Highlights
-
•
Pandemic coronavirus disease 2019 (COVID-19) has led to a worldwide vaccination.
-
•
Post- vaccine antibody responsewas correlated with age.
-
•
The reduction of antibodies response was more evidentat 12 than 4 weeks compared to 9 months.
Keywords: COVID-19, Antibody responses, Vaccine
Abstract
We collected sequential serum samples (0, 4, 12 weeks, 9 months) for the determination of S-RDB IgG levels from 103 vaccinated healthy subjects (age 45 ± 13 years; 60 women), in order to evaluate neutralizing antibody response against SARS-CoV-2 in healthy healthcare workers (HCWs) after the administration of two doses of BNT162b2 SARS-CoV-2 mRNA vaccine. Every subject received two doses of mRNA vaccine BNT162b2 (Pfizer-BioNTech), 21 days apart (January-February 2021). Furthermore, antibody titer of 14 subjects who were hospitalized for symptomatic COVID-19 was evaluated. Antibody response was (median, interquartile range) 35 U/mL (10–104) at baseline, 1960 (1241–3221) at 4 weeks, 791 (388–1179) at 12 weeks and 524 (273–931) at 6 months. Antibody response was inversely correlated with age at all timepoints (p < 0.001) while gender and Body Mass Index had no significant effect. At multivariate analysis, post-baseline values were significantly higher than baseline (p < 0.001) with a reduction at 12 weeks and 9 months (p < 0.001). Antibody response of hospitalized subjects who did not receive vaccination, symptomatic for COVID 19 infection, was 103 (25–557) U/mL, significantly higher than baseline (p = 0.007) of study population but lower than all post-baseline determinations (p < 0.001). Younger subjects showed a stronger response and a lower decrease of antibody titers compared to the classes of older subjects. SARS-CoV2 infection was excluded by performing 1017 nasopharyngeal RT-PCR swabs on the study cohort. The second dose of mRNA vaccine resulted in an antibody response effective in preventing infection in a population of healthcare professionals. The antibody level was stable through week 12, showing a reduction in the following six months.
Introduction
In the last two years, the spread of pandemic SARS-CoV-2 resulted in a great scientific mobilization in order to find out therapies and vaccines effective in reducing the impact of the disease. SARS-CoV-2 infection results in the production of antibodies against the virus spike protein (S) and nucleoprotein (N) [1], [2], [3], [4]. Several papers have analyzed the decline in antibody titer following SARS-CoV2 infection [5]. Some have shown a decline of the antibody titer in four monthswhile others have demonstrated a permanence of seropositivity of up to nine months, albeit with a decreasing titre [6], [7]. Recent studies on patients and vaccinated subjects against COVID-19 show that the disease immunizations and the vaccinations reduced the rate of SARS-CoV-2 infections [8], [9], [10]. Although the persistence of the vaccine-induced neutralizing antibodies is not yet fully known, the neutralizing antibodies induced by the infection were detectable for at least six months after the onset of symptoms [11]. Antibodies production after vaccine administration was variable depending on the type of vaccine [12], [13], [14]. Currently, the European Medicines Agency (EMA) has authorized use of four vaccines: two mRNA vaccines (BNT162b2 and mRNA-1273) and two adenoviral vector-based vaccines (ChAdOx1-S and COVID-19 Janssen) [15], [16]. All of these vaccines aremeant to generate specific antibodies against the virus spike protein and all of them showed an efficacy to induce anti-S IgG antibodies with neutralizing activity against the first pandemic variant SARS-CoV-2 Wuhan Hu-1 and the other D614G variants currently in circulation. [17], [18], [19]. The aim of the study was to evaluate neutralizing antibody responses against SARS-CoV-2, during a nine-month follow-up, in healthy healthcare workers after the administration of double dose of BNT162b2 SARS-CoV-2 mRNA vaccine.
Materials and methods
Sequential serum samples (0, 4, 12 weeks, 9 months) for the determination of S-RDB IgG levels from 103 vaccinated health care workers (HCWs), healthy and not previously affected by Covid19 were collected. The whole study population was not affected from immunological diseases and consequently did not take any immunosuppressive drugs. Just a few of study subjects were suffering from arterial hypertension and were taking common antihypertensive drugs. All participants received two doses of messenger RNA vaccine BNT162b2 (Pfizer-BioNTech) 21 days apart, (on the period of January-February 2021). The vaccine consisted of a multidose vial to be diluted before use. Each vial (0.45 mL) contained 6 doses of 0.3 mL after dilution. Each dose (0.3 mL) contained 30 µg of COVID-19 mRNA vaccine (encapsulated in lipid nanoparticles). 5 'capped single-stranded messenger RNA (mRNA), produced by cell-free transcription in vitro (cell-free) from the corresponding template DNA, encoding the SARS-CoV- viral spike protein (S) 2. The vaccine was administered intramuscularly in the deltoid region of one the upper arms, after dilution as a cycle of 2 doses (0.3 mL each) at least 21 days apart. Needles between 2.5 and 3.8 cm (1–1 and 1/2 in.) in length were usedof 22–25 gauge in caliber. The cold chain was preserved by scheduling the daily doses based on the number of subjects previously booked. The first determination of the antibody titer was performed after the first vaccine dose (baseline), after 4, 12 weeks and nine months from the second dose. In addition, the antibody titer of 14 subjects who have recovered from symptomatic COVID-19 was evaluated. The sampling of this last group of subjects was performed approximately three months after recovery and healing confirmed by a double negative nasopharyngeal swab. All subjects provided written informed consent. The study complied with the principles of the Declaration of Helsinki and was approved by the local ethics committee (approval number 6726 of the AziendaUniversitariaOspedalieraConsorziale - Policlinico of Bari, Italy). In order to verify the effective protection against infection, during the entire observation period, all study participants were subjected to periodic screening with RT-PCR anti-SARS-CoV2 molecular swab. Nasopharyngeal swabs were performed approximately every 30 days, starting from the first week after the administration of the second dose, up to the week before the last determination (ninth month), or whenever symptoms such as fever, cough, marked asthenia or the suspicion of a contact with a positive subject were found. As a whole they have been carried out 1017 nasopharyngeal swabs, averaging about 10 swabs for each participant.
Biochemical Analysis. The serum anti S-RDB IgG levels were measured on fresh samples obtained after centrifugation for 10 min at 3500g at room temperature of whole blood collected in dry tubes. The measurement was performed by indirect chemiluminescence immunoassay on Cobas e 601 instrumentation, according to the manufacturer’s instructions.
The assay is an immunoassay for the in vitro quantitative determination of antibodies (including IgG) to the SARS-CoV-2 spike (S) protein receptor binding domain (RBD) in human serum and plasma. The assay uses a recombinant protein representing the RBD of the S antigen in a double-antigen sandwich assay format, which favours detection of high affinity antibodies against SARS CoV 2. The test is intended as an aid to assess the adaptive humoral immune response to the SARS CoV 2 S protein. The nasal/oropharyngeal swab was performed with the RT-PCR method.
Statistical analysis. Data are reported as mean ± standard deviation or median with interquartile range. Difference between groups were tested with the independent sample t-test. Categorical variables, compared between groups by using the chi-squared test, were reported as number and percentage. Due to the skewed distribution, Antibody Responses was analysed after a natural logarithm transformation. Difference in Antibody Responses between repeated measures were tested with the paired sample t-test. The Spearman's coefficient was used to estimate the correlation between age and Antibody Responses. A mixed-linear regression model, with patient considered as random effects (cluster of the three repeated observations), was used to evaluate the effect of gender, age and BMI on Antibody Responses. Intra-class correlation coefficient (ICC) was estimated to evaluate the proportion of Antibody Responses variation attributed to patient. A p value of 0.05 or less was considered statistically significant. All analyses were conducted using STATA software, version 16 (Stata-Corp LP, College Station, Tex).
Results
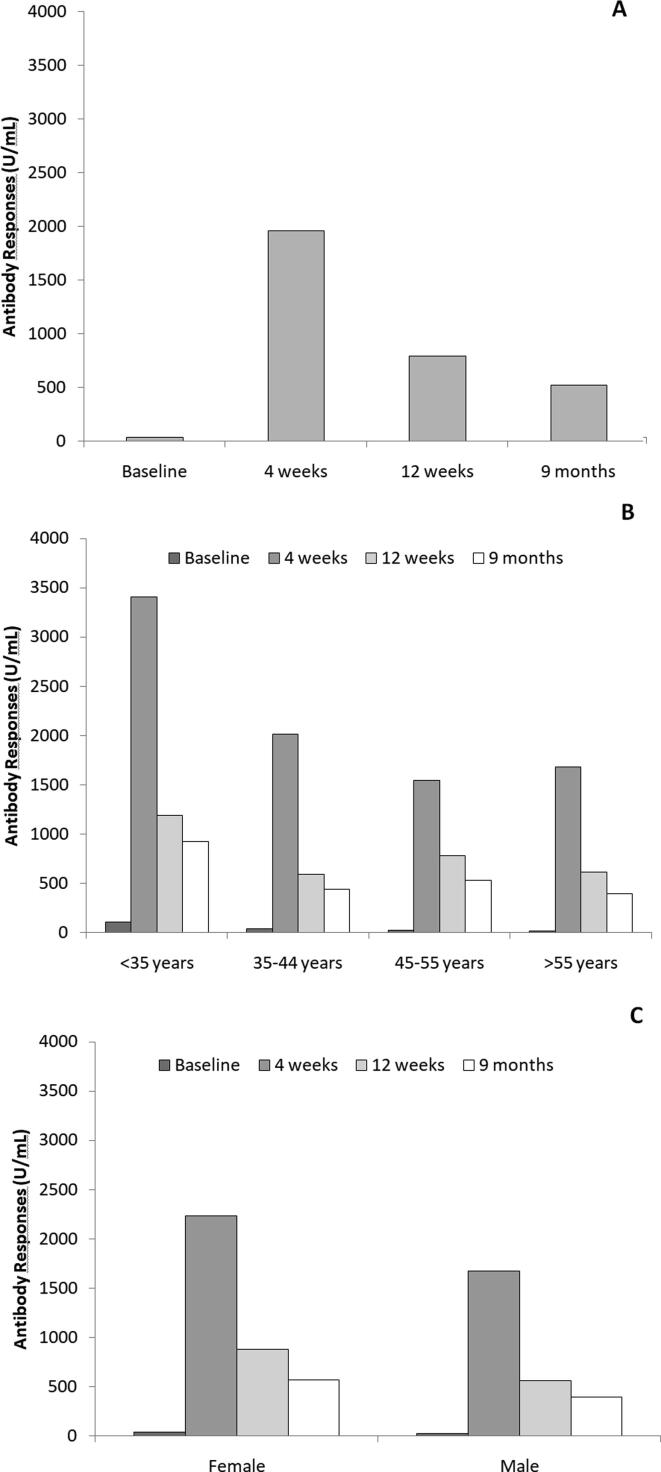
A total of 103 subjects was evaluated (age 45 ± 13 years), 60 (58%) females and 43 (42%) males. females were younger than males (40 ± 12 vs 51 ± 11 years; p < 0.001) and had a lower Body Mass Index (BMI; 23 ± 4 vs 26 ± 4 Kg/m2; p < 0.001). Fig. 1 shows median values of Antibody Responses (S-RDB IgG) at different timepoints in the overall population (panel A), by gender and age (respectively panel B and C). The determination at 4 weeks was the highest and significantly greater than the others (all p < 0.001). At 12 weeks and 6 months there was a values reduction but they stayed significantly higher than the baseline (both p < 0.001). Younger subjects and female subjects showed an higher Antibody response than older subjects and male subjects (Fig. 1, panel B and C). Table 1 reports values at each determination for the overall cohort as well as by gender and age groups.
Fig. 1.
Median of Antibody Responses (S-RDB IgG) in the overall cohort (panel A), by age (panel B) and gender (panel C).
Table 1.
Antibody Responses (S-RDB IgG) at baseline, 4 weeks, 12 weeks and 9 months.
| N | Baseline (U/mL) | 4 weeks (U/mL) | 12 weeks (U/mL) | 9 months (U/mL) | |
|---|---|---|---|---|---|
| Overall | 103 | 35 (10–104) | 1960 (1241–3221) | 791 (388–1179) | 524 (273–931) |
| Gender | |||||
| Women | 60 | 40 (17–114) | 2237 (1307–3806) | 879 (505–1322) | 573 (379–979) |
| Men | 43 | 22 (6–77) | 1672 (995–2599) | 560 (292–1045) | 398 (177–822) |
| Age (years) | |||||
| <35 | 27 | 105 (37–190) | 3408 (1883–5817) | 1188 (873–2423) | 921 (585–1872) |
| 35–44 | 27 | 35 (12–80) | 2014 (1242–2672) | 591 (374–880) | 442 (241–524) |
| 45–55 | 21 | 21 (7–55) | 1545 (991–2466) | 782 (376–1193) | 532 (236–931) |
| >55 | 28 | 18 (4–69) | 1681 (1178–2805) | 615 (252–971) | 394 (208–764) |
Median (interquartile range).
Antibody responses was inversely correlated with age at all timepoints (Spearman coefficient −0.41 at baseline, −0.35 at 4 and 12 weeks as well as at 9 months; all p < 0.001). These correlations were all confirmed in females (Spearman coefficient −0.43 at baseline, −0.44 at 4 weeks,-0.30 at 12 weeks and −0.39 at 9 months; respectively p < 0.001, p < 0.001, p = 0.029 and p = 0.005). In males, Antibody Responses inversely correlated with age only at baseline (Spearman coefficient −0.30; p = 0.047).
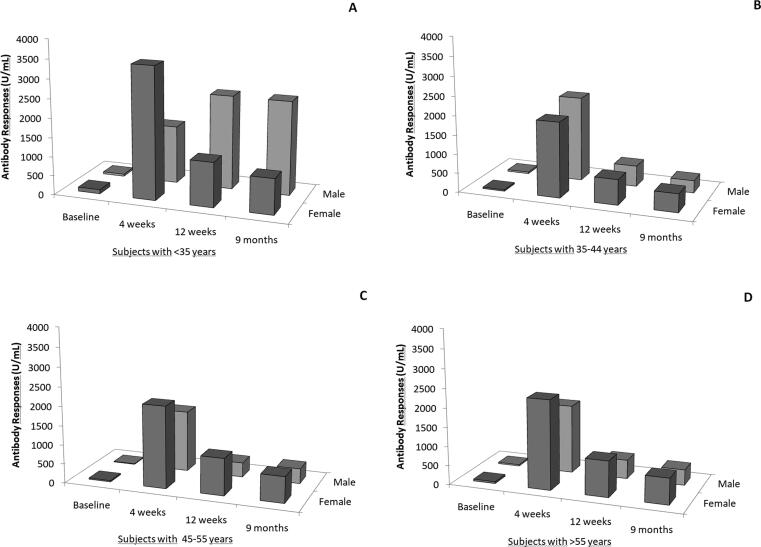
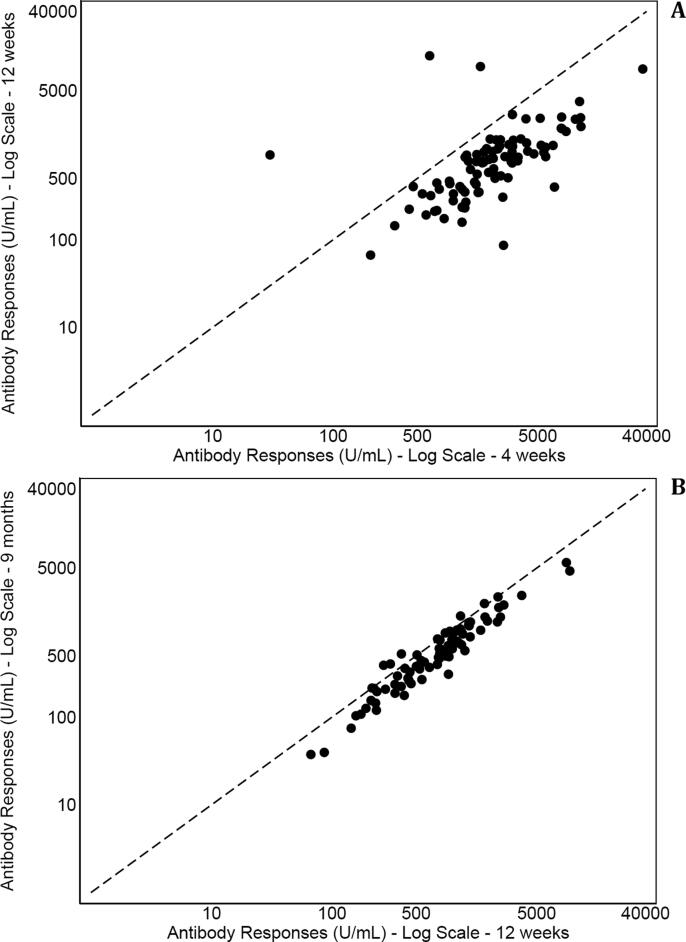
Fig. 2 depicts Antibody responses by gender in subjects with <35 years (panel A), 35–44 years (panel B), 45–55 (panel C) and more than 55 years (panel D). Within the same age group, differences between male and females were not statistically significant. At multivariate analysis, Antibody responses was inversely correlated with age (p < 0.001) with higher post-baseline values than baseline (all p < 0.001) and higher values at 4 weeks then thereafter (p < 0.001) while gender had no significant effect (p = 0.514). This model had an ICC of 0.50 indicating that 50% of total variation of Antibody responses was explained by the subject. When BMI was added to multivariate model, no significant association was found between body size and Antibody Responses (p = 0.215). Fig. 3 shows scatter plot of Antibody Responses at 4 weeks, 12 weeks and 9 months. Values were strongly correlated among consecutive measurements but the reduction of values was more marked at 12 than 4 weeks (Fig. 3, panel A) compared to 9 months (Fig. 3, panel B). The reduction at 12 weeks than 4 weeks was 1124 (663–2360) while at 9 months than 12 weeks was 181 (66–352).
Fig. 2.
Median of Antibody Responses (S-RDB IgG) by gender within age groups.
Fig. 3.
Scatter plot of Antibody Responses (S-RDB IgG) at 4 and 12 weeks (panel A) and at 12 weeks and 9 months (panel B).
Antibody responses of 14 non-vaccinated patients were compared to study population of vaccinated subjects. No difference in age was observed between patients (49 ± 9 years) and study subjects (p = 0.288). In patient’s population, 13 (93%) were males (p < 0.001) when compared to study population. Antibody responses of non-vaccinated patients was 103 (25–557) U/mL, significantly higher than baseline (p = 0.007) of study population but lower than the second and third determination (both p < 0.001). In, males that were prevalent in patients group, results were confirmed with an Antibody Responses of 114 (85–557) U/mL significantly higher than baseline (p < 0.001) of males of the study population and lower than post-baseline determinations (all p < 0.001). The execution of 1017 nasopharyngeal swabs during the observation period did not show positive cases on the whole cohort.
Discussion
Our data demonstrate that the antibody response after the administration of two doses of the BNT162b2 SARS-CoV-2 mRNA vaccine was obtained in 100% of the studied subjects. Gender does not influence extent of response. The antibody titre decreased during the observation period in the whole sample examined without any gender difference; even the BMI values did not change the trend of antibody values. Antibody Responses was inversely correlated with age at all timepoint. The subjects of the younger classes showed a stronger response and a lower decrease of the antibodies titers at the third determination compared to the older subjects. The analysis of antibody response after the first vaccine dose (and before the administration of the second one), 4, 12 weeks and nine months after the second dose showed that approximately 50% of those variations were explained by the subject and 50% by other factors. No difference emerges in the antibody response, at the first determination, from the comparison between subjects recovered from symptomatic COVID-19 (even if the sample was small) and vaccinated subjects, demonstrating a similar response in the production of antibodies to both the disease and the vaccine. The antibody titer obtained after SARS-Cov2 infection declines over a variable period of 4/9 months as has been shown by several studies. The immunity generated by the administration of the second dose of the vaccine BNT162b2 SARS-CoV2 mRNA is significantly reduced, in our study, from the ninth month, remaining significantly elevated at least until the 12th week [9], [10]. Our study sample, despite being a small one, showed a datum that has not yet completely been studied in literature [20], [21]; nine-month follow-up analysis showed a slight reduction of the antibody titer. The median at nine months, although the high individual variability, is constantly higher than the first determination (baseline), as an evidence of the permanence of the antibody titer, higher than the baseline, for at least nine months. Periodic nasopharyngeal swab did not reveal any SARS-CoV2 infection in the population tested during the observation period.
Conclusions
The aim of the study was to demonstrate the efficacy of a double-dose BNT162b2 SARS-CoV2 mRNA vaccine in producing an effective antibody response on a small sample of 103 healthy subjects, during a nine-month follow-up. Our data showed that this efficacy was confirmed by the antibody titer values (median 1690 U/mL) four weeks after administration of the second dose of vaccine. The antibody titer tends to slightly decrease over time, with a statistically significant difference between the young subjects and the older ones. Young subjects showed a stronger and more durable response. The antibody titer of the subjects hospitalized for symptomatic Covid-19 is higher, only at baseline, compared to the HCWs group. In multivariate analysis, BMI does not influence the antibody response. At present, our evidence suggests that an adequate, antibody titer, although slightly reduced, remains nine months after the administration of a complete vaccination course with BNT162b2 SARS-CoV2 mRNA vaccine. The lower responses observed in older subjects suggests an expression of an “aging” of the immune system [22]. The decline in antibody titer did not expose the subjects to infections. Further studies in order to verify and better define the antibodies protecting titers and the duration of protection against the disease in vaccinated subjects are necessary. It also appears necessary to define the need to provide for the administration of the third dose of the vaccine, and to strictly identify which are the subjects that appears the most vulnerable ones to a SARS-CoV2 infection, even after the appropriate vaccination cycle.
Funding
None.
CRediT authorship contribution statement
Franco Mastroianni: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Data curation, Writing – original draft, Writing – review & editing, Supervision. Pietro Guida: Conceptualization, Formal analysis, Data curation, Writing – review & editing, Supervision. Grazia Bellanova: Conceptualization, Methodology, Validation, Investigation, Data curation, Writing – original draft, Writing – review & editing. Edy Valentina De Nicolò: Conceptualization, Methodology, Validation, Investigation, Data curation, Writing – original draft, Writing – review & editing. Giulia Righetti: Conceptualization, Methodology, Validation, Investigation, Data curation, Writing – original draft, Writing – review & editing. Maurizio Formoso: Conceptualization, Methodology, Validation, Investigation, Data curation, Writing – original draft, Writing – review & editing. Fabrizio Celani: Conceptualization, Methodology, Validation, Investigation, Data curation, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Dong Y., Dai T., Wei Y., Zhang L., Zheng M., Zhou F. A systematic review of SARS-CoV-2 vaccine candidates. Signal Transduct Target Ther. 2020;5(1):237. doi: 10.1038/s41392-020-00352-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Okba N.M.A., Müller M.A., Li W., Wang C., GeurtsvanKessel C.H., Corman V.M., et al. Severe acute respiratory syndrome coronavirus 2-specific antibody responses in coronavirus disease patients. EmergInfectDis. 2020;26(7):1478–1488. doi: 10.3201/eid2607.200841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wajnberg A., Amanat F., Firpo A., Altman D.R., Bailey M.J., Mansour M., et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370(6521):1227–1230. doi: 10.1126/science.abd7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amanat F., Stadlbauer D., Strohmeier S., Nguyen T.H.O., Chromikova V., McMahon M., et al. A serologicalassay to detect SARS-CoV-2 seroconversion in humans. Nat Med. 2020;26(7):1033–1036. doi: 10.1038/s41591-020-0913-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fedele G, Stefanelli P, Bella A, Fiore S, Pancheri S, Benedetti E, Fabiani C, Leone P, Vacca P, Schiavoni I, Neri A, Carannante A, Simmaco M, Santino I, Zuccali MG, Bizzarri G, Magnoni R, Benetollo PP, Brusaferro S, Rezza G, Ferro A. Anti-SARS-CoV-2 antibodies persistence after natural infection: a repeated serosurvey in Northern Italy. Ann Ist Super Sanita. 2021 Oct-Dec;57(4):265-271. doi: 10.4415/ANN_21_04_01. PMID: 35076416. [DOI] [PubMed]
- 6.Varona JF, Madurga R, Peñalver F, Abarca E, Almirall C, Cruz M, Ramos E, Castellano-Vazquez JM. kinetics of anti-SARS-CoV-2 antibodies over time. Results of 10 month follow up in over 300 seropositive Health Care Workers. Eur J Intern Med. 2021 Jul;89:97-103. doi: 10.1016/j.ejim.2021.05.028. Epub 2021 May 25. PMID: 34090748; PMCID: PMC8148432. [DOI] [PMC free article] [PubMed]
- 7.Ortega N., Ribes M., Vidal M., Rubio R., Aguilar R., Williams S., et al. Seven-month kinetics of SARS-CoV-2 antibodies and role of pre-existing antibodies to human coronaviruses. Nat Commun. 2021;12(1):4740. doi: 10.1038/s41467-021-24979-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanrath A.T., Payne B.A.I., Duncan C.J.A. Prior SARS-CoV-2 infection is associated with protection against symptomatic reinfection. J Infect. 2021;82(4):e29–e30. doi: 10.1016/j.jinf.2020.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chodick G., Tene L., Patalon T., Gazit S., Ben Tov A., Cohen D., et al. Assessment of effectiveness of 1 dose of BNT162b2 vaccine for SARS-CoV-2 infection 13 to 24 days after immunization. JAMANetw Open. 2021;4(6) doi: 10.1001/jamanetworkopen.2021.15985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall VJ, Foulkes S, Charlett A, Atti A, Monk EJM, Simmons R, Wellington E, Cole MJ, Saei A, Oguti B, Munro K, Wallace S, Kirwan PD, Shrotri M, Vusirikala A, Rokadiya S, Kall M, Zambon M, Ramsay M, Brooks T, Brown CS, Chand MA, Hopkins S; SIREN Study Group. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: a large, multicentre, prospective cohort study (SIREN). Lancet. 2021 Apr 17;397(10283):1459-1469. doi: 10.1016/S0140-6736(21)00675-9. Epub 2021 Apr 9. Erratum in: Lancet. 2021;397(10286):1710. [DOI] [PMC free article] [PubMed]
- 11.Pradenas E., Trinité B., Urrea V., Marfil S., Ávila-Nieto C., Rodríguez de la Concepción M.L., et al. Stable neutralizing antibody levels 6 months after mild and severe COVID-19 episodes. Med (N Y) 2021;2(3):313–320.e4. doi: 10.1016/j.medj.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doria-Rose N, Suthar MS, Makowski M, O'Connell S, McDermott AB, Flach B, Ledgerwood JE, Mascola JR, Graham BS, Lin BC, O'Dell S, Schmidt SD, Widge AT, Edara VV, Anderson EJ, Lai L, Floyd K, Rouphael NG, Zarnitsyna V, Roberts PC, Makhene M, Buchanan W, Luke CJ, Beigel JH, Jackson LA, Neuzil KM, Bennett H, Leav B, Albert J, Kunwar P; mRNA-1273 Study Group. Antibody Persistence through 6 Months after the Second Dose of mRNA-1273 Vaccine for Covid-19. N Engl J Med. 2021;384(23):2259-2261. doi: 10.1056/NEJMc2103916. [DOI] [PMC free article] [PubMed]
- 13.Haveri A., Ekström N., Solastie A., Virta C., Österlund P., Isosaari E., et al. Persistence of neutralizing antibodies a year after SARS-CoV-2 infection in humans. Eur J Immunol. 2021 doi: 10.1002/eji.202149535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eyre D.W., Lumley S.F., Wei J., Cox S., James T., Justice A., et al. Quantitative SARS-CoV-2 anti-spike responses to Pfizer-BioNTech and Oxford-AstraZeneca vaccines by previous infection status. Clin Microbiol Infect. 2021;27(10):1516.e7–1516.e14. doi: 10.1016/j.cmi.2021.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.EMA. COVID-19 Vaccines: Authorised. Accessed 5th June 2021. https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/vaccines-covid-19/covid-19-vaccines-authorised#authorised-covid-19-vaccines-section.
- 16.Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, Bellamy D, Bibi S, Bittaye M, Clutterbuck EA, Dold C, Faust SN, Finn A, Flaxman AL, Hallis B, Heath P, Jenkin D, Lazarus R, Makinson R, Minassian AM, Pollock KM, Ramasamy M, Robinson H, Snape M, Tarrant R, Voysey M, Green C, Douglas AD, Hill AVS, Lambe T, Gilbert SC, Pollard AJ; Oxford COVID Vaccine Trial Group. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020 Aug 15;396(10249):467-478. doi: 10.1016/S0140-6736(20)31604-4. Epub 2020 Jul 20. Erratum in: Lancet. 2020 Aug 15;396(10249):466. Erratum in: Lancet. 2020;396(10266):1884.
- 17.Urbanowicz R.A., Tsoleridis T., Jackson H.J., Cusin L., Duncan J.D., Chappell J.G., et al. Two doses of the SARS-CoV-2 BNT162b2 vaccine enhance antibody responses to variants in individuals with prior SARS-CoV-2 infection. Sci Transl Med. 2021 doi: 10.1126/scitranslmed.abj0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walsh E.E., Frenck R.W., Jr, Falsey A.R., Kitchin N., Absalon J., Gurtman A., et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383(25):2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson E.J., Rouphael N.G., Widge A.T., Jackson L.A., Roberts P.C., Makhene M., et al. mRNA-1273 Study Group. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383(25):2427–2438. doi: 10.1056/NEJMoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshimura Y., Sasaki H., Miyata N., Miyazaki K., Tachikawa N. Antibody response after COVID-19 vaccine BNT162b2 on health care workers in Japan. J InfectChemother. 2021;27(12):1713–1715. doi: 10.1016/j.jiac.2021.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buonfrate D, Piubelli C, Gobbi F, Martini D, Bertoli G, Ursini T, Moro L, Ronzoni N, Angheben A, Rodari P, Cardellino C, Tamarozzi F, Tais S, Rizzi E, Degani M, Deiana M, Prato M, Silva R, Bisoffi Z. Antibody response induced by the BNT162b2 mRNA COVID-19 vaccine in a cohort of health-care workers, with or without prior SARS-CoV-2 infection: a prospective study. ClinMicrobiolInfect. 2021:S1198-743X(21)00416-X. doi: 10.1016/j.cmi.2021.07.024. [DOI] [PMC free article] [PubMed]
- 22.Pinti M., Appay V., Campisi J., Frasca D., Fülöp T., Sauce D., et al. Aging of the immune system: Focus on inflammation and vaccination. Eur J Immunol. 2016;46(10):2286–2301. doi: 10.1002/eji.201546178. [DOI] [PMC free article] [PubMed] [Google Scholar]