Abstract
Purpose:
Germline variants in Fumarate Hydratase (FH) is associate with autosomal dominant (AD) Hereditary Leiomyomatosis and Renal Cell Cancer (HLRCC) and autosomal recessive (AR) fumarase deficiency (FMRD). The prevalence and penetrance across different FH variants remains unclear.
Methods:
A database containing 120,061 records from individuals undergoing cancer germline testing was obtained. The Sherloc classification and ClinVar categorized FH variants into three categories: AD HLRCC variants, AR FMRD variants, and variants of unknown significance (VUS). Individuals with variants from these categories were compared to those with negative genetic testing.
Results:
FH variants were detected in 1.3% of individuals (0.3% AD HLRCC, 0.4% AR FMRD, 0.6% VUS). The rate of incidentally discovered AD HLRCC variants among asymptomatic individuals was 1/2668. Compared to those with negative testing, RCC prevalence was elevated with AD HLRCC variants (17.0% vs. 4.5%, p <0.0001) and VUS (6.4% vs. 4.5%, p = 0.0182) but not AD FMRD variants.
Conclusion:
The prevalence of incidentally discovered HLRCC on germline testing is similar to recent population estimates, suggesting that this is a relatively common cancer syndrome. Compared to those with a negative genetic test result, those with VUS had an elevated risk of RCC while those with AR FMRD variants did not.
Introduction
Fumarate hydratase (FH) is a Krebs cycle enzyme that catalyzes conversion of fumarate to L-malate. Specific germline pathogenic variants in FH cause the autosomal dominant (AD) cancer syndrome called Hereditary Leiomyomatosis and Renal Cell Cancer (HLRCC; MIM #150800). HLRCC is characterized by painful cutaneous leiomyomas, early-onset and highly symptomatic uterine leiomyomas, adrenal macronodular hyperplasia, and a very aggressive and histologically unique form of renal cell carcinoma (RCC).1,2 HLRCC-associated RCCs have a propensity for early spread: approximately half of patients are diagnosed with metastatic disease.3,4
Although several hundred families with HLRCC have been described, the true prevalence of this condition is unknown.5 It was previously thought that HLRCC was quite rare, with a recent prevalence estimate as low as 1 in 200,000.6,7 However, the increasing availability of genetic panel testing that includes the FH gene has identified many more patients, leading to the belief that this condition may have a higher prevalence (and consequentially a lower RCC penetrance) than previously thought. One recent analysis of exome and genome sequencing data demonstrated FH pathogenic/likely pathogenic variants (P/LP) are found in 1/902 to 1/1252 individuals.8 Further support for under-diagnosis of HLRCC is the high frequency of pathogenic germline FH variants (6.4%) when evaluating unselected metastatic non-clear cell RCC patients.9
While there are many well described FH variants associated with HLRCC, the risks of malignant or benign manifestations of the less well-established FH variants are not always clear. Many individuals with a clinical phenotype and a FH variant of unknown significance (VUS) are not causally linked to HLRCC.10 When a cancer does arise in such an individual with a FH VUS, it is often unclear whether the variant found represents an incidental finding, particularly in the setting of conflicting interpretations regarding the genotype-phenotype association in publicly available databases. Further complicating matters is fumarase deficiency (FMRD; MIM #606812), a rare disease that is caused by pathogenic autosomal recessive (AR) FH variants and is characterized by lethal neonatal neurologic complications. Heterozygous adults with such AR FH variants appear to be healthy without an elevated cancer risk.11,12 To better characterize the prevalence of FH variants and their associated manifestations in individuals undergoing genetic evaluation, we interrogated a large, well-annotated clinical database from a commercial genetic testing laboratory.
Materials and Methods
Patient and Cohort Characteristics
120,061 patients undergoing hereditary cancer testing in a commercial Clinical Laboratory Improvement Amendments (CLIA) certified laboratory (Invitae, San Francisco, California) had clinical and genomic information captured and reviewed. A wide variety of options were available for sequencing FH, including as a single gene test or on multi-gene panel tests ranging in size. The FH sequence analysis covers all coding exons and 10 or more base pairs of adjacent intronic sequences. Cases were selected when: (1) the individual had germline testing for cancer risk assessment between 2015–2020, (2) the test included FH, and (3) the test was a “proband” order (first member of the family to test). Ethnicity, age at testing, sex (male, female, or unknown), and type of cancer were self-reported. Ethnicity was categorized as Ashkenazi Jewish, Asian / Pacific Islander, Black / African American, French Canadian / Caucasian, Hispanic, Native American, Sephardic Jewish / Mediterranean, other, mixed, and unknown. IRB approval was obtained for review of de-identified data from this database (IRB#21–000887).
Genetic Testing Interpretation
All FH variants identified were classified utilizing the Sherloc framework for variant classification. This framework confidently classifies variants as Pathogenic / Likely Pathogenic (P/LP), VUS, and Likely Benign / Benign (LB/B) by summing up multiple lines of orthogonal evidence, including population frequency (e.g. gnomAD), clinical (e.g. case reports, segregation, co-occurrence), functional (e.g. molecular and enzymatic assays, large-scale mutational studies), variant effect predictions (e.g. frameshift, nonsense, splice site, missense mutations), and computational/machine-learning algorithms.13 Extending this framework, FH sequencing results were classified into four categories: pathogenic FH variant associated with an AD phenotype, pathogenic FH variant associated with an AR phenotype (AR FMRD variant), FH VUS, and negative for FH variants. If a variant has been associated with both an AD phenotype (e.g. HLRCC) and an AR recessive phenotype (e.g. FMRD), it was categorized as AD as one copy is sufficient to cause disease manifestations. Those with AD FH variants were cross-referenced on ClinVar, and further categorized specifically as an AD HLRCC variant (if clearly associated with HLRCC) or a separate AD variant that has not clearly been linked with HLRCC but may be associated with other manifestations (e.g., Paraganglioma).11 We noted when individuals had pathogenic variants in genes other than FH.
FH Variant Frequencies
Patients without HLRCC clinical features undergoing sequencing may be found with incidental FH variants. We obtained an estimate for this occurrence by identifying individuals who tested positive for a pathogenic AD FH variant and subsequently excluding those not catalogued as P/LP variants associated with HLRCC on ClinVar.11 Next, we excluded individuals with strong clinical suspicion for HLRCC based on the presence of cutaneous leiomyomas, uterine leiomyomas, renal cell carcinoma, or testing for only the FH gene. The final cohort represented individuals for whom there was no strong clinical suspicion for HLRCC, suggesting that the pathogenic FH variants associated with HLRCC identified may be incidental. The Genome Aggregation Database (https://gnomad.broadinstitute.org, gnomAD v2.1.1) was used to compare population rates for specific variants.14
Cancer Prevalence
Reporting of cancer prevalence was at time of genetic testing only, with cancer types included in the clinician-reported indication for testing field and ICD-10 codes. An annotating code was used to recognize keywords and ICD-10 codes to annotate the presence of cancer. If the two fields reported different cancer types, the individual would be annotated to both. Patients were annotated with either “no cancer” or “any cancer” as well as whether they had either one or more cancer types (Supplementary Table 1). Data regarding the cancer type that prompted genetic testing was not available. When examining cancer prevalence, we specifically excluded individuals with concurrent pathogenic variants in genes other than FH.
Estimating Potential for Reclassification of Current VUS
To approximate the percent of individuals with FH VUS that may in fact have P/LP HLRCC variants that predispose to RCC, we used the formula:
This is presuming that the percentage of P/LP AD HLRCC within the VUS category (x) and percentage of LB/B (y) variants within VUS is equal to 1 (y=1-x).
Visualization of Variants
To depict the FH variants associated with HLRCC, we created lollipop plots using the cBioPortal for Cancer Genomics v3.6.12 MutationMapper.15,16 Distinct FH variants are mapped as lollipops on a linear protein and its domains, with the height reflecting the frequency of the variant. Missense, duplication, and frameshift variants are displayed. FH variants characterized by large deletions and splice site alterations were not displayed.
Statistical Analysis
We compared the prevalence of various cancers annotated between individuals across the four FH variant categories. Most of the reporting was descriptive in nature. However, for cancer types of interest (namely RCC and Any Cancer), the prevalence was compared between FH variant classes (AD HLRCC variant, AR FMRD variant, and VUS) and those with negative genetic testing. Similarly, cancer rates for Any Cancer and RCC were compared by variant type (loss-of-function [LoF] vs. non-LoF variant among those with AD HLRCC variants), sex (male vs. female), and ethnicity. The Two Sample Test for Proportions and the Pearson’s X2 Test was used to compare categorical variables between groups when appropriate. Analyses were performed using JMP® Pro 15.0.0.
Results
Between 2015 and 2020, 120,061 proband individuals received cancer germline testing that included FH. This cohort included individuals that did or did not have a clinical suspicion for HLRCC. The median age at testing was 56.0 (interquartile range 44 to 67). Figure 1 outlines the testing results. FH variants were identified in 1,573 individuals (1.3%). Of these individuals, FH variants associated with an AD phenotype were detected in 316 (0.3%), AR FMRD variants were detected in 492 (0.4%), VUS were identified in 765 (0.6%), and the remaining 118,488 (98.7%) tested negative for FH variants.
Figure 1.
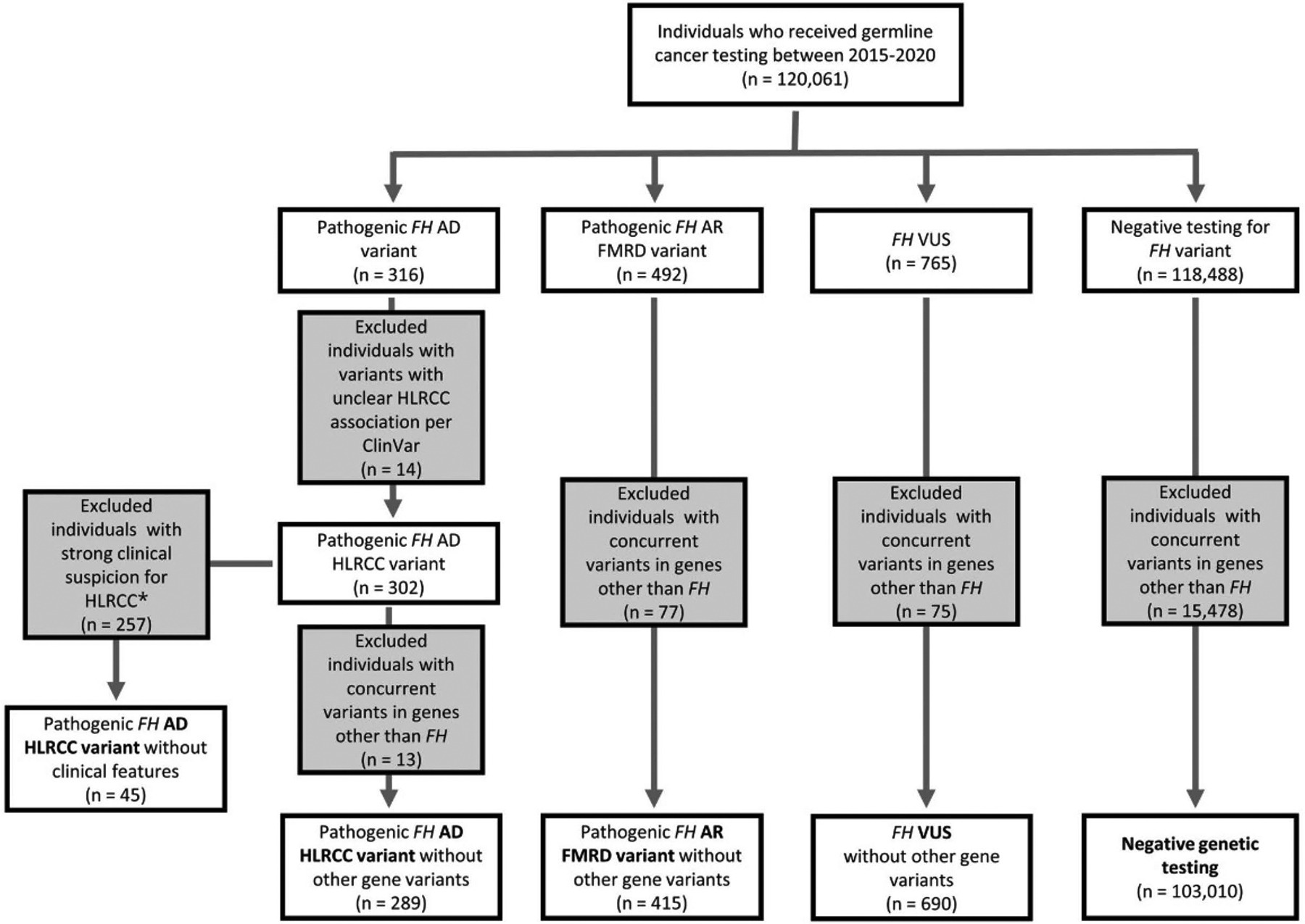
Germline testing panel results: diagram outlining results for the 120,061 individuals undergoing germline testing and variant classification. *Clinical features suggestive of HLRCC included cutaneous leiomyomas, uterine leiomyomas, renal cell carcinoma, and a genetic testing for only 1 gene (FH). AD indicates autosomal dominant; AR, autosomal recessive; FH, fumarate hydratase; FMRD, fumarase deficiency; HLRCC, hereditary leiomyomatosis and renal cell cancer; VUS, variant of unknown significance.
Of the 316 individuals carrying pathogenic FH variants associated with an AD phenotype, 302 individuals had a pathogenic variant associated with HLRCC (AD HLRCC variant), but 14 had a variant not clearly associated with classic HLRCC manifestations per ClinVar.10 One individual carried two separate variants, resulting in 303 observed AD HLRCC variants (89 unique) affecting 302 individuals. The 303 variants are depicted according to their position within the gene locus in Figure 2A. p.Arg233His was the most frequently detected variant, accounting for 17.5% (53/303) of cases.
Figure 2.
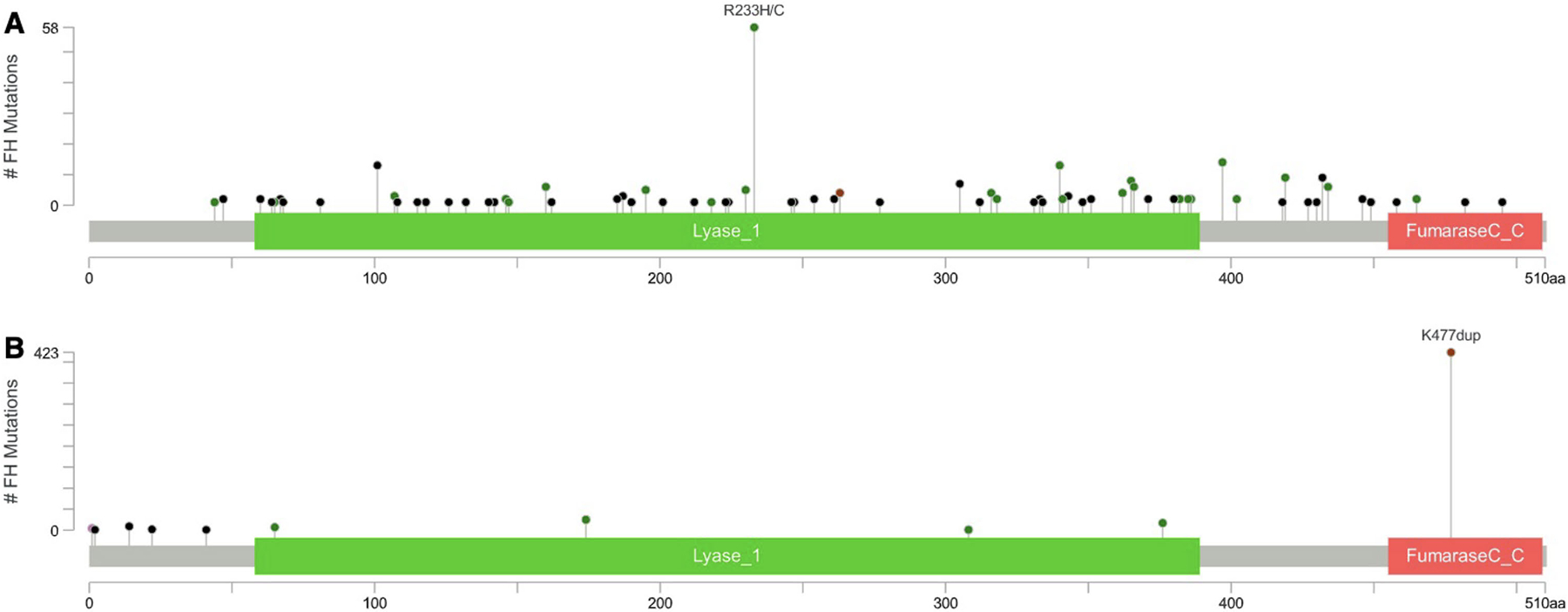
Map of AD HLRCC and AR FMRD variants. This map displays the protein domains and the positions of specific FH variants. (A) Three hundred three observed AD HLRCC variants (89 unique) affecting 302 individuals are shown. The length of the line connecting the variant annotation to the protein is indicative of the number of samples that have the variant, with p.Arg233His (R233H) being the most common variant with 53 occurrences. (B) Four hundred ninety-two individuals with AR FMRD variants (11 unique) are displayed. p.Lys477dup (K477dup) was the most common variant with 423 occurrences. AD indicates autosomal dominant; AR, autosomal recessive; FH, fumarate hydratase; FMRD, fumarase deficiency; HLRCC, hereditary leiomyomatosis and renal cell cancer.
The process estimating FH variant carrier frequency is outlined in Figure 1. Of the 302 individuals who tested positive for AD HLRCC variants, 257 were excluded given clinical suspicion for HLRCC or clinical features of HLRCC (see methods). This resulted in 45 individuals we suspected as having incidentally found HLRCC without a priori clinical features. Thus, our carrier estimate in this cohort was 0.04% (1 in 2,668 individuals).
Of those who underwent hereditary cancer testing, 66.7% (80,061/120,061) were annotated as having at least one cancer type (Any Cancer). Table 1 outlines the cancer prevalence across the four categories. Importantly, of the 120,061 individuals in the cohort, 15,644 tested positive for a pathogenic variant in a gene other than FH. The number of genes tested was similar between individuals with and without a concurrent pathogenic variant in genes other than FH, with the vast majority in both groups having 80+ gene panel testing (86.5% and 86.1% respectively) (Supplementary Table 2). Given that concurrent pathogenic variants in genes other than FH may influence cancer prevalence, we removed these individuals, resulting in 289 individuals categorized as carrying AD HLRCC variants, 415 categorized as having AR FMRD variants, 690 as having VUS, and 103,010 categorized as negative genetic testing (Figure 1).
TABLE 1.
Clinical Characteristics of Individuals Receiving Germline Testing
| AD HLRCC Variants (n = 289)a | AR FMRD Variants (n = 415)a | VUS (n = 690)a | Negative Genetic Testing (n = 103,010)a | Total (n = 120,061) | |
|---|---|---|---|---|---|
| Age, y | |||||
| Mean (SD) | 45.8 (14.4) | 56.6 (15.4) | 54.8 (15.2) | 55.3 (15.0) | 54.8 (14.9) |
| Sex, No. (%) | |||||
| Male | 82 (28.4) | 84 (20.2) | 115 (16.7) | 18,716 (18.2) | 22,535 (18.8) |
| Female | 207 (71.6) | 331 (79.8) | 575 (83.3) | 84,291 (81.8) | 97,523 (81.2) |
| Ethnicity, No. (%) | |||||
| Ashkenazi Jewish | 2 (0.7) | 26 (6.3) | 7 (1.0) | 3158 (3.1) | 3705 (3.1) |
| Asian or Pacific Islander | 14 (4.8) | 1 (0.2) | 59 (8.6) | 5120 (5.0) | 5708 (4.8) |
| Black/African American | 12 (4.2) | 5 (1.2) | 69 (10.0) | 6344 (6.2) | 7128 (5.9) |
| Hispanic | 13 (4.5) | 3 (0.7) | 33 (4.8) | 6218 (6.0) | 7227 (6.0) |
| Mediterranean or Sephardic Jewish | 1 (0.3) | 0 (0.0) | 2 (0.3) | 428 (0.4) | 493 (0.4) |
| Native American | 1 (0.3) | 1 (0.2) | 3 (0.4) | 331 (0.3) | 378 (0.3) |
| White/Caucasian or French Canadian | 208 (72.0) | 323 (77.8) | 419 (60.7) | 67,367 (65.4) | 79,075 (65.9) |
| Other or unknown | 38 (13.1) | 56 (13.5) | 98 (14.2) | 14,044 (13.6) | 16,347 (13.6) |
Abbreviations: AD, autosomal dominant; AR, autosomal recessive; FH, fumarate hydratase; FMRD, fumarase deficiency; HLRCC, hereditary leiomyomatosis and renal cell cancer; SD, standard deviation; VUS, variant of unknown significance.
Excludes individuals with germline variants in genes other than FH.
At least one cancer type was found in 46.0% of those with AD HLRCC variants, 64.6% of those with AR FMRD variants, 64.6% of those with VUS, and 66.1% of those with negative genetic testing. These rates are depicted in Figure 3A. Notably, rates of any cancer were lower when comparing those with AD HLRCC variants with those with negative genetic testing (46.0% vs. 66.1%, p < 0.0001). No statistically significant difference was found either between the AR FMRD variant group and the negative genetic testing group (64.6% vs. 66.1%, p = 0.5120) or between the VUS and the negative genetic testing group (64.6% vs. 66.1%, p = 0.4170).
Figure 3.

Prevalence of any cancer and renal cell carcinoma among FH variants. The graphs displays the prevalence of (A) any cancer and (B) RCC among different FH variants. *Statistical significance per the Pearson χ2 test. AD indicates autosomal dominant; AR, autosomal recessive; FH, fumarate hydratase; FMRD, fumarase deficiency; HLRCC, hereditary leiomyomatosis and renal cell cancer; RCC, renal cell carcinoma; VUS, variant of unknown significance.
Among the 492 individuals with AR FMRD variants, p.Lys477dup was the most common variant, representing 86.0% (423/492) of cases as depicted in Figure 2B. When specifically examining this subset compared to those with negative genetic testing, there were similar rates of any cancer (64.8% vs. 66.1%, p = 0.6066) and RCC (4.2% vs. 4.5%, p = 0.7500) (Supplemental Table 3). This p.Lys477dup variant does not appear to be enriched within our cancer-rich dataset, with a carrier frequency of 1/283 individuals (423/120,061). This carrier frequency is similar to what is reported in gnomAD, with rates of 1/195 (among Ashkenazi Jewish) and 1/677 (among European non-Finnish).14
We also examined the RCC prevalence across different FH variants. Renal cell carcinoma was present in 17.0% of those with AD HLRCC variants, 4.3% of those with AR FMRD variants, 6.4% of those with VUS, and 4.5% in those with negative genetic testing. As expected, RCC rates were higher among those with AD HLRCC variants compared to those with negative genetic testing (17.0% vs. 4.5%, p <0.0001). When these variants were further stratified by LoF versus non-LoF variants, no difference was found (20.2% vs. 15.1% p = 0. 2689) (Supplementary Table 4).
RCC was also more common among those with VUS compared the those with negative genetic testing (6.4% vs. 4.5%, p = 0.0182). To approximate the percent of individuals with FH VUS that may in fact have P/LP HLRCC variants that predispose to RCC, the above formula was used providing an estimate of 0.17x + 0.045y = 0.064. We approximate that 15% of individuals within the VUS cohort may have P/LP variants that predispose to RCC. By contrast, RCC rates among those with AR FMRD variants were no different than those with negative testing (4.3% vs. 4.5%, p = 0.8706) (Figure 3B). Also of note, as evident in Table 1, 12.8% of patients with AD HLRCC variants were noted to have “other tumors,” the majority of which were uterine leiomyomas provided by further description.
Examining the prevalence of AD HLRCC variants across ethnicities, we noted wide ethnic variability, with all groups affected: frequencies ranged from 0.05% (2/3705) in Ashkenazi Jewish individuals to 0.28% (220/79075) in French Canadian / Caucasian individuals (Supplementary Table 5).
The percentage of patients with RCC secondary to HLRCC varied by ethnic groups ranging from 0% (Ashkenazi Jewish, Sephardic Jewish / Mediterranean, and Native American groups) and 3.6% (5/141) (Asian and Pacific Islander group). Among RCC patients, 1.4% (5/355) of Black / African American patients had pathogenic FH variants compared to 0.7% (26/3719) of French Canadian / Caucasians (p = 0.1690) (Supplementary Table 6).
In the overall cohort, 81.2% of subjects were female. Given that the male/female ratio among patients with HLRCC-associated RCC is unknown, we attempted to quantify sex differences. Among the 302 patients with AD HLRCC variants, 30.5% (25/82) of males and 10.9% (24/220) of females had kidney cancer, with a relative risk ratio of 2.79 (95% CI 1.70 – 4.60). Among the overall cohort, RCC was also more common among males with 11.6% (2610/22535) of males and 2.8% (2747/97523) of females with RCC, with a relative risk ratio of 4.11 (95% CI 3.91 – 4.33). Conversely, among individuals with RCC, 0.96% (25/2610) of males and 0.87% (24/2747) of females had AD HLRCC variants (p = 0.7461) (Supplementary Table 7).
Discussion
Germline testing is more accessible due to the reduced costs, increased availability of providers, and recognition of the importance of identifying genetic alterations. Large genomic datasets and cohorts can provide important information about hereditary cancer syndromes. For newly discovered syndromes or ones that are considered rare, an individual center will have limited cases to investigate new associations, particularly for conditions with low penetrance manifestations. HLRCC is a condition recognized nearly a half-century ago, yet its genetic basis was only first discovered in 2001.2 With only a few hundred families described, our knowledge continues to be refined, such as the more recent associations with adrenal manifestations.17,18
In this report, we provide a detailed analysis of FH variants in a large cohort of patients undergoing germline cancer genetic testing. Given that FH variants were observed frequently (1.3%), clinicians must be familiar with the complexity of both AD and AR manifestations. AD HLRCC and AR FMRD variants were identified in 0.3% and 0.4% of the population, respectively. In our analysis, even after eliminating patients with suspicious features of HLRCC and including only ClinVar annotated variants, the frequency of AD HLRCC variants was 1 in 2668 (0.04%) of probands. While this estimate is not population-based, it may reflect the rate of incidentally detected HLRCC among individuals undergoing cancer genetic testing for other reasons. The true population estimate of HLRCC is unknown, but as we excluded those with suspicious features, this may serve as an underestimate of the overall population frequency of HLRCC. These results reinforce that HLRCC is a relatively common cancer syndrome and is consistent with our prior carrier estimates obtained from examining ExAC and 1000GP population databases.8 Similarly, in a separate recent analysis, investigators curated 280 likely pathogenic FH variants from ClinVar and LOVD databases. By examining these variants in publicly available databases, authors estimated carrier frequencies of 1/2,563 (BRAVO population database) and 1/3,247 (gnomAD population database).19 Such database estimates continue to confirm that the frequency of HLRCC is magnitudes higher than previously suggested.6,7 In fact, HLRCC is likely the most common hereditary kidney cancer syndrome, much higher than Von Hippel-Lindau (1/36,000) or Birt-Hogg-Dubé Syndrome (1/200,000) estimates.20,21 The previously limited identification of HLRCC may largely be due to the low penetrance and the difficulty of recognition.
In this cohort, we identified kidney cancer in 17.0% (49/289) of patients, likely due to referral bias of those with recognized manifestations. Consequentially, as expected, RCC rates were higher among those with AD HLRCC variants when compared to those with negative testing (17% vs. 4.5%, p < 0.0001). The literature has previously suggested the lifetime penetrance of RCC is 15–30%, but higher prevalence of HLRCC suggests that RCC may be less penetrant.7,22 Even with close interrogation of kidney tumors by immunohistochemistry and morphologic assessment, HLRCC accounts for a low percentage of cases.23 If the high prevalence of this syndrome is true, the common benign (uterine fibroids, cutaneous leiomyomas, adrenal nodules) may be overlooked as they are potentially subclinical or dismissed as sporadic. Clinical education and enhanced FH screening of young women with fibroids may allow for early surveillance for and identification of RCC, with the ultimate goal of offering early curative intervention.24
The overall cancer rate (any cancer) was lower among those with AD HLRCC variants compared to those with negative genetic testing (46% vs. 66.1%, p < 0.0001). This is also likely due to testing referral patterns but also suggests that this population may not have a significantly increased risk of non-HLRCC cancers compared to other cancer-rich populations. However, patients with an FH variant are still at risk for other cancers that can occur independently or could be an unrecognized HLRCC association. With increased experience managing HLRCC, we expect that new associations will emerge and additional features will be linked to this condition. Evidence of a second hit from 2-SC staining or biallelic inactivation due to loss of heterozygosity may provide more support that FH loss could play a causal role in cancer development.
The AR FMRD variants lack a strong association with a hereditary cancer syndrome but are also often incidentally found on somatic or germline testing. Our results reinforce that such FH variants are likely not associated with HLRCC and do not confer an increased risk of RCC. In our cohort, 87% of those with AR FMRD variants were the p.Lys477dup variant, known to be the most common variant. This variant has observed in a limited number of individuals with cutaneous leiomyomas and uterine pathology; therefore many centers have advocated surveillance for kidney cancers until further evidence is obtained regarding risk.25,26 However, a recent series from Zhang and colleagues found that among 24 patients with this variant, none had renal cancer, suggesting there may not be a strong RCC association.27 Our study further supports this notion, as our cohort of 361 individuals with the p.Lys477dup variant did not have an increased risk of RCC when compared to those with negative genetic testing (4.2% vs. 4.5%, p = 0.7500). Similarly, there was no increased risk of any cancer. With this data in mind, further assurance can be provided to patients that this variant does not appear to confer a much higher cancer risk than baseline.
The risk of kidney cancer was higher in the VUS population compared to those with negative genetic testing (6.4% vs. 4.5%, p = 0.0182). This introduces the possibility that many (approximately 15%) of these VUS may in fact be pathogenic for HLRCC but have not yet been linked due to the high burden to prove pathogenicity. Many of these cases need further family variant testing and/or laboratory-based enzymatic assessment. Without a clear consensus on a “clinical diagnosis,” the management of these individuals and their family members becomes unclear. Despite various guidelines to not pursue management based on a VUS, at our center we routinely consider a patient with a FH VUS and a renal tumor with morphologic or suggestive immunohistochemical features to have a clinical diagnosis HLRCC until proven otherwise. The downside of managing a VUS as pathogenic in HLRCC (entailing only surveillance imaging) is minimal compared to the upside of identifying and curing a lethal cancer.
Understanding who is at greatest risk will help personalized surveillance. Ethnicity is an important factor, with one recent series interrogating germline defects in 1,829 kidney cancer patients suggesting that HLRCC-associated RCC is more common with African ancestry. This will require further validation, as the number of patients examined was low (only 3 P/LP FH variants found among 91 patients with African ancestry.28 Our analysis yielded a similar finding; among patients with RCC, 1.4% (5/355) African American patients had a pathogenic FH variant as compared to 0.7% (26/3719) of French Canadian / Caucasians (p = 0.1690). Sex is another risk factor for kidney cancer with 2/3 of cases found in men.29 While an increase in comorbidities may play a role, there may also be biological differences modifying risk.30 For HLRCC, it has been unclear whether there are any differences in kidney cancer risk between males versus females. In other hereditary cancer predisposition syndromes, whether the male to female ratio mirrors the general population is variable. For instance, in Lynch syndrome, colorectal cancer is more common in males despite being evenly distributed in the population.31 By contrast, in hereditary paragangliomas, the male to female ratio is similar, while sporadic tumors are much more common in females.32 Within our dataset, the relative risk ratios of RCC in males compared to females was higher both among those with AD HLRCC variants and the total population. The overlapping confidence intervals of these relative risk ratios suggests that RCC also may be similarly enriched in males with HLRCC. Future work should further explore possible sex associations including whether there should be enhanced screening for males with HLRCC.
Several limitations of our analysis must be noted. Although the data provides further evidence that the FH carrier frequency is higher than previously appreciated, this cohort is not population-based and has a very high prevalence of cancer. Nonetheless, given that we excluded all individuals with potential suspicion for HLRCC, our carrier estimate may in fact be conservative. Another limitation is the method of annotating cancer history to accurately categorize and portray cancer rates. This relies heavily on the accuracy and presence of clinician-provided clinical information and could lead to misclassification or the conclusion that cancer history was not present just because was is not reported. Nonetheless, the algorithm was optimized to maximize sensitivity and specificity, with the goal of providing an accurate estimate of the prevalence of cancer.
In summary, utilizing an extensive database of 120,061 individuals from a commercial laboratory, we found that FH variants (including AD HLRCC variants, AR FMRD variants, and VUS) were present in 1.3% of the patients tested. The high frequency (1/2668) of likely incidentally discovered AD HLRCC variants reinforces that HLRCC is more common than previously appreciated. Many individuals within the VUS category may in fact also have underlying HLRCC based on the greater prevalence of RCC. Finally, we find that those with the pathogenic but AR FMRD variants (Lys477dup in particular) do not appear to be at increased risk of cancer (including RCC) when compared to the overall cohort. Taken together, our analysis helps to better characterize the spectrum of FH variants and their genotype-phenotype correlations.
Supplementary Material
TABLE 2.
Cancer Prevalence Among Individuals With FH Variants Versus Negative Genetic Testing
| Cancer Type | AD HLRCC Variants (n = 289), No. (%)a | AR FMRD Variants (n = 415), No. (%)a | VUS (n = 690), No. (%)a | Negative Genetic Testing (n = 103,010), No. (%)a | Total (n = 120,061), No. (%) |
|---|---|---|---|---|---|
| Breast | 18 (6.2) | 140 (33.7) | 231 (33.5) | 36,577 (35.5) | 42,459 (35.4) |
| Colorectal | 10 (3.5) | 26 (6.3) | 49 (7.1) | 6272 (6.1) | 7561 (6.3) |
| Renal | 49 (17.0) | 18 (4.3) | 44 (6.4) | 4639 (4.5) | 5357 (4.5) |
| Skin | 2 (0.7) | 22 (5.3) | 32 (4.6) | 4284 (4.2) | 5145 (4.3) |
| Gastrointestinal, otherb | 3 (1.0) | 19 (4.6) | 23 (3.3) | 4247 (4.1) | 5103 (4.3) |
| Prostate | 4 (1.4) | 14 (3.4) | 17 (2.5) | 4063 (3.9) | 4776 (4.0) |
| Ovarian | 4 (1.4) | 18 (4.3) | 24 (3.5) | 3926 (3.8) | 4816 (4.0) |
| Pancreatic | 0 (0.0) | 18 (4.3) | 19 (2.8) | 3329 (3.2) | 4037 (3.4) |
| Endocrine/neuroendo-crine, otherc | 5 (1.7) | 12 (2.9) | 16 (2.3) | 3174 (3.1) | 3718 (3.1) |
| Other tumors | 37 (12.8) | 8 (1.9) | 23 (3.3) | 2895 (2.8) | 3404 (2.8) |
| Gynecologic, otherd | 6 (2.1) | 9 (2.2) | 17 (2.5) | 2579 (2.5) | 3103 (2.6) |
| Hematologic | 1 (0.3) | 6 (1.4) | 12 (1.7) | 1569 (1.5) | 1901 (1.6) |
| Sarcoma | 3 (1.0) | 6 (1.4) | 7 (1.0) | 1379 (1.3) | 1638 (1.4) |
| Lung | 3 (1.0) | 4 (1.0) | 10 (1.4) | 1350 (1.3) | 1612 (1.3) |
| PGL/PCC | 1 (0.3) | 6 (1.4) | 16 (2.3) | 881 (0.9) | 1203 (1.0) |
| Brain tumor | 1 (0.3) | 0 (0.0) | 5 (0.7) | 777 (0.8) | 936 (0.8) |
| Genitourinary, othere | 1 (0.3) | 4 (1.0) | 7 (1.0) | 533 (0.5) | 694 (0.6) |
| Head and neck | 1 (0.3) | 0 (0.0) | 1 (0.1) | 84 (0.1) | 100 (0.1) |
| Uterine leiomyomas | 6 (2.1) | 0 (0.0) | 3 (0.4) | 30 (0.0) | 42 (0.0) |
| Leiomyosarcoma | 1 (0.3) | 0 (0.0) | 1 (0.1) | 2 (0.0) | 4 (0.0) |
| Any cancer | 133 (46.0) | 268 (64.6) | 446 (64.6) | 68,095 (66.1) | 80,061 (66.7) |
Abbreviations: AD, autosomal dominant; AR, autosomal recessive; FH, fumarate hydratase; FMRD, fumarase deficiency; HLRCC, hereditary leiomyomatosis and renal cell cancer; PGL/PCC, paraganglioma/pheochromocytoma; VUS, variant of unknown significance.
Excludes individuals with germline variants in genes other than FH.
Excludes colorectal and pancreatic cancer.
Excludes PGL/PCC.
Excludes ovarian and uterine leiomyomas
Excludes renal and prostate cancer.
Acknowledgments:
This work was supported by the NIH/NCI under award number P30CA016042 and by an operating grant from the National Cancer Institute Early Detection Research Network (1U01CA214194-01) to PCB.
Footnotes
Disclosure: Kathryn E. Hatchell, Sarah M. Nielsen, Edward D. Esplin, Karen Ouyang, and Keith Nykamp are employees and stockholders of Invitae Corporation, a testing laboratory that furnished the diagnostic test results used in this study. Liying Zhang reports that family members hold leadership positions and ownership interests in Decipher Medicine. Paul C. Boutros sits on the scientific advisory boards of Sage Bionetworks, Intersect Diagnostics, Inc, and BioSymetrics, Inc, and reports patents issued and pending that are unrelated to the work reported here. Brian Shuch has done consulting for Johnson & Johnson, Merck, Genentech, Veracyte, and HistoSonics. The other authors made no disclosures.
Ethics Declaration: IRB approval was obtained for review of this database (IRB#21–000887). All individual level data was de-identified.
Data Availability:
To preserve the individuals’ privacy, the raw data supporting the figures and tables in this manuscript are not publicly available, particularly as certain FH variants identified are rare and the data set includes personal identifiers (age, ethnicity, history diagnosis, etc.).
References
- 1.Merino MJ, Torres-Cabala C, Pinto P, Linehan WM. The morphologic spectrum of kidney tumors in hereditary leiomyomatosis and renal cell carcinoma (HLRCC) syndrome. Am J Surg Pathol. 2007;31(10):1578–1585. [DOI] [PubMed] [Google Scholar]
- 2.Launonen V, Vierimaa O, Kiuru M, et al. Inherited susceptibility to uterine leiomyomas and renal cell cancer. Proc Natl Acad Sci U S A. 2001;98(6):3387–3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muller M, Ferlicot S, Guillaud-Bataille M, et al. Reassessing the clinical spectrum associated with hereditary leiomyomatosis and renal cell carcinoma syndrome in French FH mutation carriers. Clin Genet. 2017;92(6):606–615. [DOI] [PubMed] [Google Scholar]
- 4.Grubb RL 3rd, Franks ME, Toro J, et al. Hereditary leiomyomatosis and renal cell cancer: a syndrome associated with an aggressive form of inherited renal cancer. J Urol. 2007;177(6):2074–2079; discussion 2079–2080. [DOI] [PubMed] [Google Scholar]
- 5.Patel VM, Handler MZ, Schwartz RA, Lambert WC. Hereditary leiomyomatosis and renal cell cancer syndrome: An update and review. J Am Acad Dermatol. 2017;77(1):149–158. [DOI] [PubMed] [Google Scholar]
- 6.Forde C, Lim DHK, Alwan Y, et al. Hereditary Leiomyomatosis and Renal Cell Cancer: Clinical, Molecular, and Screening Features in a Cohort of 185 Affected Individuals. Eur Urol Oncol. 2020;3(6):764–772. [DOI] [PubMed] [Google Scholar]
- 7.Menko FH, Maher ER, Schmidt LS, et al. Hereditary leiomyomatosis and renal cell cancer (HLRCC): renal cancer risk, surveillance and treatment. Fam Cancer. 2014;13(4):637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shuch B, Li S, Risch H, Bindra RS, McGillivray PD, Gerstein M. Estimation of the carrier frequency of fumarate hydratase alterations and implications for kidney cancer risk in hereditary leiomyomatosis and renal cancer. Cancer. 2020;126(16):3657–3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santos M, Lanillos J, Roldan-Romero JM, et al. Prevalence of pathogenic germline variants in patients with metastatic renal cell carcinoma. Genet Med. 2021;23(4):698–704. [DOI] [PubMed] [Google Scholar]
- 10.Kamihara J, Schultz KA, Rana HQ. FH Tumor Predisposition Syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al. , editors. GeneReviews(®). Seattle (WA): University of Washington, Seattle Copyright 1993–2020, University of Washington, Seattle. GeneReviews is a registered trademark of the University of Washington, Seattle. All rights reserved.; 1993. [PubMed] [Google Scholar]
- 11.Landrum MJ, Chitipiralla S, Brown GR, et al. ClinVar: improvements to accessing data. Nucleic Acids Res. 2020;48(D1):D835–d844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coman D, Kranc KR, Christodoulou J. Fumarate Hydratase Deficiency. In: Adam MP, Ardinger HH, Pagon RA, et al. , editors. GeneReviews(®). Seattle (WA): University of Washington, Seattle Copyright 1993–2020, University of Washington, Seattle. GeneReviews is a registered trademark of the University of Washington, Seattle. All rights reserved.; 1993. [PubMed] [Google Scholar]
- 13.Nykamp K, Anderson M, Powers M, et al. Sherloc: a comprehensive refinement of the ACMG-AMP variant classification criteria. Genet Med. 2017;19(10):1105–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karczewski KJ, Francioli LC, Tiao G, et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581(7809):434–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castro-Vega LJ, Buffet A, De Cubas AA, et al. Germline mutations in FH confer predisposition to malignant pheochromocytomas and paragangliomas. Hum Mol Genet. 2014;23(9):2440–2446. [DOI] [PubMed] [Google Scholar]
- 18.Shuch B, Ricketts CJ, Vocke CD, et al. Adrenal nodular hyperplasia in hereditary leiomyomatosis and renal cell cancer. J Urol. 2013;189(2):430–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Popp B, Erber R, Kraus C, et al. Targeted sequencing of FH-deficient uterine leiomyomas reveals biallelic inactivating somatic fumarase variants and allows characterization of missense variants. Mod Pathol. 2020;33(11):2341–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuroda N, Furuya M, Nagashima Y, et al. Review of renal tumors associated with Birt-Hogg-Dubé syndrome with focus on clinical and pathobiological aspects. Pol J Pathol. 2014;65(2):93–99. [DOI] [PubMed] [Google Scholar]
- 21.Kim JJ, Rini BI, Hansel DE. Von Hippel Lindau syndrome. Adv Exp Med Biol. 2010;685:228–249. [DOI] [PubMed] [Google Scholar]
- 22.Wei MH, Toure O, Glenn GM, et al. Novel mutations in FH and expansion of the spectrum of phenotypes expressed in families with hereditary leiomyomatosis and renal cell cancer. J Med Genet. 2006;43(1):18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta S, Swanson AA, Chen YB, et al. Incidence of succinate dehydrogenase and fumarate hydratase-deficient renal cell carcinoma based on immunohistochemical screening with SDHA/SDHB and FH/2SC. Hum Pathol. 2019;91:114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garg K, Rabban J. Hereditary leiomyomatosis and renal cell carcinoma syndrome associated uterine smooth muscle tumors: Bridging morphology and clinical screening. Genes Chromosomes Cancer. 2021;60(3):210–216. [DOI] [PubMed] [Google Scholar]
- 25.Martínek P, Grossmann P, Hes O, et al. Genetic testing of leiomyoma tissue in women younger than 30 years old might provide an effective screening approach for the hereditary leiomyomatosis and renal cell cancer syndrome (HLRCC). Virchows Arch. 2015;467(2):185–191. [DOI] [PubMed] [Google Scholar]
- 26.Ezgu F, Krejci P, Wilcox WR. Mild clinical presentation and prolonged survival of a patient with fumarase deficiency due to the combination of a known and a novel mutation in FH gene. Gene. 2013;524(2):403–406. [DOI] [PubMed] [Google Scholar]
- 27.Zhang L, Walsh MF, Jairam S, et al. Fumarate hydratase FH c.1431_1433dupAAA (p.Lys477dup) variant is not associated with cancer including renal cell carcinoma. Hum Mutat. 2020;41(1):103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abou Alaiwi S, Nassar AH, Adib E, et al. Trans-ethnic variation in germline variants of patients with renal cell carcinoma. Cell Rep. 2021;34(13):108926. [DOI] [PubMed] [Google Scholar]
- 29.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA: A Cancer Journal for Clinicians. 2021;71(1):7–33. [DOI] [PubMed] [Google Scholar]
- 30.Zhao H, Leppert JT, Peehl DM. A Protective Role for Androgen Receptor in Clear Cell Renal Cell Carcinoma Based on Mining TCGA Data. PLoS One. 2016;11(1):e0146505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Engel C, Vasen HF, Seppälä T, et al. No Difference in Colorectal Cancer Incidence or Stage at Detection by Colonoscopy Among 3 Countries With Different Lynch Syndrome Surveillance Policies. Gastroenterology. 2018;155(5):1400–1409.e1402. [DOI] [PubMed] [Google Scholar]
- 32.Boedeker CC, Neumann HP, Maier W, Bausch B, Schipper J, Ridder GJ. Malignant head and neck paragangliomas in SDHB mutation carriers. Otolaryngol Head Neck Surg. 2007;137(1):126–129. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
To preserve the individuals’ privacy, the raw data supporting the figures and tables in this manuscript are not publicly available, particularly as certain FH variants identified are rare and the data set includes personal identifiers (age, ethnicity, history diagnosis, etc.).


