Abstract
Cattle are an important reservoir of Shiga toxin-producing Escherichia coli (STEC) O26, O111, and O157. The fate of these pathogens in bovine feces at 5, 15, and 25°C was examined. The feces of a cow naturally infected with STEC O26:H11 and two STEC-free cows were studied. STEC O26, O111, and O157 were inoculated into bovine feces at 101, 103, and 105 CFU/g. All three pathogens survived at 5 and 25°C for 1 to 4 weeks and at 15°C for 1 to 8 weeks when inoculated at the low concentration. On samples inoculated with the middle and high concentrations, O26, O111, and O157 survived at 25°C for 3 to 12 weeks, at 15°C for 1 to 18 weeks, and at 5°C for 2 to 14 weeks, respectively. Therefore, these pathogens can survive in feces for a long time, especially at 15°C. The surprising long-term survival of STEC O26, O111, and O157 in bovine feces shows that such feces are a potential vehicle for transmitting not only O157 but also O26 and O111 to cattle, food, and the environment. Appropriate handling of bovine feces is emphasized.
Shiga toxin-producing Escherichia coli (STEC) organisms of different serotypes have been increasingly isolated from humans with disease and from healthy domestic animals (2, 3, 5, 7, 8, 10, 13, 16, 17). Many of these isolates were typical STEC belonging to serotypes O26, O111, and O157, yet most belonged to serotype O157:H7, which can cause severe disease in humans, such as hemorrhagic colitis (HC) and hemolytic-uremic syndrome (HUS) (8, 13). In Japan, STEC infection has received attention since 1990, when two kindergartners among 319 patients died from HUS in an outbreak of HC. This outbreak was attributed to well water contaminated with STEC O157:H7 (1). In 1996 and 1997 in Japan, the most predominant serotype was O157:H7 (73%), the next was O26:H11 (7.3%), and other frequently isolated serotypes were O26:H− (nonmotility), O26:HNT, O111:H−, and O111HNT. STEC O157:H7 caused 19 outbreaks involving 10 or more patients from 1996 to May 1998. Outbreaks occurring frequently in primary and nursery schools and nursery homes for the aged were presumably caused by the meals provided. STEC was isolated from specimens of salad (three cases), seafood sauce, melon, sliced raw tuna, and buckwheat noodles with topping. In a large outbreak in the city of Sakai, in 1996, three children among 7,966 HC patients died from HUS; hydroponically grown radish sprouts were suspected of being the source of infection, as determined by epidemiological investigations, although STEC was not isolated. In a diffuse outbreak in a wide area, from south Kanto to Tokai in Japan in 1997, STEC O157:H7 was isolated from hydroponically grown radish sprouts. However, the source of the contamination of foodstuff in almost all cases in Japan remain unclear (11).
Epidemiological investigations in North America and England revealed that cattle, especially young animals, are a principal reservoir of STEC O157:H7 (3, 7, 10, 16, 17). A recent study suggested that cattle are a natural reservoir for pathogenic E. coli, and cattle fed mostly grain had lower colonic pH and more acid-resistant E. coli organisms, including O157:H7, than cattle fed only hay (4). STEC O157:H7 can survive in bovine feces for a long time and can retain the potential to produce Shiga toxin (15). The long-term survival of STEC O157:H7 in ovine and bovine manure and manure slurry was also reported (9). Thus, bovine feces are a potential vehicle for transmitting O157:H7 to cattle, food, and the environment (9, 15).
Selective isolation of low levels of organisms (under 102 CFU/g) of STEC O26, O111, and O157 from bovine feces, including many other species of Enterobacteriaceae and Pseudomonas, has proven difficult. Therefore, the fate at a stage of decline of STEC O26, O111, and O157 in bovine feces has remained unclear.
Recovery of STEC O26, O111, and O157 from bovine feces, was determined according to a new selective isolation method for these organisms described by Fukushima and Gomyoda (5, 6), namely, by subjecting feces and enrichment cultures of feces in Trypticase soy broth (TSB) to hydrochloric acid treatment and then spreading them on MacConkey agar containing cefixime, tellurite, and sorbitol (CT-SMAC). The purpose of this study was to determine the ability to survive, particularly at low levels, and the growth characteristics of STEC O26, O111, and O157 in bovine feces at different temperatures.
Five strains of STEC O157:H7 (Y9, Y23, Y212, SE97029, and SE97065), two strains of O111:H− (SE97092 and SE97111), and three strains of O26 (SE97012, SE97023, and SE97024) were examined in this study. These strains were isolated from Japanese patients living in Shimane Prefecture, except for strains Y9, Y23, and Y212, which were obtained from H. Watanabe, National Institute of Infectious Disease of Japan. Each strain was grown in 2 ml of TSB for 18 h at 37°C.
Feces were obtained from three healthy beef cows, 2 (cow A), 3 (cow B), and 5 (cow C) years of age, from a local beef farm. Feces were collected from each animal just after excretion in July 1998. The feces were put into a plastic bag and kept at 4°C with ice bags during transportation. Feces were examined within 4 h after sampling. All feces were mixed well in sterile stomacher bags (18 by 30 cm) for 5 min with a model 400 stomacher (A. J. Shefard, London, United Kingdom). Before inoculation, feces from each animal were tested for the presence of O26, O111, and O157 according to the procedure described by Fukushima and Gomyoda (5, 6). In feces from cow A, 5.7 × 103 STEC O26:H11 CFU/g was detected but no STEC O157 or O111 organisms were detected; no STEC O26, O111, or O157 organisms were detected in feces from cows B and C.
Aerobic plate counts of fecal samples were determined by plating a dilution (1:102 to ∼1:108) of feces on Trypticase soy agar (TSA; BBL, Cockeysville, Md.) and desoxycholate hydrogen sulfide-lactose (DHL) agar (Nisui, Tokyo, Japan) and incubating them at 37°C for 24 h. The initial aerobic plate counts of three fecal samples were 2.0 × 109, 2.0 × 1010, and 2.0 × 109 CFU/g on TSA and 3.0 × 106, 2.6 × 106, and 3.0 × 106 CFU/g on DHL agar, respectively. The inoculum (0.1 ml) of the 10 strains of O26, O111, and O157 was added to 10 g of feces and mixed thoroughly in sterile stomacher bags for 5 min to obtain concentrations of 101, 103, and 105 CFU/g of feces. After mixing, feces were gathered and put into a closed plastic bag (3 by 5 cm).
Bovine feces in closed plastic bags were kept at 5, 15, and 25°C. STEC O157, O111, and O26 counts were determined at 0, 1, 2, 3, and 4 weeks postinoculation and thereafter at 2-week intervals until each pathogen could no longer be isolated after enrichment of samples in TSB. Fecal samples (0.2 g) were put into TSB (1.8 ml) and assayed for STEC O26, O111, and O157 by direct plating after HCl treatment and by selective enrichment. For counting viable cells in feces, a portion (0.2 ml) of a 10-fold dilution of feces was transferred to HCl solution (0.2 ml of 0.125 N HCl and 0.5% NaCl solution), mixed well, and held for 30 s; 0.2- and 0.02-ml portions of HCl-treated feces were spread onto CT-SMAC. Then, 0.02-ml portions of each HCl-treated sample were put into 1 ml of 0.067 M phosphate-buffered saline (pH 7.2). Next, a 100-fold dilution (0.01 ml) of each suspension was placed onto CT-SMAC. For selective enrichment culture, 10- and 100-fold-diluted fecal suspensions in TSB (1.8 ml) were incubated at 42°C for 6 h, and then a portion (0.02 ml) of each enrichment was transferred to HCl solution (0.02 ml), mixed well, and left for 30 s. A 0.02-ml portion of each sample subjected to these treatments was spread onto CT-SMAC. These agar plates were incubated at 37°C for 18 h. STEC O157:H7, non-sorbitol-fermenting colonies from CT-SMAC, were tested for agglutination with an antiserum against O157 (Denka-seiken, Niigata, Japan). STEC O26 and O111, sorbitol-fermenting colonies from CT-SMAC, were tested for agglutination with an antiserum against O26 or O111 (Denka-seiken).
The most significant finding of this work is that not only STEC O157 but also STEC O26 and O111 can survive for a long time in bovine feces, at low levels of organisms. The observation of STEC and background flora in fecal samples in the closed bags show their fate in the higher moisture content near the center of the specimens. The average concentrations of the background flora in bovine feces were 1010 CFU/g on TSA and 106 CFU/g on DHL agar at the start of the study. After 20 weeks, although the background flora concentrations declined to 108 to 109 CFU/g on TSA and 105 to 107 CFU/g on DHL agar at 5, 15, and 25°C, STEC O26, O111, and O157 were not detected even with enrichment culture. Although the inability of STEC O26, O111, and O157 to attain a potential maximum population in mixed cultures with nonpathogenic E. coli may be the result of metabolic crowding (6), the relative measure of survival for nonpathogenic E. coli and for the normal commensal or indigenous fecal population remains an interesting topic for further investigation.
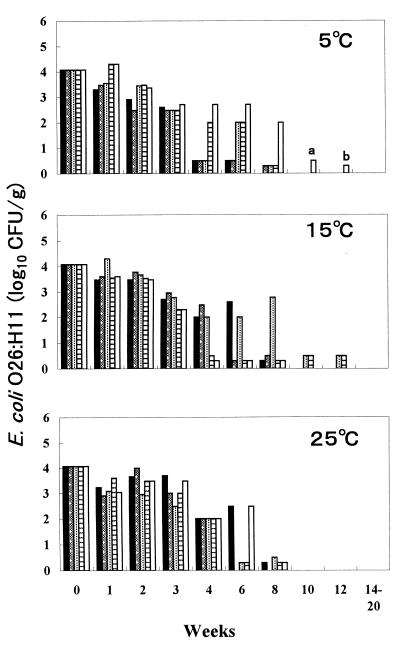
In the five divided fecal samples from cow A, which was naturally infected with STEC O26:H11 and which excreted 5.7 × 103 STEC O26:H11 CFU/g of feces, the population of STEC O26:H11 decreased gradually to a level detectable only by enrichment (<102 CFU/g) at weeks 4 to 12 at 5 and 15°C but at weeks 4 to 8 at 25°C (Fig. 1). These findings show that STEC O26:H11 in feces from a naturally infected cow can survive longer at 5 and 15°C than at 25°C.
FIG. 1.
Fate of STEC O26:H11 in feces of cow A, naturally infected with STEC O26:H11, at 5, 15, and 25°C. Five fecal samples were examined at each temperature. Bars a and b, isolation of STEC O26:H11 in enrichment cultures of 0.02 and 0.2 g of feces, respectively.
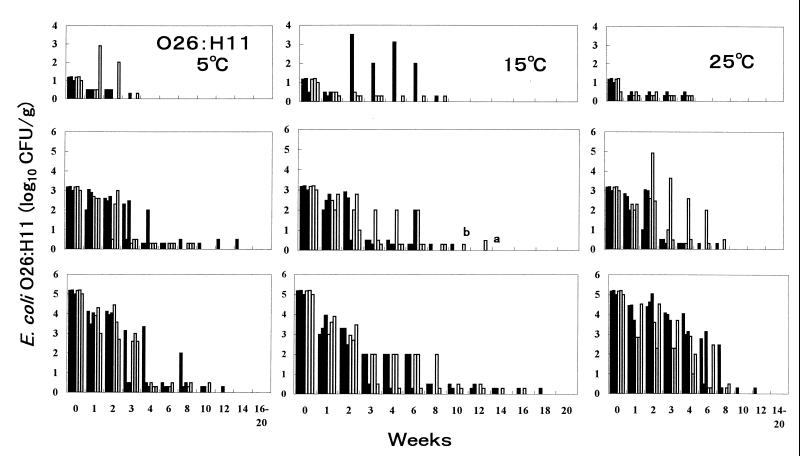
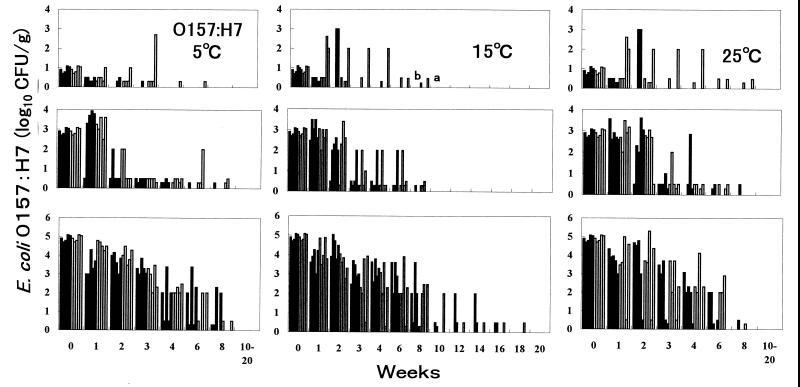
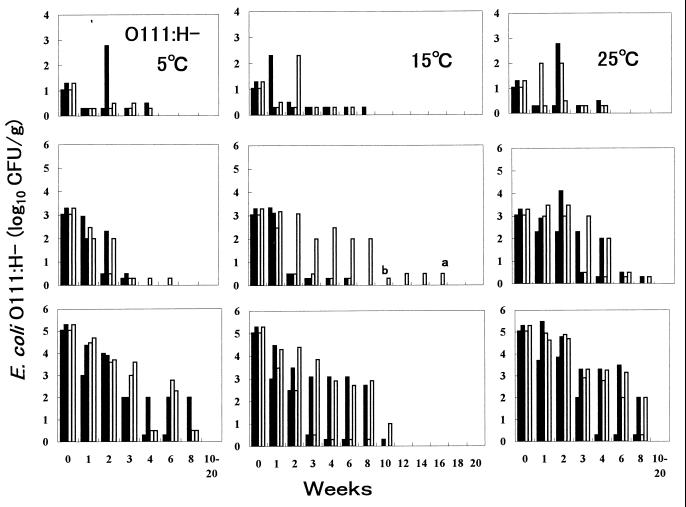
Epidemiological data from a study of cattle revealed that populations of STEC O26, O111, and O157 in bovine feces ranged from <102 to 109 CFU/g (5). Therefore, three inoculation levels—10 (low), 103 (middle), and 105 (high) CFU/g—were selected for this study. Organisms were inoculated into two fecal samples from cows B and C. Generally, STEC O26, O111, and O157 populations decreased quickly to a level detectable only by enrichment at week 1 to 3 for the low concentration, at weeks 2 to 6 for the middle concentration, and at weeks 4 to 10 for the high concentration at 5, 15, and 25°C (Fig. 2 to 4). However, the numbers of all three serotypes in some samples increased 1 or 3 log10 CFU/g 3 weeks after inoculation with the low concentration. The growth at 25°C was remarkable for STEC O157:H7. The pathogen was detectable in samples at 25°C for as long as 4 to 8 weeks. This finding seems to be the first evidence that low levels of STEC O26, O111, and O157 can survive in feces for even 8 weeks.
FIG. 2.
Fate of STEC O26:H11 in bovine feces at 5, 15, and 25°C. Three strains (from left, SE97012, SE97023, and SE97024) were inoculated into three fecal samples from cows B (■) and C (□). Bars a and b, isolation of STEC O26:H11 in enrichment cultures of 0.02 and 0.2 g of feces, respectively.
FIG. 4.
Fate of STEC O157:H7 in bovine feces at 5, 15, and 25°C. Five strains (from left, Y9, Y23, Y212, SE97029, and SE97065) were inoculated in five fecal samples from cows B (■) and C (□). Bars a and b, isolation of STEC O26:H11 in enrichment culture of 0.02 and 0.2 g of feces, respectively.
At 25°C with the middle and high concentrations, O26, O111, and O157 populations decreased gradually to under 102 CFU/g at weeks 4 to 8; these pathogens were not detected after 10 weeks, except for one case in which O26:H11 inoculated at a high concentration survived for 12 weeks. At 15°C with the middle and high concentrations, O26, O111, and O157 populations decreased gradually to under 102 CFU/g at weeks 4 to 10; these pathogens were not detected after 10 weeks, but survival of O157:H7 in three samples, of O111:H− in one sample, and of O26:H11 in four samples was evident for 12 to 18 weeks. At 5°C with the middle and high concentrations, O26, O111, and O157 populations decreased more rapidly than at 15 and 25°C, to under 102 CFU/g almost at weeks 3 to 6, and O26:H11 was detected in two samples at 12 and 14 weeks by enrichment culture. These findings show that STEC O26, O111, and O157 can survive in feces for a long time, especially at 15°C, even for 16 to 18 weeks (112 to 126 days).
Wang et al. (15) pointed out that STEC O157:H7 inoculated into bovine feces at 103 and 105 CFU/g can survive at 5°C for 63 to 70 days, at 22°C for 49 and 56 days, and at 37°C for 42 and 49 days. Although the death of STEC O157:H7 at 22 and 37°C may in part have been due to dehydration of the feces in open bags (15), our study showed short-term survival of STEC O157:H7 in bovine feces at high temperatures (25°C), even with samples in closed bags. Kudva et al. (9) also reported that STEC O157:H7 inoculated into ovine feces at 108 CFU/g can survive at 4 and 10°C for 100 days and at 23°C for 40 days even without aeration. The same phenomenon was observed for STEC O111:H− and O26:H11 in bovine feces in our study. These findings confirm that survival rates of not only STEC O157 but also STEC O26 and O111 in feces depend on temperature and the initial bacterial inoculum, regardless of dehydration.
The organism was detected by the nonenrichment culture method in samples containing over 102 CFU of STEC O26, O111, and O157 per g. However, the organisms were detected by the selective enrichment technique in samples containing under 102 CFU of STEC O26, O111, and O157 per g. In previous studies of the fate of STEC O157 in bovine and ovine feces, the pathogen was detected by enrichment culture with TSB or TSB supplemented with cefixime, potassium tellurite, and vancomycin (TSB-CTV) at 37°C for 18 h with agitation (9, 15). Although these conventional enrichment techniques are widely used for isolation of STEC O157:H7 from food and feces (3, 7, 10, 16, 17), our previous work (6) showed that TSB culture at 42°C for 6 h with no agitation is the most effective method for isolating STEC O26, O111, and O157 from food and feces. The growth of STEC O157:H7 in TSB and TSB-CTV at 37°C was poorer than at 42°C, and culturing in TSB and TSB-CTV at 37°C allowed for a more vigorous growth of other members of Enterobacteriaceae than that of STEC O157:H7. Therefore, the samples subjected to enrichment with TSB at 42°C for 6 h with no agitation were spread onto CT-SMAC agar after HCl treatment. STEC O26, O111, and O157 at under 102 CFU/g were detected at weeks 16 and 18 postinoculation after incubation at 15°C. The detection of these surviving organisms at under 102 CFU/g at temperatures between 5 and 25°C was facilitated by this new isolation method for STEC.
Dairy and beef herds have been identified as a reservoir of this pathogen (3, 7, 15, 16). Our studies revealed that low levels of organisms of not only STEC O157 but also STEC O26 and O111 can survive in bovine feces at temperatures between 5 and 25°C for longer than heretofore recognized. The long-term survival of STEC has implications for understanding the ecology of this pathogen in cattle and in the environment. Irrigating fields with manure and manure slurry may be a risk factor for transmitting the pathogen (9). Bovine feces and manure exposed in a field may become a direct or indirect source of infection with STEC when dirt, soil, or water is contaminated for a long time, because the estimated infectious dose for humans is as low as 10 bacteria (14). Direct transmission of STEC O157:H7 from calves to children via the fecal-oral route (12) and outbreaks involving contaminated vegetables (11) have been reported. Bovine feces are a potential source for the spread of STEC O157:H7 to the human food chain as well as to the environment (15). Although Wang et al. (15) pointed out that the effective control of STEC O157:H7 in dairy cattle and appropriate handling or usage of bovine feces are necessary so that contamination of the environment and food by this pathogen can be prevented, the surprising long-term survival of not only O157 but also O26 and O111 in feces of dairy and beef cattle indicates that even more strict handling may be required.
FIG. 3.
Fate of STEC O111:H− in bovine feces at 5, 15, and 25°C. Two strains (from left, SE97092 and SE97111) were inoculated in two fecal samples from cows B (■) and C (□). Bars a and b, isolation of STEC O26:H11 in enrichment cultures of 0.02 and 0.2 g of feces, respectively.
REFERENCES
- 1.Akashi S, Joh K, Tsuji A, Ito H, Hoshi H, Hayakawa T, Ihara J, Abe T, Hatori M, Mori T. A severe outbreak of haemorrhagic colitis and haemolytic uraemic syndrome associated with Escherichia coli O157:H7 in Japan. Eur J Pediatr. 1994;153:650–655. doi: 10.1007/BF02190685. [DOI] [PubMed] [Google Scholar]
- 2.Beutin L, Geier D, Steinrück H, Zimmermann S, Scheutz F. Prevalence and some properties of verotoxin (Shiga-like toxin)-producing Escherichia coli in seven different species of healthy domestic animals. J Clin Microbiol. 1993;31:2483–2488. doi: 10.1128/jcm.31.9.2483-2488.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chapman P A, Wright D J, Norman P, Fox J, Crick E. Cattle as a possible source of verocytotoxin-producing Escherichia coli O157 infections in man. Epidemiol Infect. 1993;111:439–447. doi: 10.1017/s0950268800057162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diez-Gonzalez F, Callaway T R, Kizoulis M G, Russell J B. Grain feeding and the dissemination of acid-resistant Escherichia coli from cattle. Science. 1998;281:1666–1668. doi: 10.1126/science.281.5383.1666. [DOI] [PubMed] [Google Scholar]
- 5.Fukushima H, Gomyoda M. Hydrochloric acid treatment for rapid recovery of Shiga toxin-producing Escherichia coli O26, O111, and O157 from faeces, food and environmental samples. Zentbl Bakteriol. 1999;289:285–299. doi: 10.1016/s0934-8840(99)80066-8. [DOI] [PubMed] [Google Scholar]
- 6.Fukushima, H., and M. Gomyoda. Effective, rapid and simple method for isolation of Shiga toxin-producing Escherichia coli O26, O111, and O157 from feces and food samples. Zentbl. Bakteriol., in press. [DOI] [PubMed]
- 7.Hancock D D, Besser T E, Kinsel M L, Tarr P I, Rice D H, Paros M G. The prevalence of Escherichia coli O157:H7 in dairy and beef cattle in Washington State. Epidemiol Infect. 1994;113:199–207. doi: 10.1017/s0950268800051633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karmali M A. Infection by verocytotoxin-producing Escherichia coli. Clin Microbiol Rev. 1989;2:15–38. doi: 10.1128/cmr.2.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kudva I T, Blanch K, Hovde C J. Analysis of Escherichia coli O157:H7 survival in ovine or bovine manure and manure slurry. Appl Environ Microbiol. 1998;64:3166–3174. doi: 10.1128/aem.64.9.3166-3174.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montenegro M A, Bülte M, Trumpf T, Aleksić S, Reuter G, Bulling E, Helmuth R. Detection and characterization of fecal verotoxin-producing Escherichia coli from healthy cattle. J Clin Microbiol. 1990;28:1417–1421. doi: 10.1128/jcm.28.6.1417-1421.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Institute of Infectious Disease and Infectious Disease Control Division, Ministry of Health and Welfare of Japan. Enterohemorrhagic Escherichia coli (verocytotoxin-producing E. coli) infection, 1996–April 1998. Infect Agents Surveillance Rep. 1998;19:122–123. [Google Scholar]
- 12.Renwick S A, Wilson J B, Clarke R C, Lior H, Borczyk A A, Spika J, Rahn K, McFadden K, Brouwer A, Copps A, Anderson G, Alves D, Karmali M A. Evidence of direct transmission of Escherichia coli O157:H7 infection between calves and a human. J Infect Dis. 1993;168:792–793. doi: 10.1093/infdis/168.3.792. [DOI] [PubMed] [Google Scholar]
- 13.Reily L W, Remis R S, Helgerson S D, McGee H B, Wells J G, Davis B R, Hebert R J, Olcott E S, Johnson L M, Hargrett N T, Blake P A, Cohen M L. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N Engl J Med. 1983;308:681–685. doi: 10.1056/NEJM198303243081203. [DOI] [PubMed] [Google Scholar]
- 14.Tarr P I. Escherichia coli O157:H7: clinical, diagnostic, and epidemiological aspects of human infection. Clin Infect Dis. 1995;20:1–10. doi: 10.1093/clinids/20.1.1. [DOI] [PubMed] [Google Scholar]
- 15.Wang G, Zhao T, Doyle M P. Fate of enterohemorrhagic Escherichia coli O157:H7 in bovine feces. Appl Environ Microbiol. 1996;62:2567–2570. doi: 10.1128/aem.62.7.2567-2570.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wells J G, Shipman L D, Greene K D, Sowers E G, Green J H, Cameron D N, Downes F P, Martin M L, Griffin P M, Ostroff S M, Potter M E, Tauxe R V, Wachsmuth I K. Isolation of Escherichia coli serotype O157:H7 and other Shiga-like-toxin-producing E. coli from dairy cattle. J Clin Microbiol. 1991;29:985–989. doi: 10.1128/jcm.29.5.985-989.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao T, Doyle M P, Shere J, Garber L. Prevalence of enterohemorrhagic Escherichia coli O157:H7 in a survey of dairy herds. Appl Environ Microbiol. 1995;61:1290–1293. doi: 10.1128/aem.61.4.1290-1293.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]