ABSTRACT
Background
Renal transplant recipients (RTRs) have a 6-fold higher risk of mortality than age- and sex-matched controls. Whether high consumption of ultra-processed foods is associated with survival in RTRs is unknown.
Objectives
We aimed to study the association between high consumption of ultra-processed foods and all-cause mortality in stable RTRs.
Methods
We conducted a prospective cohort study in adult RTRs with a stable graft. Dietary intake was assessed using a validated 177-item FFQ. Food items were categorized according to the NOVA classification system and the proportion ultra-processed foods comprised of total food weight per day was calculated.
Results
We included 632 stable RTRs (mean ± SD age: 53.0 ± 12.7 y, 57% men). Mean ± SD consumption of ultra-processed foods was 721 ± 341 g/d (28% of total weight of food intake), whereas the intake of unprocessed and minimally processed foods, processed culinary ingredients, and processed foods accounted for 57%, 1%, and 14%, respectively. During median follow-up of 5.4 y [IQR: 4.9–6.0 y], 129 (20%) RTRs died. In Cox regression analyses, ultra-processed foods were associated with all-cause mortality (HR per doubling of percentage of total weight: 2.13; 95% CI: 1.46, 3.10; P < 0.001), independently of potential confounders. This association was independent from the quality of the overall dietary pattern, expressed by the Mediterranean Diet Score (MDS) or Dietary Approaches to Stop Hypertension (DASH) score. When analyzing ultra-processed foods by groups, only sugar-sweetened beverages (HR: 1.21; 95% CI: 1.05, 1.39; P = 0.007), desserts (HR: 1.24; 95% CI: 1.02, 1.49; P = 0.03), and processed meats (HR: 1.87; 95% CI: 1.22, 2.86; P = 0.004) were associated with all-cause mortality.
Conclusions
Consumption of ultra-processed foods, in particular sugar-sweetened beverages, desserts, and processed meats, is associated with a higher risk of all-cause mortality after renal transplantation, independently of low adherence to high-quality dietary patterns, such as the Mediterranean diet and the DASH diet.
This trial was registered at clinicaltrials.gov as NCT02811835.
Keywords: ultra-processed foods, diet, NOVA, all-cause mortality, renal transplant recipients
See corresponding editorial on page 1455.
Introduction
Renal transplantation is the preferred treatment in patients with end-stage kidney disease (ESKD), because it improves quality of life and survival with lower medical costs compared with dialysis treatment (1, 2). Despite the success of renal transplantation, renal transplant recipients (RTRs) have a 6-fold greater risk of mortality than age- and sex-matched controls in the general population (3). It is not yet fully understood to what extent diet affects patient survival in RTRs, although it has been acknowledged that adherence to a healthy diet and lifestyle is of great importance (4).
Ultra-processed foods are industrial formulations that typically contain multiple ingredients, are made by a series of industrial processes, and contain little or no whole foods (5). They often contain additives, such as coloring chemicals and flavoring agents, and are loaded with sugars, fats, salt, and preservatives (6). They are known for their low nutritional quality and high energy density assembled in convenient (ready-to-eat), affordable, and hyper-palatable meals, and thereby are likely to replace freshly prepared dishes (5, 7). It is known that in the general population high consumption of ultra-processed foods is associated with cardiovascular disease (8), type 2 diabetes (9), cancer (10), and all-cause mortality (11–15). Although the detrimental effects of ultra-processed foods have been shown in previous studies, the consumption of ultra-processed foods also replaces the consumption of foods that are part of high-quality dietary patterns, which are mainly based on the intake of unprocessed or minimally processed foods. Scores that assess overall diet quality, such as the Mediterranean Diet Score (MDS) and the Dietary Approaches to Stop Hypertension (DASH) score, often focus on whether people eat healthy products and focus less on the consumption of unhealthy products, such as ultra-processed foods.
We already found that high adherence to a Mediterranean diet is associated with a lower risk of developing posttransplant diabetes mellitus (PTDM) and all-cause mortality (16), and leads to better kidney function outcomes in RTRs (17). Moreover, in this population a high adherence to the DASH diet is associated with a lower risk of all-cause mortality and renal function decline (18). It is known that elderly people on hemodialysis have a poorer diet quality and a higher consumption of ultra-processed foods than healthy elderly people without chronic kidney disease (19). This might (partly) be explained by the fact that most patients with ESKD are advised to consume a low-potassium and low-sodium diet, leading to restrictions in fruit, vegetable, nut, grain, and whole cereal consumption because these are the main sources of potassium. These dietary restrictions lead to patients replacing healthy foods with those that are unhealthy, characterized by ultra-processed foods that are high in energy, nonprocessed carbohydrates, and saturated fats (20). After renal transplantation there are fewer dietary restrictions, but unfortunately this does not seem to translate into an increase in fruit and vegetable intake in RTRs. A previous study showed that RTRs still have a lower intake of fruit and vegetables than the general population (21).
Despite many studies on dietary pattern scores and health outcomes in RTRs, the association of ultra-processed foods and health outcomes concerns a different pathway that, to the best of our knowledge, has not been studied in RTRs before. Whether high consumption of ultra-processed foods is associated with all-cause mortality in RTRs is not yet known. Therefore, we hypothesized that high consumption of ultra-processed foods would be associated with a higher risk of all-cause mortality in a large cohort of stable RTRs. Furthermore, we aimed to investigate whether potential associations of ultra-processed foods with all-cause mortality were independent of the MDS and DASH score. As secondary outcomes, we studied the associations of ultra-processed foods with death-censored graft failure, renal function decline, and PTDM.
Methods
Study design and population
For this observational prospective cohort study we used data from the TransplantLines Food and Nutrition Biobank and Cohort Study (NCT02811835). Adult RTRs (≥18 y old) with a functioning graft for ≥1 y, no history of alcohol and/or drug addiction, and without known malignancies or active infections were included as described previously (22, 23). Patients who visited the outpatient clinic of the University Medical Center Groningen (UMCG) between November 2008 and May 2011 were invited to participate. Of the initial 817 invited patients, we obtained the written consent of 707 (87%) RTRs. We excluded patients with missing dietary data (n = 75), leaving 632 RTRs eligible for analysis. For the analyses with PTDM, we also excluded patients with diabetes or a history of diabetes at baseline (n = 162), leaving 470 RTRs eligible for analyses (Supplemental Figure 1). Patients were advised to limit sodium intake and encouraged to lose weight in overweight individuals, but no other dietary advice was given. The Institutional Review Board approved the study protocol (METc 2008/186) and the study was performed according to the guidelines of the Declaration of Helsinki and the Declaration of Istanbul on Organ Trafficking and Transplant Tourism.
Data collection
All measurements were performed once at baseline during a morning visit to the outpatient clinic. Body weight and height were measured with patients wearing indoor clothing without shoes. BMI was calculated as weight divided by height squared (kg/m2). A semiautomatic device (Dinamap® 1846; Critikon) was used to measure blood pressure and heart rate. Relevant transplant characteristics and use of medication were retrieved from patient records. Information on smoking behavior was inquired using a questionnaire. Physical activity was assessed using the Short Questionnaire to Assess Health (SQUASH) (24). Blood was drawn after an 8- to 12-h overnight fasting period in the morning after completion of a 24-h urine collection. RTRs were instructed to discard their first morning urine specimen and then collect their urine for the next 24 h, including the next morning's first specimen of the day of their visit. The serum creatinine–based Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (25) was used to calculate the estimated glomerular filtration rate (eGFR).
Dietary intake assessment
Dietary intake was assessed at baseline using a semiquantitative FFQ, which consisted of 177 items. The FFQ was developed at Wageningen University, was reproducible, biomarker validated (26, 27), and updated several times. The questionnaire was self-administered and filled out at home to obtain information on dietary intake during the last month, taking into account seasonal variations. The frequency of consumption of each of the food items was recorded in times per day, week, or month. Expression of number of servings was in either natural units, such as a slice of bread or an apple, or household measures, for example a cup or a teaspoon. All questionnaires were checked for completeness by a trained researcher and inconsistent answers were verified with the participant. Subsequently, all dietary data were converted into total energy and nutrient intakes per day using the Dutch Food Composition Table (NEVO 2006) (28). In our cohort of RTRs, the FFQ was validated by comparing the protein intake of the FFQ with the protein intake calculated by the Maroni equation, using urinary urea excretion values (29).
NOVA classification of foods
All food items of the FFQ were categorized according to the 4 NOVA food groups (unprocessed or minimally processed foods, culinary ingredients, processed foods, and ultra-processed foods—as described in Supplemental Table 1), which is a classification system that divides food items based on the extent and purpose of industrial food processing (7, 30). In this study we focus on the last NOVA group, which contains ultra-processed foods that are largely or entirely made from industrial substances and contain little or no whole foods.
Adherence to the Mediterranean diet was assessed using an MDS as proposed by Trichopoulou et al. (31). Adherence to the DASH diet was evaluated using the DASH score (32, 33). A more detailed description of how these a priori dietary pattern scores were obtained is published elsewhere (17, 18). All patients were divided into tertiles of the overall MDS and DASH score.
Clinical endpoint
The primary outcome of this study was all-cause mortality and the secondary outcomes were death-censored graft failure, renal function decline, and PTDM. Death-censored graft failure was defined as return to dialysis treatment or retransplantation. Renal function decline was defined as doubling of serum creatinine concentration. PTDM was diagnosed according to the American Diabetes Association criteria, when ≥1 of the following criteria was met: 1) symptoms of diabetes (e.g., polyuria, polydipsia, unexplained weight loss) plus a nonfasting plasma glucose concentration ≥11.1 mmol/L (200 mg/dL), 2) fasting plasma glucose ≥7.0 mmol/L (126 mg/dL), 3) start of antidiabetes medication, or 4) plasma glycated hemoglobin (HbA1c) ≥6.5% (48 mmol/mol). Data were retrieved from patient files until the end of September 2015. RTRs were censored for PTDM at the time of graft failure or death. Because the outpatient program uses continuous surveillance systems, it guarantees correct and up-to-date information on patient status. No participants were lost to follow-up.
Statistical analysis
We estimated the consumption of unprocessed and minimally processed foods (category 1), processed culinary ingredients (category 2), processed foods (category 3), and ultra-processed foods (category 4) in RTRs by summing up the food items classified by the NOVA system in grams per day. Subsequently, the weight proportion of ultra-processed foods in the diet (percentage of total food weight in g/d) was calculated for each patient. Currently, there is no standard method to estimate intake of ultra-processed foods. A previous study from another group showed that proportion in weight was to be preferred over proportion in energy (34). Proportion in weight tends to give lower importance to energy-dense foods (often consumed in lower amounts), but higher importance to low-energy foods (often consumed in higher amounts), such as sugar-sweetened beverages. Some ultra-processed foods do not contribute to energy intake at all, such as artificial sweeteners, and therefore a weighting based on energy intake would structurally miscalculate intake of these ultra-processed foods. Analyzing ultra-processed foods using weight proportion also takes nonnutritional issues concerning food processing, such as neo-formed contaminants, into account (35, 36). Moreover, ultra-processed foods are suggested to be less satiating than minimally processed foods owing to alterations to food structures (e.g., fractioning and recombining of ingredients) (37). Both the processing and satiety characteristics of ultra-processed foods are more related to the total amount consumed rather than the energy density of foods. Normally distributed data were presented as mean ± SD, whereas skewed distributed data were expressed as median [IQR]. Categorical data were presented as n (%). Differences between sex-specific tertiles of ultra-processed foods were compared using 1-factor ANOVA tests for normally distributed variables, Kruskal–Wallis tests for skewed distributed variables, and chi-square tests for categorical variables. Correlations between ultra-processed foods and the MDS and the DASH score were assessed using Pearson correlation coefficients.
We performed Cox proportional hazards regression analyses to assess the association of ultra-processed foods with all-cause mortality in RTRs. In the Cox regression models, a doubling in the weight proportion of ultra-processed food intake in the diet was used as a continuous variable, whereas sex-specific tertiles were used as categorical variables. Sex-specific cutoffs were used, because women generally comply to a more healthy diet and have a lower food intake than men, allowing us to ensure equivalent sex ratios between tertiles. First, we performed analyses adjusted for age and sex (model 1). We further cumulatively adjusted for time between transplantation and baseline, eGFR, urinary protein excretion, primary renal disease, and donor age in model 2 and total energy intake, alcohol consumption, smoking status, and physical activity in model 3. To prevent overfitting by including too many covariates in relation to the number of events (38), we adjusted for other potential confounders in additional models based on model 3. In model 4, we included use of calcineurin inhibitors, antihypertensive drugs, and statins in addition to model 3, and in model 5 we included BMI and 24-h urinary creatinine excretion as a proxy for muscle mass in addition to model 3. To assess whether the association of ultra-processed food intake and all-cause mortality was independent of high-quality dietary patterns, we in addition adjusted model 3 for the MDS and DASH score in models 6a and 6b, respectively. Patients were censored at date of last follow-up or death. HRs and 95% CIs were given for the Cox proportional hazards analyses. Schoenfeld residuals were assessed and tested using the proportional hazard test by Grambsch and Therneau (39). The association of ultra-processed foods and all-cause mortality was visualized by fitting multivariable Cox regression analyses based on model 3. In addition, we evaluated the association of ultra-processed foods by groups with all-cause mortality.
We continued by comparing patients with the poorest diet (high consumption of ultra-processed foods and low adherence to the Mediterranean diet or DASH diet) and patients with the best diet quality (low consumption of ultra-processed foods and high adherence to the Mediterranean diet or DASH diet). Tertiles of ultra-processed food intake were reversed and summed up with the tertiles of the MDS and the DASH score, respectively. Subsequently, we performed Cox proportional hazards regression analyses to assess the association with all-cause mortality in RTRs with the poorest diet when compared with RTRs with the best diet quality. In addition, we performed these analyses based on an ultra-processed food score only including sugar-sweetened beverages, desserts, and processed meats.
As secondary analyses, we performed Cox proportional hazards regression analyses to assess the association of ultra-processed foods with death-censored graft failure, renal function decline, and PTDM in RTRs. Again, the weight proportion of ultra-processed food intake in the diet per doubling was used as a continuous variable, whereas sex-specific tertiles were used as categorical variables. Adjustments were made according to the main analysis of the article.
As sensitivity analyses and to allow for comparison with previous studies, we also calculated the proportion in energy of ultra-processed foods (percentage of total food energy in kcal/d). In the Cox regression models, a doubling in the energy proportion of ultra-processed food intake in the diet was used as a continuous variable, whereas sex-specific tertiles were used as categorical variables.
Cox proportional hazards regression analyses were performed using IBM Statistics SPSS version 23.0 (IBM Inc.). Schoenfeld residuals were checked and tested using STATA version 11.0 (StataCorp LP). GraphPad Prism 5 (GraphPad Software Inc.) was used to make a forest plot, whereas R version 3.2.3 (R Foundation for Statistical Computing) was used to perform penalized spline analyses. A 2-sided P value < 0.05 was considered statistically significant.
Results
Patient characteristics
Table 1 shows baseline characteristics of the overall RTR population and according to sex-specific tertiles of ultra-processed foods. In total, 632 patients were included at a median of 5.7 y [IQR: 1.9–12.1 y] after transplantation. Mean age was 53.0 ± 12.7 y and 357 (57%) were men. In general, RTRs with a higher consumption of ultra-processed foods were younger, had lower HDL cholesterol concentrations, used less antihypertensives and statins, had higher muscle mass measured by 24-h urinary creatinine excretion rate, and were transplanted more recently.
TABLE 1.
Baseline characteristics of the RTR population overall and according to sex-specific tertiles of UPFs
| Sex-specific tertiles | |||||
|---|---|---|---|---|---|
| UPFs | Overall RTRs (n = 632) | T1 (n = 210) | T2 (n = 211) | T3 (n = 211) | P value |
| Background variables | |||||
| Age, y | 53.0 ± 12.7 | 57.4 ± 10.1 | 54.7 ± 11.3 | 46.9 ± 14.0 | <0.001 |
| Male gender | 357 (57) | ||||
| Current smokers | 76 (12.0) | 27 (12.9) | 20 (9.5) | 29 (13.7) | 0.32 |
| Physical activity score (time × intensity) | 5230 [2423–8029] | 4920 [2040–7545] | 5760 [2760–8580] | 4940 [1800–7380] | 0.09 |
| Weight, kg | 80.3 ± 16.5 | 78.7 ± 15.4 | 80.6 ± 16.4 | 81.6 ± 17.5 | 0.20 |
| BMI, kg/m2 | 26.6 ± 4.8 | 26.2 ± 4.3 | 26.8 ± 4.8 | 26.9 ± 5.1 | 0.20 |
| eGFR, mL · min−1 · 1.73 m−2 | 52.5 ± 20.1 | 52.9 ± 19.8 | 49.2 ± 17.5 | 55.3 ± 22.4 | 0.007 |
| Circulation | |||||
| Heart rate, bpm | 68.7 ± 12.1 | 68.9 ± 13.1 | 67.7 ± 11.0 | 69.7 ± 12.0 | 0.24 |
| SBP, mm Hg | 136.2 ± 17.3 | 135.1 ± 18.5 | 137.6 ± 15.9 | 135.8 ± 17.5 | 0.32 |
| DBP, mm Hg | 82.9 ± 11.0 | 81.4 ± 10.5 | 83.5 ± 10.7 | 83.8 ± 11.6 | 0.05 |
| MAP, mm Hg | 100.7 ± 12.0 | 99.3 ± 12.2 | 101.6 ± 11.2 | 101.2 ± 12.7 | 0.12 |
| Laboratory parameters | |||||
| Triglycerides, mmol/L | 1.7 [1.2–2.3] | 1.7 [1.3–2.4] | 1.7 [1.3–2.2] | 1.7 [1.2–2.3] | 0.88 |
| Total cholesterol, mmol/L | 5.1 ± 1.1 | 5.2 ± 1.1 | 5.1 ± 1.1 | 5.1 ± 1.2 | 0.66 |
| HDL cholesterol, mmol/L | 1.4 ± 0.5 | 1.5 ± 0.5 | 1.4 ± 0.4 | 1.3 ± 0.5 | 0.04 |
| LDL cholesterol, mmol/L | 3.0 ± 0.9 | 3.0 ± 0.9 | 3.0 ± 0.9 | 3.0 ± 1.0 | 0.98 |
| Potassium, mmol/L | 4.0 ± 0.5 | 4.0 ± 0.4 | 4.0 ± 0.5 | 4.0 ± 0.5 | 0.47 |
| Phosphate, mmol/L | 1.0 ± 0.2 | 1.0 ± 0.2 | 1.0 ± 0.2 | 1.0 ± 0.2 | 0.54 |
| C-reactive protein, mg/dL | 1.6 [0.7–4.5] | 1.6 [0.7–3.9] | 1.6 [0.7–4.7] | 1.6 [0.6–5.1] | 0.92 |
| Fasting glucose, mmol/L | 5.3 [4.8–6.0] | 5.3 [4.7–6.2] | 5.2 [4.8–6.0] | 5.2 [4.8–5.9] | 0.73 |
| HbA1c, % | 6.0 ± 0.8 | 6.0 ± 0.8 | 6.0 ± 0.9 | 5.9 ± 0.8 | 0.24 |
| Urinary parameters | |||||
| Sodium excretion, mmol/24 h | 157.2 ± 61.2 | 147.0 ± 51.8 | 160.5 ± 63.3 | 164.0 ± 66.4 | 0.01 |
| Potassium excretion, mmol/24 h | 73.1 ± 24.3 | 75.9 ± 24.6 | 73.9 ± 24.5 | 69.5 ± 23.5 | 0.02 |
| Creatinine excretion, mmol/24 h | 11.7 ± 3.4 | 11.2 ± 3.0 | 11.7 ± 3.4 | 12.2 ± 3.7 | 0.01 |
| Urea excretion, mmol/24 h | 391.2 ± 114.7 | 389.5 ± 104.6 | 398.5 ± 118.9 | 385.6 ± 120.2 | 0.50 |
| Proteinuria | 138 (21.8) | 39 (18.6) | 46 (21.8) | 53 (25.1) | 0.27 |
| Primary renal disease | <0.001 | ||||
| Primary glomerulopathy | 179 (28.3) | 59 (28.1) | 72 (34.1) | 48 (22.7) | |
| Glomerulonephritis secondary to systemic disease | 46 (7.3) | 16 (7.6) | 11 (5.2) | 19 (9.0) | |
| Tubulointerstitial nephritis | 74 (11.7) | 17 (8.1) | 14 (6.6) | 43 (20.4) | |
| Polycystic kidney disease | 135 (21.4) | 55 (26.2) | 43 (20.4) | 37 (17.5) | |
| Kidney hypoplasia and dysplasia | 23 (3.6) | 7 (3.3) | 6 (2.8) | 10 (4.7) | |
| Renovascular diseases | 36 (5.7) | 12 (5.7) | 9 (4.3) | 15 (7.1) | |
| Diabetes kidney disease | 30 (4.7) | 12 (5.7) | 10 (4.7) | 8 (3.8) | |
| Other or unknown | 109 (17.2) | 32 (15.2) | 46 (21.8) | 31 (14.7) | |
| Transplant characteristics | |||||
| Transplant vintage, y | 5.7 [1.9–12.1] | 6.9 [2.6–14.4] | 5.0 [1.7–11.4] | 5.0 [1.5–10.2] | 0.009 |
| Living donor | 216 (34.2) | 62 (29.5) | 75 (35.5) | 79 (37.4) | 0.20 |
| Pre-emptive transplant | 103 (16.3) | 29 (13.8) | 35 (16.6) | 39 (18.5) | 0.43 |
| Dialysis duration, mo | 36.0 [20.0–59.0] | 30.0 [15.0–55.0] | 43.0 [30.0–56.0] | 46.0 [25.0–63.0] | 0.15 |
| Age of donor, y | 43.1 ± 15.5 | 41.4 ± 16.1 | 45.9 ± 14.5 | 42.0 ± 15.5 | 0.006 |
| Cold ischemia time, h | 15.3 [2.8–21.3] | 16.4 [3.0–22.8] | 15.0 [2.7–21.0] | 14.7 [2.6–20.7] | 0.05 |
| Warm ischemia time, min | 40.0 [33.0–50.0] | 38.0 [32.0–49.0] | 41.5 [35.0–50.0] | 40.0 [33.0–50.0] | 0.22 |
| Acute rejection | 166 (26.3) | 57 (27.1) | 60 (28.4) | 49 (23.2) | 0.45 |
| Medication | |||||
| Calcineurin inhibitor | 0.02 | ||||
| Cyclosporine | 250 (39.6) | 87 (41.4) | 89 (42.2) | 74 (35.1) | |
| Tacrolimus | 111 (17.6) | 24 (11.4) | 37 (17.5) | 50 (23.7) | |
| Proliferation inhibitor | 0.14 | ||||
| Azathioprine | 111 (17.6) | 45 (21.4) | 31 (14.7) | 35 (16.6) | |
| Mycofenol | 417 (66.1) | 130 (61.9) | 138 (65.4) | 149 (70.6) | |
| Prednisolone dose, mg | 10.0 [7.5–10.0] | 10.0 [7.5–10.0] | 10.0 [7.5–10.0] | 10.0 [7.5–10.0] | 0.78 |
| Antihypertensives | 556 (88.0) | 190 (90.5) | 192 (91.0) | 174 (82.5) | 0.01 |
| Antidiabetics | 97 (15.3) | 38 (18.1) | 31 (14.7) | 28 (13.3) | 0.37 |
| Statins | 338 (53.5) | 123 (58.6) | 122 (57.8) | 93 (44.1) | 0.004 |
Values are mean ± SD, median [IQR], or n (%). Differences were tested by ANOVA or Kruskal–Wallis test for continuous variables and with the χ2 test for categorical variables. DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; MAP, mean arterial pressure; RTR, renal transplant recipient; SBP, systolic blood pressure; T, tertile; UPF, ultra-processed food.
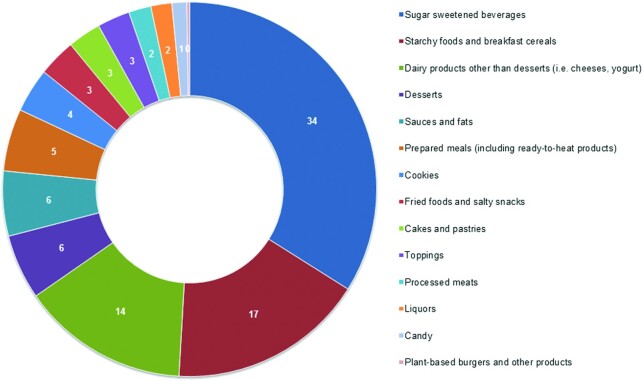
The mean consumption of ultra-processed foods was 721 ± 341 g/d (28% of total food weight per day). The intake of unprocessed and minimally processed foods, processed culinary ingredients, and processed foods accounted for 57%, 1%, and 14%, respectively, of total food weight per day (Table 2). The main food groups contributing to ultra-processed food intake were sugar-sweetened beverages (34%), starchy food and breakfast cereals (17%), dairy products other than desserts (i.e., cheese, yogurt) (14%), desserts (6%), sauces and fats (6%), and prepared meals, such as packaged ready-to-heat products (5%) (Figure 1). Table 2 shows the food groups contributing to ultra-processed food consumption and other nutritional factors of the overall RTR population and according to sex-specific tertiles of ultra-processed foods. Patients in the highest tertile of ultra-processed foods had a higher total energy intake and consumed more sugar-sweetened beverages, starchy food and breakfast cereals, dairy products other than desserts (i.e., cheese, yogurt), desserts, sauces and fats, prepared meals, cookies, fried foods and salty snacks, processed meats, and candy than patients in the lowest tertile. RTRs who consumed more ultra-processed foods consumed more carbohydrates and less proteins than patients with lower intake of ultra-processed foods, but there was no difference in total fat intake. The intake of SFAs, MUFAs, and sodium, measured by 24-h urinary sodium excretion, was higher in the RTRs who consumed more ultra-processed foods than in RTRs who consumed less ultra-processed foods. No difference was found in protein derived from animals or plants. Furthermore, RTRs in the highest tertile had a lower intake of potassium, measured by 24-h urinary potassium excretion, and total fiber, than RTRs with a low consumption of ultra-processed foods, which corresponds to a lower intake of fruit and vegetables. Adherence to both the Mediterranean diet and the DASH diet was significantly lower in RTRs with the highest consumption of ultra-processed foods. There was an inverse correlation between consumption of ultra-processed foods and the MDS (r = −0.23, P < 0.001) and the DASH score (r = −0.45, P < 0.001).
TABLE 2.
Food groups contributing to UPF consumption and other nutritional factors in the RTR population overall and according to sex-specific tertiles of UPFs1
| Sex-specific tertiles | |||||
|---|---|---|---|---|---|
| UPFs | Overall RTRs (n = 632) | T1 (n = 210) | T2 (n = 211) | T3 (n = 211) | P value |
| Total energy intake, kcal/d | 2172.7 ± 638.3 | 2033.3 ± 578.7 | 2203.5 ± 593.3 | 2280.6 ± 711.9 | <0.001 |
| Weight proportion UPFs, % g/d | |||||
| Men | 29.7 ± 11.8 | 18.1 ± 3.9 | 28.1 ± 3.2 | 43.1 ± 8.8 | <0.001 |
| Women | 26.2 ± 9.9 | 16.3 ± 3.6 | 25.1 ± 2.4 | 37.2 ± 7.5 | <0.001 |
| Energy proportion UPFs, % kcal/d | |||||
| Men | 33.9 ± 14.1 | 22.4 ± 5.6 | 31.8 ± 7.1 | 47.4 ± 14.1 | <0.001 |
| Women | 32.9 ± 13.1 | 22.7 ± 6.0 | 31.1 ± 6.6 | 45.0 ± 13.6 | <0.001 |
| NOVA classification | |||||
| Unprocessed or minimally processed foods, g/d | 1454.9 ± 471.2 | 1733.0 ± 469.5 | 1467.4 ± 368.0 | 1165.0 ± 387.4 | <0.001 |
| Percentage of total food intake | 57% | ||||
| Processed culinary ingredients, g/d | 26.3 ± 26.7 | 26.8 ± 26.4 | 24.9 ± 26.1 | 27.3 ± 27.7 | 0.62 |
| Percentage of total food intake | 1% | ||||
| Processed foods, g/d | 365.7 ± 239.8 | 411.1 ± 256.0 | 363.8 ± 235.3 | 322.4 ± 219.8 | 0.001 |
| Percentage of total food intake | 14% | ||||
| UPFs, g/d | 721.2 ± 341.1 | 453.0 ± 151.5 | 679.7 ± 193.3 | 1029.7 ± 346.8 | <0.001 |
| Percentage of total food intake | 28% | ||||
| Sugar-sweetened beverages | 163.3 [53.6–326.1] | 42.9 [8.3–123.1] | 153.6 [85.7–294.0] | 406.7 [258.4–630.9] | <0.001 |
| Starchy foods, breakfast cereals | 115.4 [84.7–149.4] | 106.4 [78.7–140.3] | 125.7 [94.7–156.7] | 114.8 [85.2–153.4] | 0.001 |
| Dairy products (not desserts) | 85.8 [28.8–145.0] | 62.1 [13.3–127.3] | 92.8 [41.4–145.0] | 104.6 [49.3–187.9] | <0.001 |
| Desserts | 20.7 [3.7–61.2] | 9.2 [0.5–33.7] | 28.3 [4.5–65.8] | 27.1 [9.2–66.7] | <0.001 |
| Sauces and fats | 38.1 [24.5–52.7] | 32.3 [19.3–47.6] | 39.9 [28.6–53.8] | 38.1 [25.4–57.3] | 0.001 |
| Prepared meals | 21.9 [5.8–51.3] | 12.9 [0.0–39.6] | 20.8 [5.8–50.5] | 40.2 [16.1–70.8] | <0.001 |
| Cookies | 21.4 [10.7–36.7] | 18.5 [8.4–29.9] | 23.8 [13.1–34.7] | 24.6 [11.8–43.5] | 0.002 |
| Fried foods and salty snacks | 16.9 [6.3–31.6] | 10.7 [2.3–21.8] | 16.3 [6.4–30.6] | 24.9 [12.5–39.6] | <0.001 |
| Cakes and pastries | 13.8 [7.9–29.1] | 13.2 [6.9–26.4] | 16.0 [7.9–34.4] | 13.0 [7.7–28.6] | 0.10 |
| Toppings | 15.0 [5.4–30.0] | 13.1 [4.5–28.8] | 16.3 [6.4–32.5] | 15.0 [4.3–29.8] | 0.07 |
| Processed meats | 11.1 [4.5–20.0] | 8.3 [1.5–16.6] | 12.2 [4.6–20.2] | 12.7 [7.3–21.4] | <0.001 |
| Liquors | 0.0 [0.0–3.7] | 0.0 [0.0–3.7] | 0.0 [0.0–4.5] | 0.0 [0.0–3.7] | 0.82 |
| Candy | 5.9 [1.6–13.0] | 4.9 [1.2–10.6] | 5.8 [1.9–11.0] | 7.1 [2.2–16.8] | 0.008 |
| Plant-based products | 0.0 [0.0–0.0] | 0.0 [0.0–0.0] | 0.0 [0.0–0.0] | 0.0 [0.0–0.0] | 0.78 |
| Nutrients | |||||
| Carbohydrates, En% | 45.9 ± 6.4 | 44.6 ± 6.5 | 45.7 ± 6.4 | 47.3 ± 6.1 | <0.001 |
| Protein, En% | 15.5 ± 2.6 | 16.2 ± 2.5 | 15.6 ± 2.6 | 14.6 ± 2.4 | <0.001 |
| Animal protein, g/d | 51.4 ± 14.9 | 50.9 ± 14.1 | 52.7 ± 15.4 | 50.5 ± 15.2 | 0.29 |
| Plant protein, g/d | 30.7 ± 9.9 | 29.5 ± 9.2 | 31.9 ± 10.3 | 30.6 ± 9.9 | 0.05 |
| Fat, En% | 35.8 [32.4–40.0] | 35.5 [32.1–39.9] | 36.1 [32.9–39.7] | 35.8 [32.2–40.5] | 0.66 |
| SFAs, g/d | 29.3 [22.7–37.7] | 26.8 [20.9–35.2] | 30.3 [24.2–38.0] | 31.6 [23.7–39.4] | <0.001 |
| MUFAs, g/d | 28.2 [21.5–35.1] | 25.2 [19.6–32.8] | 29.9 [22.6–35.3] | 29.3 [22.9–36.9] | 0.001 |
| PUFAs, g/d | 17.3 [13.0–23.1] | 16.3 [12.0–22.9] | 18.2 [13.8–23.3] | 17.5 [13.0–23.7] | 0.08 |
| Total fiber intake, g/d | 22.4 ± 6.8 | 22.6 ± 6.8 | 23.3 ± 7.3 | 21.3 ± 6.1 | 0.007 |
| Fruit consumption, g/d | 123.0 [66.3–232.0] | 167.4 [84.1–260.7] | 128.6 [70.3–232.0] | 94.4 [35.1–169.1] | <0.001 |
| Vegetables consumption,2 g/d | 112.4 [76.8–158.6] | 130.2 [87.0–171.6] | 114.7 [80.7–163.3] | 97.0 [68.4–129.9] | <0.001 |
| Alcohol consumption, g/d | 2.6 [0.0–11.1] | 4.8 [0.1–15.6] | 2.6 [0.1–9.7] | 1.1 [0.0–6.7] | 0.001 |
| Mediterranean diet score | 4.3 ± 1.7 | 4.8 ± 1.8 | 4.3 ± 1.7 | 3.8 ± 1.5 | <0.001 |
| DASH score | 23.8 ± 4.7 | 25.9 ± 4.6 | 24.2 ± 4.2 | 21.4 ± 4.1 | <0.001 |
Values are mean ± SD or median [IQR] unless indicated otherwise. Differences were tested by ANOVA or Kruskal–Wallis test for continuous variables and with the χ2 test for categorical variables. DASH, Dietary Approaches to Stop Hypertension; RTR, renal transplant recipient; T, tertile; UPF, ultra-processed food.
Including legumes.
FIGURE 1.
Contribution of food groups to the ultra-processed foods (group 4) according to the NOVA classification.
Ultra-processed foods and all-cause mortality
During a median follow-up of 5.4 y [IQR: 4.9–6.0 y], 129 (20%) RTRs died. Intake of ultra-processed foods was associated with a higher risk of all-cause mortality (HR per doubling in the weight proportion of ultra-processed foods in the diet: 1.81; 95% CI: 1.30, 2.52; P < 0.001), when adjusted for age and sex (Table 3, model 1). After adjustment for other potential confounders, including transplant characteristics, lifestyle factors, use of (immunosuppressive) medication, muscle mass, and BMI, the association remained significant (Table 3, models 2–5). We proceeded with Cox proportional hazards models for analyses of sex-specific tertiles of ultra-processed food intake and all-cause mortality. RTRs with the highest consumption of ultra-processed foods had >2 times higher risk of all-cause mortality than RTRs in the lowest tertile (HR: 2.08; 95% CI: 1.34, 3.21; P = 0.001), when adjusted for age and sex (Table 3, model 1). The association remained significant after adjustment for other potential confounders (Table 3, models 2–5). To assess whether the association of ultra-processed food intake and all-cause mortality was independent of a healthy diet, we in addition adjusted model 3 for diet quality, expressed as MDS and DASH score, respectively. There were virtually no effects on the estimates after adjustment for the MDS in model 6a (HR per doubling in the weight proportion of ultra-processed food intake in the diet: 2.12; 95% CI: 1.43, 3.12; P < 0.001) and the DASH score in model 6b (HR: 2.00; 95% CI: 1.34, 2.99; P = 0.001). The same was found when comparing tertiles of ultra-processed foods adjusted for the MDS (HR highest tertile: 2.54; 95% CI: 1.55, 4.17; P < 0.001) and also after adjustment for the DASH score (HR: 2.35; 95% CI: 1.40, 3.93; P = 0.001). Figure 2 shows the association of ultra-processed food intake and all-cause mortality in RTRs after adjustment for potential confounders, using a penalized spline adjusted for age, sex, time between transplantation and baseline, eGFR, urinary protein excretion, primary renal disease, donor age, total energy intake, alcohol consumption, smoking status, and physical activity.
TABLE 3.
Association of UPFs (percentage of total food weight in g/d) with all-cause mortality in 632 renal transplant recipients1
| Continuous (2log)2 | Sex-specific tertiles | ||||||
|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | |||||
| All-cause mortality | HR (95% CI) | P value | Reference | HR (95% CI) | P value | HR (95% CI) | P value |
| Events, n | 129 | 40 | 44 | 45 | |||
| Model 1 | 1.81 (1.30, 2.52) | <0.001 | 1.00 | 1.38 (0.90, 2.13) | 0.14 | 2.08 (1.34, 3.21) | 0.001 |
| Model 2 | 2.08 (1.45, 2.98) | <0.001 | 1.00 | 1.24 (0.80, 1.94) | 0.34 | 2.49 (1.58, 3.95) | <0.001 |
| Model 3 | 2.13 (1.46, 3.10) | <0.001 | 1.00 | 1.33 (0.83, 2.13) | 0.24 | 2.58 (1.60, 4.16) | <0.001 |
| Model 4 | 2.13 (1.46, 3.11) | <0.001 | 1.00 | 1.31 (0.81, 2.10) | 0.27 | 2.60 (1.61, 4.21) | <0.001 |
| Model 5 | 2.06 (1.42, 2.97) | <0.001 | 1.00 | 1.32 (0.82, 2.14) | 0.25 | 2.68 (1.65, 4.36) | <0.001 |
| Model 6a | 2.12 (1.43, 3.12) | <0.001 | 1.00 | 1.32 (0.81, 2.13) | 0.26 | 2.54 (1.55, 4.17) | <0.001 |
| Model 6b | 2.00 (1.34, 2.99) | 0.001 | 1.00 | 1.27 (0.78, 2.05) | 0.33 | 2.35 (1.40, 3.93) | 0.001 |
Cox proportional hazards regression analyses were performed to assess the association of UPFs with all-cause mortality. T, tertile; UPF, ultra-processed food.
HR for doubling in the weight proportion of UPF intake (percentage of total food weight in g/d). Model 1, adjustment for age and sex; model 2, model 1 + adjustment for time between transplantation and baseline, estimated glomerular filtration rate, urinary protein excretion, primary renal disease, and donor age; model 3, model 2 + adjustment for total energy intake, alcohol consumption, smoking status, and physical activity; model 4, model 3 + adjustment for use of calcineurin inhibitors, antihypertensive drugs, and statins; model 5, model 3 + adjustment for 24-h urinary creatinine excretion and BMI; model 6a, model 3 + adjustment for Mediterranean Diet Score; model 6b, model 3 + adjustment for Dietary Approaches to Stop Hypertension score.
FIGURE 2.
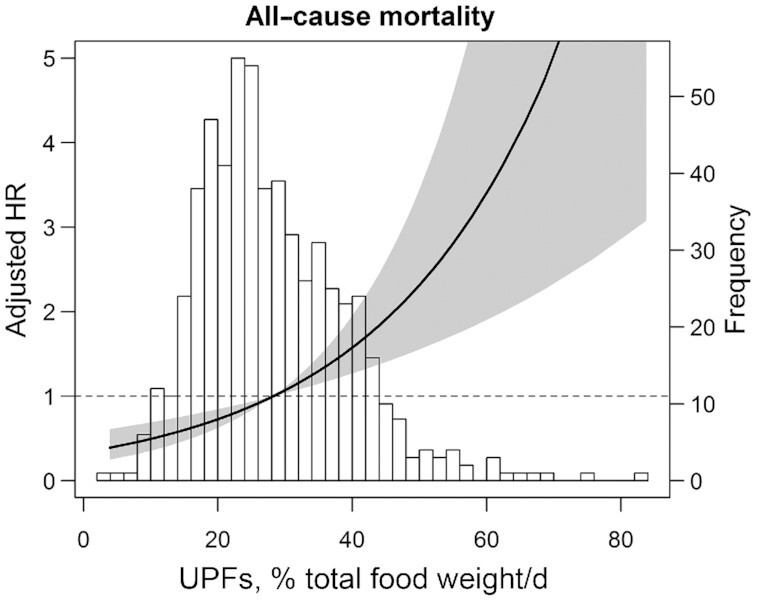
Association of UPFs (percentage of total food weight in g/d) and all-cause mortality in 632 renal transplant recipients. Data were fit by a Cox regression model based on penalized splines and adjusted for age, sex, time between transplantation and baseline, estimated glomerular filtration rate, urinary protein excretion, primary renal disease, donor age, total energy intake, alcohol consumption, smoking status, and physical activity. The black line represents the HR, whereas the gray area represents the 95% CI. The HRs were plotted relative to a value of 1.0 for the median value of UPFs as a reference. A histogram of the distribution of UPFs is plotted in the background. UPF, ultra-processed food.
We continued by analyzing the consumption of ultra-processed foods by groups. A doubling in the weight proportion of sugar-sweetened beverages (HR: 1.21; 95% CI: 1.05, 1.39; P = 0.007), desserts (HR: 1.24; 95% CI: 1.02, 1.49; P = 0.03), and processed meat (HR: 1.87; 95% CI: 1.22, 2.86; P = 0.004) was associated with a higher risk of all-cause mortality, after adjustment for potential confounders (Supplemental Table 2, model) and after adjustment for the MDS (Supplemental Table 2, model 6a). Adjustment for the DASH score (Supplemental Table 2, model 6b) had virtually no effects on the estimates of the association between desserts and all-cause mortality. The associations of sugar-sweetened beverages and processed meats with all-cause mortality did not remain significant after adjustment for the DASH score. The other groups were not associated with all-cause mortality.
Dietary patterns
Because adjustments for the MDS and DASH score had very little effect on risk estimates of ultra-processed foods, both ultra-processed foods and high-quality dietary patterns may contribute independently to mortality risk. We compared the RTRs with the poorest diet and RTRs with the best diet quality. RTRs with a low adherence to the Mediterranean diet or DASH diet and a high consumption of ultra-processed foods had a higher risk of all-cause mortality than RTRs with a high adherence to the Mediterranean diet or DASH diet and a low consumption of ultra-processed foods (HR: 3.01; 95% CI: 1.50, 6.03; P = 0.002 and HR: 3.47; 95% CI: 1.80, 6.70; P < 0.001, respectively), adjusted for age and sex (Table 4, model 1). These associations remained significant after adjustment for other potential confounders (Table 4, models 2–5). Furthermore, we performed these analyses with an ultra-processed score only including sugar-sweetened beverages, desserts, and processed meats (Table 4, models 1–5). Figure 3 shows this higher risk of all-cause mortality in RTRs with low adherence to a healthy dietary pattern and high consumption of ultra-processed foods.
TABLE 4.
Mortality risk in RTRs with the poorest diet (high UPFs and low diet quality) compared with RTRs with the best possible diet (low UPFs and high diet quality)1
| UPFs in total | UPFs based on score only including SSBs, desserts, and processed meat | |||||
|---|---|---|---|---|---|---|
| Best possible diet | Poorest possible diet | Best possible diet | Poorest possible diet | |||
| All-cause mortality | Reference | HR (95% CI) | P value | Reference | HR (95% CI) | P value |
| Diet quality based on MDS | ||||||
| Events, n | 12 | 26 | 11 | 29 | ||
| Model 1 | 1.00 | 3.01 (1.50, 6.03) | 0.002 | 1.00 | 2.98 (1.48, 6.01) | 0.002 |
| Model 2 | 1.00 | 3.39 (1.68, 6.84) | 0.001 | 1.00 | 3.01 (1.47, 6.14) | 0.002 |
| Model 3 | 1.00 | 3.11 (1.51, 6.43) | 0.002 | 1.00 | 2.71 (1.29, 5.68) | 0.008 |
| Model 4 | 1.00 | 3.21 (1.54, 6.70) | 0.002 | 1.00 | 2.66 (1.27, 5.60) | 0.01 |
| Model 5 | 1.00 | 2.66 (1.27, 5.55) | 0.009 | 1.00 | 2.39 (1.13, 5.08) | 0.02 |
| Diet quality based on DASH score | ||||||
| Events, n | 15 | 25 | 19 | 32 | ||
| Model 1 | 1.00 | 3.47 (1.80, 6.70) | <0.001 | 1.00 | 3.04 (1.70, 5.43) | <0.001 |
| Model 2 | 1.00 | 3.86 (1.97, 7.56) | <0.001 | 1.00 | 2.95 (1.61, 5.39) | <0.001 |
| Model 3 | 1.00 | 4.27 (2.13, 8.57) | <0.001 | 1.00 | 3.16 (1.70, 5.90) | <0.001 |
| Model 4 | 1.00 | 4.16 (2.07, 8.36) | <0.001 | 1.00 | 2.08 (1.65, 5.75) | <0.001 |
| Model 5 | 1.00 | 5.11 (2.48, 10.53) | <0.001 | 1.00 | 3.80 (1.96, 7.37) | <0.001 |
Cox proportional hazards regression analyses were performed to assess the risk of all-cause mortality in 632 RTRs, comparing those who have the poorest possible diet (high intake of UPFs in total and based on score only including SSBs, desserts, and processed meat combined with low adherence to the Mediterranean diet or the DASH diet) and those with the best possible diet (low intake of UPFs in total and based on score only including SSBs, desserts, and processed meat and high adherence to the Mediterranean diet or the DASH diet). HRs are for doubling in the weight proportion of UPF intake (percentage of total food weight in g/d). Model 1, adjustment for age and sex; model 2, model 1 + adjustment for time between transplantation and baseline, estimated glomerular filtration rate, urinary protein excretion, primary renal disease, and donor age; model 3, model 2 + adjustment for total energy intake, alcohol consumption, smoking status, and physical activity; model 4, model 3 + adjustment for use of calcineurin inhibitors, antihypertensive drugs, and statins; model 5, model 3 + adjustment for 24-h urinary creatinine excretion and BMI. DASH, Dietary Approaches to Stop Hypertension; MDS, Mediterranean Diet Score; RTR, renal transplant recipient; SSB, sugar-sweetened beverage; UPF, ultra-processed food.
FIGURE 3.
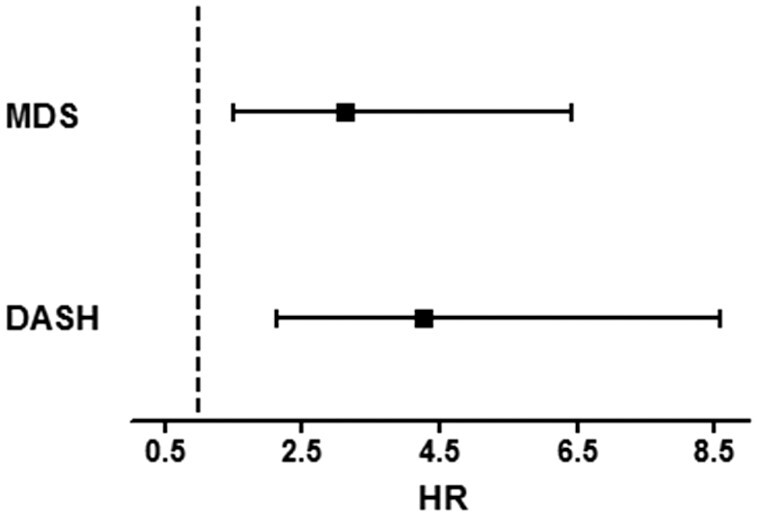
Association of UPFs (percentage of total food weight in g/d) and all-cause mortality in 632 RTRs, plotting RTRs who have a high intake of UPFs and low adherence to the MDS and DASH score against RTRs with a low intake of UPFs and high adherence to the MDS and DASH score (reference). Data were fit by a Cox regression model and adjusted for age, sex, time between transplantation and baseline, estimated glomerular filtration rate, urinary protein excretion, primary renal disease, donor age, total energy intake, alcohol consumption, smoking status, and physical activity. The error bars represent the 95% CI. DASH, Dietary Approaches to Stop Hypertension; MDS, Mediterranean Diet Score; RTR, renal transplant recipient; UPF, ultra-processed food.
Secondary analyses
Furthermore, we performed analyses of the association of the weight proportion of ultra-processed foods with death-censored graft failure, renal function decline, and PTDM. During follow-up, 76 (12%) RTRs developed graft failure, 119 (19%) RTRs had renal function decline, and 51 (11%) RTRs developed PTDM. Intake of ultra-processed foods was associated with a higher risk of renal function decline, but not with death-censored graft failure or PTDM (Supplemental Table 3, models 1–6b).
Sensitivity analyses
Sensitivity analyses were conducted by repeating the Cox regression models with the proportion of ultra-processed foods in total energy of food and beverages consumed (kcal/d). Mean consumption of ultra-processed foods in RTRs was 1138.4 ± 434.7 kcal/d (52.4% of total energy intake). The results remained similar when the ultra-processed food was weighted by energy (% kcal/d instead of % g/d): the HR per doubling in the energy proportion of ultra-processed food intake was 1.70 (95% CI: 1.23, 2.33; P = 0.001), after adjustment for age and sex (Supplemental Table 4, model 1). The association remained significant after adjustment for other potential confounders (Supplemental Table 4, models 2–6b). This also applied to the results of the Cox proportional hazards models for analyses of sex-specific tertiles with ultra-processed foods weighted by energy (Supplemental Table 4, models 1–6b).
Discussion
In this prospective study, we showed that high consumption of ultra-processed foods was associated with a higher risk of all-cause mortality in a large cohort of stable RTRs after adjustment for age, sex, and other potential confounders. Only sugar-sweetened beverages, desserts, and processed meats were significantly associated with all-cause mortality when analyzing the ultra-processed foods by groups. Furthermore, high intake of ultra-processed foods and low adherence to high-quality dietary patterns, such as the Mediterranean diet and the DASH diet, seem to contribute independently to a higher mortality risk. In secondary analyses, we found that high consumption of ultra-processed foods was associated with renal function decline, but not with death-censored graft failure and PTDM.
Our findings are consistent with previous findings in the general population. Increased consumption of ultra-processed foods was associated with a higher mortality risk among an adult population in a large observational prospective cohort study from the French NutriNet-Santé Study (13). Moreover, 2 large Spanish prospective cohorts found an association between ultra-processed food consumption and higher mortality (11, 12). One of these studies found a decrease in mortality after theoretical isocaloric substitution of ultra-processed foods by unprocessed or minimally processed foods, emphasizing the need of recommending high-quality dietary patterns (11). Likewise, a prospective study from the United States concluded a significant association between ultra-processed foods and mortality (14), and a large adult Mediterranean population-based cohort study showed an increased risk of all-cause and cardiovascular mortality in association with high consumption of ultra-processed foods (15).
There are multiple mechanisms that might explain the adverse effects of ultra-processed food consumption in RTRs. First of all, consumption of ultra-processed foods contributes to excessive intake of added sugar (40–42), which has been associated to cardiovascular deaths in the general population (15, 43). A meta-analysis indeed revealed a significant correlation between sugar-sweetened beverages and mortality (44), which is consistent with the findings of our study which showed that an increase in sugar-sweetened beverages and desserts was associated with a higher risk of all-cause mortality. Moreover, ultra-processed foods usually have a higher salt content, which has been associated with cardiovascular deaths (45). We found that RTRs with a higher consumption of ultra-processed foods had higher 24-h urinary sodium excretion values. Lower salt intake could result in lower consumption of ultra-processed foods and higher adherence to the DASH diet, both associated with a lower risk of all-cause mortality in RTRs (18). Another mechanism could be the processing of foods, leading to the production of carcinogens as a result of the Maillard reaction, which is a chemical reaction between amino acids and reducing sugars at high temperatures. This reaction is the basis for many of the flavoring industry's recipes (e.g., cooking meat) and leads to neo-formed contaminants, some of which have established carcinogenic properties (e.g., acrylamide, heterocyclic amines, and polycyclic aromatic hydrocarbons) (35, 36). Processed meat has been reported as carcinogenic and has been previously associated with stomach and colorectal cancer (46) and also with mortality in the general population (44). Moreover, ultra-processed foods contain food additives such as sodium nitrite (often used in processed meat) or titanium dioxide (TiO2, white food pigment), which have been suggested to be carcinogenic in previous animal studies and cellular models (47). Furthermore, it has been suggested that additives, such as emulsifiers (e.g., carboxymethylcellulose and polysorbate-80), result in alterations in the microbiota, leading to chronic low-grade intestinal inflammation and carcinogenesis (48). Finally, it has been postulated that some materials used in packaging of ultra-processed foods, such as bisphenol A, have carcinogenic properties after they have been in contact with foods (49).
The consumption of ultra-processed foods not only leads to excess intake of the aforementioned nutrients, but also replaces the consumption of foods that are part of high-quality dietary patterns, which are mainly based on unprocessed or minimally processed foods. It is known that higher consumption of ultra-processed foods is associated with poorer diet quality (50), which contributes to increased risk of mortality (44). The replacement of minimally processed foods in favor of ultra-processed foods warrants concern. Fruit and vegetables contain secondary plant metabolites, such as polyphenols, with antioxidant and anti-inflammatory effects (51). In our study, we found a lower consumption of fruit and vegetables and a lower adherence to high-quality dietary patterns in RTRs with a higher consumption of ultra-processed foods. Consequently, the dietary intake of antioxidants and secondary plant metabolites is likely to be affected by the intake of ultra-processed foods. A previous study showed that average intake of vitamins A, C, and E significantly decreased across quintiles of the energy contribution of ultra-processed foods (50). Furthermore, intake of protective food structures, such as dietary polyphenols and fibers, is often decreased as a result of food processing (52, 53). Indeed, we observed that RTRs with higher intake of ultra-processed foods had a lower fiber intake. Previous studies showed an inverse association between dietary fiber and mortality risk (54, 55). Another finding is the lower alcohol consumption in RTRs who have a higher intake of ultra-processed foods than in RTRs who consumed less ultra-processed foods, which might suggest a replacement of alcoholic consumptions with sugar-sweetened beverages. In our study, the association between ultra-processed foods and all-cause mortality in RTRs remained significant after adjustment for the MDS and the DASH score, suggesting an additive effect. This suggests that the high-quality dietary pattern scores do not optimally cover the effects of ultra-processed food consumption. It seems that the greatest benefit in RTRs can be achieved by not only eating more unprocessed or minimally processed foods, but also limiting consumption of ultra-processed foods.
To the best of our knowledge, this is the first prospective study to have evaluated the association between ultra-processed foods and all-cause mortality after renal transplantation. Strengths of this study include the considerable long follow-up of 5.4 y, the use of clinically relevant endpoints, and having no participants lost to follow-up. Furthermore, we used a reproducible and biomarker-validated FFQ and 24-h urinary excretion values were available. However, we do acknowledge this study has several limitations. Dietary intake was inquired once at baseline, which may not be an accurate reflection of diet longitudinally. However, the use of single baseline measurements to study associations with long-term outcomes is common, and it is known that it adversely affects the strengths of these associations with outcomes. Thus, it might have led to underestimation of associations between baseline measurements and long-term outcomes rather than overestimation (56, 57). Another limitation is that we cannot preclude misclassification of food items, although each food product was categorized into the most appropriate NOVA group. In addition, data on education and income were not available for the cohort, so it was not possible to adjust the analyses for these potential confounders. Finally, because this is a study of observational nature, drawing conclusions of causality is not possible.
In conclusion, this prospective study in a large cohort of stable RTRs showed that high consumption of ultra-processed foods was associated with a higher risk of all-cause mortality and renal function decline, independently of potential confounders. From the ultra-processed food components only sugar-sweetened beverages, desserts, and processed meat were significantly associated with all-cause mortality. In addition, high ultra-processed food consumption and poor diet quality (low adherence to the MDS and DASH score) seemed to contribute independently to a higher mortality risk. These findings suggest that good diet quality does not make up for high consumption of ultra-processed foods. The proportion of ultra-processed foods in the diet should be limited, whereas the consumption of health-promoting food products according to guidelines for a Mediterranean diet or DASH diet should be promoted. To better understand the role of ultra-processed foods on health, potential underlying mechanisms need to be delineated and therefore more studies are warranted in both experimental and epidemiologic settings.
Supplementary Material
Acknowledgments
We acknowledge the careful reading and editing of the manuscript by Josephine LC Anderson.
The authors’ responsibilities were as follows—GJN and SJLB: designed and conducted the research; MCJO and M-JD: analyzed the data and performed the statistical analysis; MCJO and EC: wrote the manuscript; M-JD, AWG-N, PCV, J-JC, CA, QC, LHD, GJN, SJLB, and EC: contributed to data interpretation, intellectual content, and manuscript revisions; GJN, SJLB, EC, and MCJO: had primary responsibility for the final content; and all authors: read and approved the final manuscript. The authors report no conflicts of interest.
Notes
Supported by Dutch Top Institute Food and Nutrition grant A-1003. M-JD received funding from the European Union's Horizon 2020 research and innovation program under Marie Skłodowska-Curie grant agreement no. 754425.
Supplemental Figure 1 and Supplemental Tables 1–4 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: DASH, Dietary Approaches to Stop Hypertension; eGFR, estimated glomerular filtration rate; ESKD, end-stage kidney disease; MDS, Mediterranean Diet Score; PTDM, posttransplant diabetes mellitus; RTR, renal transplant recipient.
Contributor Information
Maryse C J Osté, Division of Nephrology, Department of Internal Medicine, University Medical Center Groningen, University of Groningen, Groningen, Netherlands.
Ming-Jie Duan, Division of Nephrology, Department of Internal Medicine, University Medical Center Groningen, University of Groningen, Groningen, Netherlands.
Antonio W Gomes-Neto, Division of Nephrology, Department of Internal Medicine, University Medical Center Groningen, University of Groningen, Groningen, Netherlands.
Petra C Vinke, Department of Epidemiology, University Medical Center Groningen, University of Groningen, Groningen, Netherlands.
Juan-Jesus Carrero, Department of Medical Epidemiology and Biostatistics, Karolinska Institute, Stockholm, Sweden.
Carla Avesani, Department of Medical Epidemiology and Biostatistics, Karolinska Institute, Stockholm, Sweden.
QingQing Cai, Division of Nephrology, Department of Internal Medicine, University Medical Center Groningen, University of Groningen, Groningen, Netherlands.
Louise H Dekker, Department of Epidemiology, University Medical Center Groningen, University of Groningen, Groningen, Netherlands.
Gerjan J Navis, Division of Nephrology, Department of Internal Medicine, University Medical Center Groningen, University of Groningen, Groningen, Netherlands.
Stephan J L Bakker, Division of Nephrology, Department of Internal Medicine, University Medical Center Groningen, University of Groningen, Groningen, Netherlands.
Eva Corpeleijn, Department of Epidemiology, University Medical Center Groningen, University of Groningen, Groningen, Netherlands.
Data Availability
Data described in the article will be made available upon request.
References
- 1. Ponton P, Rupolo GP. Quality-of-life change after kidney transplantation. Transplant Proc. 2001;33(1–2):1887–9. [DOI] [PubMed] [Google Scholar]
- 2. Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341(23):1725–30. [DOI] [PubMed] [Google Scholar]
- 3. Oterdoom LH, de Vries APJ, van Ree RM, Gansevoort RT, van Son WJ, van der Heide JJH, Navis G, de Jong PE, Gans ROB, Bakker SJL. N-terminal pro-B-type natriuretic peptide and mortality in renal transplant recipients versus the general population. Transplantation. 2009;87(10):1562–70. [DOI] [PubMed] [Google Scholar]
- 4. Fong JVN, Moore LW. Nutrition trends in kidney transplant recipients: the importance of dietary monitoring and need for evidence-based recommendations. Front Med (Lausanne). 2018;5:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Monteiro CA, Cannon G, Levy RB, Moubarac JC, Louzada MLC, Rauber F, Khandpur N, Cediel G, Neri D, Martinez-Steele Eet al. Ultra-processed foods: what they are and how to identify them. Public Health Nutr. 2019;22(5):936–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Martínez-González MÁ, Martín-Calvo N. Ultraprocessed foods and public health: a need for education. Mayo Clin Proc. 2019;94(11):2156–7. [DOI] [PubMed] [Google Scholar]
- 7. Monteiro CA, Cannon G, Moubarac J-C, Levy RB, Louzada MLC, Jaime PC. The UN Decade of Nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr. 2018;21(1):5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Srour B, Fezeu LK, Kesse-Guyot E, Allès B, Méjean C, Andrianasolo RM, Chazelas E, Deschasaux M, Hercberg S, Galan Pet al. Ultra-processed food intake and risk of cardiovascular disease: prospective cohort study (NutriNet-Santé). BMJ. 2019;365:l1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Duan M-J, Vinke PC, Navis G, Corpeleijn E, Dekker LH. Ultra-processed food and incident type 2 diabetes: studying the underlying consumption patterns to unravel the health effects of this heterogeneous food category in the prospective Lifelines cohort. BMC Med. 2022;20(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fiolet T, Srour B, Sellem L, Kesse-Guyot E, Allès B, Méjean C, Deschasaux M, Fassier P, Latino-Martel P, Beslay Met al. Consumption of ultra-processed foods and cancer risk: results from NutriNet-Santé prospective cohort. BMJ. 2018;360:k322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blanco-Rojo R, Sandoval-Insausti H, López-Garcia E, Graciani A, Ordovás JM, Banegas JR, Rodríguez-Artalejo F, Guallar-Castillón P. Consumption of ultra-processed foods and mortality: a national prospective cohort in Spain. Mayo Clin Proc. 2019;94(11):2178–88. [DOI] [PubMed] [Google Scholar]
- 12. Rico-Campà A, Martínez-González MA, Alvarez-Alvarez I, de Deus Mendonça R, de la Fuente-Arrillaga C, Gómez-Donoso C, Bes-Rastrollo M. Association between consumption of ultra-processed foods and all cause mortality: SUN prospective cohort study. BMJ. 2019;365:l1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schnabel L, Kesse-Guyot E, Allès B, Touvier M, Srour B, Hercberg S, Buscail C, Julia C. Association between ultraprocessed food consumption and risk of mortality among middle-aged adults in France. JAMA Intern Med. 2019;179(4):490–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim H, Hu EA, Rebholz CM. Ultra-processed food intake and mortality in the USA: results from the Third National Health and Nutrition Examination Survey (NHANES III, 1988–1994). Public Health Nutr. 2019;22(10):1777–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bonaccio M, Di Castelnuovo A, Costanzo S, De Curtis A, Persichillo M, Sofi F, Cerletti C, Donati MB, de Gaetano G, Iacoviello L. Ultra-processed food consumption is associated with increased risk of all-cause and cardiovascular mortality in the Moli-sani Study. Am J Clin Nutr. 2021;113(2):446–55. [DOI] [PubMed] [Google Scholar]
- 16. Osté MCJ, Corpeleijn E, Navis GJ, Keyzer CA, Soedamah-Muthu SS, van den Berg E, Postmus D, de Borst MH, Kromhout D, Bakker SJL. Mediterranean style diet is associated with low risk of new-onset diabetes after renal transplantation. BMJ Open Diabetes Res Care. 2017;5(1):e000283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gomes-Neto AW, Osté MCJ, Sotomayor CG, van den Berg E, Geleijnse JM, Berger SP, Gans ROB, Bakker SJL, Navis GJ. Mediterranean style diet and kidney function loss in kidney transplant recipients. Clin J Am Soc Nephrol. 2020;15(2):238–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Osté MCJ, Gomes-Neto AW, Corpeleijn E, Gans ROB, de Borst MH, van den Berg E, Soedamah-Muthu SS, Kromhout D, Navis GJ, Bakker SJL. Dietary Approach to Stop Hypertension (DASH) diet and risk of renal function decline and all-cause mortality in renal transplant recipients. Am J Transplant. 2018;18(10):2523–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Martins AM, Bello Moreira AS, Canella DS, Rodrigues J, Santin F, Wanderley B, Lourenço RA, Avesani CM. Elderly patients on hemodialysis have worse dietary quality and higher consumption of ultraprocessed food than elderly without chronic kidney disease. Nutrition. 2017;41:73–9. [DOI] [PubMed] [Google Scholar]
- 20. Zelle DM, Kok T, Dontje ML, Danchell EI, Navis G, van Son WJ, Bakker SJL, Corpeleijn E. The role of diet and physical activity in post-transplant weight gain after renal transplantation. Clin Transplant. 2013;27(4):E484–90. [DOI] [PubMed] [Google Scholar]
- 21. Gomes-Neto AW, Osté MCJ, Sotomayor CG, vd Berg E, Geleijnse JM, Gans ROB, Bakker SJL, Navis GJ. Fruit and vegetable intake and risk of posttransplantation diabetes in renal transplant recipients. Diabetes Care. 2019;42(9):1645–52. [DOI] [PubMed] [Google Scholar]
- 22. van den Berg E, Engberink MF, Brink EJ, van Baak MA, Joosten MM, Gans ROB, Navis G, Bakker SJL. Dietary acid load and metabolic acidosis in renal transplant recipients. Clin J Am Soc Nephrol. 2012;7(11):1811–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van den Berg E, Geleijnse JM, Brink EJ, van Baak MA, Homan van der Heide JJ, Gans ROB, Navis G, Bakker SJL. Sodium intake and blood pressure in renal transplant recipients. Nephrol Dial Transplant. 2012;27(8):3352–9. [DOI] [PubMed] [Google Scholar]
- 24. Wendel-Vos GCW, Schuit AJ, Saris WHM, Kromhout D. Reproducibility and relative validity of the short questionnaire to assess health-enhancing physical activity. J Clin Epidemiol. 2003;56(12):1163–9. [DOI] [PubMed] [Google Scholar]
- 25. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene Tet al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Feunekes GI, Van Staveren WA, De Vries JH, Burema J, Hautvast JG. Relative and biomarker-based validity of a food-frequency questionnaire estimating intake of fats and cholesterol. Am J Clin Nutr. 1993;58(4):489–96. [DOI] [PubMed] [Google Scholar]
- 27. Feunekes GI, Van Staveren WA, Graveland F, De Vos J, Burema J. Reproducibility of a semiquantitative food frequency questionnaire to assess the intake of fats and cholesterol in The Netherlands. Int J Food Sci Nutr. 1995;46(2):117–23. [DOI] [PubMed] [Google Scholar]
- 28. Voedingscentrum, Den Haag. Stichting Nederlands Voedingsstoffenbestand, Zeist. Dutch food composition table 2006 [NEVO-tabel: Nederlands Voedingsstoffenbestand] . Bilthoven (Netherlands): RIVM; 2006. [Google Scholar]
- 29. van den Berg E, Engberink MF, Brink EJ, van Baak MA, Gans ROB, Navis G, Bakker SJL. Dietary protein, blood pressure and renal function in renal transplant recipients. Br J Nutr. 2013;109(8):1463–70. [DOI] [PubMed] [Google Scholar]
- 30. Moubarac J-C, Parra DC, Cannon G, Monteiro CA. Food classification systems based on food processing: significance and implications for policies and actions: a systematic literature review and assessment. Curr Obes Rep. 2014;3(2):256–72. [DOI] [PubMed] [Google Scholar]
- 31. Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348(26):2599–608. [DOI] [PubMed] [Google Scholar]
- 32. Fung TT, Chiuve S, McCullough M, Rexrode K, Logroscino G, Hu F. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med. 2008;168(7):713–20. [DOI] [PubMed] [Google Scholar]
- 33. Struijk EA, May AM, Wezenbeek NLW, Fransen HP, Soedamah-Muthu SS, Geelen A, Boer JMA, van der Schouw YT, Bueno-de-Mesquita HB, Beulens JWJ. Adherence to dietary guidelines and cardiovascular disease risk in the EPIC-NL cohort. Int J Cardiol. 2014;176(2):354–9. [DOI] [PubMed] [Google Scholar]
- 34. Julia C, Martinez L, Allès B, Touvier M, Hercberg S, Méjean C, Kesse-Guyot E. Contribution of ultra-processed foods in the diet of adults from the French NutriNet-Santé study. Public Health Nutr. 2018;21(1):27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Birlouez-Aragon I, Morales F, Fogliano V, Pain J-P. The health and technological implications of a better control of neoformed contaminants by the food industry. Pathol Biol (Paris). 2010;58(3):232–8. [DOI] [PubMed] [Google Scholar]
- 36. Flores M, Mora L, Reig M, Toldrá F. Risk assessment of chemical substances of safety concern generated in processed meats. Food Sci Hum Wellness. 2019;8(3):244–51. [Google Scholar]
- 37. Fardet A. Minimally processed foods are more satiating and less hyperglycemic than ultra-processed foods: a preliminary study with 98 ready-to-eat foods. Food Funct. 2016;7(5):2338–46. [DOI] [PubMed] [Google Scholar]
- 38. Peduzzi P, Concato JJ, Feinstein AR, Holford TR. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol. 1995;48(12):1503–10. [DOI] [PubMed] [Google Scholar]
- 39. Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515–26. [Google Scholar]
- 40. Martínez Steele E, Baraldi LG, da Costa Louzada ML, Moubarac J-C, Mozaffarian D, Monteiro CA. Ultra-processed foods and added sugars in the US diet: evidence from a nationally representative cross-sectional study. BMJ Open. 2016;6(3):e009892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rauber F, Louzada M, Martinez Steele E, de Rezende LFM, Millett C, Monteiro CA, Levy RB. Ultra-processed foods and excessive free sugar intake in the UK: a nationally representative cross-sectional study. BMJ Open. 2019;9(10):e027546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Machado PP, Steele EM, da Costa Louzada ML, Levy RB, Rangan A, Woods J, Gill T, Scrinis G, Monteiro CA. Ultra-processed food consumption drives excessive free sugar intake among all age groups in Australia. Eur J Nutr. 2020;59(6):2783–92. [DOI] [PubMed] [Google Scholar]
- 43. Yang Q, Zhang Z, Gregg EW, Flanders WD, Merritt R, Hu FB. Added sugar intake and cardiovascular diseases mortality among US adults. JAMA Intern Med. 2014;174(4):516–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schwingshackl L, Schwedhelm C, Hoffmann G, Lampousi A-M, Knüppel S, Iqbal K, Bechthold A, Schlesinger S, Boeing H. Food groups and risk of all-cause mortality: a systematic review and meta-analysis of prospective studies. Am J Clin Nutr. 2017;105(6):1462–73. [DOI] [PubMed] [Google Scholar]
- 45. Mozaffarian D, Fahimi S, Singh GM, Micha R, Khatibzadeh S, Engell RE, Lim S, Danaei G, Ezzati M, Powles J. Global sodium consumption and death from cardiovascular causes. N Engl J Med. 2014;371(7):624–34. [DOI] [PubMed] [Google Scholar]
- 46. Bouvard V, Loomis D, Guyton KZ, Grosse Y, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Mattock H, Straif K. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015;16(16):1599–600. [DOI] [PubMed] [Google Scholar]
- 47. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans . Carbon black, titanium dioxide, and talc. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 93. Lyon (France): International Agency for Research on Cancer; 2010. [PMC free article] [PubMed] [Google Scholar]
- 48. Viennois E, Merlin D, Gewirtz AT, Chassaing B. Dietary emulsifier–induced low-grade inflammation promotes colon carcinogenesis. Cancer Res. 2017;77(1):27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cimmino I, Fiory F, Perruolo G, Miele C, Beguinot F, Formisano P, Oriente F. Potential mechanisms of bisphenol A (BPA) contributing to human disease. Int J Mol Sci. 2020;21(16):5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Martínez Steele E, Popkin BM, Swinburn B, Monteiro CA. The share of ultra-processed foods and the overall nutritional quality of diets in the US: evidence from a nationally representative cross-sectional study. Popul Health Metr. 2017;15(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Panche AN, Diwan AD, Chandra SR. Flavonoids: an overview. J Nutr Sci. 2016;5:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chassaing B, Miles-Brown J, Pellizzon M, Ulman E, Ricci M, Zhang L, Patterson AD, Vijay-Kumar M, Gewirtz AT. Lack of soluble fiber drives diet-induced adiposity in mice. Am J Physiol Gastrointest Liver Physiol. 2015;309(7):G528–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Roopchand DE, Carmody RN, Kuhn P, Moskal K, Rojas-Silva P, Turnbaugh PJ, Raskin I. Dietary polyphenols promote growth of the gut bacterium Akkermansia muciniphila and attenuate high-fat diet–induced metabolic syndrome. Diabetes. 2015;64(8):2847–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yang Y, Zhao L-G, Wu Q-J, Ma X, Xiang Y-B. Association between dietary fiber and lower risk of all-cause mortality: a meta-analysis of cohort studies. Am J Epidemiol. 2015;181(2):83–91. [DOI] [PubMed] [Google Scholar]
- 55. Veronese N, Solmi M, Caruso MG, Giannelli G, Osella AR, Evangelou E, Maggi S, Fontana L, Stubbs B, Tzoulaki I. Dietary fiber and health outcomes: an umbrella review of systematic reviews and meta-analyses. Am J Clin Nutr. 2018;107(3):436–44. [DOI] [PubMed] [Google Scholar]
- 56. Clarke R, Shipley M, Lewington S, Youngman L, Collins R, Marmot M, Peto R. Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. Am J Epidemiol. 1999;150(4):341–53. [DOI] [PubMed] [Google Scholar]
- 57. Koenig W, Sund M, Fröhlich M, Löwel H, Hutchinson WL, Pepys MB. Refinement of the association of serum C-reactive protein concentration and coronary heart disease risk by correction for within-subject variation over time: the MONICA Augsburg studies, 1984 and 1987. Am J Epidemiol. 2003;158(4):357–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the article will be made available upon request.