ABSTRACT
Background
Both ultra-processed foods and animal-derived foods have been associated with mortality in some studies.
Objectives
We aimed to examine the association of 2 dietary factors (ultra-processed foods and animal-based foods), adjusted for each other, with all-cause mortality.
Methods
The setting is an observational prospective cohort study in North America, recruited from Seventh-day Adventist churches, comprised of 95,597 men and women, yielding an analytic sample of 77,437 participants after exclusions. The exposure of interest was diet measured by FFQ, in particular 2 dietary factors: 1) proportion of dietary energy from ultra-processed foods (other processing levels and specific substitutions in some models) and 2) proportion of dietary energy from animal-based foods (red meat, poultry, fish, and eggs/dairy separately in some models). The main outcome was all-cause mortality. Mortality data through 2015 were obtained from the National Death Index. Analyses used proportional hazards regression.
Results
There were 9293 deaths. In mutually adjusted continuous linear models of both dietary factors (ultra-processed and animal-based foods), the HR for the 90th compared with the 10th percentile of the proportion of dietary energy from ultra-processed food was 1.14 (95% CI: 1.07, 1.21, comparing 47.7% with 12.1% dietary energy), whereas for animal-based food intake (meats, dairy, eggs) it was 1.01 (95% CI: 0.95, 1.07, comparing 25.0% with 0.4% dietary energy). There was no evidence of interaction (P = 0.36). Among animal-based foods, only red meat intake was associated with mortality (HR: 1.14; 95% CI: 1.08, 1.22, comparing 6.2% with 0% dietary energy).
Conclusions
Greater consumption of ultra-processed foods was associated with higher all-cause mortality in this health-conscious Adventist population with many vegetarians. The total of animal-based food consumption (meat, dairy, eggs) was not associated with mortality, but higher red meat intake was. These findings suggest that high consumption of ultra-processed foods may be an important indicator of mortality.
Keywords: processed, ultra-processed, plant-based, animal-based, diet, dietary pattern, vegetarian, mortality
Introduction
Vegetarian dietary patterns have been associated with reduced risk of several chronic diseases and risk factors (1–3). In the Adventist Health Study—2 (AHS-2) these dietary patterns have also been associated with lower all-cause mortality (4), whereas this has not been true in EPIC-Oxford (European Prospective Investigation into Cancer and Nutrition—Oxford) (5, 6), another large study of vegetarians with differences in population characteristics, dietary details, and other aspects of lifestyle. Vegetarian (and nonvegetarian) diets may differ widely in their dietary composition and quality. The potential importance of such variation for epidemiologic associations of plant-based diets has been recognized in the development of a provegetarian index (7) and healthful and unhealthful plant-based diet indexes (8).
Food processing is one potential metric of dietary quality that is theoretically independent of the plant- or animal-based nature of the diet. Concern has particularly centered around foods categorized as ultra-processed (9–11). Consumption of these has been associated with higher mortality in some studies (12–15). In contrast, many whole foods (i.e., unprocessed or minimally processed) have been promoted as beneficial [e.g., whole grains (16)].
We hypothesized that 2 conceptually independent factors could together be used to characterize healthful compared with unhealthful diets, the first being the proportion of the diet composed of ultra-processed foods as opposed to less processed foods, and the second being the proportion of the diet from animal-based foods (comprised of meats, eggs, and dairy) as opposed to plant-based foods. Here, we examine the association of these 2 dietary factors (proportion ultra-processed and proportion animal-based) with all-cause mortality in the AHS-2, a cohort with a high percentage of persons eating plant-based diets.
Methods
Study population
AHS-2 is a cohort of 95,863 men and women recruited from Seventh-day Adventist churches in the United States and Canada between 2002 and 2007 with the primary aim of examining dietary associations with the incidence of major cancers (17–22). See Butler et al. (23) for a detailed explanation of the cohort formation and characteristics. Written informed consent was obtained from all participants upon enrollment. The study was approved by the institutional review board of Loma Linda University.
For these analyses, the following exclusions were applied in order: missing data for questionnaire return date, birth date, sex, or race (n = 874); age <25 y (n = 7); estimated energy intake (not including write-in items) <500 kcal/d or >4500 kcal/d, improbable response patterns (e.g., identical responses to all questions on a page), or >69 missing values in dietary data (n = 5081); non-US residents (n = 4093); BMI (in kg/m2) <14 or >40 (n = 176); history of a specific prior cancer diagnosis (except nonmelanoma skin cancers) (n = 6990); and loss to follow-up within 2 y of baseline (n = 938). After exclusions, there remained an analytic sample of 77,437 (Supplemental Figure 1).
Mortality data
Mortality data through 2015 were obtained from the National Death Index. The first 2 y of follow-up were excluded, to account for a possible healthy volunteer effect. International Statistical Classification of Diseases, 10th Revision (ICD-10) codes for the underlying cause of death were used to classify cause-specific mortality as follows: cardiovascular disease (CVD) deaths starting with I; cancer deaths starting with C; infectious disease deaths starting with A or B; neurologic deaths starting with G and including F01–F03; respiratory deaths starting with J; renal deaths with N; endocrine deaths with E; and “other” deaths included all other ICD codes.
Dietary data
Usual dietary intake during the previous year was assessed at baseline by a previously validated (24, 25) self-administered quantitative FFQ of >200 food items (available from: http://www.llu.edu/pages/health/documents/ahs-2.pdf). For these analyses, diet was characterized according to 2 factors.
For the processing factor we used a modification of the NOVA (not an acronym) system (9, 11). NOVA Group 2, Processed Culinary Ingredients, was not readily applicable to the AHS-2 FFQ; we therefore used a 3-level classification. Foods were placed into 3 categories: 1) unprocessed or minimally processed foods (NOVA Group 1; hereafter unprocessed), 2) moderately processed foods (representing NOVA Groups 2–3), and 3) ultra-processed foods (NOVA Group 4). Investigators considered a priori each of the food groups listed in Supplemental Table 1 and, for each food item (e.g., ice cream) or type of item (e.g., all fresh fruits) specified in the FFQ, collectively decided on the processing categorization using the principles of the NOVA system, while considering the information available from the FFQ. Supplemental Table 1 portrays the final processing classification for all individual food items or types of items listed in the FFQ. The FFQ also allowed for various write-in items, which were similarly classified but are too numerous to list. Besides using 3 categories as opposed to 4 groups, there are some differences compared to NOVA. For example, processed meats were categorized as highly processed in this analysis, whereas they are not always categorized as ultra-processed foods in NOVA [not “salted, cured, or smoked meats” (26)]; fruit juices (without additives) are considered unprocessed or minimally processed in NOVA (9, 26), whereas we classified them as moderately processed. In many cases, information about whether items were commercial/store-bought or homemade was lacking (e.g., breads, desserts), so assumptions were made about what would be most common. For food groups comprised mainly of commercially made products (e.g., meat analogs, cereals), those with fewer ingredients or less added sugar were considered moderately processed, whereas the rest were considered ultra-processed.
Each of the 3 processing categories was modeled as the proportion of total dietary energy from all foods in the category (e.g., percentage dietary energy from all ultra-processed foods). For the main models, only the proportion of dietary energy from ultra-processed foods was modeled. For some analyses, 2 of the 3 processing categories were modeled together, in which case they each modeled a substitution for the equivalent proportion of energy from the third category (at constant levels of dietary covariates).
For the animal- compared with plant-based factor: plant-based foods included all foods exclusively or primarily of plant origin (e.g., fruits, vegetables, breads). Animal-based foods included all meats (i.e., fish, poultry, red meats, etc.), dairy products, and eggs. For the main models, the proportion of dietary energy from animal-based foods was modeled. For some analyses, 4 animal-based food categories were modeled (red meat, poultry, fish, and dairy/eggs); in this case, each modeled the effect of substituting for an equal proportion of plant-based foods (at constant levels of dietary covariates).
Vegetarian status was determined according to the reported intake of foods of animal origin. Nonvegetarians consumed nonfish meats ≥1 time/mo and all meats combined (fish included) >1 time/wk; all others were defined broadly as vegetarians (comprising vegans, lacto-ovo vegetarians, pesco vegetarians, and semivegetarians, as previously defined) (4).
Covariates
Covariates were selected on an a priori basis as likely confounders based on prior literature and suspected relations. All covariates were measured by questionnaire at baseline and were included in the main analyses as follows (note that age was adjusted as the time variable, as will be described): sex (male/female); race (black and nonblack); geographic region (West, Northwest, Mountain, Midwest, East, South); education (up to high school graduate, trade school/some college/associate degree, bachelor degree, graduate degree); marital status (married/common-law, never married, widowed, divorced/separated); smoking (current smoker, quit <1 y, quit >1–5 y, quit >5–10 y, quit >10–20 y, quit >20–30 y, quit >30 y, never smoked); alcohol (nondrinker, <1.5 servings/mo, 1.5 to <4 servings/mo, 4 to <28 servings/mo, ≥28 servings/mo); exercise (“vigorous activities, such as brisk walking, jogging, bicycling, etc., long enough or with enough intensity to work up a sweat, get your heart thumping, or get out of breath”) (categories: none, ≤20 min/wk, 21–60 min/wk, 61–150 min/wk, ≥151 min/wk); sleep duration (≤4 h/night, 5 to ≥9 h/night); menopausal status of women [premenopausal (including perimenopausal), postmenopausal]; hormone replacement therapy (HRT) in postmenopausal women (not taking hormone replacement, taking hormone replacement); BMI [restricted cubic spline with knots at the 5th (19.7), 27.5th (23.4), 50th (26.1), 72.5th (29.4), and 95th (38.6) percentiles]; total energy intake (kcal/d); prevalent CVD (yes/no); and diabetes mellitus treated in the last 12 mo (yes/no). Participants self-identified their race in ≥1 of 21 specified categories. Those self-identifying at least in part as black/African American, West Indian/Caribbean, African, or other black were categorized as black for this analysis and all others as nonblack. Menopausal status and HRT were represented in models as nested covariates (i.e., sex + sex*menopause + sex*menopause*HRT). In the cause-specific analyses, smoking and alcohol were both collapsed to 2 levels because of model nonconvergence for some outcomes.
Statistical analysis
Baseline descriptive statistics were calculated according to quintiles of the proportion of dietary energy from ultra-processed foods and animal-based foods. Analyses of mortality were performed using Cox proportional hazards regression with attained age as the time variable and left-truncation by age at study entry. A joint Cox proportional hazards model (27) to deal with competing risks was used for the cause-specific mortality analysis. The variables of interest were primarily modeled in continuous linear fashion, with HRs typically reported as contrasts of the 90th and 10th percentiles of intake. For the specific animal food variables (red meat, poultry, fish, dairy/eggs), where there were many 0 intake values, a contrast between the 87.5th percentile among nonzero consumers and 0 consumption was used instead. Categorical modeling in quintiles of intake (or 0 intake and quartiles of nonzero intake for the specific animal food variables) was also done and is presented alongside the continuous model results in some figures and tables. Log likelihood testing for interaction of the 2 main dietary variables (each modeled as a continuous variable) was performed. Selected covariates (race, sex, BMI, exercise, diabetes, and CVD) were tested for possible interaction with the diet variables; there were no significant interactions. An E-value was computed to assess vulnerability to confounding for the main result (28, 29).
The proportional hazards assumption was initially evaluated using log(-log) plots and, where there was an apparent violation, further tested using attained-age interaction terms which were retained if significant. Significant nonproportionality of hazards was present for race, so an attained-age interaction term was retained in the models.
Participants with missing age, sex, race, or mortality data were excluded. Missing values for prevalent CVD and diabetes mellitus were 0 imputed (i.e., as no disease). Multiple imputation of missing values was done for the following covariates (percentage missing in parentheses): education (1.23%), BMI (2.52%), marital status (1.58%), exercise (4.61%), smoking (1.55%), sleep duration (1.66%), alcohol (2.20%), menopausal status (0.65%), and HRT (2.03%). Geographic region had no missing values. Dietary exposure variables of interest were not directly imputed (rather, the many underlying FFQ response variables used to estimate these were imputed). For these underlying FFQ data, a guided multiple imputation approach was utilized (30), because we have evidence that many commonly consumed dietary items are true nonzeroes (31). Analyses and guided multiple imputation were performed using R version 4.0.2 (32) and the Hmisc package version 3.8 (33).
Results
Among 77,437 participants followed for an mean of 7.46 y (after the first 2 excluded years) for a total of 567,303.6 person-years, there were 9293 deaths. Figure 1 shows the distributions of consumption of both ultra-processed and animal-based foods. There was 0 or very little consumption of animal-based foods among many participants (median: 9.8% of total dietary energy), whereas the intake of ultra-processed foods was more normally distributed (median: 27.4% of total dietary energy). Tables 1 and 2 compare demographic, lifestyle, and dietary descriptive statistics for the quintiles of ultra-processed and animal-based food consumption, respectively.
FIGURE 1.
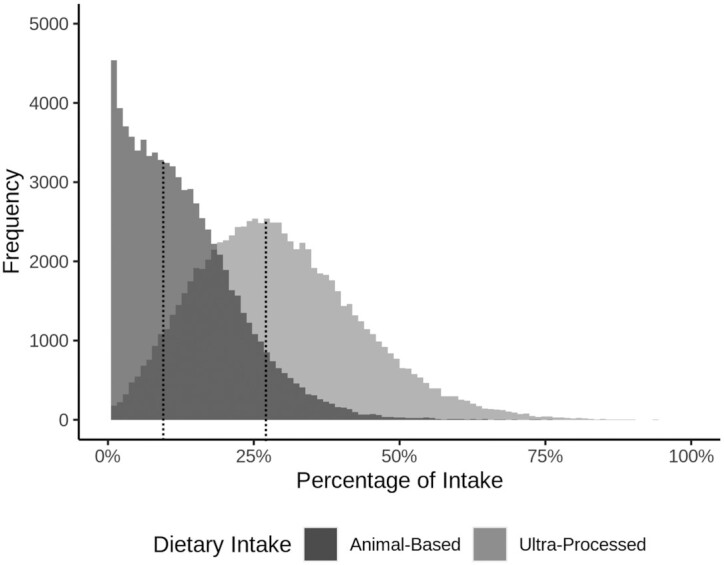
Dietary intake distributions. Population distributions for the percentage of dietary energy from ultra-processed foods (lighter shading) and from animal-based foods (darker shading; includes meat, dairy, and eggs); medians for each distribution are marked with dotted vertical lines.
TABLE 1.
Comparison of participant characteristics across quintiles of percentage of ultra-processed food intake1
| Ultra-processed foods, %kcal | ||||||
|---|---|---|---|---|---|---|
| Quintile 1 (0%–17.0%) | Quintile 2 (17.1%–24.5%) | Quintile 3 (24.6%–31.5%) | Quintile 4 (31.6%–40.5%) | Quintile 5 (40.6%–95.8%) | P values | |
| General demographics | ||||||
| Participants, n | 15,442 | 15,342 | 15,415 | 15,500 | 15,738 | |
| Deaths | 1917 (12.4) | 1961 (12.8) | 1946 (12.6) | 1765 (11.4) | 1704 (10.8) | <0.001 |
| Age at baseline, y | 61.37 ± 13.67 | 60.90 ± 13.86 | 59.85 ± 14.07 | 58.32 ± 13.94 | 55.96 ± 13.78 | <0.001 |
| Female | 10,398 (67.3) | 10,397 (67.8) | 10,179 (66.0) | 9818 (63.3) | 9472 (60.2) | <0.001 |
| Black | 3832 (24.8) | 3745 (24.4) | 3758 (24.4) | 4137 (26.7) | 5250 (33.4) | <0.001 |
| Education level | <0.001 | |||||
| High school or less | 2928 (19.0) | 2947 (19.2) | 2983 (19.4) | 3221 (20.8) | 3973 (25.2) | |
| Some college | 5814 (37.7) | 5972 (38.9) | 6099 (39.6) | 6229 (40.2) | 6564 (41.7) | |
| Bachelor's degree | 3528 (22.8) | 3384 (22.1) | 3390 (22.0) | 3345 (21.6) | 3002 (19.1) | |
| Graduate degree | 3172 (20.5) | 3039 (19.8) | 2943 (19.1) | 2705 (17.5) | 2199 (14.0) | |
| BMI | 25.24 ± 5.06 | 26.62 ± 5.51 | 27.25 ± 5.68 | 27.87 ± 5.90 | 28.91 ± 6.44 | <0.001 |
| Marital status | <0.001 | |||||
| Married/common-law | 11,006 (71.3) | 11,247 (73.3) | 11,463 (74.4) | 11,466 (74.0) | 11,029 (70.1) | |
| Divorced or separated | 1957 (12.7) | 1855 (12.1) | 1749 (11.3) | 1892 (12.2) | 2176 (13.8) | |
| Never married | 946 (6.1) | 849 (5.5) | 879 (5.7) | 901 (5.8) | 1395 (8.9) | |
| Widowed | 1533 (9.9) | 1391 (9.1) | 1324 (8.6) | 1241 (8.0) | 1138 (7.2) | |
| Health and lifestyle | ||||||
| Exercise,2 min/wk | <0.001 | |||||
| None | 2580 (16.7) | 2653 (17.3) | 2937 (19.1) | 3265 (21.1) | 4259 (27.1) | |
| ≤20 | 2273 (14.7) | 2706 (17.6) | 2918 (18.9) | 3249 (21.0) | 3786 (24.1) | |
| 21–60 | 2342 (15.2) | 2455 (16.0) | 2518 (16.3) | 2595 (16.7) | 2469 (15.7) | |
| 61–150 | 4203 (27.2) | 4180 (27.2) | 4129 (26.8) | 3800 (24.5) | 3172 (20.2) | |
| >150 | 4044 (26.2) | 3348 (21.8) | 2913 (18.9) | 2591 (16.7) | 2052 (13.0) | |
| Smoking history | <0.001 | |||||
| Current | 58 (0.4) | 85 (0.6) | 133 (0.9) | 178 (1.1) | 432 (2.7) | |
| Never | 13,000 (84.2) | 12,795 (83.4) | 12,606 (81.8) | 12,345 (79.6) | 11,756 (74.7) | |
| Quit <1 y | 23 (0.1) | 32 (0.2) | 53 (0.3) | 60 (0.4) | 150 (1.0) | |
| Quit 1–4.9 y | 130 (0.8) | 152 (1.0) | 207 (1.3) | 269 (1.7) | 406 (2.6) | |
| Quit 5–9.9 y | 179 (1.2) | 227 (1.5) | 230 (1.5) | 331 (2.1) | 402 (2.6) | |
| Quit 10–19.9 y | 490 (3.2) | 504 (3.3) | 591 (3.8) | 689 (4.4) | 838 (5.3) | |
| Quit 20–29.9 y | 634 (4.1) | 646 (4.2) | 685 (4.4) | 712 (4.6) | 830 (5.3) | |
| Quit ≥30 y | 928 (6.0) | 901 (5.9) | 910 (5.9) | 916 (5.9) | 924 (5.9) | |
| Low sleep (<5 h/d) | 4698 (30.4) | 4836 (31.5) | 4887 (31.7) | 5247 (33.9) | 5968 (37.9) | <0.001 |
| Alcohol consumption | <0.001 | |||||
| None | 14,349 (92.9) | 13,899 (90.6) | 13,810 (89.6) | 13,833 (89.2) | 13,731 (87.2) | |
| Rare | 59 (0.4) | 75 (0.5) | 128 (0.8) | 126 (0.8) | 214 (1.4) | |
| Monthly | 163 (1.1) | 255 (1.7) | 284 (1.8) | 313 (2.0) | 367 (2.3) | |
| Weekly | 583 (3.8) | 744 (4.8) | 734 (4.8) | 770 (5.0) | 862 (5.5) | |
| Daily | 288 (1.9) | 369 (2.4) | 459 (3.0) | 458 (3.0) | 564 (3.6) | |
| Cardiovascular disease3 | 947 (6.1) | 1036 (6.8) | 1033 (6.7) | 1022 (6.6) | 975 (6.2) | 0.071 |
| Diabetes4 | 746 (4.8) | 922 (6.0) | 1005 (6.5) | 983 (6.3) | 941 (6.0) | <0.001 |
| Dietary pattern | <0.001 | |||||
| Vegan | 3697 (23.9) | 1137 (7.4) | 571 (3.7) | 299 (1.9) | 184 (1.2) | |
| Lacto-ovo vegetarian | 4107 (26.6) | 4953 (32.3) | 4790 (31.1) | 4668 (30.1) | 3885 (24.7) | |
| Pesco vegetarian | 1945 (12.6) | 1704 (11.1) | 1533 (9.9) | 1255 (8.1) | 1147 (7.3) | |
| Semivegetarian | 654 (4.2) | 843 (5.5) | 918 (6.0) | 947 (6.1) | 916 (5.8) | |
| Nonvegetarian | 5039 (32.6) | 6705 (43.7) | 7603 (49.3) | 8331 (53.7) | 9606 (61.0) | |
| Nutrient intakes, g | ||||||
| Fiber | 40.54 ± 9.71 | 35.17 ± 8.28 | 31.67 ± 7.63 | 28.42 ± 7.17 | 22.56 ± 6.83 | <0.001 |
| Added sugar | 18.74 ± 9.47 | 24.97 ± 10.29 | 29.56 ± 12.67 | 35.58 ± 16.36 | 51.30 ± 33.32 | <0.001 |
| Carbohydrates | 270.95 ± 51.72 | 256.12 ± 45.26 | 246.78 ± 42.11 | 239.57 ± 40.46 | 232.07 ± 43.54 | <0.001 |
| Protein | 65.77 ± 14.05 | 68.23 ± 13.45 | 68.76 ± 13.87 | 68.61 ± 14.30 | 65.51 ± 16.32 | <0.001 |
| Fat | 58.45 ± 19.45 | 62.86 ± 16.88 | 65.66 ± 15.70 | 68.00 ± 15.26 | 69.84 ± 16.43 | <0.001 |
| Saturated fat | 12.13 ± 5.39 | 14.68 ± 5.60 | 16.26 ± 5.58 | 17.62 ± 5.53 | 19.16 ± 5.86 | <0.001 |
| Food intakes, kcal | ||||||
| Red meat | 7.91 ± 26.71 | 13.28 ± 33.26 | 18.17 ± 41.82 | 22.96 ± 47.63 | 34.01 ± 57.69 | <0.001 |
| Poultry | 12.31 ± 31.23 | 15.71 ± 31.96 | 18.18 ± 33.29 | 20.14 ± 33.58 | 23.20 ± 34.67 | <0.001 |
| Fish | 17.37 ± 39.62 | 17.44 ± 31.38 | 17.33 ± 28.38 | 15.86 ± 25.14 | 14.85 ± 22.33 | <0.001 |
| Dairy | 56.70 ± 98.54 | 83.53 ± 101.91 | 98.07 ± 102.04 | 104.21 ± 97.35 | 103.02 ± 92.85 | <0.001 |
| Eggs | 11.96 ± 28.97 | 17.06 ± 29.31 | 19.60 ± 28.28 | 21.58 ± 28.86 | 22.84 ± 28.62 | <0.001 |
| Fruit | 306.72 ± 181.13 | 238.43 ± 136.10 | 197.64 ± 116.70 | 162.43 ± 100.74 | 107.95 ± 81.59 | <0.001 |
| Vegetables | 125.59 ± 75.61 | 110.57 ± 59.49 | 101.50 ± 51.14 | 92.66 ± 46.44 | 73.96 ± 41.35 | <0.001 |
| Legumes | 104.70 ± 94.77 | 87.30 ± 70.90 | 78.07 ± 62.39 | 69.37 ± 56.83 | 51.17 ± 44.97 | <0.001 |
| Nuts and seeds | 188.20 ± 152.31 | 150.39 ± 119.62 | 127.01 ± 103.37 | 105.01 ± 86.89 | 71.46 ± 66.61 | <0.001 |
| Sweetened beverages | 86.68 ± 111.16 | 101.00 ± 109.28 | 108.98 ± 110.87 | 118.11 ± 115.45 | 149.50 ± 156.49 | <0.001 |
Values are n (%) or mean ± SD unless otherwise indicated. Statistical tests: 1-factor ANOVA for continuous variables and chi-square test for categorical variables. %kcal, percentage of total dietary energy.
Exercise defined as “vigorous activities, such as brisk walking, jogging, bicycling, etc., long enough or with enough intensity to work up a sweat, get your heart thumping, or get out of breath.”
History of coronary bypass, angioplasty/stent, carotid artery surgery, heart attack, or stroke; or angina pectoris or congestive heart failure treated in the last 12 mo.
Active or treated in the last 12 mo.
TABLE 2.
Comparison of participant characteristics across quintiles of percentage of animal-based food intake1
| Animal-based foods, %kcal | ||||||
|---|---|---|---|---|---|---|
| Quintile 1 (0%–2.4%) | Quintile 2 (2.5%–7.3%) | Quintile 3 (7.4%–12.7%) | Quintile 4 (12.8%–19.4%) | Quintile 5 (19.5%–86.5%) | P values | |
| General demographics | ||||||
| Participants, n | 15,437 | 15,479 | 15,543 | 15,429 | 15,549 | |
| Deaths | 2058 (13.3) | 1908 (12.3) | 1813 (11.7) | 1779 (11.5) | 1735 (11.2) | <0.001 |
| Age at baseline, y | 61.16 ± 14.05 | 60.19 ± 14.08 | 59.16 ± 13.89 | 58.43 ± 14.01 | 57.39 ± 13.67 | <0.001 |
| Female | 9856 (63.8) | 10,028 (64.8) | 10,145 (65.3) | 10,016 (64.9) | 10,219 (65.7) | 0.011 |
| Black | 3507 (22.7) | 4504 (29.1) | 4630 (29.8) | 4396 (28.5) | 3685 (23.7) | <0.001 |
| Education level | <0.001 | |||||
| High school or less | 2784 (18.0) | 3046 (19.7) | 3229 (20.8) | 3369 (21.8) | 3624 (23.3) | |
| Some college | 5923 (38.4) | 5854 (37.8) | 6045 (38.9) | 6271 (40.6) | 6585 (42.3) | |
| Bachelor's degree | 3574 (23.2) | 3443 (22.2) | 3372 (21.7) | 3198 (20.7) | 3062 (19.7) | |
| Graduate degree | 3156 (20.4) | 3136 (20.3) | 2897 (18.6) | 2591 (16.8) | 2278 (14.7) | |
| BMI, kg/m2 | 24.81 ± 4.87 | 26.57 ± 5.34 | 27.43 ± 5.72 | 28.12 ± 5.92 | 28.98 ± 6.48 | <0.001 |
| Marital status | <0.001 | |||||
| Married/common-law | 11,599 (75.1) | 11,399 (73.6) | 11,330 (72.9) | 11,088 (71.9) | 10,795 (69.4) | |
| Divorced or separated | 1567 (10.2) | 1784 (11.5) | 1958 (12.6) | 1996 (12.9) | 2324 (14.9) | |
| Never married | 908 (5.9) | 902 (5.8) | 989 (6.4) | 1020 (6.6) | 1151 (7.4) | |
| Widowed | 1363 (8.8) | 1394 (9.0) | 1266 (8.1) | 1325 (8.6) | 1279 (8.2) | |
| Health and lifestyle | ||||||
| Exercise,2 min/wk | <0.001 | |||||
| None | 2513 (16.3) | 2846 (18.4) | 3051 (19.6) | 3345 (21.7) | 3939 (25.3) | |
| ≤20 | 2598 (16.8) | 2816 (18.2) | 2971 (19.1) | 3260 (21.1) | 3287 (21.1) | |
| 21–60 | 2446 (15.8) | 2523 (16.3) | 2525 (16.2) | 2489 (16.1) | 2396 (15.4) | |
| 61–150 | 4216 (27.3) | 4121 (26.6) | 4118 (26.5) | 3616 (23.4) | 3413 (21.9) | |
| >150 | 3664 (23.7) | 3173 (20.5) | 2878 (18.5) | 2719 (17.6) | 2514 (16.2) | |
| Smoking history | <0.001 | |||||
| Current | 18 (0.1) | 74 (0.5) | 142 (0.9) | 226 (1.5) | 426 (2.7) | |
| Never | 13,130 (85.1) | 13,000 (84.0) | 12,638 (81.3) | 12,252 (79.4) | 11,482 (73.8) | |
| Quit <1 y | 16 (0.1) | 34 (0.2) | 62 (0.4) | 92 (0.6) | 114 (0.7) | |
| Quit 1–4.9 y | 114 (0.7) | 136 (0.9) | 198 (1.3) | 290 (1.9) | 426 (2.7) | |
| Quit 5–9.9 y | 156 (1.0) | 213 (1.4) | 250 (1.6) | 318 (2.1) | 432 (2.8) | |
| Quit 10–19.9 y | 455 (2.9) | 492 (3.2) | 580 (3.7) | 671 (4.3) | 914 (5.9) | |
| Quit 20–29.9 y | 625 (4.0) | 606 (3.9) | 710 (4.6) | 696 (4.5) | 870 (5.6) | |
| Quit ≥30 y | 923 (6.0) | 924 (6.0) | 963 (6.2) | 884 (5.7) | 885 (5.7) | |
| Low sleep (<5 h/d) | 4352 (28.2) | 5102 (33.0) | 5385 (34.6) | 5409 (35.1) | 5388 (34.7) | <0.001 |
| Alcohol consumption | <0.001 | |||||
| None | 15,169 (98.3) | 14,700 (95.0) | 14,106 (90.8) | 13,331 (86.4) | 12,316 (79.2) | |
| Rare | 13 (0.1) | 53 (0.3) | 109 (0.7) | 181 (1.2) | 246 (1.6) | |
| Monthly | 37 (0.2) | 128 (0.8) | 255 (1.6) | 343 (2.2) | 619 (4.0) | |
| Weekly | 159 (1.0) | 428 (2.8) | 711 (4.6) | 977 (6.3) | 1418 (9.1) | |
| Daily | 59 (0.4) | 170 (1.1) | 362 (2.3) | 597 (3.9) | 950 (6.1) | |
| Cardiovascular disease3 | 869 (5.6) | 986 (6.4) | 1036 (6.7) | 1053 (6.8) | 1069 (6.9) | <0.001 |
| Diabetes4 | 480 (3.1) | 799 (5.2) | 982 (6.3) | 1095 (7.1) | 1241 (8.0) | <0.001 |
| Dietary pattern | <0.001 | |||||
| Vegan | 5888 (38.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Lacto-ovo vegetarian | 7217 (46.8) | 6998 (45.2) | 4201 (27.0) | 2596 (16.8) | 1391 (8.9) | |
| Pesco vegetarian | 1439 (9.3) | 2788 (18.0) | 1753 (11.3) | 1081 (7.0) | 523 (3.4) | |
| Semivegetarian | 516 (3.3) | 1345 (8.7) | 1093 (7.0) | 789 (5.1) | 535 (3.4) | |
| Nonvegetarian | 377 (2.4) | 4348 (28.1) | 8496 (54.7) | 10,963 (71.1) | 13,100 (84.2) | |
| Nutrient intakes, g | ||||||
| Fiber | 40.84 ± 8.74 | 35.14 ± 8.35 | 31.27 ± 8.10 | 27.93 ± 7.48 | 23.02 ± 7.15 | <0.001 |
| Added sugar | 24.09 ± 13.73 | 29.83 ± 19.19 | 33.39 ± 23.26 | 36.16 ± 23.86 | 37.08 ± 24.32 | <0.001 |
| Carbohydrates | 272.83 ± 44.54 | 260.80 ± 44.27 | 251.40 ± 42.58 | 241.42 ± 40.58 | 218.80 ± 43.07 | <0.001 |
| Protein | 65.48 ± 13.88 | 65.08 ± 14.03 | 65.75 ± 14.01 | 67.64 ± 13.51 | 72.87 ± 15.58 | <0.001 |
| Fat | 58.57 ± 16.90 | 62.32 ± 16.89 | 64.67 ± 16.21 | 67.25 ± 15.94 | 72.08 ± 17.35 | <0.001 |
| Saturated fat | 10.59 ± 3.17 | 13.24 ± 3.57 | 15.57 ± 4.01 | 18.01 ± 4.45 | 22.49 ± 6.63 | <0.001 |
| Food intakes, kcal | ||||||
| Red meat | 0.12 ± 1.23 | 2.53 ± 7.82 | 10.05 ± 19.19 | 24.52 ± 35.88 | 59.27 ± 74.15 | <0.001 |
| Poultry | 0.40 ± 2.12 | 5.24 ± 11.54 | 14.76 ± 23.51 | 26.32 ± 32.97 | 42.83 ± 50.52 | <0.001 |
| Fish | 1.53 ± 5.02 | 10.37 ± 17.03 | 18.05 ± 26.60 | 23.92 ± 33.53 | 28.87 ± 43.16 | <0.001 |
| Dairy | 4.99 ± 10.36 | 36.44 ± 31.48 | 78.83 ± 51.34 | 124.74 ± 73.33 | 200.28 ± 131.83 | <0.001 |
| Eggs | 2.17 ± 5.23 | 11.64 ± 14.58 | 18.83 ± 20.05 | 24.45 ± 25.88 | 35.95 ± 47.45 | <0.001 |
| Fruit | 279.15 ± 163.73 | 230.81 ± 149.94 | 199.13 ± 135.37 | 170.36 ± 118.72 | 131.96 ± 100.01 | <0.001 |
| Vegetables | 119.30 ± 69.92 | 107.46 ± 59.46 | 101.32 ± 56.66 | 94.22 ± 51.42 | 81.49 ± 46.22 | <0.001 |
| Legumes | 103.94 ± 85.52 | 91.20 ± 76.32 | 78.21 ± 65.96 | 66.91 ± 57.30 | 49.88 ± 46.67 | <0.001 |
| Nuts and seeds | 182.36 ± 136.86 | 142.08 ± 121.81 | 122.43 ± 110.19 | 106.30 ± 97.96 | 87.85 ± 85.74 | <0.001 |
| Sweetened beverages | 77.96 ± 100.53 | 111.90 ± 126.55 | 125.79 ± 132.68 | 129.50 ± 128.76 | 119.82 ± 121.71 | <0.001 |
Values are n (%) or mean ± SD unless otherwise indicated. Statistical tests: 1-factor ANOVA for continuous variables and chi-square test for categorical variables. Animal-based included all meats, dairy products, and eggs. %kcal, percentage of total dietary energy.
Exercise defined as “vigorous activities, such as brisk walking, jogging, bicycling, etc., long enough or with enough intensity to work up a sweat, get your heart thumping, or get out of breath.”
History of coronary bypass, angioplasty/stent, carotid artery surgery, heart attack, or stroke; or angina pectoris or congestive heart failure treated in the last 12 mo.
Active or treated in the last 12 mo.
Those with higher intake of both ultra-processed foods and animal-based foods on average were younger and less educated, had higher BMI, were less likely married, exercised less, had higher rates of smoking, had more low sleep, were more likely alcohol drinkers, and had a higher prevalence of diabetes. They had much lower consumption of fiber, fruits, legumes, and nuts and seeds, and somewhat lower consumption of carbohydrates and vegetables. They had much higher consumption of added sugar, saturated fat, dairy products, eggs, and somewhat higher consumption of total fat. In addition, those with higher intake of ultra-processed foods were more likely male and black, much less likely vegan, less likely pesco vegetarian or lacto-ovo vegetarian, much more likely nonvegetarian, ate much more red meat, somewhat more poultry, much less fish, and drank much more sweetened beverages. Those with high animal-based intake had higher prevalence of CVD, somewhat higher consumption of protein and sweetened beverages, were much less likely vegans, lacto-ovo vegetarians, or pesco vegetarians, and ate much more red meat, poultry, and fish.
Figure 2 portrays the fully adjusted associations with all-cause mortality of both the proportion of dietary energy from ultra-processed foods and the proportion of dietary energy from animal-based foods (i.e., meat, dairy, and eggs), modeled separately and together. Ultra-processed food intake was significantly associated with higher mortality. The HR for the 90th compared with the 10th percentile of consumption (comparing 47.7% with 12.1% dietary energy) in a continuous linear model was 1.14 (95% CI: 1.08, 1.21). This association was largely unchanged when animal-based food consumption was added to the model (HR: 1.14; 95% CI: 1.07, 1.21). The total of animal-based food intake was not clearly associated with all-cause mortality. The HR for the 90th compared with the 10th percentile of consumption (comparing 25.0% with 0.4% dietary energy) in a continuous linear model was 1.03 (95% CI: 0.98, 1.10) when modeled separately and 1.01 (95% CI: 0.95, 1.07) when ultra-processed food intake was added to the model. There was no evidence of interaction (P = 0.36) between ultra-processed and animal-based foods. Given the different distributions of intake for ultra-processed and animal-based foods, HRs were calculated for fixed intakes to allow more direct comparison of the associations. Comparing 35% of calories with 2.5% of calories, the HR for ultra-processed was 1.14 (95% CI: 1.07, 1.20) and for animal-based was 1.02 (95% CI: 0.94, 1.10).
FIGURE 2.
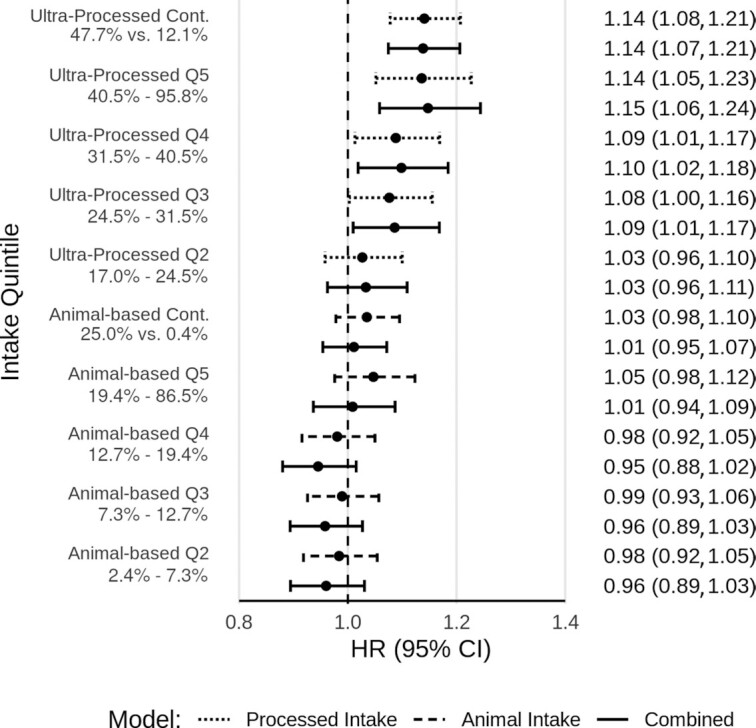
Ultra-processed food intake and animal-based food intake and all-cause mortality. Fully adjusted HRs and 95% CIs for ultra-processed food intake and animal-based food intake and all-cause mortality, from Cox proportional hazards regression (n = 77,437). Results with dotted lines are for the proportion of dietary energy from ultra-processed foods, modeled alone. Results with hashed lines are for the proportion of dietary energy from animal-based foods (meats, dairy, eggs), modeled alone. Results with solid lines are for both modeled together. Results labeled Q2–Q5 are for the second through fifth quintiles (Q1 as reference). Ranges of intake as percentages of total dietary energy are given for each quintile. Results labeled Cont. are for continuous linear models, comparing the 90th and 10th percentiles of intake. Levels of intake as percentages of total dietary energy are presented for these contrasts. Adjusted for age (i.e., attained age as time variable), sex (male, female), race (black, nonblack), geographic region (West, Northwest, Mountain, Midwest, East, South), education (up to high school graduate, trade school/some college/associate degree, bachelor degree, graduate degree), marital status (married/common-law, never married, widowed, divorced/separated), smoking (current smoker, quit <1 y, quit >1–5 y, quit >5–10 y, quit >10–20 y, quit >20–30 y, quit >30 y, never smoked), alcohol [nondrinker, rare drinker (<1.5 servings/mo), monthly drinker (1.5 to <4 servings/mo), weekly drinker (4 to <28 servings/mo), daily drinker (≥28 servings/mo)], exercise (i.e., “vigorous activities, such as brisk walking, jogging, bicycling, etc., long enough or with enough intensity to work up a sweat, get your heart thumping, or get out of breath”) (none, ≤20 min/wk, 21–60 min/wk, 61–150 min/wk, ≥151 min/wk), sleep duration (≤4 h/night, 5 to ≥9 h/night), menopause (in women) [premenopausal (including perimenopausal), postmenopausal], hormone replacement (in postmenopausal women) (not taking hormone replacement, taking hormone replacement), BMI (in kg/m2) [restricted cubic spline with knots at the 5th (19.7), 27.5th (23.4), 50th (26.1), 72.5th (29.4), and 95th (38.6) percentiles], total dietary energy (kcal/d), prevalent cardiovascular disease (coronary bypass, angioplasty/stent, carotid artery surgery, heart attack, or stroke; or angina pectoris or congestive heart failure treated in the last 12 mo) (yes, no), and diabetes mellitus active or treated in the last 12 mo (yes, no). Q, quintile.
Figure 3 portrays results of specific substitutions (i.e., ultra-processed for any other level of processing, ultra-processed for moderately processed, ultra-processed for unprocessed, and moderately processed for unprocessed, at constant levels of dietary covariates) for all participants, and then separately among vegetarians and among nonvegetarians. A positive association for ultra-processed food similar in magnitude to those reported in Figure 2 was again demonstrated, and tended to be similar, regardless of the particular substitution; however, there was no clear association when moderately processed foods were substituted for unprocessed foods. The findings for substitution models were similar among vegetarians (except that ultra-processed substituted for moderately processed was not significant) and among nonvegetarians.
FIGURE 3.
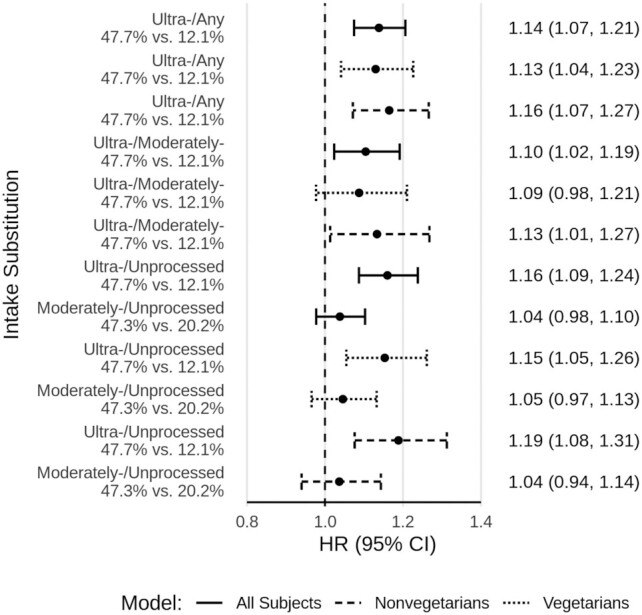
Processed food substitutions among all participants, vegetarians, and nonvegetarians. Fully adjusted HRs and 95% CIs for processed food intake substitutions and all-cause mortality, from Cox proportional hazards regression among all participants (solid line, n = 77,437), vegetarians (dotted line, n = 40,153), and nonvegetarians (hashed line, n = 37,284). All results are from continuous linear models comparing the 90th and 10th percentiles of intake. Levels of intake as percentages of total dietary energy are presented for these contrasts. All models are adjusted for the proportion of dietary energy from animal-based foods (modeled continuously). Other adjustments were for age (i.e., attained age as time variable), sex (male, female), race (black, nonblack), geographic region (West, Northwest, Mountain, Midwest, East, South), education (up to high school graduate, trade school/some college/associate degree, bachelor degree, graduate degree), marital status (married/common-law, never married, widowed, divorced/separated), smoking (current smoker, quit <1 y, quit >1–5 y, quit >5–10 y, quit >10–20 y, quit >20–30 y, quit >30 y, never smoked), alcohol [nondrinker, rare drinker (<1.5 servings/mo), monthly drinker (1.5 to <4 servings/mo), weekly drinker (4 to <28 servings/mo), daily drinker (≥28 servings/mo)], exercise (i.e., “vigorous activities, such as brisk walking, jogging, bicycling, etc., long enough or with enough intensity to work up a sweat, get your heart thumping, or get out of breath”) (none, ≤20 min/wk, 21–60 min/wk, 61–150 min/wk, ≥151 min/wk), sleep duration (≤4 h/night, 5 to ≥9 h/night), menopause (in women) [premenopausal (including perimenopausal), postmenopausal], hormone replacement (in postmenopausal women) (not taking hormone replacement, taking hormone replacement), BMI (in kg/m2) [restricted cubic spline with knots at the 5th (19.7), 27.5th (23.4), 50th (26.1), 72.5th (29.4), and 95th (38.6) percentiles], total dietary energy (kcal/d), prevalent cardiovascular disease (coronary bypass, angioplasty/stent, carotid artery surgery, heart attack, or stroke; or angina pectoris or congestive heart failure treated in the last 12 mo) (yes, no), and diabetes mellitus active or treated in the last 12 mo (yes, no). Moderately-/Unprocessed, moderately processed substituted for unprocessed foods; Ultra-/Any, ultra-processed substituted for any less processed foods; Ultra-/Moderately-, ultra-processed substituted for moderately processed foods; Ultra-/Unprocessed, ultra-processed substituted for unprocessed foods.
Figure 4 displays the association of animal-based foods with mortality with greater differentiation. Among the 4 specific categories of animal-based foods, only red meat was associated with all-cause mortality [HR: 1.14; 95% CI: 1.08, 1.22, comparing the 87.5th percentile of proportion of dietary energy from red meat among red meat consumers with 0 intake (comparing 6.2% with 0.0% dietary energy) in a continuous linear model, substituting for an equivalent proportion from plant-based foods, at constant levels of dietary covariates].
FIGURE 4.
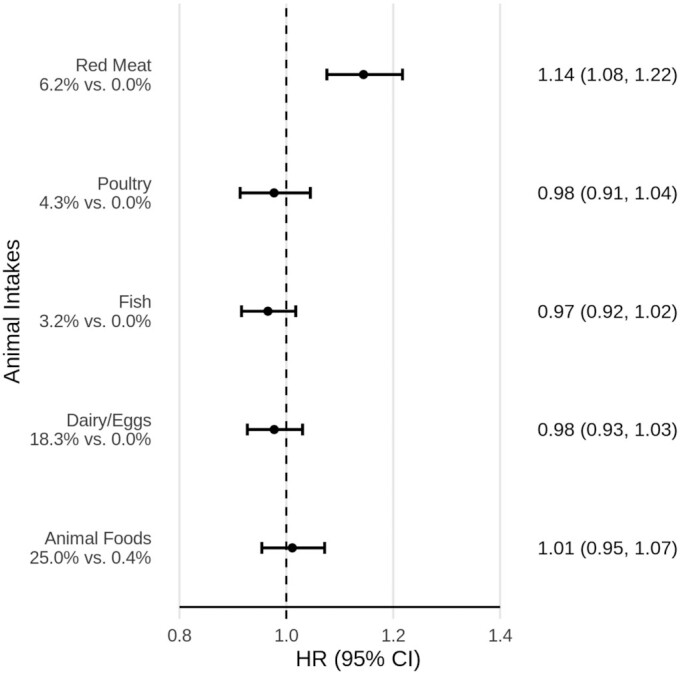
Categories of animal-based food intake and all-cause mortality. Fully adjusted HRs and 95% CIs for the proportion of dietary energy from all animal-based foods and specific animal-based foods and all-cause mortality, from Cox proportional hazards regression (n = 77,437). All results are from continuous linear models. Results for all animal-based foods combined compared the 90th and 10th percentiles of intake. The 4 specific animal-based food categories (red meat, poultry, fish, and dairy/eggs) were modeled jointly; results compared the 87.5th percentile of intake among consumers of that particular category with 0 intake for that category. Levels of intake as percentages of total dietary energy are presented for these contrasts. In all cases, substitution is for an equivalent proportion of dietary energy from plant foods. All models adjusted for the proportion of dietary energy from ultra-processed foods (modeled continuously). Other adjustments were for age (i.e., attained age as time variable), sex (male, female), race (black, nonblack), geographic region (West, Northwest, Mountain, Midwest, East, South), education (up to high school graduate, trade school/some college/associate degree, bachelor degree, graduate degree), marital status (married/common-law, never married, widowed, divorced/separated), smoking (current smoker, quit <1 y, quit >1–5 y, quit >5–10 y, quit >10–20 y, quit >20–30 y, quit >30 y, never smoked), alcohol [nondrinker, rare drinker (<1.5 servings/mo), monthly drinker (1.5 to <4 servings/mo), weekly drinker (4 to <28 servings/mo), daily drinker (≥28 servings/mo)], exercise (i.e., “vigorous activities, such as brisk walking, jogging, bicycling, etc., long enough or with enough intensity to work up a sweat, get your heart thumping, or get out of breath”) (none, ≤20 min/wk, 21–60 min/wk, 61–150 min/wk, ≥151 min/wk), sleep duration (≤4 h/night, 5 to ≥9 h/night), menopause (in women) [premenopausal (including perimenopausal), postmenopausal], hormone replacement (in postmenopausal women) (not taking hormone replacement, taking hormone replacement), BMI (in kg/m2) [restricted cubic spline with knots at the 5th (19.7), 27.5th (23.4), 50th (26.1), 72.5th (29.4), and 95th (38.6) percentiles], total dietary energy (kcal/d), prevalent cardiovascular disease (coronary bypass, angioplasty/stent, carotid artery surgery, heart attack, or stroke; or angina pectoris or congestive heart failure treated in the last 12 mo) (yes, no), and diabetes mellitus active or treated in the last 12 mo (yes, no).
Figure 5 shows associations for ultra-processed foods and animal-based foods with several categories of cause-specific mortality (all comparisons here are for the 90th and 10th percentiles of intake from continuous models). Ultra-processed foods was weakly associated with CVD mortality (HR: 1.09; 95% CI: 1.00, 1.18) and not convincingly associated with cancer mortality (HR: 0.95; 95% CI: 0.86, 1.04). Several more specific categories of mortality had associations with ultra-processed foods with substantial HR values that were statistically significant. These were as follows: respiratory mortality (HR: 1.50; 95% CI: 1.24, 1.81); neurologic mortality (HR: 1.32; 95% CI: 1.14, 1.52); and renal mortality (HR: 1.39; 95% CI: 1.05, 1.85). Infectious diseases mortality showed a modest association with ultra-processed foods but was not statistically significant (HR: 1.31; 95% CI: 0.93, 1.87). Endocrine mortality did not clearly associate with ultra-processed food consumption. Other deaths not fitting in the specified categories were associated with ultra-processed foods (HR: 1.22; 95% CI: 1.07, 1.39). Higher intake of animal-based foods was most clearly associated with higher mortality from infectious diseases (HR: 1.71; 95% CI: 1.24, 2.35) and endocrine causes (HR: 1.50; 95% CI: 1.22, 1.84). It was also associated with higher mortality from respiratory causes (HR: 1.16; 95% CI: 0.98, 1.38) and renal causes (HR: 1.24; 95% CI: 0.95, 1.61), but not statistically significantly so. Higher intake of animal-based foods was associated with lower mortality from neurologic causes (HR: 0.84; 95% CI: 0.72, 0.97). It was not clearly associated with cardiovascular, cancer, or other mortality.
FIGURE 5.
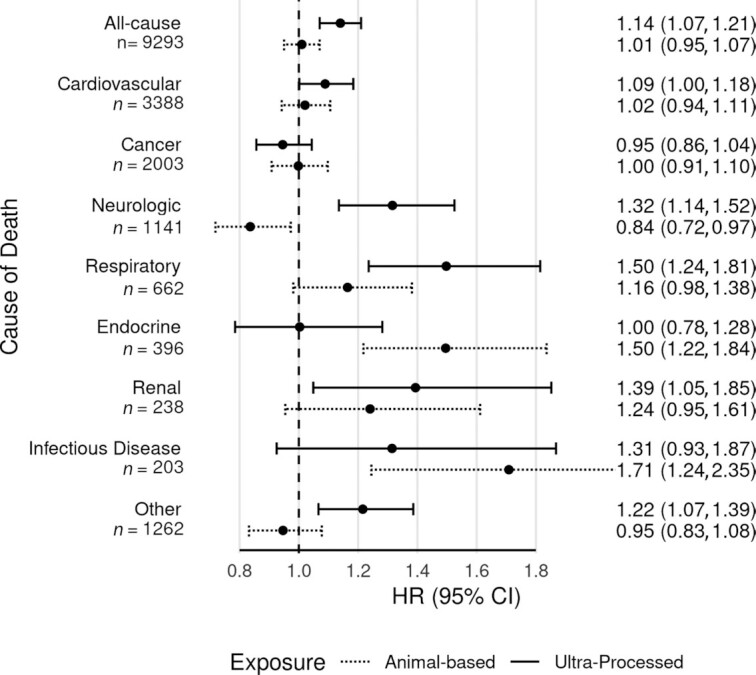
Ultra-processed food intake and animal-based food intake and cause-specific mortality. Fully adjusted HRs and 95% CIs for ultra-processed food intake and animal-based food intake and all-cause and cause-specific mortality, from Cox proportional hazards regression (n = 77,437). Results with solid lines are for the proportion of dietary energy from ultra-processed foods. Results with dotted lines are for the proportion of dietary energy from animal-based foods (meats, dairy, eggs). Results are from continuous linear models, comparing the 90th and 10th percentiles of intake (47.7% compared with 12.1% dietary energy from ultra-processed foods; 25.0% compared with 0.4% dietary energy from animal-based foods). Both dietary exposures were mutually adjusted and in addition adjusted for age (i.e., attained age as time variable), sex (male, female), race (black, nonblack), geographic region (West, Northwest, Mountain, Midwest, East, South), education (up to high school graduate, trade school/some college/associate degree, bachelor degree, graduate degree), marital status (married/common-law, never married, widowed, divorced/separated), smoking (current smoker, quit <1 y, quit >1–5 y, quit >5–10 y, quit >10–20 y, quit >20–30 y, quit >30 y, never smoked), alcohol [nondrinker, rare drinker (<1.5 servings/mo), monthly drinker (1.5 to <4 servings/mo), weekly drinker (4 to <28 servings/mo), daily drinker (≥28 servings/mo)], exercise (i.e., “vigorous activities, such as brisk walking, jogging, bicycling, etc., long enough or with enough intensity to work up a sweat, get your heart thumping, or get out of breath”) (none, ≤20 min/wk, 21–60 min/wk, 61–150 min/wk, ≥151 min/wk), sleep duration (≤4 h/night, 5 to ≥9 h/night), menopause (in women) [premenopausal (including perimenopausal), postmenopausal], hormone replacement (in postmenopausal women) (not taking hormone replacement, taking hormone replacement), BMI (in kg/m2) [restricted cubic spline with knots at the 5th (19.7), 27.5th (23.4), 50th (26.1), 72.5th (29.4), and 95th (38.6) percentiles], total dietary energy (kcal/d), prevalent CVD (coronary bypass, angioplasty/stent, carotid artery surgery, heart attack, or stroke; or angina pectoris or congestive heart failure treated in the last 12 mo) (yes, no), and diabetes mellitus active or treated in the last 12 mo (yes, no). ICD-10 codes for the underlying cause of death were used to classify cause-specific mortality as follows: CVD deaths starting with I; cancer deaths starting with C; neurologic deaths starting with G and including F01–F03; respiratory deaths starting with J; endocrine deaths with E; renal deaths with N; infectious disease deaths starting with A or B; and other deaths included all other ICD codes. The most common causes of death within each category were as follows: cardiovascular (atherosclerotic heart disease, myocardial infarction, stroke); cancer (lung, pancreatic, colon, breast, prostate); neurologic (Alzheimer dementia, unspecified dementia, Parkinson disease); respiratory (pneumonia, chronic obstructive pulmonary disease, pulmonary fibrosis); endocrine (diabetes, hyperlipidemia); renal (urinary tract infection, chronic kidney disease, kidney failure); infectious disease (sepsis, Clostridium difficile colitis, hepatitis C); and other (ill-defined or unknown cause, myelodysplastic syndrome, liver cirrhosis). CVD, cardiovascular disease; ICD-10, International Statistical Classification of Diseases, 10th Revision.
The supplementary materials contain 3 sensitivity analyses of the main results (as represented in Figure 2). Supplemental Figure 2 shows follow-up censored at 8 y (out of concern for potential attenuation due to accruing dietary misclassification over time); associations were stronger when truncated at 8 y. Supplemental Figure 3 shows results without omission of the first 2 y of follow-up; results were very similar. Supplemental Figure 4 shows results with additional exclusions for a history of diabetes mellitus and CVD (rather than model-based adjustment); results were again very similar. Supplemental Figure 5 provides results with adjustment for other chronic diseases (emphysema, chronic bronchitis, rheumatoid arthritis, osteoarthritis, ulcerative colitis, and Crohn disease), which were little changed. In Supplemental Figure 6, results are shown separately for those with and without chronic diseases at baseline (CVD, diabetes, emphysema, chronic bronchitis, rheumatoid arthritis, osteoarthritis, ulcerative colitis, and Crohn disease); the association with ultra-processed foods appears to be stronger among those with chronic diseases.
Discussion
We hypothesized that 2 conceptually independent dietary factors (proportion animal- as opposed to plant-based and proportion ultra-processed as opposed to less-processed) might be associated with mortality. In these analyses, a more ultra-processed diet was associated with higher all-cause mortality, and this association persisted after adjustment for more animal-based dietary intake. A more animal-based diet was not clearly associated with mortality overall (although those with a higher consumption of red meat had 8% increased mortality). Interestingly, ultra-processed food consumption was not associated with CVD or cancer mortality, but primarily with mortality from neurologic causes (particularly Alzheimer disease and Parkinson disease) and respiratory causes (particularly chronic obstructive pulmonary disease, even when restricted to never smokers). The association of ultra-processed food with mortality appeared stronger among those with chronic diseases at baseline, suggesting the potential for greater impact among those with higher mortality risk.
Other cohorts have demonstrated an association between ultra-processed foods and all-cause mortality (12–15), and the current findings (from one of the largest examinations of this association to date) add to this evidence. The current findings also demonstrate that such an association is specifically found among vegetarians (broadly defined) as well as nonvegetarians. Also, rather than focusing only on ultra-processed foods, we have examined 3 levels of processing and the predicted impact on mortality of substituting one level for another. However, it is interesting to note that the association with mortality depended almost entirely on the proportion of ultra-processed foods. It did not seem to matter much whether ultra-processed foods took the place of moderately processed foods or of unprocessed foods. Moderately processed foods, substituting for unprocessed foods, were not significantly associated with mortality.
The lack of a significant association with mortality for the animal-based dietary metric may seem surprising because, in previous findings from this cohort, vegetarian dietary patterns were associated with lower all-cause mortality (4). Those findings may in part reflect differing consumptions of ultra-processed foods. Vegetarians in this population not only eat less animal-derived foods by definition, but also eat comparatively less snack foods, sweets and desserts, refined grains, and nonwater beverages, but more fruits and vegetables, nuts and seeds, legumes, and whole grains (34, 35). It is thus notable that the association of more ultra-processed foods with higher mortality was present even among vegetarians.
The current findings seem to suggest that the proportion of ultra-processed foods in the diet may be more important with respect to mortality than the proportion of animal-derived foods. However, the current analysis did find that higher intake of red meat (substituting for plant-based food) was associated with higher mortality, even after adjustment for ultra-processed food intake. It is important to consider that this is a cohort with low consumption of animal-based foods (especially meats) compared with the general population. Ultra-processed foods (as here defined) are eaten much more commonly than animal-based foods in this population (Figure 1). The same animal-based scale might be more associated with mortality in populations with higher intakes of animal-based foods and red meat in particular.
Ultra-processed foods might ultimately contribute to premature mortality through several mechanisms. Ultra-processed foods are produced to “optimize” taste, texture, shelf-life, and production costs, not health. They tend to be higher in a variety of nutrients of potential concern (when consumed in excess), such as added sugars, trans fats and saturated fats, and sodium. These features are well-known to promote increased body weight, higher blood pressure, and higher serum LDL cholesterol and triglyceride (36–39). They are typically calorie dense and tend to replace significant quantities of less processed foods in the diet. They have a marked reduction in or absence of dietary fiber and a relative absence of heat-labile vitamins and other complex phytochemicals, which may promote changes in gut microbiota and inflammation (40–42). Indeed, as shown in Table 1, fiber intake was nearly cut in half for the highest compared with the lowest quintile of processed food intake. Similar nutritional factors may also affect immune system function (43–45). Most diseases of aging, such as those associated with increased risk with higher intakes of processed foods in our data, are adversely affected by these same pathophysiologic influences. Examples include diabetes (perhaps mainly through overweight) (46, 47), renal disease (often caused by diabetes and hypertension), probably dementia (48, 49), and possibly chronic obstructive lung disease (50). Hall et al.’s (51) demonstration of the potential of ultra-processed diets to lead to excess energy intake has strong implications for excess weight gain (as was seen short-term in the study) and secondarily they may lead to an increased risk of many obesity-related disease states such as insulin resistance and inflammation. The high energy density, low fiber content, and high palatability of many ultra-processed foods may all contribute to overconsumption. However, the associations we present are adjusted for BMI, suggesting the potential for weight-independent mechanisms (although baseline BMI adjustment does not fully control for adiposity-related mechanisms). The suggestion that low animal-based food intake is associated with higher rates of dementia mortality (the leading cause of the neurologic mortality category were dementias) clearly needs further investigation. Potential mechanisms may involve vitamin B-12 deficiency, especially in older persons with little to no animal-based food consumption, or conceivably their lower intakes of very-long-chain n–3 fatty acids.
Strengths of the current analysis include the large cohort and thus the relatively large number of events that increase statistical power. Despite all being Seventh-day Adventists, this is a remarkably diverse cohort in terms of sex, race, and geographical location. This gives the results greater generalizability among other populations that subscribe to broadly similar dietary patterns (vegetarian and nonvegetarian). Further, the extensive adjustments for potential confounders, although common in other studies, are not uniformly so, and this limits the probability of severe confounding. The enriched representation of those eating plant-based diets adds to the variance of dietary habits and is another means of increasing statistical power (52). We also used guided multiple imputation to fill missing data, which largely avoids biases either from excluding subjects not providing complete data, or from a 0 imputation when data are missing (31).
Despite these strengths, several important limitations remain. Dietary assessment relied on the use of an FFQ; this has limited accuracy which can lead to attenuation of association estimates (53, 54). In addition, the FFQ used in this study was not specifically designed to assess food processing and may have limited validity for this measure. We used a novel classification of food processing, which may limit comparability with other literature. Diet was measured only once at study entry, whereas dietary habits may change over time potentially leading to accumulating dietary misclassification, which would typically (assuming nondifferential misclassification) bias association estimates toward the null; we thus truncated follow-up at 8 y out of this concern in our sensitivity analysis, and association estimates were strengthened. There is also the potential for unadjusted or residual confounding, limiting causal inference. Potential confounders could include psychosocial and socioeconomic factors, health care access and quality, or aspects of physical fitness or lifestyle beyond those measured and modeled. Despite attempts to adjust for several prevalent diseases, confounding by baseline health status could still be present. Other aspects of diet correlated with the intake of ultra-processed foods (as demonstrated in Table 1) could also be potential confounders. An unadjusted confounder would have to be associated with both the exposure (highly processed foods) and outcome (mortality) with an RR of 1.54 (E-value) to nullify our main finding based on the association estimate (or an RR of 1.34 based on the CI) (28, 29). Findings may not be generalizable to dissimilar populations (e.g., differing in age, race/ethnicity, lifestyle), although we have no reason to believe that this population has biological responses differing from those of others.
In light of the current and previous findings demonstrating an association of ultra-processed foods with mortality, further study of the potential health effects of ultra-processed foods is warranted. A focus on food processing has the potential translational virtue of providing a way to approach dietary quality that is arguably easy to conceptualize and remember, as opposed to a list of disparate foods and nutrients to minimize or emphasize. That said, not all food processing is unhealthful, and not all aspects of a healthful diet (e.g., variety and nutritional adequacy) are related to food processing. Ultra-processed foods may also represent convenient and affordable food options, and the accessibility of alternatives may be limited for some populations.
We think that this approach and these findings are interesting and noteworthy. An ∼14% higher mortality rate was observed in those consuming more ultra-processed foods even in a relatively long-lived, health-conscious population with a large proportion of vegetarians. No such association was found for the total of animal-based dietary intake, although an 8% statistically significant increased risk was found for moderate consumption of red meat (i.e., among the higher consumers in this population). The current findings, together with previously published evidence, suggest that high intake of ultra-processed foods or other risk factors (such as other aspects of diet) closely related to ultra-processed food intake may be causally related to mortality.
Supplementary Material
Acknowledgments
Hannelore Bennett provided administrative support. Lars Sveen provided technical and data support. The authors’ responsibilities were as follows—GEF: had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis, obtained funding, and supervised the study; JS, KJ-S, and GEF: acquired the data; AM, MJO, JS, and GEF: analyzed and interpreted the data; MJO: drafted the manuscript; AM: performed the statistical analysis and provided administrative, technical, or material support; GEF and MJO: finalized the content; and all authors: conceived and designed the study, critically revised the manuscript for important intellectual content, and read and approved the final manuscript. The authors report no conflicts of interest.
Notes
Initial cohort support was obtained from National Cancer Institute (NCI) grant 1U01CA152939 (to GEF) and World Cancer Research Fund (WCRF) grant 2009/93 (to GEF). Support for this analysis was obtained from the Ardmore Institute of Health (AIH) (to GEF) and Loma Linda University Health (LLUH). The funding agencies had no role in the design and conduct of the study; in the collection, management, analysis, and interpretation of data; in the preparation, review, or approval of the manuscript; or in the decision to submit the manuscript for publication.
The views expressed in this article are those of the authors and do not necessarily represent the views of the NCI, WCRF, AIH, or LLUH. The ideas and opinions expressed herein are those of the authors and endorsement by the NCI, WCRF, AIH, LLUH, or their contractors or subcontractors is not intended nor should it be inferred.
Supplemental Table 1 and Supplemental Figures 1–6 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Present address of AM: Department of Community Medicine, Faculty of Health Sciences, UiT–the Arctic University of Norway, Tromsø, Norway.
Present address for UF: e-Health Group, Institute for Global Health (ISGlobal), Barcelona, Spain.
Present address for KJ-S: Clinical & Applied Science Education, School of Osteopathic Medicine, University of the Incarnate Word, 7615 Kennedy Hill Dr., San Antonio, TX.
Abbreviations used: AHS-2, Adventist Health Study—2; CVD, cardiovascular disease; HRT, hormone replacement therapy; ICD-10, International Statistical Classification of Diseases, 10th Revision.
Contributor Information
Michael J Orlich, School of Public Health, Loma Linda University, Loma Linda, CA, USA; School of Medicine, Loma Linda University, Loma Linda, CA, USA.
Joan Sabaté, School of Public Health, Loma Linda University, Loma Linda, CA, USA; School of Medicine, Loma Linda University, Loma Linda, CA, USA.
Andrew Mashchak, Adventist Health Studies, Loma Linda University Health, Loma Linda, CA, USA.
Ujué Fresán, School of Public Health, Loma Linda University, Loma Linda, CA, USA.
Karen Jaceldo-Siegl, School of Public Health, Loma Linda University, Loma Linda, CA, USA; Faculty of Graduate Studies, Loma Linda University, Loma Linda, CA, USA.
Fayth Miles, School of Public Health, Loma Linda University, Loma Linda, CA, USA; School of Medicine, Loma Linda University, Loma Linda, CA, USA.
Gary E Fraser, School of Public Health, Loma Linda University, Loma Linda, CA, USA; School of Medicine, Loma Linda University, Loma Linda, CA, USA.
Data Availability
Data described in the article, code book, and analytic code will be made available upon request pending application and approval, based on the guidelines for the use of archived data available at: https://adventisthealthstudy.org/researchers.
References
- 1. Orlich MJ, Chiu THT, Dhillon PK, Key TJ, Fraser GE, Shridhar K, Agrawal S, Kinra S. Vegetarian epidemiology: review and discussion of findings from geographically diverse cohorts. Adv Nutr. 2019;10(Supplement_4):S284–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fraser GE. Diet, life expectancy, and chronic disease: studies of Seventh-Day Adventists and other vegetarians. New York: Oxford University Press; 2003. [Google Scholar]
- 3. Orlich MJ, Fraser GE. Vegetarian diets in the Adventist Health Study 2: a review of initial published findings. Am J Clin Nutr. 2014;100(suppl_1):353S–8S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Orlich MJ, Singh PN, Sabaté J, Jaceldo-Siegl K, Fan J, Knutsen S, Beeson WL, Fraser GE. Vegetarian dietary patterns and mortality in Adventist Health Study 2. JAMA Intern Med. 2013;173(13):1230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Appleby PN, Crowe FL, Bradbury KE, Travis RC, Key TJ. Mortality in vegetarians and comparable nonvegetarians in the United Kingdom. Am J Clin Nutr. 2016;103(1):218–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Appleby PN, Key TJ, Thorogood M, Burr ML, Mann J. Mortality in British vegetarians. Public Health Nutr. 2002;5(1):29–36. [DOI] [PubMed] [Google Scholar]
- 7. Martínez-González MA, Sánchez-Tainta A, Corella D, Salas-Salvadó J, Ros E, Arós F, Gómez-Gracia E, Fiol M, Lamuela-Raventós RM, Schröder Het al. . A provegetarian food pattern and reduction in total mortality in the Prevención con Dieta Mediterránea (PREDIMED) study. Am J Clin Nutr. 2014;100(suppl_1):320S–8S. [DOI] [PubMed] [Google Scholar]
- 8. Satija A, Bhupathiraju SN, Spiegelman D, Chiuve SE, Manson JE, Willett W, Rexrode KM, Rimm EB, Hu FB. Healthful and unhealthful plant-based diets and the risk of coronary heart disease in U.S. adults. J Am Coll Cardiol. 2017;70(4):411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Monteiro CA, Cannon G, Moubarac JC, Levy RB, Louzada MLC, Jaime PC. The UN Decade of Nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr. 2018;21(1):5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Elizabeth L, Machado P, Zinocker M, Baker P, Lawrence M. Ultra-processed foods and health outcomes: a narrative review. Nutrients. 2020;12(7):1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Monteiro CA, Cannon G, Levy RB, Moubarac J-C, Louzada MLC, Rauber F, Khandpur N, Cediel G, Neri D, Martinez-Steele Eet al. . Ultra-processed foods: what they are and how to identify them. Public Health Nutr. 2019;22(5):936–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rico-Campà A, Martínez-González MA, Alvarez-Alvarez I, Mendonça RdD, de la Fuente-Arrillaga C, Gómez-Donoso C, Bes-Rastrollo M. Association between consumption of ultra-processed foods and all cause mortality: SUN prospective cohort study. BMJ. 2019;365:l1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schnabel L, Kesse-Guyot E, Alles B, Touvier M, Srour B, Hercberg S, Buscail C, Julia C. Association between ultraprocessed food consumption and risk of mortality among middle-aged adults in France. JAMA Intern Med. 2019;179(4):490–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim H, Hu EA, Rebholz CM. Ultra-processed food intake and mortality in the USA: results from the Third National Health and Nutrition Examination Survey (NHANES III, 1988–1994). Public Health Nutr. 2019;22(10):1777–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Blanco-Rojo R, Sandoval-Insausti H, López-Garcia E, Graciani A, Ordovás JM, Banegas JR, Rodríguez-Artalejo F, Guallar-Castillón P. Consumption of ultra-processed foods and mortality: a national prospective cohort in Spain. Mayo Clin Proc. 2019;94(11):2178–88. [DOI] [PubMed] [Google Scholar]
- 16. Aune D, Keum N, Giovannucci E, Fadnes LT, Boffetta P, Greenwood DC, Tonstad S, Vatten LJ, Riboli E, Norat T. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: systematic review and dose-response meta-analysis of prospective studies. BMJ. 2016;353:i2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fraser GE, Jacobsen BK, Knutsen SF, Mashchak A, Lloren JI. Tomato consumption and intake of lycopene as predictors of the incidence of prostate cancer: the Adventist Health Study-2. Cancer Causes Control. 2020;31(4):341–51. [DOI] [PubMed] [Google Scholar]
- 18. Fraser GE, Jaceldo-Siegl K, Orlich M, Mashchak A, Sirirat R, Knutsen S. Dairy, soy, and risk of breast cancer: those confounded milks. Int J Epidemiol. 2020;49(5):1526–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tantamango-Bartley Y, Knutsen SF, Jaceldo-Siegl K, Fan J, Mashchak A, Fraser GE. Independent associations of dairy and calcium intakes with colorectal cancers in the Adventist Health Study-2 cohort. Public Health Nutr. 2017;20(14):2577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tantamango-Bartley Y, Knutsen SF, Knutsen R, Jacobsen BK, Fan J, Beeson WL, Sabate J, Hadley D, Jaceldo-Siegl K, Penniecook Jet al. . Are strict vegetarians protected against prostate cancer?. Am J Clin Nutr. 2016;103(1):153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Penniecook-Sawyers JA, Jaceldo-Siegl K, Fan J, Beeson L, Knutsen S, Herring P, Fraser GE. Vegetarian dietary patterns and the risk of breast cancer in a low-risk population. Br J Nutr. 2016;115(10):1790–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Orlich MJ, Singh PN, Sabaté J, Fan J, Sveen L, Bennett H, Knutsen SF, Beeson WL, Jaceldo-Siegl K, Butler TLet al. . Vegetarian dietary patterns and the risk of colorectal cancers. JAMA Intern Med. 2015;175(5):767–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Butler TL, Fraser GE, Beeson WL, Knutsen SF, Herring RP, Chan J, Sabaté J, Montgomery S, Haddad E, Preston-Martin Set al. . Cohort profile: the Adventist Health Study-2 (AHS-2). Int J Epidemiol. 2008;37(2):260–5. [DOI] [PubMed] [Google Scholar]
- 24. Jaceldo-Siegl K, Fan J, Sabaté J, Knutsen SF, Haddad E, Beeson WL, Herring RP, Butler TL, Bennett H, Fraser GE. Race-specific validation of food intake obtained from a comprehensive FFQ: the Adventist Health Study-2. Public Health Nutr. 2011;14(11):1988–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jaceldo-Siegl K, Knutsen SF, Sabaté J, Beeson WL, Chan J, Herring RP, Butler TL, Haddad E, Bennett H, Montgomery Set al. . Validation of nutrient intake using an FFQ and repeated 24 h recalls in black and white subjects of the Adventist Health Study-2 (AHS-2). Public Health Nutr. 2010;13(6):812–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Monteiro CA, Geoffrey C, Levy R, Moubarac J-C, Jaime P, Martins AP, Canella D, Louzada M, Parra D; Ricardo C; et al. ; also withNOVA. The star shines bright. World Nutr. 2016;7(1–3):28–38. [Google Scholar]
- 27. Xue X, Kim MY, Gaudet MM, Park Y, Heo M, Hollenbeck AR, Strickler HD, Gunter MJ. A comparison of the polytomous logistic regression and joint Cox proportional hazards models for evaluating multiple disease subtypes in prospective cohort studies. Cancer Epidemiol Biomarkers Prev. 2013;22(2):275–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167(4):268–74. [DOI] [PubMed] [Google Scholar]
- 29. Mathur MB, Ding P, Riddell CA, VanderWeele TJ. Web site and R package for computing E-values. Epidemiology. 2018;29(5):e45–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fraser G, Ru Y. Guided multiple imputation of missing data: using a subsample to strengthen the missing-at-random assumption. Epidemiology. 2007;18(2):246–52. [DOI] [PubMed] [Google Scholar]
- 31. Fraser GE, Yan R, Butler TL, Jaceldo-Siegl K, Beeson WL, Chan J. Missing data in a long food frequency questionnaire: are imputed zeroes correct?. Epidemiology. 2009;20(2):289–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. R Development Core Team . R: a language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing; 2020. [Google Scholar]
- 33. Harrell FE Jr. Hmisc: Harrell miscellaneous. R package version 3.8-3.
- 34. Orlich MJ, Jaceldo-Siegl K, Sabaté J, Fan J, Singh PN, Fraser GE. Patterns of food consumption among vegetarians and non-vegetarians. Br J Nutr. 2014;112(10):1644–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Orlich M, Sabaté J, Jaceldo-Siegl K, Fan J, Singh P, Fraser G. Food consumption patterns of vegetarians and nonvegetarians in Adventist Health Study 2[Abstract 568-S]. Am J Epidemiol. 2013;177(Suppl_11):S142. [Google Scholar]
- 36. Chiavaroli L, Viguiliouk E, Nishi SK, Blanco Mejia S, Rahelić D, Kahleová H, Salas-Salvadó J, Kendall CW, Sievenpiper JL. DASH dietary pattern and cardiometabolic outcomes: an umbrella review of systematic reviews and meta-analyses. Nutrients. 2019;11(2):338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kirwan JP, Malin SK, Scelsi AR, Kullman EL, Navaneethan SD, Pagadala MR, Haus JM, Filion J, Godin JP, Kochhar Set al. . A whole-grain diet reduces cardiovascular risk factors in overweight and obese adults: a randomized controlled trial. J Nutr. 2016;146(11):2244–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li J, Guasch-Ferré M, Chung W, Ruiz-Canela M, Toledo E, Corella D, Bhupathiraju SN, Tobias DK, Tabung FK, Hu Jet al. . The Mediterranean diet, plasma metabolome, and cardiovascular disease risk. Eur Heart J. 2020;41(28):2645–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Matsumoto S, Beeson WL, Shavlik DJ, Siapco G, Jaceldo-Siegl K, Fraser G, Knutsen SF. Association between vegetarian diets and cardiovascular risk factors in non-Hispanic white participants of the Adventist Health Study-2. J Nutr Sci. 2019;8:e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Belobrajdic DP, Bird AR. The potential role of phytochemicals in wholegrain cereals for the prevention of type-2 diabetes. Nutr J. 2013;12(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Reis A, Rocha S, de Freitas V. Going “green” in the prevention and management of atherothrombotic diseases: the role of dietary polyphenols. J Clin Med. 2021;10(7):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Malesza IJ, Malesza M, Walkowiak J, Mussin N, Walkowiak D, Aringazina R, Bartkowiak-Wieczorek J, Madry E. High-fat, Western-style diet, systemic inflammation, and gut microbiota: a narrative review. Cells. 2021;10(11):3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shodja MM, Knutsen R, Cao J, Oda K, Beeson LE, Fraser GE, Knutsen S. Effects of glycosylated hemoglobin levels on neutrophilic phagocytic functions. Jacobs J Diabetes Endocrinol. 2017;8(2):9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Martínez Leo EE, Peñafiel AM, Hernández Escalante VM, Cabrera Araujo ZM. Ultra-processed diet, systemic oxidative stress, and breach of immunologic tolerance. Nutrition. 2021;91–92:111419. [DOI] [PubMed] [Google Scholar]
- 45. Ruiz-Leon AM, Lapuente M, Estruch R, Casas R. Clinical advances in immunonutrition and atherosclerosis: a review. Front Immunol. 2019;10:837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Seidell JC. Obesity, insulin resistance and diabetes—a worldwide epidemic. Br J Nutr. 2000;83(S1):S5–8. [DOI] [PubMed] [Google Scholar]
- 47. Viguiliouk E, Kendall CWC, Kahleová H, Rahelić D, Salas-Salvadó J, Choo VL, Mejia SB, Stewart SE, Leiter LA, Jenkins DJAet al. . Effect of vegetarian dietary patterns on cardiometabolic risk factors in diabetes: a systematic review and meta-analysis of randomized controlled trials. Clin Nutr. 2019;38(3):1133–45. [DOI] [PubMed] [Google Scholar]
- 48. Siervo M, Shannon OM, Llewellyn DJ, Stephan BC, Fontana L. Mediterranean diet and cognitive function: from methodology to mechanisms of action. Free Radic Biol Med. 2021;176:105–17. [DOI] [PubMed] [Google Scholar]
- 49. Dominguez LJ, Veronese N, Vernuccio L, Catanese G, Inzerillo F, Salemi G, Barbagallo M. Nutrition, physical activity, and other lifestyle factors in the prevention of cognitive decline and dementia. Nutrients. 2021;13(11):4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Furulund E, Bemanian M, Berggren N, Madebo T, Rivedal SH, Lid TG, Fadnes LT. Effects of nutritional interventions in individuals with chronic obstructive lung disease: a systematic review of randomized controlled trials. Int J Chron Obstruct Pulmon Dis. 2021;16:3145–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hall KD, Ayuketah A, Brychta R, Cai H, Cassimatis T, Chen KY, Chung ST, Costa E, Courville A, Darcey Vet al. . Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell Metab. 2019;30(1):226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. White E, Kushi LH, Pepe MS. The effect of exposure variance and exposure measurement error on study sample size: implications for the design of epidemiologic studies. J Clin Epidemiol. 1994;47(8):873–80. [DOI] [PubMed] [Google Scholar]
- 53. Freedman LS, Commins JM, Moler JE, Arab L, Baer DJ, Kipnis V, Midthune D, Moshfegh AJ, Neuhouser ML, Prentice RLet al. . Pooled results from 5 validation studies of dietary self-report instruments using recovery biomarkers for energy and protein intake. Am J Epidemiol. 2014;180(2):172–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kipnis V, Subar AF, Midthune D, Freedman LS, Ballard-Barbash R, Troiano RP, Bingham S, Schoeller DA, Schatzkin A, Carroll RJ. Structure of dietary measurement error: results of the OPEN biomarker study. Am J Epidemiol. 2003;158(1):14–21.; discussion 22–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the article, code book, and analytic code will be made available upon request pending application and approval, based on the guidelines for the use of archived data available at: https://adventisthealthstudy.org/researchers.


