Abstract
Background
Symptoms of post-acute sequelae of COVID-19 (PASC) may improve following SARS-CoV-2 vaccination. However few prospective data that also explore the underlying biological mechanism are available. We assessed the effect of vaccination on symptomatology of participants with PASC, and compared antibody dynamics between those with and without PASC.
Methods
RECoVERED is a prospective cohort study of adult patients with mild to critical COVID-19, enrolled from illness onset. Among participants with PASC, vaccinated participants were exact-matched 1:1 on age, sex, obesity status and time since illness onset to unvaccinated participants. Between matched pairs, we compared the monthly mean numbers of symptoms over a 3-month follow-up period, and, using exact logistic regression, the proportion of participants who fully recovered from PASC. Finally, we assessed the association between PACS status and rate of decay of spike- and RBD-binding IgG titers up to 9 months after illness onset using Bayesian hierarchical linear regression.
Findings
Of 349 enrolled participants, 316 (90.5%) had ≥3 months of follow-up, of whom 186 (58.9%) developed PASC. Among 36 matched pairs with PASC, the mean number of symptoms reported each month during 3 months of follow-up were comparable between vaccinated and unvaccinated groups. Odds of full recovery from PASC also did not differ between matched pairs (OR 1.57 [95%CI 0.46–5.84]) within 3 months after the matched time-point. The median half-life of spike- and RBD-binding IgG levels were, in days (95%CrI), 233 (183–324) and 181 (147–230) among participants with PASC, and 170 (125–252) and 144 (113–196) among those without PASC, respectively.
Interpretation
Our study found no strong evidence to suggest that vaccination improves symptoms of PASC. This was corroborated by comparable spike- and RBD-binding IgG waning trajectories between those with and without PASC, refuting any immunological basis for a therapeutic effect of vaccination on PASC.
Keywords: COVID-19, SARS-CoV-2, Vaccination, Long COVID, Post-acute sequelae, Therapeutic vaccine
1. Introduction
It is now well-recognised that the symptoms of coronavirus disease 2019 (COVID-19), caused by infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), can persist beyond the acute phase of infection [1], [2]. The World Health Organisation (WHO) recently defined this complex syndrome, usually called ‘long COVID’ or ‘post-acute COVID-19 syndrome’ (PASC), as the presence of COVID-19 symptoms that occurs within three months after illness onset and persists for at least 2 months [3]. An accurate estimate of the proportion of COVID-19 patients who develop PASC is still lacking, largely due to the substantial heterogeneity in study design and lack of uniform definition [2]. The aetiology of PASC also remains unclear and no treatment options currently exist.
As SARS-CoV-2 vaccination programmes are rolled out in many countries, a number of anecdotal reports and case-series from high-income countries have suggested that PASC symptoms can be alleviated after vaccination [4]. An online survey among 900 PASC patients in the United Kingdom found that over half (57.9%) of respondents reported improvement of symptoms following vaccination. Although these findings should be interpreted with caution due to selection bias and lack of a control group, the hypothesis that SARS-CoV-2 could act as a therapeutic vaccine in PASC patients gained traction due to the urgent need for treatment options for PASC. In a recent cohort study of self-referred participants with established PASC, the cumulative rate of complete remission from PASC was higher among vaccinated participants compared to propensity-score matched unvaccinated participants over a 120 day follow-up period [5]. Given that randomised clinical trials in which controls would be prevented from being vaccinated are unethical given the overwhelming benefits of vaccination for both individual and public health, robust clinical data from prospective observational studies are required to confirm or refute these early findings.
In addition, complementing clinical data with immunological findings is crucial to examining the possible biological mechanism by which vaccination would contribute to improvement of PASC symptoms. Evaluating antibody kinetics among patients with PASC and those without could help to support any observed beneficial effect of vaccination or lack thereof. For example, a recent prospective cohort study of COVID-19 patients found that IgM and IgG3 levels during primary infection and at 6 months follow-up were lower among patients with PASC compared to those without PASC [6]. Assuming a clinical benefit of vaccination, these findings imply that an effect could be mediated by boosting IgM and IgG3 antibody titers. However, no study to date has explored, within the same cohort, both the clinical effect of vaccination on PASC symptomatology and antibody kinetics between those with and without PASC over time.
Using longitudinal data from a prospective cohort study of COVID-19 patients enrolled at disease onset, we assessed the effect of vaccination on recovery from PASC symptoms. This analysis is complemented by immunological investigations to assess possible associations between antibody kinetics prior to vaccination and development of PASC.
2. Methods
2.1. Study population
RECoVERED is a cohort study of individuals with SARS-CoV-2 infection in the municipal region of Amsterdam, the Netherlands. The aims of this cohort are to describe the immunological, clinical and psychosocial sequelae of SARS-CoV-2 infection across the full spectrum of COVID-19 severity. Enrolment of study participants occurred between 11 May 2020 and 21 June 2021. Study design and procedures have been described in detail elsewhere [1]. Briefly, non-hospitalised patients were selected from notified cases of SARS-CoV-2 infection at the Public Health Service of Amsterdam, and invited to participate within 7 days of diagnosis by means of a telephone call by trained study staff. Prospectively-enrolled hospitalised participants were identified from admissions to the COVID-19 wards and enrolled within 7 days of hospital admission at the Amsterdam University Medical Centres (UMC) by means of a bedside visit on the hospital ward or a telephone call, if already discharged from hospital. Eligibility criteria included laboratory confirmation of SARS-CoV-2 infection by reverse transcriptase polymerase chain reaction (RT-PCR), age 16–85 years, residing in the municipal region of Amsterdam, and adequate understanding of Dutch or English. Individuals residing in a nursing home and those with mental disorders deemed likely to interfere to adherence to study procedures were excluded.
For the current analysis, we included RECoVERED participants with at least 3 months of follow-up after illness onset and used follow-up data collected up to 1 November 2021.
RECoVERED was approved by the medical ethical review board of the Amsterdam University Medical Centres (NL73759.018.20). All participants provided written informed consent.
2.2. Study procedures
During the first month of follow-up, trained study staff interviewed participants on the presence, severity, and start and stop dates of 20 different COVID-19 symptoms, took physical measurements (respiratory rate [RR], heart rate [HR] and oxygen saturation [SpO2]), collected biological specimens (nasopharyngeal swab for PCR, blood samples, saliva) and recorded participants’ past medical history and socio-demographic characteristics. Symptoms were based a validated questionnaire [7] and included: fatigue, cough, fever, rhinorrhoea, sore throat, dyspnoea, loss of smell and/or taste, chest pain, headache, abdominal pain, confusion, arthralgia, myalgia, loss of appetite, wheeze, skin rash, nausea and/or vomiting, diarrhoea, ear ache, spontaneous bleeding. Between months 3 to 12 of follow-up, quarterly biological sampling took place and participants completed monthly online questionnaires on the presence of the same 20 COVID-19 symptoms within the past month (yes/no). Finally, participants with PASC at first vaccination were asked at one month following first COVID-19 vaccination whether they had experienced an overall subjective change in their PASC symptoms.
From 6 January 2021, study participants were invited for SARS-CoV-2 vaccination through the national Dutch SARS-CoV-2 vaccination campaign [8]. Study participants who had not yet been vaccinated by 4 April 2021 were invited to receive two doses as part of our study, administered 28 days apart, of the BNT162b2 mRNA (Pfizer/BioNTech) vaccine [9], which were made available to RECoVERED by the Dutch Ministry of Health, Wellbeing and Sport (RIVM).
2.3. Definitions
Illness onset was defined as the earliest date upon which COVID-19 symptoms were experienced for symptomatic patients, or the date of SARS-CoV-2 diagnosis for asymptomatic patients. The definition of PASC was based on the WHO criteria as reporting at least one COVID-19 symptom that started within one month of overall illness onset and lasted beyond 3 months after illness onset. We thus considered any symptom occurring one month after the onset of COVID-19 illness unlikely to be due to COVID-19 and hence these symptoms were not deemed to be PACS symptoms [3]. Full recovery took place when all PASC symptoms had resolved (i.e., were reported as being absent). COVID-19 clinical severity was categorised according to WHO COVID-19 disease severity criteria [10]: mild disease as having a RR < 20/min and SpO2 > 94% on room air at both day 0 (D0) and day 7 (D7) study visits; moderate disease as having a RR 20–30/min and/or SpO2 90–94% or receiving oxygen therapy at D0 or D7; severe disease as having a RR > 30/min and/or SpO2 < 90% or receiving oxygen therapy at D0 or D7; critical disease as ICU admission due to COVID-19 at any point. BMI was defined in kg/m2 as: <25, underweight or normal weight; 25–29, overweight; ≥30, obese.
2.4. SARS-CoV-2 binding IgG antibody levels
Levels of Immunoglobulin G (IgG) binding to SARS-CoV-2 receptor-binding domain (RBD) and spike (S) proteins of wild type virus (Wu-1) were determined using a custom luminex assay as described previously [11], [12]. In summary, proteins were produced in HEK293F cells (Invitrogen) and purified from the cell culture supernatant using affinity chromatography with NiNTA agarose beads (Qiagen). Proteins were covalently coupled to luminex magplex beads using a two-step carbodiimide reaction. Beads were then incubated overnight with 1:100,000 diluted serum followed by detection with goat-anti-human IgG-PE (Southern Biotech) on a Magpix (Luminex) as the mean fluorescent intensity (MFI).
2.5. Neutralisation assays
Pseudovirus neutralization assay was performed as previously described [13]. Briefly, HEK293T/ACE2 cells [14] were seeded in poly-L-lysine pre-coated 96-well plates. The next day, heat-inactivated 1:100 diluted sera were 3-fold serially diluted and mixed in a 1:1 ratio with pseudovirus Wu-1 D614G (WT) [14]. After 1-hour incubation at 37 °C the mixtures were added to the cells and incubated for 48 h at 37 °C. The luciferase activity in cell lysates was measured using the Nano-Glo Luciferase Assay System (Promega) and GloMax system (Turner BioSystems). The 50% inhibitory dilution (ID50) titers were determined as the serum dilution at which infectivity was inhibited by 50% using a non-linear regression curve fit (GraphPad Prism software version 8.3).
2.6. Statistical analysis
Baseline socio-demographic and clinical characteristics of participants with at least 3 months of follow-up were compared between those who developed PASC and those who recovered from all COVID-19 symptoms within 3 months of illness onset.
We first evaluated the effect of COVID-19 vaccination on the mean numbers of PASC symptoms reported. Among participants with PASC, we matched vaccinated and unvaccinated follow-up intervals according to participants’ age group (<45 years; 45–65 years; 65+ years), sex (male/female), BMI (obese or not) and time since illness onset (in months). This was achieved by 1:1 exact matching the month in which a participant received their first vaccination to a participant who remained unvaccinated for at least one month following the matched time-point, using a coarsened exact matching (CEM) approach [15]. Follow-up was then censored at lost to follow-up, last cohort visit or at 3 months following matched time-point, whichever occurred first. Participants were allowed to contribute multiple periods of unvaccinated follow-up and could contribute to both vaccinated and unvaccinated time intervals, provided that symptom data were available for at least one follow-up time-point after the matched time-point. In order to ensure that symptom data were measured from the same baseline for each participant, we divided follow-up in monthly intervals since illness onset according to the date on which surveys were completed. We modelled the mean total number of symptoms at each time-point using linear regression, which was compared between the matched vaccinated and unvaccinated individuals using Wald χ2 tests. We bootstrapped variance estimates to ensure that variance was independent and identically distributed across participants. These variance estimates were used to calculate 95% confidence intervals (CIs) around the mean number of PASC symptoms at each time-point. Secondly, we used exact logistic regression to compare the odds of having recovered fully from PASC by the end of matched follow-up intervals between matched pairs of vaccinated and unvaccinated individuals.
We then explored the association between early antibody titers and consequent development of PASC. Among participants with at least 3 months of follow-up after illness onset, we used a Bayesian multilevel model to compare IgG antibody neutralising and binding titers (to WT-D614G spike [S] and receptor binding domain [RBD]) measured 30–60 days after illness onset between those who did and did not develop PASC. This model estimates the absolute difference in means between groups, while pooling estimates across study participants. Differences in posterior means were mean-centred such that effect sizes shown could be compared on a common scale. To assess the effect of COVID-19 severity, the analysis was repeated, this time stratifying by clinical severity group.
To assess the association between antibody kinetics and PASC status, we stratified individuals with and without PASC at 3 months after illness onset and fitted patients with at least two datapoints where spike/RBD-binding IgG measurements were made to a constant decay model. We performed Bayesian hierarchical linear regression of the log response variable (Y) against time since symptom onset (t), partially pooling decay rates across participants (i):
where and are the participant-specific decay rate and intercept.
All Bayesian models were fitted using Markov Chain Monte Carlo (MCMC) with PyMC3 [16], implementing a no-u-turn sampler. Four MCMC chains were run with at least 4000 burn-in steps and 2000 saved posterior samples. This procedure resulted in a posteriori distribution, from which its mode defined the parameter estimate and its 2.5% and 97.5 quantiles defined the 95% credible interval (CrI). If 1 was not included in the 95%CrI, the parameter estimate was considered statistically significant. Convergence for all parameters were verified by checking trace plots, ensuring their R-hat values were < 1.05 with sufficient effective sample size (>200). Full formulations of the models used are listed in the Supplementary Materials.
Statistical analyses were performed using Stata (v.15.1, StataCorp LLC, College Station, TX, USA) and the Python PyMC3 programme described above.
3. Results
3.1. Study population
Of 349 enrolled participants by 1 November 2021, 316 had at least 3 months of follow-up, of whom 186 (58.9%) developed PASC (Table 1 ). Those with PASC were older (p < 0.001) and more frequently had moderate or severe/critical COVID-19 (p < 0.001), higher BMI (p = 0.002) and were more likely to have a lower educational level (p < 0.001) compared to those who fully recovered from symptoms within 3 months of illness onset (Table 1). Whilst all participants were unvaccinated for COVID-19 prior to enrolment, the majority of participants had been vaccinated against SARS-CoV-2 by 1 November 2021.
Table 1.
Socio-demographic, clinical and study features of RECoVERED study participants with at least 3 months of follow-up, stratified by those who did and did not develop PASC.
| Total | PASC status |
p-value | ||
|---|---|---|---|---|
| No PASC | PASC | |||
| N = 316 | N = 130 | N = 186 | ||
| Sex | 0.20 | |||
| Male | 181 (57%) | 80 (62%) | 101 (54%) | |
| Female | 135 (43%) | 50 (38%) | 85 (46%) | |
| Age, years | 51.0 (36.0–62.0) | 46.0 (32.0–57.0) | 53.5 (41.0–64.0) | <0.001 |
| Clinical severity score | <0.001 | |||
| Mild | 92 (29%) | 61 (47%) | 31 (17%) | |
| Moderate | 142 (45%) | 52 (40%) | 90 (48%) | |
| Severe/critical | 82 (26%) | 17 (13%) | 65 (35%) | |
| BMI, kg/m2 | 26.0 (23.2–29.4) | 25.1 (22.9–27.7) | 26.8 (23.7–31.0) | 0.0020 |
| BMI category | 0.027 | |||
| Normal weight | 130 (41%) | 62 (48%) | 68 (37%) | |
| Overweight | 105 (33%) | 43 (33%) | 62 (33%) | |
| Obese | 71 (22%) | 20 (15%) | 51 (27%) | |
| Missing | 10 (3%) | 5 (4%) | 5 (3%) | |
| Migration background | 0.25 | |||
| Dutch | 187 (59%) | 78 (60%) | 109 (59%) | |
| Non-Dutch, OECD high-income | 39 (12%) | 20 (15%) | 19 (10%) | |
| Non-Dutch, OECD low/middle income | 74 (23%) | 26 (20%) | 48 (26%) | |
| Missing | 16 (5%) | 6 (5%) | 10 (5%) | |
| Highest level of education | <0.001 | |||
| None, primary or secondary education | 42 (13%) | 8 (6%) | 34 (18%) | |
| Vocational training | 73 (23%) | 24 (18%) | 49 (26%) | |
| University education | 181 (57%) | 93 (72%) | 88 (47%) | |
| Missing | 20 (6%) | 5 (4%) | 15 (8%) | |
| Number of COVID-19 high-risk comorbidities | 0.035 | |||
| 0 | 178 (56%) | 85 (65%) | 93 (50%) | |
| 1 | 73 (23%) | 27 (21%) | 46 (25%) | |
| 2 | 37 (12%) | 10 (8%) | 27 (15%) | |
| 3 or more | 28 (9%) | 8 (6%) | 20 (11%) | |
| PASC status | ||||
| Total | No PASC | PASC | p-value | |
| N = 316 | N = 130 | N = 186 | ||
| Place of recruitment | <0.001 | |||
| Non-hospital (PHSA) | 156 (49%) | 93 (72%) | 63 (34%) | |
| Hospital | 160 (51%) | 37 (28%) | 123 (66%) | |
| Days from illness onset to COVID-19 diagnosis | 5 (2–10) | 4 (2–8) | 6 (2–11) | 0.064 |
| Days from illness onset to inclusion in study | 12 (6–42) | 9 (5–17) | 17 (9–80) | <0.001 |
| Follow-up time from enrolment in study | 369.5 (238.5–496.0) | 363.0 (287.0–482.0) | 375.5 (216.0–502.0) | 0.65 |
| Admitted to hospital for COVID-19 | 153 (48%) | 36 (28%) | 117 (63%) | <0.001 |
| Days from illness onset to hospitalisation | 9 (7–14) | 10 (7–13) | 9 (7–14) | 0.98 |
| Admitted to ICU for COVID-19 | 42 (13%) | 9 (7%) | 33 (18%) | 0.005 |
| Days from illness onset to ICU admission | 10 (8–12) | 10 (9–12) | 10 (7–12) | 0.86 |
| Vaccinated during follow-up | 217 (69%) | 97 (75%) | 120 (65%) | 0.076 |
| Vaccine type | 0.67 | |||
| Pfizer/BioNTech (BNT162b2) mRNA | 197 (62%) | 86 (66%) | 111 (60%) | |
| Moderna mRNA | 9 (3%) | 5 (4%) | 4 (2%) | |
| AstraZeneca | 8 (3%) | 5 (4%) | 3 (2%) | |
| Janssen (Johnson & Johnson) | 2 (1%) | 1 (1%) | 1 (1%) | |
| Unvaccinated | 99 (32%) | 33 (25%) | 66 (35%) | |
| Missing | 1 (0%) | 0 (0%) | 1 (1%) | |
| Time from illness onset to first vaccination, days | 247 (144–364) | 194 (130–301) | 271 (158–387) | NA |
| Lost to follow-up | 55 | 23 | 32 | NA |
BMI = Body mass index; HIC = high-income country; HR = heart rate; LMIC = low- or middle income country; ICU = Intensive Care Unit; NA = Not applicable; OECD = Organisation for Economic Co-operation and Development; PASC = Post-acute sequelae of COVID-19; PHSA = Public Health Service of Amsterdam; RR = respiratory rate; SpO2 = oxygen saturation; UMC = University Medical Centres.
Continuous variables presented as median (IQR) and compared using the Kruskal-Wallis test; categorical and binary variables presented as n (%) and compared using the Pearson χ2 test (or Fisher exact test if n < 5).
Clinical severity groups defined as: mild as having a RR < 20/min and SpO2 on room air >94% at both D0 and D7 study visits; moderate disease as having a RR 20–30/min, SpO2 90–94% and/or receiving oxygen therapy at D0 or D7; severe disease as having a RR > 30/min or SpO2 < 90% at D0 or D7; critical disease as requiring ICU admission.
BMI categories defined as: <25 kg/m2 normal or underweight; 25–29 kg/m2 overweight; ≥30 kg/m2 obese.
Migration background was defined as Dutch and non-Dutch based on the country of birth of the participant and their parents; those of non-Dutch background were further classified as originating from a high-income (HIC) or low-/middle-income country (LMIC), according to the Organisation for Economic Co-operation and Development (OECD).
High-risk COVID-19 comorbidities are defined as listed by the WHO Clinical Management Guidelines and include: cardiovascular disease (including hypertension), chronic pulmonary disease (excluding asthma), renal disease, liver disease, cancer, immunosuppression (excluding HIV, including previous organ transplantation), previous psychiatric illness and dementia.
3.2. Changes to PASC symptoms following vaccination
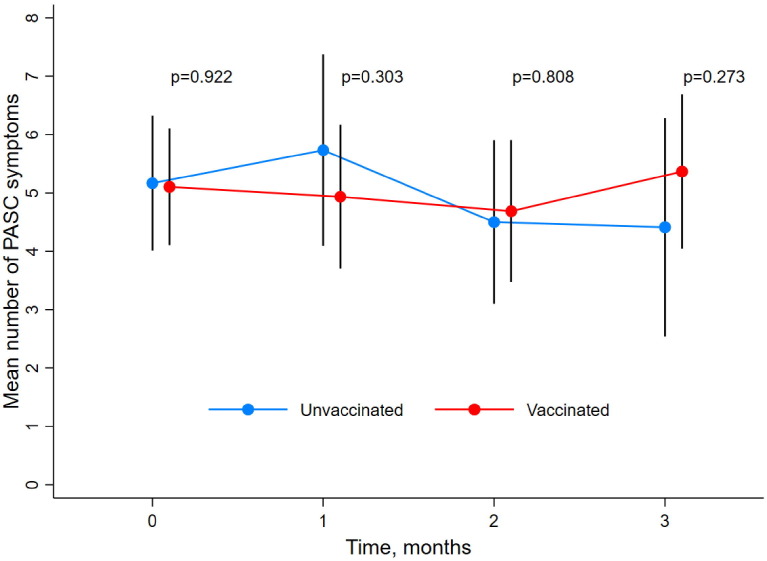
Among participants with at least 3 months of follow-up after illness onset who developed PASC (n = 186), 36 follow-up period pairs were exact matched, originating from 36 vaccinated cases and 32 unvaccinated controls (4 participants contributed two unvaccinated follow-up periods) (Supplementary Table S1; Supplementary Figure S1a). Median length of follow-up after matching did not differ between vaccinated (3 months [IQR = 3–3]) and unvaccinated (3 months [IQR 1–3]) groups. There was no statistically significant difference in the mean total number of symptoms reported between unvaccinated and vaccinated participants at each month during the 3 months following the matched time-point (Fig. 1 ). The odds of recovery from PASC did not differ between vaccinated (6 recovery events) and unvaccinated participants (4 recovery events) (OR 1.57 [95%CI 0.46–5.84], p = 0.596) in the follow-up period up to 3 months following the matched time-point. Overall, among all participants with PASC at first vaccination, the vast majority reported that they had the feeling that their PASC symptoms had not changed within a month after first vaccination, regardless of initial COVID-19 severity (Supplementary Figure S3).
Fig. 1.
Mean number of symptoms during exposure-matched unvaccinated and vaccinated follow-up intervals, among participants with PASC Black vertical bands represent 95% CIs, which were calculated from bootstrapped variance estimates to ensure that variance was independent and identically distributed across participants. Time represents nearest month since first vaccination (for vaccinated participants) or matched time-point (for unvaccinated participants) that symptom survey was completed. P-values were obtained from the Wald χ2 test comparing the mean number of symptoms in unvaccinated and vaccinated time-intervals at each month of follow-up, with bootstrapped variance estimates. PASC = Post-acute sequelae of COVID-19.
3.3. Association between early antibody titers and development of PASC
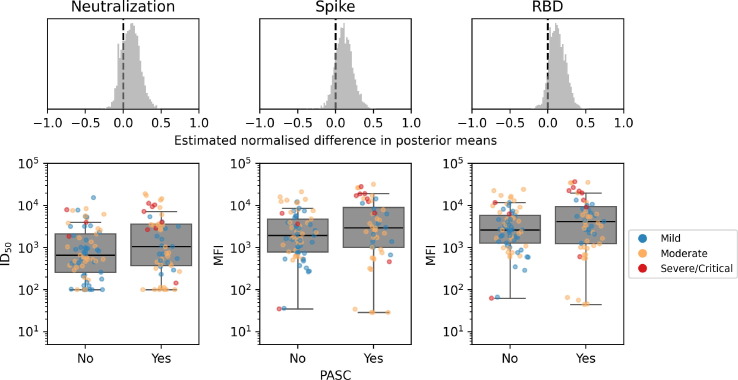
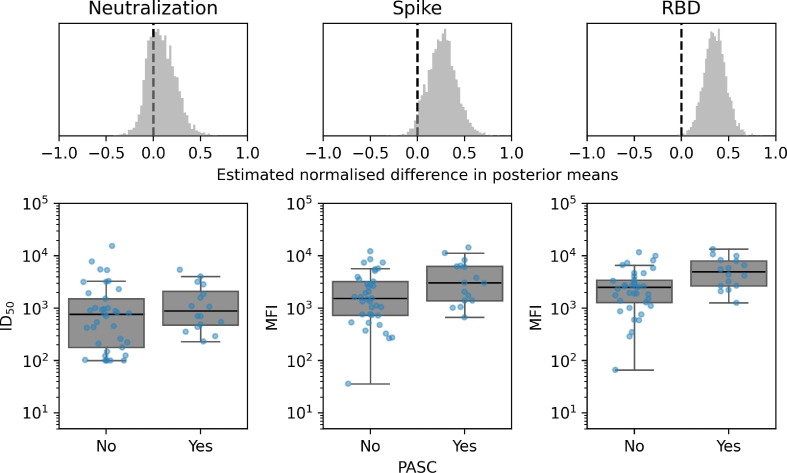
Among participants with at least 3 months of follow-up after illness onset (n = 316), no differences were observed in levels of anti-spike or anti-RBD binding IgG antibodies measured at 30–60 days after illness onset between those who developed PASC (n = 186) and those who did not (n = 130) (median difference in posterior means: 0.109 [95%CrI −0.101–0.343] and 0.093 [95%CrI −0.082–0.321], respectively) (Fig. 2 ). Levels of neutralising antibodies detected 30–60 days of illness onset were also comparable between those who developed PASC and those who did not (median difference in posterior means: 0.168 [95%CrI −0.05–0.395]). The stratified analysis was only performed in those who experienced mild and moderate COVID-19 as numbers in the severe/critical group were too low for a meaningful analysis. Results remained unchanged among those with moderate COVID-19 (Supplementary Figure S2). However, among participants with mild COVID-19, higher RBD-binding titers were observed among those who developed PASC compared to those who did not (0.324 [95%CrI 0.091–0.549]; Fig. 3 ), yet no difference in neutralising IgG levels was observed (0.147 [95%CrI −0.113–0.450]).
Fig. 2.
IgG binding and spike neutralisation at 30–60 days following illness onset among all participants who did (N = 56) and did not (N = 72) develop PASC, by COVID-19 clinical severity Difference in 30–60 day WT-D614G spike protein neutralising IgG titers and anti-spike and anti-RBD IgG binding titers between those who did and did not develop PASC. The mean effects on neutralising and IgG titers from those who did and did not develop PASC were estimated using a Bayesian multilevel model. Differences in posterior means were mean-centred such that effect sizes shown can be compared on a common scale (top row). Distributions of serum spike protein neutralising IgG titers (bottom left), spike binding (bottom middle) and RBD binding (bottom right) displayed such that each dot represents one participant, coloured according to COVID-19 clinical severity. RBD = Receptor binding domain. PASC = Post-acute sequelae of COVID-19.
Fig. 3.
IgG binding and spike neutralisation at 30–60 days following illness onset among participants with mild COVID-19 who did (N = 16) and did not (N = 36) develop PASC Difference in 30–60 day WT-D614G spike protein neutralising IgG titers and anti-spike and anti-RBD IgG binding titers between those who did and did not develop PASC with mild COVID-19. The mean effects on neutralising and IgG titers from those who did and did not develop PASC were estimated using a Bayesian multilevel model. Differences in posterior means were mean-centred such that effect sizes shown can be compared on a common scale (top row). Distributions of serum spike protein neutralising IgG titers (bottom left), spike binding (bottom middle) and RBD binding (bottom right) displayed such that each dot represents one participant. RBD = Receptor binding domain. PASC = Post-acute sequelae of COVID-19.
3.4. Association between PASC and antibody decay over time
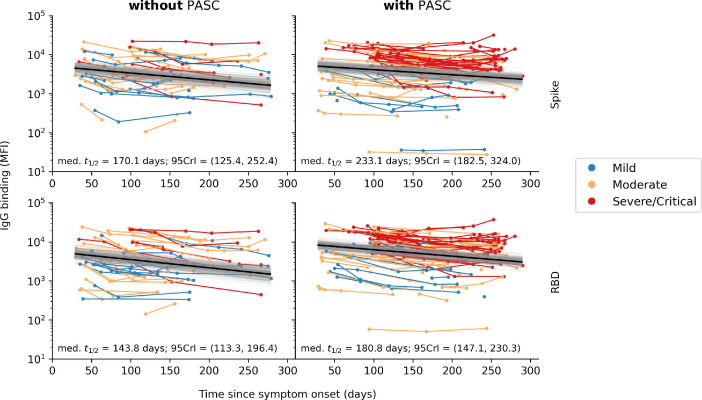
The estimated median half-life of spike- and RBD-binding IgG levels appeared to be slightly greater for participants with PASC at 3 months after illness onset, however, 95%CrI were largely overlapping for both spike- and RBD-binding IgG titers (Fig. 4 ) and therefore this difference was not statistically significant.
Fig. 4.
Rate of spike- and RBD-binding IgG decay after illness onset, by PASC status at 3 months after illness onset Figures show results of a Bayesian hierarchical linear regression of the log response variable (Y) against time since symptom onset (t), pooling decay rates across participants. Each connected line represents one study participants, coloured by COVID-19 severity. PASC groups were defined as to whether the participant continued to experience one or more symptoms at 3 months after illness onset. Estimated median half-life and the corresponding 95% credible interval (95CrI) of spike- and RBD-binding IgG levels are computed from the median (black line) and posterior distribution (gray region) of regressed lines. PASC = Post-acute sequelae of COVID-19.
4. Discussion
We assessed the effect of SARS-CoV-2 vaccination on symptoms PASC within a well-characterised prospective cohort of participants with PASC who had mild to critical COVID-19. We found no clear evidence of a beneficial effect of vaccination on PASC symptoms. These findings were corroborated by serological data, in which there was no overall difference in early neutralising antibody titers between participants with and without PASC at 3 months after illness onset, nor in antibody decay up to 9 months after illness onset.
Despite initial optimism arising from studies reporting improvement or even full recovery of PASC symptoms following SARS-CoV-2 vaccination, evidence to date is conflicting. Our study supports the evidence base that points to a lack of effect: we found no difference in the mean number of PASC symptoms reported up to 3 months after first vaccination between vaccinated cases and unvaccinated controls matched by age, sex, obesity status and months since illness onset. Additionally, vaccination had no effect on the odds of full recovery from PASC within 3 months from vaccination or matched time-point. Given the limited sample size of our matched analysis, these findings do not fully exclude a beneficial effect but do suggest that such an effect is unlikely to be substantial. Larger studies of different designs demonstrate inconsistent results, which may in part be due to differences in selection of study participants. For example, a large study (more than 450 matched pairs) reported a doubling in remission rate among vaccinated cases compared to unvaccinated controls within 120 days of follow-up [5]. However, this study suffers from selection bias, having recruited self-referred patients with PASC, and non-hospitalised patients (91.1% of participants) and women (80.5% of participants) were overrepresented, limiting generalisability of these results. Similarly, a large nationwide survey from the UK, which also included almost exclusively non-hospitalised participants with PASC, reported a small but statistically significant reduction in the odds of reporting PASC symptoms following each vaccination [17]. In contrast, other large studies corroborated the findings in our study, reporting no difference in PASC symptoms between unvaccinated and vaccinated participants [18], [19]. Overall, these conflicting results highlight the importance of prospective population-based cohorts that follow COVID-19 patients at all levels of disease severity from illness onset onwards. Vaccination remains one of the most important public health tools to avert both short- and long-term COVID-19 morbidity, and care should be taken to ensure any lack of therapeutic effect does not fuel vaccine hesitancy among PASC patients [20], [21].
Our observed lack of association between vaccination and PASC is further underscored by our neutralizing antibody analysis and the mostly lacking role of SARS-CoV-2 specific humoral responses in PASC. We did not observe an association between early (30–60 days post-illness onset) virus binding and neutralising antibody titers and the development of PASC overall, nor among PASC participants who experienced moderate COVID-19. When restricting our analyses to those with mild COVID-19, however, participants who developed PASC exhibited higher early RBD-binding antibody titers than those who did not. Importantly, neutralising IgG levels remained unchanged between PASC and non-PASC participants with mild COVID-19. These results seem to conflict with a recent report suggesting lower early IgM and IgG3 titers in PASC patients [6] but mirror a recent prospective Norwegian study of mainly non-hospitalised, mild COVID-19 patients [22]. Similar to our findings, the latter study found that spike-binding IgG titers 2 months after infection were associated with a higher number of symptoms at 6 months (aHR 1.25 [1.01–1.56], p = 0.037) [22], also when adjusting for confounding factors such as COVID-19 severity. These higher IgG levels might be explained by residual confounding due to variation in clinical severity (ranging from minimal to more substantial symptomatology) within the group classified as having mild disease, given that both antibody titers and PASC are associated with illness severity. Moreover, when we assessed longitudinal serological data, there was also no statistically significant difference in spike- and RBD-binding IgG decay over time between participants with and without PASC. Together, our findings suggest that patients with PASC do not have clearly distinct antibody kinetics during either the acute or convalescent phase, findings which are in line with the lack of effect of vaccination on symptomatology in our study. Future research may wish to investigate potential differences in cell-mediated immunity, also induced by COVID-19 vaccination, between those with and without PASC.
Our study’s strengths are its prospective design, hence avoiding bias through self-referral of PASC patients, detailed prospective symptom data, which minimises recall bias, long follow-up time and representation of the full spectrum of COVID-19 severity. However, our study also has limitations. Although we used a robust statistical method to match cases and controls, vaccination was not randomly assigned and therefore residual confounding may still exist. In addition, a large proportion of our cohort was vaccinated around 12 months after illness onset after which symptom questionnaires were no longer completed. This greatly reduced the number of participants available for matching, limiting statistical power. Another limitation faced by all PASC studies without SARS-CoV-2-negative controls is that we cannot be sure to what extent the symptoms recorded were causally related to SARS-CoV-2 infection as opposed to either underlying comorbidities (i.e., symptoms were already present before COVID-19) or the psychological and physical effects of the pandemic. This may have resulted in misclassification bias and overestimation of the proportion of participants with PASC. However, there is no reason to believe that this misclassification would be more likely to occur among participants contributing to either the vaccinated or unvaccinated matched follow-up data. Finally, as all participants in our cohort were infected with wild-type or Alpha SARS-CoV-2, results may not be generalisable to those infected with other variants.
In summary, we found no evidence of a strong therapeutic effect of SARS-CoV-2 vaccination on the mean number of symptoms among those with PASC over time, nor on odds of full recovery from PASC following first vaccination. Our findings on early and longitudinal serology among those with and without PASC at 3 months after illness onset are consistent with our clinical findings, refuting any immunological basis for a therapeutic effect. There remains a pressing need to understand the underlying biological mechanism of PASC in order to inform effective preventative measures and treatment options.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: A.B. received a grant from ANRS in the past 36 months and participated on the Data Safety Monitoring Board or Advisory Board for ZonMw for a study conducted by the Amsterdam University Medical Centers, location Amsterdam Medical Center. G.d.B. served as a paid member of the scientific advisory board of ExeVir in the past 36 months, and is a patent holder of INV 2020-039 both through their institution. All other authors report no potential conflicts.
Acknowledgments
Acknowledgments
The authors wish to thank all RECoVERED study participants and Mohamed Hoesein, Marco Hol, Melvin Angelovski and their team of the Public Health Service of Amsterdam for valuable logistical support of the vaccine sub-study.
RECoVERED Study Group members:
At the Public Health Service of Amsterdam: Ivette Agard, Jane Ayal, Floor Cavdar, Marianne Craanen, Udi Davinovich, Annemarieke Deuring, Annelies van Dijk, Ertan Ersan, Laura del Grande, Joost Hartman, Nelleke Koedoot, Tjalling Leenstra, Dominique Loomans, Agata Makowska, Tom du Maine, Ilja de Man, Amy Matser, Lizenka van der Meij, Marleen van Polanen, Maria Oud, Clark Reid, Leeann Storey, Marc van Wijk.
At the Amsterdam University Medical Centres: Joost van den Aardweg, Joyce van Assem, Marijne van Beek, Thyra Blankert, Maartje Dijkstra, Orlane Figaroa, Leah Frenkel, Jelle van Haga, Agnes Harskamp-Holwerda, Mette Hazenberg, Soemeja Hidad, Nina de Jong, Hans Knoop, Lara Kuijt, Anja Lok, Eric Moll van Charante, Pythia Nieuwkerk, Annelou van der Veen, Bas Verkaik, Gerben-Rienk Visser.
Data availability
Data supporting the findings in this manuscript are available from the corresponding author upon request.
Funding
This work was supported by the Netherlands Organization for Health Research and Development (ZonMw) (RECoVERED, grant agreement numbers 10,150,062,010,002 to M.D.d.J. and 10,430,072,110,003 to G.J. de Bree) and the Public Health Service of Amsterdam (Research & Development grant number 21–14 to M. Prins). The funders had no role in study design, data collection, data analysis, data interpretation or data reporting.
Patient consent statement
Written informed consent was obtained from each study participant. The study design was approved by the local ethics committee of the Amsterdam UMC (Medisch Ethische Toetsingscommissie [METC]; NL73759.018.20).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2022.05.090.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Wynberg E., van Willigen H.D.G., Dijkstra M., et al. Evolution of Coronavirus Disease 2019 (COVID-19) Symptoms During the First 12 Months After Illness Onset. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michelen M., Manoharan L., Elkheir N., Cheng V., Dagens A., Hastie C., et al. Characterising long COVID: a living systematic review. BMJ Global Health. 2021;6(9) doi: 10.1136/bmjgh-2021-005427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. A clinical case definition of post COVID-19 condition by a Delphi consensus. World Health Organization (WHO) clinical case definition working group on post COVID-19 condition; 2021. [DOI] [PMC free article] [PubMed]
- 4.Arnold D.T., Milne A., Samms E., Stadon L., Maskell N.A., Hamilton F.W. Symptoms After COVID-19 Vaccination in Patients With Persistent Symptoms After Acute Infection: A Case Series. Ann Intern Med. 2021;174(9):1334–1336. doi: 10.7326/M21-1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tran V-TP, Elodie; Saldanha, Julia; Pane, Isabelle; Ravaud, Philippe. Efficacy of COVID-19 Vaccination on the Symptoms of Patients With Long COVID: A Target Trial Emulation Using Data From the ComPaRe e-Cohort in France. SSRN Pre-Print; 2021.
- 6.Cervia C., Zurbuchen Y., Taeschler P., Ballouz T., Menges D., Hasler S., et al. Immunoglobulin signature predicts risk of post-acute COVID-19 syndrome. Nat Commun. 2022;13(1) doi: 10.1038/s41467-021-27797-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ISARIC. Clinical Data Collection – The COVID-19 Case Report Forms (CRFs). Available at: https://isaric.org/research/covid-19-clinical-research-resources/covid-19-crf/. Accessed 18 Feb.
- 8.Rijksoverheid. Aanpak coronavaccinatie in Nederland. Available at: https://www.rijksoverheid.nl/onderwerpen/coronavirus-vaccinatie/aanpak-coronavaccinatie. Accessed 10 Nov.
- 9.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. COVID-19 Clinical management: living guidance; 2021 25 January 2021.
- 11.Brouwer PA-O, Caniels TA-OX, van der Straten KA-O, et al. Potent neutralizing antibodies from COVID-19 patients define multiple targets of vulnerability. (1095-9203 (Electronic)). [DOI] [PMC free article] [PubMed]
- 12.van Gils MJ, van Willigen HDG, Wynberg E, et al. A Single mRNA vaccine dose in COVID-19 patients boosts neutralizing antibodies against SARS-CoV-2 and variants of concern. Cell Reports Medicine. [DOI] [PMC free article] [PubMed]
- 13.Brouwer P.J.M., Brinkkemper M., Maisonnasse P., Dereuddre-Bosquet N., Grobben M., Claireaux M., et al. Two-component spike nanoparticle vaccine protects macaques from SARS-CoV-2 infection. Cell. 2021;184(5):1188–1200.e19. doi: 10.1016/j.cell.2021.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt F, Weisblum Y, Muecksch F, et al. Measuring SARS-CoV-2 neutralizing antibody activity using pseudotyped and chimeric viruses. LID - 10.1084/jem.20201181 [doi] LID - e20201181. (1540-9538 (Electronic)). [DOI] [PMC free article] [PubMed]
- 15.Iacus S.M., King G., Porro G. Causal Inference without Balance Checking: Coarsened Exact Matching. Political Analysis. 2012;20(1):1–24. [Google Scholar]
- 16.Salvatier J., Wiecki T.V., Fonnesbeck C. Probabilistic programming in Python using PyMC3. PeerJ Computer. Science. 2016;2:e55. doi: 10.7717/peerj-cs.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ayoubkhani D, Bermingham C, Pouwels KB, et al. Changes in the trajectory of Long Covid symptoms following COVID-19 vaccination: community-based cohort study. medRxiv; 2021: 2021.12.09.21267516. [DOI] [PMC free article] [PubMed]
- 18.Taquet M, Dercon Q, Harrison PJ. Six-month sequelae of post-vaccination SARS-CoV-2 infection: a retrospective cohort study of 10,024 breakthrough infections. medRxiv; 2021: 2021.10.26.21265508. [DOI] [PMC free article] [PubMed]
- 19.Wisnivesky JP, Govindarajulu U, Bagiella E, et al. Association of Vaccination with the Persistence of Post-COVID Symptoms. (1525-1497 (Electronic)). [DOI] [PMC free article] [PubMed]
- 20.Simon MA, Luginbuhl RD, Parker R. Reduced Incidence of Long-COVID Symptoms Related to Administration of COVID-19 Vaccines Both Before COVID-19 Diagnosis and Up to 12 Weeks After. medRxiv; 2021: 2021.11.17.21263608.
- 21.Jabłońska K., Aballéa S., Toumi M. The real-life impact of vaccination on COVID-19 mortality in Europe and Israel. Public Health. 2021;198:230–237. doi: 10.1016/j.puhe.2021.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blomberg B., Mohn K.-I., Brokstad K.A., Zhou F., Linchausen D.W., Hansen B.-A., et al. Long COVID in a prospective cohort of home-isolated patients. Nat Med. 2021;27(9):1607–1613. doi: 10.1038/s41591-021-01433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting the findings in this manuscript are available from the corresponding author upon request.