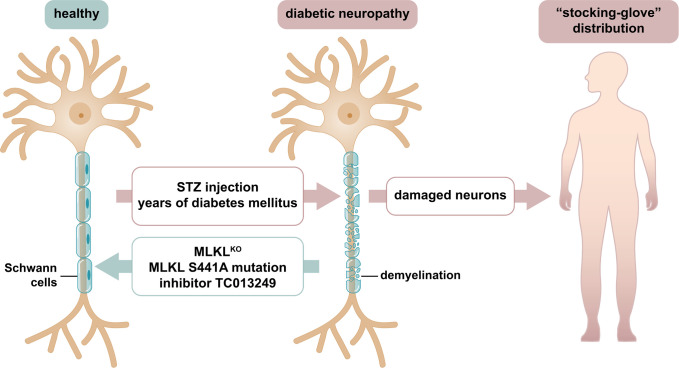
Diabetic neuropathy (DN) is clinically characterized by a “stocking and glove” pattern of symptoms such as sensory loss, numbness, pain, and burning sensations. This pattern implicates that Schwann cells, required to maintain the myelin sheath and the nodes of Ranvier, rather than the neurons themselves, represent the primary site of injury (Fig. 1). In PNAS, Guo et al. (1) explain how the necroptosis protein, mixed-lineage kinase domain–like protein (MLKL), causes Schwann cells to lose their function.
Fig. 1.
Demyelination during DN involves MLKL-mediated damage of Schwann cells. In healthy individuals, the speed at which an electrochemical impulse propagates down a neural pathway, referred to as nerve conduction velocity, requires Schwann cells for electric neuronal isolation. In DN, demyelination of Schwann cells was recognized decades ago and is well known to clinically associate with the stocking-and-glove pattern frequently observed in diabetic patients. Currently, no treatment for DN is clinically available. Guo et al. (1) set out to investigate how Schwann cells lose their function. They report a critical pathophysiological contribution of MLKL, a pseudokinase and key mediator of necroptosis. In the STZ-induced murine model of diabetes and in patients suffering from DN, Schwann cell degradation is a common feature. In that model, MLKL-deficient or MLKL-mutant mice, and wild-type mice treated with the MLKL inhibitor TC013249, exhibited less demyelination.
DN is not a classical form of demyelinating neuropathy, such as Guillain-Barré syndrome. However, chronic hyperglycemia in type 1 and type 2 diabetes mellitus patients may induce typical features of demyelination, especially in severely affected patients (2–4). Therefore, understanding the death of Schwann cells might help unravel their mechanistic role in the associated loss of the myelin sheath and clinical progression of DN.
Necroptosis is a form of regulated necrosis that is associated with phosphorylation of MLKL (5, 6), a pseudokinase (7, 8). Although activation of MLKL is not sufficient to kill a cell (9), it is required for necroptosis. The only known kinase to phosphorylate MLKL is receptor-interacting protein kinase 3 (RIPK3) (5, 6) which is controlled by death receptors through RIPK1 (10–13), Toll-like receptors through the protein TIR domain–containing adapter inducing interferon-beta (TRIF) (14–16), and nucleotide sensors such as Z-DNA-binding protein 1 (ZBP1) (13, 17–23). Interaction with these proteins requires the RIP homotypic interacting motif of RIPK3 (24, 25) which is also interfered with by viruses such as cytomegalovirus (25–27), mechanistically highlighting the well-established role for necroptosis in viral defense.
In their current work, Guo et al. (1) first establish a transmission electron microscopy–based readout system of myelin decompaction following streptozotocin (STZ)–induced loss of pancreatic beta cells, thereby inducing diabetes. They demonstrate colocalization of MLKL with a known myelin sheath marker, indicating localization in the Schwann cells. Next, the authors investigate MLKL-deficient mice which exhibit less myelin decompaction and higher nerve conduction velocity in the STZ model, indicating MLKL to mechanistically induce DN. Further, they generate Schwann cell–specific MLKL-deficient mice employing a tamoxifen-inducible Plp1 promotor and confirm the above-mentioned findings. In yet another independent approach, they replace the MLKL gene with an S441A point mutation of MLKL in mice and, once again, reverse the diabetic phenotype in this setting when compared to MLKL wild-type mice. Finally, and clinically most importantly, an MLKL inhibitor (TC013249) is tested. As this inhibitor was generated to ultimately treat humans, human MLKL knock-in mice (hMLKL-KI) are employed for this important set of experiments. The authors carefully confirm that the hMLKL-KI mice do not express traces of mouse MLKL and control for equal hMLKL expression levels compared to wild-type mice. Intraperitoneal osmotic pumps are used to continuously deliver TC013249 to the hMLKL-KI mice. Upon treatment, significantly mitigated myelin sheath decompaction is observed. This functionally correlates with significantly less STZ-induced decreases in nerve conduction velocity.
MLKL inhibition, however, should be interpreted within a bigger picture. Within the field of neurology, other conditions that include demyelination include multiple sclerosis, Marchiafava Bignami disease, central pontine myelinolysis, and others. It will be interesting to investigate the role of MLKL in these disorders. In addition, RIPK1 was demonstrated to play critical roles in diverse pathologies of the central nervous system (28). Interestingly, and somehow in contrast to a proactive role, MLKL-deficient patients may present with features of neurodegeneration (29). Beyond the field of neurology, MLKL was reported to be involved in acute conditions such as ischemia-reperfusion injury (9, 28, 30–34). In the preclinical limelight of the growing body of evidence for MLKL as a mediator of diseases, clinical trials employing MLKL inhibitors will be considered. Naturally, however, inhibiting the downstream target of necroptosis will result in the functional loss of necroptosis upon MLKL inhibition. Although most primary data indicate a viral inhibition of the kinase RIPK3 (11, 27, 35–37) rather than MLKL directly, it must be predicted that viral infections, known to be cleared by necroptosis (38), may appear more frequently. Clinical trials for testing MLKL inhibitors should therefore be designed to include more-detailed assessments than usual of safety from viral infections and associated complications.
In PNAS, Guo et al. explain how the necroptosis protein, mixed-lineage kinase domain–like protein (MLKL), causes Schwann cells to lose their function.
It is estimated that the annual cost of DN is higher than US$10 billion in the United States (39). In type 2 diabetes mellitus, improvement of glycemic control is thought to have little effect on neuropathy outcomes (39). Opioids are not recommended even in painful DN, owing to the potential for abuse. Novel treatment strategies, therefore, are of paramount clinical importance. In conclusion, the data presented by Guo et al. (1) indicate a therapeutic target in a rodent model that may be of potential future interest and clearly warrants further preclinical and clinical investigations.
Acknowledgments
Work in the Linkermann Lab is funded by German Research Foundation grants SFB-TRR 205 and SFB-TRR 127, the International Research Training Group 2251, Priority Programme 2306, the Else Kröner-Fresenius Stiftung, and the Wilhelm Sander-Stiftung. A.L. is supported by the German Research Foundation (Heisenberg-Professorship, Project 324141047).
Footnotes
The authors declare no competing interest.
See companion article, “Blockage of MLKL prevents myelin damage in experimental diabetic neuropathy,” 10.1073/pnas.2121552119.
References
- 1.Guo J., et al. , Blockage of MLKL prevents myelin damage in experimental diabetic neuropathy. Proc. Natl. Acad. Sci. U.S.A. 119, 10.1073/pnas.2121552119 (2022). [DOI] [Google Scholar]
- 2.Dunnigan S. K., et al. , Conduction slowing in diabetic sensorimotor polyneuropathy. Diabetes Care 36, 3684–3690 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gumy L. F., Bampton E. T., Tolkovsky A. M., Hyperglycaemia inhibits Schwann cell proliferation and migration and restricts regeneration of axons and Schwann cells from adult murine DRG. Mol. Cell. Neurosci. 37, 298–311 (2008). [DOI] [PubMed] [Google Scholar]
- 4.Mizisin A. P., Shelton G. D., Wagner S., Rusbridge C., Powell H. C., Myelin splitting, Schwann cell injury and demyelination in feline diabetic neuropathy. Acta Neuropathol. 95, 171–174 (1998). [DOI] [PubMed] [Google Scholar]
- 5.Sun L., et al. , Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 148, 213–227 (2012). [DOI] [PubMed] [Google Scholar]
- 6.Zhao J., et al. , Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc. Natl. Acad. Sci. U.S.A. 109, 5322–5327 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy J. M., et al. , The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity 39, 443–453 (2013). [DOI] [PubMed] [Google Scholar]
- 8.Murphy J. M., et al. , Insights into the evolution of divergent nucleotide-binding mechanisms among pseudokinases revealed by crystal structures of human and mouse MLKL. Biochem. J. 457, 369–377 (2014). [DOI] [PubMed] [Google Scholar]
- 9.von Mässenhausen A., et al. , Phenytoin inhibits necroptosis. Cell Death Dis. 9, 359 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newton K., Sun X., Dixit V. M., Kinase RIP3 is dispensable for normal NF-kappa Bs, signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and Toll-like receptors 2 and 4. Mol. Cell. Biol. 24, 1464–1469 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho Y. S., et al. , Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137, 1112–1123 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He S., et al. , Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell 137, 1100–1111 (2009). [DOI] [PubMed] [Google Scholar]
- 13.Rebsamen M., et al. , DAI/ZBP1 recruits RIP1 and RIP3 through RIP homotypic interaction motifs to activate NF-kappaB. EMBO Rep. 10, 916–922 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaiser W. J., Offermann M. K., Apoptosis induced by the Toll-like receptor adaptor TRIF is dependent on its receptor interacting protein homotypic interaction motif. J. Immunol. 174, 4942–4952 (2005). [DOI] [PubMed] [Google Scholar]
- 15.He S., Liang Y., Shao F., Wang X., Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3-mediated pathway. Proc. Natl. Acad. Sci. U.S.A. 108, 20054–20059 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaiser W. J., et al. , Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J. Biol. Chem. 288, 31268–31279 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takaoka A., et al. , DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature 448, 501–505 (2007). [DOI] [PubMed] [Google Scholar]
- 18.Kuriakose T., et al. , ZBP1/DAI is an innate sensor of influenza virus triggering the NLRP3 inflammasome and programmed cell death pathways. Sci. Immunol. 1, aag2045 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maelfait J., et al. , Sensing of viral and endogenous RNA by ZBP1/DAI induces necroptosis. EMBO J. 36, 2529–2543 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ingram J. P., et al. , ZBP1/DAI drives RIPK3-mediated cell death induced by IFNs in the absence of RIPK1. J. Immunol. 203, 1348–1355 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang D., et al. , ZBP1 mediates interferon-induced necroptosis. Cell. Mol. Immunol. 17, 356–368 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiao H., et al. , Z-nucleic-acid sensing triggers ZBP1-dependent necroptosis and inflammation. Nature 580, 391–395 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang T., et al. , Influenza virus Z-RNAs induce ZBP1-mediated necroptosis. Cell 180, 1115–1129.e13 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun X., Yin J., Starovasnik M. A., Fairbrother W. J., Dixit V. M., Identification of a novel homotypic interaction motif required for the phosphorylation of receptor-interacting protein (RIP) by RIP3. J. Biol. Chem. 277, 9505–9511 (2002). [DOI] [PubMed] [Google Scholar]
- 25.Kaiser W. J., Upton J. W., Mocarski E. S., Receptor-interacting protein homotypic interaction motif-dependent control of NF-kappa B activation via the DNA-dependent activator of IFN regulatory factors. J. Immunol. 181, 6427–6434 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Upton J. W., Kaiser W. J., Mocarski E. S., Cytomegalovirus M45 cell death suppression requires receptor-interacting protein (RIP) homotypic interaction motif (RHIM)-dependent interaction with RIP1. J. Biol. Chem. 283, 16966–16970 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Upton J. W., Kaiser W. J., Mocarski E. S., Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe 7, 302–313 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Degterev A., Ofengeim D., Yuan J., Targeting RIPK1 for the treatment of human diseases. Proc. Natl. Acad. Sci. U.S.A. 116, 9714–9722 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faergeman S. L., et al. , A novel neurodegenerative spectrum disorder in patients with MLKL deficiency. Cell Death Dis. 11, 303 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu W., et al. , RGMb protects against acute kidney injury by inhibiting tubular cell necroptosis via an MLKL-dependent mechanism. Proc. Natl. Acad. Sci. U.S.A. 115, E1475–E1484 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martens S., et al. , Sorafenib tosylate inhibits directly necrosome complex formation and protects in mouse models of inflammation and tissue injury. Cell Death Dis. 8, e2904 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newton K., et al. , RIPK3 deficiency or catalytically inactive RIPK1 provides greater benefit than MLKL deficiency in mouse models of inflammation and tissue injury. Cell Death Differ. 23, 1565–1576 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ni H. M., et al. , Receptor-interacting serine/threonine-protein kinase 3 (RIPK3)-mixed lineage kinase domain-like protein (MLKL)-mediated necroptosis contributes to ischemia-reperfusion injury of steatotic livers. Am. J. Pathol. 189, 1363–1374 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tonnus W., et al. , Rubicon-deficiency sensitizes mice to mixed lineage kinase domain-like (MLKL)-mediated kidney ischemia-reperfusion injury. Cell Death Dis. 13, 236 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daley-Bauer L. P., et al. , Mouse cytomegalovirus M36 and M45 death suppressors cooperate to prevent inflammation resulting from antiviral programmed cell death pathways. Proc. Natl. Acad. Sci. U.S.A. 114, E2786–E2795 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Omoto S., et al. , Suppression of RIP3-dependent necroptosis by human cytomegalovirus. J. Biol. Chem. 290, 11635–11648 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Upton J. W., Kaiser W. J., Mocarski E. S., DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe 11, 290–297 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mocarski E. S., Upton J. W., Kaiser W. J., Viral infection and the evolution of caspase 8-regulated apoptotic and necrotic death pathways. Nat. Rev. Immunol. 12, 79–88 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feldman E. L., et al. , Diabetic neuropathy. Nat. Rev. Dis. Primers 5, 41 (2019). [DOI] [PubMed] [Google Scholar]