Abstract
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes coronavirus disease 2019 (COVID-19). SARS-CoV-2 infection can activate innate and adaptive immune responses and result in massive inflammatory responses in the disease. A comprehensive understanding of the participation of micronutrients in the immune response to COVID-19 will allow the creation of prevention and supplementation scenarios in malnutrition states. Microelement deficiency can be decisive in the progression of diseases and their optimal levels can act as protective factors, helping to maintain homeostasis. Vitamin A, B, D, selenium, zinc, and copper, through their complementary and synergistic effects, allow the components of innate and adaptive immunity to counteract infections like those occurring in the respiratory tract.
Thus, alterations in nutritional status are related to metabolic diseases, systemic inflammation, and deterioration of the immune system that alter the response against viral infections, such as COVID-19. The aim of this review is to describe the micronutrients that play an important role as immunomodulators and its relationship between malnutrition and the development of respiratory infections with an emphasis on severe and critical COVID-19. We conclude that although an unbalanced diet is not the only risk factor that predisposes to COVID-19, a correct and balanced diet, which provides the optimal amount of micronutrients and favors an adequate nutritional status, could confer beneficial effects for prevention and improvement of clinical results. The potential usefulness of micronutrient supplementation in special cases is highlighted.
Keywords: SARS-CoV-2, COVID-19, Nutritional status, Micronutrients, Immune system, Microelements
Introduction
SARS-CoV-2 has been spreading at high speed globally and with it, the emergence of the COVID-19 pandemic since December 2019. As of May 6, 2022, the World Health Organization (WHO) reported 513,955,910 confirmed cases of COVID-19 and more than 6.2 million deaths worldwide [1]. The lack of pharmacological treatments against COVID-19 urges us to highlight the importance of micronutrient-rich diets with a preventive approach. Immune system dysfunction due a poor diet and nutrient deficiency is a major risk factor for respiratory virus infections that increase the burden of disease [2]. An optimal nutritional status is achieved through the consumption of a balanced and quality diet, which would provide all of the needed micronutrients in the appropriate quantity to guarantee an optimal response and thus prevent infections. Many studies suggest that nutrients are involved in the development of COVID-19 [2–5]; however, only a few of them directly assess nutrient deficiencies in patients with the disease, and fewer clinical assays study the influence of micronutrients in viral immune response.
There are several kinds of malnutrition: undernutrition, inadequate vitamins or minerals, overweight, obesity, and resulting diet-related non-communicable diseases [6]. Although the prevalence of malnutrition has decreased in recent decades, WHO [1] reported that 1.9 billion adults are overweight or obese, while 462 million are underweight. Obesity has increased uncontrollably and it coexists with underweight even in the same region of the world. The concept of the obesity-related double burden of malnutrition has emerged with important clinical implications. When obesity occurs, micronutrient deficiencies and clinical manifestations of malnutrition are possible and complicate its clinical course. This effect is due to a combination of a sedentary lifestyle, an inadequate diet, and alterations inherent in the pathophysiology of obesity. Among its consequences are the inability to maintain body composition and function, loss of skeletal muscle, and a negative impact on both morbidity and mortality [7].
The effect of micronutrients on immune system function and their role as regulators of oxidative stress helps to understand the greater vulnerability of the patient with obesity to SARS-CoV-2 infection. For example, diets rich in vitamin A, B, D, zinc, selenium, and copper promote a better response of the immune system against viral infections, including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [4].
In this review, we aim to describe and summarize the role of micronutrients in the immune response against SARS-CoV-2 and the development of COVID-19 disease. We hypothesize that there is a strong correlation between malnutrition and the development of severe and critical illnesses. If we consider that micronutrients fulfill various biological functions and that their deficiency has a negative influence on nutritional status, it is possible to explain the increased vulnerability of individuals to viral infection and to identify key diet components as a possible preventive and therapeutic strategy against COVID-19.
SARS-CoV-2
SARS-CoV2 is an enveloped virus of 80–120 nm size and member of the betacoronavirus family, responsible for the coronavirus disease 2019 (COVID-19). Its viral genome consists of a single-stranded RNA-positive of up to 32 kb length that encodes 4 structural proteins, 16 nonstructural proteins (NSPs), and nine accessory proteins [8]. The spike (S) protein is essential for binding to the host; it is a glycosylated homotrimer that can be cleaved by furin-like protease in S1 and S2 functional subunits. S1 protein recognizes, through a receptor-binding domain (RBD), the human angiotensin-converting enzyme 2 (ACE2) as its main receptor [8]. ACE2 is expressed in the host cellular membrane in many cell types, including alveolar, endothelial, and renal cells [9]. S2 harbors the fusion peptide (FP) which anchors to target membranes and mediates membrane fusion by connecting with lipid bilayers of the host cell, while S1 plays a key role in bringing viral and cellular membranes into close proximity to permit lipid bilayer fusion [10]. Following viral uptake and further release of RNA, translation of ORF1 and ORF2 results in pp1a and pp1b, which form the viral replication and transcription complex. Expression of NSPS and the biogenesis of viral replication organelles establish a protective microenvironment that favors viral genomic RNA replication and transcription. Structural proteins translocate into endoplasmic reticulum, where newly produced genomic RNA binds into lumen of secretory vesicular compartments, which ends in secretion of virios by exocytosis [11].
Structural nucleocapsid protein (NP) protects the viral RNA genome and packages it into a ribonucleoprotein complex [12]. Moreover, N protein has been identified in mechanisms to antagonize the host human response involving interaction and suppression of cellular antiviral-RNAi [13]. The membrane (M) protein encircles the capsid and anchors the envelope (E) and S proteins. E protein participates in virus budding by regulation of viral lysis, viral genome release, and activation of host inflammasome [8, 14]. So far, many SARS-CoV-2 mutant strains or variants have been reported and mutations located at RBD-ACE2 are associated with loss efficacy of vaccines and neutralizing antibodies [15, 16]. These strains are classified as variants of concern (VOCs) [17], as the N501Y mutation present in variants from the UK (Alfa), South Africa (Beta), and Brazil (Gamma) that has shown an increase in ACE2 affinity causing enhanced infectivity and more severe COVID-19 [8, 18].
Pathophysiology and Stages of COVID-19 Disease
COVID-19 may manifest with symptoms such as fever, dry cough, fatigue, shortness of breath, muscle pain, confusion, headache, sore throat, rhinorrhea, chest pain, diarrhea, nausea, vomiting, chills, sputum production, hemoptysis, dyspnea, bilateral pneumonia, anorexia, chest pain, leukopenia, lymphopenia, olfactory and taste disorders, and higher levels of plasma cytokines, such as IL-2, IL-7, IL-10, GSCF, IP10, MCP1, MIP1A, and TNFα in critically ill patients [19]. COVID-19 disease is classified into three levels based on the severity of the disease: mild, severe, and critical, where most patients only present mild symptoms and recover [20]. The main symptoms of the mild disease are fever, cough, fatigue, ground-glass opacities in chest CT imaging, and mild pneumonia, while in severe disease, other symptoms may also appear, such as dyspnea, blood oxygen saturation ≤ 93%, respiratory rate ≥ 30/min, arterial oxygen partial pressure to inspired oxygen fraction ratio < 300, and/or pulmonary infiltrates > 50% in 24 to 48 h. Finally, at a critical stage, acute respiratory distress syndrome (ARDS), respiratory failure, septic shock and/or multiple organ dysfunction or failure, difficult to correct metabolic acidosis, septic shock, and coagulation dysfunction may occur [19]. Nevertheless, a large number of cases with asymptomatic infection not requiring hospitalization were also reported [21].
Lung Immune Function and Response Against SARS-CoV-2
The immune response developed in the presence of a viral infection activates innate and adaptive mechanisms. As part of the innate response, the skin and all mucous membranes that form physical and chemical barriers integrate the first line of defense against the virus. Upon infection with SARS-CoV-2 and when the virus has successfully overcome the first line of defense, virus antigens that are known as pathogen-associated molecular patterns (PAMPs) activate the synthesis of antimicrobial substances in serum, such as interferons (IFN) and complement proteins. Respiratory tract epithelial cells are equipped with pathogen recognition receptors (PRRs) that recognize viral antigens and respond by producing inflammatory mediators such as IL-1β, TNFα, and type 1 IFNs. These cytokines enhance recruitment of circulating cells as neutrophils, macrophages, and NK cells, which in turn cause phagocytosis and cytokine secretion and, as an antiviral response, can promote cell death. When innate-cell barriers are surpassed and infection of epithelial cells occur, their interaction with myeloid cells contributes to activation of adaptive antiviral responses [22, 23].
Myeloid cells act as detectors of viral infections and are a key component of the antiviral response, including the coordination of immune responses and tissue homeostasis repair mechanisms. Dendritic cells (DCs), monocytes, and macrophages modulate the immune response by releasing a variety of proinflammatory cytokines, such as IL-1β, IL-6, IL-8, and TNFα, which influence the cellular and humoral response toward coronavirus infection and relate to its severity [24]. Patients with severe COVID-19 show increased numbers of mature and immature neutrophils and monocytes in the blood and a significant reduction in circulating lymphocytes as well as dendritic cells [23].
DCs are present in lung tissue; therefore, they recognize antigens from infected-tract respiratory epithelial cells and initiate adaptive immune responses by presenting antigens to naïve T lymphocytes, generating CD4 + and CD8 + T lymphocytes. While CD4 + T cell subsets participate, producing an array of cytokines to coordinate immune responses, CD8 + T cells cause apoptosis of infected cells and secrete cytokines to promote NK activation. Alveolar macrophages are able to phagocyte large numbers of microorganisms, but also produce reactive oxygen species (ROS) and can migrate to nearby lymph tissue carrying antigen for presentation [25]. B lymphocytes produce a variety of antibodies that function as opsonins to facilitate phagocytosis and promote complement activation and antibody-dependent cellular cytotoxicity by NK cells. Patients with severe COVID-19 presented antibody titer to S protein, which was previously mentioned as fundamental for viral uptake [26].
As a consequence of immune defense and cell signaling mechanisms after infection, excessively generated ROS causes oxidative stress, a condition of imbalance between free radical generation and antioxidant defenses. Free radicals generated in excess are hydroxyl radical (OH-), superoxide anion radical (O2–), hydrogen peroxide (H2O2), and peroxyl (ROO-). These are toxic to the cell because they are capable of reacting with biomolecules such as DNA, RNA, proteins, carbohydrates, and lipids, damaging cells and tissues and initiating inflammatory processes [27]. In addition, neutrophils are among the main producers of ROS and are found to be elevated in critically ill patients. The mechanisms by which oxidative stress intervenes in viral virulence are still under study. Some authors hypothesize that it may be a consequence of immune response modulation or due to an increase in the mutation rate as a result of oxidative damage [28].
The antioxidant systems that evolved to alleviate ROS-associated damage in mammals are regulated by the expression of nuclear factor erythroid 2p45-related factor 2 (Nrf2). Under normal conditions, Nrf2 is retained in the cytoplasm by a group of proteins and rapidly degrades unless activated. However, during oxidative stress, Nrf2 is activated and stimulates a variety of genes responsible for cytoprotection and detoxification. It was found that some viruses can suppress the Nrf2 pathway, thus affecting the antioxidant response in the body. In particular, respiratory viral infections were shown to be associated with inhibition of Nrf2 and activation of the nuclear factor-kappa B (NF-κB) pathway, leading to increased oxidative damage and promoting inflammation [29, 30]. Higher levels of lung inflammation caused by this abnormal proinflammatory immune response led to ARDS and worse outcomes and differential gene expression profiles identified NF-κB signaling as a potential regulator in disease severity [28]. The production of ROS is mediated by the activity of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) family, which consists of seven members: NOX1 to NOX5 and two dual oxidases, Duox1 and Duox2, expressed in most cell types. NOX4 isoform upregulates after viral infection in lung epithelial cells and is responsible for ROS generation, which activates protein kinases that favor viral ribonucleoprotein nuclear export and promote viral replication [31]. Interestingly, NOX4-derived ROS production has been shown to modulate the binding of SARS-CoV-2 to ACE2. Therefore, in COVID-19, the imbalance in the cellular redox state with an excess of free radicals (ROS) and activation of inflammatory signaling pathways cause tissue damage [27].
In older adults, Abouhashem et al. [32] found that some redox-active genes are downregulated in lung cells, when analyzing single-cell RNA sequencing data from healthy donors, compared to samples from young donors. Among them, the superoxide dismutase 3 (SOD3) gene stands out, followed by activating transcription factor 4 (ATF4) and metallothionein 2A (M2TA). These findings suggest a weakening of the cellular antioxidant systems in the lung associated with aging, which could partly explain the severity in this age group.
Another group of proteins that mediate the antiviral response, which is especially important in the context of coronavirus infection, are IFN, a group of signaling molecules that play an important role in the impaired control of viral replication. This is due to an inadequate pre-existing immunity and the delayed or inadequate type I interferon-I (IFN-I) responses that likely contribute to the pathogenesis of severe COVID-19. IFN-I antagonism is a central mechanism of virulence for many viruses, and SARS-CoV-2 is no different in this regard [24].
Antiviral memory response involves the action of T and B lymphocytes. When the infection subsides, 10% of these cells persist overtime to protect the organism against reinfection. In COVID-19, T cells and virus-specific T cells are essential to protect against viral infection; however, the memory immune response is still being studied to understand SARS-CoV-2 infection. Lymphopenia is a typical profile in patients with COVID-19 and could be a key element related to disease severity and mortality, especially in elderly sick patients in whom marked CD8 + T cell lymphopenia and increased neutrophils are reported; on the other hand, patients who overcome the disease gradually recovered their basal T cell levels [33]. A clinical trial has reported an increase of immunoglobulin G (IgG) antibody titers in patients with follow-up after 2 weeks of hospital discharge [34]. This study also found that most patients showed serum neutralizing activity in a pseudotype entry assay. Notably, there was a strong correlation between neutralizing antibody titers and the number of virus-specific T cells. Moreover, a nucleocapsid protein (NP)-specific antibody response has been reported [35]. However, virus-specific T cells and their relationship to neutralizing antibody titers in patients with COVID-19 remain uncharacterized.
In a recent study of individuals recovered from mild symptomatic COVID-19, at 3 months post-infection, the formation of virus-specific immune memory cells with the protective function was detected. Recovered individuals had increased neutralizing antibodies, classical pathogen-specific memory B cells IgG + with BCRs that formed neutralizing antibodies, Th1 cytokine-producing CXCR5 + circulating Tfh and CXCR5- non-Tfh cells, proliferating CXCR3 + CD4 + memory cells, and IFN-γ-producing CD8 + T cells [36]. Regarding CD4 + memory T cells, in patients in the study by Rodda et al. [36], they showed the ability to proliferate after re-exposure to peak protein by rapidly increasing ICOS and CD40L levels in CXCR5 + and CXCR5, as well as the expression of Th1- and Th17-associated cytokines. The duration of immune memory for SARS-CoV-2 infection is still under investigation to provide certainty on the defense mechanisms.
Impact of Nutritional Status on Immune Response and Severity of COVID-19 Disease
Recently, it has been proposed that nutrition is partly responsible for the wide differences in COVID-19 mortality rates observed between countries and even within regions of the same country [37], as is shown in Fig. 1. Nutrition can certainly affect the ability of the immune system to protect against viral infections. Malnutrition in the elderly in COVID-19 has been described in a Chinese study. This study of 182 elderly patients (age ≥ 65 years) with COVID-19 showed that 52.7% were “malnourished” and a further 27.5% were “at risk of malnutrition,” when using the Mini Nutritional Assessment score. The regression risk in the study also showed diabetes mellitus to be an independent risk factor for malnutrition (OR: 2.12; IC del 95%: 1.92–3.21; p = 0.006) [38].
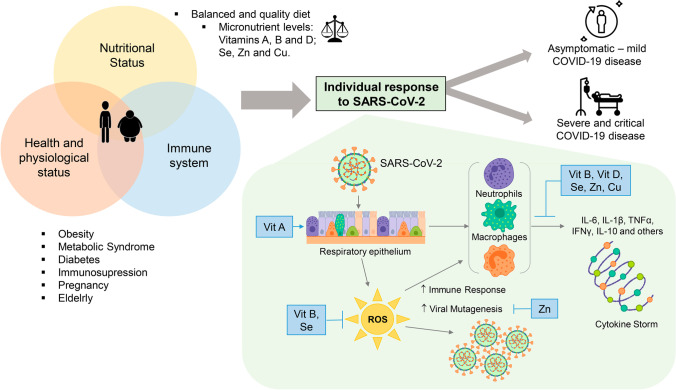
Fig. 1.
Micronutrient regulation of individual response in SARS-CoV-2 infection. An optimal state of health can be achieved when the necessary quality and quantity of micronutrients such as Vit A, B, and D, as well as Se, Zn, and Cu, are provided through the diet. This brings benefits that are reflected in an adequate nutritional state that will provide the necessary elements that allow the immune system to respond to an infection. Serious health problems such as obesity, metabolic syndrome, diabetes, and immunosuppression are a result of the imbalance in the intake of foods with high caloric content and low amount of micronutrients. Currently, with the ongoing COVID-19 pandemic, it has become clear that the most vulnerable individuals to develop the infection are those in whom the balance of the physiological-nutritional status and immune system is lost. This imbalance allows the SARS-CoV-2 virus to develop and causes the different clinical forms of the disease (asymptomatic, mild, moderate, and severe). Vit A deficiency leads to the first defense barrier lacking important components of innate immunity, which enhances the virus response. Vit B and Se play a role in the immune and antioxidant response against oxidative stress during infection. Vit B, D, Se, Zn, and Cu participate in a very important way, promoting the inhibition of the synthesis of pro-inflammatory cytokines that trigger the cytokine storm and therefore modulate the adaptive immune response by suppressing the Th1 response and promoting the production of cytokines by Th2 cells. Zn, for its part, is involved in the transduction of signals in the cell and, therefore, in the patterns of cellular and viral gene expression, thus avoiding viral mutagenesis
Optimal immune function depends on an adequate nutrition that ensures a good supply of nutrients (macro and micronutrients) for healthy immune response development and support. In this sense, different studies have associated low levels of micronutrients with the development of severe or critical disease [5, 39–42]. The main nutrients implicated in viral protection, by their antioxidant capacity or by being part of immune function, are vitamins A, C, D, E, B6, B12, folic acid, iron, zinc, copper, selenium, and magnesium. Antioxidant capacity and antioxidant response potential of the host are two of the key determinants of the outcome of SARS-CoV-2 infection. Hence, the higher antioxidant capacity of children and young adults combined with their higher expression of ACE2 is the likely explanation for the low incidence of severe infections in these age groups. This hypothesis also explains the occasional severe infections in the younger age groups because, regardless of age, anyone with a reduced antioxidant defense system would be expected to have a more severe infection. Also, an inadequate dietary intake of antioxidants, high intake of prooxidants, or excessive alcohol consumption, which decreases total antioxidant capacity, could be additional hidden risk factors in addition to age and comorbid conditions [28].
In elderly patients, there are more cases of malnutrition and comorbidities such as diabetes mellitus and cardiovascular disease, and a weakened immune system, causing an inefficient response to the virus. Micronutrient deficiency leads to an impaired immune response, with inadequate cytokine secretion, alterations in secretory antibody response, and antibody affinity, resulting in an increased susceptibility to viral infection. It is widely recognized that in conditions of obesity and overweight, there is a micronutrient deficiency, and the reason is that diets rich in ultra-processed foods, high in fat and carbohydrates, not only promote weight gain but are also often poor in vitamins and minerals [43]. Nutritional transition has led the population to consume food with a low nutritional value affecting their health, but if nutritional information is available, people could improve their diet quality. As household income decreases, diets are likely to shift towards cheaper, more energy-dense and less micronutrient-dense foods. Promotion of an adequate and balanced diet could allow the consumer to experience food safety that favors better health and directly impacts the response of the immune system to infections [44].
Obesity represents an inflammatory state associated with chronic activation of the immune system due to accumulated fat and the endocrine role of the adipocyte producing adipocytokines. Inflammation associated with obesity has been related to an altered lung function and decreased defense mechanisms and immune system response, which consequently enhance infection rates and probability of vaccination failure. Excess fat has also been correlated with an overactivation of the complement system that leads to the development of the “cytokine storm,” as well as with overexpression of ACE2, produced by mature adipocytes [45]. If the effect of the obesity-related double burden of malnutrition is added to this clinical picture, it is possible to understand more clearly the susceptibility of the obese patient to COVID-19.
Role of Micronutrients During the Viral Infection of SARS-CoV-2
Vitamin A
Vitamin A (Vit A) and carotenoids, derived from plants, have three bioactive forms: retinoic acid (RA), retinal, and retinol that carry out very important biological functions like maintenance of vision, growth, and integrity of epithelial and mucosal tissue that lines every external surface exposed to microorganisms. Thus, Vit A plays a role in the first line of defense against pathogen invasion by promoting mucin secretion and participating as a promoter of cell morphology and differentiation, especially, in the respiratory and gut epithelium.
Vit A deficiency reduces the innate immune response affecting the mechanical barrier function of epithelia and enhances respiratory and intestinal infections [46]. Mucin production in these epithelia is regulated by retinoic acid; hence, moderate-dose vitamin A supplementation improves barrier integrity by regulating gene expression of epithelial growth factors and related cytokines [47].
RA exerts its effects on skin and mucous membranes through direct mechanisms that regulate gene expression by activating the nuclear retinoic acid receptor (RAR), which forms a heterodimer with the retinoid X receptor (RXR). Once in the cell nucleus, this complex binds to specific retinoic acid response elements (RARE), which dissociate the repressors and favors the transcription of target genes [48].
RXRs and RARs are also expressed in immune cells such as B and T lymphocytes, and in dendritic cells (DCs), the most important antigen-presenting cells that activate virgin T helper cells and lead to T cell differentiation according to the presented antigen. Vit A metabolite RA has a central role in the proliferation and differentiation of innate and adaptive immune cells. At normal RA levels, the differentiation of immune cells leads to a balanced population of anti-inflammatory regulatory T cells (Treg) and proinflammatory effector T cells, which can produce interferon-gamma. RA regulates the differentiation of dendritic cells, which present antigens to CD4 + T cells that induce Th17 inflammatory responses and secrete IL-17. On the other hand, in non-inflammatory states, RA promotes Treg cells by the reduction of IL-6 secretion that negatively regulates the Th17 response and prevents an excessive immune reaction [48]. A balance between Helper T cells and Regulatory T cells is necessary for the appropriate development of immune responses.
Vit A deficiency alters the phagocytic and bactericidal activity of other cells of the innate immune system such as neutrophils and macrophages; this situation leads to further inflammation. In these cases, the number and activity of lymphoid cells decrease, such as natural killer cells, which can lead to an inefficient antiviral response [47]. Alterations in the epithelium and lung parenchyma have been described in infants with low levels of Vit A, a condition that is associated with an increased risk of respiratory diseases and pulmonary dysfunction [49]. It also has been reported that children with subclinical Vit A deficiency are more likely to have recurrent respiratory infections [50]. Critically ill COVID-19 patients have reduced levels of Tregs and interferon-gamma, so this balance is lost when SARS-CoV-2 infection occurs [51]. Thus, Vit A contributes to the maintenance of homeostasis between anti-inflammatory and proinflammatory stimuli.
In a study conducted by Tomasa-Irriguible et al. [5] in Badalona, Spain, 120 hospitalized COVID-19 patients, with ARDS criteria, were analyzed and 50 of these patients were admitted to the intensive care unit (ICU). Of them, 71.7% had low Vit A levels in plasma, with a mean value of 0.17 mg/L (normal levels > 0.3 mg/L). Low Vit A levels were associated with male sex (69% vs. 45%, p = 0.02), with the need for ICU admission (62.1% vs. 20.7%; p = 0.048), orotracheal intubation (OTI) rate (92.3% vs. 7.7%; p = 0.0001), with the need for prone decubitus (93.6% vs. 6.4%; p = 0.0001), the need for noradrenaline (92.9% vs. 7.1%; p = 0.001), and a higher rate of bacterial respiratory superinfection (95.7% vs. 4.3%; p = 0.017). All deceased patients from this study had low vitamin A levels, but a statistical association between Vit A deficiency and mortality was not found.
In contrast, in a multicenter, observational, cross-sectional, prospective analysis investigation conducted in Germany, the researchers detected a strong association between Vit A deficiency and the development of ARDS and mortality (OR 5.21 [1.06–25.5], p = 0.042). Forty hospitalized patients with SARS-CoV-2 infection and diagnosed with moderate, severe, and critical ARDS were included in the study. The control group consisted of 47 recovered patients with only mild symptoms who did not require hospitalization, and the results of the study revealed that Vit A deficiency was higher as ARDS severity increased and significantly lower in the recovered group (p < 0.01 to p < 0.001). In this study, a Vit A concentration > 2 mg/L was considered low [51] (see Table 1).
Table 1.
Summary of clinical studies findings regarding respiratory viral infections and COVID-19 in association with microelement concentrations
| Authors, year | Country | Participants/study type | Outcomes | |
|---|---|---|---|---|
| Vitamin A | Zhang et al. 2020 [50] | China |
277,064 children 0–14 years old Cross-sectional study |
Subclinical Vit A deficiency (serum Vit A < 0.2 mg/L) prevalence of 10.4% Children with recurrent respiratory infections were more vulnerable to subclinical Vit A deficiency (21.3%, 95% CI: 20.5–22.2%) |
| Tepasse et al. 2021 [51] | Germany |
40 COVID-19 hospitalized patients: -Moderate (n = 9): 54 (30–81) years old -Severe (n = 9): 50 (39–73) years old -Critical (n = 22): 58 (41–82) years old 47 age-matched convalescent persons (control group): 54 (41–70) years old Prospective, multicenter observational cross-sectional study |
Gradual association between low Vit A levels and greater severity of COVID-19. Patients with serum Vit A < 0.2 mg/L were significantly associated with the development of acute respiratory distress syndrome (OR = 5.54 [1.01–30.26]; p = 0.048), mortality (OR 5.21 CI 1.06–25.5, p = 0.042), and inflammatory markers, such as c-reactive protein, ferritin, albumin, and lymphocyte count (p < 0.001) | |
| Tomasa—Irriguible et al. 2021 [5] | Spain |
120 hospitalized COVID-19 patients, with acute respiratory distress syndrome criteria Cross-sectional descriptive study |
Low levels of Vit A in 71.7% of patients, with a mean value of 0.17 ± 0.06 mg/L (normal levels > 0.3 mg/L). Vit A deficiency was associated with male sex (69% vs. 45%, p = 0.02), with the need for ICU admission (62.1% vs. 20.7%; p = 0.048), the orotracheal intubation rate (92.3% vs. 7.7%; p = 0.000), the need for prone position (93.6% vs. 6.4%; p = 0.0001), the need for norepinephrine (92.9% vs. 7.1%; p = 0.001), and a higher rate of respiratory bacterial superinfection (92.6% vs. 7.4%; p = 0.016) | |
| Al-Saleh et al. 2022 [53] | Saudi Arabia |
155 COVID-19 patients 18–95 years old -Asymptomatic (n = 16) -Mild (n = 49) -Moderate (n = 68) -Severe (n = 22) Cross-sectional descriptive study |
Low levels of Vit A in 36.5% of patients (normal levels > 0.34 µg/mL) A 23% decrease in Vit A in patients with severe symptoms, which disappeared after adjusting for inflammatory markers |
|
| Vitamin B | Itelman et al. 2020 [58] | Israel |
162 COVID-19 patients 52 ± 20 years old -Mild (n = 92) -Moderate (n = 44) -Severe (n = 26) Cohort study |
Folic acid levels were significantly lower in severe patients when compared with moderate and mild cases (9.6 ng/mL vs. 12.9 ng/mL vs. 18.2 ng/mL, p = 0.005) A total of 80.8% of patients with severe COVID-19 were admitted to the intensive care unit (p = < 0.001). All deaths were in the severe disease subgroup (19.2%) |
| Deschasaux—Tanguy et al. 2021 [57] | France |
7766 adults -311 positive for anti-SARS-CoV-2 antibodies NutriNet-Santé web-based cohort study |
SARS-CoV-2 negative participants had a higher intake of Vit B9 when compared with positive patients (330 ± 90 µg/day and 320 ± 90 µg/day, respectively, p = 0.004). Dietary intake of Vit B9 (OR = 0.84 CI 0.72, 0.98, p = 0.02) was associated with a decreased probability of SARS-CoV-2 infection | |
| Tan et al. 2020 [59] | Singapore |
43 hospitalized COVID-19 patients > 50 years old -Intervention group: Vitamin B12, D, and Mg supplementation (n = 17) -Control group (n = 23) Cohort observational study |
Patients with Vit B12, D, and Mg supplementation had a lower need to start oxygen therapy (OR 0.13, 95% CI 0.03–0.59) and intensive care support (OR 0.20 95% CI 0.04–0.93) than the control group, during their hospital stay | |
| Vitamin D | Rodriguez et al. 2020 [68] | Mexico |
172 COVID-19 patients 51.44 ± 14 years old Cross-sectional observational study |
Vit D deficiency in 68% of patients No significant differences were found between mortality and Vit D levels (deceased 13.6 ± 6.36 ng/mL vs. survivors 17.30 ± 7.44 ng/mL, p = 0.25). Patients with Vit D < 8 ng/mL have a 3.69-fold risk of dying compared to those with levels > 8 ng/mL (OR 3.69, CI 1.62–8.37, p = 0.001) |
| Kaufman et al. 2020 [66] | USA |
191,779 patients who underwent SARS-CoV-2 testing, who had Vit D testing results from the previous 12 months Retrospective, observational study |
Patients with deficiency (< 20 ng/mL) of Vit D (n = 39,190) had a higher positivity rate for SARS-CoV-2 than those with adequate (30–34 ng/mL) Vit D levels (12.5%, CI, 12.2–12.8% vs. 8.1%, CI 7.8–8.4%) SARS-CoV-2 positivity was strongly and inversely associated with circulating Vit D (R2 = 0.96), a relationship that persists across latitudes, races/ethnicities, both sexes, and age ranges |
|
| Hastie et al. 2021 [67] | UK |
341,484 UK Biobank participants -203 died due to COVID-19 Retrospective, observational study |
Vit D serum concentration was associated with severe COVID-19 infection and mortality (HR 0.92; 95% CI 0.86–0.98; p = 0.016), but not after adjustment for confounders (HR 0.98; 95% CI 0.91–1.06; p = 0.696) | |
| Al-Saleh et al. 2022 [53] | Saudi Arabia |
155 COVID-19 patients 18–95 years old -Asymptomatic (n = 16) -Mild (n = 49) -Moderate (n = 68) -Severe (n = 22) Cross-sectional observational study |
Low levels of Vit D3 in 68% of patients (normal levels > 20.05 µg/mL) Twelve of sixteen deceased patients had Vit D3 levels < 12 µg/mL (mean 5.09 µg/mL), but without differences with Vit D3 levels in survivors (4.81 µg/mL) No association between vit D3 levels and severity, total antioxidant capacity, or superoxide dismutase was detected, after adjusting for inflammatory markers and laboratory parameters |
|
| Selenium | Moghaddam, et al. 2020 [41] | Germany |
33 COVID-19 patients 166 consecutive serum samples Cross-sectional observational study |
A total of 44.4% of COVID-19 patient samples were Se deficient (normal levels 45.7–131.6 µg/L). Serum Se levels were significantly lower in deceased COVID-19 patients vs. survivors (40.8 ± 8.1 µg/L vs. 53.3 ± 16.2, p < 0.001) A total of 39.6% of COVID-19 patient samples were deficient in SELENOP (normal levels 2.56–6.63 mg/L) Serum Se and SELENOP showed the expected strong correlation (r = 0.7758, p < 0.001) A total of 64.7% of deceased COVID-19 patients were Se deficient and 70.6% were SELENOP deficient while 39.3% and 32.6% of surviving patients were Se and SELENOP deficient, respectively |
| Kocak, et al. 2021 [77] | Turkey |
92 adults -SARS-CoV-2 infected (n = 60) -Healthy (n = 32) Observational study |
Serum Se levels were significantly lower in SARS-CoV-2 infected patients when compared with healthy adults (255.23 ± 42.67 ppb vs 255.23 ± 42.67 ppb, respectively Patients with mild, moderate, and severe disease had significantly lower selenium levels than healthy, asymptomatic patients (p < 0.001), suggesting that serum Se level is important in asymptomatic treatment of the disease |
|
| Razeghi, et al. 2021 [3] | Iran |
84 COVID-19 patients -Mild (n = 38) -Moderate (n = 27) -Severe (n = 19) Observational study |
Serum Se was as follows: 47.07 ± 20.82 ng/mL, 47.36 ± 25.6 ng/mL, 29.86 ± 11.48 ng/mL in the mild, moderate, and severe disease group, respectively Significant negative association between serum Se level and COVID-19 severity (standardized coefficient = − 0.28, p = 0.01) |
|
| Al-Saleh, et al. 2022 [53] | Saudi Arabia |
155 COVID-19 patients 18–95 years old -Asymptomatic (n = 16) -Mild (n = 49) -Moderate (n = 68) -Severe (n = 22) Cross-sectional observational study |
Thirty percent of total participants were deficient in Se (< 70.08 µg/L) Patients with severe symptoms were Se deficient in 18% of the cases Se was independently associated with COVID-19 severity (p = 0.214) |
|
| Zinc | Jothimani, et al. 2020 [88] | India |
47 COVID-19 patients 45 healthy controls Prospective study |
Low zinc levels in 57.4% of COVID-19 patients (normal reference levels: 71.8–79.6 µg/dl). COVID-19 patients had significantly lower Zn levels in comparison to the healthy controls: median 74.5 µg/dl (IQR 53.4–94.6 µg/dl) versus 105.8 µg/dl (IQR 95.65–120.90 µg/dl), p < 0.001) COVID-19 patients with ZN deficiency had a higher risk for developing complications (OR 5.54, IC del 95%: 1.56–19.6, p = 0.008) and for mortality (OR 5.48, 95% CI 0.61–49.35, p = 0.129) |
| Zeng, et al. 2021 [89] | China |
306 hospitalized COVID-19 patients -Severe cases (n = 104, 34.0%) -Non‐severe cases (n = 202, 66.0%) Retrospective cohort study |
Non-severe cases had higher Zn levels 6.61 (5.91–7.25) µg/L than severe cases 6.18 (5.67–6.79) µg/L, p < 0.001 Reference Zn normal levels: 4.3–7.8 mg/L A correlation between Zn and magnesium levels (CC 0.36 p < 0.05), as well as Zn and iron (CC 0.64 p < 0.05), was detected, suggesting a possible synergic effect in COVID-19 |
|
| Kocak, et al. 2021 [77] | Turkey |
92 adults -SARS-CoV-2 infected (n = 60) -Healthy (n = 32) Observational study |
Zn serum levels in COVID-19 patients were lower (588.17 ± 195.02 ppb) than those of healthy participants (873.4 ± 335.38 ppb, p < 0.001) A gradual decrease between Zn levels and severity were detected when assigning COVID-19 patients into groups of mild, moderate, and severe disease manifestations (p < 0.0001) |
|
| Al-Saleh, et al. 2022 [53] | Saudi Arabia |
155 COVID-19 patients 18–95 years old -Asymptomatic (n = 16) -Mild (n = 49) -Moderate (n = 68) -Severe (n = 22) Cross-sectional observational study |
Low levels of Zn in 25% of patients (Zn deficiency: < 0.693 µg/mL) No association between Zn levels and severity, vitamin E, and vitamin D3 serum levels was detected, after adjusting for inflammatory markers and laboratory parameters |
|
| Cooper | Hackler, et al. 2021 [103] | Germany |
35 hospitalized COVID-19 patients 173 consecutive serum samples |
High levels of Cu were associated with survival (Cu; 1475.9 ± 22.7 µg/L vs. 1317.9 ± 43.9 µg/L; p < 0.001), the same way its biomarker, ceruloplasmin (CP; 547.2 ± 19.5 mg/L vs. 438.8 ± 32.9 mg/L, p = 0.086) A positive linear correlation between Cu and Se levels was detected in COVID-19 patients, but not consistent during the acute phase response (R = 0.23, p = 0.003) |
| Zeng, et al. 2021 [89] | China |
306 hospitalized COVID-19 patients Severe cases (n = 104, 34.0%), non‐severe cases (n = 202, 66.0%) Retrospective cohort study |
Higher Cu levels in severe COVID-19 patients (929.73, 828.52–1080.02 µg/L) than in non-severe cases (838.55, 770.47–950.13 µg/L) Cu reference normal levels: 634.1–999.4 µg/L |
|
| Kocak, et al. 2021 [77] | Turkey |
92 adults -SARS-CoV-2 infected (n = 60) -Healthy (n = 32) Observational study |
No significant difference between COVID-19 patients Cu serum levels (952.48 ± 388.75 ppb) and healthy participants (2795.99 ± 9605.09 ppb) was detected, p > 0.05 The Zn/Cu ratio in COVID-19 patients (median ± SD, 0.68 ± 0.28) vs healthy patients (median ± SD, 0.86 ± 0.63) was not significant. However, the Zn/Cu ratio showed a significant positive correlation with hemoglobin |
|
| Al-Saleh, et al. 2022 [53] | Saudi Arabia |
155 COVID-19 patients 18–95 years old -Asymptomatic (n = 16) -Mild (n = 49) -Moderate (n = 68) -Severe (n = 22) Cross-sectional observational study |
Low levels of Cu in 3.2% of patients. Cu reference levels: 1 < 0.701 µg/mL Asymptomatic (1.30 ± 0.678 µg/mL), mild (1.31 ± 0.351 µg/mL), moderate (1.25 ± 0.341 µg/mL), and severe (1.22 ± 0.370 µg/mL) Eighty-three percent of patients having a Cu/Zn ratio > 1. This ratio is associated with COVID-19 severity when adjusted for inflammatory marker parameters (p < 0.05) |
Vit A, vitamin A; mg/L, milligrams per liter; CI, confidence interval; OR, odds ratio; COVID-19, coronavirus disease 2019; SARS-CoV-2, respiratory syndrome coronavirus 2; ICU, intensive care unit; µg/mL, micrograms per milliliter; ng/mL, nanograms per milliliter; µg/day, micrograms per day; µg/L, micrograms per liter; Vit B9, vitamin B9; Vit B12, vitamin B12; Vit D, vitamina D; Mg, magnesium; Vit B3, vitamin B3; HR, hazard ratio; SELENOP, selenoprotein P; ppb, parts per billion; Cu, cooper; Se, selenium; Zn, zinc; CP, ceruloplasmin
A theory presented by Sarohan [52] links defective metabolism of retinoic acid with COVID-19. Accordingly, the RIG-I pathway decreases, and the immune defense mechanism shifts to the activated TLR3, TLR7, TLR8, TLR9, MDA5, and UPS pathways found in neutrophil, macrophage, and dendritic cells. This causes a cytokine storm through the activation of NF-κB pathway, resulting in severe clinical presentations. In this situation, stored Vit A is quickly depleted, because of an overuse of retinoic acid in the RIG-I pathway and the IFN-I synthesis pathway, a process that is called “Retinoic Acid Depletion Syndrome” (RADS). Such theories highlight the importance of further research into Vit A and its relationship to the development and response towards viral infections. A more recent study of 155 older patients (18–95 years) showed 36.5% were Vit A deficient (< 0.343 mg/L); authors suggest that the patient with COVID-19 disease depletes serum Vit A storage. The immune response mechanism changes from innate to adaptive, preventing retinoic acids from being used, showing that inflammation balances the relationship between COVID-19 severity and Vit A levels [53].
Vitamin B
Thiamine (B1), riboflavin (B2), niacin (B3), pantothenic acid (B5), pyridoxine (B6), biotin (B7), folic acid (B9), and cobalamin (B12) are 8 compounds that form the water-soluble vitamin B (Vit B) complex that can be obtained through the diet, mainly from plants or red meat. Individual or as a complex, these vitamins can play an important role in the metabolism of proteins, lipids, and nucleic acids. They also play a role in the immune response and in the antioxidant response against oxidative stress [54].
Vit B12 is essential for DNA synthesis and regulation; its deficiency alters DNA and RNA synthesis because of its participation as a cofactor of methionine synthase. Transcobalamins exert their antioxidant mechanism facilitating the bioavailability of reduced glutathione in the cytosol and, therefore, promote the synthesis of oxidized glutathione. Cobalamin is produced by the intestinal microbiota and contributes to the regulation of the gut-brain axis, protects against intestinal dysbiosis, and favors the production of microbial metabolites in adequate proportions, with a positive impact at the metabolic level, contrary to what happens in a deficient state [55]. These processes are vital for DNA synthesis, cell homeostasis, hematopoiesis, and immunity.
Under physiological conditions, Vit B12 regulates the expression of anti-inflammatory cytokines and growth factors, which reduces systemic inflammation. In addition, by promoting the increase of NK cells and CD8 + T cells, it improves the immune response and regulates the antiviral response. Its modulatory participation in the function of phagocytic cells, interferon production, maturation of T lymphocytes, and viral replication has been described. When Vit B12 is deficient, there is a greater risk of infections and an increase in their severity [55]. Some factors such as age and the use of some drugs are associated with a higher risk of Vit B12 deficiency. In elderly, the decrease in the production of intrinsic factor results in B12 malabsorption, poor nutrition, or increased urinary and intestinal losses [38]. The administration of metformin as a treatment of type 2 diabetes has also been associated with Vit B12 malabsorption triggering deficiency. Therefore, it could make these patients more susceptible to infections [4].
Several studies have reported a possible association between deficiency of Vit B compounds with COVID-19 (Table 1) [4, 38, 54, 56]. For example, in 311 French participants of the NutriNet-Santé study, a lower consumption of Vit B9 was detected in patients positive for SARS-CoV-2 when compared to 7455 healthy participants (OR = 0.84 (0.72,0.98), p = 0.02) [57]. In Israel, 162 patients diagnosed with severe COVID-19 showed lower folate levels than moderate and mild cases (9.6 ng/mL vs 12.9 ng/mL vs 18.2 ng/mL, respectively, p = 0.005) of which 12% were immunosuppressed, 9% required non-invasive oxygenation, and 15% were intubated [58]. In another cohort of patients from Singapore, in which 43 COVID-19 patients over 50 years old participated [59], it was detected that joint supplementation of Vit B12, Vit D, and magnesium, when admitted to the hospital, was associated with a decrease in severity of the disease. In supplemented patients, there was an association with less need for oxygen therapy (17.6 vs 61.5%, p = 0.006) and a decrease in the need for intensive care, when compared to those who did not receive supplements of these micronutrients. Although these studies support the involvement of B vitamins in COVID-19 severity, clinical trials are needed to prove a causal association.
Vitamin D
Vitamin D (Vit D) levels depend on diet and exposure to UVB radiation, where 7-dehydrocholesterol 25 (OH) D is obtained and then converted to its active form, calcitriol 1,25 (OH) D, in the liver, kidneys, or other cells, including alveolar macrophages, lung epithelial cells, dendritic cells, and lymphocytes. Although, the main studied effect of this fat-soluble vitamin is involved in bone homeostasis through the regulation of calcium and phosphorus metabolism, and participates in immune function regulation. Vit D stimulates innate cellular immunity by inducing the production of antimicrobial peptides that are involved in antiviral responses such as cathelicidins, IL-37, and defensins. Vit D inhibits the cytokine storm by reducing the production of proinflammatory cytokines (IFNγ, IFNß, TNFα, and IL6) and enhancing regulatory cytokine IL-10, and it also modulates the function of immune cells by regulating adaptive immune response, suppressing the Th1 response and promoting the production of Th2 cytokines[60]. It is possible that lung production of the active form of Vit D is associated with the role of this microelement in the immune response towards respiratory infections [23].
Different mechanisms that conduct to the described effects of Vit D have been reported. For example, Vit D can induce an increase in IKBa levels, NF-κB inhibitor, in pulmonary epithelial cells that are infected with the respiratory syncytial virus. The inhibition of this transcription factor decreases the expression of target genes such as IFN-β and CXCL10, regulating the inflammatory process [61]. Vit D also reduces the effect of TGF-β on the development of fibrosis, prevents apoptosis of alveolar epithelial cells, and participates in the maintenance of the pulmonary epithelium integrity by regulating the renin-angiotensin axis. Thus, as a whole, these mechanisms protect the pulmonary epithelium and favors the resolution of ARDS, as demonstrated by Zheng et al. [62] in a murine model with LPS-induced lung injury.
Vit D is one of the most studied micronutrients in the progression of COVID-19 as shown in Table 1. Since the beginning of the pandemic, its use as a supplement has been suggested around the world, due to its potential preventive effects. These theories arise from different studies that have reported Vit D deficiency in patients with COVID-19, in association with the severity of disease and with the admission to ICU, being a more pronounced deficiency in adults older than 70 years [39, 63, 64]. In addition to age, ethnicity is another factor that has been associated with Vit D deficiency and the possibility of being a risk factor for COVID-19. Research by Hastie et al. [40] shows an association of black and South Asian ethnicity with confirmed COVID-19 (OR = 5.49, 95% CI = 3.82 to 7.88, p < 0.001; OR = 2.76, 95% CI = 1.74 to 4.39, p < 0.001, respectively) when compared with white ethnicity. However, when adjusting the model considering Vit D values, the ORs remained with minimal changes without modifying statistical significance. Therefore, despite being related, their simultaneous presence does not potentiate the risk of COVID-19.
Marcola et al. [65] evaluated the results of different studies on Vit D, seeking to determine if the associations with the reduction in the severity of COVID-19 are due to a possible causal relationship. Their review gathers evidence that meets Hill’s criteria for temporality, strength of association, dose–response relationship, consistency of findings, plausibility, accounting for alternate explanations, and consistency with known facts. However, there is a lack of clinical trials to experimentally confirm these associations, which makes associations inconclusive [39, 63, 64]. For example, in an observational study in the USA with 191,779 patients [66], a higher percentage of positivity for SARS-CoV-2 was found in those with Vit D levels below 20 ng/mL (12.5%, 95% CI, 12.2–12.8%) when compared with patients with levels above 55 ng/mL (5.9%, 95% CI, 5.5–6.4%). On the other hand, in 656 patients from the UK [67], baseline Vit D levels were evaluated in association to COVID-19 mortality. In this study, despite the fact that initially there seemed to be a clear relationship, after adjusting for confounding variables, no relationship was found with either the risk of developing COVID-19 or mortality, when taking as reference 502,624 UK Biobank participants (mortality per 10 nmol/L 25(OH)D HR 0.98; 95% CI = 0.91–1.06; p = 0.696). The same is confirmed by other research groups, one with a sample of 155 patients from Saudi Arabia [58], in which 103 participants (68.2%) had low vitamin D3 values but without association when performed bivariate analyses. Another study in 172 Mexican COVID-19 patients [68] detected an association only in those patients with Vit D levels < 8 ng/mL with a 3.69-fold risk of mortality, but without risk differences in patients with higher concentrations. Despite this, it is evident that the relationship of Vit D deficiency derived from comorbidities such as obesity, diabetes, or natural non-modifiable factors such as age and ethnicity has a relationship that adds to the nutritional status of the patient, leaving this population vulnerable to virus infection.
Selenium
Selenium (Se) is an essential micronutrient of relevance as part of the proteinogenic amino acid selenocysteine (Sec), for which an elaborate biochemical pathway has been developed and conserved in many species [69]. The main activity of Se in the organism is carried out due to its presence as a component in the structure of selenoproteins. Its functions include maintenance of the REDOX balance in cells, its antioxidant and anti-inflammatory activity, and the regulation of endoplasmic reticulum stress. A diet deficient in Se may result in a loss of immunocompetence leading to increased susceptibility to viral infections and cancer. Both its dietary restriction and the suppression in the expression of selenoproteins have been associated with higher levels of pro-inflammatory cytokines, IL-1β, IL-6, and TNF-α, in a variety of tissues, including the gastrointestinal tract, uterus, mammary gland tissues, and lung tissue [70]. Therefore, Se appears to play an important role in fighting viral diseases, such as COVID-19 [27, 71].
To date, twenty-five genes encoding selenoproteins have been identified [71], such as selenoprotein F, K, M, N, and S that fold proteins and protect against oxidative stress from the endoplasmic reticulum [72]. There is also the SELENOP antioxidant defense role, which, as it is the most abundant, is used as a biomarker of Se status [73]. Glutathione peroxidase 1 (GPX1) is one of the selenoproteins most affected by Se deficiency [74]. Considering that virus infection increases oxidative stress, GPX1 comprises a key defense against ROS by its enzymatic function catalyzing the detoxification of hydrogen peroxide into water.
Moreover, Se is necessary for phagocytic cells, which are a major component of the innate immune system. Insufficient Se intake reduces the level of phagocytic activity, which may reduce the oxidative burst as has been observed in neutrophils [30]. In mice, low Se intake has shown to inhibit the macrophage phagocytic response by significantly increasing the expression of inflammatory factors, such as iNOS, IL-1β, IL-12, IL-10, PTGe, and NF-κB; meanwhile, suppression of Se restricts macrophage production of TNFα [75].
Associations between the infection, its severity, and the reported COVID-19 cure rates with the Se status have been described (Table 1) [76] with cross-sectional studies where Se and selenoprotein P have been quantified in samples from patients who survived COVID-19, presenting higher concentrations when compared to non-survivors [41]. One of the first cross-sectional analyses quantified two important markers of Se status, SELENOP and total serum Se, both of which were significantly higher in samples from surviving COVID-19 patients compared to non-survivors (Se; 53.3 ± 16.2 vs. 40.8 ± 8.1 µg/L, SELENOP; 3.3 ± 1.3 vs. 2.1 ± 0.9 mg/L) [41]. In the study by Kocak et al. [77], significantly higher levels of Se were found in healthy controls in relation to patients with COVID-19, mostly with moderate manifestations, who had concentrations of 255.23 ± 42.6 ng/dL and with 215.34 ± 49.8 ng/dL, respectively (p > 0.001). In addition to these reports, Razeghi et al. [3] found a negative association between Se and Zn levels with respect to the degree of clinical severity of COVID-19 in a sample of 84 patients who participated in the study. They found that Se levels were below the recommended normal ranges, being the patients with severe clinical manifestations the ones that presented the lowest levels, with a mean of 29.86 ± 11.48 ng/mL. Several cross-sectional studies show that regardless of the degree of severity, once the disease is established, selenium levels do not change significantly, being the most relevant difference between healthy subjects and those infected with SARS-CoV-2 [37, 53, 78, 79]. The possible protective effects of Se against COVID-19 could be due to its role as an essential cofactor in selenoproteins that act to reduce ROS formation, as well as its anti-inflammatory properties by modulating the production of proinflammatory cytokines, since both of them are key mechanisms in the pathophysiology of this infection.
Zinc
Zinc (Zn) is an essential trace element with important physiological functions such as its participation in growth and the immune system. Body Zn deficiency is frequently due to malabsorption and increased gastrointestinal losses, in addition to deficient intake through diet [80]. The lung epithelial barrier is the first to be exposed to respiratory viruses. It has been shown that Zn deficiency alters epithelial barrier function through positive regulation of IFN-ɣ and TNF-ɑ, but also enhances FasR signaling and apoptosis so that Zn supplementation could prevent or decrease apoptosis [81]. Also, Zn deficiency modifies cellular functions that affect the immune response, for example, it affects Th1 cells and, with it, the production of IL-1, IL-2, IL-4, and IFN-ɣ, disturbing the balance of Th1/Th2 profiles which influences the isotype change from CD4 + to CD8 + [22]. Zinc signals induce tolerogenic dendritic cells by suppressing MHC-II expression and enhancing PD-L1, dampening proinflammatory Th17 and Th9 cells by Treg generation [82]. Zn is involved in the development and function of cells involved in innate immunity regulation, such as monocytes, neutrophils, dendritic cells, and NK cells, and its deficiency affects cell function and antibody production [83]. The involvement of Zn in COVID-19 is related to at least two possible non-exclusive mechanisms. First, Zn is a known structural cofactor of viral proteins, suggesting that the accessibility of Zn ions in infected cells could be a potentially limiting factor in the virus life cycle. Second, Zn ions are involved in signal transduction in the cell and, therefore, in cellular and viral gene expression patterns [84]. In patients with COVID-19, low Zn levels have been associated with advanced age, with a higher rate of ICU admission and its clinical complications [5]. Cell culture studies of SARS-CoV and equine arteritis virus (EAV) have been conducted in combination with Zn2 + and Zn ionophores such as pyrithione (PT), resulting in direct inhibition of the in vitro activity of viral RNA polymerases, preventing RNA elongation in SARS-CoV [85]. Therefore, this mechanism could be similar in cases of SARS-CoV-2 infection. It has been shown that Zinc treatment in cells infected with rhinovirus increases the production of interferon α (IFNα) by leukocytes and improves antiviral activity. Considering that downstream signaling of JAK1/STAT1 and the positive regulation of antiviral enzymes mediate the antiviral effects of IFNα, it is possible that Zn could be favoring this mechanism [42].
Zn deficiency in aging and metabolic diseases such as diabetes, obesity, and cardiovascular diseases is indirectly related to susceptibility to COVID-19 [86]. The important role of Zn in immunity and in the viral cell cycle is evident and could have the potential to be studied as an adjuvant treatment for clinical management, seeking to increase antiviral resistance. To date, clinical trials related to Zn and COVID-19 have presented mixed results (Table 1). The report by Al-Saleh et al. [53] showed a percentage of 25% of COVID-19 patients with Zn deficiency (< 0.693 µg/mL). However, when they performed a statistical adjustment to include inflammatory markers, no significant correlation was found to associate COVID-19 severity with Zn deficiency. In contrast, a cross-sectional comparative study reported that serum Zn levels decreased (median 56.61 µg/dL, n = 200) in relation to disease severity, with the most reduced values in severe COVID-19 patients [87]. This result agreed with those of Jothimani et al. [88] who found significantly lower Zn levels in COVID-19 patients (n = 47) when compared to healthy controls (n = 45) (74.5 µg/dl (IQR 53.4–94.6 µg/dl) vs. 105.8 µg/dl (IQR 95.65–120.90 µg/dl), p < 0.001). Similarly, in a retrospective cohort study conducted in China, they found a high correlation coefficient of severe COVID-19 disease with different transition metals, especially Zn, magnesium, and iron (CC: 0.64 for iron-zinc; CC: 0.55 for iron-magnesium; CC: 0.51 for iron-manganese) [89]. The aforementioned clinical studies present interesting findings that establish relevance to critical proteins involved in the antiviral immune response and containing zinc in their structure, mainly those that disrupt virus replication. Further investigation of this trace element in controlled trials will be necessary to verify the association between Zn and COVID-19.
Copper
Copper (Cu) is an essential micronutrient with two main biological functions, the first as a structural/catalytic cofactor of enzymes, and the second as a coactivator of key transcription factors [90]. Cu serves as a cofactor when present as an ion and is used in redox enzymatic reactions [91] or carrying out other functions such as the metabolism of biological amines, activation of neuropeptides, or cell respiration (cytochrome-c oxidase). Also, Cu has been linked to selenoprotein expression in animal models where it suppresses the mRNA levels of GPX1 selenoprotein and selenoprotein W, generally reducing the enzymatic activity of GPX and thyroxine reductase (TXNRD). This effect is achieved by decreasing the coding efficiency of the UGA codon in the cell but has been little explored and for this reason it is not conclusive [92]. Ceruloplasmin ferroxidase (CP) accounts for the majority of circulating Cu and serves as a transport protein. These data suggest that Cu has specific functions in viral replication in the cytoplasm, as well as in the interactions of viral proteins with the secretory pathway [93]. On the other hand, copper neutralizes toxic ROS as part of cytosolic and extracellular Cu(II)/Zn(II) superoxide dismutases (SOD1 and SOD3) [94]. Hence, its protective role against viral diseases is decisive. During the first stage of H5N1 influenza virus replication, virus-induced disruption of cytosolic Cu/Zn-SOD formation [95] unbalances the oxidative process and ATP7A, a protein that imports [Cu1 +] into trans-Golgi–derived compartments, is required for the synthesis of influenza viral RNA and proteins [96]. In vitro studies show a link between host proteins that transport Cu into the cell (CTR1) and the secretory pathway (ATP7A), with their role in influenza A (H1N1) virus protein and RNA synthesis [90].
Regarding immune function, Cu is an important factor for the proper functionality of immune cells such as B cells, T-helper cells, NK cells, neutrophils, and macrophages. Cu transport genes have been implicated in macrophage-mediated host defense [97, 98], its deficiency reduces IL-2 and T-cell proliferation, as well as reducing the number of circulating neutrophils and their capacity to generate superoxide anion [99]. On viral infections and upon initiation of the oxidative response by ROS, Cu downregulates NF-κB expression, causing suppression of inflammatory cytokines, chemokines, and adhesion molecules [100]. Cu dietary deficiency has been examined in humans and has been associated with the proliferation of peripheral blood mononuclear cells [101]. A meta-analysis of Cu consumption associated the occurrence of recurrent respiratory tract infections in Chinese children with immune function, genetic factors, and nutritional status. Among the results of this study, Zn and Cu deficiency may be contributing factors to children’s susceptibility to respiratory infections [102]. Another group of researchers determined the presence of Cu by CP quantification in serum samples from patients with COVID-19, finding that surviving patients had higher serum concentrations of Cu and CP compared to non-survivors [103]. There is a relationship between trace elements Zn and Cu due to their antagonistic behavior and competitive absorption relationship. When Zn intake is exceeded for prolonged periods, it can contribute to a Cu deficiency and vice versa [100]. Al-Saleh et al. [53] describe a 1:1 Cu/Zn ratio in healthy adults and in COVID-19 patients from their study; this ratio remained high (Cu/Zn > 1.5 + –0.63, 83%), a result that significantly correlated with C-reactive protein, an indicator of inflammation. A similar result was obtained by Skalny et al. [42] who showed an increase of up to 45% in the Cu/Zn ratio in the case of disease with mild symptoms compared to patients with moderate symptoms, suggesting that this ratio could be a biomarker associated with exacerbation of an inflammatory process derived from the infection. In contrast, in the study by Kocak et al., [76] no differences were found when comparing this ratio between people positive for SARS-CoV-2 with healthy people (see Table 1).
As a result of the COVID-19 pandemic, there was an increase in the uncontrolled consumption of multivitamins, so there is a strong possibility of Cu deficiencies in people with COVID-19 who consumed Zn supplements without supervision. As an essential micronutrient, Cu is required for the maintenance of biological mechanisms and cellular homeostasis; therefore, it is necessary to maintain a balance and avoid both its deficiency and its excess, since there are no conclusive studies relating it to the severity of COVID-19.
Perspectives
The enormous impact that the COVID-19 pandemic has had worldwide is due, in part, to the nutritional deficiency of the population. We have gone through an era of technological, epidemiological, and nutritional transition, where, regardless of the benefits, we also faced the greatest challenges in public health. The consumption of low micronutrient foods, but fat- and carbohydrate-dense, along with a sedentary lifestyle has increased the prevalence of non-communicable diseases, such as obesity, diabetes, and hypertension. In addition to the negative medium and long-term effects of these diseases, the limited consumption of micronutrients causes a weakening of the immune system function and, therefore, a greater susceptibility to developing infections. On the other hand, famine has not been eradicated; hence, energy- and micronutrient-deficient diets predispose a large amount of the world’s population to disease.
The pandemic arrived at a moment of global vulnerability, so it is important to highlight the need to acquire the habits for a healthy diet, where there is an optimal consumption of micronutrients in variety, quality, and quantity. An unbalanced diet is not the only risk factor that predisposes an individual to develop the disease, but it does have many beneficial effects regarding prevention, or it could even accelerate the recovery of patients by the use of micronutrient supplementation.
Conclusion
There is scientific evidence supporting the relationship between micronutrient status and the response against SARS-CoV-2, as a crucial factor that influences the clinical severity of COVID-19, mainly through immunomodulatory and antioxidant mechanisms. The cohort and observational studies carried out to date support a relationship between nutritional status and COVID-19, based on biological gradient, plausibility, analogy, and temporality. However, in order to confirm a causal relationship to the severity of the disease and/or mortality, it is necessary to carry out more clinical trials to demonstrate it and to explore its involvement in the antiviral response.
Acknowledgements
RBNR thanks Consejo Nacional de Ciencia y Tecnología (CONACYT) for her Ph.D. scholarship. The authors are grateful to Mejía-León AM for editing the figure.
Author Contribution
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Roldán-Bretón Nuria Renata. The first draft of the manuscript was written by Roldán-Bretón Nuria Renata and Mejía-León María Esther. González-Rascón Anna Arely and Leija-Montoya Ana Gabriela commented and participated on previous versions of the manuscript. All authors read and approved the final manuscript.
Declarations
Competing Interests
RBNR receives a CONACYT grant number 2021–000018-02NACF-06760 to pursue doctoral studies. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int. Accessed 28 Apr 2022
- 2.Alexander J, Tinkov A, Strand TA, Alehagen U, Skalny A, Aaseth J. Early nutritional interventions with zinc, selenium and vitamin D for raising anti-viral resistance against progressive COVID-19. Nutrients. 2020;12:2358. doi: 10.3390/nu12082358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Razeghi Jahromi S, Moradi Tabriz H, Togha M, et al. The correlation between serum selenium, zinc, and COVID-19 severity: an observational study. BMC Infect Dis. 2021;21:899. doi: 10.1186/s12879-021-06617-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jovic TH, Ali SR, Ibrahim N, Jessop ZM, Tarassoli SP, Dobbs TD, Holford P, Thornton CA, Whitaker IS. Could vitamins help in the fight against COVID-19? Nutrients. 2020;12:2550. doi: 10.3390/nu12092550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomasa-Irriguible T-M, Bielsa-Berrocal L, Bordejé-Laguna L, Tural-Llàcher C, Barallat J, Manresa-Domínguez J-M, Torán-Monserrat P. Low levels of few micronutrients may impact COVID-19 disease progression: an observational study on the first wave. Metabolites. 2021;11:565. doi: 10.3390/metabo11090565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fact sheets - Malnutrition. https://www.who.int/news-room/fact-sheets/detail/malnutrition. Accessed 25 Apr 2022
- 7.Barazzoni R, Gortan Cappellari G. Double burden of malnutrition in persons with obesity. Rev Endocr Metab Disord. 2020;21:307–313. doi: 10.1007/s11154-020-09578-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang H, Rao Z. Structural biology of SARS-CoV-2 and implications for therapeutic development. Nat Rev Microbiol. 2021;19:685–700. doi: 10.1038/s41579-021-00630-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devaux CA, Rolain J-M, Raoult D. ACE2 receptor polymorphism: susceptibility to SARS-CoV-2, hypertension, multi-organ failure, and COVID-19 disease outcome. J Microbiol Immunol Infect. 2020;53:425–435. doi: 10.1016/j.jmii.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santopolo S, Riccio A, Santoro MG. The biogenesis of SARS-CoV-2 spike glycoprotein: multiple targets for host-directed antiviral therapy. Biochem Biophys Res Commun. 2021;538:80–87. doi: 10.1016/j.bbrc.2020.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.V’kovski P, Kratzel A, Steiner S, Stalder H, Thiel V (2021) Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol 19:155–170 [DOI] [PMC free article] [PubMed]
- 12.Jack A, Ferro LS, Trnka MJ, et al. SARS-CoV-2 nucleocapsid protein forms condensates with viral genomic RNA. PLOS Biol. 2021;19:e3001425. doi: 10.1371/journal.pbio.3001425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mu J, Xu J, Zhang L, et al. SARS-CoV-2-encoded nucleocapsid protein acts as a viral suppressor of RNA interference in cells. Sci China Life Sci. 2020;63:1413–1416. doi: 10.1007/s11427-020-1692-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nieto-Torres JL, Verdiá-Báguena C, Jimenez-Guardeño JM, Regla-Nava JA, Castaño-Rodriguez C, Fernandez-Delgado R, Torres J, Aguilella VM, Enjuanes L. Severe acute respiratory syndrome coronavirus E protein transports calcium ions and activates the NLRP3 inflammasome. Virology. 2015;485:330–339. doi: 10.1016/j.virol.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang K-H, Chiang P-Y, Ko S-H, et al. Antibody cocktail effective against variants of SARS-CoV-2. J Biomed Sci. 2021;28:80. doi: 10.1186/s12929-021-00777-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mengist HM, Kombe Kombe AJ, Mekonnen D, Abebaw A, Getachew M, Jin T (2021) Mutations of SARS-CoV-2 spike protein: implications on immune evasion and vaccine-induced immunity. Semin Immunol 101533 [DOI] [PMC free article] [PubMed]
- 17.Yang L, Li J, Guo S, et al. SARS-CoV-2 variants, RBD mutations, binding affinity, and antibody escape. Int J Mol Sci. 2021;22:12114. doi: 10.3390/ijms222212114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu H, Wei P, Zhang Q, et al (2021) 501Y.V2 and 501Y.V3 variants of SARS-CoV-2 lose binding to bamlanivimab in vitro. mAbs 13:1919285 [DOI] [PMC free article] [PubMed]
- 19.Hu B, Guo H, Zhou P, Shi Z-L (2020) Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol 1–14 [DOI] [PMC free article] [PubMed]
- 20.Wang M-Y, Zhao R, Gao L-J, Gao X-F, Wang D-P, Cao J-M. SARS-CoV-2: structure, biology, and structure-based therapeutics development. Front Cell Infect Microbiol. 2020;10:587269. doi: 10.3389/fcimb.2020.587269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 22.Sadeghsoltani F, Mohammadzadeh I, Safari M-M, Hassanpour P, Izadpanah M, Qujeq D, Moein S, Vaghari-Tabari M (2021) Zinc and respiratory viral infections: important trace element in anti-viral response and immune regulation. Biol Trace Elem Res 1–16 [DOI] [PMC free article] [PubMed]
- 23.Vaghari-Tabari M, Mohammadzadeh I, Qujeq D, Majidinia M, Alemi F, Younesi S, Mahmoodpoor A, Maleki M, Yousefi B, Asemi Z (2021) Vitamin D in respiratory viral infections: a key immune modulator? Crit Rev Food Sci Nutr 1–16 [DOI] [PubMed]
- 24.Merad M, Subramanian A, Wang TT. An aberrant inflammatory response in severe COVID-19. Cell Host Microbe. 2021;29:1043–1047. doi: 10.1016/j.chom.2021.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braciale TJ, Sun J, Kim TS. Regulating the adaptive immune response to respiratory virus infection. Nat Rev Immunol. 2012;12:295–305. doi: 10.1038/nri3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshida S, Ono C, Hayashi H, Fukumoto S, Shiraishi S, Tomono K, Arase H, Matsuura Y, Nakagami H. SARS-CoV-2-induced humoral immunity through B cell epitope analysis in COVID-19 infected individuals. Sci Rep. 2021;11:5934. doi: 10.1038/s41598-021-85202-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khatiwada S, Subedi A. A mechanistic link between selenium and coronavirus disease 2019 (COVID-19) Curr Nutr Rep. 2021;10:125–136. doi: 10.1007/s13668-021-00354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keles ES. Mild SARS-CoV-2 infections in children might be based on evolutionary biology and linked with host reactive oxidative stress and antioxidant capabilities. New Microbes New Infect. 2020;36:100723. doi: 10.1016/j.nmni.2020.100723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kozlov EM, Ivanova E, Grechko AV, Wu W-K, Starodubova AV, Orekhov AN. Involvement of oxidative stress and the innate immune system in SARS-CoV-2 infection. Diseases. 2021;9:17. doi: 10.3390/diseases9010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laforge M, Elbim C, Frère C, Hémadi M, Massaad C, Nuss P, Benoliel J-J, Becker C. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat Rev Immunol. 2020;20:515–516. doi: 10.1038/s41577-020-0407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Checconi P, De Angelis M, Marcocci ME, Fraternale A, Magnani M, Palamara AT, Nencioni L. Redox-modulating agents in the treatment of viral infections. Int J Mol Sci. 2020;21:4084. doi: 10.3390/ijms21114084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abouhashem AS, Singh K, Azzazy HME, Sen CK. Is low alveolar type II cell SOD3 in the lungs of elderly linked to the observed severity of COVID-19? Antioxid Redox Signal. 2020;33:59–65. doi: 10.1089/ars.2020.8111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luo X-H, Zhu Y, Mao J, Du R-C. T cell immunobiology and cytokine storm of COVID-19. Scand J Immunol. 2021;93:e12989. doi: 10.1111/sji.12989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ni L, Ye F, Cheng M-L, et al. Detection of SARS-CoV-2-specific humoral and cellular immunity in COVID-19 convalescent individuals. Immunity. 2020;52:971–977.e3. doi: 10.1016/j.immuni.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodda LB, Netland J, Shehata L, et al. Functional SARS-CoV-2-specific immune memory persists after mild COVID-19. Cell. 2021;184:169–183.e17. doi: 10.1016/j.cell.2020.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Im JH, Je YS, Baek J, Chung M-H, Kwon HY, Lee J-S. Nutritional status of patients with COVID-19. Int J Infect Dis. 2020;100:390–393. doi: 10.1016/j.ijid.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wee AKH. COVID-19’s toll on the elderly and those with diabetes mellitus – is vitamin B12 deficiency an accomplice? Med Hypotheses. 2021;146:110374. doi: 10.1016/j.mehy.2020.110374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.D’Avolio A, Avataneo V, Manca A, Cusato J, De Nicolò A, Lucchini R, Keller F, Cantù M. 25-hydroxyvitamin D concentrations are lower in patients with positive PCR for SARS-CoV-2. Nutrients. 2020;12:1359. doi: 10.3390/nu12051359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hastie CE, Mackay DF, Ho F, et al. Vitamin D concentrations and COVID-19 infection in UK Biobank. Diabetes Metab Syndr. 2020;14:561–565. doi: 10.1016/j.dsx.2020.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moghaddam A, Heller RA, Sun Q, et al. Selenium deficiency is associated with mortality risk from COVID-19. Nutrients. 2020;12:E2098. doi: 10.3390/nu12072098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skalny AV, Rink L, Ajsuvakova OP, et al. Zinc and respiratory tract infections: perspectives for COVID-19 (Review) Int J Mol Med. 2020;46:17–26. doi: 10.3892/ijmm.2020.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fedele D, De Francesco A, Riso S, Collo A. Obesity, malnutrition, and trace element deficiency in the coronavirus disease (COVID-19) pandemic: an overview. Nutr Burbank Los Angel Cty Calif. 2021;81:111016. doi: 10.1016/j.nut.2020.111016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ragasa C, Lambrecht I, Mahrt K, Zhao H, Aung ZW, Scott J. Can nutrition education mitigate the impacts of COVID-19 on dietary quality? Cluster-randomised controlled trial evidence in Myanmar’s Central Dry Zone. Matern Child Nutr. 2021;17:e13259. doi: 10.1111/mcn.13259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou Y, Chi J, Lv W, Wang Y. Obesity and diabetes as high-risk factors for severe coronavirus disease 2019 (Covid-19) Diabetes Metab Res Rev. 2021;37:e3377. doi: 10.1002/dmrr.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang Z, Liu Y, Qi G, Brand D, Zheng SG. Role of vitamin A in the immune system. J Clin Med. 2018;7:258. doi: 10.3390/jcm7090258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stephensen CB, Lietz G. Vitamin A in resistance to and recovery from infection: relevance to SARS-CoV2. Br J Nutr. 2021;126:1663–1672. doi: 10.1017/S0007114521000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elmadfa I, Meyer AL. The role of the status of selected micronutrients in shaping the immune function. Endocr Metab Immune Disord Drug Targets. 2019;19:1100–1115. doi: 10.2174/1871530319666190529101816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iddir M, Brito A, Dingeo G, Fernandez Del Campo SS, Samouda H, La Frano MR, Bohn T. Strengthening the immune system and reducing inflammation and oxidative stress through diet and nutrition: considerations during the COVID-19 crisis. Nutrients. 2020;12:E1562. doi: 10.3390/nu12061562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Y, Du Z, Ma W, Chang K, Zheng C. Vitamin A status and recurrent respiratory infection among Chinese children: a nationally representative survey. Asia Pac J Clin Nutr. 2020;29:566–576. doi: 10.6133/apjcn.202009_29(3).0016. [DOI] [PubMed] [Google Scholar]
- 51.Tepasse P-R, Vollenberg R, Fobker M, Kabar I, Schmidt H, Meier JA, Nowacki T, Hüsing-Kabar A. Vitamin A plasma levels in COVID-19 patients: a prospective multicenter study and hypothesis. Nutrients. 2021;13:2173. doi: 10.3390/nu13072173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sarohan AR. COVID-19: endogenous retinoic acid theory and retinoic acid depletion syndrome. Med Hypotheses. 2020;144:110250. doi: 10.1016/j.mehy.2020.110250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Al-Saleh I, Alrushud N, Alnuwaysir H, Elkhatib R, Shoukri M, Aldayel F, Bakheet R, Almozaini M. Essential metals, vitamins and antioxidant enzyme activities in COVID-19 patients and their potential associations with the disease severity. Biometals. 2022;35:125–145. doi: 10.1007/s10534-021-00355-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lindschinger M, Tatzber F, Schimetta W, Schmid I, Lindschinger B, Cvirn G, Stanger O, Lamont E, Wonisch W. A randomized pilot trial to evaluate the bioavailability of natural versus synthetic vitamin b complexes in healthy humans and their effects on homocysteine, oxidative stress, and antioxidant levels. Oxid Med Cell Longev. 2019;2019:6082613. doi: 10.1155/2019/6082613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Batista KS, Cintra VM, Lucena PAF, Manhães-de-Castro R, Toscano AE, Costa LP, Queiroz MEBS, de Andrade SM, Guzman-Quevedo O, Aquino J de S (2022) The role of vitamin B12 in viral infections: a comprehensive review of its relationship with the muscle-gut-brain axis and implications for SARS-CoV-2 infection. Nutr Rev 80:561–578 [DOI] [PMC free article] [PubMed]
- 56.Lai Y-J, Chang H-S, Yang Y-P, Lin T-W, Lai W-Y, Lin Y-Y, Chang C-C. The role of micronutrient and immunomodulation effect in the vaccine era of COVID-19. J Chin Med Assoc. 2021;84:821–826. doi: 10.1097/JCMA.0000000000000587. [DOI] [PubMed] [Google Scholar]
- 57.Deschasaux-Tanguy M, Srour B, Bourhis L, et al. Nutritional risk factors for SARS-CoV-2 infection: a prospective study within the NutriNet-Santé cohort. BMC Med. 2021;19:290. doi: 10.1186/s12916-021-02168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Itelman E, Wasserstrum Y, Segev A, et al. Clinical characterization of 162 COVID-19 patients in Israel: preliminary report from a large tertiary center. Isr Med Assoc J IMAJ. 2020;22:271–274. [PubMed] [Google Scholar]
- 59.Tan CW, Ho LP, Kalimuddin S, et al. Cohort study to evaluate the effect of vitamin D, magnesium, and vitamin B12 in combination on progression to severe outcomes in older patients with coronavirus (COVID-19) Nutr Burbank Los Angel Cty Calif. 2020;79–80:111017. doi: 10.1016/j.nut.2020.111017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carpagnano GE, Di Lecce V, Quaranta VN, Zito A, Buonamico E, Capozza E, Palumbo A, Di Gioia G, Valerio VN, Resta O. Vitamin D deficiency as a predictor of poor prognosis in patients with acute respiratory failure due to COVID-19. J Endocrinol Invest. 2021;44:765–771. doi: 10.1007/s40618-020-01370-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hansdottir S, Monick MM, Lovan N, Powers L, Gerke A. Hunninghake GW (2010) Vitamin D decreases RSV induction of NF-κB-linked chemokines and cytokines in airway epithelium while maintaining the antiviral state. J Immunol Baltim Md. 1950;184:965–974. doi: 10.4049/jimmunol.0902840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zheng S, Yang J, Hu X, Li M, Wang Q, Dancer RCA, Parekh D, Gao-Smith F, Thickett DR, Jin S. Vitamin D attenuates lung injury via stimulating epithelial repair, reducing epithelial cell apoptosis and inhibits TGF-β induced epithelial to mesenchymal transition. Biochem Pharmacol. 2020;177:113955. doi: 10.1016/j.bcp.2020.113955. [DOI] [PubMed] [Google Scholar]
- 63.Baktash V, Hosack T, Patel N, Shah S, Kandiah P, Van Den Abbeele K, Mandal AKJ, Missouris CG (2020) Vitamin D status and outcomes for hospitalised older patients with COVID-19. Postgrad Med J postgradmedj-2020–138712 [DOI] [PMC free article] [PubMed]
- 64.Panagiotou G, Tee SA, Ihsan Y, et al (2020) Low serum 25‐hydroxyvitamin D (25[OH]D) levels in patients hospitalised with COVID‐19 are associated with greater disease severity. Clin Endocrinol (Oxf) 10.1111/cen.14276 [DOI] [PMC free article] [PubMed]
- 65.Mercola J, Grant WB, Wagner CL. Evidence regarding vitamin D and risk of COVID-19 and its severity. Nutrients. 2020;12:E3361. doi: 10.3390/nu12113361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kaufman HW, Niles JK, Kroll MH, Bi C, Holick MF. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS ONE. 2020;15:e0239252. doi: 10.1371/journal.pone.0239252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hastie CE, Pell JP, Sattar N. Vitamin D and COVID-19 infection and mortality in UK Biobank. Eur J Nutr. 2021;60:545–548. doi: 10.1007/s00394-020-02372-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rodríguez Tort A, Montelongo Mercado EA, Martínez-Cuazitl A, Puente Nieto AV, Adriana Reyes Pérez R (2020) La deficiencia de vitamina D es un factor de riesgo de mortalidad en pacientes con COVID-19. Rev Sanid Mil 74
- 69.Beck MA, Nelson HK, Shi Q, Van Dael P, Schiffrin EJ, Blum S, Barclay D, Levander OA. Selenium deficiency increases the pathology of an influenza virus infection. FASEB J Off Publ Fed Am Soc Exp Biol. 2001;15:1481–1483. [PubMed] [Google Scholar]
- 70.Avery JC, Hoffmann PR (2018) Selenium, selenoproteins, and immunity. Nutrients. 10.3390/nu10091203 [DOI] [PMC free article] [PubMed]
- 71.Taylor EW, Radding W. Understanding selenium and glutathione as antiviral factors in COVID-19: does the viral Mpro protease target host selenoproteins and glutathione synthesis? Front Nutr. 2020;7:143. doi: 10.3389/fnut.2020.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang J, Saad R, Taylor EW, Rayman MP. Selenium and selenoproteins in viral infection with potential relevance to COVID-19. Redox Biol. 2020;37:101715. doi: 10.1016/j.redox.2020.101715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bermano G, Méplan C, Mercer DK, Hesketh JE Selenium and viral infection: are there lessons for COVID-19? Br J Nutr 1–10 [DOI] [PMC free article] [PubMed]
- 74.Seale LA, Torres DJ, Berry MJ, Pitts MW. A role for selenium-dependent GPX1 in SARS-CoV-2 virulence. Am J Clin Nutr. 2020;112:447–448. doi: 10.1093/ajcn/nqaa177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu J, Gong Y, Sun Y, Cai J, Liu Q, Bao J, Yang J, Zhang Z. Impact of selenium deficiency on inflammation, oxidative stress, and phagocytosis in mouse macrophages. Biol Trace Elem Res. 2020;194:237–243. doi: 10.1007/s12011-019-01775-7. [DOI] [PubMed] [Google Scholar]
- 76.Zhang J, Taylor EW, Bennett K, Saad R, Rayman MP. Association between regional selenium status and reported outcome of COVID-19 cases in China. Am J Clin Nutr. 2020;111:1297–1299. doi: 10.1093/ajcn/nqaa095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kocak OF, Ozgeris FB, Parlak E, Kadıoglu Y, Yuce N, Yaman ME, Bakan E (2021) Evaluation of serum trace element levels and biochemical parameters of COVID-19 patients according to disease severity. Biol Trace Elem Res. 10.1007/s12011-021-02946-1 [DOI] [PMC free article] [PubMed]
- 78.Alkattan A, Alabdulkareem K, Kamel A, Abdelseed H, Almutairi Y, Alsalameen E Correlation between micronutrient plasma concentration and disease severity in COVID-19 patients. Alex J Med 57:21–27
- 79.Bagher Pour O, Yahyavi Y, Karimi A, Khamaneh AM, Milani M, Khalili M, Sharifi A. Serum trace elements levels and clinical outcomes among Iranian COVID-19 patients. Int J Infect Dis. 2021;111:164–168. doi: 10.1016/j.ijid.2021.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Livingstone C (2015) Zinc. Nutr Clin Pract 30:371–382 [DOI] [PubMed]
- 81.Bao S, Knoell DL. Zinc modulates cytokine-induced lung epithelial cell barrier permeability. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1132–1141. doi: 10.1152/ajplung.00207.2006. [DOI] [PubMed] [Google Scholar]
- 82.Wessels I, Maywald M, Rink L. Zinc as a gatekeeper of immune function. Nutrients. 2017;9:1286. doi: 10.3390/nu9121286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jarosz M, Olbert M, Wyszogrodzka G, Młyniec K, Librowski T. Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling. Inflammopharmacology. 2017;25:11–24. doi: 10.1007/s10787-017-0309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lazarczyk M, Favre M. Role of Zn2+ ions in host-virus interactions. J Virol. 2008;82:11486–11494. doi: 10.1128/JVI.01314-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.te Velthuis AJW, van den Worm SHE, Sims AC, Baric RS, Snijder EJ, van Hemert MJ. Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6:e1001176. doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mayor-Ibarguren A, Busca-Arenzana C, Robles-Marhuenda Á. A hypothesis for the possible role of zinc in the immunological pathways related to COVID-19 infection. Front Immunol. 2020;11:1736. doi: 10.3389/fimmu.2020.01736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.PVSN KK, Tomo S, Purohit P, et al (2022) Comparative analysis of serum zinc, copper and magnesium level and their relations in association with severity and mortality in SARS-CoV-2 patients. Biol Trace Elem Res 1–8 [DOI] [PMC free article] [PubMed]
- 88.Jothimani D, Kailasam E, Danielraj S, et al. COVID-19: poor outcomes in patients with zinc deficiency. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2020;100:343–349. doi: 10.1016/j.ijid.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zeng H, Yang Q, Yuan P, Wang X, Cheng L. Associations of essential and toxic metals/metalloids in whole blood with both disease severity and mortality in patients with COVID-19. FASEB J. 2021;35:e21392. doi: 10.1096/fj.202002346RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Puchkova LV, Kiseleva IV, Polishchuk EV, Broggini M, Ilyechova EYu. The crossroads between host copper metabolism and influenza infection. Int J Mol Sci. 2021;22:5498. doi: 10.3390/ijms22115498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Govind V, Bharadwaj S, Sai Ganesh MR, Vishnu J, Shankar KV, Shankar B, Rajesh R (2021) Antiviral properties of copper and its alloys to inactivate COVID-19 virus: a review. Biometals 1–19 [DOI] [PMC free article] [PubMed]
- 92.Schwarz M, Lossow K, Schirl K, Hackler J, Renko K, Kopp JF, Schwerdtle T, Schomburg L, Kipp AP. Copper interferes with selenoprotein synthesis and activity. Redox Biol. 2020;37:101746. doi: 10.1016/j.redox.2020.101746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jonathan C. Rupp ML (2017) Host cell copper transporters CTR1 and ATP7A are important for influenza A virus replication. Virol J. 10.1186/s12985-016-0671-7 [DOI] [PMC free article] [PubMed]
- 94.Linder MC. Copper homeostasis in mammals, with emphasis on secretion and excretion. a review. Int J Mol Sci. 2020;21:4932. doi: 10.3390/ijms21144932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lin X, Wang R, Zou W, Sun X, Liu X, Zhao L, Wang S, Jin M. The influenza virus H5N1 infection can induce ROS production for viral replication and host cell death in A549 cells modulated by human Cu/Zn superoxide dismutase (SOD1) overexpression. Viruses. 2016;8:13. doi: 10.3390/v8010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Influenza versus host: the role of novel copper‐related host factors in antiviral immunity - Dagdag - 2020 - The FASEB Journal - Wiley Online Library. https://faseb.onlinelibrary.wiley.com/doi/10.1096/fasebj.2020.34.s1.06545. Accessed 1 May 2022
- 97.Raha S, Mallick R, Basak S, Duttaroy AK. Is copper beneficial for COVID-19 patients? Med Hypotheses. 2020;142:109814. doi: 10.1016/j.mehy.2020.109814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stafford SL, Bokil NJ, Achard MES, Kapetanovic R, Schembri MA, McEwan AG, Sweet MJ. Metal ions in macrophage antimicrobial pathways: emerging roles for zinc and copper. Biosci Rep. 2013;33:e00049. doi: 10.1042/BSR20130014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Percival S (1998) Copper and immunity. Am J Clin Nutr. 10.1093/ajcn/67.5.1064S [DOI] [PubMed]
- 100.Rani I, Goyal A, Bhatnagar M, Manhas S, Goel P, Pal A, Prasad R. Potential molecular mechanisms of zinc- and copper-mediated antiviral activity on COVID-19. Nutr Res N Y N. 2021;92:109–128. doi: 10.1016/j.nutres.2021.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kelley DS, Daudu PA, Taylor PC, Mackey BE, Turnlund JR. Effects of low-copper diets on human immune response. Am J Clin Nutr. 1995;62:412–416. doi: 10.1093/ajcn/62.2.412. [DOI] [PubMed] [Google Scholar]
- 102.Mao S, Zhang A, Huang S. Meta-analysis of Zn, Cu and Fe in the hair of Chinese children with recurrent respiratory tract infection. Scand J Clin Lab Invest. 2014;74:561–567. doi: 10.3109/00365513.2014.921323. [DOI] [PubMed] [Google Scholar]
- 103.Hackler J, Heller RA, Sun Q, Schwarzer M, Diegmann J, Bachmann M, Moghaddam A, Schomburg L. Relation of serum copper status to survival in COVID-19. Nutrients. 2021;13:1898. doi: 10.3390/nu13061898. [DOI] [PMC free article] [PubMed] [Google Scholar]