Abstract
Objective: Wound dressings that create and maintain a moist environment provide the optimal conditions for wound healing by increasing the rate of epithelialization and angiogenesis. However, current wound dressings require periodic removal which exposes the wound to the surrounding environment, thereby increasing the likelihood for infection and drying out the wound itself. There remains an unmet medical need for the development of an absorbent, flexible, and transparent wound dressing that can conform to the irregular geometry of the wound for a long-term duration. Herein, we report the development of AFTIDerm, an Absorbent, Flexible, Transparent, and Inexpensive moisture-management wound dressing using Polyvinyl alcohol (PVA) as the host material. Methods: AFTIDerm substrates of varying glycerol concentrations (1 wt%, 3 wt%, 5 wt%, 7 wt%, and 10 wt%) were fabricated and tested. The mechanical, absorption, and biological properties of AFTIDerm were evaluated. Results: We found that 5% glycerol served as the optimal concentration for AFTIDerm. The biocompatibility, absorptive capabilities, and scalability render PVA/glycerol an ideal material composition for wound dressings. Benchtop experimentation and pre-clinical testing demonstrate AFTIDerm as a platform for use in wound dressings. Discussion/Conclusion: The development of AFTIDerm broadens the translational utility of this materials platform not only as a material for wound dressings to minimize dressing changes in low to moderate exudate environments, but also as a potential substrate material for smart bandages. Clinical and Translational Impact Statement— AFTIDerm, an absorbent, flexible, and transparent wound dressing, maintains the moist environment required for healing while enabling monitoring of healing without removal and disruption to the wound bed.
Keywords: Body fluid management, flexible substrates, polyvinyl alcohol, translational research, wound dressings
I. Introduction
Wounds vary in exudate level, depth, cleanliness, and infection and inflammation levels. A critical design factor for any wound dressing is that it protects the wound from the external environment, serving as a barrier against external bacteria as well as protection against further mechanical trauma. Bandage substrate materials should be hypoallergenic and non-toxic to ensure that the wound dressing is suitable for people with sensitive and/or delicate skin [1], [2]. In addition, clinical studies have established that a moist wound bed is essential for effective wound healing [3], [4]. The wound should be kept moist to optimize the healing process and minimize pain. However, a wound that is soaked with exudate is detrimental to wound healing [5]. Bandage changes should be minimized to avoid disruption of the delicate granulating wound bed. Furthermore, proper wound oxygenation is vital to promote healthy granulation tissue growth [6], [7]. Thus, a balance between absorption and vapor permeability is crucial for an effective dressing.
Currently there are different wound dressings for wounds of various exudate levels, depths, cleanliness, and infection and inflammation levels [8] (Table 1).
TABLE 1. Comparative Analysis of AFTIDerm Versus Standard of Care Wound Dressings.
| Wound Dressing | Advantages | Disadvantages |
|---|---|---|
| Gauze |
|
|
| Low adherent dressings |
|
|
| Semipermeable films |
|
|
| Hydrocolloids |
|
|
| Hydrogels |
|
|
| Alginates |
|
|
| Foam dressings |
|
|
| AFTIDerm |
|
|
Gauze is one of the most widely used surgical dressings [8], [9]. Gauze is highly permeable and non-occlusive, and can be used on infected and noninfected wounds [8], [9]. Gauze dressings require frequent replacement and adhere to the wound bed, thus removal causes severe detriment to the delicate wound bed and inhibits wound healing [8], [9]. Low adherent dressings allow exudate to pass through into a secondary dressing while maintaining a moist wound bed [10]. They are useful for patients with sensitive or fragile skin because they are designed to reduce adherence at the wound bed. Semipermeable films, hydrocolloids, hydrogels, alginates, and foam dressings are all classified as polymeric wound dressings [11]. Semipermeable films (e.g. HP Tegaderm™) are flexible and transparent wound coverings that are impermeable to fluids and bacteria, but are permeable to air and water vapor [8]. This makes them unable to manage large amounts of exudate. Hydrocolloids are foam sheets that form an occlusive and adhesive dressing that promotes a moist wound environment [8], [12]. They are almost entirely impermeable to water vapor and air which allows patients to bathe without risk of contaminating the wound [8]. Wounds that necessitate regular inspections should use hydrocolloid dressings with caution. Hydrogels can provide or absorb fluids to maintain a moist wound bed. Due to the partial hydration, they are not well suited for wounds with high amounts of exudate. Alginates are ideal for wounds with high levels of exudate as they can absorb 15 to 20 times their weight in fluid; however, use on wounds with little to no exudate risks adhesion to the wound surface causing pain and damage to the wound area [8]. Foam dressings are able to provide thermal insulation to the wound area while also transmitting vapor and oxygen [8]. They are highly absorbent and facilitate uniform dispersion of exudate throughout the absorbent layer and prevent exterior leakage (strike-through) due to the presence of a semipermeable backing [8].
Next generation wound dressings should provide an occlusive wound bed, absorb exudate, and be conformable to the irregular topography of the stratum corneum, while also being transparent to allow for easy inspection by clinicians without removal of the bandage. Additionally, these wound dressings should be cost efficient and avoid the use of relatively expensive doping agents such as silver nanoparticles [13], dextran [14] and/or chitosan [11]. The biocompatibility, ease of fabrication, cost, absorbing capabilities, and scalability render PVA a promising material for use in a wide array of applications including but limited to scaffold materials [15], doped hydrogels [16] for pharmaceutical applications [17], sacrificial layer in fabricating epidermal electronics [18] and as a stand-alone or composite material for wound dressings [11], [19]–[21]. There is a paucity of commercial controls utilized within studies of PVA based wound dressings to provide clinical relevance [13], [19], [22], [23]. Elucidating the mechanical and absorption properties of PVA based on varied glycerol concentrations for the fabrication of a wound dressing has not previously been studied. The biological stability of these materials and composites over a clinically relevant timeframe has not been studied on compromised skin conditions, such as ischemic wounds.
Towards addressing these technical and clinical gaps, we developed AFTIDerm, an absorbent, flexible, and transparent substrate material made with Polyvinyl Alcohol (PVA) and glycerol. The mechanical, absorption, thermal, and biological properties of this material were evaluated in benchtop and pre-clinical testing. Substrates with varied concentrations of glycerol were prepared. The resulting substrates were flexible, transparent, and absorbent and demonstrate applicability for use as wound dressings over a long-term duration (e.g. 7 days).
II. Methods and Procedures
A. Materials
PVA and glycerol were purchased from Sigma Aldrich (St. Louis, MO, USA) and used as received. Polypropylene petri dishes (Fischer Scientific #FB0875712, diameter 100 mm) were purchased and served to as the platform to cure the AFTIDerm. A commercially available medical-grade silicone acrylate adhesive (2477P, 3M Inc.) adhered the AFTIDerm to the pig skin.
B. AFTIDerm Fabrication
Each AFTIDerm sample regardless of glycerol concentration was fabricated in the same manner (Figure 1). PVA was dissolved in 90°C water under vigorous stirring. Glycerol of various percentages (1, 3, 5, 7, and 10%) was added (masses relative to that of the water). All samples, regardless of glycerol concentration, resulted in a homogenous thickness of
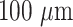 and were peeled from the dish and used for testing (Fig. 1).
and were peeled from the dish and used for testing (Fig. 1).
FIGURE 1.
AFTIDerm fabrication schematic.
C. Contact Angle
Contact angle measurements were made by placing
 of deionized water on the surface of each of the PVA composites of varied glycerol. For each sample, three samples were studied and 3 measurements for each sample were taken for statistical representation.
of deionized water on the surface of each of the PVA composites of varied glycerol. For each sample, three samples were studied and 3 measurements for each sample were taken for statistical representation.
D. Mechanical Testing
Standard uniaxial mechanical testing was conducted on samples fabricated into a uniform rectangular shape and mounted to a custom-built Uniaxial Tensile Tester. One side of each sample was mounted to a fixed stage which was connected to a commercialized force sensor (DPM-3, Transducer Techniques) to monitor applied force. The opposite side of each sample was mounted to a screw-driven movable stage which has a stepper motor that was controlled by a LabVIEW program. The apparatus measured the applied force as the sample was elongated along its principal axis. Force measurements were made in increments of
 at a frequency of 5 Hz, up to 20% applied strain. The instrument generated a force versus displacement curve for each test, and from this information, a stress versus strain curve was generated. Fitted line slopes of stress-strain curves were plotted to derive the Young’s Moduli. In order to evaluate the mechanical stability of the AFTIDerm samples under periodic loading, a cyclic tensile test was performed on AFTIDerm samples of varied glycerol percentages. This test structure was subjected to cyclic loading over a strain range of 0 to 2.5% at 5 Hz. The test was performed using the previously described tensile tester for 50 identical cycles. Trung et al. reviewed the applications of stretchable physical sensors for human performance and concluded that the required stretchability limit was < 2% and < 20% for applications on the face and hands, respectively [24]. Thus, the range of 0-2.5% strain was carried out for our benchtop testing.
at a frequency of 5 Hz, up to 20% applied strain. The instrument generated a force versus displacement curve for each test, and from this information, a stress versus strain curve was generated. Fitted line slopes of stress-strain curves were plotted to derive the Young’s Moduli. In order to evaluate the mechanical stability of the AFTIDerm samples under periodic loading, a cyclic tensile test was performed on AFTIDerm samples of varied glycerol percentages. This test structure was subjected to cyclic loading over a strain range of 0 to 2.5% at 5 Hz. The test was performed using the previously described tensile tester for 50 identical cycles. Trung et al. reviewed the applications of stretchable physical sensors for human performance and concluded that the required stretchability limit was < 2% and < 20% for applications on the face and hands, respectively [24]. Thus, the range of 0-2.5% strain was carried out for our benchtop testing.
E. Absorption Testing
AFTIDerm samples, Absorbent Tegaderm, HP Tegaderm, Telfa, latter three which served as commercial controls, were immersed in phosphate buffer saline (PBS) solution, pH 7.4 and weighed at pre-set timepoints. Samples were taken out of the solution and weighed at hourly increments for the first 5 hours and 24 hours following for up to one week. Data reported is mean ± std. deviation with six samples run per concentration group.
F. Biological Testing
The biological stability of the AFTIDerm was studied over a one-week period. Changes in pH were quantified as a sharp decrease in pH would present a cytotoxic effect. Samples were placed in PBS and the pH of the supernatant was measured at each time interval. Experiments were run in triplicate and data reported is mean ± std. deviation.
G. Biocompatibility Assessment
AFTIDerm, was evaluated for potential cytotoxic effects using an in vitro mammalian cell culture test. This study was conducted by an independent lab (NAMSA, Northfield, OH) following the guidelines of ISO 10993-5, Biological evaluation of medical devices - Part 5: Tests for in vitro cytotoxicity. A single preparation of the test article was extracted in single strength Minimum Essential Medium (IX MEM) at 37°C for 24 hours. The negative control, reagent control, and positive control were similarly prepared. L-929 mouse fibroblast cells were seeded in 10 cm2 cell culture wells, labeled with passage number and date, and incubated at 37°C in the presence of 5% CO2 to obtain sub-confluent monolayers of cells prior to use. Aseptic procedures were used in the handling of the cell cultures. Triplicate monolayers of the cells were dosed with each extract and incubated at 37°C in the presence of 5% CO2 for 48 hours. Following incubation, the monolayers were examined microscopically (100X) for abnormal cell morphology and cellular degeneration (Table 2). For the test to be valid, the reagent control and the negative control must have had a reactivity of none (grade 0), and the positive control must have been a grade 3 or 4. Percent rounding and percent cells without intracytoplasmic granules are not evaluated in the event of 100% lysis. The test article met the requirements of the test if the biological response was less than or equal to grade 2 (mild). The test would have been repeated if the controls did not perform as anticipated. The color of the test medium was observed to determine any change in pH. A color shift toward yellow would indicate an acidic pH range and a color shift toward magenta to purple would indicate an alkaline pH range.
TABLE 2. Test Scoring Following Microscopic Observation.
| Grade | Reactivity | Conditions of Cell Culture |
|---|---|---|
| 0 | None | Discrete intracytoplasmic granules, no cell lysis, no reduction of cell growth |
| 1 | Slight | Not more than 20% of the cells are round, loosely attached, or show changes in morphology; occasionally lysed cells are present; only slight inhibition observable |
| 2 | Mild | Not more than 50% of the cells are round, devoid of intracytoplasmic granules; no extensive cell lysis; not more than 50% growth inhibition observable |
| 3 | Moderate | Not more than 70% of the cell layers contain rounded cells or are lysed; cell layers not destroyed, but more than 50% growth inhibition observed. |
| 4 | Severe | Nearly complete or complete destruction of the cell layers |
H. Pre-Clinical Evaluation
A porcine infected wound model was used to assess the efficacy of AFTIDerm. One female Yorkshire pig (30–35 kg) was housed prior to surgery in steel cages with a 12- hour light dark cycle. The animal was fed antibiotic-free food and water ad libitum throughout the study, Institutional Animal Care and Use Committee (IACUC) (VA #16-071-SW-16-009 and CWRU #: 2016-0331). The pig was observed for signs of infection or altered health at least 7 days prior to surgery. On the surgery day, the pig was sedated in the cage by intramuscular injection of Telazol, 3–4 mg/kg (Wyeth Pharmaceuticals, Madison, NJ, USA) and was then transferred to the operating suite and an airway was secured with endotracheal intubation. General anesthesia was then induced, and the pig was placed in a prone position so that the entire dorsal region would be accessible for surgery. The back hair was shaved, and six wound sites were marked over the paraspinal region using a prefabricated stencil. The pig’s paraspinal region was then sterilely prepped with chlorhexidine scrub. The areas of skin to be excised were injected subcutaneously with a mixture of 1% lidocaine with 1: 100,000 epinephrine (7cc at each excision site). Bilateral full thickness excisional wounds (6 cm diameter) were created. To create an ischemic wound, a sterile double-flanged silicone block (6cm in diameter and 0.5cm high) was placed into each wound and left in situ for 14 days. Each wound was covered with a Tegaderm™dressing. The animal was wrapped in an elastic bandage (VetRap®3M Health Care, St Paul, MN) to prevent animal interference with the system. The pig was covered with a protective body jacket (Goat Tube®, Sullivan Supplies, Houston, TX) to prevent environmental contamination. The animal was awakened from general anesthesia, given post-operative. Following creation of each wound,
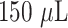 of a freshly cultured 0.5 McFarland solution of a green fluorescent protein labeled P. aeruginosa was evenly applied to each wound bed by pipette. This strain of bacteria was selected for initial testing because it is known to cause both acute and chronic infection due to the formation of stable biofilms within the wound. AFTIDerm was placed over the wound. Dressing changes for these wounds occurred on biopsy timepoint days (BTD) 1, 3, 5, 7, 10, and 14 with AFTIDerm being discarded and replaced anew. The change in mass of the AFTIDerm was monitored at each of these timepoints.
of a freshly cultured 0.5 McFarland solution of a green fluorescent protein labeled P. aeruginosa was evenly applied to each wound bed by pipette. This strain of bacteria was selected for initial testing because it is known to cause both acute and chronic infection due to the formation of stable biofilms within the wound. AFTIDerm was placed over the wound. Dressing changes for these wounds occurred on biopsy timepoint days (BTD) 1, 3, 5, 7, 10, and 14 with AFTIDerm being discarded and replaced anew. The change in mass of the AFTIDerm was monitored at each of these timepoints.
III. Results
The synthesis scheme of AFTIDerm is depicted in Fig. 2a. Song et al. showed that one glycerol molecule can supply three hydroxyl groups; thus, glycerol can act as the cross-linker for PVA chains to improve the strength and toughness of PVA hydrogels [25]. The introduction of glycerol into a PVA hydrogel provides AFTIDerm with thermoplasticity, self-healing, and long-term moisture retention and increase its low-temperature tolerance. Figure 2b plots the water contact angle on the AFTIDerm surface with respect to glycerol concentration, which demonstrated an increase in AFTIDerm hydrophilicity up to 5% glycerol, with an insignificant increase noted at 7% and 10%. Figure 2c shows images of 5% glycerol AFTIDerm sample on the skin under common material mechanics, namely under unstrained, stretched, compressed and torsion state. The images show that the sample was stretchable and remained in good contact to the skin under both compression and torsion.
FIGURE 2.
Fabrication of AFTIDerm and surface property characterization. (a) AFTIDerm synthesis. (b) Water contact angle based on varied glycerol concentrations (1%, 3%, 5%, 7%, and 10%). (c) Images showing AFTIDerm on the skin.
The mechanical and hydrophilicity of AFTIDerm (
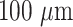 thickness) based on various glycerol percentages was first evaluated to optimize a desired weight percentage of glycerol (Table 3). Figure 3 shows the evaluation of surface and mechanical properties of AFTIDerm as a function of varied glycerol concentration. Young’s Modulus and cyclic stress were found to decrease with increasing glycerol concentration (r2 = 0.70 modeled by an exponential regression for Young’s modulus and r2 = 0.95 modeled by an exponential regression for cyclic stress) (Fig. 3a, b). While outside the scope of this study, we hypothesize that glycerol serves as a plasticizer, not only reducing the degree of crystallinity in the substrates with increased concentrations, but perhaps also lowering the crystalline melting temperatures due to defects introduced into the crystal lattice [26]. This lowered the water resistance of the plasticized PVA substrates due to coupling with the leaching of the glycerol from the substrates when immersed in water during the fabrication process [26]. The influence of glycerol on the properties of PVA substrates is related to the degree of compatibility between the plasticizer and PVA [26]. Thus, an increase in glycerol concentration had an inverse effect on the stiffness of the material. AFTIDerm at a 5% exhibited no hysteresis when subjected to cyclic stress-strain testing (380.7 ± 45.5 kPa). The stress of AFTIDerm at varied glycerol concentrations apart from 5% was also investigated (Fig. 3c). Concentrations below 5% experienced hysteresis under cyclic testing (Fig. 4). Data suggests that glycerol percentages above 5% provide long-term mechanical stability (Fig. 3d).
thickness) based on various glycerol percentages was first evaluated to optimize a desired weight percentage of glycerol (Table 3). Figure 3 shows the evaluation of surface and mechanical properties of AFTIDerm as a function of varied glycerol concentration. Young’s Modulus and cyclic stress were found to decrease with increasing glycerol concentration (r2 = 0.70 modeled by an exponential regression for Young’s modulus and r2 = 0.95 modeled by an exponential regression for cyclic stress) (Fig. 3a, b). While outside the scope of this study, we hypothesize that glycerol serves as a plasticizer, not only reducing the degree of crystallinity in the substrates with increased concentrations, but perhaps also lowering the crystalline melting temperatures due to defects introduced into the crystal lattice [26]. This lowered the water resistance of the plasticized PVA substrates due to coupling with the leaching of the glycerol from the substrates when immersed in water during the fabrication process [26]. The influence of glycerol on the properties of PVA substrates is related to the degree of compatibility between the plasticizer and PVA [26]. Thus, an increase in glycerol concentration had an inverse effect on the stiffness of the material. AFTIDerm at a 5% exhibited no hysteresis when subjected to cyclic stress-strain testing (380.7 ± 45.5 kPa). The stress of AFTIDerm at varied glycerol concentrations apart from 5% was also investigated (Fig. 3c). Concentrations below 5% experienced hysteresis under cyclic testing (Fig. 4). Data suggests that glycerol percentages above 5% provide long-term mechanical stability (Fig. 3d).
TABLE 3. Materials Properties of AFTIDerm at Varying Glycerol Concentrations.
| Glycerol (%) | Young’s Modulus (MPa) | Cyclic Stress (kPa) | Water Contact Angle (°) |
|---|---|---|---|
| 0 | 18.4 ± 0.12 | 1168 ± 338 | 53.3 ± 14 |
| 1 | 18.9 ± 0.12 | 761.0 ± 218 | 35.0 ± 6.7 |
| 3 | 9.37 ± 0.036 | 468.0 ± 99.7 | 30.6 ± 5.1 |
| 5 | 7.52 ± 0.021 | 380.7 ± 45.5 | 29.7 ± 2.2 |
| 7 | 6.39 ± 0.015 | 230.5 ± 55.2 | 34.2 ± 4.4 |
| 10 | 2.98 ± 0.0077 | 121.5 ± 30.1 | 34.2 ± 5.7 |
FIGURE 3.
Mechanical testing of the AFTIDerm substrate at varied glycerol concentrations. (a) Stress versus strain plot; (b) Young’s modulus versus glycerol concentration; (c) Cyclic stress versus glycerol concentrations at the 25th cycle; (d) Cyclic stress of the AFTIDerm substrate of 5% glycerol concentration.
FIGURE 4.
Long-term mechanical actuation of AFTIDerm samples at varied glycerol concentrations. (a) 0% glycerol; (b) 1% glycerol; (c) 3% glycerol; (d) 7% glycerol; (e) 10% glycerol.
AFTIDerm absorptive properties at various glycerol concentrations were evaluated (Fig. 5) and compared to industry products including HP Tegaderm and Absorbent Tegaderm (Fig. 6; Table 4). Samples with glycerol concentrations below 5% exhibited a decrease in absorbed mass from Day 2 to Day 7 (Fig. 5). The same was observed for both HP Tegaderm and Absorbent Tegaderm (the latter two being common wound care bandage materials used as controls in this study). The trend observed in the AFTIDerm samples less than 5% glycerol suggests that these materials reached their maximum absorption capacity prior to Day 2, reflecting the effect of a low PVA crosslinking density at lower glycerol concentrations. The decrease in absorbed mass was negligible for glycerol concentrations at 5%, 7%, or 10%, thus suggesting a glycerol concentration between 5-10% as opportune glycerol concentrations as well for AFTIDerm. When compared to commercially available control materials, specifically HP Tegaderm and Absorbent Tegaderm, 5% glycerol demonstrated consistent absorption profiles over a one-week span, indicative of glycerol stability.
FIGURE 5.
Absorption study comparing AFTIDerm at varied glycerol concentrations. Mean data reported, n = 6 trials per glycerol concentration group.
FIGURE 6.
Absorption study comparing AFTIDerm at 5% glycerol concentration, HP Tegaderm, and Absorbent Tegaderm relative to baseline values at Day 0. Data reported as mean ± std. dev, n=6 trials per glycerol concentration group.
TABLE 4. Absorption Data Comparing AFTIDerm at Varied Glycerol Percentages Against HP Tegaderm and Absorbent Tegaderm. Data Reported as Mean ± std. dev (n = 6).
| Time | 1% Glycerol | 3% Glycerol | 5% Glycerol | 7% Glycerol | 10% Glycerol | HP Tegaderm | Absorbent Tegaderm | |
|---|---|---|---|---|---|---|---|---|
| Hours | 1 | 392 ± 140 | 184 ± 29 | 86.2 ± 38 | 94.6 ± 57 | 47.5 ± 23 | 145 ± 23 | 39.1 ± 8.91 |
| 2 | 390 ± 150 | 190 ± 30 | 89.1 ± 47 | 101 ± 49 | 45.6 ± 16 | 153 ± 28 | 48.4 ± 15 | |
| 3 | 398 ± 120 | 184 ± 36 | 74.4 ± 44 | 97.1 ± 61 | 43.3 ± 19 | 207 ± 37 | 60.5 ± 12 | |
| 4 | 384 ± 84 | 185 ± 36 | 80.5 ± 43 | 102 ± 70. | 43.0 ± 13 | 225 ± 51 | 65.7 ± 20. | |
| 5 | 423 ± 150 | 178 ± 62 | 82.9 ± 55 | 106 ± 60 | 38.2 ± 17 | 215 ± 41 | 72.7 ± 15 | |
| Days | 1 | 416 ± 130 | 178 ± 46 | 78.5 ± 41 | 88.4 ± 57 | 45.3 ± 23 | 228 ± 48 | 427 ± 180 |
| 2 | 375 ± 78 | 182 ± 45 | 74.7 ± 41 | 91.2 ± 62 | 40.9 ± 18 | 217 ± 71 | 519 ± 140 | |
| 3 | 361 ± 110 | 157 ± 52 | 72.8 ± 43 | 82.5 ± 56 | 40.6 ± 20. | 203 ± 110 | 490. ± 150 | |
| 4 | 295 ± 110 | 133 ± 40 | 70.9 ± 40 | 88.8 ± 55 | 37.7 ± 20 | 171 ± 110 | 471 ± 160 | |
| 5 | 248 ± 22 | 82.9 ± 88 | 68.1 ± 38 | 85.9 ± 63 | 26.0 ± 21 | 133 ± 110 | 460. ± 180 | |
| 6 | 189 ± 200 | 65.5 ± 76 | 69.9 ± 37 | 77.5 ± 50 | 30.6 ± 22 | 109 ± 100 | 444 ± 200 | |
| 7 | 162 ± 170 | 60.3 ± 63 | 67.4 ± 45 | 57.3 ± 51 | 33.4 ± 20. | 73.3 ± 76 | 432 ± 210 |
Towards assessing their clinical utility and the biological stability, indicative of the relative change in pH when immersed in PBS, of AFTIDerm was evaluated over a one-week period, with intermediate timepoints reflecting pre-clinical benchmarks (Day 0, 1, 3, and 7) followed in our animal studies [27] (Fig. 7). No statistical difference was noted among measured pH between the various samples (Table 3). The pH found among the samples confirms their stability with and without glycerol. The testing performed was representative of that required for implantable materials as dictated in ISO-10993-12 (pH within ± 0.2) [28].
FIGURE 7.
Assessing the change in pH of samples at 5% glycerol concentration over a 7-day period. Experiments run in triplicate; data reported as mean ± std. dev.
Regarding biocompatibility, cell culture studies indicate that excessive levels of glycerol can be cytotoxic. Wiebe et al. studied the effect of the exogenous delivery of glycerol on the proliferation of several cell lines [29]. Complete proliferation suppression occurred at glycerol concentrations as low as 4% in cell culture medium for some cell lines [29]. Armitage and Mazur reported that human granulocytes are damaged by exposed to exogenously delivered glycerol concentrations of around 5% [30]. However, 5% glycerol has also been shown to be have viable therapeutic effect for dry skin [31].
Independent cytotoxicity testing to ISO 10993–5 found no cytotoxicity or cell lysis. No pH shift was observed at 48 hours. The reagent control, negative control, and the positive control performed as anticipated. The individual reactivity grades are presented in Table 5. In short, the test article extract showed no evidence of causing cell lysis or toxicity. The test article extract met the requirements of the test since the grade was less than a grade 2 (mild reactivity). These results confirm the biocompatibility of the AFTIDerm substrate for use as a wound dressing. Thus, we do not expect the AFTIDerm to pose any cytotoxic effects when placed on healthy or compromised skin.
TABLE 6. Reactivity Grades for Elution Testing.
| Well | Rounding (%) | Cells Without Intracytoplasmic Granules (%) | Lysis (%) | Grade | Reactivity |
|---|---|---|---|---|---|
| Test (1) | 0 | 0 | 0 | 0 | None |
| Test (2) | 0 | 0 | 0 | 0 | None |
| Test (3) | 0 | 0 | 0 | 0 | None |
| Negative Control (1) | 0 | 0 | 0 | 0 | None |
| Negative Control (2) | 0 | 0 | 0 | 0 | None |
| Negative Control (3) | 0 | 0 | 0 | 0 | None |
| Reagent Control (1) | 0 | 0 | 0 | 0 | None |
| Reagent Control (2) | 0 | 0 | 0 | 0 | None |
| Reagent Control (3) | 0 | 0 | 0 | 0 | None |
| Positive Control (1) | N/A | N/A | 100 | 4 | Severe |
| Positive Control (2) | N/A | N/A | 100 | 4 | Severe |
| Positive Control (3) | N/A | N/A | 100 | 4 | Severe |
TABLE 5. pH of PBS, PVA, and AFTIDerm Over a One-Week Period. Experiments Run in Triplicate; Data Reported as Mean ± std. dev.
| Days | PBS | PVA | AFTIDerm |
|---|---|---|---|
| 0 | 7.34 ± 0.041 | 7.34 ± 0.041 | 7.34 ± 0.041 |
| 0.125 | 7.34 ± 0.041 | 7.38 ± 0.033 | 7.33 ± 0.0047 |
| 1 | 7.38 ± 0.017 | 7.36 ± 0.0094 | 7.37 ± 0.0047 |
| 3 | 7.31 ± 0.0082 | 7.30 ± 0.0047 | 7.28 ± 0.0 |
| 7 | 7.35 ± 0.017 | 7.36 ± 0.0047 | 7.35 ± 0.013 |
The mechanical, absorption, and therapeutic attributes within a biological threshold provided indicate that the AFTIDerm with 5% glycerol in PVA is the optimal concentration is appropriate for in vivo or ex vivo studies.
AFTIDerm was evaluated as an absorbent wound dressing (Fig. 8a). When placed over the wound in a porcine chronic wound model, 5% glycerol AFTIDerm substrate absorbed exudate from the wound over a one-hour period (Fig. 8b). Images of wound dressing taken immediately post-biopsy, after 30 and 60 minutes (Fig. 8c). The images show the wound-dressing controlled bleeding in the first 60 minutes. To evaluate absorption, a 14-day AFTIDerm mass measurement test was carried out in the same model. The measurements show a maximum of ~44% increase in exudate absorption over a 14-day period (Fig. 8d) with no noticeable change in optical transparency.
FIGURE 8.
Translation of the AFTIDerm substrate as a wound dressing (a) Schematic detailing packaged wound dressing prior to ethylene oxide sterilization; (b) Images of the AFTIDerm wound dressing on a chronic wound; (c) Absorption of exudate by the AFTIDerm wound dressing after removal from the chronic wound (n = 4 dressing per wound; mean ± std. dev.) (d) AFTIDerm’s mass increase percentage measurements from day 1 to 14.
IV. Conclusion
This study developed an absorbent, flexible and transparent wound dressing. The mechanical, absorption, and biological properties of this material were evaluated as a platform substrate material for bioelectronic applications. The tensile and cyclic properties demonstrated a decrease in Young’s Modulus and cyclic stress with an increase in glycerol concentration. The optimal glycerol concentration was found to be 5% due to the relative stability in absorption over a one-week span compared with lower glycerol percentages, a lack of mechanical fatigue when tested over 50 cycles at 2.5%, and the alleviation of any biocompatibility concerns due to excess glycerol concentration (e.g., greater than 7%). The fabrication methodology is amenable to construction of AFTIDerm based wound dressings of varying geometries to address the diversity of clinical wounds. Benchtop pH measurements demonstrated an insignificant increase in pH suggestive of the chemical stability of the substrate with respect to glycerol concentration. Independent cytotoxicity testing to ISO 10993–5 found that AFTIDerm meets the test standard for biocompatibility. The lack of erythema, indicative of increases in skin temperature, when AFTIDerm is placed on compromised skin (wound) validates the utility of this material as a wound dressing. The technology presented herein broadens the translational utility of AFTIDerm not only as a material for wound dressings in low to moderate exudate environments, but also as a potential substrate material for epidermal electronics, in particular smart bandages.
Acknowledgment
Dhruv R. Seshadri designed and performed the experiments, analyzed all data, and wrote and edited the manuscript. Nicholas D. Bianco and Aziz N. Radwan assisted with performing experiments and analyzing the data. Christian A. Zorman and Kath M. Bogie assisted in the review of the data. All co-authors contributed to the editing and formatting of the manuscript and take responsibility for the data presented in the manuscript. The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request. The authors disclose the following IP related to this work: Intl patent pending (No. PCT/US21/26571).
Funding Statement
This work was supported by the Department of Veterans Affairs Rehabilitation Research & Development Service under Grant RX002166.
References
- [1].Scalamandré A. and Bogie K. M., “Smart technologies in wound prevention and care,” in Innovations and Emerging Technologies in Wound Care, Gefen A., Ed. New York, NY, USA: Academic, 2020, ch. 13, pp. 225–244, doi: 10.1016/B978-0-12-815028-3.00013-4. [DOI] [Google Scholar]
- [2].Brown M. S., Ashley B., and Koh A., “Wearable technology for chronic wound monitoring: Current dressings, advancements, and future prospects,” Frontiers Bioeng. Biotechnol., vol. 6, p. 47, Apr. 2018, doi: 10.3389/fbioe.2018.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Winter G. D., “Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig,” Nature, vol. 193, no. 4812, pp. 293–294, Jan. 1962, doi: 10.1038/193293a0. [DOI] [PubMed] [Google Scholar]
- [4].Winter G. D. and Scales J. T., “Effect of air drying and dressings on the surface of a wound,” Nature, vol. 197, no. 4862, pp. 91–92, Jan. 1963, doi: 10.1038/197091b0. [DOI] [PubMed] [Google Scholar]
- [5].Cutting K. F. and White R. J., “Avoidance and management of peri-wound maceration of the skin,” Prof. Nurse, vol. 18, no. 1, pp. 33 and 35–36, Sep. 2002. [PubMed] [Google Scholar]
- [6].Junker J. P. E., Kamel R. A., Caterson E. J., and Eriksson E., “Clinical impact upon wound healing and inflammation in moist, wet, and dry environments,” Adv. Wound Care, vol. 2, no. 7, pp. 348–356, Sep. 2013, doi: 10.1089/wound.2012.0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kannon G. A. and Garrett A. B., “Moist wound healing with occlusive dressings: A clinical review,” Dermatol. Surg., vol. 21, no. 7, pp. 583–590, Jul. 1995, doi: 10.1111/j.1524-4725.1995.tb00511.x. [DOI] [PubMed] [Google Scholar]
- [8].Jones V., Grey J. E., and Harding K. G., “Wound dressings,” BMJ, vol. 332, no. 7544, pp. 777–780, Apr. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dhivya S., Padma V. V., and Santhini E., “Wound dressings—A review,” BioMedicine, vol. 5, no. 4, p. 22, Nov. 2015, doi: 10.7603/s40681-015-0022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gefen A.et al. , “How should clinical wound care and management translate to effective engineering standard testing requirements from foam dressings? Mapping the existing gaps and needs,” Advances in Wound Care, Apr. 2022. Accessed: Mar. 2, 2022. [Online]. Available: https://www.liebertpub.com/doi/epdf/10.1089/wound.2021.0173, doi: 10.1089/wound.2021.0173. [DOI] [PMC free article] [PubMed]
- [11].Kamoun E. A., Kenawy E.-R.-S., and Chen X., “A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings,” J. Adv. Res., vol. 8, no. 3, pp. 217–233, May 2017, doi: 10.1016/j.jare.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Barnes H. R., “Wound care: Fact and fiction about hydrocolloid dressings,” J. Gerontol. Nursing, vol. 19, no. 6, pp. 23–26, Jun. 1993, doi: 10.3928/0098-9134-19930601-08. [DOI] [PubMed] [Google Scholar]
- [13].Azandeh S., Samadi A., Orazizadeh M., Bayati V., Rafienia M., and Karami M., “Fabrication and characterization of glycerol/chitosan/polyvinyl alcohol-based transparent hydrogel films loaded with silver nanoparticles for antibacterial wound dressing applications,” Adv. Biomed. Res., vol. 10, no. 1, p. 4, 2021, doi: 10.4103/abr.abr_211_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cascone M. G., Maltinti S., Barbani N., and Laus M., “Effect of chitosan and dextran on the properties of poly(vinyl alcohol) hydrogels,” J. Mater. Sci. Mater. Med., vol. 10, no. 7, pp. 431–435, Jul. 1999, doi: 10.1023/a:1008983215833. [DOI] [PubMed] [Google Scholar]
- [15].Yao L., Haas T. W., Guiseppi-Elie A., Bowlin G. L., Simpson D. G., and Wnek G. E., “Electrospinning and stabilization of fully hydrolyzed poly(vinyl alcohol) fibers,” Chem. Mater., vol. 15, no. 9, pp. 1860–1864, May 2003, doi: 10.1021/cm0210795. [DOI] [Google Scholar]
- [16].Jensen B. E. B., Dávila I., and Zelikin A. N., “Poly(vinyl alcohol) physical hydrogels: Matrix-mediated drug delivery using spontaneously eroding substrate,” J. Phys. Chem. B, vol. 120, no. 26, pp. 5916–5926, Jul. 2016. Accessed: Jul. 18, 2021. [Online]. Available: https://pubs.acs.org/doi/10.1021/acs.jpcb.6b01381 [DOI] [PMC free article] [PubMed] [Google Scholar]
-
[17].Hassan C. M. and Peppas N. A., “Structure and applications of poly(vinyl alcohol) hydrogels produced by conventional crosslinking or by freezing/thawing methods,” in Biopolymers
 PVA Hydrogels, Anionic Polymerisation Nanocomposites. Berlin, Germany: Springer, 2000, pp. 37–65, doi: 10.1007/3-540-46414-X_2. [DOI] [Google Scholar]
PVA Hydrogels, Anionic Polymerisation Nanocomposites. Berlin, Germany: Springer, 2000, pp. 37–65, doi: 10.1007/3-540-46414-X_2. [DOI] [Google Scholar] - [18].Addae-Mensah K. A., Reiserer R. S., and Wikswo J. P., “Poly(vinyl alcohol) as a structure release layer for the microfabrication of polymer composite structures,” J. Micromech. Microeng., vol. 17, no. 7, pp. N41–N46, May 2007, doi: 10.1088/0960-1317/17/7/N01. [DOI] [Google Scholar]
- [19].Das A., Uppaluri R., and Das C., “Feasibility of poly-vinyl alcohol/starch/glycerol/citric acid composite films for wound dressing applications,” Int. J. Biol. Macromol., vol. 131, pp. 998–1007, Jun. 2019, doi: 10.1016/j.ijbiomac.2019.03.160. [DOI] [PubMed] [Google Scholar]
- [20].Bano I., Arshad M., Yasin T., and Ghauri M. A., “Preparation, characterization and evaluation of glycerol plasticized chitosan/PVA blends for burn wounds,” Int. J. Biol. Macromol., vol. 124, pp. 155–162, Mar. 2019, doi: 10.1016/j.ijbiomac.2018.11.073. [DOI] [PubMed] [Google Scholar]
- [21].Gao T.et al. , “Patterned polyvinyl alcohol hydrogel dressings with stem cells seeded for wound healing,” Polymers, vol. 11, no. 1, p. 171, Jan. 2019, doi: 10.3390/polym11010171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lin S.-P., Lo K.-Y., Tseng T.-N., Liu J.-M., Shih T.-Y., and Cheng K.-C., “Evaluation of PVA/dextran/chitosan hydrogel for wound dressing,” Cellular Polym., vol. 38, nos. 1–2, pp. 15–30, Jan. 2019, doi: 10.1177/0262489319839211. [DOI] [Google Scholar]
-
[23].Gwon H.-J.et al. , “Characterization of PVA/glycerin hydrogels made by
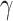 -irradiation for advanced wound dressings,” Korean J. Chem. Eng., vol. 26, no. 6, pp. 1686–1688, Nov.
2009, doi: 10.1007/s11814-009-0246-z. [DOI] [Google Scholar]
-irradiation for advanced wound dressings,” Korean J. Chem. Eng., vol. 26, no. 6, pp. 1686–1688, Nov.
2009, doi: 10.1007/s11814-009-0246-z. [DOI] [Google Scholar] - [24].Trung T. Q., Dang T. M. L., Ramasundaram S., Toi P. T., Park S. Y., and Lee N.-E., “A stretchable strain-insensitive temperature sensor based on free-standing elastomeric composite fibers for on-body monitoring of skin temperature,” ACS Appl. Mater. Interfaces, vol. 11, no. 2, pp. 2317–2327, Jan. 2019, doi: 10.1021/acsami.8b19425. [DOI] [PubMed] [Google Scholar]
- [25].Song G., Zhang L., He C., Fang D.-C., Whitten P. G., and Wang H., “Facile fabrication of tough hydrogels physically cross-linked by strong cooperative hydrogen bonding,” Macromolecules, vol. 46, no. 18, pp. 7423–7435, 2013, Accessed: Jul. 18, 2021. [Online]. Available: https://pubs.acs.org/doi/10.1021/ma401053c [Google Scholar]
- [26].Lim L. Y. and Wan L. S. C., “The effect of plasticizers on the properties of polyvinyl alcohol films,” Drug Develop. Ind. Pharmacy, vol. 20, no. 6, pp. 1007–1020, Jan. 1994, doi: 10.3109/03639049409038347. [DOI] [Google Scholar]
- [27].Bogie K. M., “The modular adaptive electrotherapy delivery system (MAEDS): An electroceutical approach for effective treatment of wound infection and promotion of healing,” Mil. Med., vol. 184, no. 1, pp. 92–96, Mar. 2019, doi: 10.1093/milmed/usy276. [DOI] [PubMed] [Google Scholar]
- [28].Biological Evaluation of Medical Devices—Part 1: Evaluation and Testing Within a Risk Management Process, Standard ISO 10993-1, Guidance for Industry and Food and Drug Administration Staff, p. 68, Aug. 2018.
- [29].Wiebe J. P. and Dinsdale C. J., “Inhibition of cell proliferation by glycerol,” Life Sci., vol. 48, no. 16, pp. 1511–1517, 1991, doi: 10.1016/0024-3205(91)90275-g. [DOI] [PubMed] [Google Scholar]
- [30].Armitage W. J. and Mazur P., “Toxic and osmotic effects of glycerol on human granulocytes,” Amer. J. Physiol.-Cell Physiol., vol. 247, no. 5, pp. C382–C389, Nov. 1984, doi: 10.1152/ajpcell.1984.247.5.C382. [DOI] [PubMed] [Google Scholar]
- [31].Korponyai C.et al. , “Effects of locally applied glycerol and xylitol on the hydration, barrier function and morphological parameters of the skin,” Acta Dermato Venereol., vol. 97, no. 2, pp. 182–187, 2017. Accessed: Jul. 19, 2021. [Online]. Available: http://www.medicaljournals.se/acta/content/html/10.2340/00015555-2493 [DOI] [PubMed] [Google Scholar]
- [32].Moshakis V., Fordyce M. J., Griffiths J. D., and McKinna J. A., “Tegadern versus gauze dressing in breast surgery,” Brit. J. Clin. Pract., vol. 38, no. 4, pp. 149–152, Apr. 1984. [PubMed] [Google Scholar]
- [33].Boateng J. S., Matthews K. H., Stevens H. N. E., and Eccleston G. M., “Wound healing dressings and drug delivery systems: A review,” J. Pharmaceutical Sci., vol. 97, no. 8, pp. 2892–2923, Aug. 2008, doi: 10.1002/jps.21210. [DOI] [PubMed] [Google Scholar]
- [34].Martin L.et al. , “The release of model macromolecules may be controlled by the hydrophobicity of palmitoyl glycol chitosan hydrogels,” J. Control Release, vol. 80, nos. 1–3, pp. 87–100, Apr. 2002, doi: 10.1016/s0168-3659(02)00005-6. [DOI] [PubMed] [Google Scholar]
- [35].Thomson T., “Foam composite,” U.S. Patent 7 048 966 B2, May 23, 2006. Accessed: Mar. 2, 2022. [Online]. Available: https://patents.google.com/patent/US7048966B2/en
- [36].Ramos-e-Silva M. and de Castro M. C. R., “New dressings, including tissue-engineered living skin,” Clin. Dermatol., vol. 20, no. 6, pp. 715–723, Dec. 2002, doi: 10.1016/s0738-081x(02)00298-5. [DOI] [PubMed] [Google Scholar]










