Abstract
Far from inert structures, our body’s epithelial boundaries engage in a dynamic crosstalk with immune cells that is vital for immune surveillance and barrier function. Using the skin and gut epithelium, two structurally distinct but critical environmental interfaces, here we review the context dependent interactions between myriad immune cells and epithelial subsets. We discuss immune communique reserved for epithelial progenitors and the enduring consequences for tissue fitness. Then, we delve into the cellular and molecular exchanges between differentiated epithelial subsets and adjacent immune cells. Therapeutically targeting stage-specific immune-epithelial interaction could boost regeneration and mitigate inflammatory pathologies.
Keywords: Epithelial Stem Cells, Differentiation, Niche, Inflammation, Regeneration
Introduction
Homeostasis, derived from the Greek word for “steady”, represents an optimal state of tissue maintenance[1]. Yet, exposure to the terrestrial environment and the resulting microbial, chemical, and physical exposures perpetually disrupt tissue homeostasis. The impact of these traumas is most pronounced at the epithelial tissues that outline our body, such as the skin and gut epithelia. Constant volleying between homeostasis and stress requires rapid epithelial sensing and responsiveness to distinct environmental stimuli. These adaptations can occur at the level of epithelial stem and progenitor cells (ESPCs) or by their differentiated progeny and rely on microenvironmental signals [2,3].
Epithelial tissues are patrolled by cells of the immune system. In addition to their anti-microbial activity, we now appreciate that immune cells contribute to epithelial function[4]. Resident and circulating immune populations include innate immune cells (macrophages, neutrophils etc.) and various lymphocyte subsets [5]. Resident immune cells vary between barrier tissues also within distinct microenvironments of tissues. The skin for example, houses unique populations of cells in the epidermis and around the hair follicle epithelia [6] (Figure 1A). Similarly, in the intestine, lymphocytes that intercalate within the epithelium are different from those residing in the lamina propria [7] (Figure 1B).
Figure 1. Epithelial and Immunological features of the Skin and Gut.
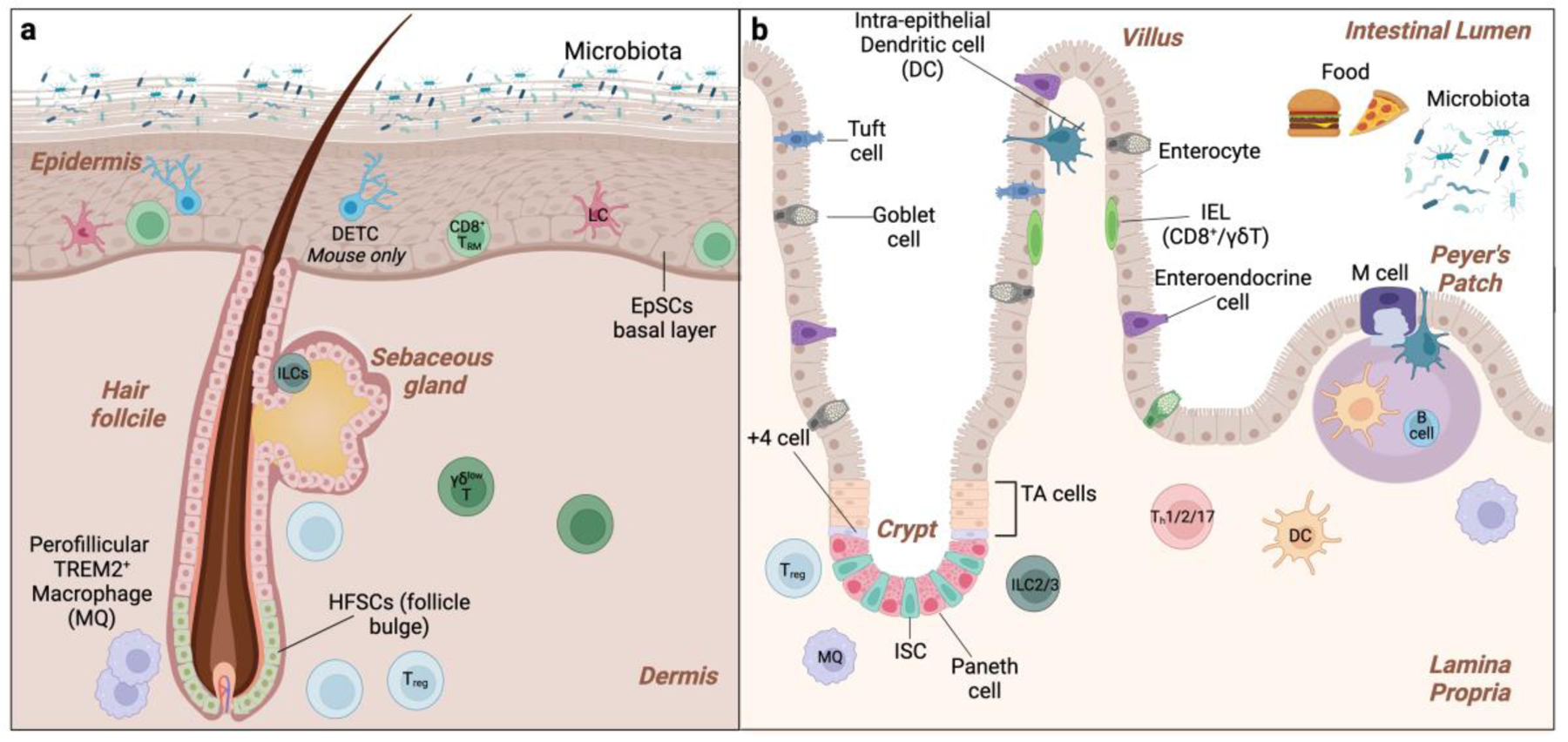
a. Basal epidermal stem and progenitor cells (EpSCs) EpSCs differentiate upward to sustain the interfollicular epidermis and hair follicle stem cells (HFSCs) residing in the bulge fuel follicle cycling. The immune niche of the epidermis includes basal Langerhans cells (LCs), CD8+ resident memory T cells (TRM), innate lymphoid cells (ILCs), and, in murine skin, suprabasal Dendritic Epidermal γδ T cells (DETCs). Regulatory T cells (Tregs) and macrophages are located in the perifollicular dermis. b. The intestinal epithelium is organized in autonomous crypt-villus structures. Intestinal stem cells (ISCs) located at the base of the crypt maintains proliferate and differentiate upward to maintain each unit. +4 cells are a reserve SC population that can substitute for ISCs upon depletion. Intestinal progenitors in the transit-amplifying (TA) region further differentiate into the six lineages of absorptive (M-cells and enterocytes) and secretory (goblet cells, enteroendocrine cells, tuft cells, and Paneth cells) intestinal cells. The intestinal immune niche includes intraepithelial lymphocytes (IELs) and DC (intraepithelial DCs), Treg, Th, γδ T cells, B cells, DCs, and macrophages. Peyer’s patch are lymphoid aggregates that are play vital role in immune surveillance. M cells sample luminal antigens and live bacteria and transcytose across the epithelial barrier to DCs.
Here we review the burgeoning literature on homeostatic and stress-dependent immune–epithelial interactions. We focus on two prominent barriers, the skin and small intestine, each with unique structures and functions that dictate epithelial-immune crosstalk. We discuss how ESPCs and their differentiated progeny dialogue with resident and recirculating immune cells and the grave consequences for tissue fitness when these conversations go awry. In so doing, we highlight opportunities to therapeutically target immune-epithelial interaction.
Overview of skin and intestinal epithelium
The skin and gut epithelium have structurally evolved to serve the needs of their respective organs. Squamous or flattened epithelia culminate in an outer layer of dead cells to cope with constant abrasion endured by the epidermis. This multi-layered barrier limits environmental agents while maintaining fluid and temperature balance. By contrast, a single layer of columnar epithelium lines the small intestine to balance barrier function and efficient nutrient uptake. Below we discuss the specific three-dimensional organization and the corresponding immune milieu of these distinct epithelial compartments. It is important to note that there are key structural and cellular distinctions in skin and gut epithelium between mouse and man (reviewed here [8,9]). Never the less, many of the insights from studying immune-epithelial crosstalk in mice have informed mechanisms of human health and disease.
The Epidermis
The epidermis is stratified into four layers: a basal layer of epidermal stem and progenitor cells (EpSCs) and differentiated spinous, granular, and cornified layers[10]. Invaginations such as hair follicles (HFs) and sebaceous glands are contiguous with the epidermal layers (Figure 1A). HFs are maintained by distinct pools of hair follicle stem cells (HFSCs) that reside in the bulge region [11]. Epidermal turnover is fueled by basal cells that constantly proliferate and differentiate outward to be shed from the skin’s surface. By contrast, HFs undergo cyclical bouts of rest (telogen) and regeneration (anagen) governed by HFSCs activation. The formation of a water-tight epidermal barrier relies on cell-cell junctions in the lower layers and lipid and protein rich upper suprabasal epidermis [12,13].
Immune cells including Langerhans cells (LCs), CD8+ resident memory T cells (TRMs), innate lymphoid cells (ILCs), and, in murine skin, Dendritic Epidermal γδ T cells (DETCs) reside in the lower layers of the epidermis [6]. Regulatory T cells (Tregs) and macrophages are enriched in the perifollicular dermis below the basement membrane and also influence epithelial cell behavior. Following injury or inflammation, innate and adaptive immune cell from systemic circulation are recruited to and dialogue with the epithelium to fuel stress responses.
Small intestinal epithelium
The columnar intestinal epithelial layer is organized into millions of autonomous crypt (invaginations)–villus (finger-like projections) structures (Figure 1B). Fueling each villus are intestinal stem cells (ISCs) that reside in the crypt base [14]. Reserve SCs that compensate for ISCs loss are located 4 cells away from the crypt base [15]. ISCs proliferate and differentiate upward to generate transit-amplifying progenitors, which further divide and differentiate to the six lineages of absorptive (M-cells and enterocytes with brush villi) and secretory (goblet cells, enteroendocrine cells, tuft cells and Paneth cells) cells [14]. Remarkably, this entire cell compartment turns over in 3–5 days as epithelia slough off the villi surface into the lumen.
Chronic exposure to food and microbial antigens necessitates a robust immune presence at the intestinal interface. Intraepithelial lymphocytes (IELs) coordinate responses with epithelial cells to ward off luminal pathogens [16]. Specialized epithelial M-cells and goblet cells surveil the barrier by sampling and transcytosing microbial and food antigens from the lumen to dendritic cells (DCs) [17]. Epithelial-dwelling and lamina propria DCs also directly monitor luminal contents [18] **. The lamina propria houses innate-like lymphocytes including ILCs and γδ T cells as well as helper T cells (Th) and Tregs that supply growth factors and cytokines [19]. Lamina propria macrophages, eosinophils and other innate immune cells also have direct and circuitous effects on epithelial function. Similar to the skin, perturbations to homeostasis (inflammation, injury, infection, malnutrition) dynamically modulate the intestinal immune apparatus and enhance immune-epithelial interactions. We discuss the cell and organ-specific underpinnings of this crosstalk below.
ESPCs-immune communication underlies long-term tissue adaptation
Epithelial stem and progenitor cells (ESPCs) sustain the skin and gut epithelium throughout our lifetime. By taking cues from their microenvironment or “niche”, these long-live cells adapt to oscillating demands of the tissue [2]. Omics technologies have been instrumental in unbiasedly defining the hematopoietic stem cell niche [20]. By contrast, ESPC niche components are incompletely understood, particularly in disease states. Nevertheless, targeted functional and imaging studies have revealed that immune cells are bone-fide members of the ESPC niches and contextually drive ESPC quiescence, expansion, differentiation, and memory (Figure 2A,C) [2].
Figure 2. Mechanisms of immune-epithelial crosstalk.
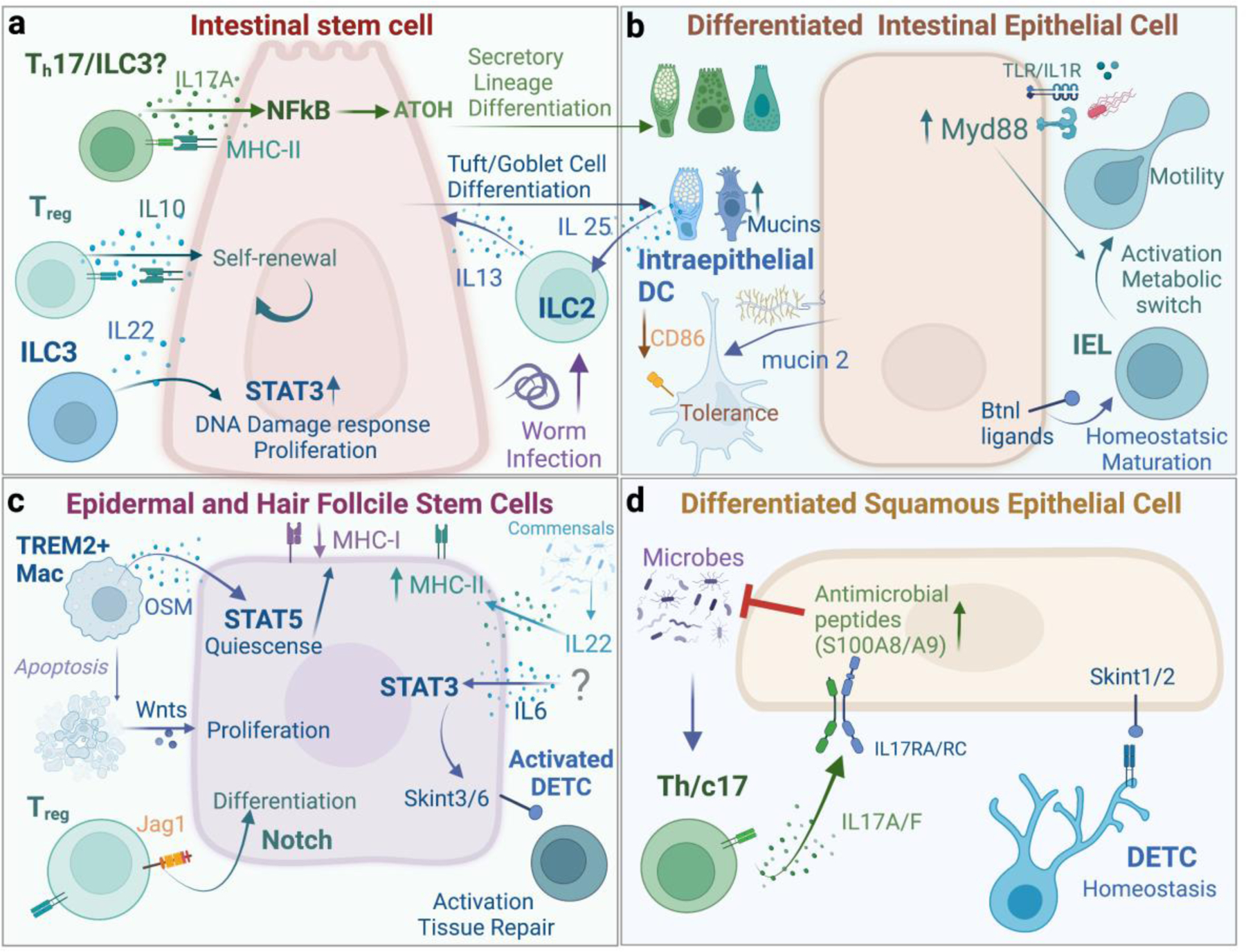
The crosstalk with immune niche with a. Intestinal stem cells, b. Intestinal differentiated epithelial cells, c. Epidermal and Hair follicle stem cells, and d. Differentiated squamous epithelial cells.
Immune cells direct ESPC quiescence and activation.
Immune signals control HFSC quiescence and activation. TREM2+ macrophages accumulate around the resting hair follicle bulge[21]. These perifollicular macrophages secrete Oncostatin M (OSM) which sustains HFSC quiescence via STAT5 activation. TREM2+ macrophages and OSM expression gradually decline as telogen progress, presumably to facilitate anagen entry. Indeed, in late telogen, perifollicular macrophages undergo apoptosis and secrete Wnts to activate HFSCs and induce hair growth[22]. Intestinal macrophages are dispersed throughout the lamina propria from crypt to villus. Global macrophage depletion by CSF1R antibody treatment impairs ISC proliferation, and Paneth and M-cell differentiation[23]. If macrophages directly impact ISCs or do so circuitously via Paneth cells, which are known to supply remains to be seen. Additionally, if certain subsets are uniquely equipped to engage ISCs, similar to TREM2+ skin macrophages that activate HFSCs requires elucidation.
HFSCs can be coaxed into activation by hair depilation. Leveraging this model, Ali et al uncovered a role for perifollicular Tregs in HFSCs activation and HF cycling via the notch ligand Jag-1 [24]. Interestingly, inhibiting JAK signaling promotes anagen independently of Tregs suggesting a divergent homeostatic and post-depilation Tregs repair responses[21]. Under duress HFs are capable of sending distress signals to recruit macrophages and Tregs [25,26]. If and how perifollicular macrophage populations are instructed by the follicle or the surrounding mesenchyme during the homeostatic hair cycle warrants further investigation.
The transcription factor STAT3 has emerged as a particularly powerful activator of ISCs and can entirely substitute for classical mitogens (Wnts) to drive ISC expansion [27]. This signaling axis is induced by many inflammatory cytokines emanating from distinct cellular sources. For instance, ILC3 derived interleukin (IL)-22 can induce ISCs activation via STAT3 independently of Paneth and other niche cells [27]. IL-22-STAT3 signaling also augments the DNA damage response to protect ISCs from genotoxic stress [28]. Upon wounding IL-6 potently activates STAT3 in wound edge epithelial progenitors to express a suite of immune activating Skint molecules [29]. The age-related loss of IL-6-STAT3-Skint axis significantly impairs wound repair but can be revered by supplying recombinant IL-6. Similarly, the ectopic expression of a constitutively active IL-6 receptor, gp130, in the gut epithelium induces ISC expansion and protects from intestinal erosion [30].
ESPC-immune interactions at the crossroads of stem cell fate and plasticity
Immune derived signals fine tune stem cell output and drive distinct fate choices. A recent study by Lin and colleagues unveiled that IL-17A signaling in ISCs induces Atoh gene expression via the transcription factor NF-κB and drives differentiation of secretory lineages [31]. Loss of IL17RA on ISCs results in a profound loss of Paneth, enteroendocrine, and goblet cells. Following worm infection, lamina propria ILC2s secrete IL-13 which induces differentiation of tuft and goblet cells. Consequently, goblet cells augment mucus production to enable worm clearance and tuft cells produce IL-25 to propagate the intestinal immune response in a feed-forward circuit [32].
Consistent with signal driven ISC fate choice, intestinal organoids stimulated with cytokines that typify distinct types of immune response (Th1, Th2, and Th17) resulted in unique cellular outputs and differentiation trajectories. Interestingly, the anti-inflammatory cytokine IL-10 retained organoid ISCs self-renewal, underscoring the importance of immune dampening factors in preserving ISCs [33]. Many of these cytokines are expressed simultaneously, raising the question of how these diverse signals are parsed out and enacted in vivo.
Immune cells can also direct fate switching between different subsets of stem cells. Wounding draws HFSCs out of their niche to help repair the interfollicular epidermal barrier by converting to EpSCs[34]. Interestingly, Tregs facilitate the fate switch between follicle and EpSCs by curbing exuberant inflammatory IL-17A responses [35]. Crypt resident +4 cells exhibit similar plasticity and function as ISCs by migrating downward to the crypt base. If immune signals similarly facilitate plasticity between stem cell populations in the intestine has not been explored.
Following damage, or during stages of physiological activation, stem cells both draw in lymphocytes to their vicinity by expressing chemokines and modulate lymphocyte-mediated immune surveillance by upregulate expression of antigen presenting molecules such as Major histocompatibility complex I (MHCI) and II (MHCII) [25,36]. A number of recent studies have implicated microbial driven-IL-22 and dietary metabolites in controlling expression of antigen-presentation machinery in stem cells, loss of which predisposes these cells to tumorigenesis [33,37]. Intriguingly, expression of MHCII is tightly-linked to cell proliferation, suggesting that stem cells have forged an evolutionary alliance with their immune brethren which is essential for monitoring their proliferative activity and limiting tissue overgrowth [36].
ESPCs remember inflammation
One of the most unexpected features of tissue stem cell longevity is their recently discovered capacity to learn from their stress encounters [38]. Following skin inflammation, we found that EpSCs encode a memory of this encounter by maintaining accessibility at key inflammatory loci. This enables their rapid responses to subsequent stressors. Deepening this picture, Larsen et al. beautifully unearthed the molecular mechanism responsible for encoding EpSCs inflammatory memory [39]**. Once again, STAT3 emerged as a central player inducing chromatin accessibility at stress response genes in EpSCs. STAT3 recruits FOS-JUN to these loci during active inflammation. Upon resolution, JUN and other homeostatic TFs remain in memory loci and facilitate rapid secondary responses. Retaining a memory of inflammation could be a double-edged sword. On the one hand it enables rapid repair, but on the other hand epithelial memory has also been shown to predispose tissues to cancer or recurrent inflammatory pathology [40,41].
Elegant studies by Lim et al. demonstrated that exposure to inflammatory mediators in utero can entrain developing fetal epithelial progenitors with lasting consequences for the off-springs [42] **. Maternal Yersinia pseudotuberculosis infection results in systemic release of IL-6 that crosses the placental barrier and induce inflammatory memory in off-spring ISCs. Functionally, ISC inflammatory memory protected off-springs from Salmonella infection but also rendered them more vulnerable to inflammatory colitis. These fascinating and early glimpses suggest that maternal inflammatory environment sensitize off-spring and may underlie childhood inflammatory diseases such as atopic dermatitis and allergy. Yet, many questions remain. Are certain windows of lifespan more sensitive to imprinting ESPCs with memory than others? Examining memory at the book ends of life, during development and aging may reveal novel means of manipulation ESPCs to increase tissue fitness.
Differentiated progeny crosstalk with immunity
Terminally differentiated epithelial cells provide key selection and survival signals to their immune brethren (Figure 2B, D). Murine enterocytes, for instance, promote the maturation and differentiation of the Vγ7+ subset of IELs via Butyrophilin-like 1[43]. Human intestinal epithelial BTNL3 and 8 regulate Vγ4+ IELs, underscoring an evolutionary conserved role for Butyrophilin family ligands in regulating epithelial-IEL crosstalk[44]. Intestinal epithelia also drive anti-inflammatory or tolerogenic immune responses to limit aberrant responses to food and commensal antigens[18]. Goblet cell derived mucin, for instance, conditions intraepithelial DCs to dampen immune activating receptor CD86 and adopt a tolerogenic phenotype. How inflammation-induced expansion or loss of goblet cells may re-wire the DC sentinel network remains to be seen.
Just as epithelia control healthy immune function, so to do immune-derived signals govern the nutrient sensing and uptake function of absorptive epithelia. Sullivan et. al uncovered this unexpected role of intestinal ᵞᵟ T Cells in modulating epithelial expression of enzymes and transporters that mediate digestion and absorption of carbohydrates [45]**. Similarly, in healthy skin, commensal colonization augments expression of the epithelial anti-microbial peptides s100A8/9 [46]. However, this adaptive response is not a result of autonomous microbial sensing by epithelia, but requires the IL-17A from commensal specific Tc17 cells.
Injury, inflammation, pathogenic, or noxious stimuli induce dynamic changes in immune-epithelial interactions with profound consequences for barrier fitness and function. In healthy skin DETCs localize to suprabasal epidermal layers. They form dendritic synapses via their T cell receptor (TCR) at squamous epithelial tight junctions [47]. Under duress, DETCs reorient their junctions to the basal epidermis, suggesting that steady state normalcy sensing relies on structural features of the barrier. Localized between enterocytes and the underlying basement membrane of the upper villus structure, IELs also alter their behavior and epithelial engagement swiftly under duress [7]. Sophisticated intravital imaging studies by van Konijnenburg and colleagues revealed that IELs constitutively scan the basolateral epithelial surface at steady state. However, following Salmonella thyphimurium infection IELs intercalate between enterocytes, scanning for the pathogens to mount anti-microbial responses. This “flossing-like” IEL behavior is instigated by adjacent My88- activated epithelial cells and results in the glycolytic rewiring of IELs. There is a broad appreciation for IELs in mounting epithelial repair and anti-pathogen responses by supplying growth and anti-microbial factors and recruiting accessory immune cells [48,49]. However, the precise contribution of IEL behavioral changes, for instance “flossing” or junctional reorientation, to epithelial cell function requires clarification.
Type 17 immune responses are essential for pathogen protection, but when unchecked causes devastating autoimmune diseases[50]. For instance, Psoriasis is driven by IL-17 and accordingly and-IL-17 therapies have been highly effective in treating moderate-to-severe disease [51]. IL-17RC is dominantly expressed by differentiated suprabasal epithelium in the skin [52]. A massive single cell transcriptomics survey of inflammatory skin diseases revealed a concomitant enrichment differentiated epithelia and inflammatory transcripts associated with IL-17 signaling, suggesting that differentiated suprabasal not basal epithelial subsets are the primary disease target of IL-17 in the skin. Indeed, Aggor et al. demonstrated a direct engagement of squamous epithelia by IL-17 following oral infection with Candida albicans [53]. By contrast, in the intestine, IL-17RA/RC expressed by ISCs are essential for homeostatic differentiation of epithelial subsets [31]. Perhaps one reason IL-17 blocking therapies have been successful in psoriasis, but failed in inflammatory bowel disease is because of their divergent functions in stem cells in the intestine and differentiated squamous epithelium. Further understanding the unique cell and tissue specific actions of immune mediators will help fine tune reparative therapies and mitigate inflammatory disease.
Perspectives and therapeutic implications
An evolutionary alliance between epithelial and immune cells underlies skin and intestinal barrier responses. As tissue immunologists and epithelial biologists unravel the mechanistic underpinnings of these conversations, they reveal a myriad of therapeutic targets to manipulate barrier function and regeneration. Epithelial organoids or tissue “avatars” are powerful tools to actualize these inquiries in human tissues [54]. Indeed, a number of groups have now use organoids cultured in the presence of immune cells or their ligands to define mechanisms of communication. Additionally, organoid-based studies have also yielded insights into microbial and metabolic influences and how they dynamically shift immune-epithelial crosstalk.
One critical facet to consider is the specific preservation of fitness boosting aspects of this communication and curb any exuberant inflammatory reactions. Balancing the tight rope between heightening barrier function, while avoiding autoimmunity or cancer will require a more precise understanding of signaling events downstream of immune mediators and their induced programs. For instance, IL-22 induces both regenerative STAT3 signaling and inflammatory STAT1 signaling. Saxton and colleagues cleverly designed partial IL-22 agonists that induce STAT3 to promote ISC regeneration, but do not induce pro-inflammatory STAT1 [55]**. Synthetic biology approaches, such as these, will unleash the potential of immunomodulation to direct epithelial responses in chronic non-healing conditions such as skin ulcers or inflammatory diseases such as psoriasis and inflammatory bowel disease.
Acknowledgments
We apologize to our friends and colleagues whose work we could not include due to space constraints. Figures were generated with Biorender. S. N. is a NYSCF Robertson Stem Cell Investigator and is funded by grants from National Institutes of Health (1DP2AR079173–01, R01-AI168462), the Pew Foundation (00034119), and the Packard Foundation. D.R. is an International Human Frontier Science Program (HFSP) postdoctoral fellow, LT000839/2021-L.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
SN is on the SAB of Seed Inc and consults for BiomX. SN has received funding from Takeda.
Disclosures
SN is on the SAB of Seed Inc., is a consultant for BiomX, and receives research funding from Takeda Pharmaceuticals.
References
- 1.Chovatiya R, Medzhitov R: Stress, inflammation, and defense of homeostasis. Mol Cell 2014, 54:281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xing Y, Naik S: Under pressure: Stem cell-niche interactions coordinate tissue adaptation to inflammation. Curr Opin Cell Biol 2020, 67:64–70. [DOI] [PubMed] [Google Scholar]
- 3.Naik S, Larsen SB, Cowley CJ, Fuchs E: Two to Tango: Dialog between Immunity and Stem Cells in Health and Disease. Cell 2018, 175:908–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bukhari S, Mertz AF, Naik S: Eavesdropping on the conversation between immune cells and the skin epithelium. Int Immunol 2019, 31:415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan X, Rudensky AY: Hallmarks of Tissue-Resident Lymphocytes. Cell 2016, 164:1198–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kobayashi T, Naik S, Nagao K: Choreographing Immunity in the Skin Epithelial Barrier. Immunity 2019, 50:552–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoytema van Konijnenburg DP, Reis BS, Pedicord VA, Farache J, Victora GD, Mucida D: Intestinal Epithelial and Intraepithelial T Cell Crosstalk Mediates a Dynamic Response to Infection. Cell 2017, 171:783–794 e713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zomer HD, Trentin AG: Skin wound healing in humans and mice: Challenges in translational research. J Dermatol Sci 2018, 90:3–12. [DOI] [PubMed] [Google Scholar]
- 9.Thi Loan Anh Nguyen SV-S, Adrian Liston , Jeroen Raes How informative is the mouse for human gut microbiota research? Disease Modeling and Mechanisms 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schepeler T, Page ME, Jensen KB: Heterogeneity and plasticity of epidermal stem cells. Development 2014, 141:2559–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morita R, Sanzen N, Sasaki H, Hayashi T, Umeda M, Yoshimura M, Yamamoto T, Shibata T, Abe T, Kiyonari H, et al. : Tracing the origin of hair follicle stem cells. Nature 2021, 594:547–552. [DOI] [PubMed] [Google Scholar]
- 12.Quiroz FG, Fiore VF, Levorse J, Polak L, Wong E, Pasolli HA, Fuchs E: Liquid-liquid phase separation drives skin barrier formation. Science 2020, 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sumigray KD, Lechler T: Cell adhesion in epidermal development and barrier formation. Curr Top Dev Biol 2015, 112:383–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gehart H, Clevers H: Tales from the crypt: new insights into intestinal stem cells. Nat Rev Gastroenterol Hepatol 2019, 16:19–34. [DOI] [PubMed] [Google Scholar]
- 15.Takeda N, Jain R, LeBoeuf MR, Wang Q, Lu MM, Epstein JA: Interconversion between intestinal stem cell populations in distinct niches. Science 2011, 334:1420–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheroutre H, Lambolez F, Mucida D: The light and dark sides of intestinal intraepithelial lymphocytes. Nat Rev Immunol 2011, 11:445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allaire JM, Crowley SM, Law HT, Chang SY, Ko HJ, Vallance BA: The Intestinal Epithelium: Central Coordinator of Mucosal Immunity: (Trends in Immunology 39, 677–696, 2018). Trends Immunol 2019, 40:174. [DOI] [PubMed] [Google Scholar]
- 18. Rivera CA, Randrian V, Richer W, Gerber-Ferder Y, Delgado MG, Chikina AS, Frede A, Sorini C, Maurin M, Kammoun-Chaari H, et al. : Epithelial colonization by gut dendritic cells promotes their functional diversification. Immunity 2022, 55:129–144 e128. **This very interesting study demostrates that food and differentiated epithelial derived signals orchestrate the epithelial colonization and reprograming of cDC2 cells to adopt a tolerogenic pheotype.
- 19.Mowat AM, Agace WW: Regional specialization within the intestinal immune system. Nat Rev Immunol 2014, 14:667–685. [DOI] [PubMed] [Google Scholar]
- 20.Tikhonova AN, Dolgalev I, Hu H, Sivaraj KK, Hoxha E, Cuesta-Dominguez A, Pinho S, Akhmetzyanova I, Gao J, Witkowski M, et al. : The bone marrow microenvironment at single-cell resolution. Nature 2019, 569:222-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang ECE, Dai Z, Ferrante AW, Drake CG, Christiano AM: A Subset of TREM2(+) Dermal Macrophages Secretes Oncostatin M to Maintain Hair Follicle Stem Cell Quiescence and Inhibit Hair Growth. Cell Stem Cell 2019, 24:654–669 e656. [DOI] [PubMed] [Google Scholar]
- 22.Castellana D, Paus R, Perez-Moreno M: Macrophages contribute to the cyclic activation of adult hair follicle stem cells. PLoS Biol 2014, 12:e1002002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sehgal A, Donaldson DS, Pridans C, Sauter KA, Hume DA, Mabbott NA: The role of CSF1R-dependent macrophages in control of the intestinal stem-cell niche. Nat Commun 2018, 9:1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ali N, Zirak B, Rodriguez RS, Pauli ML, Truong HA, Lai K, Ahn R, Corbin K, Lowe MM, Scharschmidt TC, et al. : Regulatory T Cells in Skin Facilitate Epithelial Stem Cell Differentiation. Cell 2017, 169:1119–1129 e1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lay K, Yuan S, Gur-Cohen S, Miao Y, Han T, Naik S, Pasolli HA, Larsen SB, Fuchs E: Stem cells repurpose proliferation to contain a breach in their niche barrier. Elife 2018, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen CC, Wang L, Plikus MV, Jiang TX, Murray PJ, Ramos R, Guerrero-Juarez CF, Hughes MW, Lee OK, Shi S, et al. : Organ-level quorum sensing directs regeneration in hair stem cell populations. Cell 2015, 161:277–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindemans CA, Calafiore M, Mertelsmann AM, O’Connor MH, Dudakov JA, Jenq RR, Velardi E, Young LF, Smith OM, Lawrence G, et al. : Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature 2015, 528:560–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gronke K, Hernandez PP, Zimmermann J, Klose CSN, Kofoed-Branzk M, Guendel F, Witkowski M, Tizian C, Amann L, Schumacher F, et al. : Interleukin-22 protects intestinal stem cells against genotoxic stress. Nature 2019, 566:249–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keyes BE, Liu SQ, Asare A, Naik S, Levorse J, Polak L, Lu CP, Nikolova M, Pasolli HA, Fuchs E: Impaired Epidermal to Dendritic T Cell Signaling Slows Wound Repair in Aged Skin. Cell 2016, 167:1323-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taniguchi K, Wu LW, Grivennikov SI, de Jong PR, Lian I, Yu FX, Wang K, Ho SB, Boland BS, Chang JT, et al. : A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature 2015, 519:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin X, Gaudino SJ, Jang KK, Bahadur T, Singh A, Banerjee A, Beaupre M, Chu T, Wong HT, Kim C-K, et al. : IL-17RA-signaling in Lgr5+ intestinal stem cells induces expression of transcription factor ATOH1 to promote secretory cell lineage commitment. Immunity [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.von Moltke J, Ji M, Liang HE, Locksley RM: Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature 2016, 529:221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biton M, Haber AL, Rogel N, Burgin G, Beyaz S, Schnell A, Ashenberg O, Su CW, Smillie C, Shekhar K, et al. : T Helper Cell Cytokines Modulate Intestinal Stem Cell Renewal and Differentiation. Cell 2018, 175:1307–1320 e1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ge Y, Gomez NC, Adam RC, Nikolova M, Yang H, Verma A, Lu CP, Polak L, Yuan S, Elemento O, et al. : Stem Cell Lineage Infidelity Drives Wound Repair and Cancer. Cell 2017, 169:636–650 e614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mathur AN, Zirak B, Boothby IC, Tan M, Cohen JN, Mauro TM, Mehta P, Lowe MM, Abbas AK, Ali N, et al. : Treg-Cell Control of a CXCL5-IL-17 Inflammatory Axis Promotes Hair-Follicle-Stem-Cell Differentiation During Skin-Barrier Repair. Immunity 2019, 50:655–667 e654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agudo J, Park ES, Rose SA, Alibo E, Sweeney R, Dhainaut M, Kobayashi KS, Sachidanandam R, Baccarini A, Merad M, et al. : Quiescent Tissue Stem Cells Evade Immune Surveillance. Immunity 2018, 48:271–285 e275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tamoutounour S, Han SJ, Deckers J, Constantinides MG, Hurabielle C, Harrison OJ, Bouladoux N, Linehan JL, Link VM, Vujkovic-Cvijin I, et al. : Keratinocyte-intrinsic MHCII expression controls microbiota-induced Th1 cell responses. Proc Natl Acad Sci U S A 2019, 116:23643–23652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naik S, Larsen SB, Gomez NC, Alaverdyan K, Sendoel A, Yuan S, Polak L, Kulukian A, Chai S, Fuchs E: Inflammatory memory sensitizes skin epithelial stem cells to tissue damage. Nature 2017, 550:475–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Larsen SB, Cowley CJ, Sajjath SM, Barrows D, Yang Y, Carroll TS, Fuchs E: Establishment, maintenance, and recall of inflammatory memory. Cell Stem Cell 2021. **This very interesting study comprehensively decodes the mechanisms by which EPSCs encode inflammatory memory.
- 40.Ordovas-Montanes J, Dwyer DF, Nyquist SK, Buchheit KM, Vukovic M, Deb C, Wadsworth MH 2nd, Hughes TK, Kazer SW, Yoshimoto E, et al. : Allergic inflammatory memory in human respiratory epithelial progenitor cells. Nature 2018, 560:649–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Del Poggetto E, Ho IL, Balestrieri C, Yen EY, Zhang S, Citron F, Shah R, Corti D, Diaferia GR, Li CY, et al. : Epithelial memory of inflammation limits tissue damage while promoting pancreatic tumorigenesis. Science 2021, 373:eabj0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lim AI, McFadden T, Link VM, Han SJ, Karlsson RM, Stacy A, Farley TK, Lima-Junior DS, Harrison OJ, Desai JV, et al. : Prenatal maternal infection promotes tissue-specific immunity and inflammation in offspring. Science 2021, 373. **This very interesting study is the first to uncover that maternal exposure to a pathogen induces inflammatory memory in off-springs ISCs. Inflammatory training protects offpsring from future oral bacterial infection, but renders them more vulnerable to inflammatory pathology
- 43.Di Marco Barros R, Roberts NA, Dart RJ, Vantourout P, Jandke A, Nussbaumer O, Deban L, Cipolat S, Hart R, Iannitto ML, et al. : Epithelia Use Butyrophilin-like Molecules to Shape Organ-Specific gammadelta T Cell Compartments. Cell 2016, 167:203–218 e217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Willcox CR, Vantourout P, Salim M, Zlatareva I, Melandri D, Zanardo L, George R, Kjaer S, Jeeves M, Mohammed F, et al. : Butyrophilin-like 3 Directly Binds a Human Vgamma4(+) T Cell Receptor Using a Modality Distinct from Clonally-Restricted Antigen. Immunity 2019, 51:813–825 e814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sullivan ZA, Khoury-Hanold W, Lim J, Smillie C, Biton M, Reis BS, Zwick RK, Pope SD, Israni-Winger K, Parsa R, et al. : gammadelta T cells regulate the intestinal response to nutrient sensing. Science 2021, 371. **This very interesting study is the first to uncover that nutrient sensing by γδ T cells triggers epithelial immune crosstalk that regulates tissue transcriptional adaptation to dietary changes.
- 46.Naik S, Bouladoux N, Linehan JL, Han SJ, Harrison OJ, Wilhelm C, Conlan S, Himmelfarb S, Byrd AL, Deming C, et al. : Commensal-dendritic-cell interaction specifies a unique protective skin immune signature. Nature 2015, 520:104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chodaczek G, Papanna V, Zal MA, Zal T: Body-barrier surveillance by epidermal gammadelta TCRs. Nat Immunol 2012, 13:272–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.MacLeod AS, Hemmers S, Garijo O, Chabod M, Mowen K, Witherden DA, Havran WL: Dendritic epidermal T cells regulate skin antimicrobial barrier function. J Clin Invest 2013, 123:4364–4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jameson J, Ugarte K, Chen N, Yachi P, Fuchs E, Boismenu R, Havran WL: A role for skin gammadelta T cells in wound repair. Science 2002, 296:747–749. [DOI] [PubMed] [Google Scholar]
- 50.McGeachy MJ, Cua DJ, Gaffen SL: The IL-17 Family of Cytokines in Health and Disease. Immunity 2019, 50:892–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ghoreschi K, Balato A, Enerback C, Sabat R: Therapeutics targeting the IL-23 and IL-17 pathway in psoriasis. Lancet 2021, 397:754–766. [DOI] [PubMed] [Google Scholar]
- 52.Hughes MHWI Travis K, Gierahn Todd M, Do Tran, Weiss David, Andrade Priscilla R., Ma Feiyang, de Andrade Silva Bruno J., Shao Shuai, Tsoi Lam C, Ordovas-Montanes Jose, Gudjonsson Johann E, Modlin Robert L, Love J Christopher, Shalek Alex K: Highly Efficient, Massively-Parallel Single-Cell RNA-Seq Reveals Cellular States and Molecular Features of Human Skin Pathology. bioRxiv 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aggor FEY, Break TJ, Trevejo-Nunez G, Whibley N, Coleman BM, Bailey RD, Kaplan DH, Naglik JR, Shan W, Shetty AC, et al. : Oral epithelial IL-22/STAT3 signaling licenses IL-17-mediated immunity to oral mucosal candidiasis. Sci Immunol 2020, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kretzschmar K, Clevers H: Organoids: Modeling Development and the Stem Cell Niche in a Dish. Dev Cell 2016, 38:590–600. [DOI] [PubMed] [Google Scholar]
- 55. Saxton RA, Henneberg LT, Calafiore M, Su L, Jude KM, Hanash AM, Garcia KC: The tissue protective functions of interleukin-22 can be decoupled from pro-inflammatory actions through structure-based design. Immunity 2021, 54:660–672 e669. **This very interesting study uses structure-based design to provide insights into cytokine pleiotropy and the synthetic design of IL-22 agonists that decouple inflammatory signaling from regenerative activation of the IL-22 receptor


