Abstract
Background
According to available research, there have been no head-to-head studies comparing the effect of glucagon-like peptide 1 (GLP-1) agonists and sodium-glucose cotransporter 2 (SGLT-2) inhibitors on cardiovascular outcomes among patients with type 2 diabetes not reaching glycemic goal with metformin.
Methods
Relevant studies were identified through electronic searches of PubMed and EMBASE published up to January 15, 2020. Efficacy outcomes of interest included the composite of cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke, its individual components, all-cause death, and hospitalization for heart failure (HF). Safety outcomes included all suggested side effects of both agents previously reported.
Results
Eleven studies, including 94,727 patients were used for the analysis. The risk of composite end point was significantly lower in both groups compared to the control group (hazard ratio [HR] 0.88, 95% confidence interval [CI] 0.85–0.92, p < 0.001). The risk of hospitalization for HF was significantly lower in both groups but the magnitude of the effect was more pronounced in the SGLT-2 inhibitors group (HR 0.68, 95% CI 0.60–0.76, p < 0.001) than the GLP-1 agonists group (HR 0.92, 95% CI 0.84–0.99, p = 0.03). Patients treated with GLP-1 agonists discontinued trial medications more frequently compared to conventionally treated patients because of serious side effects.
Conclusions
Both GLP-1 agonists and SGLT-2 inhibitors showed comparable cardiovascular outcomes in patients with type 2 diabetes. However, the SGLT-2 inhibitors were associated with more pronounced reduction of hospitalization for HF and lower risk of treatment discontinuation than GLP-1 agonists.
Keywords: diabetes mellitus, sodium-glucose transporter 2 inhibitors, glucagon-like peptide 1 receptor, cardiovascular disease
Introduction
Use of appropriate antidiabetic drugs has become an important issue in diabetic patients with atherosclerotic cardiovascular disease (CVD) and those with multiple risk factors [1, 2]. Although metformin is generally recommended and widely used as a first-line therapy due to its cardioprotective effects, selection of a subsequent antidiabetic agent among type 2 diabetic patients who failed to reach their glycemic goal has been debated [3, 4].
Several classes of antidiabetic agents have been effective in glycemic control when added to metformin and these include the incretin-based dipeptidyl peptidase 4 (DPP-4) inhibitors, glucagon-like peptide 1 (GLP-1) agonists [5], and sodium-glucose cotransporter 2 (SGLT-2) inhibitors. Current guidelines recommend addition of either SGLT-2 inhibitors or GLP-1 agonists in type 2 diabetes patients who failed to achieve their glycemic goal with metformin monotherapy or even as a first-line therapy for patients with atherosclerotic CVD [4, 6, 7].
Contrary to recent trials of DPP-4 inhibitors that did not show benefits or harms, several GLP-1 agonists and SGLT-2 inhibitors were effective in terms of cardiovascular outcomes [8–17]. Understanding cardiovascular outcomes of second-line antidiabetic agents in high-risk diabetic patients could help physicians to select treatment strategy after failure of metformin-based antidiabetic management. However, there were no head-to-head studies comparing the efficacy of both classes of antidiabetic agents. The purpose of this study was to investigate the effectiveness of GLP-1 agonists and SGLT-2 inhibitors and their safety profiles.
Methods
Data sources
Relevant studies were identified through electronic searches of PubMed and EMBASE published up to January 15, 2020. Medical subject headings and keyword searches included the terms ‘empagliflozin’, ‘canagliflozin’, ‘dapagliflozin’, ‘ertugliflozin’, ‘lixisenatide’, ‘exenatide’, ‘liraglutide’, ‘semaglutide’, ‘albiglutide’, ‘dulaglutide’, ‘heart infarction’, ‘myocardial infarction’, ‘cerebrovascular accident’, ‘stroke’, ‘death’, ‘major adverse cardiac event’, ‘mace’, ‘major adverse cardiovascular event’, ‘heart failure’, ‘controlled study’, ‘random’, and ‘placebo’. Reference lists of selected articles were systematically reviewed for other potentially relevant citations. No language restriction was enforced.
Study selection
Two investigators (S.-H.L. and J.-S.J.) independently conducted the literature search, data extraction, and quality assessment by using a standardized approach. Selected publications were reviewed by the same investigators to assess if studies met the inclusion criteria: (1) randomized allocation; (2) all participants with type 2 diabetes mellitus; (3) comparison of GLP-1 agonist or SGLT-2 inhibitor with a control group; (4) follow-up of more than 1 year.
Data extraction
Two reviewers (Y.-M.L. and J.-S.J.) extracted relevant information from the articles including study treatment, study period, patient characteristics (mean age, gender distribution, duration of diabetes, history of atherosclerotic CVD and heart failure [HF]), sample size, estimated glomerular filtration rates. Reviewers were not blinded to the articles, publication sites, and affiliation of authors.
End points
Efficacy end points of this study were the composite of cardiovascular death, non-fatal myocardial infarction (MI), or non-fatal stroke, its individual components, all-cause death, and hospitalization for HF. Safety end points of interest included pancreatitis, pancreatic cancer, retinopathy, genital and urinary tract infection, diabetic ketoacidosis, lower limb amputations, fractures, acute kidney injury, any malignancy, severe hypoglycemia, and discontinuation of study medications.
Data synthesis and analysis
Hazard ratios (HRs) were pooled with 95% confidence intervals (CIs) for the effect of randomizing treatment allocation on the outcomes across trials and the adjusted risk estimates were pooled after logarithmic transformation according to fixed-effects models with the generic inverse variance method. For safety outcomes, random-effects models producing across-study summary risk ratios (RRs) with 95% CIs were used. All p values were 2-tailed, with statistical significance set at 0.05. Included studies were well performed and the Cochrane tool for the assessment of risk bias in randomized clinical trials revealed low risk of bias in all studies [18]. Statistical heterogeneity between trials was assessed with I2 statistic, which is derived from the Cochran’s Q and the degree of freedom [100 × (Q − df)/Q]] [19]. I2 values lesser than 25%, greater than 25%, 50%, and 75% were considered as evidence of no, low, moderate, and severe statistical heterogeneity, respectively. If significant heterogeneity was noted across the studies, we then performed sensitivity analyses, serially excluding studies to determine the source of heterogeneity. Additionally, sensitivity analysis based on the different backbone across GLP-1 agonist studies were conducted to examine the heterogeneity between exendin-4 analogues and human GLP-1 analogues. Publication bias was examined by visual inspection of constructed funnel plots. All statistical analyses were performed using the Review Manager version 5.2 (The Nordic Cochrane Center, Copenhagen, Denmark). Meta-analysis was performed according the statement of Preferred Reporting Items for Systematic reviews and meta-analysis [20].
Results
The search strategy identified 309 potential articles, of which 32 were read in full text and 11 clinical studies were included into the final analysis. Among them, 7 studies were phase 3, double-blind, placebo-controlled trials comparing GLP-1 agonists and standard treatment [9–11, 13, 21–23], while 4 trials were phase 3, double-blind, placebo-controlled trials comparing SGLT-2 inhibitors and control group [12, 17, 24, 25]. Table 1 summarizes characteristics of the included studies. Of the 94,727 patients, 27,977 patients received GLP-1 agonists, 21,266 patients received SGLT-2 inhibitors and 45,484 patients were managed with conventional treatment. To compare different studies, regimen of study treatment, duration of diabetes, glycated hemoglobin level, proportion of patients with atherosclerotic CVD and HF, and glomerular filtration rates were extracted (Table 1). Of the 7 GLP-1 agonists studies, only one of the latest studies [23] used oral regimen, instead of subcutaneous injection. Human GLP-1 analogues were used in 5 GLP-1 agonist studies [10, 11, 21–23] while exendin-4 analogues were used in 2 studies [9, 13]. Primary end point of the included studies was composite of cardiovascular mortality, MI, or non-fatal stroke except for one study [24] reporting composite of renal outcomes and mortality.
Table 1.
Characteristics of included studies.
| Study | Study treatment | Year | Randomization period | Patients | Median follow-up [years] | Age [years] | Women (%) | Diabetes duration [years] | HbA1c [%] | ASCVD [%] | Heart failure [%] | eGFR [mL/min/1.73 m2] | eGFR < 60 mL/min/1.73 m2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GLP-1 agonists | |||||||||||||
| ELIXA | Lixisenatide (up to 20 μg sc daily) | 2015 | 2010–2013 | 3034/3034 | 2.1 | 60/61 | 30/31 | 9.3 / 9.5 | 7.7/7.6 | 100/100 | 23/22 | 77/75 | 22/25 |
| LEADER | Liraglutide (up to 1.8 mg sc daily) | 2016 | 2010–2012 | 4668/4672 | 3.5 | 64/64 | 35/36 | 13/13 | 8.7/8.7 | 82/81 | 18/18 | – | 24/22 |
| SUSTAIN-6 | Semaglutide (0.5 mg/1.0 mg sc weekly) | 2016 | 2013 | 1648/1649 | 2.1 | 65/65 | 39/40 | 14/14 | 8.7/8.7 | 60/61 | 23/24 | – | 28/29 |
| EXSCEL | Extended–release exenatide (2 mg sc weekly) | 2017 | 2010–2015 | 7356/7396 | 3.2 | 62/62 | 38/38 | 12/12 | 8.0/0.0 | 73/73 | 16/17 | 77/76 | 21/22 |
| Harmony Outcomes | Albiglutide (30–50 mg sc weekly) | 2018 | 2015–2016 | 4731/4732 | 1.6 | 64/64 | 30/31 | 14/14 | 8.8/8.7 | – | 20/20 | 79/79 | 11/11 |
| REWIND | Dulaglutide (1.5 mg sc weekly) | 2019 | 2011–2013 | 4949/4952 | 5.4 | 66/66 | 47/46 | 11/11 | 7.3/7.4 | 32/31 | 9/9 | 75/75 | 22/23 |
| PIONEER 6 | Semaglutide (14 mg po daily) | 2019 | 2017 | 1591/1592 | 1.3 | 66/66 | 32/31 | 15/15 | 8.2/8.2 | 85/85 | 12/13 | 74/74 | 27/27 |
| SGLT-2 inhibitors | |||||||||||||
| EMPA-REG OUTCOME | Empagliflozin (10 mg/25 mg po daily) | 2015 | 2010–2013 | 4687/2333 | 3.1 | 63/63 | 29/28 | – | 8.1/8.1 | 100/100 | 10/11 | 74/74 | 26/26 |
| CANVAS Program | Canagliflozin (100–300 mg po daily) | 2017 | 2009–2017 | 5795/4347 | 2.4 | 63/63 | 35/37 | 14/14 | 8.2/8.2 | 71/74 | 14/15 | 77/76 | – |
| DECLARE-TIMI 58 | Dapagliflozin (10 mg po daily) | 2018 | 2013–2015 | 8582/8578 | 4.2 | 64/64 | 37/38 | 11/10 | 8.3/8.3 | 41/41 | 10/10 | 85/85 | 7/8 |
| CREDENCE | Canagliflozin (100 mg po daily) | 2019 | 2014–2017 | 2202/2199 | 2.6 | 63/63 | 35/33 | 16/16 | 8.3/8.3 | 51/50 | 15/15 | 56/56 | 59/59 |
Data are presented as second line agents/control. HbA1c — glycated hemoglobin; ASCVD — atherosclerotic cardiovascular disease; eGFR — estimated glomerular filtration rate; GLP-1 — glucagon-like peptide 1; ELIXA — evaluation of lixisenatide in acute coronary syndrome; LEADER — liraglutide effect and action in diabetes: evaluation of cardiovascular outcome results; SUSTAIN-6 — trial to evaluate cardiovascular and other long-term outcomes with semaglutide in subjects with type 2 diabetes; EXSCEL — exenatide study of cardiovascular event lowering study group; REWIND — researching cardiovascular events with a weekly incretin in diabetes; SGLT-2 — sodium glucose cotransporter 2; CANVAS — canagliflozin cardiovascular assessment study; DECLARE-TIMI 58 — dapagliflozin effect on cardiovascular events–thrombolysis in myocardial infarction 58; CREDENCE — canagliflozin and renal events in diabetes with established nephropathy clinical evaluation
Efficacy outcomes
Composite of cardiovascular death, non-fatal MI, or non-fatal stroke
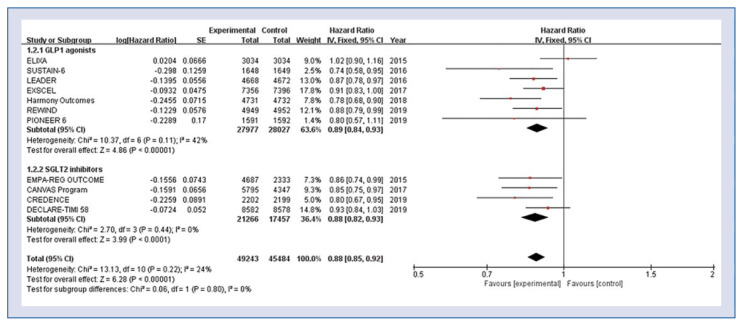
Eleven studies including 94,727 patients were used for the analysis of composite end point. The risk for the composite end point of cardiovascular death, non-fatal MI, or non-fatal stroke was significantly lower in both GLP-1 agonists group and SGLT-2 inhibitors group compared to the control group (HR 0.88, 95% CI 0.85–0.92, p < 0.001, Fig. 1). There was evidence of low statistical heterogeneity among the included studies (heterogeneity χ2 = 13.13, I2 = 24%, p = 0.22).
Figure 1.
Hazard ratios for composite of cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke stratified by classes of anti-diabetic agent; CI — confidence interval.
Mortality, non-fatal MI, non-fatal stroke
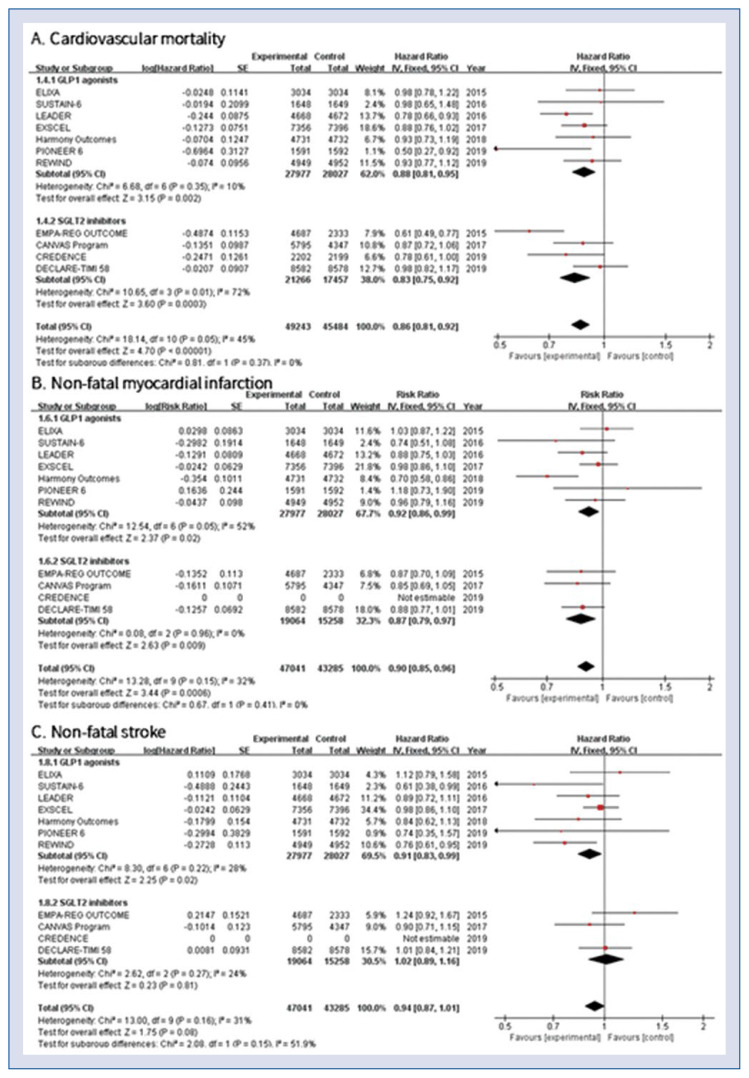
Pooled effects of cardiovascular mortality showed significantly lower rates in the GLP-1 agonists and SGLT-2 inhibitors group compared to conventional treatment (HR 0.86, 95% CI 0.81–0.92, p < 0.001, Fig. 2A). Low statistical heterogeneity was found among the included studies (heterogeneity χ2 = 18.14, I2 = 45%, p = 0.05). All-cause death was also significantly lower in patients treated with both GLP-1 agonists and SGLT-2 inhibitors compared with the control group (HR 0.87, 95% CI 0.83–0.91, p < 0.001). Both GLP-1 agonists and SGLT-2 inhibitors were associated with significantly lower rates of non-fatal MI (HR 0.90, 95% CI 0.85–0.96, p < 0.001, Fig. 2B). Risk of non-fatal stroke was significantly lower with the use of GLP-1 agonists (HR 0.91, 95% CI 0.83–0.99, p = 0.02) but not with the SGLT-2 inhibitors (HR 1.02, 95% CI 0.89–1.16, p = 0.81, Fig. 2C).
Figure 2.
Hazard ratios stratified by classes of anti-diabetic agent; A. Cardiovascular mortality; B. Non-fatal myocardial infarction; C. Non-fatal stroke; CI — confidence interval.
Hospitalization for heart failure
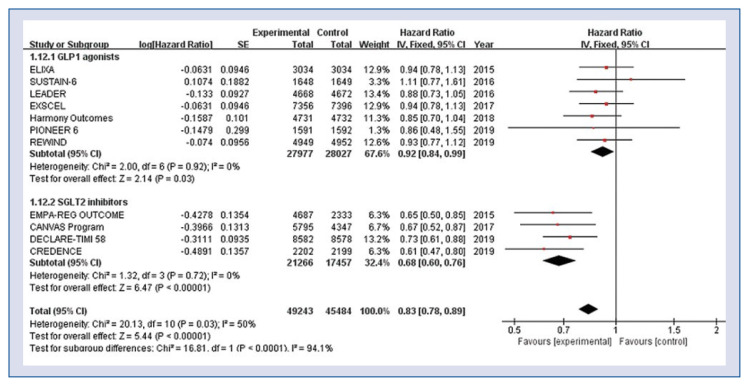
There was a substantial disparity between GLP-1 agonists and SGLT-2 inhibitors in the risk of hospitalization for HF. The risk of hospitalization for HF was significantly lower in both GLP-1 agonists group and SGLT-2 inhibitors group as compared to the control group (HR 0.83, 95% CI 0.78–0.89, p < 0.001, Fig. 3), but the magnitude of effect was more pronounced in the SGLT-2 inhibitors group (HR 0.68, 95% CI 0.60–0.76, p < 0.001) compared with the GLP-1 agonists group (HR 0.92, 95% CI 0.84–0.99, p = 0.03). There was no evidence of statistical heterogeneity among the included studies (GLP-1 agonists; heterogeneity χ2 = 2.00, I2 = 0%, p = 0.92, SGLT-2 inhibitors; heterogeneity χ2 = 1.32, I2 = 0%, p = 0.72).
Figure 3.
Hazard ratios for hospitalization for heart failure stratified by classes of anti-diabetic agent; CI — confidence interval.
Safety outcomes
In the analysis of safety outcomes, use of GLP-1 agonists was associated with a significantly increased risk of gastrointestinal events (RR 1.47, 95% CI 1.06–2.02, p = 0.02), but did not influence the rates of pancreatitis (RR 0.72, 95% CI 0.37–1.43, p = 0.35), pancreatic cancer (RR 1.17, 95% CI 0.74–1.85, p = 0.51) and retinopathy (RR 1.07, 95% CI 0.88–1.29, p = 0.50) (Suppl. Fig. S1). Patients treated with SGLT-2 inhibitors showed a significantly increased risk of genital infection compared with the patients on conventional treatment (RR 4.50, 95% CI 3.32–6.10, p < 0.001), but showed a similar risk of urinary tract infection (RR 1.03, 95% CI 0.96–1.10, p = 0.38). Use of SGLT-2 inhibitors tended to increase the risk of amputation (RR 1.31, 95% CI 1.00–1.70, p = 0.05), but the exclusion of studies with canagliflozin demonstrated a similar risk of amputation between the canagliflozin and the conventional treatment group (RR 1.08, 95% CI 0.90–1.28, p = 0.41) (Suppl. Fig. S2). The SGLT-2 inhibitors decreased the risk of acute kidney injury compared with the conventional treatment (RR 0.75, 95% CI 0.62–0.89, p = 0.001).
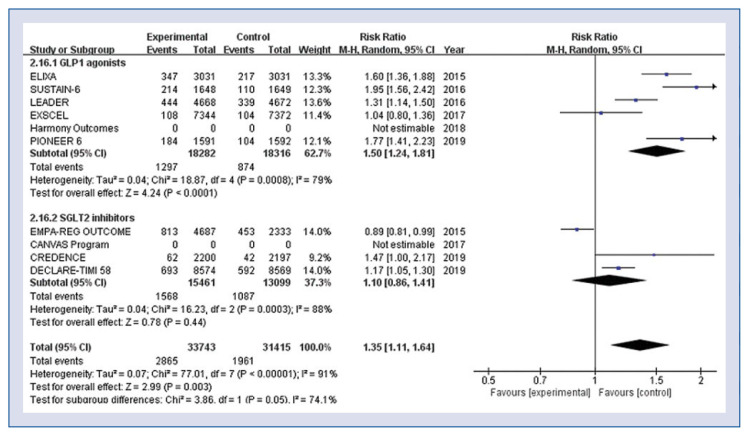
Patients treated with GLP-1 agonists discontinued trial medication more frequently compared to conventionally treated patients because of serious side effects (RR 1.50, 95% CI 1.24–1.81, p < 0.001, Fig. 4), but SGLT-2 inhibitors did not increase the rates of withdrawal (RR 1.10, 95% CI 0.86–1.41, p = 0.44, Fig. 4).
Figure 4.
Hazard ratios for adverse events leading to discontinuation of study medication stratified by classes of antidiabetic agent; CI — confidence interval.
Sensitivity analysis of different GLP-1 agonist studies
Stratified analysis according to the different GLP-1 agonists demonstrated significantly lower rates of the composite end point of cardiovascular death, non-fatal MI, or non-fatal stroke compared to the control group in the studies of human GLP-1 analogues (HR 0.84, 95% CI 0.79–0.90, p < 0.001), but not in studies using exendin-4 analogues (HR 0.95, 95% CI 0.88–1.02, p = 0.16, Suppl. Fig. S3). Human GLP-1 analogue was associated with significantly lower rates of non-fatal MI (HR 0.85, 95% CI 0.77–0.94, p = 0.001) and non-fatal stroke (HR 0.80, 95% CI 0.70–0.91, p < 0.001) than conventional regimen, whereas no significant differences were found in exendin-4 analogues group. Moreover, the risk of hospitalization for HF was significantly lower in the human GLP-1 analogues group as compared to the control group (HR 0.90, 95% CI 0.81–1.00, p = 0.005), but not in the exendin-4 analogues group (HR 0.94, 95% CI 0.82–1.07, p = 0.35).
Publication bias
Assessment of publication bias using RR of composite end point of cardiovascular death, non-fatal MI, or non-fatal stroke of the included studies showed a symmetric funnel plot with little evidence of publication bias.
Discussion
In this systematic review and meta-analysis of the 11 trials enrolling 94,727 patients with type 2 diabetes, both GLP-1 agonists and SGLT-2 inhibitors showed comparable efficacy in the reduction of composite end point of cardiovascular death, non-fatal MI, or non-fatal stroke as compared with the conventional antidiabetic treatment. There was also a comparably significant reduction in the respective risk of all-cause death, cardiovascular death and non-fatal MI in both groups. Also found was significantly lower risk of hospitalization for HF in both classes of experimental medications, especially more pronounced effect with SGLT-2 inhibitors than GLP-1 agonists. Regarding safety outcomes, it was found that the GLP-1 agonists did not increase risk of pancreatitis, pancreatic cancer, or retinopathy. The SGLT-2 inhibitors showed a tendency toward increased risk of genital infection, but did not increase urinary tract infection compared with control group. Risk of non-fatal stroke was significantly lower with the use of GLP-1 agonists, but not with the use of SGLT-2 inhibitors. However, discontinuation of trial medication due to serious side effects was more frequently observed in patients receiving GLP-1 agonists treatment.
There have been no randomized clinical trials that directly compared the efficacy of GLP-1 agonists and SGLT-2 inhibitors to improve cardiovascular outcomes. Despite limitations of observational studies, the CVD-REAL study demonstrated that use of SGLT-2 inhibitors lowered all-cause mortality compared with other medications [26, 27]. In the EMPA-REG OUTCOME (Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes) trial, which is a randomized, double-blind, placebo-controlled trial of 7,020 patients with type 2 diabetes, composite risk of MI, stroke, and cardiovascular death was significantly reduced with empagliflozin (HR 0.86, 95% CI 0.74–0.99) over a median follow-up of 3.1 years [17]. Cardiovascular death (HR 0.62, 95% CI 0.49–0.77) and all-cause death were reduced in a similar magnitude (HR 0.68, 95% CI 0.57–0.82) and there was also significant reduction in hospitalization for HF in the empagliflozin group (HR 0.65, 95% CI 0.50–0.85). However, a reduction in hospitalizations for HF and cardiovascular death in the empagliflozin group was observed consistently across patients who have HF or did not have HF at baseline [28].
The mechanism of beneficial effects for HF of SGLT-2 inhibitors has not been definitely defined [29]. Recent studies of SGLT-2 inhibitors for prevention of cardiovascular and renal outcomes suggest that the renoprotective effects related with the natriuresis comprise a part of the reasons for the improvement in hospitalization for HF [30, 31]. The natriuresis induced by SGLT-2 inhibition is a stimulating factor for tubuloglomerular feedback, which, in turn causes afferent renal arteriolar vasoconstriction. After the vasoconstriction of afferent arterioles of the kidney, resultant intraglomerular pressure reduction is caused and reduced intraglomerular pressure provides a renoprotective effect [32]. Furthermore, renoprotective effects and natriuresis are especially beneficial in patients with impaired renal function at baseline who have substantial risk for hospitalization for HF [29]. A 32% reduction in the risk of hospitalization for HF and a 25% reduction in the rates of acute kidney injury in the present study might explain the reduced risk of cardiovascular death. Ongoing trials are assessing cardioprotective effects of SGLT-2 inhibitors on hospitalization for HF in non-diabetic patients with HF as well as diabetic patients with HF [33].
In the present study, the SGLT-2 inhibitors were relatively well tolerated with lower incidence of serious adverse events compared with the GLP-1 agonists that were associated with higher risk of adverse events leading to withdrawal of the study drug. As previously reported [34], gastrointestinal side effects were reported as the main side effect by GLP-1 agonists leading to participant withdrawal of study drug in our analysis. We think that SGLT-2 inhibitors have an advantage over GLP-1 agonists in terms of lower rates of treatment discontinuation and this might help physicians to make a better treatment plan for diabetic patients who have failed to achieve their glycemic target with metformin monotherapy.
Two studies with exendin-4 analogues (exenatide and lixisenatide) did not reveal superiority over control regimen with respect to clinical outcomes [9, 13]. In our analysis, human GLP-1 analogues showed a greater effect on the composite end point of cardiovascular death, non-fatal MI, or non-fatal stroke compared to the control group but exendin-4 analogues did not. Differences in structure of GLP-1 agonists and subsequent different immunogenicity might be responsible for the better clinical outcomes in studies using human GLP-1 analogues than exendin-4 analogues [35]. Further studies are needed to elucidate how different molecular structure could affect diverse cardiovascular outcomes [36].
Limitations of the study
There are several limitations in this study. First, aggregated study-level data for meta-analysis was used instead of patient-level data. Therefore, a further subgroup-level study and quantified cumulative follow-up time for end point events and safety events could not be investigated. Second, all of the trial did not use exactly the same definition of events, inclusion and exclusion criteria. But most of the definitions of events are very similar to each other, there would not have been a significant difference in event discrimination and there was no significant statistical heterogeneity among the included studies for analyses. Third, most of the included studies showed median follow-up duration shorter than 4 years. Because risk factors for CVD is chronic diseases and some of side effects may occur later over time, there is a strong need for long-term follow-up. Fourth, this study lacks evidence for diabetic patients with low cardiovascular risk. Most of the included study targeted diabetic patients with established CVD or high-risk patients. Until now there is no definite treatment option about second-line antidiabetic agents improving cardiovascular outcomes for diabetic patients with low cardiovascular risk. Finally, this study is not a direct head-to-head study but compared SGLT-2 inhibitors with GLP-1 agonists indirectly. Despite being an indirectly comparative study, consistent results of efficacy and safety outcomes were found across most of the included studies. Furthermore, the results of this study add to a growing body of evidence in the literature aggregating individual studies that compared SGLT-2 inhibitors and GLP-1 agonist as second-line antidiabetic agents with conventional therapy.
Conclusions
Both GLP-1 agonists and SGLT-2 inhibitors showed comparable efficacy in reducing composite cardiovascular outcomes, mortality, and MI as compared to conventional antidiabetic medications in patients with type 2 diabetes. Safety analyses of the included studies revealed increased risk of genital infections by SGLT-2 inhibitors, and use of GLP-1 agonists were associated with a higher risk of adverse events leading to medication withdrawal.
Supplementary Information
Funding Statement
This work was supported by 2019 Inje University Busan Paik Hospital research grant.
Footnotes
Conflict of interest: None declared
Funding
This work was supported by 2019 Inje University Busan Paik Hospital research grant.
References
- 1. Campbell PT, Newton CC, Patel AV, et al. Diabetes and cause-specific mortality in a prospective cohort of one million U.S. adults. Diabetes Care. 2012;35(9):1835–1844. doi: 10.2337/dc12-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American Diabetes Association. 8. Pharmacologic Approaches to Glycemic Treatment. Diabetes Care. 2018;41(Suppl 1):S73–S85. doi: 10.2337/dc18-S008. [DOI] [PubMed] [Google Scholar]
- 3. Nesti L, Natali A. Metformin effects on the heart and the cardiovascular system: A review of experimental and clinical data. Nutr Metab Cardiovasc Dis. 2017;27(8):657–669. doi: 10.1016/j.numecd.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 4. Garber AJ, Abrahamson MJ, Barzilay JI, et al. American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm — 2018. Executive Summary Endocr Pract. 2018;24(1):91–120. doi: 10.4158/CS-2017-0153. [DOI] [PubMed] [Google Scholar]
- 5. Liu J, Li L, Deng Ke, et al. Incretin based treatments and mortality in patients with type 2 diabetes: systematic review and meta-analysis. BMJ. 2017;357:j2499. doi: 10.1136/bmj.j2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. American Diabetes Association. 9. Pharmacologic Approaches to Glycemic Treatment. Diabetes Care. 2019;42(Suppl 1):S90–S102. doi: 10.2337/dc19-S009. [DOI] [PubMed] [Google Scholar]
- 7. Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41(2):255–323. doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 8. Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11–22. doi: 10.1056/NEJMoa1411892. [DOI] [PubMed] [Google Scholar]
- 9. Holman RR, Bethel MA, Mentz RJ, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377(13):1228–1239. doi: 10.1056/NEJMoa1612917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 11. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2016;375(4):311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 13. Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373(23):2247–2257. doi: 10.1056/NEJMoa1509225. [DOI] [PubMed] [Google Scholar]
- 14. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369(14):1317–1326. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 15. White W, Kupfer S, Zannad F, et al. Cardiovascular mortality in patients with type 2 diabetes and recent acute coronary syndromes from the EXAMINE trial. Diabetes Care. 2016;39(7):1267–1273. doi: 10.2337/dc16-0303.. [DOI] [PubMed] [Google Scholar]
- 16. Zheng SL, Roddick AJ, Aghar-Jaffar R, et al. Association between use of sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 agonists, and dipeptidyl peptidase 4 inhibitors with all-cause mortality in patients with type 2 diabetes: a systematic review and meta-analysis. JAMA. 2018;319(15):1580–1591. doi: 10.1001/jama.2018.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 18. Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394(10193)(19):121–130. 31149. doi: 10.1016/S0140-6736. [DOI] [PubMed] [Google Scholar]
- 22. Hernandez AF, Green JB, Janmohamed S, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392(10157):1519–29. doi: 10.1016/S0140-6736(18)32261-X. [DOI] [PubMed] [Google Scholar]
- 23. Husain M, Birkenfeld AL, Donsmark M, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381(9):841–851. doi: 10.1056/NEJMoa1901118. [DOI] [PubMed] [Google Scholar]
- 24. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 25. Wiviott S, Raz I, Bonaca M, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–357. doi: 10.1056/nejmoa1812389.. [DOI] [PubMed] [Google Scholar]
- 26. Birkeland KI, Jørgensen ME, Carstensen B, et al. Cardiovascular mortality and morbidity in patients with type 2 diabetes following initiation of sodium-glucose co-transporter-2 inhibitors versus other glucose-lowering drugs (CVD-REAL Nordic): a multinational observational analysis. Lancet Diabetes Endocrinol. 2017;5(9)(17):709–717. 30258. doi: 10.1016/S2213-8587. [DOI] [PubMed] [Google Scholar]
- 27. Kosiborod M, Cavender MA, Fu AZ, et al. Lower risk of heart failure and death in patients initiated on sodium-glucose cotransporter- 2 inhibitors versus other glucose-lowering drugs: the CVD-REAL study (comparative effectiveness of cardiovascular outcomes in new users of sodium-glucose cotransporter-2 inhibitors) Circulation. 2017;136(3):249–259. doi: 10.1161/CIRCULATIONAHA.117.029190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fitchett D, Butler J, van de Borne P, et al. Effects of empagliflozin on risk for cardiovascular death and heart failure hospitalization across the spectrum of heart failure risk in the EMPA-REG OUTCOME® trial. Eur Heart J. 2018;39(5):363–370. doi: 10.1093/eurheartj/ehx511. [DOI] [PubMed] [Google Scholar]
- 29. Zelniker TA, Braunwald E. Cardiac and renal effects of sodium-glucose co-transporter 2 inhibitors in diabetes: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72(15):1845–1855. doi: 10.1016/j.jacc.2018.06.040. [DOI] [PubMed] [Google Scholar]
- 30. Heerspink HJL, Perkins BA, Fitchett DH, et al. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016;134(10):752–772. doi: 10.1161/CIRCULATIONAHA.116.021887. [DOI] [PubMed] [Google Scholar]
- 31. Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393(10166):31–39. doi: 10.1016/S0140-6736(18)32590-X. [DOI] [PubMed] [Google Scholar]
- 32. Verma S, McMurray JJV. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia. 2018;61(10):2108–2117. doi: 10.1007/s00125-018-4670-7. [DOI] [PubMed] [Google Scholar]
- 33. Butler J, Hamo CE, Filippatos G, et al. The potential role and rationale for treatment of heart failure with sodium-glucose cotransporter 2 inhibitors. Eur J Heart Fail. 2017;19(11):1390–1400. doi: 10.1002/ejhf.933. [DOI] [PubMed] [Google Scholar]
- 34. Shyangdan DS, Royle P, Clar C, et al. Glucagon-like peptide analogues for type 2 diabetes mellitus: systematic review and meta-analysis. BMC Endocr Disord. 2010;10(10):20. doi: 10.1186/1472-6823-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Madsbad S. Review of head-to-head comparisons of glucagon-like peptide-1 receptor agonists. Diabetes Obes Metab. 2016;18(4):317–332. doi: 10.1111/dom.12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Uccellatore A, Genovese S, Dicembrini I, et al. Comparison review of short-acting and long-acting glucagon-like peptide-1 receptor agonists. Diabetes Ther. 2015;6(3):239–256. doi: 10.1007/s13300-015-0127-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.