Keywords: high-fat diet, kidney, kidney damage, obesity, tubuloglomerular feedback
Abstract
Obesity increases the risk of renal damage, but the mechanisms are not clear. Normally, kidneys autoregulate to keep the glomerular capillary pressure (PGC), renal blood flow, and glomerular filtration rate in a steady state. However, in obesity, higher PGC, renal blood flow, and glomerular filtration rate are noted. Together, these may lead to glomerular damage. PGC is controlled mainly by afferent arteriole resistance, which, in turn, is regulated by tubuloglomerular feedback (TGF), a vasoconstrictor mechanism. High fat-induced obesity causes renal damage, and this may be related to increased PGC. However, there are no studies as to whether high-fat diet (HFD)-induced obesity affects TGF. We hypothesized that TGF would be attenuated in obesity caused by HFD feeding (60% fat) in Sprague–Dawley rats. Sprague–Dawley rats fed a normal-fat diet (NFD; 12% fat) served as the control. We studied 4 and 16 wk of HFD feeding using in vivo renal micropuncture of individual rat nephrons. We did not observe significant differences in body weight, TGF response, and mean arterial pressure at 4 wk of HFD feeding, but after 16 wk of HFD, rats were heavier and hypertensive. The maximal TGF response was smaller in HFD-fed rats than in NFD-fed rats, indicating an attenuation of TGF in HFD-induced obesity. Baseline PGC was higher in HFD-fed rats than in NFD-fed rats and was associated with higher glomerulosclerosis. We conclude that attenuated TGF and higher PGC along with hypertension in HFD-fed obese Sprague–Dawley rats could explain the higher propensity of glomerular damage observed in obesity.
NEW & NOTEWORTHY Reduced tubuloglomerular feedback, higher glomerular capillary pressure, and hypertension in combination may explain the higher glomerular damage observed in high-fat diet-induced obesity.
INTRODUCTION
Obesity is now a global pandemic (1) and a cause of “loss of health” worldwide. Nearly 70% of the adult United States population is either overweight or obese. A recent report by the Centers for Disease Control and Prevention indicated that in the United States alone, almost 42.4% of people are obese (2). The Centers for Disease Control and Prevention also pointed out that parallel to the increase in obesity prevalence, kidney damage is also on the rise (2), and currently almost 4 million people have obesity-related kidney pathology in the United States alone in the absence of diabetes and hypertension (3). In addition, according to the National Institutes of Health and the United States Renal Data System, obesity is strongly linked to the two top causes of end-stage renal damage, i.e., diabetes and hypertension (4). Almost 90% of the diabetic population and 70% of the hypertensive population are either overweight or obese (5–7); thus, obesity should be considered one of the most important causes of renal damage. Kidney damage associated with both diabetes and hypertension costs approximately $127 billion annually to the United States, i.e., almost 19% of all-cause medical service cost (8). Once the kidney is damaged, there is no treatment, and most of the patients either must go for dialysis for their lifetime or their kidney needs to be replaced (9, 10). Therefore, in the absence of any treatment for obesity-related kidney disease and its increasing prevalence, it is critical to understand the pathogenesis of this disease.
Maladaptation in renal hemodynamics has been implicated as one of the key factors for the renal damage observed in individuals with obesity (11–13). In obesity, alterations in renal hemodynamics result in higher glomerular capillary pressure (PGC), renal blood flow (RBF), and glomerular filtration rate (GFR), but the mechanism behind this is unclear. These alterations in renal hemodynamics and higher PGC may expose fragile glomerular cells to higher pressure and cause glomerular barotrauma, which can lead to renal damage.
In a normal kidney, RBF is autoregulated by mechanisms such as tubuloglomerular feedback (TGF), connecting tubule glomerular feedback, and the myogenic response (14). The renal artery supplies the blood to the kidneys, and within the kidneys, each nephron receives blood from the arteriole called the afferent arteriole. The afferent arteriole branches out to the glomerular capillaries, and finally blood leaves the nephron through the efferent arteriole (14). Since the afferent arteriole, glomerular capillaries, and efferent arteriole are all arranged in series, their dynamics are closely interconnected (15). The afferent arteriole and efferent arteriole regulate inflow and outflow, respectively, and regulate both PGC and single-nephron GFR (15). Afferent arteriole constriction results in lower PGC and glomerular plasma flow downstream and may decrease GFR (15). In contrast, efferent arteriole constriction can increase PGC and single-nephron GFR (16, 17). TGF causes afferent arteriole constriction in response to increased NaCl in the macula densa via Na+-K+-2Cl− cotransporter isoform 2 (15, 17). A decrease in TGF may cause afferent arteriole dilation, and this can reduce renal vascular resistance. Reduced renal afferent arteriole resistance, in turn, can lead to increased RBF and increased transmission of systemic pressure to the glomerulus, causing higher PGC (18).
Previous studies by us and others have shown that there is increased PGC and a decreased TGF response (measured using the stop-flow pressure method) in a monogenic model of obesity like the Zucker obese rat (19, 20). However, whether TGF is attenuated in a high-fat diet (HFD)-induced obesity in Sprague–Dawley (SD) rats is not known. We hypothesized that HFD-induced obesity impairs the TGF response and decreases whole kidney GFR and RBF. SD rats were fed with a HFD (60% fat) or normal-fat diet (NFD; 12% fat) for 4 or 16 wk. We used in vivo renal micropuncture using the stop-flow technique and also measured renal hemodynamics parameters (RBF and GFR) to understand whole kidney hemodynamics.
MATERIALS AND METHODS
All animal experiments were approved by the Henry Ford Health System Institutional Animal Care and Use Committee and were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Renal Micropuncture Experiments
Male SD rats were obtained from Charles River Laboratories. We conducted in vivo renal micropuncture experiments to evaluate the TGF response at two time points, the first at 4 wk of HFD feeding and the second at 16 wk of HFD feeding, as previously described (15, 17). Briefly, thiobutabarbital was used intraperitoneally (125 mg/kg body wt) to anesthetize the rats, and additional dosages of thiobutabarbital were administered to HFD-fed rats especially at 16 wk of HFD to reach the plane of anesthesia to conduct renal micropuncture experiments. The femoral artery and jugular vein were cannulated to measure mean arterial pressure (MAP) and to infuse 0.154 M NaCl solution (1.5 mL/h to maintain euvolemia), respectively (21). The abdomen was cut open through the left lateral side, and a lucite cup was used to place the left kidney. Afterward, saline-soaked cotton or animal fat was used to immobilize the kidney. Thirty to forty-five minutes were allowed for equilibration before the start of the renal micropuncture experiments. First, stained dye was injected into surface tubules, permitting the detection of tubule loops with a finding pipette. This helps in the identification of the course of the tubules. Grease was then injected into an early segment of the proximal tubule causing a tubule blockage; afterward, two pipettes were inserted inside the same tubule. First, a perfusion pipette was inserted downstream from the grease block and attached to the infusion pump. Second, a pipette for measuring stop-flow pressure (PSF) was inserted upstream of the grease block and was attached to a micropressure system (model 900 A, World Precision 99 Instruments, Sarasota, FL). To generate a PSF curve, the late proximal perfusion (in an orthograde manner) rate was increased stepwise from 0 to 40 nL/min while PSF was measured. Each of the perfusion rates was maintained until we observed stable PSF. TGF was calculated as the decrease in PSF caused by an increase in nephron perfusion (15, 22, 23).
RBF Measurement
After 14 wk of HFD feeding, SD rats were fasted overnight but allowed free access to drinking water. On the experiment day, rats were anesthetized with the use of an intraperitoneal injection of thiobutabarbital (125 mg/kg body wt) and placed on a heated surgical table to maintain constant body temperature. Tracheotomy was done using PE-240 tubing to allow free breathing of room air. The femoral artery and vein were cannulated with PE-50 catheters for MAP measurement and supplementation of 2 mL/h of 2% BSA (Sigma-Aldrich), respectively. A midventral abdominal incision was performed, and the intestines were wrapped in moist gauze and moved to the side of the peritoneal cavity to expose the left kidney. The renal artery was carefully isolated from the vein and fitted with a Doppler flow probe (Transonic, Ithaca, NY) connected to a transit-time perivascular flowmeter (model TS-420, Transonic) to record RBF. The rat was draped, and 30 min were allowed for stabilization before RBF measurements, as described elsewhere (24).
Measurement of GFR in Conscious Rats
GFR was measured in conscious and unrestrained rats (16-wk HFD-fed rats) using a high-throughput method that involves the detection of fluorescent FITC-labeled inulin (TdB Consultancy, Uppsala, Sweden) clearance from blood, as described elsewhere (25). Basically, predialyzed 20 mg/mL FITC-inulin solution in saline (2 μL of 2% solution per 1 g body wt) was administered in bolus via tail vein injection to rats briefly anesthetized with isoflurane. Anesthesia was discontinued after the administration of inulin, and the animals regained consciousness. Afterward, 10 μL of blood were collected at 3, 5, 8, 16, 25, 40, 60, 80, 100, and 120 min after the injection by bleeding the tail. Thereafter, plasma was separated, and inulin clearance was quantified using FITC intensity. Fluorescence was measured using a NanoDrop 3300 Fluorospectrometer (Thermo Fisher Scientific, Waltham, MA). GFR was calculated from the observed decrease in FITC fluorescence using a two-compartment model (the initial fast decay representing the redistribution of FITC-inulin from the intravascular compartment to the extracellular fluid and the slower phase reflecting clearance from plasma). GFR curves were approximated with a biexponential decay function using OriginPro 9.0 (Origin Lab, Northampton, MA) software, and GFR values (in mL/min) were obtained from the fitting parameters using the previously described equation (25).
Intraperitoneal Glucose Tolerance Test
After 14 wk of HFD feeding, SD rats were used to evaluate glucose tolerance. Rats were fasted overnight, blood samples were taken from the tail vein, and glucose was measured using a glucometer (Bayer Contour Blood glucose meter) at 0 (fasting), 15, 30, 60, 90, and 120 min after an intraperitoneal injection of glucose (2 g/kg) was given.
Measurement of Albuminuria
Albuminuria was measured every 4 wk starting from 8 to 16 wk of HFD feeding. After 24 h of adaptation to metabolic cages, both HFD- and NFD-fed rats underwent urine collection for 24 h. The total volume of collected urine was measured, and aliquots were prepared and centrifuged twice at 1,200 g at 4°C for 10 min. The supernatants were then filtered and stored at −80°C until further analysis. Albuminuria was measured using an ELISA kit (GenWay Biotech ELISA) following the manufacturer’s instructions; 24-h albuminuria was calculated as urine albumin concentration multiplied by 24-h urine volume output, as described elsewhere (14).
Collection of Kidneys for Histological Experiments
Animals were anesthetized with thiobutabarbital (125 mg/kg body wt), and a catheter was placed in the abdominal aorta below the renal artery. Blood was collected, and plasma was then isolated for the measurement of insulin and leptin hormones. The aorta was then clamped above the renal artery, and the kidneys were perfused using 0.9% saline followed by fixation by 4% paraformaldehyde solution. Fixed kidneys were sent to the histology core at Henry Ford Health System for further processing for periodic acid-Schiff staining and hematoxylin and eosin staining. Unstained and charged slides were used for the measurements of glomerulosclerosis.
Statistical Analysis
All data are expressed as means ± SE; n represents the number of animals. We used ANOVA for repeated measures for dependent data and standard ANOVA for independent data. With two-way designs, an interaction effect was also tested. However, our primary interest was in the comparison of the replicates at each flow rate level. We used a set of paired t tests, where our multiple testing adjustments were Hochberg’s method. When our interest shifted to two group comparisons, we used a group of Student’s t tests, one at each flow level. Again, a Hochberg’s correction for multiple testing was used. Individual group comparisons were examined using unpaired Student’s t tests.
RESULTS
Effect of HFD Feeding on Body Weight and Glucose Tolerance
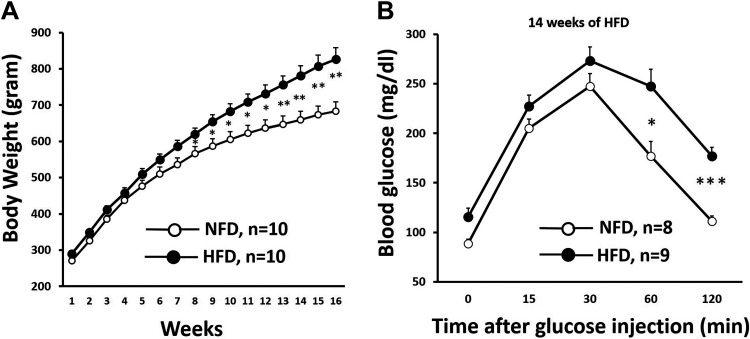
HFD-fed SD rats gradually increased their weight. HFD-fed rats were significantly heavier compared with NFD-fed rats starting at week 8 (NFD: 587 ± 21 g vs. HFD: 654 ± 19 g, P < 0.05; Fig. 1A).
Figure 1.
A: body weight after high-fat diet (HFD) feeding. HFD-fed Sprague–Dawley (SD) rats weighed significantly higher compared with normal-fat diet (NFD)-fed rats after 8 wk of HFD feeding. Body weight for the NFD-fed group vs. body weight for the HFD-fed group: *P < 0.05 and **P < 0.01. B: glucose tolerance after HFD feeding. HFD-fed SD rats exhibited glucose intolerance after 14 wk of HFD feeding. Blood glucose was significantly increased in HFD-fed SD rats than in NFD-fed rats at baseline, 60 min after intraperitoneal glucose injection, and 120 min after intraperitoneal glucose injection. Blood glucose in the NFD-fed group vs. blood glucose in the HFD-fed group: *P < 0.05 and ***P < 0.001.
The blood glucose level was slightly, but significantly, higher in HFD-fed rats at the baseline level and remained elevated even after 2 h of intraperitoneal glucose administration (Fig. 1B), indicative of glucose intolerance at 14 wk of HFD feeding.
TGF Responses at 4 and 16 wk of HFD Feeding
To determine whether HFD-induced obesity was associated with changes in the TGF response, late proximal tubules were perfused with artificial tubular fluid, while PSF was measured at two time points, before obesity developed (at 4 wk) or after rats became fully obese at 16 wk of HFD feeding. During the experiment, the perfusion rate was increased from 0 to 40 nL/min stepwise to initiate TGF. The TGF response was similar in NFD- and HFD-fed rats after 4 wk of diet (Fig. 2A) but was significantly attenuated at 16 wk of HFD feeding (Fig. 2B). At 16 wk of HFD feeding, PSF was significantly higher even at the baseline level when the perfusion rate was 0 nL/min (NFD: 38.7 ± 0.5 mmHg vs. HFD: 41.6 ± 0.8 mmHg, P < 0.05). The maximum TGF response (calculated by subtracting the PSF value from 0 nL/min perfusion rate to 40 nL/min perfusion rate) was similar between NFD- and HFD-fed rats at 4 wk of HFD feeding (Fig. 2C) but was significantly attenuated at 16 wk of HFD feeding (Fig. 2D). These data indicate a progressive decline in the TGF response upon HFD feeding.
Figure 2.
A: tubuloglomerular feedback (TGF) responses at 4 wk of high-fat diet (HFD) feeding were similar in both HFD- and normal-fat diet (NFD)-fed Sprague–Dawley (SD) rats and HFD-fed SD rats. B: TGF responses at 16 wk of HFD feeding was significantly lower in HFD-fed rats than in NFD-fed SD rats. TGF response in the NFD-fed group vs. TGF response in the HFD-fed group: *P < 0.05, **P < 0.01, and ***P < 0.001. C: comparison of maximum TGF response in both HFD- and NFD-fed rats at 4 wk of feeding. D: comparison of maximum TGF response in both HFD- and NFD-fed rats at 16 wk of feeding. Maximum TGF response in the NFD-fed group vs. maximum TGF response in the HFD-fed group: *P < 0.05.
MAP at 4 and 16 wk of HFD Feeding
To determine whether HFD feeding is associated with an increase in MAP, we measured MAP in thiobutabarbital anesthetized rats using a femoral catheter during in vivo renal micropuncture. We did not observe a significant difference in MAP at 4 wk of HFD feeding, but at 16 wk of feeding, MAP was significantly higher in HFD-fed rats (anesthetized) than in NFD-fed rats (Fig. 3, A and B), indicating a gradual increase in blood pressure upon HFD feeding.
Figure 3.
A: mean arterial pressure was similar in high-fat diet (HFD)-fed Sprague–Dawley (SD) rats compared with normal-fat diet (NFD)-fed SD rats after 4 wk of HFD feeding measured under thiobutabarbital anesthesia using an intrafemoral catheter. B: mean arterial pressure was significantly higher in HFD-fed SD rats than in NFD-fed SD rats after 16 wk of HFD feeding measured under thiobutabarbital anesthesia using an intrafemoral catheter. Mean arterial pressure in the NFD-fed group vs. the HFD-fed group: *P < 0.05.
RBF and GFR at 16 wk of HFD Feeding
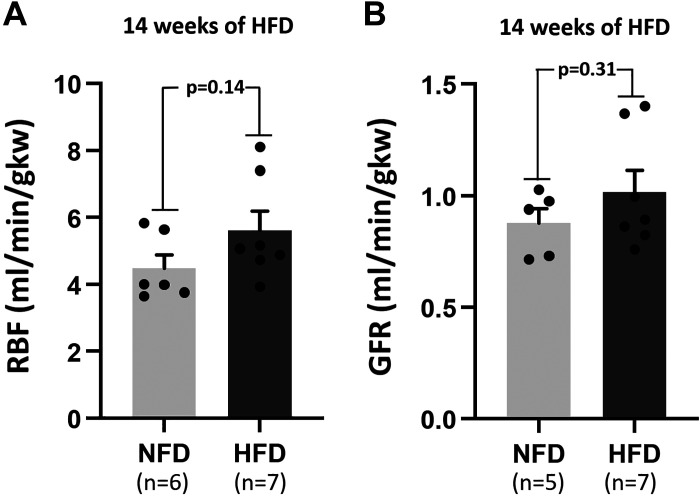
We investigated whether there was any change in RBF upon HFD feeding. Although we observed a trend toward higher RBF in HFD (Fig. 4A), it did not reach statistical significance.
Figure 4.
A: renal blood flow (RBF) in high-fat diet (HFD)-fed Sprague–Dawley (SD) rats compared with normal-fat diet (NFD)-fed SD rats after 14 wk of HFD feeding. B: glomerular filtration rate (GFR) in HFD-fed SD rats compared with NFD-fed SD rats after 14 wk of HFD feeding. gkw, grams kidney weight.
We investigated whether obesity was associated with hyperfiltration in HF-fed rats, and for that, we measured GFR after 16 wk of HFD feeding. We observed a trend toward higher GFR in HFD-fed rats (Fig. 4B), but it failed to reach statistical significance.
Measurement of Renal Damage in HFD-Fed Rats
Albuminuria (a marker of renal damage) was not found to be significantly higher in HFD-fed rats even after 16 wk of HFD feeding (Fig. 5A). However, we found that glomerulosclerosis was significantly higher after 16 wk of HFD feeding, indicative of higher renal damage (Fig. 5B).
Figure 5.
A: albuminuria, a marker of renal damage, was not found to be higher in high-fat diet (HFD)-fed Sprague–Dawley rats even after 16 wk of feeding. B: glomerulosclerosis was significantly higher in HFD-fed rats compared with normal-fat diet (NFD)-fed rats after 16 wk of feeding. *P < 0.05, NFD-fed group vs. HFD-fed group.
DISCUSSION
The mechanisms by which HFD-induced obesity causes renal damage are not well understood. In this study, we investigated whether HFD feeding in normal SD rats blunts the TGF response and increases PGC and if this change at the single nephron level is accompanied by changes in whole kidney renal hemodynamic parameters, such as RBF, GFR, and renal damage. We measured the TGF response at two time points: at 4 wk of HFD feeding (before the development of obesity) and at 16 wk of HFD feeding (when HFD-fed rats weighed significantly more). Our results show that the TGF response was unaltered at 4 wk of HFD feeding but was significantly attenuated at 16 wk of HFD feeding. At 16 wk of HFD feeding, RBF and GFR remained unchanged, whereas blood pressure was higher. There was no difference in proteinuria between NFD- and HFD-fed rats, but glomerulosclerosis was significantly higher in HFD-fed rats after 16 wk of feeding.
HFD-fed SD rats gradually started weighing more compared with NFD-fed SD rats, which became significant after 8 wk of feeding, and this difference further increased over time (Fig. 1). In addition to the increased body weight upon HFD feeding, these rats developed insulin resistance observed after 14 wk of HFD feeding similar to what has been previously reported (26). Higher PGC could be detrimental for the fragile glomerular microvessels in the long term and has been implicated in causing glomerular barotrauma leading to renal damage in various renal disease models such as high-protein diet (27, 28), subtotal nephrectomy (29), and genetic models of obesity like Zucker rats (15, 22). The in vivo micropuncture experiments conducted in this study revealed no elevation of PGC after 4 wk of feeding in the HFD-fed group, but this was significantly higher after 16 wk of HFD feeding even when the perfusion rate was zero, i.e., in the absence of TGF. This finding is similar to our previous finding in Zucker obese rats, wherein we found higher PGC in the absence of any tubular perfusion (14). We do not know what changes occurred in afferent glomerular microvessels or efferent arterioles between 4 wk of HFD feeding and 16 wk of HFD feeding, but these may be related to either a reduced myogenic response or increased efferent arteriole constriction (14). A decrease in the myogenic response could allow systemic pressure to get transmitted to the glomerulus and could elevate PSF. A recent study by Polichnowski et al. (30) suggested that the myogenic response remains unaltered after 10 wk in 45% HFD-fed rats using transfer function analysis. However, in the present study, we fed SD rats with 60% of fat diet and for 16 wk as opposed to 10 wk; thus, we may still speculate that the chronic HFD feeding, such as of 16 wk, could have decreased the intrinsic myogenic contractility in response to the higher systemic pressure, which could have increased PSF. In fact, a previous study has shown that the myogenic response is significantly reduced in a monogenic model of obesity such as Zucker obese rats (31). Another rationale for increased PSF after 16 wk of HFD feeding could be further constriction of the efferent arteriole in 16 wk of HFD-fed rats as hypothesized in obesity (32, 33). To our knowledge, no study exists that has directly explored the role of the efferent arteriole in alterations of renal hemodynamics in the context of HFD-induced obesity. However, in Zucker obese rats, constriction of the efferent arteriole has been previously reported (34). In addition, it is also interesting to note that there was no significant difference in MAP between the NFD- and HFD-fed groups after 4 wk of feeding. However, after 16 wk, MAP was significantly higher in the HFD-fed group. This could be another reason for higher pressure transmission to the glomerulus in this group, especially when renal autoregulation is decreased. We have previously reported that TGF has a vasodilatory effect on the efferent arteriole (at least in vitro; 35), and therefore one may speculate that since TGF is attenuated in the HFD-fed group, the efferent arteriole might have constricted resulting into higher PGC.
Our data show attenuated TGF at 16 wk of HFD along with high blood pressure. To our knowledge, a relationship between high blood pressure and differences in TGF has not been established. We and others have measured the TGF response in various hypertensive animal models and failed to establish a directional cause-effect relationship. For example, TGF is attenuated in hypertensive Dahl salt-sensitive rats on a high-salt diet, whereas TGF is enhanced in spontaneously hypertensive rats or Milan hypertensive rats (22, 36, 37). The TGF response is attenuated in normotensive uninephrectomized rats and mildly hypertensive obese Zucker rats (14, 15). In some cases, HFD was shown to enhance the renin-angiotensin system (38, 39), and this may indirectly affect the TGF response. In an elegant study, Müller-Suur et al. (40) altered renal renin content up to 1,000-fold using various animal models (heminephrectomized rats, two-kidney Goldblatt hypertensive rats, and DOCA and salt-loaded rats) but failed to find a correlation with TGF responses. Our group has previously shown that in normotensive rabbits, angiotensin II enhances TGF (41). Therefore, we would expect that the HFD, through potential actions on the renin-angiotensin system, would enhance the TGF response, but we observed the opposite. These data suggest that the HFD is likely blunting TGF through mechanisms that are independent from the increased blood pressure.
We decided to study TGF in the HFD-induced obesity model because this model resembles more closely what is observed in humans with obesity with respect to its polygenic metabolic and cardiovascular response to excessive caloric intake from fats (42). Our finding that a HFD induces hypertension in SD rats is in sync with other previously reported findings (30, 43).
Our results also demonstrated, for the first time, that TGF remains unchanged at 4 wk of HFD feeding but was significantly attenuated by 16 wk on HFD in SD rats. The mechanisms behind this observation are largely unclear. TGF is initiated by Na+ reabsorption by Na+-K+-2Cl− cotransporters at the macula densa segment of the nephron, which, in turn, releases adenosine (44). We and others have previously shown that the released adenosine could cause vascular constriction by acting on the adenosine type A1 receptor (A1R) present on the wall of the afferent arteriole and elicits TGF (35, 44). It is possible that adenosine-A1R signaling gets impaired upon HFD feeding, and that could have resulted into reduced TGF, but, to our knowledge, there is no study showing alterations of this TGF signaling pathway by HFD. We have previously reported that in Zucker obese rats, another vasodilatory renal intrinsic feedback mechanism, the connecting tubule glomerular feedback mechanism, was significantly higher. This could be elevated also in HFD-fed rats and possibly have attenuated the TGF response. The connecting tubule glomerular feedback mechanism is initiated by the epithelial Na+ channel in the connecting tubule, and its expression was found to be increased in HFD-fed mouse kidneys (45), but it is unclear whether this occur in the rat. Numerous studies have also suggested that nitric oxide synthesized by the macula densa suppresses TGF (46). Nitric oxide inhibition by Nω-nitro-l-arginine methyl ester administration in conscious, chronically instrumented high fat-fed, obese-prone SD rats increased renal vascular resistance by 39% and decreased RBF by 16%, indicating higher renal nitric oxide production (30). In contrast, minimal effects of Nω-nitro-l-arginine methyl ester were seen in obese resistant SD rats, suggesting that higher nitric oxide production in the macula densa could be another player in the attenuated TGF response observed in our 16-wk HFD-fed group. These hypotheses of a reduced TGF response in the HFD-fed model need to be tested in future studies.
Although we have observed a reduction in the TGF response as well as an elevation in PGC, we failed to observe any significant elevation of either RBF or GFR, and the reason behind this is not clear. However, it is worth noting that we only studied TGF responses or PGC using accessible surface cortical nephrons, and it is possible that the renal cortical and medullary circulation are affected unequally in HFD-fed SD rats. This may explain why we did not see increases in either RBF or GFR at whole kidney level. This possibility also needs further investigation.
Although we did not observe any increase in RBF or GFR despite an elevation of PGC and a reduction in the TGF response, we did observe significantly higher glomerulosclerosis in HFD-fed SD rats, while proteinuria was similar. Others have also shown podocyte effacement (47) and mesangial expansion in HFD-induced obesity in rodent models (38, 48,49). This indicates that an attenuated TGF response and higher PGC may be sufficient to cause glomerular damage in the absence of whole kidney hyperfiltration or hyperperfusion similar to what has been previously proposed (30).
In summary, our study provides the first direct evidence of TGF resetting in HFD-induced obesity. Sixteen weeks of HFD in SD rats also showed higher PGC and MAP in association with higher renal damage, indicating that these factors in combination could potentially contribute to renal damage.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant RO1DK107263 (to P.A.O.).
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or Henry Ford Hospital.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.R.M. and P.A.O. conceived and designed research; S.R.M., H.W., D.L.P., and T.-D.L. performed experiments; S.R.M., H.W., D.L.P., and P.A.O. analyzed data; S.R.M., H.W., and P.A.O. interpreted results of experiments; S.R.M. prepared figures; S.R.M. drafted manuscript; S.R.M. and P.A.O. edited and revised manuscript; S.R.M., H.W., D.L.P., T.-D.L., and P.A.O. approved final version of manuscript.
REFERENCES
- 1.Meldrum DR, Morris MA, Gambone JC. Obesity pandemic: causes, consequences, and solutions-but do we have the will? Fertil Steril 107: 833–839, 2017. doi: 10.1016/j.fertnstert.2017.02.104. [DOI] [PubMed] [Google Scholar]
- 2.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief 1–8, 2020. [32487284] [PubMed] [Google Scholar]
- 3.D'Agati VD, Chagnac A, de Vries AP, Levi M, Porrini E, Herman-Edelstein M, Praga M. Obesity-related glomerulopathy: clinical and pathologic characteristics and pathogenesis. Nat Rev Nephrol 12: 453–471, 2016. [DOI] [PubMed] [Google Scholar]
- 4.National Institute of Diabetes and Digestive and Kidney Diseases. Overweight & Obesity Statistics (Online). https://www.niddk.nih.gov/health-information/health-statistics/overweight-obesity. [Accessed 6 May 2020].
- 5.Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res 116: 991–1006, 2015. doi: 10.1161/CIRCRESAHA.116.305697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maniecka-Bryla I, Szymocha M, Bryla M. Overweight and obesity as risk factors in hypertension–study of the working population. Med Lav 102: 523–538, 2011. [PubMed] [Google Scholar]
- 7.Zeve JL, Tomaz CA, Nassif PA, Lima JH, Sansana LR, Zeve CH. Obese patients with diabetes mellitus type 2 undergoing gastric bypass in Roux-en-Y: analysis of results and its influence in complications. Arq Bras Cir Dig 26: 47–52, 2013. doi: 10.1590/s0102-67202013000600011. [DOI] [PubMed] [Google Scholar]
- 8.Laliberte F, Bookhart BK, Vekeman F, Corral M, Duh MS, Bailey RA, Piech CT, Lefebvre P. Direct all-cause health care costs associated with chronic kidney disease in patients with diabetes and hypertension: a managed care perspective. J Manag Care Pharm 15: 312–322, 2009. doi: 10.18553/jmcp.2009.15.4.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moon KH, Ko IK, Yoo JJ, Atala A. Kidney diseases and tissue engineering. Methods 99: 112–119, 2016. doi: 10.1016/j.ymeth.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 10.Naganuma H, Nishinakamura R. From organoids to transplantable artificial kidneys. Transpl Int 32: 563–570, 2019. doi: 10.1111/tri.13404. [DOI] [PubMed] [Google Scholar]
- 11.Bondar IA, Klimontov VV, Simakova AI. Obesity and chronic kidney disease [in Russian]. Ter Arkh 83: 66–70, 2011. [PubMed] [Google Scholar]
- 12.Bosma RJ, Krikken JA, Homan van der Heide JJ, de Jong PE, Navis GJ. Obesity and renal hemodynamics. Contrib Nephrol 151: 184–202, 2006. doi: 10.1159/000095329. [DOI] [PubMed] [Google Scholar]
- 13.Leggio M, Lombardi M, Caldarone E, Severi P, D'Emidio S, Armeni M, Bravi V, Bendini MG, Mazza A. The relationship between obesity and hypertension: an updated comprehensive overview on vicious twins. Hypertens Res 40: 947–963, 2017. doi: 10.1038/hr.2017.75. [DOI] [PubMed] [Google Scholar]
- 14.Monu SR, Maheshwari M, Peterson EL, Carretero OA. Role of connecting tubule glomerular feedback in obesity related renal damage. Am J Physiol Renal Physiol 315: F1708–F1713, 2018. doi: 10.1152/ajprenal.00227.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monu SR, Ren Y, Masjoan-Juncos JX, Kutskill K, Wang H, Kumar N, Peterson EL, Carretero OA. Connecting tubule glomerular feedback mediates tubuloglomerular feedback resetting after unilateral nephrectomy. Am J Physiol Renal Physiol 315: F806–F811, 2018. doi: 10.1152/ajprenal.00619.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ren Y, Garvin JL, Carretero OA. Efferent arteriole tubuloglomerular feedback in the renal nephron. Kidney Int 59: 222–229, 2001. doi: 10.1046/j.1523-1755.2001.00482.x. [DOI] [PubMed] [Google Scholar]
- 17.Wang H, D'Ambrosio MA, Ren Y, Monu SR, Leung P, Kutskill K, Garvin JL, Janic B, Peterson EL, Carretero OA. Tubuloglomerular and connecting tubuloglomerular feedback during inhibition of various Na transporters in the nephron. Am J Physiol Renal Physiol 308: F1026–F1031, 2015. doi: 10.1152/ajprenal.00605.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baylis C, Ichikawa I, Willis WT, Wilson CB, Brenner BM. Dynamics of glomerular ultrafiltration. IX. Effects of plasma protein concentration. Am J Physiol Renal Physiol 232: F58–F71, 1977. doi: 10.1152/ajprenal.1977.232.1.F58. [DOI] [PubMed] [Google Scholar]
- 19.Park SK, Kang SK. Renal function and hemodynamic study in obese Zucker rats. Korean J Intern Med 10: 48–53, 1995. doi: 10.3904/kjim.1995.10.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park SK, Meyer TW. The effect of hyperglycemia on glomerular function in obese Zucker rats. J Lab Clin Med 125: 501–507, 1995. [PubMed] [Google Scholar]
- 21.Araujo M, Welch WJ. Cyclooxygenase 2 inhibition suppresses tubuloglomerular feedback: roles of thromboxane receptors and nitric oxide. Am J Physiol Renal Physiol 296: F790–F794, 2009. doi: 10.1152/ajprenal.90446.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H, D’Ambrosio MA, Garvin JL, Ren Y, Carretero OA. Connecting tubule glomerular feedback in hypertension. Hypertension 62: 738–745, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang H, Garvin JL, D'Ambrosio MA, Ren Y, Carretero OA. Connecting tubule glomerular feedback antagonizes tubuloglomerular feedback in vivo. Am J Physiol Renal Physiol 299: F1374–F1378, 2010. doi: 10.1152/ajprenal.00403.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gordish KL, Beierwaltes WH. Resveratrol induces acute endothelium-dependent renal vasodilation mediated through nitric oxide and reactive oxygen species scavenging. Am J Physiol Renal Physiol 306: F542–F550, 2014. doi: 10.1152/ajprenal.00437.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rieg T. A High-throughput method for measurement of glomerular filtration rate in conscious mice. J Vis Exp e50330, 2013. doi: 10.3791/50330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tasyurek HM, Altunbas HA, Balci MK, Griffith TS, Sanlioglu S. Therapeutic potential of lentivirus-mediated glucagon-like peptide-1 gene therapy for diabetes. Hum Gene Ther 29: 802–815, 2018. doi: 10.1089/hum.2017.180. [DOI] [PubMed] [Google Scholar]
- 27.Carlström M, Wilcox CS, Arendshorst WJ. Renal autoregulation in health and disease. Physiol Rev 95: 405–511, 2015. doi: 10.1152/physrev.00042.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyer TW, Anderson S, Rennke HG, Brenner BM. Reversing glomerular hypertension stabilizes established glomerular injury. Kidney Int 31: 752–759, 1987. doi: 10.1038/ki.1987.62. [DOI] [PubMed] [Google Scholar]
- 29.Hostetter TH, Olson JL, Rennke HG, Venkatachalam MA, Brenner BM. Hyperfiltration in remnant nephrons: a potentially adverse response to renal ablation. Am J Physiol Renal Physiol 241: F85–F93, 1981. doi: 10.1152/ajprenal.1981.241.1.F85. [DOI] [PubMed] [Google Scholar]
- 30.Polichnowski AJ, Licea-Vargas H, Picken M, Long J, Bisla R, Williamson GA, Bidani AK, Griffin KA. Glomerulosclerosis in the diet-induced obesity model correlates with sensitivity to nitric oxide inhibition but not glomerular hyperfiltration or hypertrophy. Am J Physiol Renal Physiol 309: F791–F799, 2015. doi: 10.1152/ajprenal.00211.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayashi K, Kanda T, Homma K, Tokuyama H, Okubo K, Takamatsu I, Tatematsu S, Kumagai H, Saruta T. Altered renal microvascular response in Zucker obese rats. Metabolism 51: 1553–1561, 2002. doi: 10.1053/meta.2002.36311. [DOI] [PubMed] [Google Scholar]
- 32.Tuttle KR. Back to the future: glomerular hyperfiltration and the diabetic kidney. Diabetes 66: 14–16, 2017. doi: 10.2337/dbi16-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zoccali C. Overweight, obesity and metabolic alterations in chronic kidney disease. Prilozi 30: 17–31, 2009. [PubMed] [Google Scholar]
- 34.Roos MH, Eringa EC, van Rodijnen WF, van Lambalgen TA, Ter Wee PM, Tangelder GJ. Preglomerular and postglomerular basal diameter changes and reactivity to angiotensin II in obese rats. Diabetes Obes Metab 10: 898–905, 2008. doi: 10.1111/j.1463-1326.2007.00827.x. [DOI] [PubMed] [Google Scholar]
- 35.Ren Y, Garvin JL, Liu R, Carretero OA. Possible mechanism of efferent arteriole (Ef-Art) tubuloglomerular feedback. Kidney Int 71: 861–866, 2007. doi: 10.1038/sj.ki.5002161. [DOI] [PubMed] [Google Scholar]
- 36.Boberg U, Persson AE. Increased tubuloglomerular feedback activity in Milan hypertensive rats. Am J Physiol Renal Physiol 250: F967–F974, 1986. doi: 10.1152/ajprenal.1986.250.6.F967. [DOI] [PubMed] [Google Scholar]
- 37.Welch WJ, Tojo A, Wilcox CS. Roles of NO and oxygen radicals in tubuloglomerular feedback in SHR. Am J Physiol Renal Physiol 278: F769–F776, 2000. doi: 10.1152/ajprenal.2000.278.5.F769. [DOI] [PubMed] [Google Scholar]
- 38.Dobrian AD, Davies MJ, Prewitt RL, Lauterio TJ. Development of hypertension in a rat model of diet-induced obesity. Hypertension 35: 1009–1015, 2000. doi: 10.1161/01.hyp.35.4.1009. [DOI] [PubMed] [Google Scholar]
- 39.Teien G, Ulsaker GA. Identification of chloroform formed by disinfection with sodium hypochlorite and spirit. Acta Pharm Nord 1: 311, 1989. [PubMed] [Google Scholar]
- 40.Müller-Suur R, Gutsche HU, Samwer KF, Oelkers W, Hierholzer K. Tubuloglomerular feedback in rat kidneys of different renin contents. Pflugers Arch 359: 33–56, 1975. doi: 10.1007/BF00581276. [DOI] [PubMed] [Google Scholar]
- 41.Wang H, Garvin JL, Carretero OA. Angiotensin II enhances tubuloglomerular feedback via luminal AT1 receptors on the macula densa. Kidney Int 60: 1851–1857, 2001. doi: 10.1046/j.1523-1755.2001.00999.x. [DOI] [PubMed] [Google Scholar]
- 42.Nilsson C, Raun K, Yan FF, Larsen MO, Tang-Christensen M. Laboratory animals as surrogate models of human obesity. Acta Pharmacol Sin 33: 173–181, 2012. doi: 10.1038/aps.2011.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Erdos B, Broxson CS, Cudykier I, Basgut B, Whidden M, Landa T, Scarpace PJ, Tümer N. Effect of high-fat diet feeding on hypothalamic redox signaling and central blood pressure regulation. Hypertens Res 32: 983–988, 2009. doi: 10.1038/hr.2009.129. [DOI] [PubMed] [Google Scholar]
- 44.Wei J, Zhu J, Zhang J, Jiang S, Qu L, Wang L, Buggs J, Tan X, Cheng F, Liu R. Aging impairs renal autoregulation in mice. Hypertension 75: 405–412, 2020. doi: 10.1161/HYPERTENSIONAHA.119.13588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quadri SS, Culver S, Ramkumar N, Kohan DE, Siragy HM. (Pro)Renin receptor mediates obesity-induced antinatriuresis and elevated blood pressure via upregulation of the renal epithelial sodium channel. PLoS One 13: e0202419, 2018. doi: 10.1371/journal.pone.0202419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Welch WJ, Wilcox CS. Role of nitric oxide in tubuloglomerular feedback: effects of dietary salt. Clin Exp Pharmacol Physiol 24: 582–586, 1997. doi: 10.1111/j.1440-1681.1997.tb02095.x. [DOI] [PubMed] [Google Scholar]
- 47.Chen JY, Jian DY, Lien CC, Lin YT, Ting CH, Chen LK, Hsu TC, Huang HM, Wu YT, Kuan TT, Chao YW, Wu LY, Huang SW, Juan CC. Adipocytes play an etiological role in the podocytopathy of high-fat diet-fed rats. J Endocrinol 231: 109–120, 2016. doi: 10.1530/JOE-16-0064. [DOI] [PubMed] [Google Scholar]
- 48.Danda RS, Habiba NM, Rincon-Choles H, Bhandari BK, Barnes JL, Abboud HE, Pergola PE. Kidney involvement in a nongenetic rat model of type 2 diabetes. Kidney Int 68: 2562–2571, 2005. doi: 10.1111/j.1523-1755.2005.00727.x. [DOI] [PubMed] [Google Scholar]
- 49.Deji N, Kume S, Araki S, Soumura M, Sugimoto T, Isshiki K, Chin-Kanasaki M, Sakaguchi M, Koya D, Haneda M, Kashiwagi A, Uzu T. Structural and functional changes in the kidneys of high-fat diet-induced obese mice. Am J Physiol Renal Physiol 296: F118–F126, 2009. doi: 10.1152/ajprenal.00110.2008. [DOI] [PubMed] [Google Scholar]