Abstract
According to the US Center for Disease Control, cancer deaths are the second most common cause of mortality in both adults and children. Definitive treatment of solid tumors involves surgical resection with or without systemic chemotherapy and radiation. The advent of local drug delivery presents a unique treatment modality that can offer substantial benefits in cancer management. Local drug delivery offers targeted drug delivery to cancer tissues while minimizing side effects of the medications. Three main phases in solid tumor management exist for the treating physician: initial diagnosis with tissue biopsy, surgical resection with or without chemotherapy, and management of metastatic disease. Image guided studies, using modalities such as MRI, computerized tomography, and ultrasound to sample tumors have been described. The initial diagnosis phase offers a treatment window for local drug delivery with the aid of image guidance. After the diagnosis of malignancy is made, surgical resection can become an important part of tumor management. Currently, FDA approved local drug delivery systems are being used in concert with resection for intracranial glioma. Many other applications of implantation of local drug delivery at the time of surgery in other tumors, including breast and neuroblastoma, are being investigated. Finally, for patients who present with or progress to single sites of metastatic disease, such as brain or liver metastasis, studies have shown potential applications for local drug delivery as well. This review will discuss the current state of local drug delivery in the treatment of solid tumors and possible future directions.
Keywords: Local drug delivery, solid tumor, resection, metastasis
INTRODUCTION:
In the year 2015, it is estimated that 1,658,370 people will be diagnosed with cancer, and 589,430 deaths in the US alone (American cancer society). Types of solid tumors are numerous and affect both the pediatric as well as adult populations. In adults, prostate cancer is the most commonly diagnosed tumor in men, with a rate of 1.4 million worldwide, and has the second highest cancer death rate behind lung cancer.[1] Breast cancer accounts for 1.8 million-cancer diagnosis annually in women.[1] In the pediatric population, neuroblastoma, Wilm’s tumor, and osteosarcoma represent some of the more common solid tumors, and account for up to 30% of all pediatric cancer related deaths.
Current management of solid tumors largely depends on tumor stage, and can range from surgical resection alone, to combination surgery with chemotherapy or radiation treatment, or radiation and chemotherapy alone. [2–4] The use of systemic chemotherapy, however, is not without side effects. Side effects can range from mild nausea to cardiotoxicity, nephrotoxicity and the risk of secondary malignancy. [5] Due to these complications, many groups have investigated outcomes with lower dosages, with the findings that some decrease in dosage can result in equivalent outcomes. [6] An additional problem that is specific to solid tumors is the ability of chemotherapy to completely penetrate the tumor. [7] Multiple studies have demonstrated that penetration of solid tumors is reduced based upon abnormal tumor vasculature, elevated interstitial fluid pressure within the tumor, and a dense extracellular matrix surrounding the tumor. [7–9] It is because of this inability to penetrate the tumors that the need for higher dosing chemotherapy has arisen. One of the investigational mechanisms to combat this problem is the use of nanoparticles and liposomes. Waite et. al. described transportation of nanoparticles into solid tumors be means of diffusion or binding to receptors on cell surfaces (Figure 1)[7]. These function by using the principle of enhanced permeability and retention effect (EPR effect)[10]. This principle is based upon the fact that the vasculature of solid tumors is deregulated and results in abnormal vascular permeation as well as small pores within the vasculature. This combination of these effects results in longer duration of interstitial fluid retention within these tissues[10]. Because of this, nanoparticles and liposomes can concentrate within these tissues to provide local tumor therapy.
Figure 1:
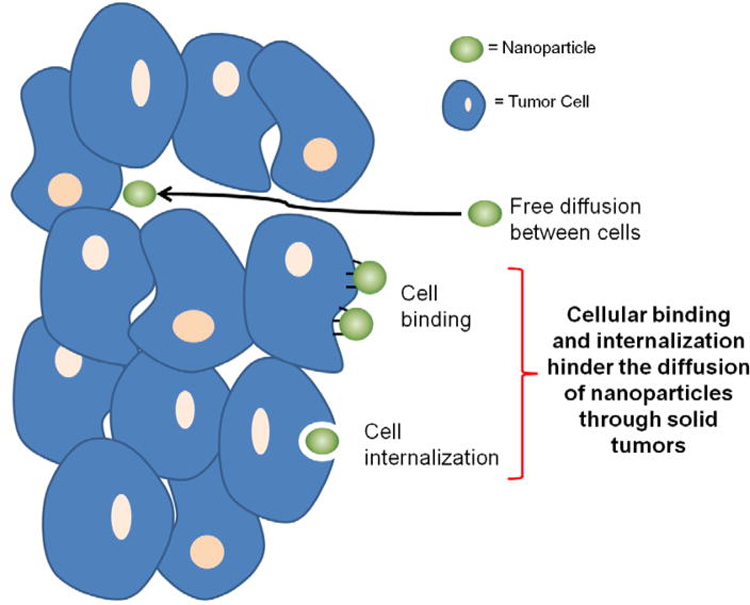
Mechanism of transport of nanoparticles through solid tumors: free diffusion through cells or binding by cellular receptors.[7]
Due to these tumor characteristics and the multiple toxic side effects of systemic chemotherapy, many investigation drugs and drug delivery modalities are being studied. One solution is the use of nanotechnology. This allows the delivery of chemotherapy and other agents that targets tumor specific tissue, minimizing systemic effects of these agents. [11,12] These can be administered systemically, either by inhalation or intravenously. Additionally, subcutaneous injections of nanoparticles have demonstrated promise in tumor treatment as well [1]. Injections of expansile nanoparticles into fat pads to treat breast cancer as well as subcutaneous injection of hyaluronic linked doxorubicin tumor with drainage and uptake within the local lymphatic channels demonstrates potential loco-regional metastatic treatment of breast cancers [2–4]. Two recent reports have shown toxicity to normal lung parenchyma as well as the ability of some nanoparticles to cross the blood brain barrier and cause unwanted central nervous system toxicity. [13,14] Therefore, the ability to deliver drug therapy locally to the tumor still remains as an important therapeutic option for solid tumors. Additionally, mechanisms of delivery that utilize biodegradable products as vehicles for drug delivery potentially can help minimize the effects of foreign material in the body.
This review will focus on the clinical application of local drug delivery systems in three important phases of tumor management. Investigational as well as approved methods of local drug delivery at time of initial tissue biopsy, surgical resection, and management of metastatic disease for solid tumors will be described.
DRUG DELIVERY AT THE TIME OF TUMOR BIOPSY
The initial step after the identification of a potential solid tumor is biopsy of the tumor allowing for accurate tissue diagnosis, as well as tissue specific treatment. Current biopsy strategies vary based upon the tumor type, but most employ percutaneous methods of tissue biopsy. Image guided biopsy has become a popular method of tumor sampling due to the less invasive nature of the biopsy when compared to surgical biopsy. For example, either ultrasound or computed tomography (CT) are being instituted to aid in diagnosis of ovarian cancer [15,16]. These modalities offer several advantages including ability to sample tumor in patients with poor performance status and could otherwise not tolerate a large open surgery. Additionally image guided biopsies are able to provide a definitive diagnosis of cancer type and aid with decisions regarding neoadjuvant treatment prior to definitive resection [15]. CT guided imaging in pancreatic tumors can yield a diagnosis in 98.1% of the cases, making it a reliable method of biopsy [17]. Finally, ultrasound and CT have been found to be not only safe, but also highly accurate, with sensitivities of 96.5%, and specificity of 100%, in biopsy of pulmonary lesions. [18] With the increasing use of image guided biopsy techniques, there is a unique treatment opportunity for the patients that could employ the same non-invasive techniques to delivery neoadjuvant therapy at the time of diagnosis[19].
Pre-Clinical Research:
Multiple image guidance modalities have been used in delivering drugs directly into the solid tumor. One such modality is positron emission therapy (PET). PET is an imagining modality that involves injection of positron emitting radionuclides that localize to tissues of high metabolic activity such as cancer cells [20]. Often after tumor diagnosis, PET imaging is used to determine the extent of the disease as well as identify any metastatic areas [20]. The ability to localize to tumor cells specifically using PET imaging has been a popular area of investigation. Liposomes can be loaded with biomolecules and used in drug delivery. Due to their hydrophobic lipid bilayer, liposomes offer an ability to load higher drug concentration without systemic effects, by maintaining loaded material within the liposome until it fuses with the intended targeted cell membrane [21]. Coupling liposomes with a radioisotope used in PET scans, was first described by Seo in 2008 [22]. Paoli et al found that using liposomes delivered via injection into the tail vein coupled with radioisotopes used in PET scanning to deliver treatment to met-1 cancer cells in mice there was a 101 fold increase of drug concentration within the tumor compared to giving the drug by itself [23]. This demonstrates that targeted therapies combined with PET scanning is a viable option for delivering high concentrations of drug to the targeted tissues for the treatment of solid tumors.
In addition to linking liposomes with radioisotopes, heat application can also aid in targeting specific sites for delivery. Heat has long been shown that when applied to liposomes, the release of drug from this platform is increased [24]. In a recent study by Ranjan et al, they developed a low temperature sensitive liposome that was loaded with doxorubicin [25]. After applying heat to site specific targets using magnetic resonance, they were able a higher concentration of doxorubicin from the liposomes to the Vx2 cell line compared to liposomes without heat application (Figure 2) [25]. Although there is a higher affinity for the treatment agent to accumulate in the tumor using liposomes, there remains a systemic absorption component, as demonstrated by the accumulation of liposomes within the liver, kidney, and spleen in addition to the primary tumor. [23].
Figure 2:
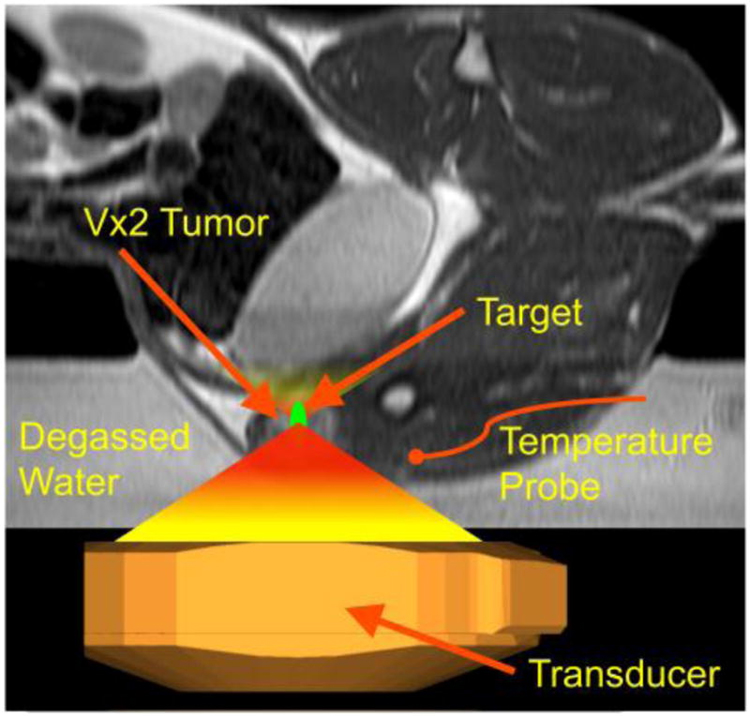
Targeted Delivery of Liposomal Therapy: Image guided hyperthermia was used in the hindlimb of rabbits to deliver target specific liposomes by submerging in degassed water and targeting the center of tumor.[25]
The use of percutaneous administration of various treatment agents directly into the targeted site is being investigated in both animal and human studies. An animal study done by Zhang et al, has shown that the use of MRI can aid in delivery of 5-FU to the common bile duct of swine, without evidence of extravasation from the delivery site [26]. This method of delivery greatly decreases the systemic absorption of chemotherapy and increases local tumor concentration. Given that MRI is a common imaging modality for biopsy, this could potentially be employed at the same time to both diagnose and begin early treatment in the future. Studies have demonstrated the feasibility of percutaneous injection of TNF alpha hydrogel into S180 sarcoma cell line in mice. Not only did they find that intra tumoral injections were more effective with lower doses than systemic administration, but side effects were also minimized [27]. For deeper, non palpable tumors, image guidance could be used to aid in administration of this treatment at the time of biopsy.
Initial tumor biopsy yields helpful information for tumor management such as tumor type, biomarkers, cellular and molecular signatures, susceptibilities and more importantly resistances to various drugs [28]. One of the more challenging clinical conundrums is a multidrug resistant tumor. This limits the success of chemotherapy and overall survival of the patients. Extensive research is being done in the field of multidrug resistant tumors to identify possible treatment options to overcome this. Conde et al studied a MDR breast cancer in a mouse. They created hydrogel that was embedded with dark-gold nanoparticles that were directly implanted into the tumor. The function of these nanoparticles was to silence the multidrug resistance protein 1 [29]. They found that after administration, a 90% tumor reduction was achieved using 5- FU, chemotherapy to which the tumor had been previously shown resistance. This is a very exciting advancement that could be considered during the initial treatment stage. The ability to improve tumor susceptibility to chemotherapy in a previously unresectable and difficult to treat tumor allows for broader treatment options and possibility for cure.
Clinical Investigation:
Image guidance for tumor treatment is currently being utilized in clinical practice. One of the most common types of solid tumor to use these modalities is hepatocellular carcinoma (HCC). Due to the advanced disease typically present at the time of diagnosis, only 10–15% of patients are surgical candidates [30]. Chemotherapy is often the first line of treatment in these cancers. Additionally, delivery of chemotherapy via localized methods for HCC has been investigated. The majority of these interventions are done via intra-arterial administration (TACE). TACE utilizes normal liver anatomy to deliver drugs to the tumor; the majority of hepatic tumors are fed via the hepatic artery while the liver receives the majority of its blood supply from the portal vein. TACE has been shown to have superior outcomes compared to conservative treatment alone [31,32]. However, a major draw back of this treatment is acute liver failure following administration and only effective against lower grade tumors [33].
Use of a local drug delivery system via image guidance has blossomed from TACE treatment. Drug-eluting beads that are delivered via percutaneous access to the hepatic artery can deliver chemotherapy immediately upon injection, similar to TACE. However, an advantage to these beads compared to the single dosing of TACE, is the continued sustained drug release that allows prolonged drug delivery to cancerous cells [34]. This sustained exposure to chemotherapeutic agent is important in maintaining tumor suppression and cellular necrosis. This treatment offers a new approach for oncologist in the management of intermediate stage HCC. This could potentially be done in concert with initial tumor biopsy in a relatively non-invasive manner.
Intra-tumoral drug delivery has been a topic of investigation over several years. Firusiam et al used P-32 chromic phosphate injections into varying solid tumors, including breast, colon, hepatocellular, and squamous cell carcinomas and found a tumor response rate of 71%, with complete remission seen in 41% of patients [35]. Additionally, intra-tumoral injection of non-small cell lung cancer with cisplatin was examined recently. Using ultrasound guidance, the authors performed serial intra-tumoral injections in patients with either stage IIIb or IV lung cancer [36]. This in combination with systemic chemotherapy yielded a 50% decrease in tumor volume. However, a major draw back of this is the need for recurrent administration of intra-tumoral injections, and anesthesia is required for each procedure. Finally, an intratumoral application that is FDA approved is used in brain tumors. These can be implanted either with or without tumor resection, and aid in the delivery of cytotoxic dosing of therapy to tumors otherwise shielded by the blood brain barrier [37] As investigation moves forward, simultaneous biopsy with intra-tumoral implantation of a sustained release treatment agent, either a chemotherapeutic or an immunomodulation, could become an effective option for patients with solid tumors.
DRUG DELIVERY AT THE TIME OF SOLID TUMOR RESECTION
Definitive management, and the only hope for true cure, for solid tumors involves complete surgical resection [38]. However, even though a tumor appeared to be resectable on preoperative imaging, at the time of operation, involvement or close proximity to major blood vessels or vital structures could prevent complete resection. The surgeons would have to choose between an incomplete resection or abort the procedure entirely. Radical surgical debulking of tumors, especially in ovarian tumors, has been shown to improve outcomes compared to no surgical resection at all, even if residual disease remains [39,40]. Without achieving a complete resection, or with positive margins, patients are required to undergo systemic adjuvant chemotherapy. Local drug delivery therapy is a unique advantage to surgeons at the time of the resection. This allows them to not only resect as much tumor as possible, but also begin therapy of residual disease immediately [41].
Pre-Clinical Studies:
With the success of Gliadel in treatment of glioma, as well as the concept of local drug delivery, several investigational studies have been conducted to determine the application of local drug delivery therapies for various solid tumors in concert with surgical resection. In one study, combination of subtotal resection of neuroblastoma tumors with implantation of a silk film loaded with sustained release doxorubicin was performed [41]. Animals treated with the film compared with control had significantly longer survival without overt systemic toxicity. Additionally, it was shown that these films could also be loaded with targeted drug therapy, crizotinib. These locally applied drug load films were shown to have improved outcomes in tumor suppression when compared with systemic administration of the same drug [42]. Applications of a sustained release silk film have also been tested in breast cancer tumors in mice, showing again decreased tumor growth [43]. While this study did not combine the application of silk film with resection this most certainly would be a viable treatment strategy.
Investigation into combining surgical resection with intra operative drug administration in the resection bed has also been performed in head and neck tumors. In a rat model, Wang et al completed a partial resection of squamous cell carcinoma, followed by immediate treatment of liposomes load with beta-emission rhenium-186 [44]. They found that compared to control, there was halted tumor growth in this treatment group. This study demonstrated that in otherwise unresectable tumors, a combination of surgical resection plus local drug delivery is a viable treatment option that resulted in slowed tumor growth when compared to resection alone.
Clinical Application:
Currently local drug delivery has been greatly utilized for patients with primary brain malignancies. Advantages to local drug delivery therapy in central nervous system tumors is largely due to the inability for many medications to cross the blood brain barrier [37]. Intra-tumoral injection of treatment agents has been described [45,46]. However, one of the biggest challenges in solid tumors is penetration of the entire treatment area. In a study described by Sampson et al, Convection-enhanced delivery (CED) was used in an attempt to increase the volume of distribution of human–murine chimeric mAb Ch81C6 [47]. They found that when CEDs were administered to the resection bed of patients with recurrent malignant glioblastoma multiforme the volume of distribution was greater than just intra-tumoral injection [47].
Another currently FDA approved local drug delivery therapy for the treatment of malignant glioma is Gliadel wafer [37,48]. The wafers contain carmustine, which is released over a period of 2 to 3 weeks, and up to eight wafers can be implanted at the time of resection [49]. In randomized placebo controlled double blinded trial, use of Gliadel wafers showed a 29% reduction in risk of death when compared to placebo [49]. The use of the wafer offers direct drug application. While side effects do occur, most commonly cerebral edema, the systemic effects of chemotherapy are minimized.
APPLICATION OF LOCAL DRUG DELIVERY IN METASTATIC DISEASE
Metastatic disease in solid tumors presents a significant challenge for the treating physicians. Metastatic disease can be found at the initial diagnosis or identified as a recurrence, and the mortality of patients with metastatic disease is the highest of any clinical stages. In patients with rhabdomyosarcoma, the five-year survival rate of patients with progressive or recurrent disease is only 17% [3]. Investigations of mechanisms to prolong survival have been an area of active research. It has been shown that in patients with liver metastasis from primary colon cancer, the five-year survival can be as high as 72% in some studies [50]. In this study by Nikfarjam, a combination of surgical resection, radiofrequency ablation, neoadjuvant or adjuvant chemotherapy was used.
Complete resection of the metastatic disease is a significant predictor in survival following metastasectomy [51]. Osteosarcoma, a common solid tumor, most frequently metastasizes to the lungs [38]. Numerous studies have shown that aggressive metastasectomy of these lesions can lead to overall prolonged survival [52–54]. Neoadjuvant treatments are viable options to try to decrease the tumor burden prior to treatment. Some of these include radiofrequency ablation of liver metastasis, irreversible electroporation, as well as systemic chemotherapy [50,55]. Additionally, management of liver tumors is limited by the amount of liver that can be resected without causing liver failure. Techniques, including portal vein ligation and partitioning of the liver to encourage hepatic regeneration of the unaffected liver, have shown promising outcomes for otherwise large, unresectable liver metastasis [56] Using local drug delivery, either chemotherapeutics or biologic agents, as a method to treat solid tumors with metastatic foci is another potential technique for tumor management.
Pre-Clinical Studies:
Spread of cancer cells can be via either hematogenous or lymphatic route [38,57]. In those cancers that have lymphatic spread, such as lung, breast, or melanoma, often the first site of disease beyond the primary tumor is a lymph node. Sato et al investigated the use of nano/microbubbles loaded with cis-diamminedichloroplatinum delivered intra-lymphatically to treat lymph node metastasis in breast cancer [58]. They used these microbubbles in combination with ultrasound to enhance delivery of the drug to treat breast cancer cells both in vitro and in mice. They found that this combination achieved higher therapeutic effect, without injuring adjacent normal tissues.
Once disease spreads beyond the lymph nodes, tumors can be established in many different organs. Metastatic disease involving the brain is a particularly challenging area to treat with traditional systemic chemotherapy. The blood-brain barrier prevents transfusion of many different chemicals, making it difficult to achieve a concentration high enough to kill cancer cells [59]. Because of this, local drug delivery therapy to the brain is being investigated to bypass the challenges of the blood-brain barrier. Ten to 20% of patients with metastatic breast cancer will have foci within the brain [60]. Ewend et al tested the outcomes of local drug delivery with controlled release polymers loaded with carboplatin directly implanted into intracranial metastatic breast tumors in mice [48]. They found that using their biodegradable polymer loaded with carboplatin, survival was significantly improved in this group when compared with control. They found that this model worked well for patients that had one to two lesions within the brain and found there was minimal added morbidity to the treatment groups [48]. Another vehicle for local drug delivery for metastatic brain tumors are microcapsules. Upadhyay et al investigated loading temozolomide and doxorubicin onto microcapsules to treat metastatic breast cancer in mice [61]. They found that overall survival was prolonged when compared to systemic administration and that temozolomide capsules induced higher rates of apoptosis.
Another very common location for metastatic disease is the liver. As mentioned previously, surgical resection of liver disease is important for long term survival of patients with these tumors [62]. However, of those presenting with metastatic liver disease, only 15% are resectable at the time of diagnosis [63]. Due to this fact, local drug delivery is an important tool to manage liver metastasis to either completely treat, or decrease the tumor burden to enable surgical resection. Zhao et al investigated the use of doxorubicin-loaded liposomes in the treatment of both human colon cancer cell lines as well as hepatocellular carcinoma cell lines [64]. These liposomes localized to tumors established within the liver of mice. Liposomes in this experiment could be accurately targeted to hepatic metastatic disease specifically and significantly slowed progression of the metastatic disease[64]. The ability to suppress tumor growth in the liver could be used in combination with surgical resection offering potentially complete resection of the tumor and prolong overall survival.
Lastly, metastasis involving bones, more specifically the spinal cord, can be challenging as well as severely impacting the quality of life of the patient [65]. Current management of spinal metastasis is generally targeted toward palliation and symptom control. A feared complication of spinal metastasis is paralysis. Using a nanocarrier loaded with indocyanine green with near-infrared fluorescence, Funayama et al investigated the effects on hind limb paralysis in rats [66]. They found that animals that received the combination therapy had delayed progression to total hind limb paralysis, demonstrating that local treatment of spinal metastasis was feasible and effective. This offers a treatment option to patients that otherwise could not tolerate an extensive spinal operation and aid in symptom management and quality of life improvement.
Clinical Experience:
Much of the current clinical application for local drug delivery focuses on brain metastasis. Surgical resection with local drug delivery application at the time of surgery, with and without whole brain radiation, has been studied. Twenty-five patients with solitary metastasis to the brain, from lung, breast, renal cancer and melanoma, underwent surgical resection, implantation of carmustine polymer wafers in the resection bed, and external beam radiation therapy [67]. Two patients experienced side effects attributed to the local drug delivery therapy. However, the most impressive was a 0% local recurrence rate at 33 months post treatment [67]. In another series of 59 patients, Brem et al studied the outcomes of surgical resection in conjunction with carmustine wafers for metastatic lesions in the brain without whole brain radiation [68]. The goal of this study was to determine if local drug delivery would decrease the rate of neurocognitive functional decline. They found that not only was neurocognitive function better preserved with local drug delivery but that the rates of local recurrence were similar to that of resection with whole brain radiation. These studies show that local delivery of chemotherapy is both feasible for treating intracranial metastatic disease and results in less side effects than surgical resection and whole brain radiation.
CONCLUSION
Solid tumors are among the most commonly diagnosed cancer and have a high rate of morbidity and mortality. And as such, many investigational therapies have been devised to improve overall outcome and tumor free survival. Current clinical application of local drug delivery therapies have been shown to halt tumor growth, manage incomplete resections or residual microscopic disease, or treat metastatic lesions. Many types of local drug delivery still requires further investigation before they can become standard of care for management of solid tumors. Minimizing the side effects of the treatment and being able to more precisely target the tumor cells are important challenges to overcome in designing local drug delivery therapies. These modalities have the potential to be a power tool for improving the overall survival of patients affected with cancer.
Table 1:
Summary of Literature review using local drug delivery. Both pre-clinical as well as clinical investigational studies during three phases of treatment, diagnosis, resection, and metastatic disease, were reviewed. The summary of the study design and outcomes are outlined in the table.
| Author | Year Published | Animal/ Human study | Mode of Delivery | Tumor Type | Tumor Size/ Timing at Delivery | Drug | |
|---|---|---|---|---|---|---|---|
| At Tumor Diagnosis | |||||||
|
| |||||||
| Pre-Clinical | |||||||
| Yatvin | 1978 | in-vitro | Liposomes | N/a | N/a | Neomycin | |
| Lewis | 2006 | in-vitro | DC Bead | N/a | N/a | Doxorubicin | |
| Seo | 2008 | Mouse | Liposomes injected via tail vein | N/a | N/a | Cu-specific chelator, 6-[p-(bromoacetamido)benzyl]-1,4,8,11-tetraazacyclotetradecane-N,N’,N”,N”‘-tetraacetic acid (BAT) | |
| Paoli | 2010 | Mouse | Liposomes injected via tail vein | Met-1 tumor | 0.5 cm | hydrophilic model drug | |
| Xu | 2015 | Mouse | Intratumoral injection | Breast | N/a | IL-21 | |
| Conde | 2015 | Mouse | Bare-Gold nanoparticles implanted peri tumorally | Triple Negative Breast Cancer | 100 mm3 | 5-fluorouracil (5-FU)-intercalated nanobeacons | |
| Ranjan | 2012 | Rabbit | Low temperature sensitive liposomes via | Vx2 tumor | 1cm | Doxorubicin | |
| Zhang | 2015 | Pigs | Image guided percutaneous injection into target | N/a, Common Bile duct | N/a | Motexafin gadolinium (MGd) and trypan blue dye as well as 5-fluorouracil (5-Fu) | |
| Clinical Investigation | |||||||
| Sampath | 1998 | Human | Implantable biodegradable polymer within tumor | Glioma | N/a | Carmustine (BCNU)-impregnated | |
| Firusian | 1999 | Human | Direct Intratumoral injection | Solid Tumors (hepatoma, GI malignancy, breast, and thyroid) | N/a | P-32 chromic phosphate | |
| Hohenforst-Schmidt | 2013 | Human | Intratumoral-regional and intravenous administration | Non-small cell lung cancer | Stage 3a or 4 | Cisplatin analogues | |
|
| |||||||
| At Time of Resection | |||||||
|
| |||||||
| Pre-Clinical | |||||||
| Ewend | 1998 | Mouse | Controlled release polymers in surgical resection bed | Breast | 5 days post inoculation | Carmustine (BCNU), carboplatin, and camptothecin | |
| Zaharoff | 2010 | Mouse | Intratumoral biodegradable polysaccharide chitosan | Colon and Pancreatic | 7 days post inoculation | IL-12 | |
| Seib | 2012 | Mouse | Controlled release silk film at tumor | Breast | 12 days post inoculation | Doxorubicin | |
| Chiu | 2014 | Mouse | Controlled release silk film at tumor resection bed | Neuroblastoma | 300 mm3 | Doxorubicin | |
| Seib | 2015 | Mouse | Controlled release silk film at tumor | Neuroblastoma | 70 mm3 | Doxorubicin and Crizotinib | |
| Wang | 2008 | Rat | Intratumoral injection of liposome | squamous cell carcinoma | 2–3 cm3 | Beta-emission rhenium-186 | |
| Clinical Investigation | |||||||
| Westphal | 2003 | Human | Implantable biodegradable wafer in tumor resection bed | Malignant Glioma | N/a | 1,3-bis (2-chloroethyl)-1-nitrosourea (BCNU) | |
| Sampson | 2006 | Human | Convection-enhanced delivery via stereotactic catheter | Recurrent Glioblastoma Multiforme | N/a | Antitenascin mAb 81C6 | |
| Roberts | 2014 | Human | Intratumoral injection | Retroperitoneal leiomyosarcoma | N/a | Clostridium novyi | |
|
| |||||||
| Metastatic Disease | |||||||
|
| |||||||
| Pre-Clinical | |||||||
| Ewend | 1998 | Mouse | Controlled release polymers in surgical resection bed | Breast | 5 days post inoculation | carmustine (BCNU), carboplatin, and camptothecin | |
| Zhao | 2013 | Mouse | Drug-loaded conventional liposomes injected into portal vein | Colon metastasized to the liver | 7 days post inoculation | Doxorubicin | |
| Sato | 2015 | Mouse | Intralymphatic delivery with nano/ microbubbles in combination with ultrasound | Mammary and hepatoma | Day 3,4 post inoculation | cis-diamminedichloroplatinum | |
| Funayama | 2013 | Rat | Tumor-selective photodynamic therapy (PDT) with nanocarrier | Spinal cord metastasis | N/a | Indocyanine green | |
| Upadhyay | 2014 | Rat | Intracranial implanted microcapsules | Breast | 0,6,13 days post inoculation | Temozolomide and Doxorubicin | |
| Clinical Investigation | |||||||
| Ewend | 2007 | Human | Polymer wafer in tumor resection bed | Metastatic brain lesions: lung, melanoma, breast, renal | N/a | Carmustine | |
| Brem | 2013 | Human | Polymer wafer in tumor resection bed | Single or multiple brain metastasis | N/a | Carmustine | |
Footnotes
Conflict Of Interest: The authors have nothing to disclose
References:
- 1.Global Burden of Disease Cancer, C.; Fitzmaurice C; Dicker D; Pain A; Hamavid H; Moradi-Lakeh M; MacIntyre MF; Allen C; Hansen G; Woodbrook R; Wolfe C; Hamadeh RR; Moore A; Werdecker A; Gessner BD; Te Ao B; McMahon B; Karimkhani C; Yu C; Cooke GS; Schwebel DC; Carpenter DO; Pereira DM; Nash D; Kazi DS; De Leo D; Plass D; Ukwaja KN; Thurston GD; Yun Jin K; Simard EP; Mills E; Park EK; Catala-Lopez F; deVeber G; Gotay C; Khan G; Hosgood HD 3rd; Santos IS; Leasher JL; Singh J; Leigh J; Jonas J; Sanabria J; Beardsley J; Jacobsen KH; Takahashi K; Franklin RC; Ronfani L; Montico M; Naldi L; Tonelli M; Geleijnse J; Petzold M; Shrime MG; Younis M; Yonemoto N; Breitborde N; Yip P; Pourmalek F; Lotufo PA; Esteghamati A; Hankey GJ; Ali R; Lunevicius R; Malekzadeh R; Dellavalle R; Weintraub R; Lucas R; Hay R; Rojas-Rueda D; Westerman R; Sepanlou SG; Nolte S; Patten S; Weichenthal S; Abera SF; Fereshtehnejad SM; Shiue I; Driscoll T; Vasankari T; Alsharif U; Rahimi-Movaghar V; Vlassov VV; Marcenes WS; Mekonnen W; Melaku YA; Yano Y; Artaman A; Campos I; MacLachlan J; Mueller U; Kim D; Trillini M; Eshrati B; Williams HC; Shibuya K; Dandona R; Murthy K; Cowie B; Amare AT; Antonio CA; Castaneda-Orjuela C; van Gool CH; Violante F; Oh IH; Deribe K; Soreide K; Knibbs L; Kereselidze M; Green M; Cardenas R; Roy N; Tillman T; Li Y; Krueger H; Monasta L; Dey S; Sheikhbahaei S; Hafezi-Nejad N; Kumar GA; Sreeramareddy CT; Dandona L; Wang H; Vollset SE; Mokdad A; Salomon JA; Lozano R; Vos T; Forouzanfar M; Lopez A; Murray C; Naghavi M The Global Burden of Cancer 2013. JAMA Oncol 2015, 1 (4), 505–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maris JM; Hogarty MD; Bagatell R; Cohn SL Neuroblastoma. Lancet 2007, 369 (9579), 2106–2120. [DOI] [PubMed] [Google Scholar]
- 3.Malempati S; Hawkins DS Rhabdomyosarcoma: review of the Children’s Oncology Group (COG) Soft-Tissue Sarcoma Committee experience and rationale for current COG studies. Pediatric blood & cancer 2012, 59 (1), 5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdulkarim BS; Cuartero J; Hanson J; Deschenes J; Lesniak D; Sabri S Increased risk of locoregional recurrence for women with T1–2N0 triple-negative breast cancer treated with modified radical mastectomy without adjuvant radiation therapy compared with breast-conserving therapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2011, 29 (21), 2852–2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee JS; DuBois SG; Boscardin WJ; Wustrack RL; Goldsby RE Secondary malignant neoplasms among children, adolescents, and young adults with osteosarcoma. Cancer 2014, 120 (24), 3987–3993. [DOI] [PubMed] [Google Scholar]
- 6.Baker DL; Schmidt ML; Cohn SL; Maris JM; London WB; Buxton A; Stram D; Castleberry RP; Shimada H; Sandler A; Shamberger RC; Look AT; Reynolds CP; Seeger RC; Matthay KK; Children’s Oncology G Outcome after reduced chemotherapy for intermediate-risk neuroblastoma. The New England journal of medicine 2010, 363 (14), 1313–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waite CL; Roth CM Nanoscale drug delivery systems for enhanced drug penetration into solid tumors: current progress and opportunities. Crit Rev Biomed Eng 2012, 40 (1), 21–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tannock IF; Lee CM; Tunggal JK; Cowan DS; Egorin MJ Limited penetration of anticancer drugs through tumor tissue: a potential cause of resistance of solid tumors to chemotherapy. Clinical cancer research : an official journal of the American Association for Cancer Research 2002, 8 (3), 878–884. [PubMed] [Google Scholar]
- 9.Minchinton AI; Tannock IF Drug penetration in solid tumours. Nat Rev Cancer 2006, 6 (8), 583–592. [DOI] [PubMed] [Google Scholar]
- 10.Nehoff H; Parayath NN; Domanovitch L; Taurin S; Greish K Nanomedicine for drug targeting: strategies beyond the enhanced permeability and retention effect. Int J Nanomedicine 2014, 9, 2539–2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gong C; Deng S; Wu Q; Xiang M; Wei X; Li L; Gao X; Wang B; Sun L; Chen Y; Li Y; Liu L; Qian Z; Wei Y Improving antiangiogenesis and anti-tumor activity of curcumin by biodegradable polymeric micelles. Biomaterials 2013, 34 (4), 1413–1432. [DOI] [PubMed] [Google Scholar]
- 12.Wu Q; Deng S; Li L; Sun L; Yang X; Liu X; Liu L; Qian Z; Wei Y; Gong C Biodegradable polymeric micelle-encapsulated quercetin suppresses tumor growth and metastasis in both transgenic zebrafish and mouse models. Nanoscale 2013, 5 (24), 12480–12493. [DOI] [PubMed] [Google Scholar]
- 13.Fattal E; Grabowski N; Mura S; Vergnaud J; Tsapis N; Hillaireau H Lung toxicity of biodegradable nanoparticles. J Biomed Nanotechnol 2014, 10 (10), 2852–2864. [DOI] [PubMed] [Google Scholar]
- 14.Leite PE; Pereira MR; Granjeiro JM Hazard effects of nanoparticles in central nervous system: Searching for biocompatible nanomaterials for drug delivery. Toxicol In Vitro 2015, 29 (7), 1653–1660. [DOI] [PubMed] [Google Scholar]
- 15.Spencer JA; Anderson K; Weston M; Wilkinson N; Hewitt M Image guided biopsy in the management of cancer of the ovary. Cancer Imaging 2006, 6, 144–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thabet A; Somarouthu B; Oliva E; Gervais DA; Hahn PF; Lee SI Image-guided ovarian mass biopsy: efficacy and safety. Journal of vascular and interventional radiology : JVIR 2014, 25 (12), 1922–1927 e1921. [DOI] [PubMed] [Google Scholar]
- 17.Tyng CJ; Almeida MF; Barbosa PN; Bitencourt AG; Berg JA; Maciel MS; Coimbra FJ; Schiavon LH; Begnami MD; Guimaraes MD; Zurstrassen CE; Chojniak R Computed tomography-guided percutaneous core needle biopsy in pancreatic tumor diagnosis. World journal of gastroenterology : WJG 2015, 21 (12), 3579–3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Busso M; Sardo D; Garetto I; Righi L; Libero G; Vavala T; Ardissone F; Novello S; Papotti M; Veltri A Safety and diagnostic performance of image-guided lung biopsy in the targeted therapy era. Radiol Med 2015. [DOI] [PubMed] [Google Scholar]
- 19.Morrison J; Haldar K; Kehoe S; Lawrie TA Chemotherapy versus surgery for initial treatment in advanced ovarian epithelial cancer. Cochrane Database Syst Rev 2012, 8, CD005343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chakravarty R; Hong H; Cai W Positron emission tomography image-guided drug delivery: current status and future perspectives. Mol Pharm 2014, 11 (11), 3777–3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goyal P; Goyal K; Vijaya Kumar SG; Singh A; Katare OP; Mishra DN Liposomal drug delivery systems--clinical applications. Acta Pharm 2005, 55 (1), 1–25. [PubMed] [Google Scholar]
- 22.Seo JW; Zhang H; Kukis DL; Meares CF; Ferrara KW A novel method to label preformed liposomes with 64Cu for positron emission tomography (PET) imaging. Bioconjugate chemistry 2008, 19 (12), 2577–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paoli EE; Kruse DE; Seo JW; Zhang H; Kheirolomoom A; Watson KD; Chiu P; Stahlberg H; Ferrara KW An optical and microPET assessment of thermally-sensitive liposome biodistribution in the Met-1 tumor model: Importance of formulation. Journal of controlled release : official journal of the Controlled Release Society 2010, 143 (1), 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yatvin MB; Weinstein JN; Dennis WH; Blumenthal R Design of liposomes for enhanced local release of drugs by hyperthermia. Science 1978, 202 (4374), 1290–1293. [DOI] [PubMed] [Google Scholar]
- 25.Ranjan A; Jacobs GC; Woods DL; Negussie AH; Partanen A; Yarmolenko PS; Gacchina CE; Sharma KV; Frenkel V; Wood BJ; Dreher MR Image-guided drug delivery with magnetic resonance guided high intensity focused ultrasound and temperature sensitive liposomes in a rabbit Vx2 tumor model. Journal of controlled release : official journal of the Controlled Release Society 2012, 158 (3), 487–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang F; Bai Z; Shi Y; Wang J; Li Y; Yang X Interventional MRI-guided local delivery of agents into swine bile duct walls using MR-compatible needle-integrated balloon catheter system. NMR in biomedicine 2015, 28 (6), 679–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu M; Liu M; Du X; Li S; Li H; Li X; Li Y; Wang Y; Qin Z; Fu YX; Wang S Intratumoral Delivery of IL-21 Overcomes Anti-Her2/Neu Resistance through Shifting Tumor-Associated Macrophages from M2 to M1 Phenotype. J Immunol 2015, 194 (10), 4997–5006. [DOI] [PubMed] [Google Scholar]
- 28.Abi-Jaoudeh N; Duffy AG; Greten TF; Kohn EC; Clark TW; Wood BJ Personalized oncology in interventional radiology. Journal of vascular and interventional radiology : JVIR 2013, 24 (8), 1083–1092; quiz 1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conde J; Oliva N; Artzi N Implantable hydrogel embedded dark-gold nanoswitch as a theranostic probe to sense and overcome cancer multidrug resistance. Proceedings of the National Academy of Sciences of the United States of America 2015, 112 (11), E1278–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geschwind JF Chemoembolization for hepatocellular carcinoma: where does the truth lie? Journal of vascular and interventional radiology : JVIR 2002, 13 (10), 991–994. [DOI] [PubMed] [Google Scholar]
- 31.Seinstra BA; van Delden OM; van Erpecum KJ; van Hillegersberg R; Mali WP; van den Bosch MA Minimally invasive image-guided therapy for inoperable hepatocellular carcinoma: What is the evidence today? Insights Imaging 2010, 1 (3), 167–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Llovet JM; Bruix J Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology 2003, 37 (2), 429–442. [DOI] [PubMed] [Google Scholar]
- 33.Lau WY; Lai EC Treatment of unresectable hepatocellular carcinoma with transarterial radioembolization: iodine-131-lipiodol. ANZ J Surg 2008, 78 (5), 331–332. [DOI] [PubMed] [Google Scholar]
- 34.Lewis AL; Gonzalez MV; Lloyd AW; Hall B; Tang Y; Willis SL; Leppard SW; Wolfenden LC; Palmer RR; Stratford PW DC bead: in vitro characterization of a drug-delivery device for transarterial chemoembolization. Journal of vascular and interventional radiology : JVIR 2006, 17 (2 Pt 1), 335–342. [DOI] [PubMed] [Google Scholar]
- 35.Firusian N; Dempke W An early phase II study of intratumoral P-32 chromic phosphate injection therapy for patients with refractory solid tumors and solitary metastases. Cancer 1999, 85 (4), 980–987. [DOI] [PubMed] [Google Scholar]
- 36.Hohenforst-Schmidt W; Zarogoulidis P; Darwiche K; Vogl T; Goldberg EP; Huang H; Simoff M; Li Q; Browning R; Turner FJ; Le Pivert P; Spyratos D; Zarogoulidis K; Celikoglu SI; Celikoglu F; Brachmann J Intratumoral chemotherapy for lung cancer: re-challenge current targeted therapies. Drug Des Devel Ther 2013, 7, 571–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sampath P; Brem H Implantable Slow-Release Chemotherapeutic Polymers for the Treatment of Malignant Brain Tumors. Cancer control : journal of the Moffitt Cancer Center 1998, 5 (2), 130–137. [DOI] [PubMed] [Google Scholar]
- 38.Dasgupta R; Rodeberg DA Update on rhabdomyosarcoma. Semin Pediatr Surg 2012, 21 (1), 68–78. [DOI] [PubMed] [Google Scholar]
- 39.Aletti GD; Dowdy SC; Gostout BS; Jones MB; Stanhope CR; Wilson TO; Podratz KC; Cliby WA Aggressive surgical effort and improved survival in advanced-stage ovarian cancer. Obstet Gynecol 2006, 107 (1), 77–85. [DOI] [PubMed] [Google Scholar]
- 40.La Quaglia MP; Kushner BH; Su W; Heller G; Kramer K; Abramson S; Rosen N; Wolden S; Cheung NK The impact of gross total resection on local control and survival in high-risk neuroblastoma. Journal of pediatric surgery 2004, 39 (3), 412–417; discussion 412–417. [DOI] [PubMed] [Google Scholar]
- 41.Chiu B; Coburn J; Pilichowska M; Holcroft C; Seib FP; Charest A; Kaplan DL Surgery combined with controlled-release doxorubicin silk films as a treatment strategy in an orthotopic neuroblastoma mouse model. British journal of cancer 2014, 111 (4), 708–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seib FP; Coburn J; Konrad I; Klebanov N; Jones GT; Blackwood B; Charest A; Kaplan DL; Chiu B Focal therapy of neuroblastoma using silk films to deliver kinase and chemotherapeutic agents in vivo. Acta biomaterialia 2015, 20, 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seib FP; Kaplan DL Doxorubicin-loaded silk films: drug-silk interactions and in vivo performance in human orthotopic breast cancer. Biomaterials 2012, 33 (33), 8442–8450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang SX; Bao A; Herrera SJ; Phillips WT; Goins B; Santoyo C; Miller FR; Otto RA Intraoperative 186Re-liposome radionuclide therapy in a head and neck squamous cell carcinoma xenograft positive surgical margin model. Clinical cancer research : an official journal of the American Association for Cancer Research 2008, 14 (12), 3975–3983. [DOI] [PubMed] [Google Scholar]
- 45.Zaharoff DA; Hance KW; Rogers CJ; Schlom J; Greiner JW Intratumoral immunotherapy of established solid tumors with chitosan/IL-12. J Immunother 2010, 33 (7), 697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roberts NJ; Zhang L; Janku F; Collins A; Bai RY; Staedtke V; Rusk AW; Tung D; Miller M; Roix J; Khanna KV; Murthy R; Benjamin RS; Helgason T; Szvalb AD; Bird JE; Roy-Chowdhuri S; Zhang HH; Qiao Y; Karim B; McDaniel J; Elpiner A; Sahora A; Lachowicz J; Phillips B; Turner A; Klein MK; Post G; Diaz LA Jr.; Riggins GJ; Papadopoulos N; Kinzler KW; Vogelstein B; Bettegowda C; Huso DL; Varterasian M; Saha S; Zhou S Intratumoral injection of Clostridium novyi-NT spores induces antitumor responses. Sci Transl Med 2014, 6 (249), 249ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sampson JH; Akabani G; Friedman AH; Bigner D; Kunwar S; Berger MS; Bankiewicz KS Comparison of intratumoral bolus injection and convection-enhanced delivery of radiolabeled antitenascin monoclonal antibodies. Neurosurg Focus 2006, 20 (4), E14. [DOI] [PubMed] [Google Scholar]
- 48.Ewend MG; Sampath P; Williams JA; Tyler BM; Brem H Local delivery of chemotherapy prolongs survival in experimental brain metastases from breast carcinoma. Neurosurgery 1998, 43 (5), 1185–1193. [DOI] [PubMed] [Google Scholar]
- 49.Westphal M; Hilt DC; Bortey E; Delavault P; Olivares R; Warnke PC; Whittle IR; Jaaskelainen J; Ram Z A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro-oncology 2003, 5 (2), 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nikfarjam M; Shereef S; Kimchi ET; Gusani NJ; Jiang Y; Avella DM; Mahraj RP; Staveley-O’Carroll KF Survival outcomes of patients with colorectal liver metastases following hepatic resection or ablation in the era of effective chemotherapy. Ann Surg Oncol 2009, 16 (7), 1860–1867. [DOI] [PubMed] [Google Scholar]
- 51.Tronc F; Conter C; Marec-Berard P; Bossard N; Remontet L; Orsini A; Gamondes JP; Louis D Prognostic factors and long-term results of pulmonary metastasectomy for pediatric histologies. Eur J Cardiothorac Surg 2008, 34 (6), 1240–1246. [DOI] [PubMed] [Google Scholar]
- 52.Salah S; Fayoumi S; Alibraheem A; Massad E; Abdel Jalil R; Yaser S; Albadainah F; Albaba H; Maakoseh M The influence of pulmonary metastasectomy on survival in osteosarcoma and soft-tissue sarcomas: a retrospective analysis of survival outcomes, hospitalizations and requirements of home oxygen therapy. Interact Cardiovasc Thorac Surg 2013, 17 (2), 296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Okiror L; Peleki A; Moffat D; Bille A; Bishay E; Rajesh P; Steyn R; Naidu B; Grimer R; Kalkat M Survival following Pulmonary Metastasectomy for Sarcoma. Thorac Cardiovasc Surg 2015. [DOI] [PubMed] [Google Scholar]
- 54.Zarroug AE; Hamner CE; Pham TH; Houghton SG; Stavlo P; Moir CR; Rodeberg DA Bilateral staged versus bilateral simultaneous thoracotomy in the pediatric population. Journal of pediatric surgery 2006, 41 (4), 647–651; discussion 647–651. [DOI] [PubMed] [Google Scholar]
- 55.Scheffer HJ; Nielsen K; de Jong MC; van Tilborg AA; Vieveen JM; Bouwman AR; Meijer S; van Kuijk C; van den Tol PM; Meijerink MR Irreversible electroporation for nonthermal tumor ablation in the clinical setting: a systematic review of safety and efficacy. Journal of vascular and interventional radiology : JVIR 2014, 25 (7), 997–1011; quiz 1011. [DOI] [PubMed] [Google Scholar]
- 56.Hasselgren K; Sandstrom P; Bjornsson B Role of associating liver partition and portal vein ligation for staged hepatectomy in colorectal liver metastases: a review. World journal of gastroenterology : WJG 2015, 21 (15), 4491–4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kleppe M; Kraima AC; Kruitwagen RF; Van Gorp T; Smit NN; van Munsteren JC; DeRuiter MC Understanding Lymphatic Drainage Pathways of the Ovaries to Predict Sites for Sentinel Nodes in Ovarian Cancer. Int J Gynecol Cancer 2015, 25 (8), 1405–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sato T; Mori S; Sakamoto M; Arai Y; Kodama T Direct delivery of a cytotoxic anticancer agent into the metastatic lymph node using nano/microbubbles and ultrasound. PloS one 2015, 10 (4), e0123619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eichler AF; Chung E; Kodack DP; Loeffler JS; Fukumura D; Jain RK The biology of brain metastases-translation to new therapies. Nat Rev Clin Oncol 2011, 8 (6), 344–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parker SL; Tong T; Bolden S; Wingo PA Cancer statistics, 1996. CA Cancer J Clin 1996, 46 (1), 5–27. [DOI] [PubMed] [Google Scholar]
- 61.Upadhyay UM; Tyler B; Patta Y; Wicks R; Spencer K; Scott A; Masi B; Hwang L; Grossman R; Cima M; Brem H; Langer R Intracranial microcapsule chemotherapy delivery for the localized treatment of rodent metastatic breast adenocarcinoma in the brain. Proceedings of the National Academy of Sciences of the United States of America 2014, 111 (45), 16071–16076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takahashi M; Hasegawa K; Oba M; Aoki T; Sakamoto Y; Sugawara Y; Kokudo N Repeat resection leads to long-term survival: analysis of 10-year follow-up of patients with colorectal liver metastases. Am J Surg 2015. [DOI] [PubMed] [Google Scholar]
- 63.Curing patients with liver metastases from colorectal cancer. Drug Ther Bull 2011, 49 (4), 42–45; quiz i-ii. [DOI] [PubMed] [Google Scholar]
- 64.Zhao C; Feng Q; Dou Z; Yuan W; Sui C; Zhang X; Xia G; Sun H; Ma J Local targeted therapy of liver metastasis from colon cancer by galactosylated liposome encapsulated with doxorubicin. PloS one 2013, 8 (9), e73860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cole JS; Patchell RA Metastatic epidural spinal cord compression. Lancet Neurol 2008, 7 (5), 459–466. [DOI] [PubMed] [Google Scholar]
- 66.Funayama T; Tsukanishi T; Hara I; Ozeki E; Sakane M Tumor-selective near-infrared photodynamic therapy with novel indocyanine green-loaded nanocarrier delays paralysis in rats with spinal metastasis. Photodiagnosis Photodyn Ther 2013, 10 (4), 374–378. [DOI] [PubMed] [Google Scholar]
- 67.Ewend MG; Brem S; Gilbert M; Goodkin R; Penar PL; Varia M; Cush S; Carey LA Treatment of single brain metastasis with resection, intracavity carmustine polymer wafers, and radiation therapy is safe and provides excellent local control. Clinical cancer research : an official journal of the American Association for Cancer Research 2007, 13 (12), 3637–3641. [DOI] [PubMed] [Google Scholar]
- 68.Brem S; Meyers CA; Palmer G; Booth-Jones M; Jain S; Ewend MG Preservation of neurocognitive function and local control of 1 to 3 brain metastases treated with surgery and carmustine wafers. Cancer 2013, 119 (21), 3830–3838. [DOI] [PMC free article] [PubMed] [Google Scholar]


