresholds for identification of ischemic core in hyperacute stroke. Stroke. 2019;50(suppl 1):ATP66. [Google Scholar]
- 16.Wintermark M, Flanders AE, Velthuis B, et al. Perfusion-CT assessment of infarct core and penumbra. Stroke. 2006;37(4):979-985. [DOI] [PubMed] [Google Scholar]
- 17.Sarraj A, Mlynash M, Savitz SI, et al. Outcomes of thrombectomy in transferred patients with ischemic stroke in the late window: a subanalysis from the DEFUSE 3 trial. JAMA Neurol. 2019;76(6):682-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarraj A, Hassan AE, Savitz S, et al. Outcomes of endovascular thrombectomy vs medical management alone in patients with large ischemic cores: a secondary analysis of the optimizing patient's selection for endovascular treatment in acute ischemic stroke (SELECT) study. JAMA Neurol. 2019;76(10):1147-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wheeler HM, Mlynash M, Inoue M, et al. Early diffusion-weighted imaging and perfusion-weighted imaging lesion volumes forecast final infarct size in DEFUSE 2. Stroke. 2013;44(3):681-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albers GW, Goyal M, Jahan R, et al. Ischemic core and hypoperfusion volumes predict infarct size in SWIFT PRIME. Ann Neurol. 2016;79(1):76-89. [DOI] [PubMed] [Google Scholar]
- 21.Boned S, Padroni M, Rubiera M, et al. Admission CT perfusion may overestimate initial infarct core: the ghost infarct core concept. J Neurointerv Surg. 2017;9(1):66. [DOI] [PubMed] [Google Scholar]
- 22.Laredo C, Renú A, Tudela R, et al. The accuracy of ischemic core perfusion thresholds varies according to time to recanalization in stroke patients treated with mechanical thrombectomy: a comprehensive whole-brain computed tomography perfusion study. J Cereb Blood Flow Metab. 2019;40(5):966-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiu W, Kuang H, Lee TY, et al. Confirmatory study of time-dependent computed tomographic perfusion thresholds for use in acute ischemic stroke. Stroke. 2019;50(11):3269-3273. [DOI] [PubMed] [Google Scholar]
- 24.Bivard A, Kleinig T, Miteff F, et al. Ischemic core thresholds change with time to reperfusion: a case control study. Ann Neurol. 2017;82(6):995-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Najm M, Al-Ajlan FS, Boesen ME, et al. Defining CT perfusion thresholds for infarction in the golden hour and with ultra-early reperfusion. Can J Neurol Sci. 2018;45(3):339-342. [DOI] [PubMed] [Google Scholar]
- 26.Rodrigues GM, Mohammaden MH, Haussen DC, et al. Ghost infarct core following endovascular reperfusion: a risk for computed tomography perfusion misguided selection in stroke. Int J Stroke. Epub 2021 Nov 19. [DOI] [PubMed]
- 27.Jones TH, Morawetz RB, Crowell RM, et al. Thresholds of focal cerebral ischemia in awake monkeys. J Neurosurg. 1981;54(6):773-782. [DOI] [PubMed] [Google Scholar]
- 28.Nagaraja N, Forder JR, Warach S, Merino JG. Reversible diffusion-weighted imaging lesions in acute ischemic stroke. Neurology. 2020;94(13):571. [DOI] [PubMed] [Google Scholar]
- 29.Lakomkin N, Pan J, Stein L, Malkani B, Dhamoon M, Mocco J. Diffusion MRI reversibility in ischemic stroke following thrombolysis: a meta-analysis. J Neuroimaging. 2020;30(4):471-476. [DOI] [PubMed] [Google Scholar]
- 30.Cereda CW, Christensen S, Campbell BCV, et al. A benchmarking tool to evaluate computer tomography perfusion infarct core predictions against a DWI standard. J Cereb Blood Flow Metab. 2016;36(10):1780-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Austein F, Riedel C, Kerby T, et al. Comparison of perfusion CT software to predict the final infarct volume after thrombectomy. Stroke. 2016;47(9):2311-2317. [DOI] [PubMed] [Google Scholar]
- 32.Martins N, Aires A, Mendez B, et al. Ghost infarct core and admission computed tomography perfusion: redefining the role of neuroimaging in acute ischemic stroke. Interv Neurol. 2018;7(6):513-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simonsen CZ, Mikkelsen IK, Karabegovic S, Kristensen PK, Yoo AJ, Andersen G. Predictors of infarct growth in patients with large vessel occlusion treated with endovascular therapy. Front Neurol. 2017;8:574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsai JP, Mlynash M, Christensen S, et al. Time from imaging to endovascular reperfusion predicts outcome in acute stroke. Stroke. 2018;49(4):952-957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarraj A, Hassan AE, Grotta J, et al. Early infarct growth rate correlation with endovascular thrombectomy clinical outcomes: analysis from the SELECT study. Stroke. 2021;52(1):57-69. [DOI] [PubMed] [Google Scholar]
- 36.Hoving JW, Marquering HA, Majoie CBLM, et al. Volumetric and spatial accuracy of computed tomography perfusion estimated ischemic core volume in patients with acute ischemic stroke. Stroke. 2018;49(10):2368-2375. [DOI] [PubMed] [Google Scholar]
- 37.d'Esterre CD, Boesen ME, Ahn SH, et al. Time-dependent computed tomographic perfusion thresholds for patients with acute ischemic stroke. Stroke. 2015;46(12):3390-3397. [DOI] [PubMed] [Google Scholar]
- 38.Christensen S, Obi C, Albers G, Lansberg M. Ultra-acute CT perfusion imaging: a stroke in the scanner. Neurology. 2015;85(19):1725-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rao VL, Mlynash M, Christensen S, et al. Collateral status contributes to differences between observed and predicted 24-h infarct volumes in DEFUSE 3. J Cereb Blood flow Metab. 2020;40(10):1966-1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arakawa S, Wright PM, Koga M, et al. Ischemic thresholds for gray and white matter: a diffusion and perfusion magnetic resonance study. Stroke. 2006;37(5):1211-1216. [DOI] [PubMed] [Google Scholar]
- 41.Chen C, Bivard A, Lin L, Levi CR, Spratt NJ, Parsons MW. Thresholds for infarction vary between gray matter and white matter in acute ischemic stroke: a CT perfusion study. J Cereb Blood flow Metab. 2019;39(3):536-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Falcao ALE, Reutens DC, Markus R, et al. The resistance to ischemia of white and gray matter after stroke. Ann Neurol. 2004;56(5):695-701. [DOI] [PubMed] [Google Scholar]
- 43.van Horn N, Kniep H, Leischner H, et al. Predictors of poor clinical outcome despite complete reperfusion in acute ischemic stroke patients. J Neurointerv Surg. 2021;13(1):14. [DOI] [PubMed] [Google Scholar]
- 44.Federau C, Mlynash M, Christensen S, et al. Evolution of volume and signal intensity on fluid-attenuated inversion recovery MR images after endovascular stroke therapy. Radiology. 2016;280(1):184-192. [DOI] [PubMed] [Google Scholar]
- 45.Federau C, Christensen S, Mlynash M, et al. Comparison of stroke volume evolution on diffusion-weighted imaging and fluid-attenuated inversion recovery following endovascular thrombectomy. Int J Stroke. 2017;12(5):510-518. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.