ABSTRACT
Background
Intrauterine exposure to maternal vitamin D status <50 nmol/L of serum 25-hydroxyvitamin D [25(OH)D] may adversely affect infant body composition. Whether postnatal interventions can reprogram for a leaner body phenotype is unknown.
Objectives
The primary objective was to test whether 1000 IU/d of supplemental vitamin D (compared with 400 IU/d) improves lean mass in infants born with serum 25(OH)D <50 nmol/L.
Methods
Healthy, term, breastfed infants (Montréal, Canada, March 2016–2019) were assessed for serum 25(OH)D (immunoassay) 24–36 h postpartum. Infants with serum 25(OH)D <50nmol/L at 24–36 h were eligible for the trial and randomly assigned at baseline (1 mo postpartum) to 400 (29 males, 20 females) or 1000 IU/d (29 males, 20 females) of vitamin D until 12 mo. Infants (23 males, 18 females) with 25(OH)D ≥50 nmol/L (sufficient) formed a nonrandomized reference group provided 400 IU/d. Anthropometry, body composition (DXA), and serum 25(OH)D concentrations were measured at 1, 3, 6, and 12 mo.
Results
At baseline, mean ± SD serum 25(OH)D concentrations in infants allocated to the 400 and 1000 IU/d vitamin D groups were 45.8 ± 14.1 and 47.6 ± 13.4, respectively; for the reference group it was 69.2 ± 16.4 nmol/L. Serum 25(OH)D concentration increased on average to ≥50 nmol/L in the trial groups at 3–12 mo. Lean mass varied differently between groups over time; at 12 mo it was higher in the 1000 IU/d vitamin D group than in the 400 IU/d group (mean ± SD: 7013 ± 904.6 compared with 6690.4 ± 1121.7 g, P = 0.0428), but not the reference group (mean ± SD: 6715.1 ± 784.6 g, P = 0.19). Whole-body fat mass was not different between the groups over time.
Conclusions
Vitamin D supplementation (400 or 1000 IU/d) during infancy readily corrects vitamin D status, whereas 1000 IU/d modestly increases lean mass by 12 mo. The long-term implications require further research. This trial was registered at clinicaltrials.gov as NCT02563015.
Keywords: infant, vitamin D status, vitamin D supplementation, lean mass, randomized controlled trial
Intrauterine exposure to maternal vitamin D status <50 nmol/L of serum 25-hydroxyvitamin D (25(OH)D) may adversely impact infant body composition. This study uniquely screened for newborn vitamin D status in a diverse sociodemographic population, followed by a trial that tested whether postnatal interventions using 1000 IU/d of supplemental vitamin D (vs. 400 IU/d) improves lean mass in breastfed infants born with serum 25(OH)D <50 nmol/L. Vitamin D supplementation at either 400 or 1000 IU/d readily supported achievement of vitamin D status above the cut-point for sufficiency of 50 nmol/L from 3 to 12 months of age, whereas 1000 IU/d modestly increasedlean mass by 12 months.The finding that vitamin D has a role in establishing a healthy body composition in infants, specifically a leaner body phenotype, suggests that vitamin D supplementation merits investigation as an intervention strategy to help prevent childhood obesity in future studies. In addition, this study showed that for healthy breastfed infants vitamin D supplementation of 400 IU/d adequately compensates for insufficient vitamin D status at birth, reinforcing both Dietary Reference Intake values and public health policy recommendations in North America.
Introduction
Vitamin D is required for growth and development (1), with a well-established role in bone health through calcium and phosphate homeostasis (2). There is an emerging body of evidence beyond bone indicating that vitamin D status is implicated in programming of body composition (3–5). Recent evidence from large pregnancy cohort studies in India, New Zealand, and the United Kingdom (6–8) suggests that intrauterine exposure to insufficient vitamin D status, defined as serum 25-hydroxyvitamin D [25(OH)D] <50nmol/L, associates with elevated percentage fat mass and lower percentage lean mass in the offspring at 4–9.5 y of age. In addition in the Netherlands, maternal severe vitamin D deficiency, defined as serum 25(OH)D <25nmol/L, has been associated with lower percentage lean mass in children at 6 y of age (9). This implies that fetal exposure to insufficient maternal vitamin D status may have a long-lasting impact on body composition. According to the developmental origins of pediatric obesity, the etiology of excessive adiposity and its metabolic consequences can begin very early in life (10), suggesting that early-life interventions may reprogram body composition.
A recent systematic review and meta-analysis of randomized controlled trials on the effects of vitamin D supplementation early in life on children's body composition proposed that vitamin D supplementation in pregnancy and early during the neonatal period shows a consistent trend of decreasing adiposity early in life (11). The Institute of Medicine (IOM) expert committee set an Adequate Intake (AI) value for vitamin D at 400 IU (10 µg)/d across infancy to maintain vitamin D status in the range of 40–50 nmol 25(OH)D/L (12) and to support bone health outcomes. To the best of our knowledge, the only dose-response study reporting body composition according to vitamin D supplementation is in infants with plasma 25(OH)D on average ≥50 nmol/L at baseline (2). Plasma 25(OH)D concentrations were positively associated with higher percentage lean body mass and lower fat mass at 12 mo (3). In Canada, insufficient vitamin D status is prevalent among newborns (13–15). For instance, based on a large pregnancy cohort study in Quebec City, 24% of infants had vitamin D insufficiency (14). To date, no study has determined a dosage of postnatal vitamin D supplementation that mitigates vitamin D insufficiency and also results in a lean body phenotype to overcome exposures to low maternal–fetal transfer of vitamin D in utero.
The primary objective of this trial was to test whether correction of insufficient vitamin D status early in the neonatal period improves whole-body lean mass in infancy. It was hypothesized that neonates born with insufficient vitamin D status and provided the standard of care, 400 IU vitamin D3/d, would have lower lean mass and lean mass accretion by 3 mo of age and thereafter by 12 mo than infants provided with a higher dosage of 1000 IU vitamin D3/d. A secondary objective tested whether correction of insufficient vitamin D status early in infancy using 1000 IU vitamin D/d would also improve infant growth and fat mass accretion rate. In addition, we compared insulin-like growth factor-1 (IGF-1) and its main carrier protein insulin-like growth factor binding protein 3 (IGFBP-3) among groups because these have established functions in growth and development of skeletal muscle across infancy.
Methods
Study design and participants
This was a double-blinded, randomized, controlled parallel group trial comparing 1000 with 400 IU/d of supplemental vitamin D in infants from 1 to 12 mo of age. Healthy, term, breastfed infants (n = 139; 81 males, 58 females) were recruited at the Lakeshore General Hospital, located in greater Montréal (Québec, Canada), from March 2016 through March 2019. The inclusion criteria for the trial were healthy, term, singleton infants of appropriate weight for gestational age [AGA; 10th–90th percentile, according to Canadian birth reference values (16)] born to healthy mothers who intended to breastfeed for ≥3 mo. For the reference group, infants were recruited if their mothers had a prepregnancy BMI (in kg/m2) between 18.5 and 27.0 to help limit maternal excess adiposity preconception as a confounding factor associated with adverse infant body composition (17), whereas prepregnancy BMI was not an exclusion criterion for infants in the trial groups. The exclusion criteria for the trial and the reference groups were maternal smoking, as well as maternal comorbidities including type 1, type 2, and gestational diabetes; hypertension; pre-eclampsia; malabsorption syndromes such as celiac disease; Crohn's disease; as well as taking any medication that alters vitamin D metabolism except for vitamin/mineral supplements.
Before discharge from hospital, infant capillary blood samples were collected by heel lance within 24–36 h postnatally for measurement of serum 25(OH)D using a chemiluminescence immunoassay (CLIA; Liaison, Diasorin Inc.). Blood samples were collected at the same time as the routine blood collection for newborn screening (e.g., phenylketonuria). Families subsequently attended the Mary Emily Clinical Nutrition Research Unit, McGill University (greater Montréal, Québec, Canada) for a baseline visit at 1 mo (range: 0.2–1.5 mo) of age and were followed at 3, 6, and 12 mo of age. At the baseline visit, infants born with serum 25(OH)D <50nmol/L (n = 98) were randomly assigned to receive either 400 or 1000 IU/d of vitamin D supplementation until 12 mo of age. Randomization was stratified by infants’ measured skin tone using a spectrophotometer (CM-700d/600d, Konica Minolta) at baseline. Infants born with 25(OH)D ≥50 nmol/L (n = 41) formed a nonrandomized reference group and received 400 IU/d, the standard of care, with all of the follow-up measurements identical to those of the trial. Participant enrollment and assignment to the trial groups based on skin tone blocks were performed by the research team.
Ethics approval and trial registration
The research protocol was reviewed and approved by St. Mary's Hospital Research Ethics Committee which oversees research approvals of the Lakeshore General Hospital. Trial registration was completed before the beginning of recruitment (NCT02563015). Before collection of data or blood samples at the hospital or at the baseline visit, parents provided written informed consent in either official language (English or French). The trial was also reviewed and approved by the Health Canada Research Ethics Board (REB 2019-033H) and Privacy Management Division (HC-PR-2019-000024).
Demographic data/obstetric history and lifestyle survey
Demographic information was surveyed including maternal age, maternal self-reported population group (white/all other groups), maternal education level (elementary/high school, college/vocational school, university), maternal country of birth (Canada/all other countries), and family income [<70,000, ≥70,000 Canadian dollars (CAD), or not reported] according to the median income for Canadians (18). Type of delivery (cesarean/vaginal), parity, infant gestational age at birth, and birth weight were obtained from hospital records. Weight-for-age and -sex z scores were calculated using the growth standards from the WHO (19).
Prepregnancy weight and weight at delivery were extracted from the medical record. Gestational weight gain was also calculated by subtracting prepregnancy weight from weight measured at delivery. Maternal use of vitamin supplements in the 3 mo before conception and during pregnancy were surveyed separately, along with frequency of use. Lifestyle factors surveyed included smoking history (never, past, current), alcohol consumption during pregnancy (yes/no), and physical activity in the 3 mo before conception and separately during pregnancy (yes/no) along with frequency/intensity of activity. Sun exposure including the time spent outdoors between 10:00 and 16:00 with hands and/or face exposed (yes/no), and the use of sun protection factor (SPF) products including make-up creams (yes/no) in the last trimester, were also surveyed according to questions adapted from the Canadian Health Measures Survey (20). In view of the potential for endogenous synthesis of vitamin D, date of birth was converted into a theoretical vitamin D synthesizing/nonsynthesizing period based on solar UVB radiation strength (1 April–31 October/1 November–31 March) for Canada (21) and season of birth as defined by equinox and solstice dates. Study data were collected and managed using REDCap (Research Electronic Data Capture) version 7.4.
Skin tone
Skin tone of the infant was measured at the research facility by taking the mean of 3 measurements at the inner upper arm for constitutive pigmentation (basal skin color) using a spectrophotometer (CM-700d/600d, Konica Minolta). Individual typological angle (ITA°) was calculated with the L* and b* values using published equations (22). Infants were classified into 2 skin tone types (F I–III; F IV–VI) based on Fitzpatrick descriptions (23, 24).
Supplements, randomization, masking, and adherence
Study products containing 400 or 1000 IU vitamin D3 (cholecalciferol) were formulated by Europharm (Europharm International Canada Inc.). The products were externally verified (Sandoz, Novartis Division) to be within 5% of the target and stable for ≤12 mo. All supplements for the trial and reference groups were provided in identical bottles (50 mL), with identical color (brown liquid), taste (cherry flavor), smell, and texture. Each bottle had a unique code to enable tracing back to the product dosage in the case of an adverse event [e.g., 25(OH)D concentrations ≥225 nmol/L associated with hypercalcemia] without unblinding the study. Both study products were blinded to participating families and all researchers across the entire study and only unblinded after all data had been double-audited.
Allocation occurred at the end of the initial baseline visit and parents were educated by a registered nurse on how to properly give the supplement in a 1-mL/d volume using a dropper provided by the company. Randomization (allocation ratio 1:1) was set according to block sizes of 4 (2 × 400 IU and 2 × 1000 IU), using Random Allocation Software (http://mahmoodsaghaei.tripod.com/Softwares/randalloc.html) with stratification based on measured skin tone (F I–III and F IV–VI) to create 2 trial arms. At the baseline visit, families were provided with a vitamin D compliance calendar in order to record the number of missed dosages; this was reviewed and recorded at each follow-up visit to estimate adherence to supplementation.
Body composition assessment
Body composition was assessed in infants using a fan-beam dual-energy X-ray absorptiometer (DXA; APEX version 13.3:3, Hologic 4500A Discovery Series) and using infant whole-body mode. At each study visit, infants were scanned while wearing a single light gown with no metal or plastic components and a diaper. To minimize the potential for movement artifacts, infants were wrapped in a single receiving blanket, and most were scanned asleep. Output from the whole-body scan included lean mass (g), fat mass (g and %), and total body mass (g). For quality control purposes, a spine phantom (Hologic phantom; No. 14774) was used and the CVs (%) for bone mineral content, bone mineral density, and bone area were <1% across the study. Values for lean mass and fat mass accretion (g/mo) were calculated as change in lean mass or fat mass from baseline to 3 mo, 3 to 6 mo, or 6 to 12 mo and adjusted for time between visits. Lean and fat percentage (%) were calculated using lean (excluding bone mass) and fat mass (kg) divided by total mass (kg) times 100. Lean mass index (LMI) and fat mass index (FMI) were also calculated using lean and fat mass relative to length (kg/m2).
Anthropometric measurements and dietary assessments
Infant nude weight was measured to the nearest gram using an electronic scale with a dynamic weighing program (Mettler-Toledo Inc.). Crown–heel length was measured to the nearest 0.1 cm using an infant length board (Infantometer; O'Leary Length Boards, Ellard Instrumentation Ltd.). Head circumference was measured to the nearest 0.1 cm using a nonstretchable tape (Perspective Enterprises). Weight, length, and head circumference z scores for age and sex were calculated using WHO software (WHO AnthroPlus). Maternal anthropometric measurements included weight using a balance-beam scale (Detecto; Webb) to the nearest 0.1 kg while wearing light clothing and no shoes, and height to the nearest 0.1 cm using a wall-mounted stadiometer (Seca Medical Scales and Measuring Systems). Maternal prepregnancy weight from the medical record and measured height at baseline were used to calculate prepregnancy BMI.
All infants were discharged from the hospital with a prescription for vitamin D supplements (400 IU/d) from their physician and compliance was surveyed at the baseline visit. At each study visit, information regarding breastfeeding status (yes/no) and the type of breastfeeding (exclusively/mixed), as well as infant's age at the introduction of solid food and when they started crawling or walking, were surveyed. Infant dietary intake over the study period was assessed using 3-d diet records completed by parents after each study visit. To estimate nutrient intake from breast milk, total number of feeds for each infant over 24 h was multiplied by the mean feed volume depending on infant age (1, 3, 6, or 12 mo) and based on test-weighing as previously reported (25). Nutrient intake was created using Nutritionist Pro software version 5.4.0 (Axxya Systems LLC) and the 2010b Canadian Nutrient File database (Health Canada) (26). Moreover, maternal supplement use over pregnancy was surveyed.
Biochemistry measurements
Capillary blood samples (0.4–0.5 mL; Capiject, Terumo Corp.) were collected at newborn screening (between 24 and 36 h after birth) and thereafter at baseline (1 mo), 3, 6, and 12 mo of age via infant heel/finger lance into micro tubes, 1 with and 1 without heparin (Becton Dickinson). Samples were centrifuged (4000 × g for 20 min at 6°C) in order to obtain plasma and serum. Serum that was not analyzed immediately and all plasma samples were stored at −80°C until batch analysis at McGill University. One 5-mL venous sample was taken from mothers at the baseline visit (nonfasted state) for measurement of serum 25(OH)D. Total serum 25(OH)D was measured using an automated CLIA (25 µL; Liaison, DiaSorin Inc.). Newborn samples (n = 2) with 25(OH)D below the lower limit of quantification of 10 nmol/L were assigned a value of 5 nmol/L (27); concentrations at baseline and thereafter were all >10 nmol/L. The laboratory obtained a certificate of proficiency from the Vitamin D External Quality Assessment Scheme to facilitate comparison with other laboratories. For quality assurance purposes, vitamin D control samples from the National Institute of Standards and Technology (NIST) were implemented in routine quality control measures. The interassay CV for NIST972a (levels 1–4) was on average <10% and the accuracy was 97.4%. The interassay CV% for an internal laboratory control human serum sample (62.8 nmol/L) was 8.2% across all assays. In addition, in a subset of mothers and infants (n = 83) total serum 25(OH)D was in agreement (mean difference = −0.8 nmol/L) with LC–tandem MS (Queen's University, Kingston, Ontario, Canada), as certified by the Vitamin D Standardization-Certification Program.
Whole-blood ionized calcium was measured immediately after a blood draw at each study visit using a portable blood gas analyzer (65 µL; ABL80 FLEX Radiometer Medical A/S) and values were compared to the age-specific (2.5th–97.5th percentile) reference ranges of 1.32–1.47 mmol/L for 1 mo, 1.31–1.46 mmol/L for 3 mo, 1.29–1.41 mmol/L for 6 mo, and 1.25–1.39 mmol/L for 12 mo of age (28). Plasma samples were used to measure IGF-1 and IGFBP-3 using the quantitative sandwich enzyme immunoassay technique (10 μL; Quantikine® ELISA R&D Systems, Inc.; CAT# DG100B and SG100B, respectively). An internal laboratory pooled sample was implemented for quality assurance yielding intra-assay CV% of <8% and <7% for IGF-1 and IGFBP-3, respectively. The minimum detectable concentrations of human IGF-1 and IGFBP-3 were 0.004 and 0.02 ng/mL, respectively.
Sample size estimation
As indicated in the trial registration, the aim was to recruit ≤74 infants per trial group in order to account for possible dropouts or missing data (no whole-body scan owing to movement artifacts or failure to cooperate) or the maximum sample recruited over 3 y to reflect all seasons equally. The minimum estimated sample size (n = 46) per trial arm was based on the primary objective, lean mass (g) at 3 mo of age, and an effect size of 0.59, SD of 670 g, an allocation ratio of 1:1, power of 80%, and α of 5%. The estimated effect size of 0.59 was based on a subgroup analysis of a vitamin D dose-response trial of breastfed infants with healthy status, supplemented with 400 IU/d and who increased lean mass between 1 and 3 mo by 670 g with a mean change of 400 g (3). We aimed to recruit a similar number of infants to the nonrandomized reference group in order to help guide interpretation of the data; over the 3 y of recruitment n = 41 agreed to participate.
Statistical analyses
Characteristics at birth and continuous data for outcome measurements are expressed as n (%) or mean ± SD, unless otherwise noted. The effect of vitamin D dosage on lean body mass evaluated among groups (trial 400 IU/d and 1000 IU/d, and reference group) over time was the primary analysis using a linear mixed-effect model (SAS PROC MIXED). This procedure provides unbiased estimates using the method of restricted/residual maximum likelihood and uses all available data, thus missing data do not result in omission of an infant's existing data (29). In evaluating our primary outcome, differences between groups over time were tested using the existing data and without imputation. As stipulated a priori, baseline was included as a time point (as part of the outcome vector), yielding 4 repeated measurements (baseline, 3, 6, and 12 mo). This was important because the nonrandomized reference group had different inclusion criteria by design (i.e., sufficient vitamin D status at birth). The mixed-effect model tested for fixed effects of group-by-time interaction, sex (male, female) (30, 31), and skin tone (F I–III, F IV–VI) (32) based on design, as well as covariates selected using the disjunctive cause criterion (33) on the basis of participant characteristics known to relate to infant body composition (outcome) or the exposure (vitamin D status). Covariates included: infant actual age at each visit (30, 34), parity (34) (first child, second, third and more), maternal (35) and paternal age at delivery (36), maternal BMI preconception (17) (<25, ≥25 kg/m2), and family annual income (35) (<70,000, ≥70,000 CAD, or not reported). Additional variables known to relate to infant body composition or vitamin D status that were considered as fixed were self-reported population group (31) (white, all other groups), education (36) (elementary/high school, college/vocational school, or university), as well as breastfeeding status (37) (yes, no). These variables did not improve the model as judged by Akaike information criterion, thus they were removed from the final model. Each model included the random effect of an individual infant (ID) modeled as variance covariance, as well as a repeated statement for time using first-order autoregressive or AR(1) covariance structure selected based on the correlation matrix and the lowest Akaike information criterion (29). In order to test for effects within group*time the SLICE statement was used to test for differences of least-square means followed by Tukey-Kramer post hoc adjustment for multiple comparisons. For each model normality of the residuals was evaluated using normality tests (Kolmogorov–Smirnov and Shapiro–Wilk), the histogram, and Q-Q plots. Homogeneity of variances was checked using Levene or Bartlett tests. The same model was used for the analysis of the secondary outcomes including fat mass, anthropometry, and serum biochemistry. Sensitivity analyses were also conducted for the trial groups and the reference group in a subgroup with maternal prepregnancy BMI 18.5–27.0 kg/m2 as per the inclusion criterion for the reference group; the model was otherwise the same.
A post hoc analysis of our primary outcome was conducted using ANCOVA with adjustment for baseline values of whole-body lean mass. In this model, the repeated measures modeled were thus 3 time points (3, 6, and 12 mo) with baseline lean mass as a covariate (38).
Differences in categorical data between groups over time, including the proportion meeting the sufficient cutoff of 50 nmol/L (20 ng/mL) of 25(OH)D according to the IOM definition for individuals (12), adherence to infant vitamin D supplementation (proportion of supplements taken), and breastfeeding status (yes/no), were compared at each time point using chi-square (χ2) and Fisher's exact tests. All data were analyzed using Statistical Analysis System (SAS) version 9.4 (SAS Institute Inc.). Data were interpreted according to P < 0.05, including after adjustment for multiple comparisons where applicable.
Results
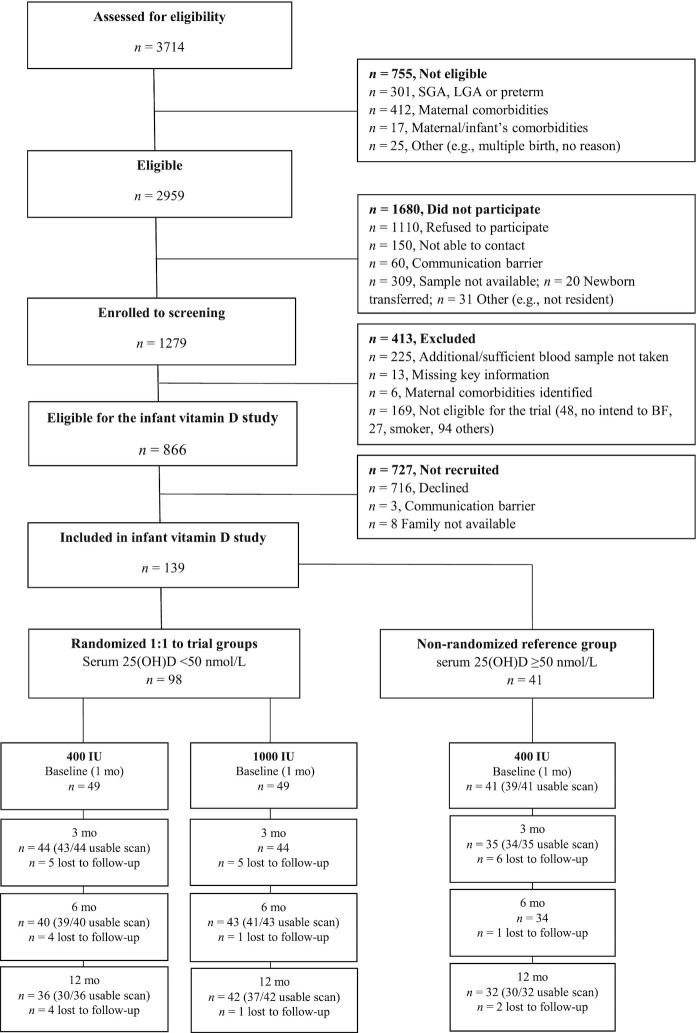
Of the mother–infant dyads screened for eligibility, 2959 were eligible for assessment of newborn vitamin D status. Of 866 with serum 25(OH)D concentration available and being eligible for the trial, 139 families consented to the infant vitamin D study. Ninety-eight infants with serum 25(OH)D <50nmol/L at 24–36 h were eligible for the trial and randomly assigned at baseline to 400 (29 males, 20 females) or 1000 IU vitamin D/d (29 males, 20 females) until 12 mo. Infants (23 males, 18 females) with 25(OH)D ≥50 nmol/L formed the nonrandomized reference group provided 400 IU/d (Figure 1). Table 1 shows maternal and neonatal characteristics for the groups at birth and at baseline.
FIGURE 1.
Consolidated Standards of Reporting Trials (CONSORT) flow diagram. BF, breastfeed; LGA, large for gestational age; SGA, small for gestational age; 25(OH)D, 25-hydroxyvitamin D.
TABLE 1.
Neonatal and maternal characteristics at birth and at baseline1
| Parameters | Reference 400 IU/d (n = 41) | Trial 400 IU/d (n = 49) | Trial 1000 IU/d (n = 49) |
|---|---|---|---|
| Infants | |||
| At birth (24–36 h postpartum) | |||
| Sex | |||
| Male | 23 (56.1) | 29 (59.2) | 29 (59.2) |
| Female | 18 (43.9) | 20 (40.8) | 20 (40.8) |
| UVB period2 | |||
| Synthesizing period | 25 (61.0) | 30 (61.2) | 26 (53.1) |
| Nonsynthesizing period | 16 (39.0) | 19 (38.8) | 23 (46.9) |
| Gestational age, wk | 39.6 ± 1.0 | 39.7 ± 1.0 | 39.6 ± 1.1 |
| Weight, kg | 3.5 ± 0.3 | 3.4 ± 0.4 | 3.4 ± 0.4 |
| Weight-for-age z score | 0.3 ± 0.7 | 0.1 ± 0.8 | 0.1 ± 0.8 |
| Serum 25(OH)D, nmol/L | 68.0 ± 13.2 | 30.8 ± 9.2 | 34.4 ± 12.0 |
| At baseline (1 mo postpartum) | |||
| Skin tone3 | |||
| F I–III | 38 (92.7) | 35 (71.4) | 35 (71.4) |
| F IV–VI | 3 (7.3) | 14 (28.6) | 14 (28.6) |
| Weight, kg | 4.1 ± 0.5 | 3.9 ± 0.5 | 3.9 ± 0.5 |
| Weight-for-age z score | 0.03 ± 0.8 | −0.2 ± 0.9 | −0.1 ± 0.7 |
| Length, cm | 53.5 ± 2.0 | 52.7 ± 1.8 | 52.9 ± 2.2 |
| Length-for-age z score | 0.1 ± 1.0 | −0.2 ± 0.9 | 0.03 ± 0.9 |
| HC, cm | 36.5 ± 1.1 | 36.6 ± 1.2 | 36.1 ± 1.3 |
| HC-for-age z score | 0.1 ± 0.9 | 0.3 ± 0.9 | −0.02 ± 0.8 |
| Serum 25(OH)D, nmol/L | 69.2 ± 16.4 | 45.8 ± 14.1 | 47.6 ± 13.4 |
| Mothers | |||
| At delivery (24–36 h postpartum) | |||
| Mother's age, y | 32.3 ± 4.0 | 32.8 ± 4.3 | 31.2 ± 4.8 |
| Father's age, y | 35.0 ± 4.9 | 35.8 ± 5.4 | 33.2 ± 5.3 |
| Self-reported population group | |||
| White | 31 (75.6) | 22 (44.9) | 24 (49.0) |
| All other groups4 | 10 (24.4) | 27 (55.1) | 25 (51.0) |
| Family income, CAD | |||
| ≥70,000 | 29 (70.7) | 27 (55.1) | 22 (44.9) |
| <70,000 | 7 (17.1) | 15 (30.6) | 18 (36.7) |
| Not reported | 5 (12.2) | 7 (14.3) | 9 (18.4) |
| Education | |||
| Elementary/high school | 1 (2.4) | 8 (16.3) | 4 (8.2) |
| College/vocational school | 10 (24.4) | 8 (16.3) | 11 (22.4) |
| University | 30 (73.2) | 33 (67.4) | 34 (69.4) |
| Prepregnancy BMI, kg/m2 | 23.1 ± 2.6 | 24.6 ± 4.4 | 25.8 ± 5.9 |
| Supplement use5 | 38 (92.7) | 44 (89.8) | 46 (93.9) |
| Parity | |||
| Primiparous | 13 (31.7) | 12 (24.5) | 19 (38.8) |
| Multiparous (i.e., ≥2) | 28 (68.3) | 37 (75.5) | 30 (61.2) |
| At baseline (1 mo postpartum) | |||
| Serum 25(OH)D, nmol/L | 94.3 ± 23.3 | 51.6 ± 14.5 | 60.0 ± 22.9 |
Values are mean ± SD or n (%). CAD, Canadian dollar; F, Fitzpatrick; HC, head circumference; 25(OH)D, 25-hydroxyvitamin D.
Infants born in vitamin D–synthesizing period: 1 April–31 October or vitamin D–nonsynthesizing period: 1 November–31 March.
Based on Fitzpatrick descriptions (F I–III or F IV–VI).
Including South Asian, Chinese, black, Filipino, Latin American, Arab, Southeast Asian, West Asian, Korean, Japanese, and other.
Maternal multivitamin use during pregnancy (yes/no).
Effect of vitamin D supplementation on infant body composition and anthropometry
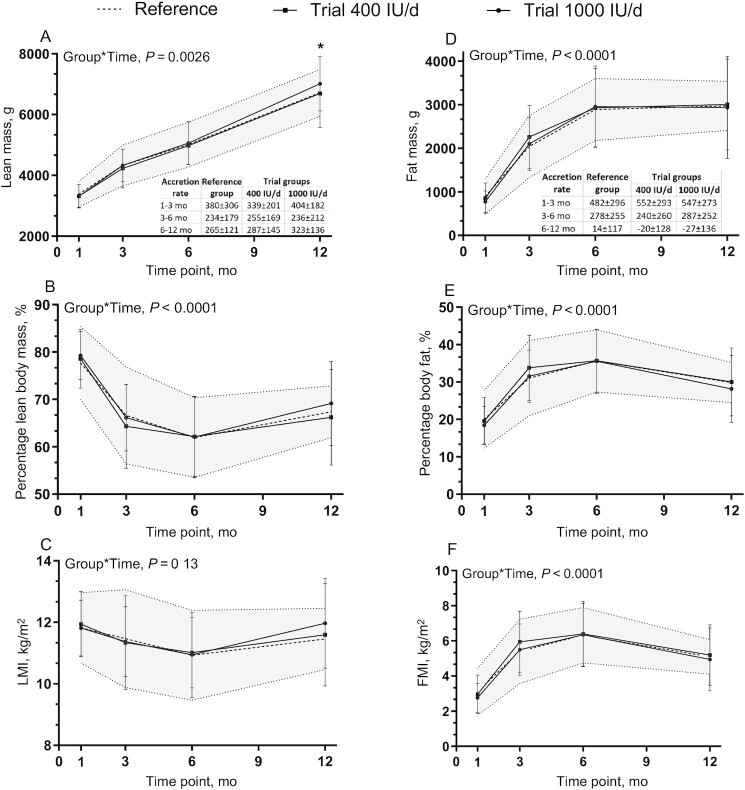
Lean body mass of the infants differed between groups over time (Figure 2A); at 12 mo of age, lean body mass was higher in the 1000 IU/d trial group than in the 400 IU/d trial group (7013 ± 905 compared with 6690 ± 1122 g; P = 0.0428; 4.8% difference), but not different from the reference group (7013 ± 905 compared with 6715 ± 785 g; P = 0.19). Lean body mass was also observed to be higher in male infants than in females (P < 0.0001) (Supplemental Figure 1A, B) and in infants born to mothers with prepregnancy BMI ≥25 kg/m2 than in those born to mothers with BMI <25 kg/m2 (P = 0.0222). In a sensitivity analysis according to maternal prepregnancy BMI (18.5–27.0 kg/m2) as per the inclusion criterion for the reference group, the interpretation was the same in terms of the difference in lean body mass between the trial 1000 IU/d and trial 400 IU/d groups at 12 mo (7049 ± 870 compared with 6448 ± 965 g; P = 0.0005) but no difference from the reference group (7049 ± 870 compared with 6715 ± 785 g; P = 0.11). Post hoc analyses of the primary outcome at 12 mo with adjustment for baseline lean body mass as a covariate did not change the interpretation with respect to a difference between the trial 1000 IU/d and trial 400 IU/d groups in lean body mass (P = 0.0486) and no difference from the reference group (P = 0.37) (Table 2). No difference was observed between groups over time for LMI and percentage lean body mass (Figure 2B, C).
FIGURE 2.
Infant body composition over time. (A) Lean mass and lean mass accretion, (B) percentage lean mass, (C) LMI, (D) fat mass and fat mass accretion, (E) percentage fat mass, and (F) FMI among groups over time. Data are mean ± SD. Sample sizes are as follows: reference group (baseline, n = 39; 3 mo, n = 34; 6 mo, n = 34; 12 mo, n = 30); 400 IU/d group (baseline, n = 49; 3 mo, n = 43; 6 mo, n = 39; 12 mo, n = 30); and 1000 IU/d group (baseline, n = 49; 3 mo, n = 44; 6 mo, n = 41; 12 mo, n = 37). Data were compared using a mixed-effect model (SAS PROC MIXED) for fixed effects of group × time, sex, skin tone, infant actual age at each visit, gravida, parental age at delivery, maternal BMI preconception, and family income. Participant (ID) modeled as a random effect. P values reflect Tukey's post hoc tests with Tukey-Kramer adjustment for multiple comparisons. The shaded area reflects reference ± SD. *P < 0.05, 1000 IU/d group compared with 400 IU/d group at 12 mo for lean mass; no other differences between groups over time were observed. FMI, fat mass index; LMI, lean mass index.
TABLE 2.
Lean mass of infants at 3, 6, and 12 mo in response to 1000 compared with 400 IU/d of supplemental vitamin D, post hoc ANCOVA1
| Lean mass, g | 1000 IU/d minus 400 IU/d | 400 IU/d minus Reference | 1000 IU/d minus Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Time point | Reference | 400 IU/d | 1000 IU/d | Difference (95% CI) | P value | Difference (95% CI) | P value | Difference (95% CI) | P value |
| 3 mo | 4327.0 ± 682.7 | 4225.1 ± 640.0 | 4326.3 ± 534.9 | 170.6 (−174.0, 515.1) | 0.47 | −252.3 (−624.7, 120.1) | 0.25 | −81.7 (−454.3, 290.8) | 0.86 |
| 6 mo | 5010.5 ± 754.0 | 4975.4 ± 608.9 | 5062.2 ± 707.4 | 161.5 (−193.3, 516.2) | 0.53 | −199.6 (−579.3, 180.1) | 0.43 | −38.2 (−415.4, −339.1) | 0.97 |
| 12 mo | 6715.1 ± 784.6 | 6690.4 ± 1121.7 | 7012.5 ± 904.6 | 389.3 (−1.8, 776.7) | 0.0486 | −162.1 (−573.8, 249.6) | 0.62 | 227.2 (−167.8, 622.1) | 0.37 |
Data were tested using a linear mixed-effects model for lean mass (g) as the primary outcome. Infant (ID) modeled as a random effect, followed by post hoc Tukey's tests with Tukey-Kramer adjustment for multiple comparisons. Adjusted for sex, skin tone, parity, maternal prepregnancy BMI, parental age at delivery, and family income.
There were no differences in fat mass, FMI, as well as fat percentage between groups at any time across the study (Figure 2D–F), with no sex differences in fat mass and percentage body fat after accounting for multiple comparison tests and adjusting for covariates (Supplemental Figure 1C, D). Fat mass increased over time from baseline to 6 mo of age and then remained at a plateau thereafter. Similarly, FMI and fat percentage values increased over time, but the values slightly decreased after 6 mo of age.
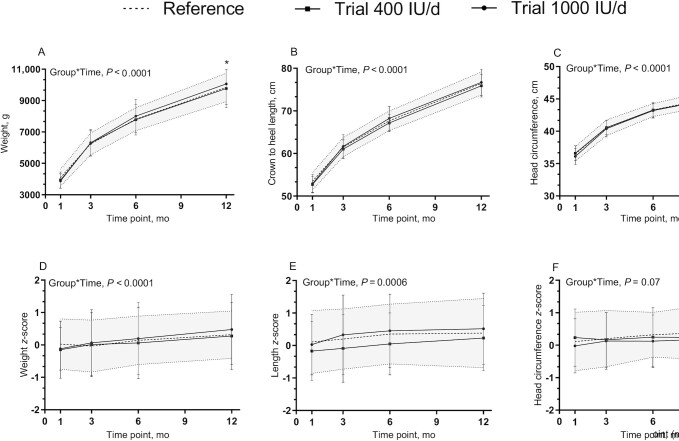
All of the infants were healthy, born at term, AGA, and growing well. Infant weight, length, and head circumference increased in all groups over time. Weight-, length-, and head-circumference-for-age and -sex z scores were within normal ranges according to the WHO growth standards. Overall, there were no interaction effects of group and time on all standard anthropometry measurements except body weight over the course of the study; body weight was higher in the 1000 IU/d group by 12 mo (296.05 g; 3.0% difference; P = 0.0298) than in the 400 IU/d group and not different from the reference group (P = 0.37) (Figure 3).
FIGURE 3.
Infant growth over time. (A) Weight, (B) crown to heel length, and (C) head circumference measurements, (D) weight-for-age, (E) length-for-age, and (F) head-circumference-for-age z scores, among groups over time. Data are mean ± SD. Sample sizes are as follows: reference group (baseline, n = 41; 3 mo, n = 35; 6 mo, n = 34; 12 mo, n = 32); 400 IU/d group (baseline, n = 49; 3 mo, n = 44; 6 mo, n = 40; 12 mo, n = 36); and 1000 IU/d group (baseline, n = 49; 3 mo, n = 44; 6 mo, n = 43; 12 mo, n = 42). Data were compared for weight, length, and head circumference measurements using a mixed-effect model (SAS PROC MIXED) tested for fixed effects of group × time, sex, skin tone, infant actual age at each visit, gravida, parental age at delivery, maternal BMI preconception, and family income. Participant (ID) modeled as a random effect; and for weight-, length-, and head-circumference-for-age z scores sex and infant actual age at each visit were removed from the mixed model. P values reflect Tukey's post hoc tests with Tukey-Kramer adjustment for multiple comparisons. The shaded area reflects reference ± SD. *P < 0.05, 1000 IU/d compared with 400 IU/d at 12 mo for body weight; no other differences in growth between groups over time were observed.
Effect of vitamin D supplementation on infant serum 25(OH)D concentration
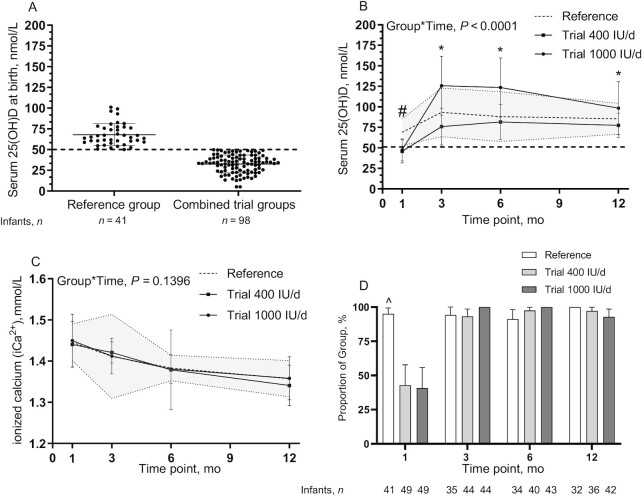
At birth, by design, on average the reference group had a serum 25(OH)D concentration ≥50 nmol/L and different from the pool of infants eligible for the trial (68.0 ± 13.7 nmol/L compared with 32.6 ± 10.8 nmol/L) (Figure 4A). Similarly, measurements done at the baseline visit showed that infants in the reference group on average had higher serum 25(OH)D concentrations than those in the 400 and 1000 IU/d vitamin D supplement groups (69.2 ± 16.4 nmol/L compared with 45.8 ± 14.1 and 47.6 ± 13.4 nmol/L, respectively; P < 0.0001). Thereafter, the trial group receiving 1000 IU vitamin D/d had higher concentrations than the reference group and the trial group receiving 400 IU/d at each time from 3 to 12 mo of the study (Figure 4B). Across the trial, ionized calcium was within normal limits (Figure 4C). At the baseline visit, in both trial 400 and 1000 IU/d groups, 42.9% and 40.8% of infants had sufficient concentrations of 25(OH)D ≥50 nmol/L, respectively, whereas from 3 to 12 mo of the study the percentage of infants achieving the cutoff for sufficiency of vitamin D increased over time (Figure 4D). Only in the 1000 IU/d group did 100% of infants achieve sufficient 25(OH)D concentrations at 3 and 6 mo.
FIGURE 4.
Infant serum 25(OH)D concentrations of the reference group (n = 41) and those in the pool for the trial (n = 98) at birth (24–36 h postpartum) (A), infant serum 25(OH)D concentrations among groups over the first 12 mo of life (baseline, 3, 6, and 12 mo) (B), infant whole-blood ionized calcium among groups (C), and proportions of infants in the reference, trial 400 IU/d, and trial 1000 IU/d groups achieving the cutoff for sufficiency for vitamin D status [25(OH)D ≥50 nmol/L] at each time point (D). (A–C) Data are mean ± SD, (D) data are mean and 95% CIs. Sample sizes are as follows: reference group (baseline, n = 41; 3 mo, n = 35; 6 mo, n = 34; 12 mo, n = 32); 400 IU/d group (baseline, n = 49; 3 mo, n = 44; 6 mo, n = 40; 12 mo, n = 36); and 1000 IU/d group (baseline, n = 49; 3 mo, n = 44; 6 mo, n = 43; 12 mo, n = 42). Continuous data were compared using a mixed-effect model (SAS PROC MIXED) tested for fixed effects of group × time, sex, skin tone, infant actual age at each visit, gravida, parental age at delivery, maternal BMI preconception, and family income. Participant (ID) modeled as a random effect. P values reflect Tukey's post hoc tests with Tukey-Kramer adjustment for multiple comparisons. The shaded area is reference ± SD. #P < 0.05, reference group compared with trial 400 and 1000 IU/d groups; *P < 0.05, trial 1000 IU/d compared with the trial 400 IU/d and reference groups. Categorical data were compared at each time point using chi-square analyses: ^P < 0.05, reference group compared with the trial 400 IU/d and 1000 IU/d groups at baseline only. 25(OH)D, 25-hydroxyvitamin D.
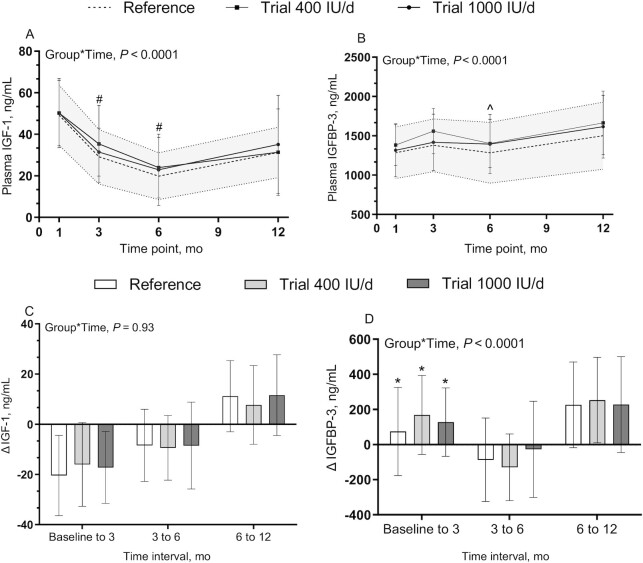
Effect of vitamin D supplementation on plasma IGF-1 and IGFBP-3
There was no difference between groups in IGF-1 and IGFBP-3 at baseline or during the trial, regardless of whether they were shown as concentrations (Figure 5A, B) or the change between each time point (Figure 5C, D). IGF-I and IGFBP-3 concentrations had different developmental patterns over the first 12 mo of life; IGF-1 concentration declined from baseline to 6 mo, with an increase with age thereafter. However, a less prominent change was observed in the IGFBP-3 concentrations over time. The main effect of sex was not significant for IGF-1 (P = 0.41) or IGFBP-3 (P = 0.0721) (Supplemental Figure 2).
FIGURE 5.
Infant plasma IGF-1 (A), IGFBP-3 concentrations (B), Δ IGF-1 (C), and Δ IGFBP-3 (D). Data are mean ± SD. Sample sizes are as follows: reference group (baseline, n = 41; 3 mo, n = 35; 6 mo, n = 34; 12 mo, n = 32); 400 IU/d group (baseline, n = 49; 3 mo, n = 44; 6 mo, n = 40; 12 mo, n = 36); and 1000 IU/d group (baseline, n = 49; 3 mo, n = 44; 6 mo, n = 43; 12 mo, n = 42). Data were compared using a mixed-effect model (SAS PROC MIXED) tested for fixed effects of group × time, sex, skin tone, infant actual age at each visit, gravida, parental age at delivery, maternal BMI preconception, and family income. Participant (ID) modeled as a random effect. P values reflect Tukey's post hoc tests with Tukey-Kramer adjustment for multiple comparisons. The shaded area is reference ± SD. #P < 0.05, baseline compared with 3 mo and 6 mo for all groups; ^P < 0.05, 3 mo compared with 6 mo for all groups except trial 1000 IU/d; *P < 0.05, baseline to 3 mo compared with 3 mo–6 mo for all groups. IGFBP-3, insulin-like growth factor binding protein 3; IGF-1, insulin-like growth factor 1.
Compliance with vitamin D supplementation and nutritional data
Between birth and the baseline visit, all of the infants in the reference group received 400 IU/d of vitamin D supplementation, whereas those in the pool for the trial were 96.4% adherent. For the trial and reference groups, the median compliance with supplementation was ≥91% from baseline until 3 mo, ≥92% from 3 to 6 mo, and ≥85% from 6 to 12 mo follow-up (9).
In the trial groups, all of the infants were predominantly breastfed and 81.6% in the trial 400 IU/d group and 85.7% in the trial 1000 IU/d group were exclusively breastfed at the initial baseline visit. At 3 mo of age >90% received breast milk, >80% did at 6 mo of age, and 40% at 12 mo were still receiving some breast milk (Supplemental Table 2). The median age at solid food introduction was 5 mo (IQR: 4–5.5 mo) with no difference between groups. Likewise, the median ages for crawling and walking were 6 and 10 mo (IQR: 5–7 and 9–11 mo for age at crawling and walking, respectively) with no difference between groups. No differences were observed in nutrient intakes, including energy, carbohydrate, fat, vitamin D, and calcium, between the vitamin D supplementation groups over time; however, protein intake was higher in the reference group than in the trial 400 IU/d group and not different from the 1000 IU/d group; overall, the values for all nutrient intakes increased over time (Supplemental Table 3).
Among trial groups, 78 families completed the 12-mo trial, and 20 families were lost to follow-up mainly because of family circumstances (e.g., time constraints or moving out from the area); furthermore, owing to movement artifacts, 4.6% of the whole-body scans (16 of 348) were missing over the course of the trial. Similarly, in the reference group 9 families were lost to follow-up and 3.5% of the whole-body scans (5 of 142) were missing across the study. Supplemental Table 4 shows neonatal and maternal characteristics of completers and dropouts. Among trial groups a higher proportion of the dropouts were born in the vitamin D–synthesizing period (P = 0.0238) and were multiparous than for completers (P = 0.0290). Family income was lower in dropouts than in completers (P = 0227), and dropouts had lower gestational weight gain than completers (P < 0.0001).
Discussion
This trial was designed to test whether infants who were born with serum 25(OH)D <50nmol/L would benefit from a higher dosage of vitamin D supplementation. Interestingly, body composition did not vary in neonates according to vitamin D status at birth, whereas divergent patterns were observed later in infancy. Specifically, a dosage of 1000 IU/d was shown to modestly increase whole-body lean mass (322.1 g; 4.8% difference compared with 400 IU/d) without altering weight or length z scores for age and sex. Thus, our research hypothesis was not fully accepted, because a difference in lean body mass was evident only at 12 mo and not at 3 mo of age. No differences were observed in whole-body fat mass across the trial, likely due to normal developmental variation in infants and the limitations of the technology in estimating fat mass.
In accordance with a recent systematic review and meta-analysis of intervention trials (39), in our study achieving the AI of 400 IU vitamin D/d through supplementation supported recovery of vitamin D status in infants born with serum 25(OH)D <50nmol/L. This research is novel in that infants allocated to 1000 IU vitamin D/d not only had increased serum 25(OH)D but also had greater lean body mass, the magnitude of which parallels that of Hazell et al. (3). Unlike our trial, most infants in Hazell et al.’s study had 25(OH)D ≥50 nmol/L at inception. Therefore, they used a higher cutoff of 75 nmol/L (40) and reported that plasma 25(OH)D was positively associated with percentage lean mass at 12 mo (∆ 3.89%; 5.41% difference; P = 0.006). In the present trial, lack of differences in fat mass and a higher body weight of the 1000 IU vitamin D/d group at 12 mo (296.05 g; 3.0% difference compared with 400 IU/d) suggest increments in lean body mass are more prominent than decreases in fat mass. This agrees with observations in female weanling rats with vitamin D deficiency (5). Rats that received 4 IU vitamin D3/g diet had an increase in 25(OH)D from 24.1 to 60.3 nmol/L within 8 wk and had higher lean mass (250.4 compared with 219.6 g; P = 0.01) than the control (1 IU vitamin D3/g diet). These controlled studies in human infants and weanling rats suggest that increased dietary vitamin D modestly improves lean body mass (5) and complement the aforementioned cohorts in pregnancy where maternal vitamin D sufficiency associates with a lean body phenotype of the child (6, 8, 9). Collectively, the evidence is consistent for sufficient vitamin D status having a role to play in healthy body composition of children.
In line with reports of body composition in healthy term infants (30, 41), fat mass increased rapidly during the first 6 mo. The plateau observed in fat mass after 6 mo can be ascribed to postnatal fat mass serving as an energy reserve. As infants accomplish energy-demanding developmental milestones such as crawling and walking, they require additional energy to support growth (30, 42). Because age at crawling and walking and nutrient intakes were not different between groups, this might help to explain lack of differences in fat mass.
The mechanisms that explain enhanced lean mass include a mutual engagement in skeletal muscle growth and metabolism exerted by vitamin D and IGF-1; this is through molecular and cellular pathways that regulate cell differentiation and growth in skeletal muscle (43). In weanling rats (5), higher dietary vitamin D3 maintained plasma IGF-1 longer during growth than in the control group, suggesting a potential mechanism by which IGF-1 and vitamin D stimulate lean mass accretion. Consistent with another report in healthy infants (44), different patterns were observed in terms of IGF-1 and IGFBP-3 concentrations. IGF-1 declined over time from baseline to 6 mo in a well-accepted physiological pattern, followed by an increase with age (from 6 to 12 mo); this could be ascribed to increased dietary protein intake as a strong positive predictor of IGF-1 concentration (45). Less notable changes were observed in IGFBP-3 with relatively higher concentrations than IGF-1. Both trial groups overlapped in IGF-1 and IGFBP-3 across the study and the values were within the anticipated range judged by our reference data.
In line with other reports where sexual dimorphisms in IGF-1 and IGFBP-3 concentrations appear at birth (46), or from 3 to 12 mo (47), we observed similar patterns, but these only appeared to diverge at 12 mo. Lack of statistical differences between the sexes could be explained by our smaller sample size. Higher concentrations of IGF-1 and IGFBP-3 are usually observed in female infants than in males owing to differentials in the IGF-1/GH axis over the first 12 mo of life. Early postnatally, IGF-1 is responsive to nutritional intake (breastfeeding status) and insulin secretion, whereas later in infancy growth hormone is the predominant regulator of IGF-1 (48). Growth hormone sensitivity to changes in IGF-1 is more pronounced in females than in males (46). Overall, the results suggest that increments in lean body mass are not necessarily ascribed to plasma IGF-1 and IGFBP-3, hence other mechanisms need to be explored considering the dynamics of vitamin D status and free IGF-1 utilization for tissue (lean mass) growth in infancy.
The major implication of our trial is that vitamin D has a role in establishing a healthy body composition in infants and specifically a leaner body phenotype. Vitamin D supplementation of 400 IU/d for healthy breastfed infants is suitable to compensate for serum 25(OH)D <50nmol/L at birth. Increasing vitamin D intakes to 1000 IU/d is not required to support serum 25(OH)D in the range of 50–125 nmol/L ( 12). This counters some professional society recommendations (40, 49). Whether the modest benefits to lean mass evident at 12 mo would extend into childhood with possible implications in reduced risk of excess adiposity requires further research with longer-term follow-up. In doing so, unraveling epigenetic mechanisms such as DNA methylation of the genes involved in vitamin D metabolism (vitamin D receptor, VDR; vitamin D binding protein DBP;retinoid X receptor RXR; cytochrome P450 CYP), would aid in understanding how improving vitamin D status with a higher dosage of supplementation results in a change in lean body phenotype.
We acknowledge the limitations of this study. We recruited our minimum sample size needed to ensure the specified precision and although we observed differences in lean mass, the dropout rate may have biased our results. Nonetheless, by using a mixed model all the available data were used in our analyses including sociodemographic covariates. In addition, numerous assessments were tested across 4 time points, posing a risk of type I errors. To alleviate this concern, post hoc testing was limited to differences between groups over time and adjusted for multiple comparisons. Serum 25(OH)D was measured using an immunoassay which is not a gold-standard technique, although the assay manufacturer is certified by the Vitamin D Standardization-Certification Program (50). In addition, we implemented rigorous quality assurance measures across the study using the Vitamin D External Quality Assessment Scheme as well as the NIST standard reference materials. Moreover, we verified in a subgroup of mothers and infants (n = 83) that total serum 25(OH)D was in agreement (mean difference = −0.8 nmol/L) with LC–tandem MS. Another limitation is that normative data for body composition in infancy are limited (30) to infants with different demographic characteristics from the present study. To help overcome this, patterns of growth and body composition in the trial groups were compared with a reference group tracked at the same time. Lastly, parent self-reported compliance was a limitation and pre and post weighing of used bottles of supplement was not feasible; those that were returned had often been cleaned for recycling. Nonetheless, the biological response to supplementation was monitored using serum 25(OH)D concentration.
Overall, this trial provides a high level of evidence suggesting that postnatal vitamin D supplementation (400 and 1000 IU/d) with high adherence compensates for low maternal–fetal transfer of vitamin D in otherwise healthy AGA infants. Increasing supplemental vitamin D intakes to 1000 IU/d appears to have implications in programming of a leaner body phenotype without altering other patterns of growth. Further investigations are required to explore the underlying mechanisms, with possible implications for child and public health.
Supplementary Material
Acknowledgments
We thank the following people for their contributions toward the success of this work: Katherine Gray-Donald, McGill University, for her involvement in designing the study; Roger Cue, McGill University, for his valuable guidance and insightful advice throughout the statistical analyses; Sherry Agellon, Paula Lavery, and Maggie Yuan from McGill University for their long-standing assistance in laboratory measurements across the study; and Veronique Menard, Erika de Risi, Kristina Mullahoo, Laura Glenn, Sharina Patel, and Zahra Farahnak for their involvement in recruitment at the Lakeshore General Hospital.
The authors’ responsibilities were as follows—HAW, SQW, DM, FR, GJ, and SK: designed the study; HAW: supervised the study; AK: designed the product, performed the external testing, and managed the randomization scheme; HAW and CAV: managed the study project; HAW, CAV, MR, NG, and OFS: collected the data; MR, NG, and OFS: performed the laboratory analyses; HAW and MR: performed the statistical analysis; MR: wrote the final manuscript with the intellectual aid and comments of HAW; and all authors: read and approved the final manuscript. The authors report no conflicts of interest.
Notes
Supported by Canadian Institutes of Health Research grant #MOP-142391 (to HAW), and Canada Foundation for Innovation Project 202820 (to HAW) which provided funding for infrastructure. At the time of the research, HAW was funded by Canada Research Chairs Program 2015–2018 salary award 950-230633; MR and NG received Graduate Excellence Fellowships from McGill University. The funding agencies were not involved in the study design; collection, management, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Supplemental Figures 1 and 2 and Supplemental Tables 1–4 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: AGA, appropriate weight for gestational age; AI, Adequate Intake; CAD, Canadian dollars; CLIA, chemiluminescence immunoassay; F, Fitzpatrick; FMI, fat mass index; IGFBP-3, insulin-like growth factor binding protein 3; IGF-1, insulin-like growth factor 1; IOM, Institute of Medicine; LMI, lean mass index; NIST, National Institute of Standards and Technology; 25(OH)D, 25-hydroxyvitamin D.
Contributor Information
Maryam Razaghi, School of Human Nutrition, McGill University, Ste-Anne-de-Bellevue, Québec, Canada.
Nathalie Gharibeh, School of Human Nutrition, McGill University, Ste-Anne-de-Bellevue, Québec, Canada.
Catherine A Vanstone, School of Human Nutrition, McGill University, Ste-Anne-de-Bellevue, Québec, Canada.
Olusola F Sotunde, School of Human Nutrition, McGill University, Ste-Anne-de-Bellevue, Québec, Canada.
Ali Khamessan, Quality & Regulatory Affairs, Europharm International Canada Inc., Montréal, Québec, Canada.
Shu Q Wei, Quebec National Institute of Public Health (INSPQ), Montréal, Québec, Canada.
Dayre McNally, Department of Pediatrics, Children's Hospital of Eastern Ontario, University of Ottawa, Ottawa, Ontario, Canada.
Frank Rauch, Shriners Hospital for Children, Montréal, Québec, Canada.
Glenville Jones, School of Medicine, Department of Biomedical and Molecular Sciences, Queen's University, Kingston, Ontario, Canada.
Sarah Kimmins, Animal Sciences, Faculty of Agricultural & Environmental Sciences, McGill University, Ste-Anne-de-Bellevue, Québec, Canada.
Hope A Weiler, School of Human Nutrition, McGill University, Ste-Anne-de-Bellevue, Québec, Canada; Nutrition Research Division, Bureau of Nutritional Sciences, Food Directorate, Health Products and Food Branch, Health Canada, Ottawa, Ontario, Canada.
Data Availability
The data described in the article will not be made available because permission to share data was not requested at the time of obtaining participant consent.
References
- 1. Bi WG, Nuyt AM, Weiler H, Leduc L, Santamaria C, Wei SQ. Association between vitamin D supplementation during pregnancy and offspring growth, morbidity, and mortality: a systematic review and meta-analysis. JAMA Pediatr. 2018;172(7):635–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gil A, Plaza-Diaz J, Mesa MD. Vitamin D: classic and novel actions. Ann Nutr Metab. 2018;72(2):87–95. [DOI] [PubMed] [Google Scholar]
- 3. Hazell TJ, Gallo S, Berzina L, Vanstone CA, Rodd C, Weiler HA. Plasma 25-hydroxyvitamin D, more so than its epimer, has a linear relationship to leaner body composition across infancy in healthy term infants. Appl Physiol Nutr Metab. 2014;39(10):1137–43. [DOI] [PubMed] [Google Scholar]
- 4. Hazell T, Gallo S, Vanstone C, Agellon S, Rodd C, Weiler H. Vitamin D supplementation trial in infancy: body composition effects at 3 years of age in a prospective follow-up study from Montreal. Pediatr Obes. 2017;12(1):38–47. [DOI] [PubMed] [Google Scholar]
- 5. Razaghi M, Djekic-Ivankovic M, Agellon S, Mak I, Lavery P, Weiler HA. Lean body mass accretion is elevated in response to dietary vitamin D: a dose–response study in female weanling rats. Nutr Res. 2019;68:92–100. [DOI] [PubMed] [Google Scholar]
- 6. Krishnaveni GV, Veena SR, Winder NR, Hill JC, Noonan K, Boucher BJ, Karat SC, Fall CH. Maternal vitamin D status during pregnancy and body composition and cardiovascular risk markers in Indian children: the Mysore Parthenon Study. Am J Clin Nutr. 2011;93(3):628–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boyle VT, Thorstensen EB, Thompson JM, McCowan LM, Mitchell EA, Godfrey KM, Poston L, Wall CR, Murphy R, Cutfield W. The relationship between maternal 25-hydroxyvitamin D status in pregnancy and childhood adiposity and allergy: an observational study. Int J Obes. 2017;41(12):1755–60. [DOI] [PubMed] [Google Scholar]
- 8. Harvey NC, Moon RJ, Sayer AA, Ntani G, Davies JH, Javaid MK, Robinson SM, Godfrey KM, Inskip HM, Cooper C. Maternal antenatal vitamin D status and offspring muscle development: findings from the Southampton Women's Survey. J Clin Endocrinol Metab. 2014;99(1):330–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miliku K, Felix JF, Voortman T, Tiemeier H, Eyles DW, Burne TH, McGrath JJ, Jaddoe VW. Associations of maternal and fetal vitamin D status with childhood body composition and cardiovascular risk factors. Matern Child Nutr. 2019;15(2):e12672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Benyshek DC. The developmental origins of obesity and related health disorders – prenatal and perinatal factors. Coll Antropol. 2007;31(1):11–17. [PubMed] [Google Scholar]
- 11. Ma K, Wei SQ, Bi WG, Weiler HA, Wen SW. Effect of vitamin D supplementation in early life on children's growth and body composition: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2021;13(2):524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weiler H, Fitzpatrick-Wong S, Veitch R, Kovacs H, Schellenberg J, McCloy U, Yuen CK. Vitamin D deficiency and whole-body and femur bone mass relative to weight in healthy newborns. Can Med Assoc J. 2005;172(6):757–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Morgan C, Dodds L, Langille DB, Weiler HA, Armson BA, Forest J-C, Giguère Y, Woolcott CG. Cord blood vitamin D status and neonatal outcomes in a birth cohort in Quebec, Canada. Arch Gynecol Obstet. 2016;293(4):731–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aghajafari F, Field CJ, Kaplan BJ, Maggiore JA, O'Beirne M, Hanley DA, Eliasziw M, Dewey D, Ross S, Rabi D. The high prevalence of vitamin D insufficiency in cord blood in Calgary, Alberta (APrON-D Study). J Obstet Gynaecol Can. 2017;39(5):347–53.e1. [DOI] [PubMed] [Google Scholar]
- 16. Kramer MS, Platt RW, Wen SW, Joseph K, Allen A, Abrahamowicz M, Blondel B, Bréart G, Fetal/Infant Health Study Group of the Canadian Perinatal Surveillance System . A new and improved population-based Canadian reference for birth weight for gestational age. Pediatrics. 2001;108(2):e35. [DOI] [PubMed] [Google Scholar]
- 17. Hull HR, Dinger MK, Knehans AW, Thompson DM, Fields DA. Impact of maternal body mass index on neonate birthweight and body composition. Am J Obstet Gynecol. 2008;198(4):416.e1–e6. [DOI] [PubMed] [Google Scholar]
- 18. Statistics Canada . Table 11-10-0190-01. Market income, government transfers, total income, income tax and after-tax income by economic family type. 2015–2019. [Internet]. Ottawa, Canada: Statistics Canada; 2021. Available from: 10.25318/1110019001-eng. Accessed 15 Jul 2021. [DOI] [Google Scholar]
- 19. World Health Organization . WHO AnthroPlus for personal computers manual: software for assessing growth of the world's children and adolescents[Internet]. Geneva, Switzerland: WHO; 2009. Available from: http://www.who.int/growthref/tools/en/. Accessed 20 Aug 2021. [Google Scholar]
- 20. Brooks SPJ, Greene-Finestone L, Whiting S, Fioletov VE, Laffey P, Petronella N. An analysis of factors associated with 25-hydroxyvitamin D levels in white and non-white Canadians. J AOAC Int. 2017;100(5):1345–54. [DOI] [PubMed] [Google Scholar]
- 21. Webb AR, DeCosta BR, Holick MF. Sunlight regulates the cutaneous production of vitamin D3 by causing its photodegradation. J Clin Endocrinol Metab. 1989;68(5):882–7. [DOI] [PubMed] [Google Scholar]
- 22. Chardon A, Cretois I, Hourseau C. Skin colour typology and suntanning pathways. Int J Cosmet Sci. 1991;13(4):191–208. [DOI] [PubMed] [Google Scholar]
- 23. Del Bino S, Sok J, Bessac E, Bernerd F. Relationship between skin response to ultraviolet exposure and skin color type. Pigm Cell Res. 2006;19(6):606–14. [DOI] [PubMed] [Google Scholar]
- 24. Reeder AI, Hammond VA, Gray AR. Questionnaire items to assess skin color and erythemal sensitivity: reliability, validity, and “the dark shift”. Cancer Epidemiol Biomarkers Prev. 2010;19(5):1167–73. [DOI] [PubMed] [Google Scholar]
- 25. Gallo S, Comeau K, Vanstone C, Agellon S, Sharma A, Jones G, L'Abbé M, Khamessan A, Rodd C, Weiler H. Effect of different dosages of oral vitamin D supplementation on vitamin D status in healthy, breastfed infants: a randomized trial. JAMA. 2013;309(17):1785–92. [DOI] [PubMed] [Google Scholar]
- 26. Health Canada . Canadian Nutrient File (CNF)[Internet]. Ottawa, Canada: Government of Canada; 2016[cited 15 July, 2021]. Available from: https://food-nutrition.canada.ca/cnf-fce/index-eng.jsp. [Google Scholar]
- 27. Cohen MA, Ryan PB. Observations less than the analytical limit of detection: a new approach. J Air Waste Manage Assoc. 1989;39(3):328–9. [Google Scholar]
- 28. Gallo S, Comeau K, Sharma A, Vanstone CA, Agellon S, Mitchell J, Weiler HA, Rodd C. Redefining normal bone and mineral clinical biochemistry reference intervals for healthy infants in Canada. Clin Biochem. 2014;47(15):27–32. [DOI] [PubMed] [Google Scholar]
- 29. Fitzmaurice GM, Laird NM, Ware JH. Applied longitudinal analysis. Hoboken, NJ: John Wiley & Sons; 2012. [Google Scholar]
- 30. Sotunde OF, Gallo S, Vanstone CA, Weiler HA. Normative data for lean mass and fat mass in healthy predominantly breast-fed term infants from 1 month to 1 year of age. J Clin Densitom. 2020;23(2):264–70. [DOI] [PubMed] [Google Scholar]
- 31. Weiler HA, Vanstone CA, Razaghi M, Gharibeh N, Patel S, Wei SQ, McNally D. Disparities in vitamin D status of newborn infants from a diverse sociodemographic population in Montreal, Canada. J Nutr. 2022;152(1):255–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Carpenter TO, Herreros F, Zhang JH, Ellis BK, Simpson C, Torrealba-Fox E, Kim GJ, Savoye M, Held NA, Cole DE. Demographic, dietary, and biochemical determinants of vitamin D status in inner-city children. Am J Clin Nutr. 2012;95(1):137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. VanderWeele TJ. Principles of confounder selection. Eur J Epidemiol. 2019;34(3):211–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Harvey NC, Poole J, Javaid MK, Dennison EM, Robinson S, Inskip HM, Godfrey KM, Cooper C, Sayer AA. Parental determinants of neonatal body composition. J Clin Endocrinol Metab. 2007;92(2):523–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Millette M, Sharma A, Weiler H, Sheehy O, Bérard A, Rodd C. Programme to provide Quebec infants with free vitamin D supplements failed to encourage participation or adherence. Acta Paediatr. 2014;103(10):e444–9. [DOI] [PubMed] [Google Scholar]
- 36. Moschonis G, Kaliora AC, Karatzi K, Michaletos A, Lambrinou C-P, Karachaliou AK, Chrousos GP, Lionis C, Manios Y. Perinatal, sociodemographic and lifestyle correlates of increased total and visceral fat mass levels in schoolchildren in Greece: the Healthy Growth Study. Public Health Nutr. 2017;20(4):660–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bell KA, Wagner CL, Feldman HA, Shypailo RJ, Belfort MB. Associations of infant feeding with trajectories of body composition and growth. Am J Clin Nutr. 2017;106(2):491–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ritz C. Statistical analysis of continuous outcomes from parallel-arm randomized controlled trials in nutrition—a tutorial. Eur J Clin Nutr. 2021;75(1):160–71. [DOI] [PubMed] [Google Scholar]
- 39. Zittermann A, Pilz S, Berthold HK. Serum 25-hydroxyvitamin D response to vitamin D supplementation in infants: a systematic review and meta-analysis of clinical intervention trials. Eur J Nutr. 2020;59(1):359–69. [DOI] [PubMed] [Google Scholar]
- 40. Godel JC, Canadian Paediatric Society, First Nations, Inuit and Métis Health Committee . Vitamin D supplementation: recommendations for Canadian mothers and infants. Paediatr Child Health. 2007;12(7):583–9. [PMC free article] [PubMed] [Google Scholar]
- 41. Orsso CE, Colin-Ramirez E, Field CJ, Madsen KL, Prado CM, Haqq AM. Adipose tissue development and expansion from the womb to adolescence: an overview. Nutrients. 2020;12(9):2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Admassu B, Ritz C, Wells JC, Girma T, Andersen GS, Belachew T, Owino V, Michaelsen KF, Abera M, Wibaek R. Accretion of fat-free mass rather than fat mass in infancy is positively associated with linear growth in childhood. J Nutr. 2018;148(4):607–15. [DOI] [PubMed] [Google Scholar]
- 43. Hamilton B. Vitamin D and human skeletal muscle. Scand J Med Sci Sports. 2010;20(2):182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yüksel B, Özbek MN, Mungan NÖ, Darendeliler F, Budan B, Bideci A, Çetinkaya E, Berberoğlu M, Evliyaoğlu O, Yeşilkaya E. Serum IGF-1 and IGFBP-3 levels in healthy children between 0 and 6 years of age. J Clin Res Pediatr Endocrinol. 2011;3(2):84–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Madsen AL, Larnkjær A, Mølgaard C, Michaelsen KF. IGF-I and IGFBP-3 in healthy 9 month old infants from the SKOT cohort: breastfeeding, diet, and later obesity. Growth Horm IGF Res. 2011;21(4):199–204. [DOI] [PubMed] [Google Scholar]
- 46. Geary MP, Pringle PJ, Rodeck CH, Kingdom JC, Hindmarsh PC. Sexual dimorphism in the growth hormone and insulin-like growth factor axis at birth. J Clin Endocrinol Metab. 2003;88(8):3708–14. [DOI] [PubMed] [Google Scholar]
- 47. Ong KK, Langkamp M, Ranke MB, Whitehead K, Hughes IA, Acerini CL, Dunger DB. Insulin-like growth factor I concentrations in infancy predict differential gains in body length and adiposity: the Cambridge Baby Growth Study. Am J Clin Nutr. 2009;90(1):156–61. [DOI] [PubMed] [Google Scholar]
- 48. de Jong M, Cranendonk A, Twisk JWR, van Weissenbruch MM. IGF-I and relation to growth in infancy and early childhood in very-low-birth-weight infants and term born infants. PLoS One. 2017;12(2):e0171650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vidailhet M, Garabédian M. Vitamin D requirements for French children. Arch Pediatr. 2010;17(6):808–9. [DOI] [PubMed] [Google Scholar]
- 50. DiaSorin Inc . LIAISON® 25 OH Vitamin D TOTAL assay[Internet]. Saluggia, Italy: DiaSorin Inc; 2012; [cited 1 July, 2021]. Available from: https://www.diasorin.com/sites/default/files/allegati_prodotti/ese_25_oh_vit_m0870004213_e_low.pdf. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data described in the article will not be made available because permission to share data was not requested at the time of obtaining participant consent.