Abstract
As the hemp industry continues to develop in the United States, there is an increasing interest in feeding byproducts of industrial hemp production to livestock. A completely randomized design experiment using crossbred finishing heifers (initial body weight [BW] ± SE = 494 ± 10 kg) was conducted to determine the effects of feeding hempseed cake in a corn-based finishing diet (10% forage) formulated to meet or exceed ruminally degradable and metabolizable protein requirements on growth performance, carcass characteristics, feeding behavior, and plasma parameters. Dietary treatments were the inclusion of 20% (dry matter [DM] basis) of dried corn distillers grains plus solubles (DDGS, n = 16) or hempseed cake (HEMP, n = 15). Cattle were housed in two pens, had ad libitum access to feed and water, and individual intakes and feeding behavior were monitored using the Insentec feeding system. Cattle were fed treatment diets for 111 d, and every 14 d BW were measured and blood samples were collected. Blood plasma was analyzed for glucose, urea nitrogen, and individual amino acids, and results were analyzed using repeated-measures analysis in SAS. Final BW, average daily gain, gain:feed, and hot carcass weight decreased (P ≤ 0.05) by 2.3%, 7.7%, 7.7%, and 2.6%, respectively, in heifers fed the HEMP diet than in heifers fed the DDGS diet. Net energy for maintenance and gain (Mcal/kg of feed, DM basis), estimated based on heifer intake and performance, were greater (P = 0.02) for the DDGS diet than for the HEMP diet. All other performance and carcass characteristics were not different (P ≥ 0.20) between treatments. Heifers fed the HEMP diet had greater (P < 0.05) plasma urea nitrogen concentration in samples from each collection day compared with heifers fed the DDGS diet, although there was a treatment-by-day interaction (P < 0.01) because of variability in the magnitude of treatment differences over time. Plasma glucose concentration was not influenced (P = 0.17) by dietary treatment. Plasma concentrations of total amino acids, nonessential amino acids, and essential amino acids were not different between treatments (P ≥ 0.09), although there were several interactions between treatment and day (P ≤ 0.04) for individual amino acids. These data suggest that hempseed cake has a lower net energy for maintenance and gain relative to DDGS when adequate metabolizable protein is supplied, while still providing adequate nutrition to support the acceptable performance of finishing cattle.
Keywords: animal performance, finishing cattle, hempseed cake, nutrition, plasma metabolites
Hempseed cake has the potential for use as an alternative feedstuff for cattle finishing diets.
Introduction
Industrial hemp has been produced for thousands of years, largely for its fiber, but production decreased in favor of cheaper alternatives (Fike, 2016). The Marihuana Tax Act of 1937 stopped industrial hemp production in the United States and placed hemp on the Schedule 1 list of the Controlled Substances Act (USDA, Agricultural Marketing Service, 2021). In recent years, there has been renewed interest in industrial hemp production after its removal from the list of US Drug Enforcement Agency Schedule 1 drugs as a result of the 2018 Agricultural Improvement Act. A series of pilot studies were allowed under the 2014 Agricultural Improvement Act with the goal to reinstate industrial hemp production. By statute, industrial hemp must contain less than 0.3% delta-9-tetrahydrocannabinol (THC), which is the psychoactive component of the hemp plant, and is the differentiating component between hemp and marijuana (USDA, Agricultural Marketing Service, 2021). Cannabis with a THC level exceeding 0.3% (dry matter [DM] basis) remains a Schedule 1 controlled substance. Industrial hemp is produced for its fibers that are used in papers, textiles, and many other products that value the fiber strength, and more recently for the oil found in the hempseed that is used for medicines, paints, detergents, cooking, and other uses (Fike, 2016). Processing of the hempseed for oil extraction has increased with the rise in demand for hemp oil for human use (Mark et al., 2020). Hempseed oil extraction creates a byproduct that is high in neutral detergent fiber (NDF), acid detergent fiber (ADF), and crude protein (CP; 51%, 39%, and 30%, respectively) but limited markets are available for it because hemp and hemp byproducts cannot be fed to livestock or pets without FDA approval through the Association of American Feed Control Officials (Kleinhenz et al., 2020).
Because of its relatively high CP and ADF concentration, hempseed cake could be a useful feed ingredient for ruminants because of its potential as a dietary protein and energy source. Furthermore, hemp and hemp byproducts could have therapeutic benefits when fed to livestock because of the 3:1 ratio of linoleic to linolenic omega polyunsaturated fats (Kleinhenz et al., 2020), which is thought to be optimal for human health (Leizer et al., 2000). While most of the seed oil is removed during pressing, hempseed cake contains roughly 7% oil, which could be beneficial if used as a feedstuff (Table 1). Although feeding hemp byproducts is legal in the European Union (among other places), there are relatively few data on the nutritive value of hempseed cake as a feed ingredient for beef cattle (Hessle et al., 2008). Hessle et al. (2008) reported that feeding hempseed cake does not negatively influence DM intake (DMI) but performance metrics such as average daily gain (ADG) and gain:feed (G:F) are decreased when fed to growing bulls at 20% of diet DM. In the same manuscript, these authors reported that feeding hempseed cake to finishing steers at 9% of diet DM did not influence DMI, ADG, G:F, or final body weight (BW). Karlsson and Martinsson (2011) fed hempseed cake to ewe lambs at 22% of diet DM and observed a decrease in ADF, G:F, and final BW while not influencing DMI. Currently, corn distillers grains are the most common byproduct fed in finishing diets in the United States and have similarities in nutrient composition to hempseed cake (CP, NDF, and ether extract; Samuelson et al., 2016; Table 1). Furthermore, the most common inclusion rate for corn distillers grains is between 10% and 30% of diet DM when used as the primary byproduct (Samuelson et al., 2016). Feeding hempseed cake in comparison to corn distillers grains at a common inclusion rate in finishing cattle diets has not been evaluated. Therefore, the objectives of this study were to determine the effect of including hempseed cake, as compared with dried corn distillers grains plus solubles (DDGS), in finishing diets on growth performance, carcass characteristics, feeding behavior, and plasma metabolites in heifers.
Table 1.
Diet ingredients and nutrient composition of diets containing dried corn distillers grains plus solubles or hempseed cake
| Ingredient, % of diet DM | Treatments1 | Byproducts2 | ||
|---|---|---|---|---|
| DDGS | Hemp | DDGS | Hemp | |
| Dry-rolled corn | 55 | 55 | - | - |
| Dried distillers grains plus solubles | 20 | 0 | - | - |
| Hempseed cake | 0 | 20 | - | - |
| Corn silage | 20 | 20 | - | - |
| Supplement | 5 | 5 | - | - |
| Fine ground corn | 1.82 | 1.82 | - | - |
| Limestone | 2 | 2 | - | - |
| Salt | 0.1 | 0.1 | - | - |
| Urea | 1 | 1 | - | - |
| Vitamin premix3 | 0.01 | 0.01 | - | - |
| Trace mineral premix4 | 0.05 | 0.05 | - | - |
| Rumensin-905 | 0.02 | 0.02 | - | - |
| Nutrient analyses, %6 | ||||
| Dry matter | 66.0 | 65.1 | 88.8 | 90.9 |
| Ash | 5.79 | 6.39 | 5.49 | 8.52 |
| Starch | 43.7 | 43.2 | 6.93 | 1.14 |
| Crude protein | 14.8 | 15.8 | 29.6 | 33.9 |
| Ether extract | 3.47 | 3.38 | 5.70 | 7.39 |
| NDF | 29.1 | 30.4 | 49.6 | 50.4 |
| ADF | 11.4 | 16.3 | 15.6 | 36.3 |
| Calcium | 0.69 | 0.78 | 0.02 | 0.19 |
| Phosphorus | 0.44 | 0.53 | 0.92 | 1.68 |
| Calcium:phosphorus | 1.56 | 1.48 | 0.02 | 0.11 |
Treatment nutrient analyses for the complete diet.
Byproduct nutrient analyses for the individual byproducts (DDGS and Hemp).
Contained 48,510 kIU per kg vitamin A and 4,630 kIU per kg vitamin D.
Contained 3.62% calcium, 2.56% copper, 16% zinc, 6.5% iron, 4% manganese, 1,050 mg/kg iodine, and 250 mg/kg cobalt.
Formulated to supply monensin (Rumensin-90, Elanco Animal Health, Greenfield, IN, USA) at 40 mg/kg.
Average of samples taken weekly.
Materials and Methods
All animal care and management practices were approved by the North Dakota State University Institutional Animal Care and Use Committee prior to the initiation of the study.
Animals, experimental design, and dietary treatments
A 111-d finishing study was conducted at the North Dakota State University Beef Cattle Research Complex in Fargo, ND, USA. A total of 31 angus-crossbred finishing heifers (initial BW ± SE = 494 ± 10 kg; average age = 19 mo) were used. Heifers were weighed two consecutive days at the initiation of the experiment and were assigned randomly to one of two dietary treatments with 16 heifers per pen and 1 pen per treatment. The DDGS contained 55% dry-rolled corn, 20% corn silage, 20% DDGS, and 5% supplement (DM basis). The hempseed cake treatment (HEMP) contained the same ingredients except hempseed cake replaced DDGS (DM basis; Table 1). The DDGS were sourced from Dakota Spirit Midwest Ag Energy (Spiritwood, ND, USA) and the hempseed cake was sourced from Healthy Oilseeds, LLC (Carrington, ND, USA). The 20% inclusion level for DDGS and hempseed cake was selected as this is a common inclusion level for similar byproducts feeds used in practice (Samuelson et al., 2016). Cattle were adapted to the finishing diet over a 20-d step-up period. This was accomplished with byproduct (hempseed cake or DDGS) held constant at 20% with corn silage at 60% for step 1 and displaced by dry-rolled corn until corn silage constituted 20% of the diet. Treatments were formulated to meet or exceed ruminally degradable protein intake and metabolizable protein requirements (NASEM, 2016). The supplement was formulated to provide 40 mg/kg of monensin (Rumensin, Elanco Animal Health, Greenfield, IN, USA). Urea was added to both diets at 1% of diet DM to ensure ruminally degradable protein requirements were met (NASEM, 2016).
On day 1, heifers were implanted with 140 mg trenbolone acetate and 14 mg estradiol (Revalor H, Merck Animal Health, Kenilworth, NJ, USA). BWs were collected on days 0, 1, 2, 3, 7, 14, and then every 14 d until day 98, and a final BW at slaughter (days 112 to 120). Carcass data were collected after cattle were slaughtered at the North Dakota State University Meat Laboratory via captive bolt stunning and exsanguination. Cattle were slaughtered on 5 d across a 9-d period to examine the effects of the withdrawal period (0, 1, 4, 7, and 8 d) from feeding hempseed cake on cannabinoid residues in plasma and tissues (Chakrabarty et al., 2021b). All cattle were offered the DDGS diet for all days on feed after day 111. Cattle were assigned randomly to slaughter date with equal treatment representation each day. Final BW was collected at slaughter, and regression analysis was used to calculate final BW before the withdrawal periods began (day 111). Day 111 was used as the final BW for all growth performance measures. The carcass was chilled for a minimum of 24 h and fat thickness, longissimus muscle (LM) area, and USDA marbling scores were recorded, and yield grade was calculated according to USDA standards. Because hemp is not currently approved to be fed to cattle entering the human food chain, all cattle fed hempseed cake were harvested and disposed of at the completion of the experiment.
Feeding behavior measurement
Feeding behavior data were collected daily over the course of the 111-d feeding period (November to February) using the Insentec BV Feeding System (Hokafarm Group, Marknesse, The Netherlands). Each heifer received a radio frequency identification tag in the right ear before the initiation of the experiment to allow for use of the Insentec automated feeding system (Hokofarm B.V.). This system allows for monitoring of feeding behavior characteristics, quantified as the number of visits to the bunk, meals (defined as visits separated by ≤7 min combined into one), time eating (per visit, meal, and day), and eating rate (per visit, meal, and minute; Montanholi et al., 2010). DMI and feeding behavior data were summarized as the average of each heifer over the entire feeding period, including the adaptation period. Each visit to the feed bunk was captured and used to quantify feeding behavior.
Feed sample analyses
Feed samples were collected weekly for DM analysis and dried at 60 °C in a forced-air oven for 48 h and then ground to pass a 1-mm screen. Ground aliquots were analyzed for DM, organic matter, nitrogen (N), and ether extract (AOAC, 1990). NDF and ADF (assayed with heat-stable amylase and sodium sulfite) were quantified as described by Van Soest et al. (1991). CP concentration was calculated as 6.25 × N. Samples were also analyzed for starch concentration (Herrera-Saldana and Huber, 1989). Cannabinoid concentrations in hempseed cake were measured using electrospray ionization rapid screening using a similar methodology as those described by Chakrabarty et al. (2021a). Total concentrations of 10 cannabinoids (including cannabinol and THC) were 13 mg/kg of hempseed cake (DM basis). Heifers fed the HEMP diet had an average DMI of 14.13 kg, 20% of which was hempseed cake, resulting in an average daily total cannabinoid intake of 36.7 mg per hd per d.
Blood sample collection and analyses
Blood samples were collected from all heifers before feed delivery in the morning via jugular venipuncture into tubes containing sodium heparin (Becton Dickinson, Rutherford, NJ, USA). Blood sample collection was performed on days 0, 2, 3, 7, 14, 28, 42, 56, 70, 84, and 98. Immediately upon collection, plasma was isolated by centrifugation (3,000 × g at 4 °C) and stored at −20 °C until later analysis. Plasma samples were analyzed for amino acid concentrations on samples from days 0, 7, 56, and 98. Plasma samples were analyzed for glucose and urea N (PUN) concentrations on all days of plasma collection.
Plasma amino acid concentrations were analyzed by reversed-phase ultra-performance liquid chromatography after precolumn derivatization of amino acids with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate (Salazar et al., 2012; Lemley et al., 2013) and using ethylene bridged hybrid C18 column (2.1 × 150 mm; 1.7 μm; Waters Corp., Milford, MA, USA). Total amino acids, total essential amino acids, and total nonessential amino acids were calculated by summing the amino acid concentrations within each category for each heifer. Essential amino acids consisted of histidine, arginine, threonine, lysine, methionine, valine, isoleucine, leucine, phenylalanine, and tryptophan. Nonessential amino acids consisted of asparagine, glutamic acid, glutamine, glycine, aspartic acid, serine, alanine, proline, and tyrosine. Total amino acids consisted of all essential and nonessential amino acids listed previously.
Plasma glucose concentration was analyzed using the hexokinase/glucose-6-phosphate dehydrogenase method (Farrance, 1987) using the Infinity glucose hexokinase kit (Thermo Trace, Louisville, KY, USA). PUN was determined using the Urease/Berthelot procedure (Fawcett and Scott, 1960; Chaney and Marbach, 1962) using the QuantiChrom urea assay kit (BioAssay Systems, Hayward, CA, USA).
Dietary calculations
Rumen degradable protein (RDP) balance was modeled using NASEM (2016) empirical level of solution. Because RDP of hempseed cake was not known, only the DDGS diet was evaluated to determine RDP balance. Using BW and DMI from this experiment, the DDGS diet provided an estimated excess of 77 g/d of RDP. Dietary net energy for maintenance (NEm) and NE for gain (NEg) were calculated using the Galyean (2009) net energy calculator, which is based on NRC (1996) net energy equations. The calculator inputs are initial BW, final BW, target endpoint with choice quality grade assumed, DMI and ADG. The NEm and NEg of hempseed cake were calculated assuming NEm and NEg values of DDGS (2.21 and 1.52 Mcal/kg, respectively; NASEM, 2016) and based on the inclusion rate of DDGS and hempseed cake at 20% of diet DM. To calculate the NEm of hempseed cake, the following formula was used to establish the NEm (represented by × in this equation) of the base diet (byproduct removed): NEm, Mcal/kg of the total diet = (0.2 × 2.21 Mcal/kg) + (0.8 × x). The HEMP diet NEm was set equal to 0.8 × 1.825 (base diet NEm, Mcal/kg calculated in the previous step) + (0.2 × x), where × is equal to the NEm of hempseed cake. Calculations for dietary NEm and NEg were done for individual heifers.
Statistical analysis
Data were analyzed using the MIXED procedure in SAS 9.4 (SAS Inst Inc., Cary, NC, USA) as a completely randomized design. One heifer was removed from the analyses from the HEMP treatment group dataset because it was pregnant. Initial BW was used as a covariate for performance and carcass data. Amino acid, glucose, and PUN data were analyzed as repeated measures using the MIXED procedure of SAS, with day as the repeated variable. Five covariance structures (autoregressive 1, compound symmetry, Toeplitz, unstructured, and ante-dependence 1) were compared for each analysis using repeated measures, with the lowest fit statistic type selected (compound symmetry or ante-dependence 1 were found to be the lowest fit statistics for all measures). Heifer was the experimental unit (n = 16 for DDGS; n = 15 for HEMP). Treatment and day (for plasma metabolites and amino acids) were included in the model as fixed effects and treatment-by-day interactions were tested. When treatment-by-day interactions were present, interactive means were compared using the least significant difference method. Treatment differences were considered significant when P ≤ 0.05.
Results and Discussion
Growth performance
Heifers fed the DDGS diet had greater (P ≤ 0.05) final BW, ADG, and G:F than heifers fed the HEMP diet (Table 2), while DMI was not influenced (P = 0.94) by treatment. The observed lack of effect on DMI is similar to what Mustafa et al. (1998) also reported whereby DMI was not influenced in sheep fed hempseed meal at 20% of the diet. However, Hessle et al. (2008) reported an increase in DMI when hempseed cake is included at 20% of the diet DM in comparison to a mixture of soybean meal and rolled barley in calf diets. Furthermore, when hempseed cake was included in dairy rations up to 31% of diet DM (displaced a compound pellet comprised largely of barley), DMI increased (Karlsson et al., 2010).
Table 2.
Performance and carcass characteristics of heifers fed diets containing dried corn distillers grains plus solubles or hempseed cake
| Treatments1 | SEM | P-value | ||
|---|---|---|---|---|
| DDGS | Hemp | |||
| Performance2 | ||||
| Initial BW, kg | 493 | 497 | 17 | 0.80 |
| Final BW, kg | 699 | 683 | 7.4 | 0.05 |
| DMI, kg | 14.16 | 14.13 | 0.36 | 0.95 |
| ADG, kg | 1.83 | 1.69 | 0.07 | 0.05 |
| G:F | 0.130 | 0.120 | 0.004 | 0.02 |
| NEm, Mcal/kg feed | 1.92 | 1.82 | 0.04 | 0.02 |
| NEg, Mcal/kg feed | 1.28 | 1.19 | 0.04 | 0.02 |
| Carcass characteristics | ||||
| HCW, kg | 422 | 411 | 4.9 | 0.03 |
| Dressing, % | 60.44 | 60.51 | 0.5 | 0.90 |
| LM area, cm2 | 96.6 | 94.0 | 2.8 | 0.37 |
| Fat thickness, cm | 1.74 | 1.66 | 0.16 | 0.61 |
| Marbling score3 | 512 | 498 | 21 | 0.48 |
| Calculated YG4 | 3.41 | 3.35 | 0.24 | 0.81 |
Treatments consisted of 20% DDGS or 20% Hemp (DM basis) in a finishing ration.
Performance measures were analyzed over the 111-d feeding period.
Marbling score: 400 = Slight00, 450 = Slight50, 500 = Small, etc.
Yield grade (YG) = 2.50 + (0.9843 × rib fat thickness, cm) + (0.2 × 2.5% kidney, pelvic, and heart fat) + (0.0084 × hot carcass weight) − (0.496 × LM area, cm2; USDA, 2017).
The observed decrease in ADG and G:F in the present experiment is similar to what Hessle (2008) observed when hempseed cake was included in diets for growing cattle, and differs from what Gibb (2005) observed where final BW, DMI, and G:F were not different between steers fed barley-based diets with or without the inclusion of dry-rolled hempseed. Whole hempseed contains more oil than hempseed cake (28.4% vs. 7.4% oil) and could explain the lack of difference in performance reported by Gibb (2005). Similarly, when hempseed cake was included at 22% of a barley-based diet (DM basis) as a protein source for lambs, no differences in final BW, ADG, or G:F were observed when compared with a barley-based diet without an additional protein source (Karlsson and Martinsson, 2011). These authors indicate that a high insoluble fiber concentration and low RUP digestibility could have played a role in the lack of performance response observed by feeding hempseed cake. Discrepancies between experiments evaluating hempseed byproducts could be because of potential associative effects when fed with differing combinations of nutrients, and also could be because of the feedstuff being displaced by the hemp byproduct. The decrease in ADG and G:F observed in the current experiment could be a consequence of the increase in ADF concentration in the HEMP diet as it had approximately 5 percentage units greater ADF than the DDGS diet (Table 1), which may have resulted in lower digestibility and reduced available energy (Karlsson and Martinsson, 2011).
The average NEm and NEg for hempseed cake were calculated to be 1.73 and 1.10 Mcal/kg. Although caution should be used when comparing estimates between studies, our estimated NEm and NEg concentrations of hempseed cake are comparable to canola meal, which was calculated to have NEm and NEg values of 1.81 and 1.18 Mcal/kg, respectively, when included at 20% of diet DM (Nair et al., 2015). Dietary NEm and NEg (Mcal/kg of feed, DM basis) were greater (P = 0.02) for DDGS compared with HEMP diets (Table 2). Predicted dietary energy was lower for the HEMP diet than the DDGS diet, and NEg values for both HEMP and DDGS diets were lower than the most common industry recommendation of averages of 1.50 Mcal/kg NEg (Samuelson et al., 2016), likely because of the greater initial BW of the heifers used in this experiment compared with typical initial BW which may have negatively influenced growth potential over the feeding period. The reduced predicted net energy of hempseed cake relative to DDGS indicates that finishing cattle performance could be reduced compared with cattle receiving typical finishing rations containing DDGS at the current inclusion rate of 20% (DM basis). Diets were formulated to meet or exceed ruminally degradable protein and metabolizable protein requirements, so the estimated net energy value of hempseed cake could be more dependent on the quality of dietary energy more than on dietary protein.
Feeding behavior
No differences were observed (P ≥ 0.32) in feeding behavior between treatments (Table 3). While the effect of hempseed cake on cattle feeding behavior has not been reported elsewhere, the lack of effect may not be surprising because of the observed lack of response in DMI. However, data from other species have suggested that cannabinoids from hemp can influence feed intake and feeding behavior (Engali, 2012). Additionally, some have reported that differences in dietary fiber concentration (Swanson et al., 2017) and fatty acid profile and concentration (Benson et al., 2001) can influence feed intake and feeding behavior. The cannabinoid concentration and differences in dietary fiber or fatty acid profile likely were not great enough in the current experiment to elicit changes in feed intake or feeding behavior.
Table 3.
Feeding behavior of heifers fed diets containing dried corn distillers grains plus solubles or hempseed cake
| Item | Treatments1 | SEM | P-value | |
|---|---|---|---|---|
| DDGS | Hemp | |||
| Events, per d | ||||
| Visits2 | 56.5 | 51.2 | 5.5 | 0.35 |
| Meals3 | 10.3 | 10.2 | 0.6 | 0.87 |
| Time eating, min | ||||
| Per visit | 2.64 | 2.82 | 0.31 | 0.56 |
| Per meal | 13.6 | 13.4 | 0.9 | 0.75 |
| Per day | 138 | 135 | 8 | 0.67 |
| Eating rate, kg | ||||
| Per visit | 0.27 | 0.30 | 0.03 | 0.32 |
| Per meal | 1.40 | 1.44 | 0.08 | 0.68 |
| Per min | 0.11 | 0.11 | 0.01 | 0.79 |
Treatments consisted of 20% DDGS or 20% Hemp (DM basis) in a finishing ration.
Visit is defined as each time the Insentec system detected a heifer at the bunk.
Meal is defined as eating periods combined if the break between was not longer than 7 min.
Carcass characteristics
Hot carcass weight (HCW) was greater (P = 0.03) in heifers fed the DDGS diet than in the HEMP diet while all other carcass characteristics were not different (P ≥ 0.37). The decreased HCW measured in HEMP heifers is likely because of the decrease in ADG and its subsequent influence on decreased final BW. The observation, that other carcass characteristics were not influenced by treatment, is consistent with previous research comparing diets with and without the inclusion of hemp products (Gibb et al., 2005; Hessle et al., 2008).
Plasma parameters
Amino acid concentrations in plasma are influenced by many factors including dietary amino acid supply, ruminal fermentation, feed digestibility, amino acid absorption, amino acid metabolism, protein deposition, and tissue protein turnover (Hammond, 1983; LaPierre et al., 2006; Bergen, 2008). Essential amino acids, nonessential amino acids, and total plasma amino acid concentrations were not different across treatments (P ≥ 0.09; Table 4). The lack of differences between treatments may indicate that feeding hempseed cake did not result in substantial changes in amino acid availability or utilization compared with DDGS, although plasma concentrations of many individual amino acids were influenced by treatment or treatment-by-day interaction.
Table 4.
Plasma amino acid concentrations of heifers fed diets containing dried corn distillers grains plus solubles or hempseed cake
| Item, µM | Treatments1 | SEM | P-value3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DDGS2 | Hemp2 | |||||||||||
| Day | Day | Trt | Day | TxD | ||||||||
| 0 | 7 | 56 | 98 | 0 | 7 | 56 | 98 | |||||
| EAA | ||||||||||||
| Arginine | 86.3a | 76.5a | 109.3bc | 98.3abc | 92.5ab | 99.5abc | 111.0c | 129.0d | 7.9 | <0.01 | L | 0.04 |
| Histidine | 66.2 | 58.1 | 90.2 | 73.8 | 67.3 | 56.3 | 73.0 | 83.0 | 5.6 | 0.49 | L | 0.06 |
| Isoleucine | 113a | 172b | 145b | 152b | 118a | 102a | 119a | 143b | 14 | <0.01 | L | <0.01 |
| Leucine | 219b | 123a | 308c | 323c | 226b | 134a | 188b | 225b | 20 | <0.01 | L | <0.01 |
| Lysine | 84.6b | 55.3a | 120.4d | 113.5d | 90.6b | 92.8bc | 114.3cd | 141.9e | 9.8 | <0.01 | L | 0.01 |
| Methionine | 25.5a | 43.2e | 38.3cde | 34.9cd | 27.8ab | 25.4ab | 32.6bc | 40.0de | 3.8 | 0.07 | L | <0.01 |
| Phenylalanine | 71.9bc | 69.7b | 94.0d | 94.9d | 68.5b | 50.5a | 68.1b | 80.2c | 5.3 | <0.01 | L | 0.01 |
| Threonine | 66.4 | 57.3 | 86.3 | 75.0 | 72.4 | 66.2 | 81.5 | 84.3 | 7.1 | 0.25 | L | 0.53 |
| Tryptophan | 47.4 | 54.1 | 72.4 | 69.9 | 46.4 | 42.3 | 59.8 | 67.4 | 4.3 | 0.03 | Q | 0.09 |
| Valine | 282 | 209 | 379 | 418 | 290 | 245 | 308 | 372 | 27 | 0.24 | L | 0.06 |
| Total EAA | 1,063 | 918 | 1,442 | 1,453 | 1,099 | 913 | 1,155 | 1,366 | 93 | 0.09 | L | 0.21 |
| NEAA | ||||||||||||
| Alanine | 233 | 177 | 220 | 214 | 253 | 224 | 208 | 249 | 13 | 0.02 | ND | 0.11 |
| Asparagine | 48.8 | 45.4 | 64.0 | 51.1 | 54.4 | 48.3 | 57.2 | 60.2 | 3.7 | 0.33 | Q | 0.15 |
| Aspartic acid | 14.1c | 7.1a | 11.8b | 14.0c | 13.2bc | 11.7b | 7.1a | 14.3c | 1.4 | 0.80 | Q | <0.01 |
| Glutamine | 363a | 330a | 429bc | 375a | 363a | 365a | 377ab | 454c | 19 | 0.26 | L | <0.01 |
| Glutamic acid | 70.1 | 46.8 | 49.2 | 56.4 | 66.7 | 48.3 | 42.8 | 65.3 | 3.8 | 0.95 | Q | 0.07 |
| Glycine | 405 | 341 | 326 | 241 | 380 | 363 | 315 | 297 | 22 | 0.58 | L | 0.18 |
| Proline | 94.8c | 77.2ab | 119.9de | 118.9e | 102.5cd | 70.3a | 91.2abc | 93.7c | 9.5 | 0.01 | L | 0.03 |
| Serine | 113.8 | 95.1 | 125.9 | 111.8 | 117.2 | 100.9 | 117.5 | 123.8 | 10.4 | 0.57 | L | 0.65 |
| Tyrosine | 62.8bc | 56.2ab | 101.7e | 79.7d | 65.6bc | 51.1a | 70.0bcd | 65.4bc | 6.9 | <0.01 | Q | 0.03 |
| Total NEAA | 1,405 | 1,175 | 1,447 | 1,261 | 1,415 | 1,283 | 1,286 | 1,422 | 89 | 0.54 | ND | 0.17 |
| Total AA | 2,468 | 2,093 | 2,889 | 2,714 | 2,514 | 2,196 | 2,441 | 2,788 | 90 | 0.54 | L | 0.28 |
Treatments consisted of 20% DDGS or 20% Hemp (DM basis) in a finishing ration.
Control and Hemp treatment mean by day sharing the same superscript (a - e) do not differ (P ≤ 0.05).
Standard error of the mean (SEM) for the treatment-by-day (Trt × Day) interaction. Significant (P < 0.05) linear (L) and quadratic (Q) day effects denoted with L and Q. Not different (ND) indicates nonsignificant linear or quadratic effect.
Plasma tryptophan concentration was lower (P = 0.04) and plasma alanine concentration was greater (P = 0.02) in heifers fed the HEMP diet compared with heifers fed the DDGS diet. There were treatment-by-day interactions (P ≤ 0.04) for plasma concentrations of the essential amino acids: arginine, isoleucine, leucine, lysine, methionine, and phenylalanine because treatment did not influence concentration on day 0 but did on other days and because of variability in concentrations over time. When examining dietary treatment effects within each sampling day, plasma arginine concentration was greater (P = 0.04) on day 98 in heifers fed the HEMP diet than in heifers fed the DDGS diet. Plasma isoleucine concentrations were lower (P < 0.01) on days 7 and 56, and plasma methionine concentrations were lower (P < 0.01) on day 7 for heifers fed the HEMP diet compared with heifers fed the DDGS diet. Plasma leucine concentration was lower (P < 0.01) on days 56 and 98 for heifers fed the HEMP diet compared with heifers fed the DDGS diet. Plasma lysine concentration was lower (P = 0.01) on days 7 and 98 for heifers fed the HEMP diet compared with heifers fed the DDGS diet. Hempseed cake has a greater lysine concentration than DDGS (1.07% vs. 0.85%; Liu, 2011) and likely explains the observed increase in plasma lysine concentration. Lysine is typically the first limiting amino acid in corn-based diets (NASEM, 2016), so further research to better define the contribution of lysine in hempseed cake to cattle metabolizable lysine requirements is warranted. Plasma phenylalanine concentration was lower (P = 0.02) on days 7, 56, and 98 for heifers fed the HEMP diet compared with heifers fed the DDGS diet. There were treatment-by-day interactions (P ≤ 0.04) for plasma concentrations of the nonessential amino acids: aspartic acid, glutamine, proline, and tyrosine because of variability in concentrations after day 0. Heifers receiving the HEMP diet had greater (P < 0.01) day 7, and lower (P < 0.01) day 56 plasma aspartic acid concentration compared with heifers fed the DDGS diet. Plasma glutamine concentration was greater (P < 0.01) on day 98 for heifers fed the HEMP diet compared with the DDGS diet. Lastly, plasma proline and tyrosine concentrations were lower (P = 0.03) on days 56 and 98 for heifers fed the HEMP diet compared with heifers fed the DDGS diet. Many of the interactions likely resulted from differing amino acid intakes, profiles, and digestibility between hempseed cake and DDGS. Because of the many interactions of hempseed cake supplementation over time, further research is needed to better examine the biological relevance of changes in plasma amino acid concentrations resulting from feeding hempseed cake.
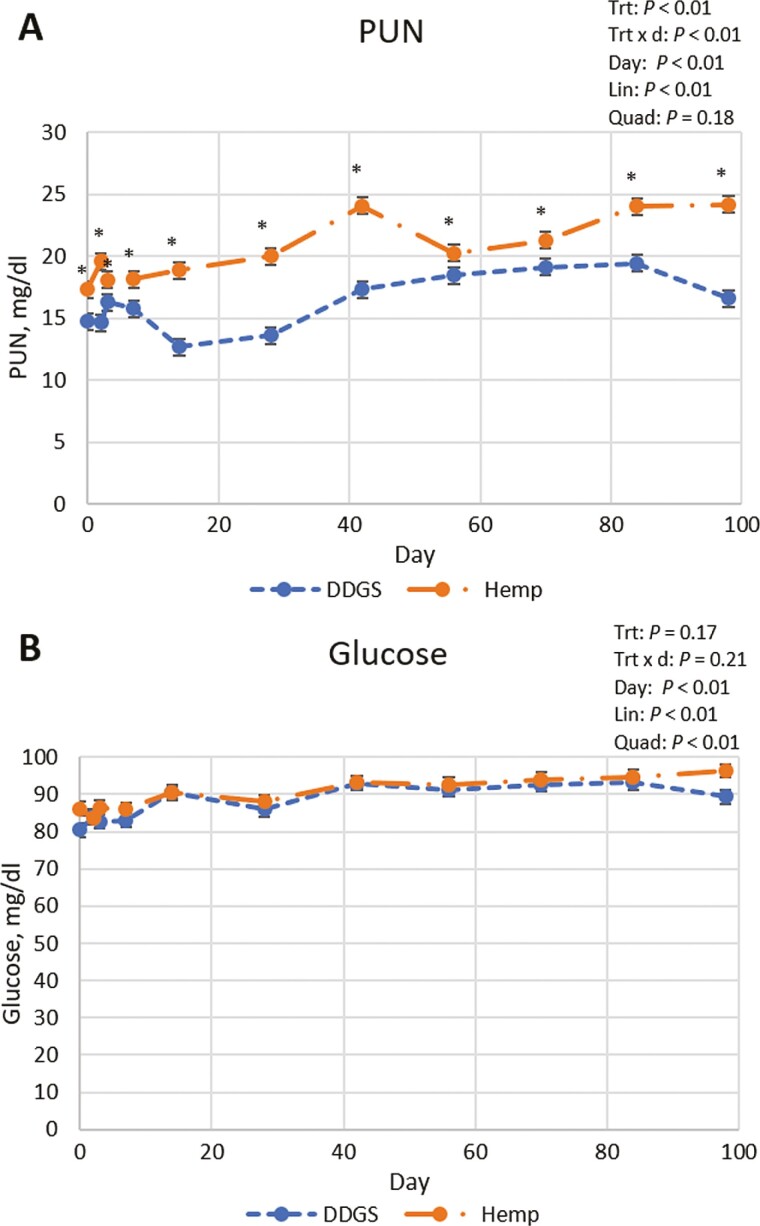
Plasma glucose concentration was not different (P = 0.17) between treatments but was affected quadratically (P < 0.01) by day with glucose concentration increasing until day 42 and then plateauing (Figure 1). Joy et al. (2017) also reported an increase and plateau (quadratic effect) as the finishing period progressed. Interaction between treatment and day was observed for PUN (P < 0.01) because of variability in the magnitude of differences between treatments over time, as heifers fed the HEMP diet had greater (P < 0.05) PUN on all collection days. PUN was affected quadratically (P = 0.04) by day. The observed greater (P ≤ 0.05) PUN in heifers fed the HEMP diet was likely because the HEMP diet had roughly 1 percentage unit more CP in the diet. Potential site of CP digestion differences between treatments could influence PUN as well. Furthermore, both treatment diets likely provided excess MP relative to requirements which can lead to greater urea production (Jennings et al., 2018). Ruminal degradability of protein within hempseed cake has been reported to be high (71% to 74%; Karlsson and Martinsson, 2011; Karlsson et al., 2012), while others have reported low ruminal degradability of hempseed meal (22.6%; Mustafa et al., 1998). Concentrations of PUN linearly increased (P < 0.01) throughout the feeding period. This response was expected as MP requirements decrease over the feeding period resulting in greater PUN concentrations (Simpfendorfer, 1974).
Figure 1.
Plasma urea N (A) and glucose (B) of heifers fed diets containing dried corn distillers grains plus solubles or hempseed cake. Means within day that differ between treatments are denoted with an asterisk (P < 0.05).
Conclusions
Heifers fed the HEMP diet had reduced final BW, ADG, G:F, and HCW. The lack of difference in DMI between treatments suggests that diets containing hempseed cake are readily consumed by finishing heifers. Because diets were formulated to exceed MP requirements, the observed decrease in ADG and G:F may have been because of differential utilization of the nonprotein component in hempseed cake. Acid detergent fiber concentrations are greater in hempseed cake than DDGS, which potentially results in decreased overall digestibility, and possibly growth performance. Further understanding of how hempseed cake influences cattle growth performance is necessary to better define the nutritional quality of hempseed cake for use in cattle diets. Data on total tract digestibility, postruminal nutrient flow, ruminal function, and immune function are needed to better understand how hempseed cake can best be utilized in cattle diets. Although hempseed cake may have lower NEm and NEg concentrations and potentially result in marginally lower growth performance than DDGS when adequate metabolizable protein is supplied, it could be a viable alternative feed source for ruminants depending on availability and cost.
Acknowledgments
This project was partially supported by a cooperative agreement between North Dakota State University and the United States Department of Agriculture, Agricultural Research Service. We would like to thank the employees of the NDSU Beef Cattle Research Complex and the Nutrition, Physiology, and Meat Science Laboratories in the Department of Animal Science at North Dakota State University (Fargo, ND) for assistance with the project.
Glossary
Abbreviations
- AA
amino acids
- ADF
acid detergent fiber
- ADG
average daily gain
- BW
body weight
- CP
crude protein
- DM
dry matter
- DMI
dry matter intake
- EAA
essential amino acids
- G:F
gain:feed
- HCW
hot carcass weight
- LM
longissimus muscle
- NDF
neutral detergent fiber
- NEAA
nonessential amino acids
- NEg
net energy for gain
- NEm
net energy for maintenance
- OM
organic matter
- PUN
plasma urea nitrogen
- THC
delta-9-tetrahydrocannabinol
Contributor Information
Thomas M Winders, Department of Animal Sciences, North Dakota State University, Fargo, ND 58108-6050, USA.
Eric M Serum, USDA ARS-ETSARC, Fargo, ND 58102, USA.
David J Smith, USDA ARS-ETSARC, Fargo, ND 58102, USA.
Bryan W Neville, Carrington Research Extension Center, Carrington, ND 58421-0219, USA.
Golam K Mia, Department of Animal Sciences, North Dakota State University, Fargo, ND 58108-6050, USA.
Samat Amat, Department of Microbiological Sciences, North Dakota State University, Fargo, ND 58108-6050, USA.
Carl R Dahlen, Department of Animal Sciences, North Dakota State University, Fargo, ND 58108-6050, USA.
Kendall C Swanson, Department of Animal Sciences, North Dakota State University, Fargo, ND 58108-6050, USA.
Conflict of Interest Statement
The authors declare that they have no known financial, nonfinancial, or personal interest that could influence the subject matter or materials discussed in this paper in any way.
LITERATURE CITED
- AOAC. 1990. Official methods of analysis. Arlington (VA): AOAC. [Google Scholar]
- Benson, J. A., Reynolds C. K., Humphries D. J., Rutter S. M., and Beever D. E.. . 2001. Effects of abomasal infusion of long-chain fatty acids on intake, feeding behavior, and milk production in dairy cows. J. Dairy Sci. 84:1182–1191. doi: 10.3168/jds.S0022-0302(01)74579-1 [DOI] [PubMed] [Google Scholar]
- Bergen, W. G. 2008. Measuring in vivo intracellular protein degradation rates in animal systems. J. Anim. Sci. 86:E3–E12. doi: 10.2527/jas.2007-0430 [DOI] [PubMed] [Google Scholar]
- Chakrabarty, S., Shelver W. L., and Smith D. J.. . 2021a. Electrospray ionization inlet tandem mass spectrometry: a hyphenated method for the sensitive determination of chemicals in animal tissues and body fluids. J. Mass. Spectrom. 32:14–20. doi: 10.1021/jasms.9b00114 [DOI] [PubMed] [Google Scholar]
- Chakrabarty, S., Winders T. M., Serum E. M., Swanson K. C., Dahlen C. R., Kleinhenz M. D., and Smith D. J.. . 2021b. Rapid quantification of cannabinoids in bovine plasma using electrospray ionization mass spectrometry sans LC column. In: Proceedings of the American Society for Mass Spectrometry Conference on Mass Spectrometry and Allied Topics (abstract). Oct. 31 - Nov. 4. Philadelphia, PA.
- Chaney, A. L., and Marbach E. P.. . 1962. Modified reagents for determination of urea and ammonia. Clin. Chem. 8:130–132. doi: 10.1093/clinchem/8.2.130 [DOI] [PubMed] [Google Scholar]
- Engali, S. 2012. Central and peripheral cannabinoid receptors as therapeutic targets in the control of food intake and body weight. Handb. Exp. Pharmacol. 209:357–381. doi: 10.1007/978-3-642-24716-3_17 [DOI] [PubMed] [Google Scholar]
- Farrance, I. 1987. Plasma glucose methods, a review. Clin. Biochem. Rev. 8:55–68. [Google Scholar]
- Fawcett, J. K., and Scott J. E.. . 1960. A rapid and precise method for the determination of urea. J. Clin. Path. 13:156–159. doi: 10.1136/jcp.13.2.156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fike, J. 2016. Industrial hemp: renewed opportunities for an ancient crop. Crit. Rev. Plant Sci. 35:406–424. doi: 10.1080/07352689.2016.1257842 [DOI] [Google Scholar]
- Galyean, M. 2009. NEm and NEg calculations. The home page of Michael Galyean. [accessed August 9, 2021]. https://www.depts.ttu.edu/afs/home/mgalyean/. [Google Scholar]
- Gibb, D. J., Shah M. A., Mir P. S., and McAllister T. A.. . 2005. Effect of full-fat hemp seed on performance and tissue fatty acids of feedlot cattle. Can. J. Anim. Sci. 85:223–230. doi: 10.4141/A04-078 [DOI] [Google Scholar]
- Hammond, A. C. 1983. The use of blood urea nitrogen concentration as an indicator of protein status in cattle. Bov. Pract. 18:114–118. doi: 10.21423/bovine-vol1983no18p114-118 [DOI] [Google Scholar]
- Herrera-Saldana, R., and Huber J. T.. . 1989. Influence of varying protein and starch degradabilities on performance of lactating cows. J. Dairy Sci. 72:1477–1483. doi: 10.3168/jds.S0022-0302(89)79257-2 [DOI] [PubMed] [Google Scholar]
- Hessle, A., Eriksson M., Nadau E., Turner T., and Johansson B.. . 2008. Cold-pressed hempseed cake as a protein feed for growing cattle. Acta Agric. Scand. A Anim. Sci. 58:136–145. doi: 10.1080/09064700802452192 [DOI] [Google Scholar]
- Jennings, J. S., Meyer B. E., Guiroy P. J., and Cole N. A.. . 2018. Energy costs of feeding excess protein from corn-based by-products to finishing cattle. J. Anim. Sci. 96:653–669. doi: 10.1093/jas/sky021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joy, F., McKinnon J. J., Hendrick S., Gorka P., and Penner G. B.. . 2017. Effect of dietary energy substrate and days on feed on apparent total tract digestibility, ruminal short-chain fatty acid absorption, acetate and glucose clearance, and insulin responsiveness in finishing feedlot cattle. J. Anim. Sci. 95:5606–5616. doi: 10.2527/jas2017.1817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson, L., Finell M., and Martinsson K.. . 2010. Effects of increasing amounts of hempseed cake in the diet of dairy cows on the production and composition of milk. Animal 4:1854–1860. doi: 10.1017/S1751731110001254 [DOI] [PubMed] [Google Scholar]
- Karlsson, L., and Martinsson K.. . 2011. Growth performance of lambs fed different protein supplements in barley-based diets. Livest. Sci. 138:125–131. doi: 10.1016/j.livsci.2010.12.010 [DOI] [Google Scholar]
- Karlsson, L., Ruiz-Moreno M., Stern M. D., and Martinsson K.. . 2012. Effects of temperature during moist heat treatment on ruminal degradability and intestinal digestibility of protein and amino acids in hempseed cake. Asian-Australas. J. Anim. Sci. 25:1559–1567. doi: 10.5713/ajas.2012.12213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhenz, M. D., Magnin G., Ensley S. E., Griffen J. J., Goeser J., Lynch E., and Coetzee J. F.. . 2020. Nutrient concentrations, digestibility, and cannabinoid concentrations of industrial hemp plant components. Appl. Anim. Sci. 36:489–494. doi: 10.15232/aas.2020-02018 [DOI] [Google Scholar]
- Lapierre, H. D. P., Berthiaume R., Ouellet D. R., Schwab C. G., Dubreuil P., Holtrop G., and Lobley G. E.. . 2006. What is the true supply of amino acids for a dairy cow? J. Dairy Sci. 89:E1–E14. doi: 10.3168/jds.S0022-0302(06)72359-1 [DOI] [PubMed] [Google Scholar]
- Leizer, C., Ribnicky D., Poulev A., Dushenkov S., and Raskin I.. . 2000. The composition of hemp seed oil and its potential as an important source of nutrition. J. Nutr. Funct. Med. Foods 2:35–53. doi: 10.1300/J133v02n04_04 [DOI] [Google Scholar]
- Lemley, C. O., Camacho L. E., Meyer A. M., Kapphahn M., Caton J. S., and Vonnahme K. A.. . 2013. Dietary melatonin supplementation alters uteroplacental amino acid flux during intrauterine growth restriction in ewes. Animal 7:1500–1507. doi: 10.1017/S1751731113001006 [DOI] [PubMed] [Google Scholar]
- Liu, K. 2011. Chemical composition of distillers grains, a review. J. Agrc. Food Chem. 59:1508–1526. doi: 10.1021/jf103512z [DOI] [PubMed] [Google Scholar]
- Mark, T., Shepherd J., Olson D., Snell W., Proper S., and Thornsbury S.. . 2020. Economic viability of industrial hemp in the United States: a review of state pilot programs, EIB-217. United States Department of Agriculture. Economic Research Service. Washington, DC. [Google Scholar]
- Montanholi, Y. R., Swanson K. C., Palme R., Schenkel F. S., McBride B. W., Lu D., and Miller S. P.. . 2010. Assessing feed efficiency in beef steers through feeding behavior, infrared thermography and glucocorticoids. Animal 4:692–701. doi: 10.1017/S1751731109991522 [DOI] [PubMed] [Google Scholar]
- Mustafa, A. F., McKinnon J. J., and Christensen D. A.. . 1998. The nutritive value of hemp meal for ruminants. Can. J. Anim. Sci. 79:91–95. doi: 10.4141/A98-031 [DOI] [Google Scholar]
- Nair, J., Penner G., Yu P., Lardner H. A., McCallister T., Damiran D., and McKinnon J. J.. . 2015. Evaluation of canola meal derived from Brassica juncea and Brassica napus seed as an energy source for feedlot steers. Can. J. Anim. Sci. 95:599–607. doi: 10.1139/CJAS-2015-0184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NASEM. 2016. Nutrient requirements of beef cattle, 8th rev. ed. Washington (DC): The National Academies Press. doi: 10.17226/19014 [DOI] [Google Scholar]
- NRC. 1996. Nutrient requirements of beef cattle, 8th rev. ed. Washington (DC): The National Academies Press. [Google Scholar]
- Salazar, C., Armenta J. M., and Shulaev V.. . 2012. An UPLC-ESI-MS/MS assay using 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate derivatization for targeted amino acid analysis: application to screening of Arabidopsis thaliana mutants. Metabolites 2:398–428. doi: 10.3390/metabo2030398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelson, K. L., Hubbert M. E., Galyean M. L., and Loest C. A.. . 2016. Nutritional recommendations of feedlot consulting nutritionists: The 2015 New Mexico and Texas Tech University survey. J. Anim. Sci. 94:2648–2663. doi: 10.2527/jas.2016-0282 [DOI] [PubMed] [Google Scholar]
- Simpfendorfer, S. 1974. Relationship of body type, size, sex, and energy intake to the body composition of cattle [Ph.D. dissertation]. Ithaca (NY): Cornell University. [Google Scholar]
- Swanson, K. C., Carlson Z. E., Ruch M. C., Gilbery T. C., Underdahl S. R., Keomanivong F. E., Bauer M. L., and Islas A.. . 2017. Influence of forage source and forage inclusion level on growth performance, feeding behavior, and carcass characteristics in finishing steers. J. Anim. Sci. 95:1325–1334. doi: 10.2527/jas2016.1157 [DOI] [PubMed] [Google Scholar]
- USDA, Agricultural Marketing Service. 2021. Establishment of a domestic hemp production program, final rule. 7CFR Part 990. Fed. Resist. 86:5596–5691. [Google Scholar]
- USDA. 2017. United states standards for grades of carcass beef. United states department of agriculture, agricultural marketing service, livestock, poultry, and seed program. Washington, DC. [Google Scholar]
- Van Soest, P. J., Robertson J. B., and Lewis B. A.. . 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2 [DOI] [PubMed] [Google Scholar]