Abstract
Objectives
In the context of the ongoing overdose crisis, a stark increase in toxic drug deaths from the unregulated street supply accompanied the onset of the COVID-19 pandemic. Injectable opioid agonist treatment (iOAT – hydromorphone or medical-grade heroin), tablet-based iOAT (TiOAT), and safer supply prescribing are emerging interventions used to address this crisis in Canada. Given rapid clinical guidance and policy change to enable their local adoption, our objectives were to describe the state of these interventions before the pandemic, and to document and explain changes in implementation during the early pandemic response (March–May 2020).
Methods
Surveys and interviews with healthcare providers comprised this mixed methods national environmental scan of iOAT, TiOAT, and safer supply across Canada at two time points. Quantitative data were summarized using descriptive statistics; interview data were coded and analyzed thematically.
Results
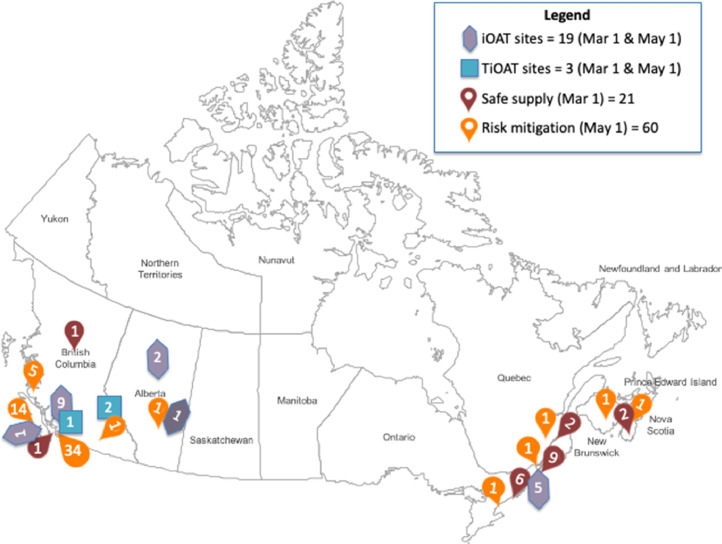
103 sites in 6 Canadian provinces included 19 iOAT, 3 TiOAT and 21 safer supply sites on March 1, 2020; 60 new safer supply sites by May 1 represented a 285% increase. Most common substances were opioids, available at all sites; most common settings were addiction treatment programs and primary care clinics, and onsite pharmacies models. 79% of safer supply services were unfunded. Diversity in service delivery models demonstrated broad adaptability. Qualitative data reinforced the COVID-19 pandemic as the driving force behind scale-up.
Discussion
Data confirmed the capacity for rapid scale-up of flexible, community-based safer supply prescribing during dual public health emergencies. Geographical, client demographic, and funding gaps highlight the need to target barriers to implementation, service delivery and sustainability.
Keywords: Safer supply, Substance use, Addiction, Harm reduction, Injectable opioid agonist treatment, Service delivery models, COVID-19 pandemic, Canada
Introduction
On March 11, 2020, the World Health Organization declared COVID-19 a global pandemic (World Health Organization, 2020), prompting governments around the world to close borders, redeploy healthcare providers to prioritize COVID-19-related needs, and shutter or decrease in-person capacity for healthcare and social services (Berglöf, 2020; Canadian Community Epidemiology Network on Drug Use, 2020; Luković & Stojković, 2020; Public Health Agency of Canada, 2020). These restrictions inadvertently exacerbated the ongoing North American overdose crisis, leading to increased toxicity in unregulated drug supplies in many regions, and more people using alone (Canadian Community Epidemiology Network on Drug Use, 2020; Genberg et al., 2021; Public Health Agency of Canada, 2020). In the United States, over 94,000 people died of apparent toxic drug poisoning in the 12 months leading up to January 2021, the highest annual figures on record (Ahmad, Rossen, & Sutton, 2021). In Canada, which has also been hard-hit by the ongoing overdose crisis, 5148 apparent opioid toxicity deaths occurred from April-December 2020, representing an 89% increase from the same period in 2019 (2722 deaths) (Public Health Agency of Canada, 2021). Fentanyl is a major driver in this surge even before the pandemic (Ivsins, Boyd, Beletsky, & McNeil, 2020), as well as stimulants more recently, with 87% and 59% of opioid toxicity deaths in the first half of 2021 involving fentanyl and stimulants, respectively (Government of Canada, 2021b).
In autumn 2020, the Canadian government endorsed a spectrum of treatment and harm reduction approaches to address the crisis, including prioritizing “safer supply” as a primary focus to reduce harm and improve health outcomes for people who use drugs (PWUD) (Hajdu, 2020; Woo, 2020). Safer supply is defined by Health Canada as a pharmaceutical-grade alternative to the unregulated drug supply (Hajdu, 2020). The Canadian Association of People Who Use Drugs, a national organization of PWUD, includes the terms “legal” and “regulated” in its definition, and extends the goals of safer supply from harm reduction and improved outcomes, to include furthering human rights and social justice (Canadian Association of People who Use Drugs, 2019). Health Canada's medical model of prescribed pharmaceuticals formed the basis of pre-pandemic safer supply services in Canada. Pre-pandemic government action provided over $33 million toward safer supply and drug checking programs (Health Canada, 2019, 2020a, 2020b, 2020c), which had recently been or were in the process of being implemented. However, the Canadian government avoided action toward decriminalization despite longstanding calls from PWUD, local and provincial medical health officers and police (Hajdu, 2020; Woo, 2020).
The rapid publication of interim provincial risk mitigation guidance by the British Columbia (BC) Centre on Substance Use in March 2020 to address the dual COVID-19 and overdose public health emergencies marked a key shift in safer supply provision. The goals of the so-called “risk mitigation” prescribing were to ameliorate COVID-19-related harms by supporting PWUD to follow physical distancing recommendations e.g., during hotel self-isolation; and to reduce overdose risk, by circumventing the increasingly toxic unregulated drug supply and associated deaths observed early in the pandemic (Ahamad et al., 2020). Provincial and regional bodies in Ontario and Québec also released or updated guidance documents for safer supply and/or COVID-19 risk mitigation prescribing in the months immediately following the declaration of the COVID-19 pandemic (Canadian Research Initiative in Substance Misuse [CRISM], 2019a; Goyer, Hudon, Plessis-Bélair, & Ferguson, 2020; Hales et al., 2019). To our knowledge, no other provinces and territories initiated similar actions, despite death rates increasing nationally (Government of Canada, 2021b), with the highest rates observed in BC, Alberta and Ontario (Government of Canada, 2021b).
Policy changes were also enacted in the province of BC that authorized registered and psychiatric nurses to prescribe certain controlled substances, as a means of accelerating the implementation of risk mitigation prescribing (British Columbia Ministry of Mental Health and Addictions, 2020). The BC government also released a new prescribed safer supply policy in July 2021, with allocated funding for its implementation (British Columbia Ministry of Mental Health and Addictions, 2021). These swift changes in response to COVID-19 transmission risk and to exceptionally high observed overdose rates, highlighted the need to monitor their impacts on implementation nationally in order to inform the need for further supports. Our team's annual national environmental scan of injectable opioid agonist treatment (iOAT) and tablet-based iOAT (TiOAT) afforded the infrastructure to pursue this work.
Members of our team from the Canadian Research Initiative in Substance Misuse network began conducting national environmental scans in 2018 to monitor the emergence of evidence-based iOAT for addiction (Canadian Research Initiative in Substance Misuse [CRISM], 2019b). iOAT involves supervised dosing of prescribed injectable hydromorphone or diacetylmorphine (i.e., pharmaceutical-grade heroin), typically with access to wrap-around health and social services, to reduce risk of overdose and death (Eydt et al., 2020; Fairbairn et al., 2019). Our second scan (conducted in 2019) identified an emerging iOAT adaptation at one of the iOAT programs: supervised tablet-based (TiOAT) hydromorphone taken according to client preference (e.g., intravenously, orally) (Eydt et al., 2020). This 2020 scan adds safer supply (unsupervised take-home doses) (Bonn et al., 2020) of a wide range of prescribed pharmaceutical medications and other substances (e.g., alcohol), for a more fulsome picture of the changing landscape.
Terminology was variable across the country, with many providers, particularly in BC, using the term ‘risk mitigation’ to refer primarily to prescribing for the purpose of decreasing risk of COVID-19, despite the stated goals of BC's Risk Mitigation guidance document also including harm reduction from overdose or toxic drug poisoning. This inconsistent use of terms makes differentiating between the terms ‘risk mitigation’ and ‘safer supply’ prescribing challenging. Safer supply conceptualization in Canada and the U.S. can even include iOAT and TiOAT, when its goal is to reduce overdose risk (Csete & Elliott, 2021; Ivsins et al., 2020). For the purposes of this article, we use the terms iOAT and TiOAT to describe supervised dosing of prescribed injectab injectable and tablet opioids, and ‘safer supply’ as an umbrella term for prescribing unsupervised doses of pharmaceutical alternatives to the unregulated drug supply (including but not limited to opioids), which includes prescribing for COVID-19 risk mitigation. We differentiate between safer supply and risk mitigation only in the context of direct participant quotes.
The purposes of this article were to: 1) describe the operational and clinical characteristics of iOAT, TiOAT and safer supply sites across Canada before and early on in the COVID-19 pandemic; and 2) explain the reasons for the emerging changes in safer supply implementation during the pandemic's first wave. Additional data on the barriers to implementation and potential mitigating strategies will be addressed elsewhere.
Methods
Study design
This national, mixed methods environmental scan used a single cross-sectional survey alongside qualitative interviews about operations at two time points (March 1 and May 1, 2020), to examine prescribing practices and services from the provider perspective. The University of British Columbia/Providence Healthcare Joint Research Ethics Board approved the project, under the purview of program evaluation. The Checklist for Reporting of Survey Studies (CROSS) was used to guide reporting.
Recruitment
Recruitment through convenience sampling involved email and public and private social media invitations based on a scoping web, primary, and grey literature search for authors and funding recipients working on relevant research and/or clinical projects, and provincial/national professional associations, research networks, clinical guideline committees, PWUD/advocacy groups, health and social service providers, federal and provincial governments, and health authorities across Canada. Snowball sampling and word of mouth reached additional participants. Inclusion criteria were providers at any iOAT/TiOAT and/or safer supply site as of March 1 and/or May 1, 2020, offering prescription opioids, stimulants, benzodiazepines, alcohol, cannabis, and/or nicotine products, with clinical and operational knowledge of the services. Prospective participants were invited to contact the study team to indicate their interest in taking part, in order to receive an email with the survey. Each participant could report on any number of sites at which they provided services through a single survey or interview. Sites with more than one prospective participant were asked to nominate one respondent based on their knowledge of the information asked on the survey or interview, respectively. No compensation was offered for participation.
Data gathering
The 9-section survey (up to 61 questions depending on the site's service scope) was adapted from our iOAT scans (Appendix 1) (Eydt et al., 2020) and emailed to participants as a Word document. Questions included requests for quantitative data, as well as open-text responses about program characteristics, changes in response to COVID-19, lessons learned, and barriers, gaps and strengths (see Appendix 1). Retrospective data were gathered from participants beginning in May 2020 through January 2021 by the second author. Data on client characteristics were gathered for time point 1 (March 1) in keeping with previous annual scan protocols. Data on service delivery models, substances, prescribing practices, barriers/facilitators of implementation, and operational changes in response to COVID-19 for each service (iOAT, TiOAT, safer supply) related to time point 2 (May 1).
Individual 10-60-minute (mode ∼30) semi-structured telephone or Zoom interviews were used to clarify survey responses if needed (e.g., elaborating on responses to open-ended questions, such as barriers/facilitators; confirming that quantitative data referred to a specific site when more than one site was represented), and participant capacity permitting, to also gather qualitative data on factors influencing implementation. The interview guide was structured based on the theoretical domains of the Consolidated Framework for Implementation Research (Damschroder et al., 2009), to probe barriers and facilitators of implementation. Data on operational changes in response to COVID-19 (McCrae et al., 2022), on the impact of safer supply, and on barriers and mitigating strategies for implementation will be reported elsewhere.
All participants were invited to complete both the survey and interview; however, the context of dual public health emergencies necessitated flexibility to support participation (e.g., completing only one, based on participant-determined feasibility, having different site representatives complete the survey versus interview, and offering telephone survey administration). Participants could also omit or estimate survey responses when data were not readily available. For sites with interview-only data, participants were asked priority survey questions if time permitted (e.g., services and substances offered). All participants were asked to consult with wider program staff (where applicable) to confirm data accuracy and broaden the range of perspectives represented in the survey data. Participants were offered the opportunity to verify the accuracy of their answers before publication. Interviewer training/experience (2nd author) included 2019 scan administration and orientation to 2020 changes. Data were stored on a secure, password-protected university server.
Analysis
Anonymized quantitative data were summarized using descriptive statistics in Excel. “Sites” included: 1) each setting where a participant was offered a given service (iOAT; TiOAT; safer supply, including COVID-19 “risk mitigation”); and 2) each service, when multiple services were offered at one location. Transcribed interviews were analyzed in NVivo (QSR International, 2019). Safer supply and risk mitigation models were reported together (as “safer supply”) because of dynamic shifts in early-pandemic prescribing aims, which at times made differentiating between them challenging. Interviews were audio recorded and transcribed; data specific to survey questions or resulting from clarifying survey responses were extracted and combined with survey data for analysis by two authors to address objective 1. An interpretive epistemological lens guided the qualitative analysis (Merriam & Grenier, 2019). Qualitative data were initially coded to the theoretically-derived constructs of the Consolidated Framework for Implementation Research (Damschroder et al., 2009) and combined with survey data on barriers and facilitators of implementation (reported elsewhere). Additional coding of data on reasons for service implementation (and on COVID-19, largely reported elsewhere) followed by two authors to address objective 2, using directed content analysis (Hsieh & Shannon, 2005). The resulting categories were reviewed with a third author alongside the coded data.
Results
Sample
The 50 participants from 103 sites included 2 survey-only respondents (covering 3 sites), 17 interview-only respondents (39 sites), and 31 completing both (60 sites). This sample yielded both survey and interview data for 64 sites (on account of different respondents nominated for survey versus interview), survey-only for 3 sites, and interview-only for 35 sites. Each participant reported on between one and 14 sites, while two reported on all sites across a given health region, accounting in part for the large number of sites with interview-only data. Participants included: 16 physicians, 7 nurse practitioners; 2 medical residents; 8 nurses; 5 directors/leads (1 counted as a physician above); 6 managers; 6 coordinators; and 1 knowledge translation and evaluation officer. Forty-four (85%) participants were female, the remainder male. Survey participants consulted 116 colleagues, including medical students; nursing assistants; pharmacists; pharmacy technicians; directors; regional harm reduction coordinators; community action teams; outreach, harm reduction, and overdose prevention sites; and mental health, social, overdose outreach, community liaison, personal support and peer workers; along with many of the roles listed for participants. Thirty-four (68%) respondents consulted with colleagues to confirm data accuracy during data gathering, while 39 (75%) personally verified the data for accuracy with the data collector prior to publication.
Program characteristics (from survey and interview findings)
Services provided at each time point
Fig. 1 presents site locations, by type of service. Between March 1 and May 1, 2020, sixty participating sites reported having introduced safer supply between March 1 and May 1, 2020 (i.e., a 285% increase from the 21 sites in our sample reporting offering it as of March 1), while iOAT/TiOAT numbers remained constant; no sites had discontinued pre-pandemic services at the May 1 time point.
Fig. 1.
Participating sites.
Client demographics
Client demographics were available at March 1, 2020 only, and are presented in Table 1 .
Table 1.
Client demographic data at time point 1 (Mar 1), by service type.
|
Service |
iOAT |
TiOAT |
Safer supply |
Total |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Client characteristics | # | Sites reporting |
# | Sites reporting |
# | Sites reporting |
# | Sites reporting |
|||||
| # | % | # | % | # | % | # | % | ||||||
| Total starts | 1041 | 18 | 95% | 123 | 2 | 67% | 298 | 19 | 90% | 1468 | 39 | 91% | |
| Active clients | 401 | 19 | 100% | 82 | 2 | 67% | 255 | 17 | 81% | 738 | 38 | 88% | |
| Waitlist count | 441 | 5 | 26% | 42 | 1 | 33% | 228 | 5 | 6% | 711 | 11 | 26% | |
| Mean age [range] | 46[17-70] | 8[12] | 42%[63%] | 40[21-26] | 2[2] | 67%[67%] | 42[18-63] | 7[16] | 33%[76%] | 44[17-70] | 17[30] | 40[70%] | |
| Gender | % M | 68% | 15 | 79% | 60% | 2 | 67% | 63% | 15 | 71% | 69% | 32 | 74% |
| % W | 32% | 40% | 36% | 33% | |||||||||
| % T | <1% | 0% | 2% | <1% | |||||||||
| % Indigenous | 29% | 9 | 47% | Unknown | 0 | 0 | 23% | 15 | 71% | 26% | 24 | 56% | |
| % Non-Indigenous | 71% | 82% | 71% | ||||||||||
| Total # of sites | 19 | 3 | 21 | 43 | |||||||||
Note: Total starts = # clients ever accessing prescriptions; # active clients = # clients accessing services within 1 week of the scan reference date; M = man; W = woman; T = transgender, non-binary, Two-Spirit, genderfluid. Missing data were omitted when calculating means, ranges and proportions.
Substances offered
At both time points, all 19 iOAT sites offered injectable hydromorphone (278 clients, 69.3% of all iOAT clients); two sites also provided diacetylmorphine (123 clients, 30.7% of all iOAT clients); one iOAT site also offered TiOAT. All TiOAT sites provided tablet hydromorphone. Safer supply/risk mitigation substances varied between provinces, though all sites provided opioids (Table 2 ). While treatment approaches like iOAT and TiOAT employed individualized titration for each client, some (but not all) safer supply services use more protocolized dosing (i.e., set dose across clients), while still enabling the tailoring of dosing to meet clients’ needs more effectively (e.g., adjusting dosing for concurrent opioid and benzodiazepine use, services not discontinued if days were missed).
Table 2.
Available safer supply/risk mitigation substances by region.
| Available Substance |
TOTAL |
British Columbia |
Alberta |
Ontario |
Québec |
Atlantic Provinces |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time point | Mar | May | Mar | May | Mar | May | Mar | May | Mar | May | Mar | May |
| Opioids | 21 | 81 | 2 | 56 | 0 | 1 | 15 | 17 | 2 | 3 | 2 | 4 |
| Benzodiazepines | 4 | 24 | 0 | 17 | 0 | 0 | 0 | 1 | 2 | 3 | 2 | 3 |
| Stimulants | 5 | 56 | 1 | 47 | 0 | 1 | 2 | 2 | 0 | 3 | 0 | 3 |
| Cannabis | 2 | 14 | 0 | 2 | 0 | 0 | 0 | 9 | 0 | 3 | 0 | 0 |
| Alcohol | 8 | 27 | 0 | 14 | 0 | 0 | 8 | 9 | 0 | 3 | 0 | 1 |
| Nicotine products | 9 | 25 | 0 | 13 | 0 | 0 | 9 | 9 | 0 | 3 | 0 | 0 |
| # of sites | 21 | 81 | 2 | 56 | 0 | 1 | 15 | 17 | 2 | 3 | 2 | 4 |
Note: Bold font denotes increases by second scan reference date. Opioids included hydromorphone (Dilaudid, generic, contin), morphine (Kadian, M Eslon), methadone, Sublocade, Suboxone, fentanyl (patch, IV), and oxycodone. Specified benzodiazepines included Lorazepam, Clonazepam, and Diazepam. Specified stimulants included dextroamphetamine and methylphenidate. Alcohol included beer and wine. Nicotine products included nicotine patches and gum, cigarettes, and nicotine cessation medication.
Table 3 reports on other quantifiable service delivery model components at participating sites, including settings, pharmacy model, frequency of care, and funding sources. Differences between time points 1 and 2 are indicated, where data were available. These characteristics are described below.
Table 3.
Service delivery model components, by service type.
| # (%)* reporting, by type of service | iOAT | TiOAT | Safer Supply | Total | |
|---|---|---|---|---|---|
| Service delivery model/setting | Mar & May 1 (no change) | Mar & May 1 (no change) | Mar 1 | New as of May 1 | Combined |
| Comprehensive, dedicated iOAT clinic | 3 (16%) | 1 (33%) | 0 | 2 (3%) | 6 (6%) |
| Hospital-based | 2 (11%) | 0 | 2 (10%) | 1 (2%) | 5 (5%) |
| Pharmacy-based | 0 | 0 | 0 | 0 | 0 |
| Dedicated safer supply clinic | 0 | 0 | 1 (5%) | 0 | 1 (1%) |
| Prescriber's private practice | 0 | 0 | 4 (19%) | 5 (8%) | 9 (%) |
| Mobile outreach | 0 | 0 | 1 (5%) | 1 (2%) | 2 (2%) |
| COVID-19 isolation unit | 0 | 0 | 0 | 2 (3%) | 2 (2%) |
| Vending machine dispensing | 0 | 0 | 1 (5%) | 0 | 1 (1%) |
| Embedded in existing services | – | – | – | – | – |
| Housing | 5 (26%) | 0 | 3 (%) | 4 (7%) | 12 (12%) |
| Hospice | 1 (5%) | 0 | 1 (5%) | 0 | 2 (1%) |
| Shelter | 3 (16%) | 0 | 4 (19%) | 0 | 7 (7%) |
| Primary care clinic | 0 | 0 | 1 (5%) | 19 (32%) | 20 (%) |
| Community health centre | 4 (22%) | 0 | 5 (24%) | 3 (5%) | 12 (12%) |
| Addiction treatment program (e.g., oral OAT clinic) | 0 | 0 | 0 | 25 (42%) | 25 (19%) |
| Harm reduction program (e.g., overdose prevention/supervised consumption site) | 2 (11%) | 2 (67%) | 0 | 3 (5%) | 7 (7%) |
| Intensive case management team | 0 | 0 | 0 | 4 (7%) | 4 (4%) |
| Youth program | 0 | 0 | 0 | 2 (3%) | 2 (2%) |
| Mental health program | 0 | 0 | 0 | 7 (12%) | 7 (7%) |
| Perinatal clinic | 0 | 0 | 0 | 1 (2%) | 1 (1%) |
| Number reporting | 19/19(100%) | 3/3(100%) | 21/21 (100%) | 60/60 (100%) | 103/103(100%) |
| Pharmacy model | May 1 (no change from Mar 1 reported) | ||||
| # (%)* reporting, by type of service | iOAT | TiOAT | Safer Supply | Total | |
| Onsite pharmacy | 8 (44%) | 2 (67%) | 24 (55%) | 34 (52%) | |
| Hospital pharmacy partner | 2 (11%) | 0 | 4 (9%) | 6 (9%) | |
| Community pharmacy partner | 5 (28%) | 1 (33%) | 5 (11%) | 11 (17%) | |
| Informal relationship with some pharmacies | 0 | 0 | 1 (2%) | 1 (2%) | |
| Patient choice | 3 (17%) | 0 | 26 (59%) | 29 (45%) | |
| Delivery available | 5 (28%) | 0 | 15 (%) | 20 (31%) | |
| Number reporting | 18/19(95%) | 3/3(100%) | 44/81(54%) | 65/103(63%) | |
| Frequency seen by prescriber | May 1 (3 sites reported decreased frequency by May 1) | ||||
| # (%)* reporting, by type of service | iOAT | TiOAT | Safer Supply | Total | |
| Every 6 weeks or less | 0 | 0 | 4 (11%) | 4 (7%) | |
| Every 4 weeks | 0 | 2 (100%) | 21 (60%) | 23 (41%) | |
| Every 2 weeks | 0 | 1 (50%) | 16 (46%) | 18 (32%) | |
| Weekly | 0 | 0 | 21 (60%) | 21 (38%) | |
| More than once per week | 19 (100%) | 0 | 2 (6%) | 21 (38%) | |
| Number reporting | 19/19(100%) | 2/3(67%) | 35/81(43%) | 56/103(54%) | |
| Funding sources | May 1 (No change reported for Mar 1 sites) | ||||
| # (%)* reporting, by type of service | iOAT | TiOAT | Safer Supply | Total | |
| Federal government | 1 (5%) | 0 | 3 (4%) | 4 (4%) | |
| Provincial government Ministries | 9 (47%) | 0 | 0 | 9 (9%) | |
| Health authorities | 12 (63%) | 3 (100%) | 10 (12%) | 25 (24%) | |
| Community partners | 6 (32%) | 1 (33%) | 2 (2%) | 9 (9%) | |
| Private donations | 0 | 0 | 2 (2%) | 2 (2%) | |
| Patient fees | 0 | 0 | 2 (2%) | 2 (2%) | |
| No funding | 0 | 0 | 64 (79%) | 64 (62%) | |
| Number reporting | 19/19(100%) | 3/3(100%) | 81/81(100%) | 103/103 (100%) | |
Note: iOAT=injectable opioid agonist treatment; TiOAT=tablet-based iOAT; % add to more than 100% where more than one model was reported at a site; % represent only the proportion of those reporting.
Service delivery models
Existing safer supply sites on March 1 included dedicated private practices, mobile outreach, machine-dispensed, hospital-based, and embedded services in hospices, shelters, primary care clinics, community health centres, addiction treatment, and harm reduction programs. By May 1, 2020, the survey data identified that new safer supply sites were also embedded in youth or mental health services, perinatal care clinics, overdose prevention/supervised consumption sites, oral OAT, iOAT and TiOAT clinics, and intensive case management teams.
Frequency of care
Many safer supply/risk mitigation sites offered flexibility in care frequency, ranging from weekly to monthly prescriber check-ins to monitor client goals and health, based on client stability and prescribed medication(s), while the TiOAT sites reported once or twice per month appointments. Three sites reported decreased frequency of care with the onset of the pandemic, as well as shifting to virtual care for some or all appointments. This approach differed significantly from iOAT programs where supervised dosing occurred two to five times per day.
Pharmacy models
Pharmacies included onsite pharmacies (e.g., in hospital settings), partnered (formal or informal partnerships established by the service to provide a single location that either provided the medications to the service site, or accepted clients to go and receive their doses), or client-selected (i.e., their preferred local pharmacy in the community). One of the new sites (for risk mitigation) had all medications delivered to clients quarantined in a motel. The machine-dispensed service offered prescribed opioids for up to 15 clients without the barriers of daily witnessed doses or check-ins, which had been the norm for most programs pre-pandemic:
“They don't need to wait in line, no one needs to know that they're actually picking up, when they're picking up. Someone can come in and grab their meds for the day in the morning and then go to the work for the rest of the day. They don't have to leave work 3 times a day. So, there's the dignity in choice in giving people back their schedule and their life, and also just trusting that those who are accessing it know what's best for them.”
Participant 42
Shifts in prescriber approaches
Prescribing in response to COVID-19 and its impact on risks to people who use drugs emerged organically, at times “flying under the radar” [Participant 40] to avoid clinic oversight. One prescriber referred to their pandemic practice as “guerilla-style” prescribing:
“The model is basically not in the clinic. It started off really guerilla style at the homeless encampment that was set up in April/May to encourage some degree of isolation, and so we were just there in a pop-up tent. Luckily it was warm, and now that it's getting colder, it's done out of one of the mental health outreach [programs] – it's not really associated with my clinic.”
Participant 32
Lack of clear endorsement by regulatory bodies, and the perceived unacceptability of safer supply by colleagues, shifted somewhat with the pandemic, as described by another prescriber when asked when they began safer supply prescribing:
“Well, honestly, without telling anybody, like three years ago maybe…I've always been really wanting to do more harm reduction and fearful of my license or other people knowing about what I'm doing because of the [political] climate, but I think there's way bigger, way bigger appetite [since the pandemic]. It's kind of ‘cooler’ now.”
Participant 36
Others described informal networks collaborating to provide safer supply/risk mitigation in the absence of formal training for what they considered to be a new approach:
“Because it's a novel program, we're coming up with never-before-seen circumstances or marginal cases that need discussion, so it really helps a lot [to be connected]. And having a variety of different perspectives. I mean we have [nurse practitioners] and the balance of the prescribers are [nurse practitioners], but there's a couple of physicians on board as well, and we have nurses and we have harm reduction workers from all over the city which really allows us to get a broader picture of what the community needs, as well as getting different perspectives on questions.”
Participant 12
Funding
Dedicated pre-pandemic funding for iOAT and TiOAT programs was typical, and was obtained from provincial health ministries, health authorities and/or community partners, with one program receiving federal funding. In contrast, pervasive lack of dedicated funding for safer supply meant new safer supply services were typically folded into existing funding structures, causing significant resource strain: “It's the funding pieces that have been like the hardest. So, like funding for staff, funding for a supervised model which we still don't have, funding for the medication.” [Participant 1]. Another participant [13] reported “I think we could run a lot more efficiently if we had more support, like an [electronic medical record], more staff, more time, more clinic time available, just everything would help. So, we're working within that.”
Unfunded programs accepted new safer supply clients despite full caseloads for their other services (e.g., iOAT, OAT), or relied on donations and volunteer support to operate:
“There's been a lot of in-kind support or we couldn't have done this. But no, nothing [in terms of funding], even simple things like paying for the rent of the room we had…No, it's been a struggle to get any kind of resources.“
Participant 43
This lack of funding had implications for providing services: “It's just heartbreaking when you know that there are so many people that could benefit from this, but I just don't have enough funding to let everybody on it.” [Participant 10].
Existing funding requirements also impeded providers’ ability to deliver the level of care they felt was important and required, with respect to safer supply:
“Any of those changes have to go through our funder and we have to build up quite a case for why we want to do this and you know, what the evidence behind that is and what this will look like, and there's a lot of things to work through, and so it creates a lot of difficulty in being able to accomplish the kind of care that we want to accomplish with people, and to build and evolve the program into something that's going to better meet people's needs.”
Participant 18
Where they did exist, safer supply funding sources included provincial health ministries, health authorities, community partners, federal government, private donations, and patient fees. This new funding, in some cases long-sought, was only available because of the pandemic:
“Funding has been a challenging piece to obtain, for sure, and it wasn't until the pandemic that that was approved. And with funding comes all of the resources that were needed. We knew that we needed a more comprehensive wrap around care model and we weren't able to do that without the funding.”
Participant 16
Ancillary services
Ancillary services and staffing ranged from one prescriber with minimal supports (more common for safer supply services), to onsite wraparound health and social services (more prevalent in iOAT/TiOAT services). Fourteen (13.6%) of the 103 sites represented in the scan reported staffing a peer support worker, navigator, or collaborator at time point 2. Available referrals were reported for harm reduction, primary care, allied and mental health, emergency care, intensive and specialty care (e.g., dental, wound, infectious disease), lab testing, outreach, shelters, spiritual/cultural services, and other social services.
Reasons for rapid scale-up (qualitative findings)
Qualitative data reinforced the COVID-19 pandemic as the predominant driver of safer supply/risk mitigation scale-up in Canada during this period, because of (a) the need to address health risks from COVID-19 itself; (b) the increasingly toxic unregulated drug supply; (c) new pandemic-specific clinical guidance that was released in some jurisdictions to address these factors; and (d) pandemic-specific funding opportunities that became available. These factors are discussed below, with supporting participant quotes.
(a) Changing perceptions about the relative advantage of safer supply (i.e., benefits with respect to preventing COIVD-19 infection, versus perceived risks of providing access to safer supply) from leadership served as enablers:
“With COVID…the hesitation from leadership in the health authority went away…because of concerns about an outbreak in the shelter system… they saw that we would absolutely have to provide [safer supply] to help people stay in one spot. The risks and benefits of the prescription shifted.”
Participant 39
(b) The target of this urgency transitioned quickly toward reducing overdose risk, given the increased mortality observed by providers even before it was acknowledged by authorities:
“Right from the very beginning we thought it was a dual pandemic, because we saw these trends early, in April. We were seeing high numbers of overdoses week to week in our practices, so I think that hasn't changed. I think, yes, probably for the first couple weeks, like the second half of March, we were thinking of COVID risk mitigation, but probably by April we were already seeing the toxic drug supply even before it was declared, so we really thought about it in those terms and that hasn't changed, it's just gotten worse.”
Participant 34, resident
(c) Clinical guidelines facilitated adoption for some participants. This guidance documentation provided prescribers with formal approval to proceed whereas without it, many had been hesitant because of the professional risk associated with offering safer supply without regulatory or government approval. “People have been working on [safer supply] for quite a while in the background, but COVID hit, and the guidelines came out, and people were given the freedom to immediately start doing this.” [Participant 6, nurse practitioner]. Associated with service changes were also shifts in attitudes about harm reduction, towards greater appreciation of its utility as an approach to care moving forward:
“I think harm reduction has been rolling along and becoming more and more accepted for a long time, and then I think COVID just kind of opened a can whoop-ass. I think it was also coincidence, but I think it definitely did push it into [being] more acceptable.”
Participant 36, nurse practitioner
(d) Pandemic-related funding also created opportunities to implement long-planned harm reduction services (e.g., managed alcohol program; outreach service) that were previously unfeasible; pandemic conditions also averted planned TiOAT at multiple sites, potentially shaping services moving forward:
“There're definitely groups in Ontario and Quebec who were…in the planning stages [for TiOAT], but COVID derailed all that, unfortunately…As safe supply continues, there's less need for [TiOAT] sites…They still play a role, absolutely, for the people who really need that connection [to wrap-around care], but for people who are stable, who can go to a pharmacy every day and get carries, then that's the best option for them.”
Participant 19, clinic manager
And although for many prescribers COVID-19 risk mitigation was the stimulus for adjusting their prescribing practices, three indicated plans to discontinue post-pandemic in an effort to return to previous organizational protocols, or in anticipation of challenges maintaining adequate levels of care:
“I think we might end up not doing long term safe supply programs per se. Because we're concerned about sustainability. I guess typically in [clinic] when someone is stable, we try and transition them to another clinic…but there's nowhere to transition people so then we're going to have all these people who are on safe supply with nowhere to go. So that's the biggest barrier. It…feels like a barrier. We're concerned that we'll just have too many people and we'll do a bad job.”
Participant 13
Another prescriber described their current approach to easing off safer supply services in anticipation of the end of the pandemic, to align care with previous services, which prioritized OAT:
“We are also making a concerted effort to actually start actively weaning, especially those who are on 8 mg 14 tablets a day, and…I started pretty well only prescribing safe supply in addition to opioid agonist therapy and never on its own.”
Participant 17
Other participants clearly intended (or hoped to be able) to continue with safer supply prescribing after the pandemic was over, based on the benefits they had observed, and the influence that pandemic prescribing had on normalizing safer supply among their colleagues. As one prescriber participant noted:
“For most of our clinicians, it's quite clear that you can't just withdraw the work the pandemic has helped us increase. As long as the black market is contaminated, we don't see why we should stop that…when you get used to it and you do it and you see the effect on your patient, you never want that to go away… so we are confident that it's really just because it's a new practice, but we all discussed together that, yeah, there's no plan on withdrawing the practice when COVID is finished.”
Participant 41
Discussion
The ambiguity we encountered in the terminologies and definitions the participants used to describe their services, including the ways their perceptions about risk mitigation and safer supply changed during the early pandemic, led us to describe iOAT, TiOAT and safer supply (including risk mitigation) as a spectrum of service options for people who use drugs. Differences between the treatment-based options (iOAT/TiOAT) and safer supply as it emerged during this time, related primarily to the supervision of doses, how engaged clients were with ancillary services, and how individualized vs. protocolized the dosing was. In essence, these services can be seen asoptions along a continuum within a medical model, from aiming to reduce substance use to reducing risk, and from high to low barrier for clients. As these services continue to evolve, careful attention to the language and perceptions of both providers and clients will be needed.
iOAT/TiOAT programs with witnessed consumption are resource-intensive, which partially explains their gradual scale-up (from 11 in 2018 to 19 in 2020) (Eydt et al., 2020). In contrast, the rapid and significant safer supply expansion over two months was likely enabled by lower operational costs (i.e., no requirement for observed dosing) and ease of tailoring to local contexts. Service delivery models for iOAT/TiOAT in Canada are currently limited to four discrete models, namely: 1) comprehensive, dedicated programs; 2) programs embedded in existing services (5 settings [see Table 3]); 3) pharmacy-based (no longer in operation); and 4) hospital-based (Eydt et al., 2020). However, safer supply sites displayed a far broader range of community settings and medication options, as well as less demanding care frequency than iOAT, suggesting greater capacity for versatility in where and how services are provided provi (although this flexibility was only available after SARS-CoV2 exposure at some sites). The innovative machine dispensing model, established before the pandemic, was particularly low-barrier, cost-effective, and enabled physical distancing with minimal human resources (Tyndall, 2020).
The congruency in the proportion of male clients accessing sites as of March 1 and those who received emergency medical responses for suspected opioid-related overdoses (68% versus 73%) suggests that these harm reduction services are targeting the highest priority population in terms of risk (Government of Canada, 2021b). In the absence of client characteristic data at time point 2, we are not able to speak to changes associated with the pandemic. However, the meager national coverage through the identified services, and the toxic drug death projections for 2022 at or above current levels if harm reduction interventions are not increased (Government of Canada, 2021a), indicate the urgency of rapid scale-up. Quantifying the unmet demand warrants further research. Our partial waitlist data suggest inadequate intake capacity, although many sites did not report because of response burden, while other sites combined waitlists for safer supply and other services, kept no waitlists and/or reported avoided promoting their service because of limited intake capacity.
The relatively higher proportions of Indigenous clients reported by participating sites relative to the Canadian population (at 5%) (Government of Canada, 2018) may be attributed in part to priority access policies at four Ontario sites. While current data on rates of unregulated substance use by Indigenous people in Canada is difficult to find, rates in the U.S. were reported at 13%, compared to 8% of white people (Substance Abuse and Mental Health Services Administration, 2018). Priority intake policies can address health outcome disparities, such as death rates among PWUD who are Indigenous observed at five times higher than for others (Lavalley, Kastor, Valleriani, & McNeil, 2018). Evidence suggests that people identifying as Indigenous are less likely to receive substance use care, and more likely to discontinue it (Urbanoski, 2017). Recognition of the impacts of colonialism and ongoing racism in Canada necessitate culturally tailored supports (Nutton & Fast, 2015).
Lack of direct funding for safer supply was compounded by the urgency of COVID-19-driven implementation. The disproportionate pandemic safer supply expansion in BC relative to other regions was likely influenced by a favorable political climate; provinces with governments more resistant to harm reduction, such as Alberta and Ontario (Gibson, 2020; Smith, 2020), saw far less uptake. Continuing programming without dedicated funding is unsustainable and will be a barrier to safer supply expansion across Canada if not addressed.
The low reported rates of staffing of people with lived/living experience at safer supply sites warrants examination. Regional variations in terminology may have resulted in missed data; the rapid scale-up of safer supply and lack of funding (including for these roles) may also be contributing factors. However, a large proportion of sites did not report having any people with lived/living experience employed at their sites. The benefits of these “peer” workers include increased capacity to operate services, and ability to reach structurally vulnerable PWUD, in addition to fostering a safe sense of community for PWUD, supporting transitions to treatment, and providing low-barrier work opportunities (Kennedy et al., 2019). However, low wages, burnout from chronic stressors, including job precarity, trauma and grief, and limited psychological supports are prevalent, and should be addressed as scale-up continues (Bardwell et al., 2018; Olding, Boyd, Kerr, & McNeil, 2021).
Limitations
We are aware of safer supply providers who did not participate; for some, increased pandemic and workload demands prevented participation, while for others, unwillingness related to fear of retribution from colleagues, regulatory bodies, and/or regional governments. Because of the ambiguous definition of safer supply within the prescriber community, others may have been uncertain as to whether or not their prescribing of unsupervised doses would be considered “safer supply” (e.g., for those prescribing take-home doses of opioids for PWUD to manage chronic pain or treat addiction, rather than to mitigate SARS-CoV2 exposure risk or to reduce risk of overdose). Several sites were excluded because their services launched after May 1, 2020. Future research should be done using other data sources when available, such as administrative data sets (e.g., the coordinated national prescription monitoring program that exists in the United States), to more accurately estimate prescribing rates by region.
Not all participants completed both surveys and interviews, leading to an incomplete data set. Potential for inaccuracies resulted from only three quarters of participants verifying the finalized survey data, although colleague consultation assisted in confirming its accuracy. Some client demographic data were too burdensome to collect in the absence of established site evaluation procedures. Caution in interpreting quantitative data is required, although broad colleague consultation and diversity of participant roles enhanced its generalizability.
Conclusion
This environmental scan provides the only national examination of the rise in safer supply/risk mitigation prescribing at a critical early phase in Canada's COVID-19 response. This study highlighted the swift increase in an adaptable and flexible form of harm reduction-based prescribing during a time when PWUD were experiencing unprecedented increases in harms from unregulated drug use. Given the rapid changes in both addiction treatment and harm reduction practices and policies, our findings highlight the value of further research, including longitudinal studies to monitor changes in access to and delivery of safer supply in the country.
Funding statement
This work was funded in part by the Canadian Institutes of Health Research (CIHR) through a Canadian Research Initiative on Substance Misuse (CRISM) grant. Dr. Glegg is supported by a CIHR Fellowship Award (CIHR-FRN #415293). Dr. Kolla is supported by a Canadian Network on Hepatitis C (CanHepC) Postdoctoral Fellowship and a CIHR Banting Postdoctoral Fellowship. Dr. Brothers is supported by a CIHR Fellowship Award (CIHR-FRN #171259) and a Dalhousie University Internal Medicine Research Foundation Fellowship Award. Dr. Le Foll is supported by the Centre for Addiction and Mental Health (CAMH) and a clinician-scientist award from the department of Family and Community Medicine of the University of Toronto and an Addiction Psychiatry Chair from the department of Psychiatry of the University of Toronto. Dr. Fairbairn is supported by a Philip Owen Professorship in Addiction Medicine at the University of British Columbia, and a Scholar Award from the Michael Smith Foundation for Health Research/St. Paul's Hospital Foundation. The funding organizations had no roles in the project's design, implementation or analysis.
Ethics Approvals
All procedures were performed in compliance with relevant laws and institutional guidelines. The University of British Columbia/Providence Healthcare Joint Research Ethics Board approved the project, under the purview of program evaluation.
Declarations of Interest
Dr. Le Foll has obtained funding from Pfizer (GRAND Awards, including salary support) for investigator-initiated projects. Dr. Le Foll has some in-kind donations of cannabis products from Aurora, a medication donation from Pfizer and Bioprojet, was provided a coil for TMS study from Brainsway, and has been a consultant for Shionogi. Dr. Le Foll has obtained industry funding from Canopy (through research grants handled by CAMH or University of Toronto), Bioprojet, ACS and Alkermes, and in-kind donations of nabiximols from GW Pharma for past studies funded by CIHR and the National Institutes of Health (NIH). The other authors have no competing interests to declare.
Acknowledgments
The authors thank the participants for their time and insights, the project collaborators (Health Canada, Canadian Association of People Who Use Drugs, Canadian Drug Policy Coalition) for their contributions to the scope of data gathering, and knowledge translation planning, as well as the funders for their support.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.drugpo.2022.103742.
Appendix. Supplementary materials
References
- Ahamad K., Bach P., Brar R., Chow N., Coll N., Compton M., Yau S. British Columbia Centre on Substance Use.; 2020. Risk mitigation in the context of dual health emergencies v1.5; p. 25. Retrieved from https://www.bccsu.ca/risk-mitigation-in-the-context-of-dual-public-health-emergencies-v1-5/ [Google Scholar]
- Ahmad F.B., Rossen L.M., Sutton P. National Center for Health Statistics.; 2021. Provisional drug overdose death counts. Retrieved October 4, 2021, from website https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm. [Google Scholar]
- Bardwell G., Anderson S., Richardson L., Bird L., Lampkin H., Small W., McNeil R. The perspectives of structurally vulnerable people who use drugs on volunteer stipends and work experiences provided through a drug user organization: Opportunities and limitations. International Journal of Drug Policy. 2018;55(1):40–46. doi: 10.1016/j.drugpo.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglöf E. Vol. 1. Project Syndicate.; 2020. (A pandemic strategy as global as COVID-19). Retrieved from https://www.project-syndicate.org/commentary/covid19-pandemic-g20-cooperation-by-erik-berglof-2020-03. [Google Scholar]
- Bonn M., Palayew A., Bartlett S., Brothers T.D., Touesnard N., Tyndall M. Addressing the syndemic of hiv, hepatitis c, overdose, and covid-19 among people who use drugs: The potential roles for decriminalization and safe supply. Journal of Studies on Alcohol and Drugs. 2020;81(5):556–560. doi: 10.15288/jsad.2020.81.556. [DOI] [PubMed] [Google Scholar]
- British Columbia Ministry of Mental Health and Addictions . BC Government News Release.; 2020. New public health order to help slow B.C.’s overdose crisis. Retrieved from https://archive.news.gov.bc.ca/releases/news_releases_2017-2021/2020MMHA0051-001754.htm. [Google Scholar]
- British Columbia Ministry of Mental Health and Addictions . BC Government News Release.; 2021. B.C. introduces new prescribed safer supply policy, a Canadian first. Retrieved July 21, 2021, from website https://news.gov.bc.ca/releases/2021MMHA0035-001375. [Google Scholar]
- Canadian Association of People who Use Drugs . 2019. Safe supply concept document. Retrieved from https://vancouver.ca/files/cov/capud-safe-supply-concept-document.pdf. [Google Scholar]
- Canadian Community Epidemiology Network on Drug Use . 2020. Changes related to COVID-19 in the illegal drug supply and access to services, and resulting health harms. Retrieved from https://www.ccsa.ca/sites/default/files/2020-05/CCSA-COVID-19-CCENDU-Illegal-Drug-Supply-Alert-2020-en.pdf. [Google Scholar]
- Canadian Research Initiative in Substance Misuse (CRISM) CRISM website.; 2019. National injectable opioid agonist treatment for opioid use disorder operational guidance. Retrieved from https://crism.ca/wp-content/uploads/2019/09/CRISM_National_IOAT_Operational_Guideline-17Sept2019-English-FINAL.pdf. [Google Scholar]
- Canadian Research Initiative in Substance Misuse (CRISM) 2019. National injectable opioid agonist treatmetn for opioid use disorder clinical guideline. Retrieved from https://crism.ca/wp-content/uploads/2019/09/CRISM_National_IOAT_Clinical_Guideline-10-Sep2019-English-FINAL.pdf. [Google Scholar]
- Csete J., Elliott R. Consumer protection in drug policy: The human rights case for safe supply as an element of harm reduction. International Journal of Drug Policy. 2021;91 doi: 10.1016/j.drugpo.2020.102976. [DOI] [PubMed] [Google Scholar]
- Damschroder L.J., Aron D.C., Keith R.E., Kirsh S.R., Alexander J.A., Lowery J.C. Fostering implementation of health services research findings into practice: A consolidated framework for advancing implementation science. Implementation Science. 2009;7(4):50. doi: 10.1186/1748-5908-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eydt E., Glegg S., Sutherland C., Meador K., Trew M., Perreault M., Fairbairn N. Service delivery models for injectable opioid agonist treatment in Canada: An environmental scan. CMAJ: Canadian Medical Association Journal. 2020;9(1):E115–E124. doi: 10.9778/cmajo.20200021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbairn N., Ross J., Trew M., Meador K., Turnbull J., MacDonald S., Sutherland C. Injectable opioid agonist treatment for opioid use disorder: A national clinical guideline. Canadian Medical Association Journal. 2019;191(38):E1049–E1056. doi: 10.1503/cmaj.190344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genberg B.L., Astemborski J., Piggott D.A., Woodson-Adu T., Kirk G.D., Mehta S.H. The health and social consequences during the initial period of the COVID-19 pandemic among current and former people who inject drugs: A rapid phone survey in Baltimore, Maryland. Drug and Alcohol Dependence. 2021;221(August 2020) doi: 10.1016/j.drugalcdep.2021.108584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson V. IPolitics.; 2020. Ontario not considering “safe supply” measures, despite spike in suspected overdoses. Retrieved from https://ipolitics.ca/2020/05/04/ontario-not-considering-safe-supply-measures-despite-spike-in-suspected-overdoses/ [Google Scholar]
- Government of Canada . Statistics Canada.; 2018. Annual demographic estimates: Canada, Provinces and Territories, 2018. Analysis: Population by age and sex. Retrieved May 1, 2021, from website https://www150.statcan.gc.ca/n1/pub/91-215-x/2018002/sec2-eng.htm. [Google Scholar]
- Government of Canada . 2021. Modelling opioid-related deaths during the COVID-19 outbreak. Retrieved from https://www.canada.ca/en/health-canada/services/opioids/data-surveillance-research/modelling-opioid-overdose-deaths-covid-19.html. [Google Scholar]
- Government of Canada . 2021. Opioid- and stimulant-related harms in Canada (December 2021) Retrieved from https://health-infobase.canada.ca/substance-related-harms/opioids-stimulants/ [Google Scholar]
- Goyer M.-È., Hudon K., Plessis-Bélair M.-C., Ferguson Y. Institut Universaires sur les Dépendances.; 2020. Substance replacement therapy in the context of the COVID-19 pandemic in Québec: Clinical guidance for prescribers; p. 78. Retrieved from http://dependanceitinerance.ca/wp-content/uploads/2020/10/Guide-Pharmaco-COVID_ANG-VF.19.10.20.pdf. [Google Scholar]
- Hajdu P. 2020. Letter from the Minister of Health regarding treatment and safer supply. Retrieved from https://www.canada.ca/en/health-canada/services/substance-use/minister-letter-treatment-safer-supply.html. [Google Scholar]
- Hales J., Kolla G., Man T., O'Reilly E., Rai N., Sereda A. 2019. Safer opioid supply programs (SOS): A harm reduction informed guiding document for primary care teams-April 2020 update. Retrieved from https://bit.ly/3dR3b8m. [Google Scholar]
- Health Canada . Health Canada.; 2019. Backgrounder: New measures to address the opioid crisis and emerging drug threats. Retrieved April 22, 2020, from news release website https://www.canada.ca/en/health-canada/news/2019/07/backgrounder-new-measures-to-address-the-opioid-crisis-and-emerging-drug-threats.html. [Google Scholar]
- Health Canada . Government of Canada.; 2020. Government of Canada supports safer supply pilot project in B.C. Retrieved October 13, 2020, fromNews Release website https://www.canada.ca/en/health-canada/news/2020/07/government-of-canada-supports-safer-supply-pilot-project-in-bc.html. [Google Scholar]
- Health Canada . Cision News.; 2020. Government of Canada supports a safer drug supply project in Toronto. Retrieved from https://www.newswire.ca/news-releases/government-of-canada-supports-a-safer-drug-supply-project-in-toronto-845437678.html. [Google Scholar]
- Health Canada . Government of Canada.; 2020. Government of Canada highlights support for safer drug supply projects in Ontario. Retrieved October 13, 2020, fromNews Release website https://www.canada.ca/en/health-canada/news/2020/09/government-of-canada-highlights-support-for-safer-drug-supply-projects-in-ontario.html. [Google Scholar]
- Hsieh H.-F., Shannon S.E. Three approaches to qualitative content analysis. Qualitative Health Research. 2005;15(9):1277–1288. doi: 10.1177/1049732305276687. [DOI] [PubMed] [Google Scholar]
- Ivsins A., Boyd J., Beletsky L., McNeil R. Tackling the overdose crisis: The role of safe supply. International Journal of Drug Policy. 2020;80 doi: 10.1016/j.drugpo.2020.102769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M.C., Boyd J., Mayer S., Collins A., Kerr T., McNeil R. Peer worker involvement in low-threshold supervised consumption facilities in the context of an overdose epidemic in Vancouver, Canada. Social Science & Medicine Medicine. 2019;225(1):60–68. doi: 10.1016/j.socscimed.2019.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavalley J., Kastor S., Valleriani J., McNeil R. Reconciliation and Canada’s overdose crisis: Responding to the needs of Indigenous Peoples. Canadian Medical Association Journal. 2018;190(50):E1466–E1467. doi: 10.1503/cmaj.181093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luković S., Stojković D. Covid-19 pandemic and global tourism. Hotel and Tourism Management. 2020;8(2):79–88. doi: 10.5937/menhottur2002079l. [DOI] [Google Scholar]
- Merriam, S. B., & Grenier, R. S. (2019). Qualitative research in practice: Examples for discussion and analysis (2nd ed.; S. B. Merriam & R. S. Gernier, Eds.). San Francisco, CA: Jossey-Bass.
- Nutton J., Fast E. Historical trauma, substance use, and Indigenous peoples: Seven generations of harm from a “Big Event”. Substance Use and Misuse. 2015;50(1):839–847. doi: 10.3109/10826084.2015.1018755. [DOI] [PubMed] [Google Scholar]
- Olding M., Boyd J., Kerr T., McNeil R. And we just have to keep going”: Task shifting and the production of burnout among overdose response workers with lived experience. Social Science & Medicine. 2021;270(1) doi: 10.1016/j.socscimed.2020.113631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Public Health Agency of Canada . Government of Canada.; 2020. Statement from the Chief Public Health Officer of Canada on COVID-19. Retrieved June 16, 2020, from website https://www.canada.ca/en/public-health/news/2020/05/statement-from-the-chief-public-health-officer-of-canada-on-covid-198.html. [Google Scholar]
- Public Health Agency of Canada . Government of Canada.; 2021. Special advisory committee on the epidemic of opioid overdoses. Opioid- and stimulant-related harms in Canada. Retrieved July 19, 2021, from website https://health-infobase.canada.ca/substance-related-harms/opioids-stimulants/ [Google Scholar]
- QSR International . QSR International.; 2019. NVivo. [Google Scholar]
- Smith A. Calgary Herald.; 2020. Province won't consider safe supply programs to address spiking overdose deaths. Retrieved from https://calgaryherald.com/news/politics/province-wont-consider-safe-supply-programs-to-address-spiking-overdose-deaths. [Google Scholar]; https://ipolitics.ca/2020/05/04/ontario-not-considering-safe-supply-measures-despite-spike-in-suspected-overdoses/
- Substance Abuse and Mental Health Services Administration. (2018). Results from the 2017 National survey on drug use and health: Detailled tables. Rockville, Marylannd.
- Tyndall M. Safer opioid distribution in response to the COVID-19 pandemic. International Journal of Drug Policy. 2020;83(1) doi: 10.1016/j.drugpo.2020.102880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanoski K.A. Need for equity in treatment of substance use among Indigenous people in Canada. Cmaj. 2017;189(44):E1350–E1351. doi: 10.1503/cmaj.171002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo A. The Globe and Mail.; 2020. Trudeau says focus is on safe supply, not decriminalization as overdose deaths spike. Retrieved from https://www.theglobeandmail.com/canada/british-columbia/article-trudeau-says-focus-is-on-safe-supply-not-decriminalization-as/ [Google Scholar]
- World Health Organization . 2020. Listing of WHO’s response to COVID-19. Retrieved February 16, 2021, from website https://www.who.int/news/item/29-06-2020-covidtimeline. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.