ABSTRACT
Background
Epidemiologic observations suggest increased potato consumption correlates with weight gain, adiposity, and diabetes risk, whereas nut consumption is associated with weight control and metabolic health. Randomized controlled trial (RCT) data indicate humans respond to changes in energy intake in single dietary components and compensate for extra energy consumed.
Objectives
We completed an RCT testing whether increased daily potato consumption influences energy balance [specifically, fat mass (FM)] compared with calorie-matched almond consumption.
Methods
A 30-d RCT of 180 adults prescribed calorie-matched (300 kcal/d, n = 60 participants/group) than consumed 1 of the following: 1) almonds (almond group), 2) French fries (potato group), or 3) French fries with herb/spices mix (potato + herb/spices group). Baseline and 30-d FM were measured by DXA (primary outcome), with secondary outcomes including body weight and carbohydrate metabolism markers [glycated hemoglobin (HbA1c), fasting blood glucose and insulin, HOMA-IR)]. A subset of 5 participants/group participated in a postprandial meal-based tolerance test.
Results
A total of 180 participants were randomly assigned [gender: 67.8% female; mean ± SD age: 30.4 ± 8.7 y; BMI (in kg/m2): 26.1 ± 4.2; and weight: 75.6 ± 15.4 kg], with 12 dropouts and 3 terminations. No significantly different FM changes were observed between almond and potato consumption [combined ± herb/spices; mean ± SE almond: 230.87 ± 114.01 g; potato: 123.73 ± 86.09 g; P = 0.443], fasting glucose (P = 0.985), insulin (P = 0.082), HOMA-IR (P = 0.080), or HbA1c (P = 0.269). Body weight change was not significantly different in the potato groups combined compared with the almond group (P = 0.116), but was significantly different among the 3 groups (P = 0.014; almond: 0.49 ± 0.20 kg; potato: –0.24 ± 0.20 kg; potato + herb/spices: 0.47 ± 0.21 kg). In meal tests, significantly lower post-prandial glucose and insulin responses to almonds compared with potatoes were observed (P = 0.046, P = 0.006, respectively), with potato + herb/spices having intermediate effects.
Conclusion
There were no significant differences in FM or in glucoregulatory biomarkers after 30 d of potato consumption compared with almonds. Results do not support a causal relation between increased French fried potato consumption and the negative health outcomes studied. This trial was registered at clinicaltrials.gov as NCT03518515.
Keywords: carbohydrates, nuts, French fries, potato chips, adipose, glycemia, herb/spices
Introduction
Although humans have consumed potatoes in a variety of forms across cultures for millennia, their inclusion in human diets has periodically been controversial since the introduction of potatoes to Europe from the New World centuries ago (1). The modern rise of obesity in Westernized cultures has brought new scrutiny to the composition of diets, with potato consumption noted as a correlate of weight gain and diabetes risk (2–5). Published observational studies have suggested that increasing baseline potato consumption by 1 additional serving/d is associated with an increase in body weight over a 4-y period. This weight gain (positive energy balance) is proposed despite the relatively small contribution of potatoes to mean daily energy intake, where the change in average consumption of potatoes is <1 full serving every 5 d for 95% of the population reported (3, 6).
Despite the known and admitted limitations of nonrandomized evidence in nutrition research, a causative role for observed dietary associations—e.g., potato consumption and weight gain—is at times accepted in the face of existing counterevidence or presumed true even absent any probative study (7). Randomized controlled trial (RCT) data indicate that healthy humans often respond to changes in energy intake, including in single components of the diet, compensating for extra energy consumed (through dietary intake modification and/or energy expenditure changes) (8). This compensation results in substantially less change in body energy stores than would be predicted (8). Considering this, observational data describing an association may be valuable for developing hypotheses (as has been described for potato consumption and weight gain), but invite the use of randomized evidence to explicitly test for causation. The importance of this testing is highlighted by the fact that RCTs of specific nutrient interventions have often not only failed to support the predicted hypothesis, but at times have countered the predicted hypothesis in the opposite direction of effect (7, 9).
Perhaps this failure to validate observational findings in single nutrient/food items in RCTs is not so surprising when considering the biochemical complexity of the human diet. Whereas nutrition research has focused at times on single food items, diets (and thus nutrition) are inherently complex from a biochemical diversity perspective.
Noncaloric, bioactive compounds are found in many plants, including herb/spices that have been consumed in the human diet for thousands of years and are often paired with carbohydrate-rich root vegetables like potatoes (10–16). It is possible that herbs and spices interact with dietary carbohydrates via carbohydrate metabolism-modifying properties to delay ingestion and absorption, influencing glycemia and further enhancing the dietary fiber and satiety signals of potato consumption (10–16).
To test the hypotheses raised by association studies related to potato consumption and energy balance, we performed an RCT. We tested the hypothesis that there is no difference in weight gain (specifically FM change) with increased daily potato consumption (e.g., French fries) versus an isoenergetic (i.e., calorie-matched) portion of almonds, a food item with reported benefits for weight and glycemia control (17–21), in a 30-d RCT. We further asked whether a herb/spice mix consumed with potatoes might have significant (positive) effects on glycemic control.
Subjects and Methods
Randomized Controlled Trial
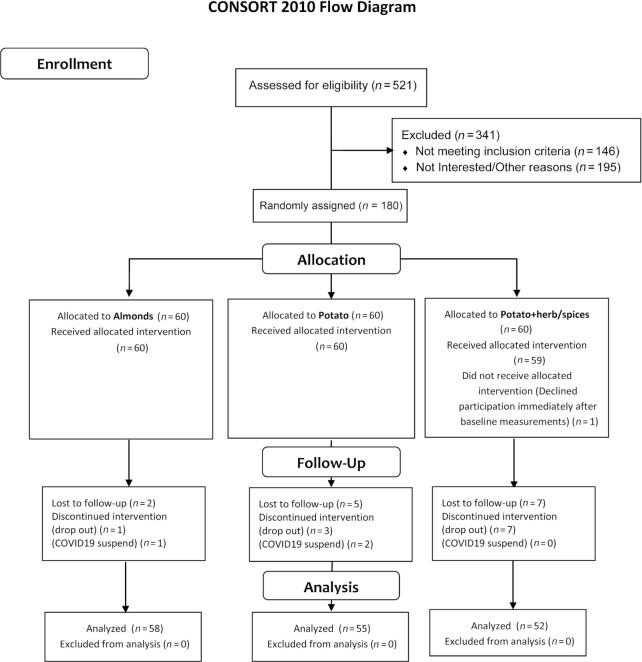
The study design followed an RCT with parallel randomization. Study participants were recruited from the University of Alabama at Birmingham (UAB) campus, the greater Birmingham, Alabama area, and surrounding communities through a combination of advertisements, including fliers and social media. In total, 180 study participants were randomly assigned to 1 of the 3 treatment groups using block random assignment with block sizes of 12, using PROC PLAN in SAS 9.4. Random assignments were sealed in numbered envelopes and provided to the study coordinator to be opened after eligibility verification and enrollment consent. Random assignment was to a “study group” in which participants consumed 1 of the 3 foods (calorie matched) each day for a 30-d period as described in the CONSORT diagram in Figure 1. The 3 arms included ∼300 kcal/d (Supplemental Table 1) from 1 of the 3 food items: 1) nonpotato food [as the “control group,” in the form of almonds, selected for comparison as a dietary component considered for its “health food” status in regard to energy-balance, body composition, and inherent low glycemic index (17–21), Wonderful® almonds, roasted and salted], 2) standard white potato French fries (potato group; Tater Pals®, Ovenable Crinkle Cut Fries, Simplot Foods), 3) white potato French fries with herb/spice mix [potato + herb/spice; a combination of oregano, basil, garlic, onion, and rosemary provided with single-use defined portion measures [approximately one teaspoon (5 cc) fixed amount per potato serving, Tater Pals® with herb/spice mix provided]. Instructions were provided regarding storage and preparation methods of food items for all participants. Food items were provided in “single day” (300 kcal) portion sizes weekly for the 30-d study, including frozen French fries and room temperature almonds. The method of preparation and timing of the daily consumption of the food items was left up to the study participants. Compliance in consumption of the food items was monitored by asking participants about their consumption of the food items, with some participants returning empty food packages and/or photographs thereof. Participants were asked to incorporate the specific food item into their normal daily diet with no other instruction or request for other dietary modification. Due to the differences in food items (visual, olfactory, gustatory), blinding was limited to assessors for outcome collection and analyses (e.g., research staff such as technician and phlebotomist).
FIGURE 1.
Study diagram. CONSORT flow diagram of study progress through the phases of the randomized controlled trial.
Ethics
The Human Subjects Protocol was approved by the UAB Institutional Review Board and performed in accordance with ethical standards of responsible research and conduct. This trial was registered at clinicaltrials.gov as NCT03518515.
Study eligibility
Participation in the study was dependent on meeting predefined criteria determined during screening by the study coordinator. These eligibility criteria were initially assessed during a phone interview and were verified during the in-person screening. Participation criteria are provided in Table 1.
TABLE 1.
Study participant eligibility criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| Gender: any | Nut or food allergy |
| Age: 18–50 y | Diabetes, type 1 or type 2 |
| Ethnicity: any | History of bariatric surgery |
| BMI (kg/m2): 20–35 | Pregnancy, anticipating pregnancy or lactation |
| Diet: no dietary prohibitions/allergies | Consumes >1 serving of potatoes daily before enrollment |
| Weight stable | Weight loss or gain >5% within past 6 mo |
| Medical conditions or medications that would prevent the ability to comply with treatment assignment and/or affect energy balance |
Body composition
Body composition (total FM, fat-free mass, and total lean mass) was determined by DXA with assistance from the Diabetes Research Center and Nutrition Obesity Research Center Metabolism Core. Change (Δ) in total “body fat” mass served as the primary outcome and was determined by participant scans at baseline and after 30-d study completion (22, 23).
Body weight
Body weight was determined at baseline and at study completion along with height for calculating individual subject BMI (in kg/m2), with change (Δ) in body weight (30-d treatment minus baseline) calculated from the collected values for analysis.
Fasting blood chemistries
Blood collection was performed at baseline and at study completion by trained research nursing personnel at the Clinical Research Unit, and blood was analyzed for factors related to glucose metabolism, including blood glucose, insulin, and glycated hemoglobin (HbA1c). Fasting glucose and fasting insulin were used to estimate insulin sensitivity relative to the general population using the HOMA-IR (24).
Meal-based tolerance test
More specific effects of the assigned treatment contributions to glucose dynamics were assessed using a meal-based tolerance test (MBTT) in a random subset of 5 participants in each group at the end of the 30-d study. Eight participants in each group were randomized for MBTT in the creation of the treatment allocation schedule, where the first 5 of 8 to schedule the follow-up visit completed the MBTT. Participants were required to fast for 12 h before the day of testing. At time “zero,” participants were asked to consume the assigned treatment food they had consumed over the last 30 d, and intake was completed within 15 min of meal initiation. Blood samples were collected at baseline (time point 0, 2 samples) and at 15, 30, 45, 60, 90, and 120 min. Sera was processed and stored at –85°C until analyzed. Samples were measured for glucose, insulin, and c-peptide (TOSOH Bioscience AIA900) with assistance from the DRC Human Physiology Core as previously described (24).
Power and sample size calculations
To determine group sample size for the primary study outcome (body fat), estimates based on published association relations of potato consumption and body weight gain (3) were used along with weight-gain prediction models (25), with the assumption of no compensation of assigned treatment energy content (∼300 kcal/d) and variance observed with the feeding study regarding body fat and weight change (SD: 0.9–1.8) (8, 22, 26). A sample size of n = 50/treatment was determined to provide sufficient power (0.8) to determine a moderate effect size (d: 0.57) with significant difference (α: 0.05; 2-tailed) of ∼0.67 kg (SD: 1.18) change in total body FM. A 20% increase in sample size (n = 60/group; n = 180 total) was recruited to accommodate potential study dropout.
Statistical analysis
Descriptive statistics for baseline characteristics are provided for study participants. Study data from baseline and 30-d completion were used to calculate “change” values for individual outcomes of interest. Data were analyzed with 1-way ANCOVA on changes from baseline with preselected characteristics [baseline pre-randomization age, gender (self-reported), and BMI (or FM) as covariates]. Analyses were performed once with the 2 potato groups combined and once across all 3 groups. Assumptions of normality and homoscedasticity of residuals were evaluated and satisfied by visual inspection of the residual plots (QQ-plots and residuals compared with predicted values) and measures of skewness and SDs (27, 28). The assumption of homogeneity of slopes in ANCOVA was evaluated with significance tests of interaction terms between covariates and group, where a significant interaction between group and baseline BMI was identified for total FM and is described below. All statistical analyses were performed with 2-tailed tests with 0.05 α level to determine statistical significance. P values are unadjusted for the multiple analyses performed such that the chance of a type I error across all results is > 0.05 overall. Following an omnibus F-test in the 3-group comparison, pairwise testing between groups was performed with Tukey adjustment [Tukey's honestly significant difference test (HSD)] for multiple comparisons to control the family-wise α level. Bayes factors (BFs) were calculated for each model to measure the evidence of the null hypothesis of no group effect, where BF >1 is evidence for the null hypothesis, and BF <1 is evidence against the null (29). Primary ANCOVA analyses included 165 participants who completed the study. To test the robustness of our findings, sensitivity analyses were performed as “intention to treat” (ITT) analysis including all 180 randomly assigned participants, with the use of ANCOVA and with missing data handled by multiple imputation as well as linear mixed models on outcomes at each time point (baseline/follow-up), where differences in changes among groups were tested with group × time interactions. Multiple-chain Markov chain Monte Carlo (MCMC) methods were used for multiple imputation in SAS (v.9.4), where the percentage of incomplete cases was used as the number of imputed datasets (m = 9). Additional sensitivity analyses were performed adjusting for baseline fat-free mass or lean mass in addition to age, gender, and BMI.
Results
Study population
A total of 180 participants were successfully recruited and randomly assigned to the study intervention groups. Descriptive statistics are provided in Table 2, revealing the similarity of baseline characteristics across the 3 study groups. There were n = 12 study dropouts and n = 3 study protocol terminations (due to Coronavirus Disease 2019–related suspension of research study protocols, Figure 1), representing 8.3% of the total study sample size, as shown in Table 2 (completers, Figure 1). All participants completing the study returned for final measures (n = 165), where each participant self-reported consumption of the assigned group food items. However, detailed records of daily or weekly consumption were not obtained for verification of adherence.
TABLE 2.
Participant characteristics and baseline measures for randomized participants (n = 180)1
| Almonds (n = 60) | Potatoes (n = 60) | Herb/spice mix (n = 60) | Total (n = 180) | |
|---|---|---|---|---|
| Age, y | 30.48 ± 8.39 | 30.27 ± 9.58 | 30.30 ± 8.32 | 30.35 ± 8.74 |
| Gender | ||||
| Female | 40 (66.67%) | 40 (66.67%) | 42 (70.00%) | 122 (67.78%) |
| Male | 20 (33.33%) | 20 (33.33%) | 18 (30.00%) | 58 (32.22%) |
| Race2 | ||||
| White | 30 (50.00%) | 40 (66.67%) | 35 (58.33%) | 105 (58.33%) |
| Black | 17 (28.33%) | 12 (20.00%) | 16 (26.67%) | 45 (25.00%) |
| Asian | 8 (13.33%) | 2 (3.33%) | 5 (8.33%) | 15 (8.33%) |
| Hispanic | 1 (1.67%) | 0 (0.00%) | 0 (0.00%) | 1 (0.56%) |
| Other | 4 (6.67%) | 6 (10.00%) | 4 (6.67%) | 14 (7.78%) |
| BMI | 25.71 ± 3.93 | 25.78 ± 3.85 | 26.83 ± 4.61 | 26.11 ± 4.15 |
| Total FM, g | 24326.08 ± 7835.04 | 24292.81 ± 9209.05 | 26591.88 ± 10081.22 | 25070.26 ± 9102.19 |
| Total fat-free mass, g | 49740.81 ± 11529.56 | 50833.33 ± 9900.57 | 49894.71 ± 11220.75 | 50156.28 ± 10856.23 |
| Total lean mass, g | 46980.11 ± 11029.42 | 48050.61 ± 9454.17 | 47157.04 ± 10704.95 | 47395.92 ± 10370.62 |
| Weight, kg | 74.39 ± 15.73 | 75.50 ± 14.10 | 76.78 ± 16.54 | 75.56 ± 15.44 |
| Glucose, mg/dL | 95.00 ± 7.19 | 97.08 ± 9.04 | 97.48 ± 8.77 | 96.52 ± 8.40 |
| Insulin, uU/mL | 10.97 ± 7.54 | 8.90 ± 6.23 | 9.83 ± 6.19 | 9.90 ± 6.70 |
| HbA1c, % | 5.18 ± 0.32 | 5.23 ± 0.33 | 5.20 ± 0.35 | 5.20 ± 0.33 |
| HOMA-IR | 2.64 ± 1.96 | 2.18 ± 1.68 | 2.43 ± 1.79 | 2.42 ± 1.81 |
Values presented as means ± SDs or n (%) of patients unless otherwise indicated. HbA1c, glycated hemoglobin.
Race as self-identified
Change in Body Composition (Total FM)
The primary outcome (change in total body FM) for both potato groups (with and without herb/spices) was not significantly different from that for the almond group (P = 0.443, Table 3). Similarly, change in total FM was not significantly different among the 3 treatment groups (Figure 2, Table 4), including adjustment for age, gender, and baseline BMI (P = 0.375) or baseline FM (P = 0.371). A BF value of 6.78 indicates evidence in favor of the null hypothesis of no true differences between groups. Sensitivity analyses also adjusting for baseline fat-free mass or lean mass provided similar results (no significant differences among the 3 treatment groups). Additionally, when testing model assumptions for homogeneous slopes in ANCOVA models, a significant interaction was identified with “group” by “baseline BMI” on change in “total FM” (P = 0.025). Participants with higher baseline BMI gained less weight in the potato group than the other 2 groups. Additional analyses of change in body composition outcomes from DXA, including fat-free mass and lean mass, were similarly not significantly different with a 2-group (almond compared with potato combined) assessment (P = 0.564 and P = 0.572, respectively, Table 3) or among the 3 groups (P = 0.156 and P = 0.154, respectively, Table 4). Analyses with imputation for those n = 15 participants lost to follow-up revealed a similar pattern of findings, namely, no significant difference in change in total FM (Supplemental Tables 2–5) or change in total fat free or lean mass within the 2-group comparison (Supplemental Tables 2 and 4), but a nearly statistically significant result among the 3 group comparison with mixed models (P = 0.060 and P = 0.057, respectively) wherein the potato group was lower in fat free mass and lean mass (Supplemental Table 5).
TABLE 3.
ANCOVA comparing 2 groups, combining potatoes with and without herb/spice mix (n = 165)1
| Almonds (n = 58) | Potatoes2 (n = 107) | ANCOVA P-value | |
|---|---|---|---|
| Changes from baseline | |||
| Total FM, g | 230.87 ± 114.01 | 123.73 ± 86.09 | 0.443 |
| Total fat free mass, g | 137.50 ± 155.00 | 27.94 ± 117.05 | 0.564 |
| Total lean mass, g | 140.64 ± 155.23 | 33.33 ± 117.22 | 0.572 |
| Weight, kg | 0.49 ± 0.20 | 0.10 ± 0.15 | 0.116 |
| Glucose, mg/dL | –0.44 ± 1.08 | –0.42 ± 0.82 | 0.985 |
| Insulin, uU/mL | –1.17 ± 0.69 | 0.31 ± 0.52 | 0.082 |
| HbA1c, % | 0.03 ± 0.02 | –0.005 ± 0.02 | 0.269 |
| HOMA-IR | –0.32 ± 0.19 | 0.09 ± 0.15 | 0.080 |
Values presented as means ± SEs unless otherwise indicated. ANCOVA models on change scores among groups were adjusted for age, gender, and baseline BMI; n = 165 who completed the trial. FM, fat mass; HbA1c, glycated hemoglobin.
Potatoes group combined with and without herb/spice mix.
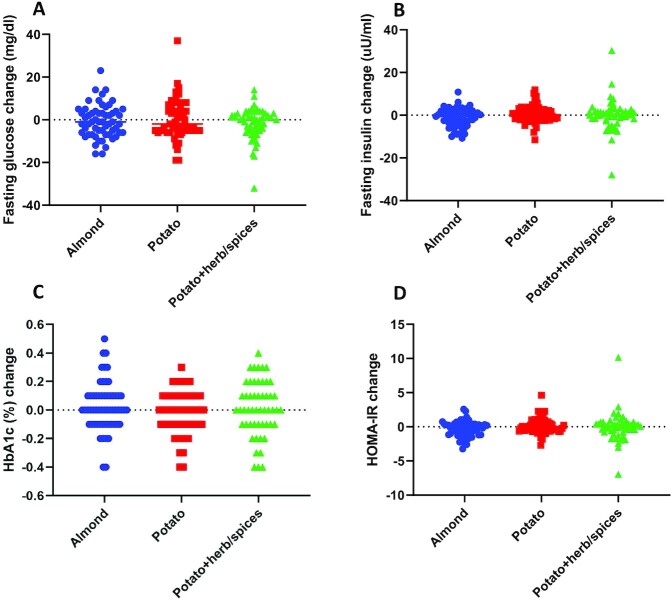
FIGURE 2.
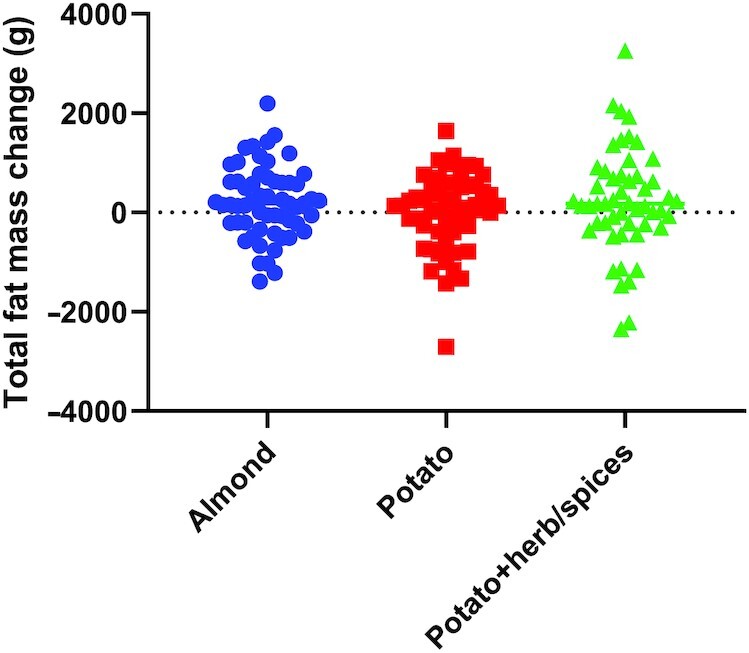
Change in total FM. Change in the primary outcome, total FM (in grams), as measured by DXA between baseline and study completion. Groups: almond—circles (n = 58); potato—squares (n = 55); potato + herb/spices—triangles (n = 52). Individual data shown in each diet treatment group. ANCOVA, adjusted for age, gender, and baseline BMI: P = 0.375; adjusted for age, gender, and baseline FM: P = 0.371. FM, fat mass.
TABLE 4.
ANCOVA comparing changes from baseline in 3 groups (n = 165)1
| Almonds (n = 58) | Potatoes (n = 55) | Herb/spice mix (n = 52) | ANCOVA P-value | Almonds vs. potatoes P-value2 | Almonds vs. herb/spice P-value2 | Potatoes vs. herb/spice P-value2 | BF | |
|---|---|---|---|---|---|---|---|---|
| Total FM, g | 231.27 ± 113.87 | 30.85 ± 116.83 | 225.16 ± 121.87 | 0.375 | 0.425 | 0.999 | 0.470 | 6.78 |
| Total fat free mass, g | 138.34 ± 153.84 | –169.64 ± 157.84 | 243.69 ± 164.65 | 0.156 | 0.331 | 0.882 | 0.157 | 3.16 |
| Total lean mass, g | 141.49 ± 154.05 | –165.84 ± 158.05 | 250.81 ± 164.86 | 0.154 | 0.334 | 0.874 | 0.153 | 3.40 |
| Weight, kg | 0.49 ± 0.20 | –0.24 ± 0.20 | 0.47 ± 0.21 | 0.014 | 0.025 | 0.999 | 0.035 | 0.35 |
| Glucose, mg/dL | –0.44 ± 1.08 | 0.58 ± 1.11 | –1.50 ± 1.16 | 0.418 | 0.780 | 0.775 | 0.384 | 8.93 |
| Insulin, uU/mL | –1.17 ± 0.70 | 0.41 ± 0.71 | 0.21 ± 0.74 | 0.217 | 0.243 | 0.353 | 0.979 | 4.57 |
| HbA1c, % | 0.03 ± 0.02 | –0.02 ± 0.02 | 0.01 ± 0.02 | 0.391 | 0.361 | 0.864 | 0.696 | 6.86 |
| HOMA-IR | –0.32 ± 0.19 | 0.13 ± 0.20 | 0.05 ± 0.21 | 0.208 | 0.220 | 0.374 | 0.955 | 3.84 |
Values presented as means ± SEs unless otherwise indicated. ANCOVA models on change scores among groups were adjusted for age, gender, and baseline BMI; n = 165 who completed the trial. BF, Bayes factor; FM, fat mass; HSD, honestly significant difference test; vs., versus.
P values from pairwise comparisons are adjusted with Tukey's HSD.
Change in body weight
Comparing between the potato (with and without herb/spices) and almond groups revealed a nonsignificant difference in weight gain, despite the small numeric value of weight change [0.49 kg compared with 0.10 kg, respectively, P = 0.116, Table 3, with imputation (Supplemental Table 2), and mixed models (Supplemental Table 4)]. In contrast with body composition, change in body weight was significantly different among the 3-group assessment (P = 0.014), with the potato group losing a significantly different amount of weight (–0.24 kg) than either the almond group (+0.49 kg, Tukey P = 0.025 compared with potato) or potato + herb/spices groups (+0.47 kg, Tukey P = 0.035 compared with potato), respectively [Figure 3, Table 4, with imputation (Supplemental Table 3), and mixed models (Supplemental Table 5)].
FIGURE 3.
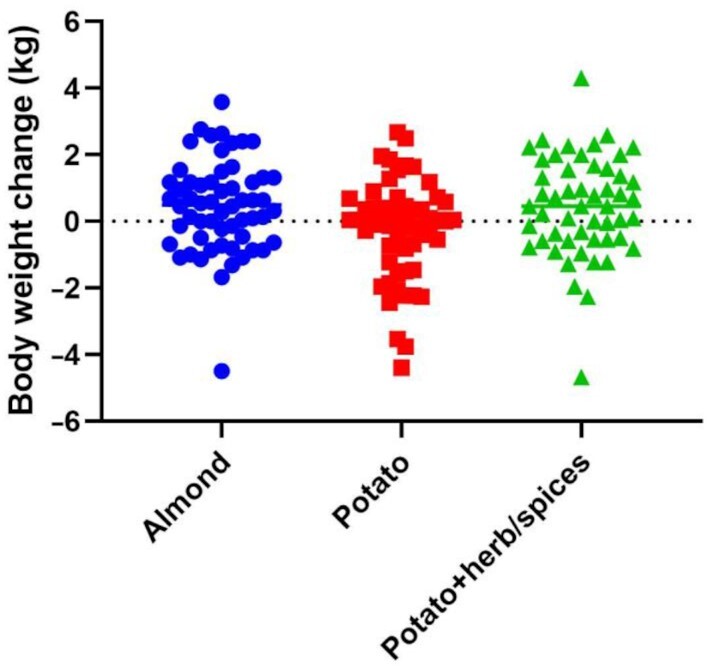
Change in body weight. Change in the body weight (in kg) measured between baseline and study completion. Groups: almond—circles (n = 58); potato—squares (n = 55); potato + herb/spices—triangles (n = 52). Individual data shown in each diet treatment group. ANCOVA adjusted for age, gender, and baseline BMI: P = 0.014.
Change in glucoregulatory biomarkers
Despite the observed small but significant difference in body weight change among the groups, there were no significant differences comparing potato with and without herb/spices with almonds for fasting glucose: P = 0.985; insulin: P = 0.082; or HbA1c: P = 0.269 (Table 3, Supplemental Tables 2 and 4). Similarly, there were no significant differences for change in fasting glucose: P = 0.418; insulin: P = 0.217; or HbA1c: P = 0.391, among the 3 treatment groups (Figure 4, Table 4, Supplemental Tables 3 and 5). HOMA-IR was also not significantly different between the almond and potato (combined) groups (P = 0.080), nor among groups in the 3-group comparison at the 0.05 level (Figure 4; Tables 3 and 4, Supplemental Tables 2–5).
FIGURE 4.
Change in glucoregulatory biomarkers. Change in fasting glucose (mg/dL) (A). Change in fasting insulin (uU/mL) (B). Change in HOMA-IR (C). Change in HbA1c (%) as measured between baseline and study completion (D). Groups: almond—circles (n = 58); potato—squares (n = 55); potato + herb/spices—triangles (n = 52). Individual data shown in each diet treatment group. ANCOVA adjusted for age, gender, and baseline BMI: glucose, P = 0.418; insulin, P = 0.217; HbA1c, P = 0.391; HOMA-IR, P = 0.208. HbA1c, glycated hemoglobin.
Meal-based tolerance tests
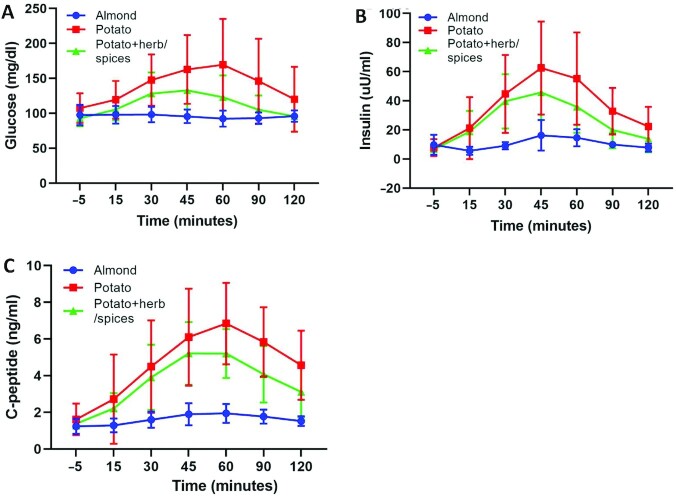
Acute effects on postprandial responses to each group treatment food item were examined on a randomly selected subsample of the total group (n = 5/group of the n = 60 total). Despite there being no significant difference for change in fasting values for glucose and insulin as noted above, the postprandial response of mean “peak” glucose was significantly different among groups (P = 0.014, Figure 5, Table 5), with the 120-min AUC for glucose being nearly statistically significant (P = 0.054) in the 3-group analysis, with significant between-group differences in the almond group compared with the potato group with no herb/spices (Tukey P = 0.046, Table 5, Figure 5), but no significant difference in AUC for the almond compared with the potato + herb/spices group (Tukey P = 0.601). Both peak and AUC values for insulin were significantly different among groups (Table 5), with the almond group exhibiting a lower postprandial response relative to the potato group which did not include herb/spices (Tukey P = 0.007). C-peptide, a marker of acute insulin secretion, was also significantly different among the 3 treatment groups for peak values, where almond group was < potato group (Tukey P < 0.001) and almond group was < potato + herb/spices group (Tukey P = 0.009) (Table 5). The C-peptide AUC was also significantly lower for the almond group relative to either potato group, with potato + herb/spices exhibiting intermediate levels that were not significantly different from the potato no herb/spices group (Table 5, Figure 5).
FIGURE 5.
Meal-based tolerance test. Glucose (mg/dL) (A). Insulin (uU/mL) (B). C-peptide (ng/mL) postprandial measures collected during a meal-based tolerance test following consumption of the group-assigned food item (C). Groups: Almond—circles; potato—squares; potato + herb/spices—triangles; (n = 5/treatment group; means ± SDs. ANCOVA adjusted for age, gender, and baseline BMI: AUC for glucose, P = 0.054; insulin, P = 0.008; C-peptide, P = 0.001. AUC, area under the curve.
TABLE 5.
Meal-based tolerance test: peak values and AUC1
| Almonds (n = 5) | Potatoes (n = 5) | Herb/spice mix (n = 5) | ANOVA P-value | Almonds vs. potatoes P-value2 | Almonds vs. herb/spice P-value2 | Potatoes vs. herb/spice P-value2 | |
|---|---|---|---|---|---|---|---|
| Glucose, mg/dL | |||||||
| Peak time, min | 41 | 51 | 42 | NA | |||
| Peak value | 101.00 ± 15.90 | 180.40 ± 15.90 | 144.20 ± 15.90 | 0.014 | 0.011 | 0.175 | 0.279 |
| AUC | 11907.00 ± 1541.72 | 17820.00 ± 1541.72 | 14051.50 ± 1541.72 | 0.054 | 0.046 | 0.601 | 0.235 |
| Insulin, uU/mL | |||||||
| Peak time, min | 60 | 54 | 42 | NA | |||
| Peak value | 17.08 ± 9.86 | 69.18 ± 9.86 | 49.32 ± 9.86 | 0.009 | 0.007 | 0.093 | 0.360 |
| AUC | 1323.40 ± 604.30 | 4621.10 ± 604.30 | 3291.05 ± 604.30 | 0.008 | 0.006 | 0.094 | 0.301 |
| C-peptide, ng/mL | |||||||
| Peak time, min | 69 | 69 | 60 | NA | |||
| Peak value | 2.03 ± 0.68 | 7.75 ± 0.68 | 5.56 ± 0.68 | <0.001 | <0.001 | 0.009 | 0.099 |
| AUC | 206.98 ± 58.08 | 620.43 ± 58.08 | 475.43 ± 58.08 | 0.001 | <0.001 | 0.017 | 0.222 |
Values presented as means ± SEs unless otherwise indicated. Peak and AUC values were first calculated for each participant and averaged across participants, using ANOVA for among group analyses for peak value and AUC. AUC, area under the curve; HSD, honestly significant difference test; NA, not applicable; vs., versus.
P values from pairwise comparisons are adjusted with Tukey's HSD.
Discussion
Potatoes remain the most highly consumed vegetable in the American diet and are a staple food providing nutrition to many cultures throughout the world (6, 30). In addition to complex carbohydrates, potatoes are a dietary source of multiple micronutrients, providing as much or more fiber and potassium (even on a per gram weight basis) than do other commonly consumed vegetables (6, 30). Despite the multiple epidemiologic studies associating dietary factors with health or disease risk that have brought attention to both potatoes and almonds, most often for their opposing relations to study outcomes, fewer randomized, controlled studies have been performed to directly test hypotheses regarding potential negative effects of potato consumption or positive effects of nut consumption. Notwithstanding the general agreement among prior observational studies, prospective RCTs have not supported negative effects on cardiometabolic risk factors related to potato consumption compared with other food items (31, 32).
The results of the present study further support the homeostatic response of humans to changes in dietary intake, which resulted in a lack of significant difference in body-fat gain among the groups. Similarly, the impact of the treatment assignments on additional outcomes related to fasting blood biomarkers of glucose homeostasis indicate that increasing the intake of potatoes by 300 kcal/d is no better or worse than consuming a caloric equivalent of nuts. The changes in body weight are more nuanced in that the potato consumers (without herb/spices) lost a small but statistically significant amount of body weight (including lean or fat-free mass) relative to the almond consumer group or the potato with herb/spices group. Although this change in body weight was not hypothesized and is the opposite of the predicted body-weight effects based on previous observations, the lack of body weight gain suggests factors contributing to energy balance were sufficient to compensate for the daily increased intake of calories from the potatoes.
The results of the meal-based tolerance tests provide additional insights to complement the standard pre-post measures of fasting blood markers. Although these measures were from a small, randomized subsample of the overall treatment groups, the resulting outcomes on blood glucose, insulin, and C-peptide highlight the potential importance for understanding study outcomes. Differences in outcomes may result from acute meal responses compared with the pre– and post–30-d measures and the acute effects that components in herbs/spices may have on postprandial glucose excursions. Findings from the meal-based tolerance test raise additional questions regarding the specific components within the herb/spices mix used as contributing to the glycemia response, whether increasing the amount of herb/spices per serving has additional effects and whether interactions between the individual herbs/spices used might be further enhanced (or suppressed) in the context of ingredients used in dishes that included potatoes or other carbohydrate-rich vegetables. Some of the most concentrated dietary sources of noncaloric phytochemicals are the herbs and spices broadly used in cuisine throughout history (33). Many of these herbs/spices also possess polyphenols, for which some have reported health benefits ranging from antioxidant to blood pressure effects. Notably, subclasses of polyphenols possess biophysical properties (α-glucosidase inhibition, with relevance to carbohydrate digestion and absorption) resembling that of the type 2 diabetes medication acarbose (34). Similar to other food items, noncaloric biochemical compounds are also present in types of potatoes (35, 36), with the complexity and number of phytochemicals differing between varieties of potatoes, with pigmented or colored potatoes (e.g., purple) containing high levels of polyphenols (10). Future studies focusing on herb/spice–derived phytochemicals at specific concentrations, singularly or in combination and relative to the type or complexity of carbohydrate consumed, will be necessary to assess any direct role on carbohydrate metabolism.
The completed RCT was implemented as a free-living trial, wherein participants were assigned to consume a daily caloric amount of the particular food item. This setting contrasts with an inpatient ward where greater control over the conditions of the study participants is available and where compliance of intake can be directly observed by study personnel (37). The hypotheses regarding potato consumption and weight gain are derived from observations of free-living, real-world conditions and rely on self-reported food intake, in line with the trial we have performed. Additionally, this experiment was not an overfeeding study in which participants are assigned to eat a diet containing their basal caloric requirements and then required to eat additional calories in excess of their requirement from the assigned food item. Study participants self-reported inclusion of the additional experimental group food items in their daily diets, and although adherence to their individual dietary standard practices was requested, participants were not prohibited from making voluntary changes in their complementary diet or activity behaviors that could contribute to overall energy balance during the study period. In contrast, the meal-based tests were performed in a clinical setting and confined to the experimental group daily assigned food items, preventing dietary compensation or variable activity during the acute period of collected measures. Similarly, the choice of a comparator group (control) in nutrition presents multiple challenges such as macronutrient differences, caloric density, and hedonic properties to name a few. In the present study, 2 food items with disparate associations to health outcomes were utilized, including the primary outcome of body fat and multiple secondary outcomes.
Despite these specific study design choices that were more likely to result in limitations compared with other potential design elements, this study also has multiple strengths to consider. In contrast with the observational reports, this RCT did not focus on a subset of individuals who voluntarily increased their daily consumption of potatoes, but rather randomly assigned participants to an intervention of increased daily consumption of potatoes or almonds. This design reduces issues related to unknown confounding, multiple collinearities, multiple testing with complex data, and other inherent design aspects from epidemiological studies. Additionally, previous studies have suggested that the specific form of potatoes consumed was less important than the food item itself for overall significance in association with health-related outcomes. We chose to utilize the white potato in a “French fry” as an oven-ready, prepared form for the current study, as it was a middle choice option between the polar dietary forms of a simple baked potato compared with potato chips. The differences in overall moisture content, frequency of consumption in modern dietary behaviors, relative contribution of the potato compared with preparation-associated calories (fat from frying), standardization of study food item preparation/presentation, and convenience were all factors in the potato food selection. Additionally, the addition of the selected herb/spices mix allowed an overview assessment of common dietary pairing of ingredients in dishes with potatoes and/or other starch-containing foods, highlighting the potential glucoregulatory properties that herb/spices may possess. Despite our matching the kilocalorie intake across groups, inherent differences in caloric density, moisture, fiber content, specific types of macronutrients, etc. remained among the food items utilized.
A weakness of the study relates to its short-term nature (i.e., 30 d). We cannot address whether these dietary changes could have impacted health over years or decades. Specifically, the meal test employing almond consumption was associated with lower glucose and insulin responses, and whether these differences would have more long-term consequences that were not observed with the 30-d trial is unknown. Additionally, in contrast with recent work comparing potato and rice consumption in participants who had diabetes (31), this study did not include participants with diabetes and utilized a different dietary form of potatoes, limiting extrapolation of findings from our work to individuals with diabetes. Nevertheless, the completed study provides experimental data testing a hypothesis generated from observational associations and finds no evidence that increased daily consumption of potatoes results in significantly greater FM, body weight gain, or changes in fasting blood biomarkers related to glucose metabolism, than an energy-matched consumption of almonds.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—DBA and DLS: conceived, designed, and supervised the study and developed and oversaw the protocol; RLH, SLD, and DLS: assisted with data collection; SLD, DBA, and DLS: assisted with interpretation; SLD and XC: implemented statistical analyses and prepared tables under the supervision of DBA and DLS; DLS: prepared initial versions of the manuscript; and all authors: read and approved the final manuscript. In the 36 mo prior to this report, DBA has received personal payments or promises for same from Amin Talati Wasserman and Glanbia; Arnold; Big Sky Health, Inc.; Biofortis Innovation Services (Merieux NutriSciences); California Walnut Commission; Clark Hill PLC; Kaleido Biosciences; Law Offices of Ronald Marron; Nestec/Nestle; Sports Research Corporation; and WW (formerly Weight Watchers International, LLC). Donations to a foundation have been made on his behalf by the Northarvest Bean Growers Association/Communique. DBA's institution, Indiana University, and the Indiana University Foundation have received funds or donations to support his research or educational activities from: American Egg Board; California Walnut Commission, Almond Board; Peanut Institute; Herbalife International; Mars, Incorporated; Mondelez; National Cattlemen's Beef Association; Reckitt Benckiser Group PLC; Alliance for Potato Research and Education; Dairy Management Inc.; Arnold Ventures LLC; and numerous other for-profit and nonprofit organizations to support the work of the School of Public Health and the university more broadly. SLD and XC's institution, Indiana University, has received funds to support their work from NIH; Reckitt Benckiser Group PLC; American Federation for Aging Research; Arnold Ventures LLC; Soleno Therapeutics; National Cattlemen's Beef Association; Whistle Labs, Inc.; and Alliance for Potato Research and Education. WTG has served as site PI for clinical trials sponsored through the University of Alabama at Birmingham and funded by Novo Nordisk, Pfizer, Eli Lilly, Lexicon, and Epitomee. He has served on advisory bords for Boehringer-Ingelheim, Novo Nordisk, Pfizer, JAZZ Pharmaceuticals, and the Milken Institute but has not accepted any compensation and so there is no financial relation. DLS's institution, University of Alabama at Birmingham, has received funds to support research from the National Institutes of Health, McCormick Science Institute, and the Alliance for Potato Research and Education. All other authors report no conflicts of interest.
Notes
This study was supported in part by a grant from the Alliance for Potato Research and Education (APRE) to DBA and DLS, by Core services through NIH grant awards P30DK056336 and P60DK079626 and the donation of study food items by JR Simplot Company. The opinions expressed are those of the authors and do not necessarily represent those of the APRE, NIH, or any other organization.
Supplemental Tables 1–5 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: APRE, Alliance for Potato Research and Education; BF, Bayes factor; FM, fat mass; HbA1c, glycated hemoglobin; HSD, honestly significant difference test; MBTT, Meal-Based Tolerance Test; MCMC, Markov Chain Monte Carlo; RCT, randomized controlled trial; UAB, University of Alabama at Birmingham.
Contributor Information
Daniel L Smith, Jr, Department of Nutrition Sciences, University of Alabama at Birmingham, Birmingham, AL, USA; Nutrition Obesity Research Center, University of Alabama at Birmingham, Birmingham, AL, USA; Integrative Center for Aging Resaerch, University of Alabama at Birmingham, Birmingham, AL, USA; Nathan Shock Center of Excellence in the Biology of Aging, University of Alabama at Birmingham, Birmingham, AL, USA.
Rebecca L Hanson, Department of Nutrition Sciences, University of Alabama at Birmingham, Birmingham, AL, USA.
Stephanie L Dickinson, Department of Epidemiology and Biostatistics, School of Public Health—Bloomington, Indiana University, Bloomington, IN, USA.
Xiwei Chen, Department of Epidemiology and Biostatistics, School of Public Health—Bloomington, Indiana University, Bloomington, IN, USA.
Amy M Goss, Department of Nutrition Sciences, University of Alabama at Birmingham, Birmingham, AL, USA; Nutrition Obesity Research Center, University of Alabama at Birmingham, Birmingham, AL, USA.
John B Cleek, Department of Nutrition Sciences, University of Alabama at Birmingham, Birmingham, AL, USA.
W Timothy Garvey, Department of Nutrition Sciences, University of Alabama at Birmingham, Birmingham, AL, USA; Nutrition Obesity Research Center, University of Alabama at Birmingham, Birmingham, AL, USA.
David B Allison, Department of Epidemiology and Biostatistics, School of Public Health—Bloomington, Indiana University, Bloomington, IN, USA.
Data Availability
Data described in the manuscript, code book, and analytic code will be made publicly and freely available without restriction at https://doi.org/10.5967/ppbv-f434It's.
References
- 1. De Jong H. Impact of the potato on society. Am J Potato Res. 2016;93(5):415–29. [Google Scholar]
- 2. Halton TL, Willett WC, Liu S, Manson JE, Stampfer MJ, Hu FB. Potato and French fry consumption and risk of type 2 diabetes in women. Am J Clin Nutr. 2006;83(2):284–90. [DOI] [PubMed] [Google Scholar]
- 3. Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364(25):2392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Muraki I, Rimm EB, Willett WC, Manson JE, Hu FB, Sun Q. Potato consumption and risk of type 2 diabetes: results from three prospective cohort studies. Diabetes Care. 2016;39(3):376–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bao W, Tobias DK, Hu FB, Chavarro JE, Zhang C. Pre-pregnancy potato consumption and risk of gestational diabetes mellitus: prospective cohort study. BMJ. 2016;352:h6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Storey ML, Anderson PA. Contributions of white vegetables to nutrient intake: NHANES 2009–2010. Adv Nutr. 2013;4(3):335S–44S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Young SS, Karr A. Deming, data and observational studies. A process out of control and needing fixing. Significance. 2011;8(3):116–20. [Google Scholar]
- 8. Dhurandhar EJ, Kaiser KA, Dawson JA, Alcorn AS, Keating KD, Allison DB. Predicting adult weight change in the real world: a systematic review and meta-analysis accounting for compensatory changes in energy intake or expenditure. Int J Obes. 2015;39(8):1181–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dhurandhar EJ, Dawson J, Alcorn A, Larsen LH, Thomas EA, Cardel M, Bourland AC, Astrup A, St-Onge MP, Hill JOet al. . The effectiveness of breakfast recommendations on weight loss: a randomized controlled trial. Am J Clin Nutr. 2014;100(2):507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ramdath DD, Padhi E, Hawke A, Sivaramalingam T, Tsao R. The glycemic index of pigmented potatoes is related to their polyphenol content. Food Funct. 2014;5(5):909–15. [DOI] [PubMed] [Google Scholar]
- 11. Linderborg KM, Salo JE, Kalpio M, Vuorinen AL, Kortesniemi M, Griinari M, Viitanen M, Yang B, Kallio H. Comparison of the postprandial effects of purple-fleshed and yellow-fleshed potatoes in healthy males with chemical characterization of the potato meals. Int J Food Sci Nutr. 2016;67(5):581–91. [DOI] [PubMed] [Google Scholar]
- 12. El-Beshbishy H, Bahashwan S. Hypoglycemic effect of basil (Ocimum basilicum) aqueous extract is mediated through inhibition of alpha-glucosidase and alpha-amylase activities: an in vitro study. Toxicol Ind Health. 2012;28(1):42–50. [DOI] [PubMed] [Google Scholar]
- 13. Kim SH, Jo SH, Kwon YI, Hwang JK. Effects of onion (Allium cepa L.) extract administration on intestinal alpha-glucosidases activities and spikes in postprandial blood glucose levels in SD rats model. Int J Mol Sci. 2011;12(6):3757–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moradabadi L, Montasser KS, Fehresti SM. Hypoglycemic effects of three medicinal plants in experimental diabetes: inhibition of rat intestinal alpha-glucosidase and enhanced pancreatic insulin and cardiac glut-4 mRNAs expression. Iran J Pharm Res. 2013;12(3):387–97. [PMC free article] [PubMed] [Google Scholar]
- 15. Kumar R, Chhatwal S, Arora S, Sharma S, Singh J, Singh N, Bhandari V, Khurana A. Antihyperglycemic, antihyperlipidemic, anti-inflammatory and adenosine deaminase-lowering effects of garlic in patients with type 2 diabetes mellitus with obesity. Diabetes Metab Syndr Obes. 2013;6:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Matsui T, Ueda T, Oki T, Sugita K, Terahara N, Matsumoto K. alpha-Glucosidase inhibitory action of natural acylated anthocyanins. 2. alpha-Glucosidase inhibition by isolated acylated anthocyanins. J Agric Food Chem. 2001;49(4):1952–6. [DOI] [PubMed] [Google Scholar]
- 17. Hollis J, Mattes R. Effect of chronic consumption of almonds on body weight in healthy humans. Br J Nutr. 2007;98(3):651–6. [DOI] [PubMed] [Google Scholar]
- 18. Li SC, Liu YH, Liu JF, Chang WH, Chen CM, Chen CY. Almond consumption improved glycemic control and lipid profiles in patients with type 2 diabetes mellitus. Metabolism. 2011;60(4):474–9. [DOI] [PubMed] [Google Scholar]
- 19. Tan SY, Mattes RD. Appetitive, dietary and health effects of almonds consumed with meals or as snacks: a randomized, controlled trial. Eur J Clin Nutr. 2013;67(11):1205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Berryman CE, West SG, Fleming JA, Bordi PL, Kris-Etherton PM. Effects of daily almond consumption on cardiometabolic risk and abdominal adiposity in healthy adults with elevated LDL-cholesterol: a randomized controlled trial. J Am Heart Assoc. 2015;4(1):e000993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hunter SR, Considine RV, Mattes RD. Almond consumption decreases android fat mass percentage in adults with high android subcutaneous adiposity but does not change HBA1C in a randomised controlled trial. Br J Nutr. 2021:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goss AM, Goree LL, Ellis AC, Chandler-Laney PC, Casazza K, Lockhart ME, Gower BA. Effects of diet macronutrient composition on body composition and fat distribution during weight maintenance and weight loss. Obesity. 2013;21(6):1139–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goss AM, Chandler-Laney PC, Ovalle F, Goree LL, Azziz R, Desmond RA, Wright BG, Gower BA. Effects of a eucaloric reduced-carbohydrate diet on body composition and fat distribution in women with PCOS. Metabolism. 2014;63(10):1257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gower BA, Chandler-Laney PC, Ovalle F, Goree LL, Azziz R, Desmond RA, Granger WM, Goss AM, Bates GW. Favourable metabolic effects of a eucaloric lower-carbohydrate diet in women with PCOS. Clin Endocrinol. 2013;79(4):550–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thomas DM, Martin CK, Lettieri S, Bredlau C, Kaiser K, Church T, Bouchard C, Heymsfield SB. Can a weight loss of one pound a week be achieved with a 3500-kcal deficit? Commentary on a commonly accepted rule. Int J Obes. 2013;37(12):1611–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sabate J, Cordero-MacIntyre Z, Siapco G, Torabian S, Haddad E. Does regular walnut consumption lead to weight gain?. Br J Nutr. 2005;94(5):859–64. [DOI] [PubMed] [Google Scholar]
- 27. Casella G, Berger RL. Statistical Inference. Belmont (CA): Duxbury; 2002. [Google Scholar]
- 28. Glass GV, Peckham PD, Sanders JR. Consequences of failure to meet assumptions underlying the fixed effects analyses of variance and covariance. Rev Educ Res. 1972;42(3):237–88. [Google Scholar]
- 29. Morey RD, Rouder JN. BayesFactor: Computation of Bayes Factors for Common Designs. R package version 0.9.12-4.2. 2018 [Internet]. Available from: https://CRAN.R-project.org/package=BayesFactor.
- 30. Weaver C, Marr ET. White vegetables: a forgotten source of nutrients: Purdue roundtable executive summary. Adv Nutr. 2013;4(3):318S–26S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Devlin BL, Parr EB, Radford BE, Hawley JA. Lower nocturnal blood glucose response to a potato-based mixed evening meal compared to rice in individuals with type 2 diabetes. Clin Nutr. 2021;40(4):2200–9. [DOI] [PubMed] [Google Scholar]
- 32. Johnston EA, Petersen KS, Kris-Etherton PM. Daily intake of non-fried potato does not affect markers of glycaemia and is associated with better diet quality compared with refined grains: a randomised, crossover study in healthy adults. Br J Nutr. 2020;123(9):1032–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Perez-Jimenez J, Neveu V, Vos F, Scalbert A. Identification of the 100 richest dietary sources of polyphenols: an application of the phenol-explorer database. Eur J Clin Nutr. 2010;64:S3, S112–20. [DOI] [PubMed] [Google Scholar]
- 34. Williamson G. Possible effects of dietary polyphenols on sugar absorption and digestion. Mol Nutr Food Res. 2013;57(1):48–57. [DOI] [PubMed] [Google Scholar]
- 35. Chong ES, McGhie TK, Heyes JA, Stowell KM. Metabolite profiling and quantification of phytochemicals in potato extracts using ultra-high-performance liquid chromatography-mass spectrometry. J Sci Food Agric. 2013;93(15):3801–8. [DOI] [PubMed] [Google Scholar]
- 36. Liu RH. Health-promoting components of fruits and vegetables in the diet. Adv Nutr. 2013;4(3):384S–92S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hall KD. Challenges interpreting inpatient and outpatient human nutrition studies. Cell Metab. 2019;30(2):227–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made publicly and freely available without restriction at https://doi.org/10.5967/ppbv-f434It's.