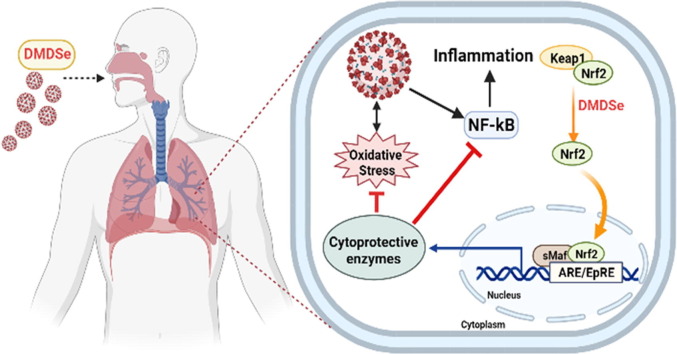
Graphical abstract
Keywords: Selenium, Dimethyldiselenide, COVID-19 mortality, Environmental exposure, Nrf2
Abstract
Environmental selenium (Se) distribution in the US is uneven, yet US residents appear to have a relatively narrow range of serum Se concentrations, according to the NHANES III survey data; this is probably due to the modern food-distribution system. In the US, Se concentration in alfalfa leaves has been used as a proxy for regional Se exposure (low, medium or high, corresponding to ≤ 0.05, 0.06–0.10 and ≥ 0.11 ppm respectively). Se in plants, soil, water, and bacteria can be transformed into volatile dimethyldiselenide, which can be inhaled and excreted via the lung. Hence, pulmonary Se exposure may be different in states with different atmospheric Se levels. We found a significantly higher death rate from COVID-19 in low-Se states than in medium-Se or high-Se states, though the case densities of these states were not significantly different. Because inhaled dimethyldiselenide is a potent inducer of nuclear-factor erythroid 2 p45-related factor 2 (Nrf2), exposure to higher atmospheric dimethyldiselenide may increase Nrf2-dependent antioxidant defences, reducing the activation of NFκB by SARS-CoV-2 in the lung, thereby decreasing cytokine activation and COVID-19 severity. Atmospheric dimethyldiselenide may thereby play a role in COVID-19 mortality, although the extent of its involvement is unclear.
1. Introduction
Numerous studies have been published linking selenium (Se) status with the ability to resist viral infections. SARS-CoV-2 infection may be thwarted by the action of selenoproteins and redox-active Se species on virus-triggered oxidative stress, excessive inflammatory response and immune-system dysfunction (Zhang et al., 2020a). Thus, there is evidence that higher Se status is linked to better recovery from COVID-19. A number of studies found Se status to be significantly higher in serum samples from surviving than non-surviving COVID-19 patients (Fakhrolmobasheri et al., 2021, Heller et al., 2021, Moghaddam et al., 2020), while a positive association was found between the cure rate of Chinese patients with COVID-19 and regional Se status (Zhang et al., 2020b).
Up to now, no one has found a link between Se status in the US and recovery from COVID-19. Based on NHANES III 1988–1994, the only one of the NHANES studies that split US residents into four geographic regions and measured their serum Se, there was little regional difference in Se status (Niskar et al., 2003). Specifically, mean (95% confidence interval, CI) serum Se concentrations of the Northeast, Midwest, South and West regions were 124.4 (121.6, 127.9), 128.3 (122.4, 135.0), 120.5 (119.2, 122.4) and 128.7 (124.0, 132.7) μg/L, respectively. Moreover, taking the lowest lower and the highest upper 95% CI showed a relatively narrow range of serum Se concentrations, i.e., 119.2 μg/L to 135.0 μg/L. Given the uneven environmental Se distribution in the US (see below), the unusually narrow serum Se range is probably due to the modern US food-distribution system. Such a narrow serum Se range largely excludes the likelihood of observing an association between death caused by COVID-19 in the US and human Se status as measured in the serum, plasma or blood.
Nonetheless, environmental Se levels in the US are diverse (Cowgill, 1997). Se concentration in alfalfa leaves has been used as a proxy for regional Se exposure (Cowgill, 1997). Accordingly, different states in the US have been categorized as low-Se, medium-Se and high-Se according to Se concentrations in alfalfa, a forage crop (Cowgill, 1997). Se in plant, soil, water and bacteria can be transformed into volatile dimethyldiselenide (Haygarth, 1994), thus atmospheric dimethyldiselenide levels may be different in states with different Se concentrations in alfalfa leaves. As atmospheric dimethyldiselenide is subject to pulmonary inhalation (al-Bayati et al., 1992), local Se levels in the lungs may be different in states with different Se concentrations in alfalfa leaves.
Dimethyldiselenide has been reported to be the strongest inducer of nuclear factor erythroid 2 p45-related factor 2 (Nrf2)-associated proteins among a range of Se compounds (Xiao and Parkin, 2006). Dimethyldiselenide can double quinone-reductase activity at concentrations as low as 0.16 μM, being more potent than sulforaphane, the well-recognized Nrf2 inducer (Xiao and Parkin, 2006). Inducers of Nrf2 have been regarded as giving effective protection against COVID-19 (Bousquet et al., 2020, Cuadrado et al., 2020, Olagnier et al., 2020, Ordonez et al., 2021, Sun et al., 2021). Exposure to higher atmospheric dimethyldiselenide levels may increase Nrf2 defence and reduce SARS-CoV-2-induced activation of NFκB in the lung, thereby lowering the risk of cytokine activation that can increase COVID-19 severity. Thus we speculated that environmental Se distribution may play a role in COVID-19 mortality. The current study was undertaken to examine this possibility.
2. Materials and methods
States were divided into low-, medium- and high-Se according to Se concentrations in alfalfa of ≤ 0.05 ppm, 0.06–0.10 ppm and ≥ 0.11 ppm, respectively (Cowgill, 1997). States having ≤ 0.05 ppm Se in their alfalfa are Connecticut, Delaware, Florida, Illinois, Indiana, Maine, Maryland, Massachusetts, Michigan, New Hampshire, New Jersey, New York, Ohio, Pennsylvania, Rhode Island, Vermont, West Virginia, and Wisconsin. States with 0.06–0.10 ppm Se in their alfalfa are California, Georgia, Idaho, Kentucky, Minnesota, North Carolina, Oregon, South Carolina, Tennessee, Virginia, and Washington. States having ≥ 0.11 ppm Se in their alfalfa are Alabama, Arizona, Colorado, Iowa, Kansas, Louisiana, Mississippi, Missouri, Montana, Nebraska, Nevada, New Mexico, North Dakota, Oklahoma, South Dakota, Texas, Utah, and Wyoming.
The data from August 27, 2020 on COVID-19 case number and case mortality in US states is available from the CDC website of the US (https://www.cdc.gov/coronavirus/2019-ncov/covid-data/previouscases.html). Land areas are from the US Census summary 2010, Tables 1 and 18 (Bureau, 2012).
Data for death rate and case density are presented as mean ± SEM (standard error of the mean). The differences between groups were analyzed using GraphPad Software (Prism version 5, San Diego, California, USA) by one-way ANOVA post hoc Tukey's Multiple Comparison Test. A p value of <0.05 was considered statistically significant. Stata (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC) was used to fit logistic regression model to the data, with mortality yes/no as the binary outcome. Se status was included in the model as a 3-level factor explanatory variable with low Se concentration as the reference category, and case density was included as a continuous explanatory variable.
3. Results and discussion
Although NHANES III divided the US into four regions and saw little difference in their Se status as assessed by serum Se levels, we have looked at 46 States which gives us a much greater possibility of seeing notable environmental differences between them.
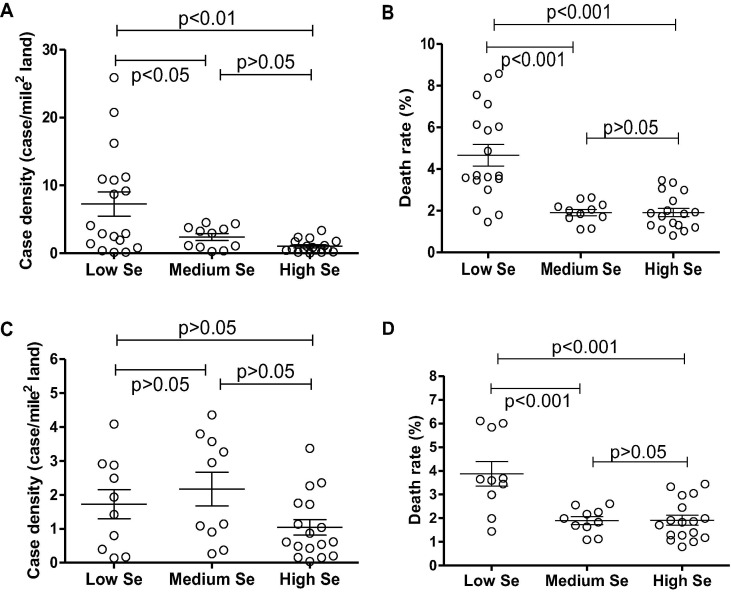
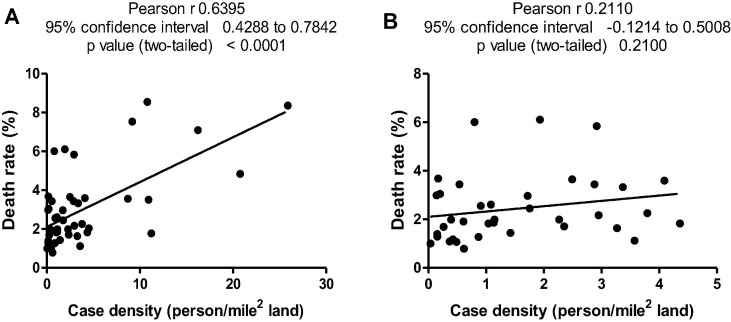
States with low, medium and high Se concentrations in alfalfa are shown in Table 1 . Case density (case number/land area) and death rate (case mortality/case number) are also listed in Table 1. Case density and death rate were all significantly higher in the low-Se region than those in the medium-Se and high-Se regions (Fig. 1 A and B). A high case density may lead to a larger death rate due to insufficient medical facilities as reflected in the current study, i.e., the Pearson r of case density and death rate of all states without exclusion was 0.6395 (p < 0.0001, n = 46) (Fig. 2 A). We thus excluded states with case density of over 4.5 person/mile2 to maintain case density of low-Se, medium-Se and high-Se states at equivalent levels without significant differences (p > 0.05) (Fig. 1C). Under such conditions, case density and death rate did not exhibit significant correlations (Pearson r = 0.211, p = 0.21 and n = 37) (Fig. 2B). Accordingly, death rate in the low-Se region was 3.88%, whereas death rates in medium-Se and high-Se regions were 1.90% and 1.92%, respectively. The death rate for low-Se regions was significantly higher than those of both medium-Se and high-Se regions (p < 0.001) (Fig. 1D).
Table 1.
Death rate and case density in low (L)-, medium (M)-, and high (H)-Se regions (L ≤ 0.05 ppm, M 0.06–0.10 ppm and H ≥ 0.11 ppm alfalfa Se concentrations (Cowgill, 1997)).
| Location | Se status | Case | Case mortality | Death rate (%) | Land area (mile2) | Case density (person/mile2 land) |
|---|---|---|---|---|---|---|
| New Jersey | L | 190,306 | 15,914 | 8.36 | 7354.22 | 25.877 |
| Rhode Island | L | 21,454 | 1041 | 4.85 | 1033.81 | 20.752 |
| Connecticut | L | 52,220 | 4463 | 8.55 | 4842.36 | 10.784 |
| Massachusetts | L | 126,503 | 8987 | 7.10 | 7800.06 | 16.218 |
| Maryland | L | 106,063 | 3722 | 3.51 | 9707.24 | 10.926 |
| New York | L | 432,458 | 32,610 | 7.54 | 47126.40 | 9.177 |
| Delaware | L | 16,976 | 604 | 3.56 | 1948.54 | 8.712 |
| Florida | L | 602,113 | 10,733 | 1.78 | 53624.76 | 11.228 |
| Pennsylvania | L | 130,536 | 7624 | 5.84 | 44742.70 | 2.917 |
| Ohio | L | 117,584 | 4044 | 3.44 | 40860.69 | 2.878 |
| Illinois | L | 227,044 | 8163 | 3.60 | 55518.93 | 4.089 |
| Indiana | L | 89,359 | 3259 | 3.65 | 35826.11 | 2.494 |
| New Hampshire | L | 7159 | 430 | 6.01 | 8952.65 | 0.800 |
| Michigan | L | 109,480 | 6690 | 6.11 | 56538.90 | 1.936 |
| Wisconsin | L | 77,092 | 1108 | 1.44 | 54157.80 | 1.423 |
| West Virginia | L | 9540 | 190 | 1.99 | 24038.21 | 0.397 |
| Vermont | L | 1577 | 58 | 3.68 | 9216.66 | 0.171 |
| Maine | L | 4416 | 132 | 2.99 | 30842.92 | 0.143 |
| California | M | 679,099 | 12,407 | 1.83 | 155779.22 | 4.359 |
| Virginia | M | 116,579 | 2527 | 2.17 | 39490.09 | 2.952 |
| North Carolina | M | 158,985 | 2606 | 1.64 | 48617.91 | 3.270 |
| Georgia | M | 260,590 | 5311 | 2.04 | 57513.49 | 4.531 |
| Tennessee | M | 147,353 | 1648 | 1.12 | 41234.90 | 3.574 |
| South Carolina | M | 114,093 | 2573 | 2.26 | 30060.70 | 3.795 |
| Kentucky | M | 45,230 | 902 | 1.99 | 39486.34 | 1.145 |
| Washington | M | 72,161 | 1880 | 2.61 | 66455.52 | 1.086 |
| Minnesota | M | 72,390 | 1855 | 2.56 | 79626.74 | 0.909 |
| Oregon | M | 25,571 | 433 | 1.69 | 95988.01 | 0.266 |
| Idaho | M | 30,780 | 337 | 1.09 | 82643.12 | 0.372 |
| Texas | H | 592,137 | 11,805 | 1.99 | 261231.71 | 2.267 |
| Alabama | H | 119,254 | 2045 | 1.71 | 50645.33 | 2.355 |
| Louisiana | H | 145,661 | 4851 | 3.33 | 43203.90 | 3.371 |
| Missouri | H | 78,062 | 1449 | 1.86 | 68741.52 | 1.136 |
| Mississippi | H | 80,695 | 2399 | 2.97 | 46923.27 | 1.720 |
| Arizona | H | 199,459 | 4896 | 2.45 | 113594.08 | 1.756 |
| Iowa | H | 58,215 | 1066 | 1.83 | 55857.13 | 1.042 |
| Oklahoma | H | 59,715 | 763 | 1.28 | 68594.92 | 0.871 |
| Colorado | H | 55,994 | 1927 | 3.44 | 103641.89 | 0.540 |
| Kansas | H | 39,937 | 426 | 1.07 | 81758.72 | 0.488 |
| Utah | H | 50,870 | 402 | 0.79 | 82169.62 | 0.619 |
| Nevada | H | 66,938 | 1279 | 1.91 | 109781.18 | 0.610 |
| Nebraska | H | 32,727 | 386 | 1.18 | 76824.17 | 0.426 |
| New Mexico | H | 24,732 | 755 | 3.05 | 121298.15 | 0.204 |
| South Dakota | H | 11,571 | 162 | 1.40 | 75811.00 | 0.153 |
| North Dakota | H | 10,800 | 139 | 1.29 | 69000.80 | 0.157 |
| Wyoming | H | 3684 | 37 | 1.00 | 97093.14 | 0.038 |
Fig. 1.
Comparison of death rates in states located in low-, medium- and high-Se regions. (A) Case density without exclusion of states with case density of over 4.5 person/mile2; (B) Death rate without exclusion of states with case density of over 4.5 person/mile2; (C) Case density excluding those states with case density higher than 4.5 person/mile2; (D) Death rate excluding those states with case density higher than 4.5 person/mile2.
Fig. 2.
Correlation of case density and death rate. (A) Case density without exclusion of states with case density of over 4.5 person/mile2; (B) Case density excluding those states with case density higher than 4.5 person/mile2.
The odds of death, estimated using a logistic regression model, were approximately halved in states with medium- or high-Se concentrations than in those with low-Se concentrations [adjusted OR 0.46 95% CI (0.46 to 0.47); p < 0.001 in medium Se areas; adjusted OR 0.56 95% CI (0.55 to 0.56); p < 0.001 in high Se areas]. Case density was also a significant predictor of outcome, with the odds of mortality increasing by 3% (p < 0.001) for each unit increase in case density. If we combine medium- and high-Se into a single category, the odds of mortality are 50% lower in high- or medium-Se states compared to low-Se states [adjusted OR 0.50 95% CI (0.50 to 0.51); p < 0.001].
Consistent with our finding on the US data, a Chinese ecological study that investigated the association between COVID-19 mortality and the Se content in crops also showed a significant link between environmental Se and COVID-19 mortality (Zhang et al., 2021). Specifically, the case fatality rates gradually increased from 1.17% in non-Se-deficient areas (>0.06 ppm Se in crops) to 1.28% in moderate-Se-deficient areas (0.03–0.06 ppm Se in crops), and further to 3.16% in severe-Se-deficient areas (<0.03 ppm Se in crops) (p = 0.002). The zero-inflated negative binomial regression model showed a significantly higher fatality risk in severe-Se-deficient cities than non-Se-deficient cities, with an incidence-rate ratio of 3.88 (95% CIs: 1.21, 12.52). The two studies found the same phenomenon, i.e., COVID-19 patients in regions with Se concentrations in plants above 0.06 ppm had a lower mortality.
The mechanism by which Se may mediate a protective role needs to be understood given the little variation in serum Se levels of US residents. A potential mechanism may involve environmental volatile dimethyldiselenide released from plants and inhaled by the lung.
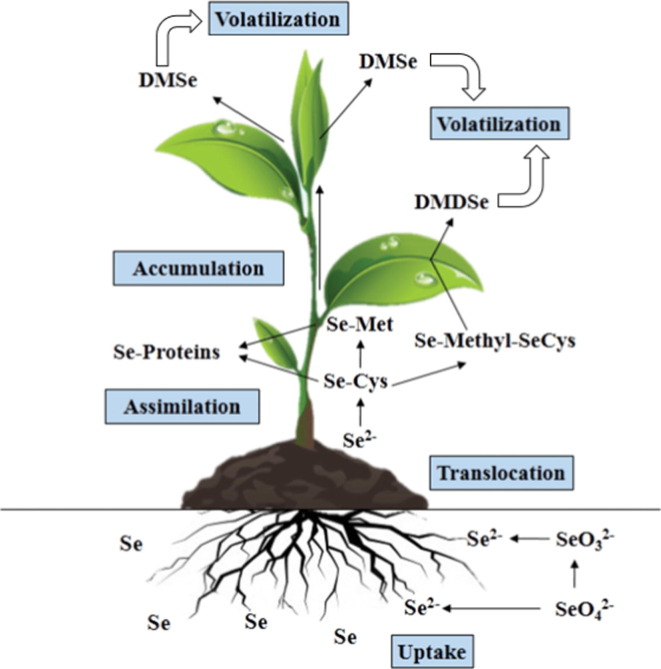
The major species of Se in plant sources are selenite, selenate, selenomethione, selenocysteine, Se-containing proteins where methionine and cysteine have been replaced by selenomethione and selenocysteine, Se-methyl-selenocysteine, and γ-glutamyl-Se-methyl-selenocysteine (Dumont et al., 2006). However, plants can volatilise significant amounts of Se as dimethylselenide and dimethyldiselenide (Fig. 3 ) (Chomchan et. al., 2017). It was estimated that total flux of Se into the atmosphere each year was estimated to be 13,000 or 19,000 tons (Mosher and Duce, 1987, Nriagu and Pacyna, 1988). A higher environmental Se, as reflected by Se concentrations in plants like alfalfa, probably generates more volatile dimethylselenide and dimethyldiselenide (Haygarth, 1994). Dimethylselenide is not a redox-active Se compound and is considered to be non-toxic and biologically-inert since it is >500-times less toxic than selenite in rats (McConnell and Portman, 1952, Wilber, 1980). However, like many diselenide compounds such as synthetic diphenyldiselenide, diethyldiselenide, dipropyldiselenide and dibutyldiselenide (Álvarez-Pérez et al., 2018, Barbosa et al., 2017) as well as naturally occurring selenocystine, dimethyldiselenide generates reactive oxygen species in the presence of glutathione and thus is a redox-active Se compound (Spallholz et al., 2001). The low ionization energy of dimethyldiselenide (7.9 ± 0.1 eV) suggests that it can react with many radical cations by electron transfer (Thoen et al., 1996). Following dietary intake of Se (by selenomethionine, Se-methyl-selenocysteine or selenite) at levels above those required for selenoprotein biosynthesis, small molecular weight Se compounds including methylselenol, hydrogen selenide and dimethyldiselenide are accumulated (Zhang et al., 2020a). Dimethyldiselenide can be expelled in the breath (Zhang et al., 2020a). The true dimethydiselenide levels in the lung are influenced by atmospheric dimethyldiselenide and dimethydiselenide generated by the body. Dimethydiselenide formed in vivo may be exhaled in a poor Se environment with low levels of atmospheric dimethyldiselenide; by contrast, the lung may inhale dimethydiselenide in a high Se environment with a good concentration of atmospheric dimethyldiselenide. A loss of Se via exhalation down a concentration gradient is likely in low Se regions, where the partial pressure of volatile Se species is reduced. Loss of pulmonary dimethydiselenide via exhalation may help explain a significantly higher death rate from COVID-19 in states with low environmental (atmospheric) Se concentrations.
Fig. 3.
Se metabolism in plants. This depiction of Se metabolism in plants was modified from Chomchan et al. (2017) with the use of broad arrows indicating volatile Se species. Abbreviations: Se-Proteins, seleno-proteins; Se-Met, seleno-methionine; Se-Cys, seleno-cysteine; Se-Methyl-SeCys, seleno-methylselenocysteine; DMSe, dimethylselenide; DMDSe, dimethyldiselenide.
In addition to upregulating selenoproteins at optimal nutritional levels, Se at a level beyond the requirement for selenoprotein biosynthesis also increases Nrf2-associated antioxidant and detoxifying proteins (Xiao and Parkin, 2006). Significantly, Se was found to be elevated only in the lung in rats exposed to dimethyldiselenide (al-Bayati et al., 1992). Among 27 Se compounds examined, dimethyldiselenide is distinct owing to its capacity to double quinone-reductase activity at an exceptionally low concentration (0.16 μM) in murine hepatoma cells (Xiao and Parkin, 2006). Such a concentration is only slightly higher than the requirement for optimal biosynthesis of selenoproteins including glutathione peroxidase 1 and thioredoxin reductase 1 (normally 0.1 μM in cell culture). Moreover, dimethyldiselenide was found to be more potent than sulforaphane, an extensively characterized Nrf2 activator extracted from broccoli (Xiao and Parkin, 2006). The activity of Se compounds in inducing quinone-reductase was thought to be associated with a putative selenol-generating potential (Xiao and Parkin, 2006). In this context, dimethyldiselenide with low ionization energy (Thoen et al., 1996) only undergoes a one-step reduction by glutathione to form methylselenol (Dauplais et al., 2021a, Dauplais et al., 2021b). It has been suggested that Nrf2 activation could be deployed against COVID-19 (Bousquet et al., 2020, Cuadrado et al., 2020, Olagnier et al., 2020, Ordonez et al., 2021, Sun et al., 2021). This mechanism provides cytoprotection by restoring redox and protein homeostasis, countering the hyperinflammatory cytokine activation associated with COVID-19 and facilitating tissue repair (Bousquet et al., 2020, Cuadrado et al., 2020, Olagnier et al., 2020, Ordonez et al., 2021, Sun et al., 2021).
Moreover, heme oxygenase 1 upregulated mainly by Nrf2 normally exerts an antiviral effect against many viruses including influenza, respiratory syncytial virus, and Ebola (Cuadrado et al., 2020). Heme oxygenase 1 catalyzes the degradation of heme into biliverdin which can inhibit viral proteases. 3CLpro (Mpro) and PLpro share high homology with other viral proteases, thus biliverdin is expected to inhibit SARS-CoV-2 3CLpro and PLpro that promote replication of SARS-CoV-2 (Cuadrado et al., 2020). Indeed, Nrf2 agonists, 4-octyl-itaconate and the clinically-approved dimethyl fumarate, not only limit host inflammatory responses to SARS-CoV-2 infection, but also inhibit the replication of SARS-CoV-2 (Olagnier et al., 2020). The Nrf2 activators, bardoxolone and bardoxolone-methyl, also promote the resolution of inflammation and inhibit the replication of SARS-CoV-2 (Sun et al., 2021). Sulforaphane, a well-recognized robust Nrf2 inducer, exhibits in vitro and in vivo antiviral activity against SARS-CoV-2 (Ordonez et al., 2021). An adaptation strategy against COVID-19 via intake of Nrf2-interacting nutrients has been proposed (Bousquet et al., 2020). Atmospheric dimethyldiselenide, which is more potent than sulforaphane, may activate Nrf2 in the lung, the major battlefield against SARS-CoV-2. Residents in low-Se regions may be more vulnerable to SARS-CoV-2 due to a lack of dimethyldiselenide-induced pulmonary Nrf2.
SARS-CoV-2 has been found in the environment, including air, water, cold-chain, biota, soil and object surfaces (Shao et al., 2021). Aerosols contain SARS-CoV-2 which is mainly distributed in two size ranges (0.25–1.0 μm and > 2.5 μm). Aerosol transmission in closed environments has a large impact on the COVID-19 pandemic (Shao et al., 2021). A study based on mathematical and statistical relationship between temperature and viral transmission suggests that human transmission of SARS-CoV-2 is intrinsically linked to temperature; at the threshold of 16.92 °C, the infection rate was found to be highest (Liu, 2022). The current study attempts to find a potential relationship between dimethyldiselenide and death rate caused by COVID-19, rather than the potential relationship between dimethyldiselenide and COVID-19 infection rate. Thus, the relationship of aerosol particles or temperature with dimethyldiselenide as well as the combined influence (aerosol particles/dimethyldiselenide or temperature/dimethyldiselenide) on SARS-CoV-2 infection are beyond the scope of this study.
4. Significance and limitation summaries
Based on the regional divisions used in NHANES III, only a small difference in serum Se between these four divisions was found, probably due to the modern US food-distribution system. Thus, Se status in the US has not been linked to COVID-19 mortality. Previously, environmental Se exposure was estimated by its concentration in alfalfa leaves where it can be transformed into volatile dimethyldiselenide, absorbable by the lung. Using known alfalfa Se concentration in 37 states which were divided into low-, medium- and high-Se states with comparable case-densities, the odds of mortality were significantly lower in high- or medium-Se states than in low-Se states [OR 0.50, 95 %CI (0.50–0.51)]. Higher atmospheric dimethyldiselenide may increase Nrf2 defence in the lung, thereby lowering the risk of cytokine activation that may increase COVID-19 severity, potentially triggering death.
Though we have identified a link between State-linked environmental Se exposure and COVID-19 mortality with large effect sizes that are highly statistically significant, one should be cautious of the interpretation of these results. Because of the very high case numbers, it is almost inevitable that if there are any real effects, these will emerge as statistically significant due to the high number of observations included in the model, as we have seen here. Additional sensitivity analyses, however, showed that Se status remained significant, albeit with wider confidence intervals. It is important to note that the total explanatory effect of the model is small; there is much more required to explain the differences in mortality rates than has been discussed here. The analysis carried out here makes no attempt to demonstrate causation and has no ability to verify or refute the biological plausibility of the hypotheses discussed.
Whether atmospheric dimethyldiselenide might really be involved is unclear, as exact levels of atmospheric dimethyldiselenide and how much dimethyldiselenide is inhaled are not known. The potential relationship found in this study may provide a pointer for future studies.
CRediT authorship contribution statement
Jinsong Zhanga: Conceptualization, Methodology, Investigation, Resources, Validation, Formal analysis, Writing – Original Draft, Review & Editing. Ethan Will Taylorb: Conceptualization, Methodology, Investigation, Resources, Writing – Original Draft, Review & Editing. Kate Bennett: Validation, Formal analysis, Writing – Original Draft, Review & Editing. Margaret P. Rayman: Conceptualization, Methodology, Investigation, Resources, Writing – Original Draft, Review & Editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The study was funded by the National Natural Science Foundation of China (31771971) to J.Z. and by an unrestricted gift from the Dr. Arthur and Bonnie Ennis Foundation, Decatur, IL, to E.W.T.
References
- Al‐Bayati M.A., Raabe O.G., Teague S.V. Effect of inhaled dimethylselenide in the Fischer 344 male rat. J Toxicol Environ Health. 1992;37(4):549–557. doi: 10.1080/15287399209531692. [DOI] [PubMed] [Google Scholar]
- Álvarez-Pérez M., Ali W., Marć M., Handzlik J., Domínguez-Álvarez E. Selenides and Diselenides: A Review of Their Anticancer and Chemopreventive Activity. Molecules. 2018;23(3):628. doi: 10.3390/molecules23030628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa N.V., Nogueira C.W., Nogara P.A., de Bem A.F., Aschner M., Rocha J.B.T. Organoselenium compounds as mimics of selenoproteins and thiol modifier agents. Metallomics. 2017;9(12):1703–1734. doi: 10.1039/c7mt00083a. [DOI] [PubMed] [Google Scholar]
- Bousquet, J., Cristol, J.P., Czarlewski, W., Anto, J.M., Martineau, A., Haahtela, T., Fonseca, S.C., Iaccarino, G., Blain, H., Fiocchi, A., Canonica, G.W., Fonseca, J.A., Vidal, A., Choi, H.J., Kim, H.J., Le Moing, V., Reynes, J., Sheikh, A., Akdis, C.A., Zuberbier, T., group, A., 2020. Nrf2-interacting nutrients and COVID-19: time for research to develop adaptation strategies. Clin Transl Allergy 10, 58. [DOI] [PMC free article] [PubMed]
- Bureau, U.S.C., 2012. 2010 Census of Population and Housing, Population and Housing Unit Counts, CPH-2-1. United States Summary U.S. Government Printing Office, Washington, DC.
- Chomchan R., Siripongvutikorn S., Puttarak P. Selenium bio-fortification: an alternative to improve phytochemicals and bioactivities of plant foods. Functional Foods in Health and Disease. 2017;7(4):263. [Google Scholar]
- Cowgill U.M. The distribution of selenium and mortality owing to acquired immune deficiency syndrome in the continental United States. Biol Trace Elem Res. 1997;56(1):43–61. doi: 10.1007/BF02778983. [DOI] [PubMed] [Google Scholar]
- Cuadrado A., Pajares M., Benito C., Jiménez-Villegas J., Escoll M., Fernández-Ginés R., Garcia Yagüe A.J., Lastra D., Manda G., Rojo A.I., Dinkova-Kostova A.T. Can Activation of NRF2 Be a Strategy against COVID-19? Trends Pharmacol Sci. 2020;41(9):598–610. doi: 10.1016/j.tips.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauplais M., Bierla K., Maizeray C., Lestini R., Lobinski R., Plateau P., Szpunar J., Lazard M. Methylselenol Produced In Vivo from Methylseleninic Acid or Dimethyl Diselenide Induces Toxic Protein Aggregation in Saccharomyces cerevisiae. IJMS. 2021;22(5):2241. doi: 10.3390/ijms22052241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauplais M., Mahou P., Plateau P., Lazard M. Exposure to the Methylselenol Precursor Dimethyldiselenide Induces a Reductive Endoplasmic Reticulum Stress in Saccharomyces cerevisiae. IJMS. 2021;22(11):5467. doi: 10.3390/ijms22115467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont E., Vanhaecke F., Cornelis R. Selenium speciation from food source to metabolites: a critical review. Anal. Bioanal. Chem. 2006;385(7):1304–1323. doi: 10.1007/s00216-006-0529-8. [DOI] [PubMed] [Google Scholar]
- Fakhrolmobasheri M., Mazaheri-Tehrani S., Kieliszek M., Zeinalian M., Abbasi M., Karimi F., Mozafari A.M. COVID-19 and Selenium Deficiency: a Systematic Review. Biol Trace Elem Res. 2021 doi: 10.1007/s12011-021-02997-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haygarth P.M. Marcel-Dekker; New York: 1994. Global Importance and Cycling of Selenium. [Google Scholar]
- Heller R.A., Sun Q., Hackler J., Seelig J., Seibert L., Cherkezov A., Minich W.B., Seemann P., Diegmann J., Pilz M., Bachmann M., Ranjbar A., Moghaddam A., Schomburg L. Prediction of survival odds in COVID-19 by zinc, age and selenoprotein P as composite biomarker. Redox Biol. 2021;38:101764. doi: 10.1016/j.redox.2020.101764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L.u. The dynamics of early-stage transmission of COVID-19: A novel quantification of the role of global temperature. Gondwana Res. 2022 doi: 10.1016/j.gr.2021.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell K.P., Portman O.W. Toxicity of dimethyl selenide in the rat and mouse. Proc. Soc. Exp. Biol. Med. 1952;79(2):230–231. doi: 10.3181/00379727-79-19333. [DOI] [PubMed] [Google Scholar]
- Moghaddam A., Heller R.A., Sun Q., Seelig J., Cherkezov A., Seibert L., Hackler J., Seemann P., Diegmann J., Pilz M., Bachmann M., Minich W.B., Schomburg L. Selenium Deficiency Is Associated with Mortality Risk from COVID-19. Nutrients. 2020;12 doi: 10.3390/nu12072098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher B.W., Duce R.A. A global atmospheric selenium budget. J Geophys Res-Atmos. 1987;92(D11):13289. doi: 10.1029/JD092iD11p13289. [DOI] [Google Scholar]
- Niskar A.S., Paschal D.C., Kieszak S.M., Flegal K.M., Bowman B., Gunter E.W., Pirkle J.L., Rubin C., Sampson E.J., Mcgeehin M. Serum selenium levels in the US population: Third National Health and Nutrition Examination Survey, 1988–1994. Biol Trace Elem Res. 2003;91(1):1–10. doi: 10.1385/BTER:91:1:1. [DOI] [PubMed] [Google Scholar]
- Nriagu J.O., Pacyna J.M. Quantitative assessment of worldwide contamination of air, water and soils by trace metals. Nature. 1988;333(6169):134–139. doi: 10.1038/333134a0. [DOI] [PubMed] [Google Scholar]
- Olagnier D., Farahani E., Thyrsted J., Blay-Cadanet J., Herengt A., Idorn M., Hait A., Hernaez B., Knudsen A., Iversen M.B., Schilling M., Jorgensen S.E., Thomsen M., Reinert L.S., Lappe M., Hoang H.D., Gilchrist V.H., Hansen A.L., Ottosen R., Nielsen C.G., Moller C., van der Horst D., Peri S., Balachandran S., Huang J., Jakobsen M., Svenningsen E.B., Poulsen T.B., Bartsch L., Thielke A.L., Luo Y., Alain T., Rehwinkel J., Alcami A., Hiscott J., Mogensen T.H., Paludan S.R., Holm C.K. SARS-CoV2-mediated suppression of NRF2-signaling reveals potent antiviral and anti-inflammatory activity of 4-octyl-itaconate and dimethyl fumarate. Nat Commun. 2020;11:4938. doi: 10.1038/s41467-020-18764-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordonez, A.A., Bullen, C.K., Villabona-Rueda, A.F., Thompson, E.A., Turner, M.L., Davis, S.L., Komm, O., Powell, J.D., D'Alessio, F.R., Yolken, R.H., Jain, S.K., Jones-Brando, L., 2021. Sulforaphane exhibits in vitro and in vivo antiviral activity against pandemic SARS-CoV-2 and seasonal HCoV-OC43 coronaviruses. bioRxiv. [DOI] [PMC free article] [PubMed]
- Shao L., Ge S., Jones T., Santosh M., Silva L.F.O., Cao Y., Oliveira M.L.S., Zhang M., BéruBé K. The role of airborne particles and environmental considerations in the transmission of SARS-CoV-2. Geosci. Front. 2021;12(5):101189. doi: 10.1016/j.gsf.2021.101189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spallholz J.E., Shriver B.J., Reid T.W. Dimethyldiselenide and methylseleninic acid generate superoxide in an in vitro chemiluminescence assay in the presence of glutathione: implications for the anticarcinogenic activity of L-selenomethionine and L-Se-methylselenocysteine. Nutr. Cancer. 2001;40(1):34–41. doi: 10.1207/S15327914NC401_8. [DOI] [PubMed] [Google Scholar]
- Sun Q., Ye F., Liang H., Liu H., Li C., Lu R., Huang B., Zhao L., Tan W., Lai L. Bardoxolone and bardoxolone methyl, two Nrf2 activators in clinical trials, inhibit SARS-CoV-2 replication and its 3C-like protease. Signal Transduct Target Ther. 2021;6:212. doi: 10.1038/s41392-021-00628-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoen K.K., Beasley B.J., Smith R.L., Kenttämaa H.I. Distinguishing conventional and distonic radical cations by using dimethyl diselenide. J Am Soc Mass Spectrom. 1996;7(12):1245–1250. doi: 10.1016/S1044-0305(96)00107-9. [DOI] [PubMed] [Google Scholar]
- Wilber C.G. Toxicology of selenium: a review. Clin. Toxicol. 1980;17(2):171–230. doi: 10.3109/15563658008985076. [DOI] [PubMed] [Google Scholar]
- Xiao H., Parkin K.L. Induction of phase II enzyme activity by various selenium compounds. Nutr Cancer. 2006;55(2):210–223. doi: 10.1207/s15327914nc5502_13. [DOI] [PubMed] [Google Scholar]
- Zhang H.Y., Zhang A.R., Lu Q.B., Zhang X.A., Zhang Z.J., Guan X.G., Che T.L., Yang Y., Li H., Liu W., Fang L.Q. Association between fatality rate of COVID-19 and selenium deficiency in China. BMC Infect. Dis. 2021;21:452. doi: 10.1186/s12879-021-06167-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Saad R., Taylor E.W., Rayman M.P. Selenium and selenoproteins in viral infection with potential relevance to COVID-19. Redox Biol. 2020;37:101715. doi: 10.1016/j.redox.2020.101715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Taylor E.W., Bennett K., Saad R., Rayman M.P. Association between regional selenium status and reported outcome of COVID-19 cases in China. Am. J. Clin. Nutr. 2020;111(6):1297–1299. doi: 10.1093/ajcn/nqaa095. [DOI] [PMC free article] [PubMed] [Google Scholar]