Abstract
The specialized pro-resolving lipid mediator Maresin 1 (MaR1) is involved in the resolution phase of tissue inflammation. It was hypothesized that exogenous administration of MaR1 would attenuate abdominal aortic aneurysm (AAA) growth in a cytokine-dependent manner via LGR6 receptor signaling and macrophage-dependent efferocytosis of smooth muscle cells (SMCs). AAAs were induced in C57BL/6 wild-type (WT) mice and smooth muscle cell specific TGF-β2 receptor knockout (SMC-TGFβr2−/−) mice using a topical elastase AAA model. MaR1 treatment significantly attenuated AAA growth as well as increased aortic SMC α-actin and TGF-β2 expressions in WT mice, but not SMC-TGFβr2−/− mice, compared to vehicle-treated mice. In vivo inhibition of LGR6 receptors obliterated MaR1-dependent protection in AAA formation and SMC α-actin expression. Furthermore, MaR1 upregulated macrophage-dependent efferocytosis of apoptotic SMCs in murine aortic tissue during AAA formation. In vitro studies demonstrate that MaR1-LGR6 interaction upregulates TGF-β2 expression and decreases MMP2 activity during crosstalk of macrophage-apoptotic SMCs. In summary, these results demonstrate that MaR1 activates LGR6 receptors to upregulate macrophage-dependent efferocytosis, increases TGF-β expression, preserves aortic wall remodeling and attenuate AAA formation. Therefore, this study demonstrates the potential of MaR1-LGR6 mediated mitigation of vascular remodeling through increased efferocytosis of apoptotic SMCs via TGF-β2 to attenuate AAA formation.
INTRODUCTION
Abdominal aortic aneurysms (AAA) were identified as the primary cause of 9,923 deaths in the United States in 2018 (1). When left untreated, the natural progression of AAA is rupture, which is reported to occur in 12,000–15,000 patients each year in the US with an associated mortality of 50–80% (2). Although screening and subsequent repair of AAA greater than 5.5cm has reduced the mortality rate by nearly half (3), there remains no effective medical therapy for the treatment of AAA.
While the exact pathophysiology of AAA formation remains to be fully elucidated, inflammation of the aortic wall is known to play a central role (4, 5). The infiltration of the aortic wall by immune cells with subsequent production of pro-inflammatory cytokines i.e. IL-1β, IL-17, MCP-1, and HMGB1, leads to a series of events including elastin degradation, smooth muscle cell (SMC) apoptosis, and vascular remodeling (6–9) The end result is disruption of vascular wall integrity and subsequent aortic dilatation.
Specialized pro-resolving lipid mediators (SPMs) are a novel class of bioactive derivatives of ω−3 and ω−6 fatty acids that have recently been shown to regulate resolution of chronic inflammation in various diseases (10–13). Maresin 1 (MaR1) is an endogenous SPM derived from the omega-3 fatty acid, docosahexanoic acid (DHA) that has been shown to modulate leucine-rich repeat-containing G protein–coupled receptor 6 (LGR6) and modulate tissue inflammation (12, 14). LGR6 is present in multiple tissues and is known to promote repair and regeneration. The biogenesis of SPMs, including MaR1, occurs as a natural part of the resolution phase of inflammation and has been shown to have protective and homeostatic effects in several inflammatory disease models, including colitis, tissue regeneration, pain control, acute lung injury, and lung ischemia/reperfusion injury (15–18). Specifically related to the vasculature, in a carotid artery ligation model, MaR1 demonstrated protective effects on neointimal hyperplasia formation (19).
Mechanistically, the protective role of MaR1/LGR6 signaling in modulating smooth muscle activation and attenuating TGF-β1 mediated vascular inflammation remains undescribed. The process of phagocytosis of apoptotic cells, termed ‘efferocytosis’ is known to play a central role in tissue homeostasis, allowing for clearance of apoptotic cells and resulting in resolution of inflammation in surrounding tissues (20). As such, efferocytosis is vital not only in attenuating the inflammatory response, but also in aiding in the resolution of inflammation (13, 21). Although defective efferocytosis has been implicated in several chronic inflammatory diseases (22), its role in the development of AAA has not been well studied, especially in the context of MaR1-mediated efferocytosis of vascular smooth muscle cells.
By employing the pro-resolving effects of MaR1, it was hypothesized that MaR1 would inhibit the formation of AAA by targeting pathologic inflammation. In this study, the immunomodulation of vascular inflammation and remodeling during AAA formation by MaR1 and LGR6 signaling was investigated using an established topical elastase-treatment model of experimental murine AAA. Furthermore, the role of MaR1 in upregulating the process of macrophage-mediated efferocytosis, i.e. uptake of apoptotic/necrotic SMCs, and its contribution to smooth muscle cell remodeling via TGF-β signaling was delineated.
MATERIALS AND METHODS
Animals
Eight to 12-week old wild-type C57BL/6 male mice (Jackson Laboratory, Bar Harbor, ME) and SMC-TGF-βr2 (produced by crossing Tgfbr2f/f with Myh11-CreER strains) (23) were housed and maintained at 70°F, 50% humidity, in 12-hour light-dark cycles as per institutional animal protocols. Mice were provided drinking water and standard chow diet ad libitum. All experiments were approved by and conducted in accordance to the Institutional Animal Care and Use Committee of the University of Florida (protocol # 201910902).
Human aortic tissue analysis
Collection of human aortic tissue was approved by the University of Florida’s Institutional Review Board (#IRB201902782). Consent was obtained from all patients before surgery. Aortic tissue from male and female patients was resected during open surgical AAA repair as well as during organ transplant donor surgery (controls). Tissue was homogenized in Trizol, and RNA was purified per manufacturer’s protocol (Qiagen, Valencia, CA). cDNA was synthesized using the iScript cDNA Synthesis Kit (BioRad, Hercules, CA). Quantitative (real-time) RT-PCR was performed with primer sets (MWG/Operon, Huntsville, AL) in conjunction with SsoFast EvaGreen Supermix (BioRad, Hercules, CA). Gene expression was calculated by using the relative quantification method according to the following equation: 2(−ΔCT), where ΔCT=(Average gene of interest)−(Average reference gene), where GAPDH was used as the reference gene.
Elastase treatment model of abdominal aortic aneurysms
AAA formation was induced using a previously described topical elastase model (24). In summary, the infrarenal aorta was exposed circumferentially from just distal the renal arteries to the aortic bifurcation. A micropipette was used to apply 5μl of porcine pancreatic elastase (Sigma-Aldrich, St. Louis, MO; 0.3mg protein/mL, 7 units/mg protein) topically to the exposed aortic adventitia for 5 minutes. The abdominal aorta was exposed and dissected free from surrounding tissue from the left renal vein proximally to the aortic bifurcation distally. A small puncture was made in the suprarenal aorta and blood evacuated from the vessel lumen. Aortic diameters were measured by video micrometry using NIS-Elements D5.10.01 software attached to the microscope (Nikon SMZ-25; Nikon Instruments, Melville, NY). Aortic dilation percentage was determined by [(maximal AAA diameter − self-control aortic diameter)/ (self-control aortic diameter)] × 100. Aortic dilation of ≥ 100% was considered positive for AAA (25). The aortas were collected and either flash frozen in −80°F for protein extraction, or incubated overnight in paraformaldehyde solution for histology and immunohistochemistry.
Treatment of mice with MaR1
To evaluate the preventive aspect of MaR1 in AAA formation, mice received intraperitoneal injections (i.p.) with 0.2ml vehicle (0.1% ethanol in 0.9% saline) or MaR1 (4ng/g or 40 ng/g bodyweight; Cayman Chemicals; Ann Arbor, MI) (19) on post-operative days 1, 3, 5, and 7 and aortas were harvested on post-operative day 14. A separate group of animals were analyzed to assess the effect of MaR1 treatment of pre-formed AAAs. In this group, mice underwent i.p. injections with either vehicle (0.1% ethanol in 0.9% saline) or MaR1 (4ng/g bodyweight) administered on post-operative days 7, 9, 11, and 13 and aortas were harvested on post-operative day 14 for further analyses.
LGR6 expression quantification
In order to evaluate expression of LGR6 receptor, mice underwent AAA induction surgery as described above with topical elastase or heat inactivated elastase as a control, and aortas were harvested on days 7 or 14. RNA was isolated from murine and human aortas using Total Exosome RNA and Protein Isolation Kit (Thermo Fisher Scientific) The following primers were used for murine analysis: LGR6 Fwd: GAGGACGGCATCATGCTGTC, LGR6 Rev: GCTCCGTGAGGTTGTTCATACT, β-Actin Fwd: GGCTGTATTCCCCTCCATCG and β-Actin Rev: CCAGTTGGTAACAATGCCATGT. To evaluate expression of LGR6 receptor in humans with and without AAA, aortic tissue was obtained as described above and RNA was isolated. The following primers were used: LGR6 Fwd: ACGGCTTACCTGGACCTCA, LGR6 Rev: TGCTTGTCCTGGGATGTGTG, GAPDH Fwd: TGACGGCTTACCTGGACCTCA, GAPDH Rev: AGAGAATGCTTGTCCTGGGATG. cDNA was synthesized via the iScript Reverse Transcription supermix for RT-qPCR (BioRad) and the PCR was carried out using the SYBRgreen master mix. Each PCR reaction was carried out in triplicate, and the relative quantification of gene expression was analyzed using the ΔΔCT method with β-Actin (mice) and GAPDH (humans) as the endogenous reference.
In vivo knockdown of LGR6
Mice were injected i.p. with siRNA for mouse LGR6 (10μg; catalog no. E- 044056-00-0010, Dharmacon Accell siRNA pool) or nontarget siRNA nontarget siRNA (10 μg; nontargeting siRNA no. 1; catalog no. D-001910-01-05), as previously described (14). One day later, both groups of mice underwent AAA induction surgery as described above with active elastase. Quantification of LGR6 expression by RT-PCR was performed in purified peritoneal macrophages from LGR6 siRNA and control siRNA treated mice. LGR6-siRNA-injected mice reduced LGR6 expression by 52.3±1.9% compared with nontarget siRNA-injected mice. Mice received i.p. injections with MaR1 (4ng/g bodyweight) on post-operative days 1, 3, 5, and 7 and aortas were harvested on post-operative day 14. Aortic dilation percentage was measured and aortic tissue was collected for immunohistochemistry.
Histology
Harvested aortic tissue was fixed in 4% buffered formaldehyde overnight, transferred to 70% ethanol, and embedded in paraffin. Aortic cross sections were prepared and stained for alpha SMCs (anti-mouse alpha smooth muscle actin, 1:1000). Images were acquired with 20X magnification by an Olympus microscope equipped with a digital camera (Olympus America, Center Valley, PA, USA) and ImagePro software (Media Cybernetics, Rockville, MD, USA). For grading, the positive staining area of entire aortic tissue sample was selected and measured using integrated optical density of each section.
Enzyme Linked Immunosorbent Assay
MaR1 was quantified by ELISA using a commercially available ELISA kit as per the manufacturer’s instructions (Cayman Chemicals, Ann Arbor, MI).
Flow cytometry
Murine aortic tissue was harvested from WT mice after undergoing AAA induction and being treated with i.p. injections of either 4ng/g MaR1 or vehicle (0.2ml; 0.1% ethanol in 0.9% saline) on days 1, 3, 5, and 7 and harvested on day 7 or day 14. Aortic tissue from mice was minced and incubated for 15 min at 37°C with collagenase type IA (Sigma Aldrich; St. Louis, MO) in PBS with 0.5% BSA and 2mM EDTA. The cell suspension was prepared for flow cytometry analysis using the following antibodies: APC–Cy7–labeled CD45, CD11b-PerCP-Cy5.5 and SMα-actin-Alexa Flour 488 (eBioscience; San Diego, CA). To facilitate intracellular staining, cells were fixed with flow cytometry fixation buffer and permeabilized with flow cytometry permeabilization/wash buffer (R&D Systems), and incubated with either an unconjugated mouse IgG1 or SMα-actin-Alexa Flour 488 antibodies. Nonspecific staining was assessed by using isotype controls for intracellular staining in place of the primary antibodies. FACS data was analyzed using FlowJo software v9.
Cell culture experiments
Primary aortic smooth muscle cells (SMCs) were purified from WT mice (26) and RAW264.7 macrophages (ATCC, Manassas, VA) were cultured (0.5 × 106 cells in 12-well plates), as per manufacturer’s recommendations. SMCs were exposed to transient elastase treatment for 30 min or staurosporine to induce apoptosis and was followed by washing with PBS and replacement of the medium. Separately, macrophage cultures were incubated with/without MaR1 (100nM) for 1hr at 37°C. For knockdown of LGR6, macrophages were transfected with LGR6 siRNA or scramble-control siRNA (ThermoFisher, Waltham, MA) prior to treatment with/without MaR1. Transfection of RAW264.7 macrophages with LGR6 siRNA demonstrated an 82.3±2.9% decrease in LGR6 expression compared to control siRNA. Following the respective treatments, apoptotic SMCs and macrophages (1:2 ratio) were co-cultured for 24hrs and supernatants were analyzed for TGF-β2 and MMP2 expressions. Controls consisted of co-cultures of untreated SMCs and macrophages and supernatants were collected after 24 hrs. Flow cytometry analysis was done on apoptotic SMCs and macrophages to analyze efferocytosis, as described above.
TGF-β2 and MMP2 expression assay
Murine aortic tissue and cell culture supernatants were analyzed for the quantification of TGF-β2 expression using an ELISA kit, as suggested by the manufacturer’s instructions (R&D Systems, Minneapolis, MN). MMP2 expression was measured using a luminex bead array assay per the manufacturer’s assay (Millipore Sigma, Burlington, MA).
Statistical analysis
Statistical analysis was completed with GraphPad 7 (GraphPad Software, La Jolla, CA) software. One-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test was used to compare means between groups three or more groups. An unpaired students t-test with nonparametric Mann-Whitney or Wilcoxon rank sum test was also used for pairwise comparison of groups. Results are displayed as mean ± standard error of mean and p<0.05 was considered statistically significant.
RESULTS
Maresin-1 prevents experimental murine AAA formation
Using the topical elastase model, mice were treated with either elastase or heat-inactivated elastase on day 0 and injected with vehicle or MaR1 (4ng/g bodyweight) on post-operative days 1, 3, 5, 7 and harvested on day 14 (Figure 1A). A significant increase in mean aortic diameter was noted in elastase treated mice compared to heat-inactivated elastase treated control mice (132.1±6.1% vs. 1.28±0.64%; p<0.001). When mice were administered MaR1 (4ng/g bodyweight), elastase-treated mice demonstrated significantly decreased mean aortic dilation compared to mice treated with vehicle alone (95.1±5.3% vs. 132.1±6.1%; p<0.001, Figure 1B–C). The expression of TGF-β2 in aortic tissue was significantly increased after MaR1 treatment compared to untreated controls (44.4±10.4 vs. 14.2±2.4 pg/ml; p=0.01; Figure 1D). Conversely, the expression of MMP2 was significantly attenuated in MaR1-treated mice compared to untreated elastase controls (181.7±29 vs. 351.9±39.9 pg/ml; p=0.003; Figure 1E). Additionally, MaR1 treated mice demonstrated significantly increased expression of smooth muscle alpha actin (SM-αA) compared to mice treated with vehicle alone (18.5 ± 1.1% vs. 13.8% ± 0.9%; p=0.02, Figure 1F–G).
Figure 1.
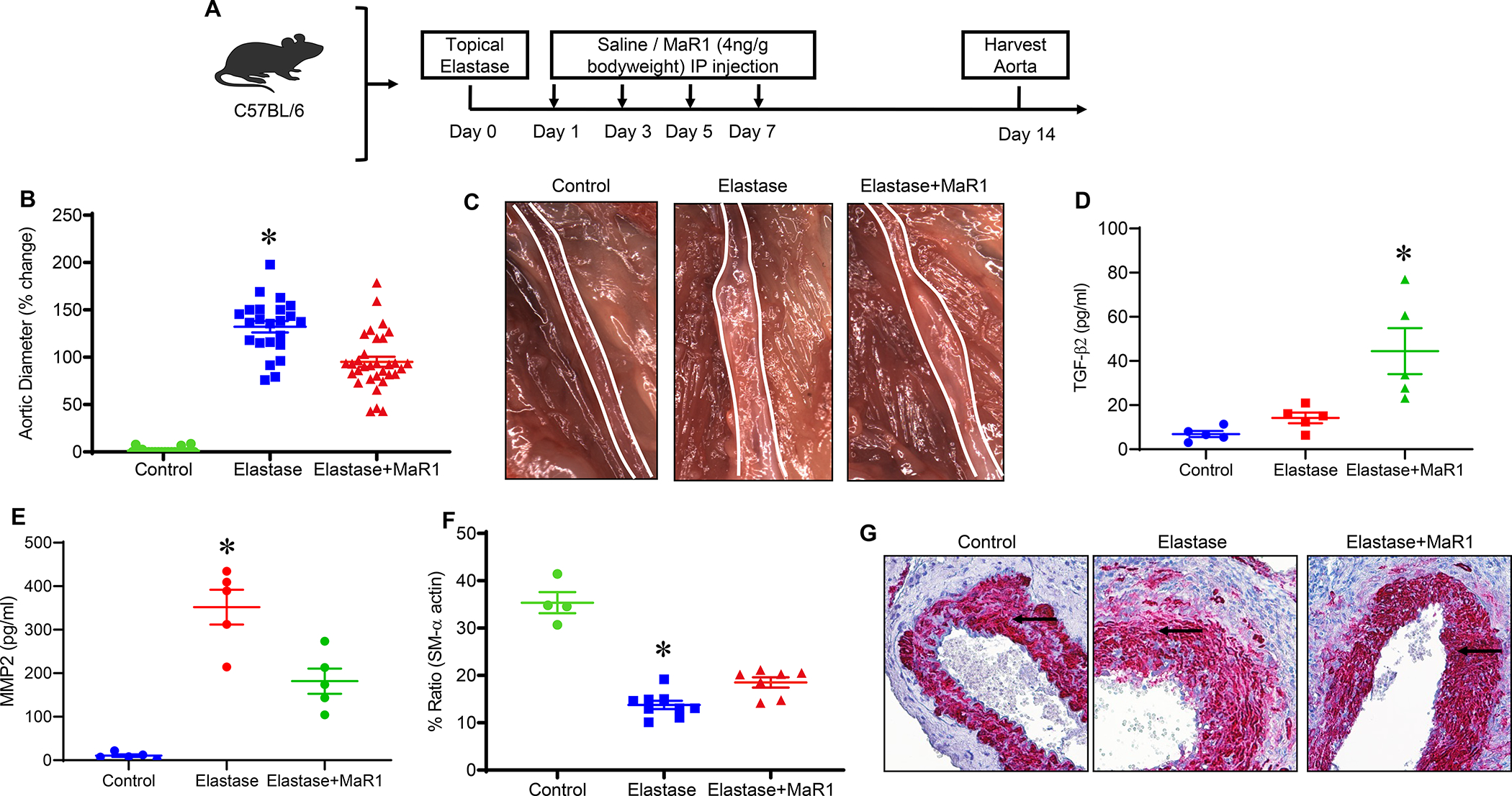
MaR1 administration attenuates AAA formation and preserves smooth muscle-α actin within the aortic wall. A, Schematic description of topical elastase treatment design. Mice were divided into three groups and treated with either heat inactivated elastase or elastase on day 0. Mice were then administered either vehicle or MaR1 on days 1, 3, 5, and 7. Aortic diameter was measured on day 14 and tissue harvested for additional analysis. B, MaR1 treated mice demonstrated a significant decrease in aortic diameter compared to vehicle treated mice; *p<0.001 vs. other groups; n=20–32 per group). C, Representative images of aortic phenotype in the respective groups. D, TGF-β2 expression in aortic tissue was significantly increased after MaR1 treatment in elastase-treated WT mice compared to untreated controls (*p<0.05 vs. other groups; n=5/group). E, Expression of MMP2 in aortic tissue was significantly attenuated in MaR1-treated mice compared to untreated controls (*p<0.01 vs. other groups; n=5/group). F, Expression of smooth muscle-α actin is significantly increased in mice treated with MaR1 compared to mice treated with vehicle alone (*p<0.02 vs. other groups; n=4–9 per group). G, Representative histological images of smooth muscle-α actin staining in the respective groups. Arrows indicate areas of immunostaining.
To test the dose-dependent effect of MaR1 on AAA formation, a separate group of elastase treated mice were administered vehicle or higher dose MaR1 (40ng/g bodyweight) on post-operative days 1, 3, 5, and 7 and harvested on day 14 (Supplement Figure S1A). MaR1 (higher dose) treated mice again demonstrated a significantly decreased mean aortic dilation compared to vehicle treated mice (99.2 ± 4.8% vs. 124.1 ± 9%; p=0.01, Supplement Figure S1B). However, there was no significant difference in mean aortic dilation between mice receiving MaR1 (lower dose) vs. MaR1 (higher dose) (95.1±5.3% vs. 99.2±4.8%; p=0.81, Supplement Figure S1C).
Maresin-1 attenuates the growth of pre-formed AAAs
To test the potential of MaR1 in mitigating clinically applicable scenario of preformed AAAs, mice were administered either vehicle or MaR1 beginning on days 7, 9, 11, and 13 and harvested on day 14 (Figure 2A). Mice treated with MaR1 demonstrated significantly attenuated mean aortic dilation compared to mice treated with vehicle alone (113±5.6% vs. 165±9.4%; p<0.001, Figure 2B and 2C). MaR1 treated mice demonstrated significantly increased expression of SM-α actin compared to mice treated with vehicle alone (27.1±3.4% vs. 14.2 ± 2.4%, respectively; p=0.03, Figure 2D–E).
Figure 2.
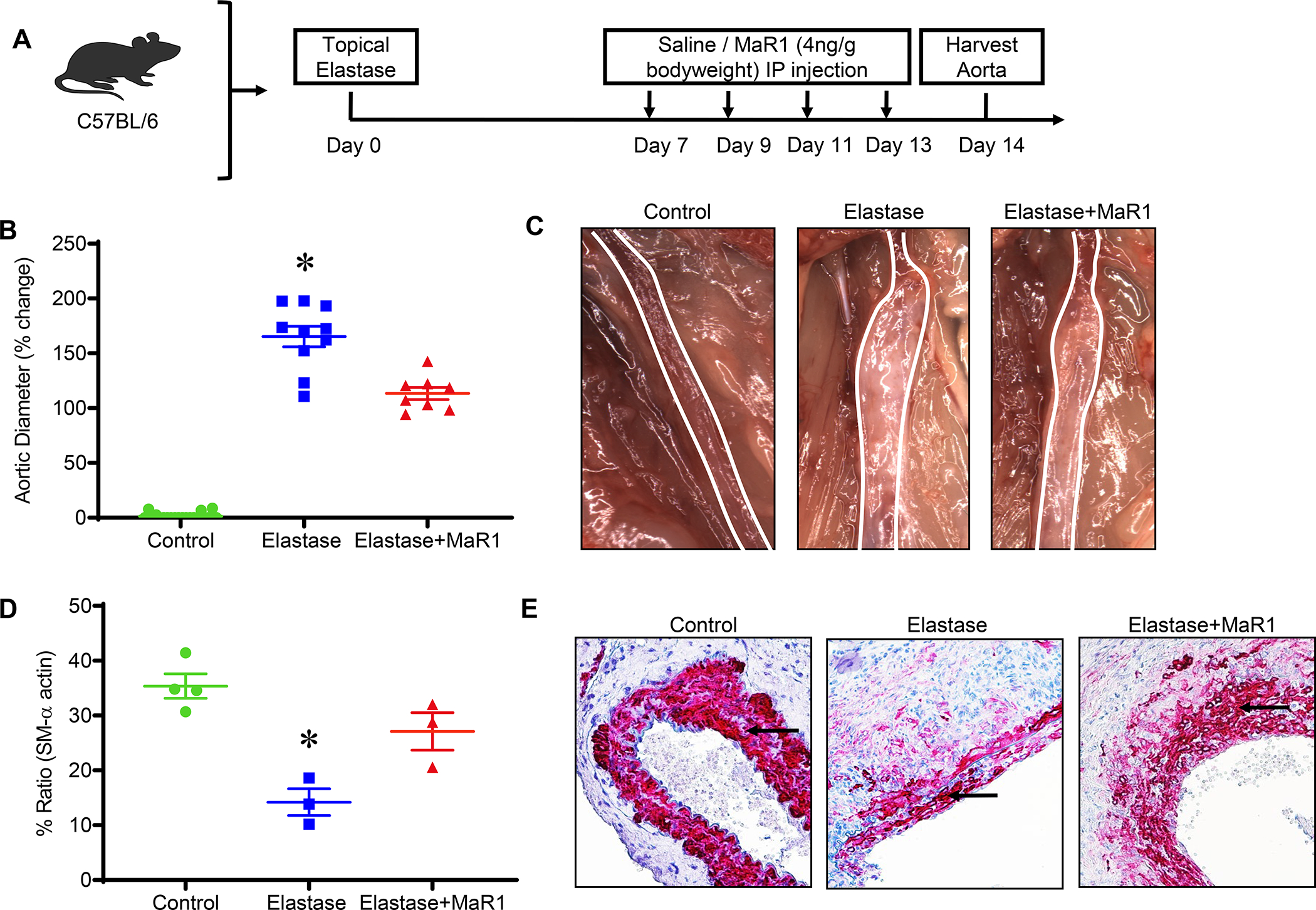
MaR1 attenuates the growth of pre-formed experimental murine AAA and preserves smooth muscle-α actin expression. A, Schematic description of treatment design. B, MaR1 treated mice demonstrated a significant decrease in aortic diameter compared to vehicle treated mice (*p<0.001 vs. other groups; n=8–10 per group). C, Representative images of aortic phenotype in the respective groups. D, Expression of smooth muscle-α actin is significantly increased in mice treated with MaR1 compared to mice treated with vehicle alone (*p<0.03 vs. Elastase+MaR1; n=3–4 per group). E, Representative histological images of smooth muscle-α actin staining in the respective groups is shown. Arrows indicate areas of immunostaining.
Maresin-1 mediated mitigation of aortic remodeling is regulated by TGF-β signaling
To investigate the effects of MaR1 on SMC activation and aortic remodeling via TGF-β signaling, AAA formation was analyzed in tamoxifen-treated SMC-Tgfβr2−/− mice (Figure 3A). There was no significant difference in aortic diameter in elastase-treated SMC-Tgfβr2−/− mice compared to elastase-treated WT mice (119.1± 8.5% vs. 133.9±11.9%; p=0.59; Figure 3B–C). MaR1 administration failed to attenuate AAA formation in elastase-treated SMC-Tgfβr2−/− mice compared to vehicle administered and elastase-treated SMC-Tgfβr2−/− mice (131.6± 12% vs. 119.1± 8.5%; p=0.410). Similarly, no significant difference was observed in expression of SM-α actin after MaR1 treatment in elastase-treated SMC-Tgfβr2−/− compared to vehicle-treated SMC-Tgfβr2−/− mice (Figure 3D–E).
Figure 3.
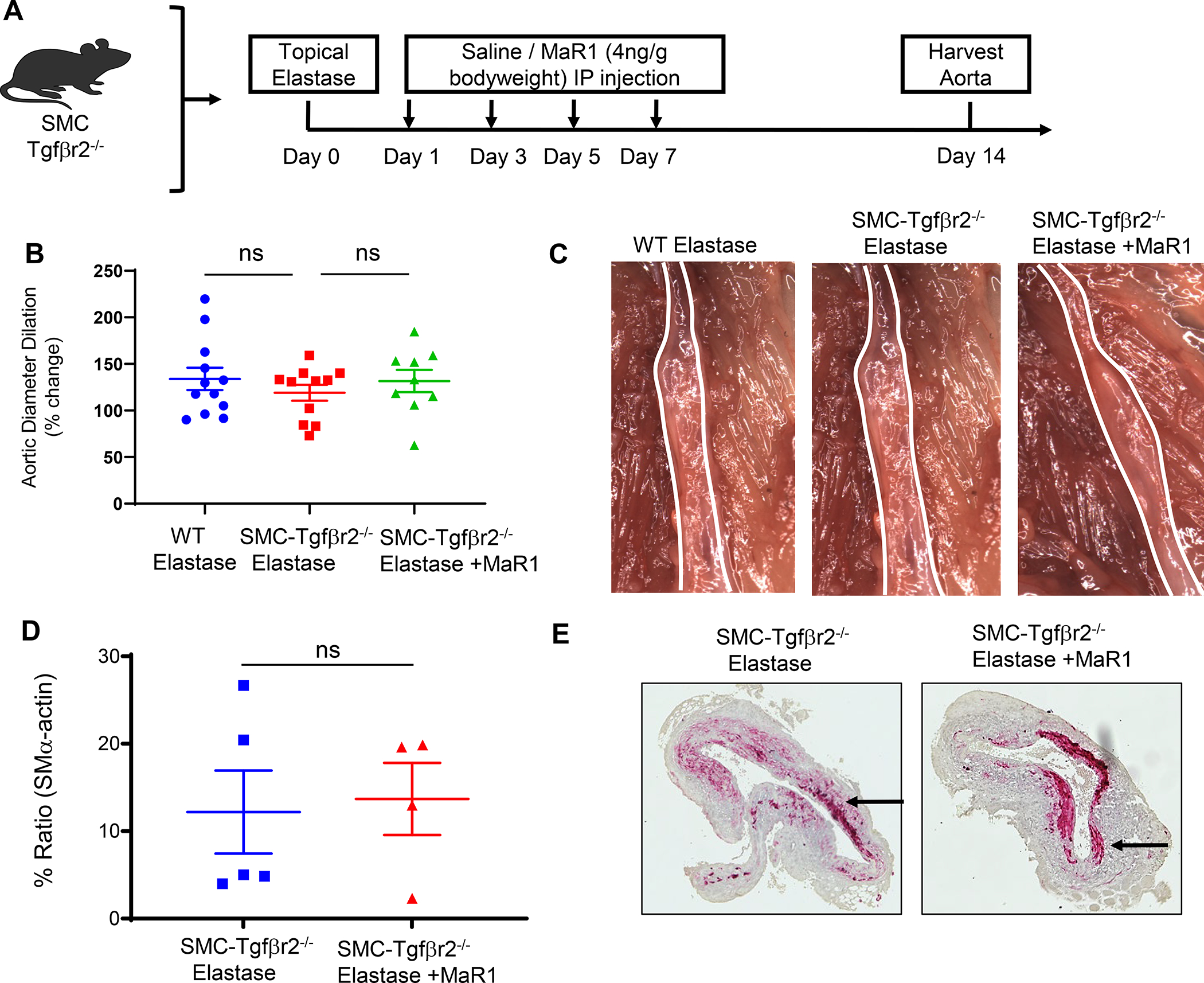
Maresin-1 mitigates AAA formation via smooth muscle cell-dependent TGF-β2 signaling. A, Schematic description of elastase AAA model in tamoxifen-treated SMC-Tgfβr2−/− mice. B, MaR1 treated SMC-Tgfβr2−/− mice showed no difference in aortic diameter compared to vehicle treated SMC-Tgfβr2−/− mice or elastase-treated WT mice (n=9–11 mice per group; ns, not significant). C, Representative images of aortic phenotype in respective groups. D, Expression of smooth muscle cell-α actin is similar in SMC-Tgfβr2−/− mice treated with MaR1 compared to mice treated with vehicle alone (n=4–5/group; ns, not significant). E, Representative histological images of smooth muscle-α actin staining in respective groups. Images were acquired using 20X magnification.
Maresin 1-dependent protection against AAA formation is mediated by LGR6 receptors
In order to assess the molecular target and ligand-receptor interactions of MaR1, the effect of in vivo knockdown of endogenous murine LGR6 was examined. Treatment with LGR6 siRNA obliterated the protective effect of MaR1 as seen by increased aortic diameter as compared with mice that were administered with control siRNA+MaR1 (108.0 ± 11.5% vs. 71.4±9.9%; p=0.04, Figure 4A–B) Expression of smooth muscle-α actin was decreased in mice administered with MaR1 and treated with siRNA for LGR6 compared to control siRNA+MaR1 (Figure 4C). Endogenous MaR1 levels in murine aortic tissue after topical elastase and heat-inactivated (control) elastase treatment were measured at days 1, 3, 7 and 14 in mice and were found to be the highest on day 7 corresponding with the largest progression of aortic dilation (Supplement Figure S2A–S2B). The expression of LGR6 receptor in murine aortic tissue was significantly downregulated in AAA tissue compared to respective controls on days 7 and 14 (Supplement Figure S2C–S2D). Similarly, LGR6 expression in human aortic tissue from AAA patients was also significantly decreased as compared to controls (Supplement Figure S3).
Figure 4.
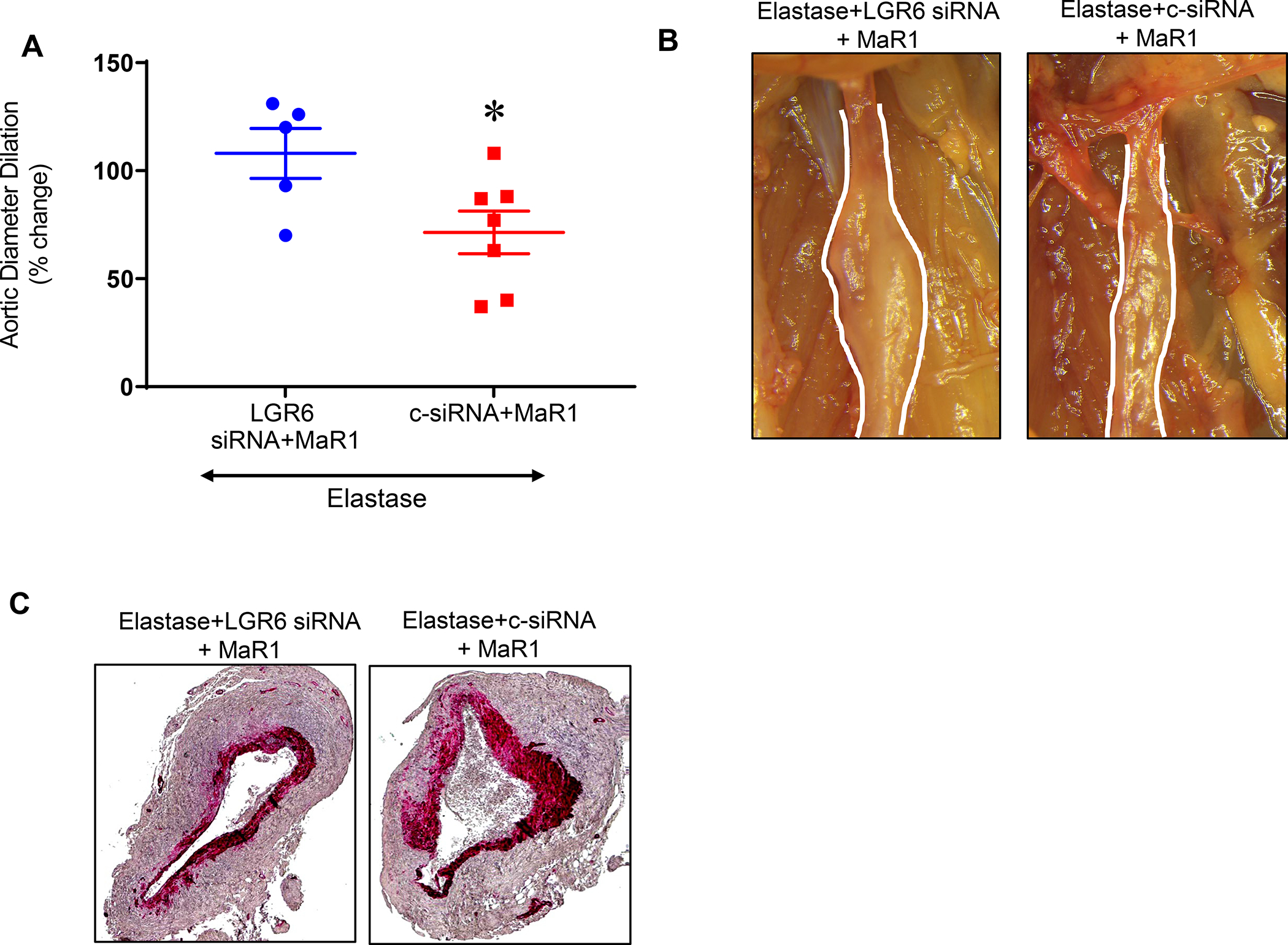
In vivo LGR6 knockdown reduces protective effect of MaR1. A, LGR6-siRNA treatment of WT mice demonstrated a significant increase in aortic diameter as compared to control (c)-siRNA treated mice after administration of MaR1 in respective groups (*p=0.04, n=5–7 per group). B, Representative images of aortic phenotype in respective groups. Images were acquired using 20X magnification. C, Expression of smooth muscle-α actin is decreased in mice administered with MaR1 and treated with LGR6-siRNAcompared to control-siRNA (n=5 per group).
Efferocytosis of SMCs is increased by Maresin-1 in aortic wall of murine AAAs
Previous studies have shown that murine and human aneurysm tissues is accompanied by biochemical, morphological and molecular changes consistent with SMC apoptosis. To investigate the clearance of apoptotic SMCs by phagocytic macrophages i.e. efferocytosis, and its modulation by MaR1, we analyzed murine aortic tissue in the elastase AAA model. Flow cytometry analysis of murine aortic tissue demonstrated increased levels of co-expression CD11b+SM-αA+ cell population in MaR1 treated mice compared to mice treated with vehicle alone on post-operative day 7 (46.4±2.6% vs. 17.2±3%; p<0.001, Figure 5A) and post-operative day 14 (50.3±1.2% vs. 32.8±4.2%; p=0.01, Figure 5B). These results suggest that MaR1 upregulates macrophage-dependent uptake of SMCs during AAA formation.
Figure 5.
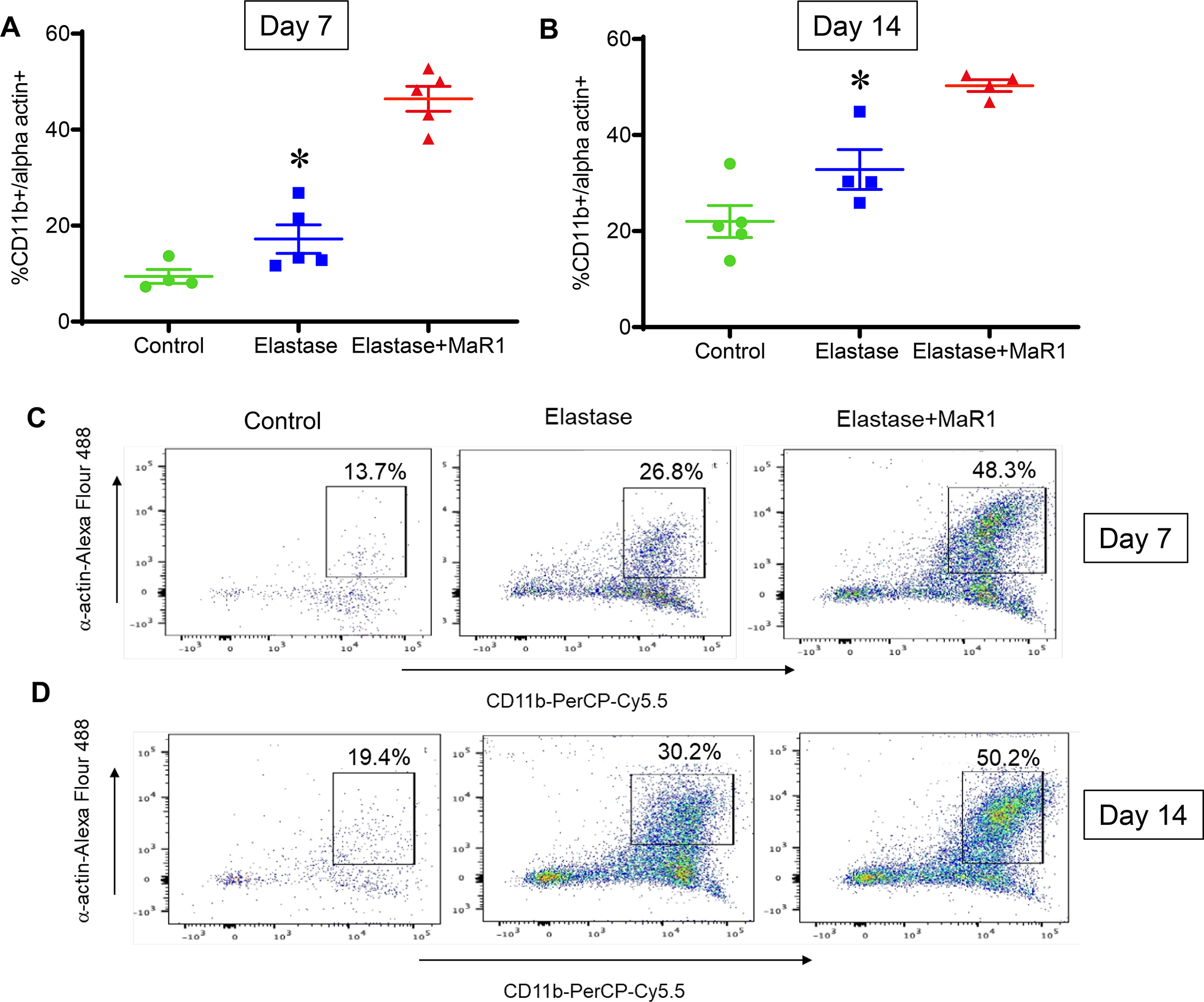
MaR1 increases efferocytosis of SMCs in aortic tissue of murine AAA. Flow cytometry analysis of murine aortic tissue demonstrated increased levels of co-expression CD11b+SM-αA+ cell population in MaR1 treated mice compared to mice treated with vehicle alone at (A) post-operative day 7 (*p<0.001, n=4–5 per group) and (B) post-operative day 14 (*p=0.01, n=4–5 per group). Representative flow cytometry panels of day 7 (C) and day 14 (D) murine aortic tissue analysis.
Maresin-1 interacts with LGR6 on macrophages to upregulate TGF-β secretion during phagocytosis of apoptotic SMCs
To investigate the mechanistic crosstalk between MaR1-activated macrophages and apoptotic smooth muscle cells, we used in vitro studies involving RAW264.7 macrophages and murine SMCs. Transient elastase-treatment (30 minutes) treatment induced apoptosis in SMCs after 6hrs as measured by Annexin V/FITC analysis (data not shown). Co-culture of elastase-induced apoptotic SMCs with macrophages (separately treated with/without MaR1) were performed for 24 hrs and culture supernatants were analyzed for TGF-β2 and MMP2 expression. A significantly increased TGF-β2 secretion was observed in supernatants from co-cultures treated with MaR1 compared to untreated controls (252.2±30.1 vs. 86.9±14.5 pg/ml; p<0.01; Figure 6A). Moreover, expression of MMP2 was significantly increased in elastase-induced apoptotic SMCs compared to controls (254.4±36.2 vs. 44.2±4.8 pg/ml; p<0.01; Figure 6B). However, MaR1-treated macrophages significantly decreased MMP2 expression in supernatants of co-cultures with apoptotic SMCs compared to untreated controls (138.7±13.5 vs. 255.5±33.3 pg/ml; Figure 6B). Similar to the in vivo results, a significant increase in efferocytosis was observed in MaR1-treated macrophages for the uptake of apoptotic SMCs (Supplement Figure S4). Furthermore, the MaR1-dependent increase in TGF-β2 was abolished when pre-treatment with siRNA for LGR6 was performed and these macrophages were co-cultured with apoptotic SMCs, compared to pre-treatment with control siRNA (51.1±8.3 vs. 178.5±27.2 pg/ml; p<0.01; Figure 6C). Finally, LGR6 blockade of macrophages abolished the MaR1-mediated decrease in MMP2 expression when co-cultured with apoptotic SMCs, compared to pre-treatment of macrophages with control siRNA (284.2±31.3 vs. 133.3±15.2 pg/ml; p<0.01; Figure 6D). Taken together, the in vivo and in vitro results suggest that MaR1-LGR6 signaling on macrophages upregulates efferocytosis of SMCs and increases TGF-β signaling to mitigate SMC activation and vascular remodeling leading to mitigation of AAA formation (Figure 7).
Figure 6.
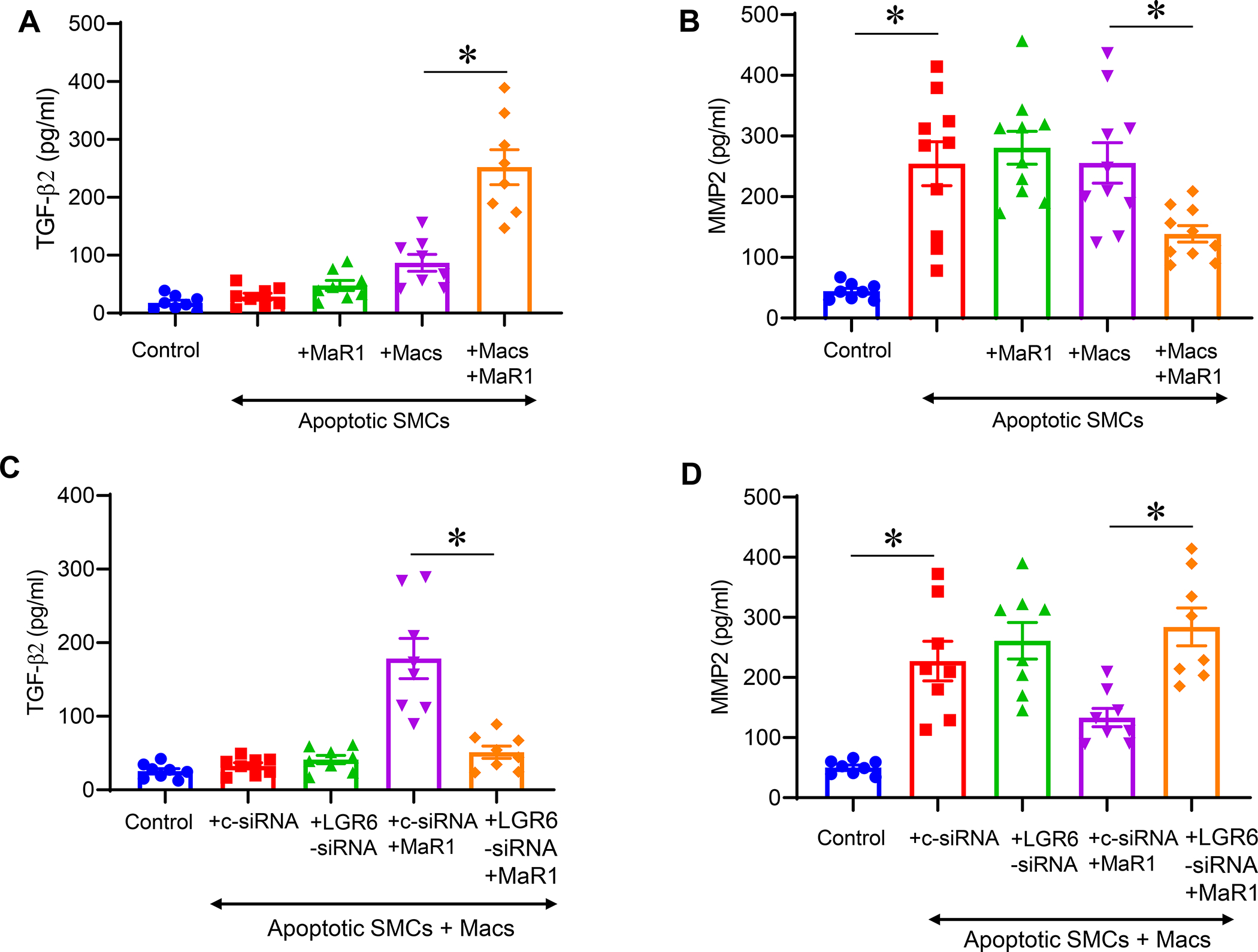
Maresin-1 upregulates TGF-β2 secretion and decreases MMP2 expression. A, Co-culture of apoptotic SMCs with MaR1-treated macrophages upregulates TGF-β2 secretion compared to co-cultures of untreated macrophages and SMCs. B, Elastase-induced apoptotic SMCs demonstrate increased MMP2 expression which was inhibited by co-cultures with MaR1-treated macrophages. C, MaR1-treated macrophages upregulate TGF-β2 secretion via LGR6 receptors when co-cultured with apoptotic SMCs. MaR-1 dependent increase in TGF-β2 was abolished with LGR6-siRNA blockade of macrophages and co-culture with apoptotic SMCs, compared to pre-treatment with control siRNA. D, Inhibition of MMP2 expression by MaR1-treated macrophages is dependent on LGR6 receptors. Macrophages treated with LGR6-siRNA+MaR1 abolished the decrease in MMP2 expression observed in macrophages treated with control siRNA+MaR1. *p<0.05; n=8–10/group.
Figure 7.
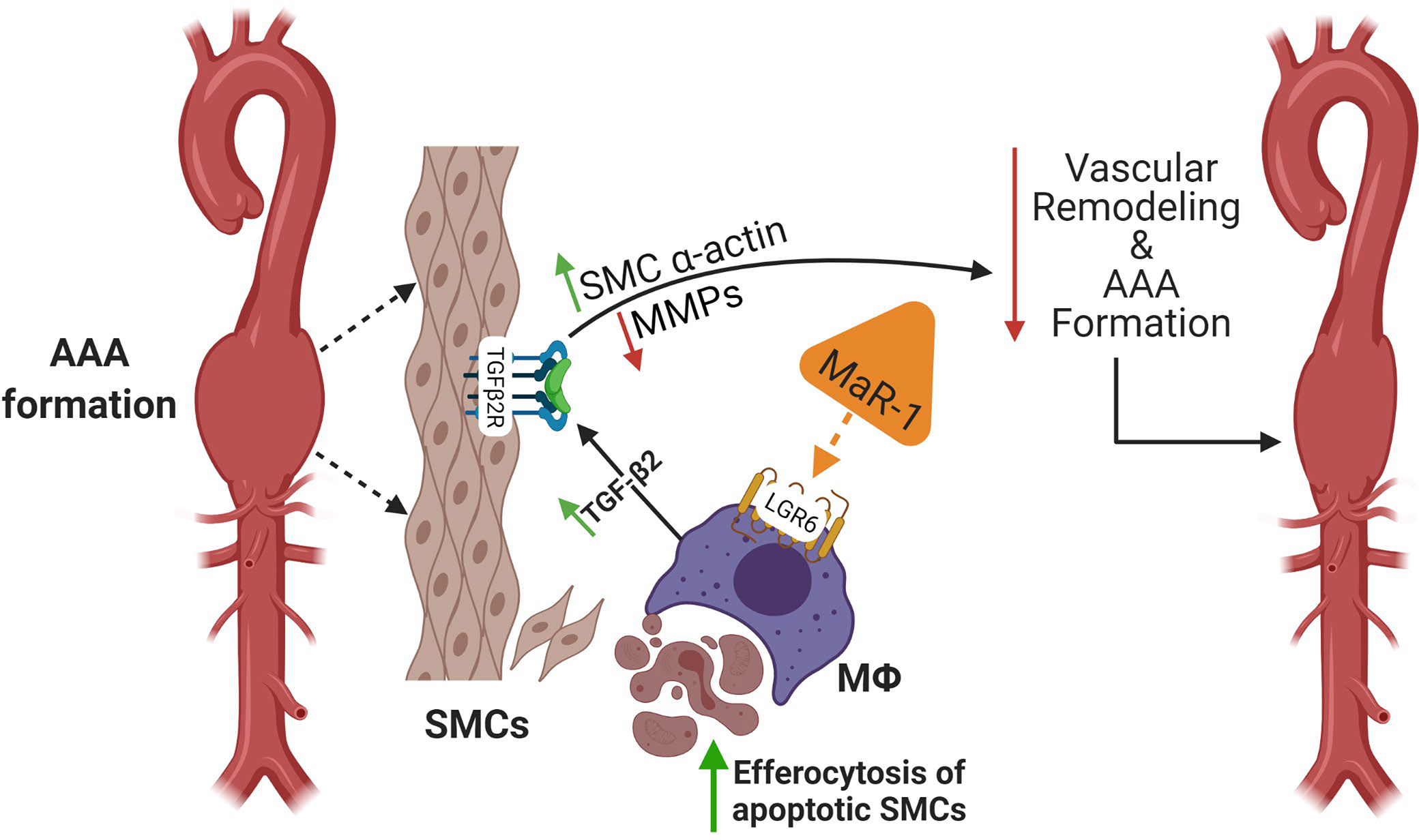
Schematic description of MaR1 mediated attenuation of vascular remodeling during AAA formation. The crosstalk between immune cells and aortic SMCs involves pro- and anti-inflammatory pathways that modulates vascular remodeling during AAA formation. Apoptosis and transformation of SMC architecture is a hallmark of aortic inflammation and remodeling that can be immunomodulated by SPMs. MaR1 acts on LGR6 receptors on macrophages to upregulate efferocytosis of apoptotic SMCs during AAA formation. This process upregulates TGF-β2 secretion that leads to SMC preservation, decrease in MMP2 activation and subsequent aortic remodeling as well as AAA formation. MaR1, maresin-1; LGR6, leucine-rich repeat-containing G-protein coupled receptor 6; SMCs; smooth muscle cells; MФ; macrophages; MMP; matrix metalloproteinases.
DISCUSSION
In this study, it was demonstrated that treatment with the SPM isoform, MaR1, prevented AAA formation as well as attenuated the growth of small pre-formed aneurysms in an experimental murine AAA model. The MaR1-dependent mitigation of AAA formation and SMC preservation is abolished in SMC-TGFβ2r−/− mice suggesting an association between MaR1 and SMC-dependent TGF-β2 signaling. In vivo knockdown of LGR6, a previously reported target of MaR1 (14), diminished the protective effects of MaR1 on AAA growth, suggesting that the therapeutic effects of MaR1 are mediated by this glycoprotein receptor. Histologic staining demonstrated that AAA attenuation was accompanied by increased levels of SMC preservation within the aortic wall. Additionally, increased efferocytosis of apoptotic SMCs in aortic tissue was observed in MaR1 treated mice, indicating that MaR1 can upregulate macrophage-specific uptake of apoptotic SMCs leading to mitigation of aortic inflammation. In vitro studies reveal that the process of macrophage-dependent efferocytosis of apoptotic SMCs upregulates TGF-β2 and is dependent on MaR1-LGR6 interactions. By using knockdown of LGR6, we uncovered a previously undescribed role for LGR6 in mediating MaR1’s pro-resolving actions in vascular inflammation and remodeling in an experimental AAA murine model. These results provide a novel mechanism by which MaR1 prevents and attenuates AAA formation through the promotion of efferocytosis of apoptotic vascular SMCs, mediated by the LGR6 receptor.
Once thought as a passive process, the resolution of inflammation is now known to be an active process involving endogenous SPMs, including Lipoxins, Resolvins, Protectins, and Maresins (27). Each of these SPMs has been shown to alter inflammation in a variety of disease models involving many different organ systems. In AAA disease, SPMs resolvin D1 (RvD1) and D2 (RvD2) were shown to both prevent and treat murine AAA. We previously reported that RvD1 and RvD2 treatment resulted in decreased pro-inflammatory cytokines, decreased immune cell infiltration, reduction of MMP activity, and promoted macrophage polarization toward an M2 phenotype (10). The result was preservation of elastin and overall reduction of AAA diameter when compared with controls. Additionally, our studies demonstrated that RvD1 decreased AAA formation through inhibition of neutrophil extracellular traps (NETosis) which is a downstream event of IL-1β mediated neutrophil activation and infiltration in the aortic wall (9, 11). The mechanistic aspects of SPMs can be mediated via specific receptors that elucidate anti-inflammatory activities in a cell- and tissue-specific manner (14, 28–31). RvD1 has been shown to exhibit its pro-resolving function by signaling through two G protein-coupled receptors (GPCRs), A lipoxin/formyl peptide receptor 2 (ALX/FPR2) and GPR32 resulting in decreased PMN infiltration as well as stimulating macrophage phagocytic function (32). In fact, Petri et al showed that the deletion of ALX/FRP2 resulted in exacerbation of angiotensin II induced murine AAA (29). More recently, MaR1 was shown to bind LGR6, expressed in phagocytes with downstream effects of promoting phagocytic and efferocytic activity (14). Our results also demonstrated similar protective benefit of MaR1 via LGR6 in attenuating AAA disease suggesting the mechanistic process of ligand-receptor interactions of this isoform of SPMs.
Maresins have previously been shown to mitigate inflammation through various mechanisms in several disease processes. In models of experimental colitis, MaR1 was shown to inhibit the NFκB pathway resulting in downregulation of multiple inflammatory cytokines including IL-1β, TNF-α, IL-6, and IFN-γ, while promoting macrophage class switching to M2 phenotype (15). In an LPS induced acute lung injury model, MaR1 decreased the pro-inflammatory cytokines TNF-α, IL-1β, and IL-6 while also decreasing neutrophil infiltration and decreasing pulmonary myeloperoxidase activity (17). Serhan et al. demonstrated the role of MaR1 in resolution of inflammation, stimulating tissue regeneration in planaria, and controlling neuropathic pain in mice (16). Specific to vascular disease, MaR1 has been shown to promote vascular homeostasis through limiting both neutrophil and macrophage recruitment to the site of vascular injury as well as promoting increased macrophage polarization to an M2 phenotype (19). MaR1 has also been shown in multiple studies to promote efferocytosis in both infectious and injury induced inflammation, a key characteristic of resolution physiology in limiting ongoing both direct effects of neutrophil activity as well as downstream effects of apoptotic signaling (12, 33).
However, MaR1 did not appear to confer its protective effects on AAA formation through classically described inflammatory cytokines, but rather primarily affected SMC preservation via increased TGF-β2 and mitigation of MMP2 to attenuate vascular remodeling. The process of SMC apoptosis can trigger a cytotoxic milieu thereby attracting immune cell mediated infiltration and activation leading to further damage and remodeling of the aortic wall (34, 35). This led us to examine how MaR1 may affect the clearance of apoptotic SMCs within the aortic wall. Apoptosis of vascular SMCs is a key characteristic in AAA pathogenesis (35). This loss not only contributes to weakening of vascular wall integrity, but also decreases the connective tissue repair function directed by vascular SMCs (36). Clearance of these apoptotic cells is important in limiting the inflammatory response generated by dying cells and the resultant preservation of existing cell populations. Kojima et al. suggest that defective efferocytosis contributes to AAA formation as evidenced by the upregulation of the anti-phagocytic marker CD47 in aneurysmal tissue of both humans and mice. Further, they demonstrated that restoration of efferocytic function through administration with anti-CD47 antibodies resulted in attenuation of murine AAA development through clearance of apoptotic bodies within the aortic wall (37). Our data suggests that MaR1 confers protective effects on AAA development through the increase in efferocytosis of apoptotic smooth muscle within the aortic wall thereby preserving the vascular architecture and preventing aortic remodeling. This process allows for both the preservation of SMCs by limiting progressive necrotic dysfunction as well as the regeneration and proliferation of vascular SMCs resulting in stabilization of the aortic wall and AAA prevention and formation.
The role of TGF-β signaling in modulation of aortic aneurysms remains controversial. Several previous studies have reported that SMC TGF-β signaling can be the primary cause of aortic aneurysms and disruption of TGF-βR1 or TGF-βR2 has been reported to lead to dilatation of the aorta and enhance aneurysm development (23, 38–41). A recent study by Chen et al. demonstrate that abrogation of TGF-β signaling in SMCs in combination with hypercholesterolemia results in formation of aortic aneurysms due to reprograming of normal SMCs into mesenchymal-like stem cells as well as macrophage-like cells (42). Moreover, AngII-induced aortic rupture has been shown to be enhanced by TGF-β neutralization providing further evidence of the immunomodulation of aortic aneurysm progression by TGF-β signaling (43). However, the crosstalk between macrophages and SMCs in vascular pathologies i.e. aortic aneurysms, via TGF-β had not been previously elucidated. Macrophages have the unique ability to inhibit inflammation via autocrine or paracrine mechanisms involving TGF-β after ingesting apoptotic cells (44). Our findings provide a previously undescribed mechanistic pathway wherein ingestion of apoptotic SMCs by MaR1/LGR6 activated macrophages stimulate TGF-β signaling that protects SMC activation and MMP2-mediated vascular remodeling. The implications of this study further supports the effects of disrupted SMC TGF-β signaling which can be enhanced by SPMs and prevent SMC reprogramming to accelerate resolution of aneurysm formation.
Several limitations to our study exist. Translation to human subjects remains to be determined in a large animal model as clinical trials for pharmacotherapy based on murine data alone are rare. Additionally, the effect of SPMs on chronic AAA and subsequent aortic rupture remains to be elucidated and will be the next logical step to determine the immunomodulation of aortic remodeling in our recently described murine aortic rupture models (45, 46). Furthermore, a combined therapeutic strategy using various bioactive isoforms of SPMs will be more prudent as an effector strategy to mitigate aortic inflammation and vascular remodeling. The synergistic effect of RvD1 on the aortic inflammation and MaR1 on apoptotic SMC uptake underscores the multifaceted approach of combined therapeutic intervention by bioactive isoforms of SPMs that will be deciphered in our recently described large animal porcine model as well as chronic aortic aneurysm and aortic rupture models (25, 45, 47).
In conclusion, treatment with MaR1 can significantly prevent, as well as slow the growth of small preformed AAAs in a murine model. The mechanistic approach of MaR1 mediated mitigation of aortic inflammation is conferred through increased efferocytosis of apoptotic vascular SMCs within the aortic wall via LGR6-MaR1 interaction. This provides a newly described mechanism by which SPMs have the potential to inhibit AAA formation. Further research is needed in order to decipher the combined therapy of various SPM bioactive isoforms to provide insight into a clinically translatable approach for patients with aortic aneurysms.
Supplementary Material
Acknowledgements:
The authors have no relevant conflicts of interest. This study was supported by NIH R01 HL153341 (G.R.U. and A.K.S.). A.C.F. received a grant that was supported by the American College of Surgeons Resident Research Scholarship. Schematic figure was made using www.biorender.com.
Abbreviations:
- AAA
Abdominal aortic aneurysm
- HMGB1
High mobility group box protein 1
- LGR6
Leucine-rich repeat containing G protein-coupled receptor 6
- MaR1
Maresin 1
- TGF-β2
Transforming growth factor-beta 2
- α-SMA
Alpha-smooth muscle cell actin
- SPMs
Specialized pro-resolving lipid mediators
REFERENCES:
- 1.Underlying Cause of Death 1999–2018 on CDC WONDER Online Database. United States, Centers for Disease Control and Prevention, http://wonder.cdc.gov/ucd-icd10.html. Accessed 10/14/2020. [Google Scholar]
- 2.Harris LM, Faggioli GL, Fiedler R, Curl GR, and Ricotta JJ (1991) Ruptured abdominal aortic aneurysms: factors affecting mortality rates. Journal of vascular surgery 14, 812–818; discussion 819–820 [DOI] [PubMed] [Google Scholar]
- 3.Thompson SG, Ashton HA, Gao L, Buxton MJ, and Scott RAP (2012) Final follow-up of the Multicentre Aneurysm Screening Study (MASS) randomized trial of abdominal aortic aneurysm screening. Br J Surg 99, 1649–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dale MA, Ruhlman MK, and Baxter BT (2015) Inflammatory cell phenotypes in AAAs; their role and potential as targets for therapy. Arterioscler Thromb Vasc Biol 35, 1746–1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimizu K, Mitchell RN, and Libby P (2006) Inflammation and cellular immune responses in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol 26, 987–994 [DOI] [PubMed] [Google Scholar]
- 6.Bhamidipati CM, Mehta GS, Lu G, Moehle CW, Barbery C, DiMusto PD, Laser A, Kron IL, Upchurch GR Jr., and Ailawadi G (2012) Development of a novel murine model of aortic aneurysms using peri-adventitial elastase. Surgery 152, 238–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah PK (1997) Inflammation, metalloproteinases, and increased proteolysis: an emerging pathophysiological paradigm in aortic aneurysm. Circulation 96, 2115–2117 [DOI] [PubMed] [Google Scholar]
- 8.Newman KM, Jean-Claude J, Li H, Ramey WG, and Tilson MD (1994) Cytokines that activate proteolysis are increased in abdominal aortic aneurysms. Circulation 90, Ii224–227 [PubMed] [Google Scholar]
- 9.Meher AK, Spinosa M, Davis JP, Pope N, Laubach VE, Su G, Serbulea V, Leitinger N, Ailawadi G, and Upchurch GR Jr. (2018) Novel Role of IL (Interleukin)-1beta in Neutrophil Extracellular Trap Formation and Abdominal Aortic Aneurysms. Arterioscler Thromb Vasc Biol 38, 843–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pope NH, Salmon M, Davis JP, Chatterjee A, Su G, Conte MS, Ailawadi G, and Upchurch GR Jr. (2016) D-series resolvins inhibit murine abdominal aortic aneurysm formation and increase M2 macrophage polarization. FASEB J 30, 4192–4201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spinosa M, Su G, Salmon M, Lu G, Cullen JM, Fashandi AZ, Hawkins RB, Montgomery W, Meher AK, Conte MS, Sharma AK, Ailawadi G, and Upchurch GR (2018) Resolvin D1 Decreases Abdominal Aortic Aneurysm Formation by Inhibiting NETosis in a Mouse Model. Journal of vascular surgery 68, 93S–103S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF, Oh SF, and Spite M (2009) Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. In J Exp Med Vol. 206 pp. 15–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serhan CN (2014) Pro-resolving lipid mediators are leads for resolution physiology. Nature 510, 92–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiang N, Libreros S, Norris PC, de la Rosa X, and Serhan CN (2019) Maresin 1 activates LGR6 receptor promoting phagocyte immunoresolvent functions. J Clin Invest 129, 5294–5311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marcon R, Bento AF, Dutra RC, Bicca MA, Leite DF, and Calixto JB (2013) Maresin 1, a proresolving lipid mediator derived from omega-3 polyunsaturated fatty acids, exerts protective actions in murine models of colitis. Journal of immunology (Baltimore, Md. : 1950) 191, 4288–4298 [DOI] [PubMed] [Google Scholar]
- 16.Serhan CN, Dalli J, Karamnov S, Choi A, Park CK, Xu ZZ, Ji RR, Zhu M, and Petasis NA (2012) Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. Faseb j 26, 1755–1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gong J, Wu ZY, Qi H, Chen L, Li HB, Li B, Yao CY, Wang YX, Wu J, Yuan SY, Yao SL, and Shang Y (2014) Maresin 1 mitigates LPS-induced acute lung injury in mice. British journal of pharmacology 171, 3539–3550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun Q, Wu Y, Zhao F, and Wang J (2017) Maresin 1 Ameliorates Lung Ischemia/Reperfusion Injury by Suppressing Oxidative Stress via Activation of the Nrf-2-Mediated HO-1 Signaling Pathway. Oxid Med Cell Longev 2017, 9634803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akagi D, Chen M, Toy R, Chatterjee A, and Conte MS (2015) Systemic delivery of proresolving lipid mediators resolvin D2 and maresin 1 attenuates intimal hyperplasia in mice. FASEB J 29, 2504–2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arandjelovic S, and Ravichandran KS (2015) Phagocytosis of apoptotic cells in homeostasis. Nat Immunol 16, 907–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwab JM, Chiang N, Arita M, and Serhan CN (2007) Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature 447, 869–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doran AC, Yurdagul A, and Tabas I (2019) Efferocytosis in health and disease. Nature Reviews Immunology 20, 254–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang P, Schmit BM, Fu C, DeSart K, Oh SP, Berceli SA, and Jiang Z (2016) Smooth muscle cell-specific Tgfbr1 deficiency promotes aortic aneurysm formation by stimulating multiple signaling events. Sci Rep 6, 35444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laser A, Lu G, Ghosh A, Roelofs K, McEvoy B, DiMusto P, Bhamidipati CM, Su G, Zhao Y, Lau CL, Ailawadi G, Eliason JL, Henke PK, and Upchurch GR Jr. (2012) Differential gender- and species-specific formation of aneurysms using a novel method of inducing abdominal aortic aneurysms. J Surg Res 178, 1038–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu G, Su G, Davis JP, Schaheen B, Downs E, Roy RJ, Ailawadi G, and Upchurch GR (2017) A novel chronic advanced staged abdominal aortic aneurysm murine model. Journal of vascular surgery 66, 232–242 e234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spinosa M, Lu G, Su G, Bontha SV, Gehrau R, Salmon MD, Smith JR, Weiss ML, Mas VR, Upchurch GR Jr., and Sharma AK (2018) Human mesenchymal stromal cell-derived extracellular vesicles attenuate aortic aneurysm formation and macrophage activation via microRNA-147. FASEB J, fj201701138RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Serhan CN (2017) Discovery of specialized pro-resolving mediators marks the dawn of resolution physiology and pharmacology. Molecular aspects of medicine 58, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Serhan CN, Krishnamoorthy S, Recchiuti A, and Chiang N (2011) Novel anti-inflammatory--proresolving mediators and their receptors. Curr Top Med Chem 11, 629–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petri MH, Thul S, Andonova T, Lindquist-Liljeqvist M, Jin H, Skenteris NT, Arnardottir H, Maegdefessel L, Caidahl K, Perretti M, Roy J, and Bäck M (2018) Resolution of Inflammation Through the Lipoxin and ALX/FPR2 Receptor Pathway Protects Against Abdominal Aortic Aneurysms. JACC Basic Transl Sci 3, 719–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nelson JW, Leigh NJ, Mellas RE, McCall AD, Aguirre A, and Baker OJ (2014) ALX/FPR2 receptor for RvD1 is expressed and functional in salivary glands. Am J Physiol Cell Physiol 306, C178–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krishnamoorthy S, Recchiuti A, Chiang N, Fredman G, and Serhan CN (2012) Resolvin D1 receptor stereoselectivity and regulation of inflammation and proresolving microRNAs. Am J Pathol 180, 2018–2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee CH, Yang R, Petasis NA, and Serhan CN (2010) Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc Natl Acad Sci U S A 107, 1660–1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Francos-Quijorna I, Santos-Nogueira E, Gronert K, Sullivan AB, Kopp MA, Brommer B, David S, Schwab JM, and Lopez-Vales R (2017) Maresin 1 Promotes Inflammatory Resolution, Neuroprotection, and Functional Neurological Recovery After Spinal Cord Injury. J Neurosci 37, 11731–11743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rowe VL, Stevens SL, Reddick TT, Freeman MB, Donnell R, Carroll RC, and Goldman MH (2000) Vascular smooth muscle cell apoptosis in aneurysmal, occlusive, and normal human aortas. Journal of vascular surgery 31, 567–576 [PubMed] [Google Scholar]
- 35.Thompson RW, Liao S, and Curci JA (1997) Vascular smooth muscle cell apoptosis in abdominal aortic aneurysms. Coron Artery Dis 8, 623–631 [DOI] [PubMed] [Google Scholar]
- 36.Lopez-Candales A, Holmes DR, Liao S, Scott MJ, Wickline SA, and Thompson RW (1997) Decreased vascular smooth muscle cell density in medial degeneration of human abdominal aortic aneurysms. Am J Pathol 150, 993–1007 [PMC free article] [PubMed] [Google Scholar]
- 37.Kojima Y, Werner N, Ye J, Nanda V, Tsao N, Wang Y, Flores AM, Miller CL, Weissman I, Deng H, Xu B, Dalman RL, Eken SM, Pelisek J, Li Y, Maegdefessel L, and Leeper NJ (2018) Proefferocytic Therapy Promotes Transforming Growth Factor-beta Signaling and Prevents Aneurysm Formation. Circulation 137, 750–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li W, Li Q, Jiao Y, Qin L, Ali R, Zhou J, Ferruzzi J, Kim RW, Geirsson A, Dietz HC, Offermanns S, Humphrey JD, and Tellides G (2014) Tgfbr2 disruption in postnatal smooth muscle impairs aortic wall homeostasis. J Clin Invest 124, 755–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Angelov SN, Hu JH, Wei H, Airhart N, Shi M, and Dichek DA (2017) TGF-beta (Transforming Growth Factor-beta) Signaling Protects the Thoracic and Abdominal Aorta From Angiotensin II-Induced Pathology by Distinct Mechanisms. Arterioscler Thromb Vasc Biol 37, 2102–2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gallo EM, Loch DC, Habashi JP, Calderon JF, Chen Y, Bedja D, van Erp C, Gerber EE, Parker SJ, Sauls K, Judge DP, Cooke SK, Lindsay ME, Rouf R, Myers L, ap Rhys CM, Kent KC, Norris RA, Huso DL, and Dietz HC (2014) Angiotensin II-dependent TGF-beta signaling contributes to Loeys-Dietz syndrome vascular pathogenesis. J Clin Invest 124, 448–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lindsay ME, Schepers D, Bolar NA, Doyle JJ, Gallo E, Fert-Bober J, Kempers MJ, Fishman EK, Chen Y, Myers L, Bjeda D, Oswald G, Elias AF, Levy HP, Anderlid BM, Yang MH, Bongers EM, Timmermans J, Braverman AC, Canham N, Mortier GR, Brunner HG, Byers PH, Van Eyk J, Van Laer L, Dietz HC, and Loeys BL (2012) Loss-of-function mutations in TGFB2 cause a syndromic presentation of thoracic aortic aneurysm. Nat Genet 44, 922–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen PY, Qin L, Li G, Malagon-Lopez J, Wang Z, Bergaya S, Gujja S, Caulk AW, Murtada SI, Zhang X, Zhuang ZW, Rao DA, Wang G, Tobiasova Z, Jiang B, Montgomery RR, Sun L, Sun H, Fisher EA, Gulcher JR, Fernandez-Hernando C, Humphrey JD, Tellides G, Chittenden TW, and Simons M (2020) Smooth Muscle Cell Reprogramming in Aortic Aneurysms. Cell Stem Cell 26, 542–557 e511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y, Ait-Oufella H, Herbin O, Bonnin P, Ramkhelawon B, Taleb S, Huang J, Offenstadt G, Combadiere C, Renia L, Johnson JL, Tharaux PL, Tedgui A, and Mallat Z (2010) TGF-beta activity protects against inflammatory aortic aneurysm progression and complications in angiotensin II-infused mice. J Clin Invest 120, 422–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, and Henson PM (1998) Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest 101, 890–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fashandi AZ, Hawkins RB, Salmon MD, Spinosa MD, Montgomery WG, Cullen JM, Lu G, Su G, Ailawadi G, and Upchurch GR Jr. (2017) A novel reproducible model of aortic aneurysm rupture. Surgery [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu G, Su G, Davis JP, Schaheen B, Downs E, Roy RJ, Ailawadi G, and Upchurch GR Jr. (2017) A novel chronic advanced stage abdominal aortic aneurysm murine model. Journal of vascular surgery 66, 232–242 e234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cullen JM, Lu G, Shannon AH, Su G, Sharma A, Salmon M, Fashandi AZ, Spinosa MD, Montgomery WG, Johnston WF, Ailawadi G, and Upchurch GR Jr. (2019) A novel swine model of abdominal aortic aneurysm. Journal of vascular surgery 70, 252–260 e252 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


