Abstract
The integral membrane, Kunitz-type serine protease inhibitors HAI-1 and HAI-2, can suppress the proteolytic activity of the type 2 transmembrane serine protease matriptase with high specificity and potency. High levels of extracellular matriptase proteolytic activity have, however, been observed in some neoplastic B-cells with high levels of endogenous HAI-2, indicating that HAI-2 may be an ineffective matriptase inhibitor at the cellular level. The different effectiveness of the HAIs in the control of extracellular matriptase proteolytic activity is examined here. Upon inducing matriptase zymogen activation in the HAI Teton Daudi Burkitt lymphoma cells, which naturally express matriptase with very low levels of HAI-2 and no HAI-1, nascent active matriptase was rapidly inhibited or shed as an enzymatically active enzyme. With increasing HAI-1 expression, cellular matriptase-HAI-1 complex increased, and extracellular active matriptase decreased proportionally. Increasing HAI-2 expression, however, resulted in cellular matriptase-HAI-2 complex levels reaching a plateau, while extracellular active matriptase remained high. In contrast to this differential effect, both HAI-1 and HAI-2, even at very low levels, were shown to promote the expression and cell-surface translocation of endogenous matriptase. The difference in the suppression of extracellular active matriptase by the two closely related serine protease inhibitors could result from the primarily cell surface expression of HAI-1 compared to the mainly intracellular localization of HAI-2. The HAIs, therefore, resemble one another with respect to promoting matriptase expression and surface translocation but differ in their effectiveness in the control of extracellular matriptase enzymatic activity.
Keywords: HAI-1, HAI-2, Matriptase, Neoplastic B-cells, Proteolytic activity
Introduction
The integral membrane Kunitz-type serine protease inhibitors, HAI-1 and HAI-2, were initially identified from the conditioned medium of gastric cancer cells by their potent inhibitory activity against the blood-borne serine protease, hepatocyte growth factor activator (HGFA), in solution, and were named the HGF activator inhibitors (HAIs).1,2 The HAIs contain two Kunitz domains, which structurally resemble aprotinin. Aprotinin, also known as bovine pancreatic trypsin inhibitor (BPTI), serves as the prototype for 27 such domains identified in 18 Kunitz domain-containing proteins from the human genome.3 Kunitz inhibitors suppress a protease via a well understood standard mechanism (known alternatively as the canonical or Laskowski mechanism) through the insertion of a reactive loop into the active site of the protease in a “lock-and-key” fashion.4 Therefore, the specificity and potency of a Kunitz inhibitor against a given protease are highly dependent on the amino acid sequence in the reactive loop. The almost complete identity of sequences of the reactive loops in the Kunitz domains 1 of HAI-1 and HAI-2 (VGRCRGS versus VGRCRAS, with the second Arg serving as the P1 site) suggests that HAI-1 and HAI-2 resemble one another regarding their selectivity and potency for their target proteases, at least in solution. Furthermore, this level of sequence similarity is unique for the HAIs out of the 27 Kunitz domains identified in the human genome, particularly with respect to the P3 site with a positively charged residue.5 This shared structural similarity provides the basis for the shared biological functions between the HAIs. For example, both HAIs exhibit potent inhibition of not only HGFA but also the serine proteases, matriptase and prostasin. The functional partnership between the HAIs and the latter two membrane-bound serine proteases has been observed at the cellular level and in physiological settings. Enzyme-inhibitor complexes containing matriptase or prostasin with HAI-1 or HAI-2 have been identified and isolated from human body fluids.6 Studies with animal models in which defects associated with HAI deletion can be negated by simultaneous deletion of matriptase support not only the physiological relationship between matriptase and the HAIs but also the shared physiological function of HAI-1 and HAI-2 in the control of matriptase activity.7, 8, 9, 10 The relationship between the HAIs and matriptase is apparently more than the conventional relationship between a protease and protease inhibitor. In early studies, HAI-1 was shown to be required for normal matriptase synthesis and trafficking out of the endoplasmic reticulum (ER) and the Golgi apparatus.11 This unconventional function for HAI- 1 in matriptase synthesis and intracellular trafficking has also been reported for HAI-2.12
There is increasing evidence, however, that also indicates that there are significant differences between HAI-1 and HAI-2 with respect to some of their functions. While the protease inhibitory activity of the HAIs depends on their highly homologous Kunitz domains, the modes of action for the Kunitz domains appear to be different between the HAIs. Structural and enzymatic studies have indicated that the activity of HAI-1 Kunitz domain 1 is greatly affected by the other surrounding protein domains, which can either positively or negatively regulate the inhibitory potency of the domain against serine proteases.13, 14, 15, 16, 17 Although the two Kunitz domains in HAI-1 are separated by an LDL receptor class A domain, they are in fact quite close to one another due to the unusually compact conformation of the HAI-1 extracellular domains, by which the reactive sites of both Kunitz domains are blocked by the surrounding protein domains (auto-inhibition).13 An extended conformation is apparently induced by the presence of active matriptase, and the potent inhibitory activity of HAI-1 against matriptase probably results not only from the structural complementarity between the matriptase serine protease domain and Kunitz domain 1 but also through interaction between the second matriptase CUB domain and HAI-1 Kunitz domain 2. While the detailed three-dimensional structure of HAI-2 remains largely unexplored, the two Kunitz domains appear to be well separated, and both may be functional. This notion is based on the detection of stable matriptase-HAI-2 complexes with a greater mass than would be expected by a simple matriptase-HAI-2 complex, suggesting that Kunitz domain 2 might also be occupied by another serine protease.18 Regarding the normal physiological target proteases for these inhibitors, despite their almost ubiquitous co-expression by epithelial/carcinoma cells, HAI-1 appears to represent the primary matriptase inhibitor. The inhibition of matriptase by HAI-2 is cell-type selective and largely depends on whether HAI-2 is co-localized with matriptase.18 Similarly, while HAI-2 represents the most significant inhibitor for prostasin in Caco-2 human colon adenocarcinoma cells and normal human intestinal tissues, HAI-2 does not appear to play an important role in prostasin inhibition in 184 A1N4 human mammary epithelial cells or HaCaT human keratinocytes.19,20 Furthermore, HAI-1 is expressed as two splice variants that differ by only 16 amino acids located between Kunitz domain 1 and the LDL receptor class A domain,21 whereas an HAI-2 splice variant found in rodents lacks the functionally important Kunitz domain 1.22 On the other hand, human HAI-2 undergoes post-translational modification into two different forms based on differential glycosylation: one form appears to have high-mannose-type N-glycan modification whereas the other appears to have extensive N-glycan branching.5 Regardless of their glycosylation state, both HAI-2 forms are detected predominantly inside the cell with distinct subcellular localization in epithelial/carcinoma cells. In contrast, while HAI-1 can be intracellularly detected, HAI-1 is also detected at high levels on the cell periphery. Interestingly, the HAI-2 species with intensive N-glycan branching was the form that was initially identified and isolated from the conditioned medium from gastric cancer cells, which demonstrates that HAI-2 can be secreted into the extracellular milieu.2 It appears, therefore, that the nature and regulation of translocation to the plasma membrane and shedding from cell surface differ for HAI-2 and HAI-1, at least, in epithelial/carcinoma cells.
Matriptase zymogen was recently reported to be sufficient to rescue the defects associated with the targeted deletion of matriptase in mice.23 The proteolytic activity of the enzyme is, however, still most likely the primary mechanism for mediating its physiological function in humans, based on the presence of activated matriptase in complexes with the HAIs and serpins in the human body fluids.6,24,25 Matriptase is almost universally co-expressed with the HAIs.5,18,26 So the levels and duration of enzymatically active matriptase will largely depend on how fast and the extent to which the HAIs bind to and inhibit active matriptase generated through the zymogen activation process. The subtleties of the different properties and behavior of the two HAIs make it a challenge to precisely pin down how and to what degree the individual HAIs contribute to matriptase regulation in matriptase-expressing cells that also co-express both HAI-1 and HAI-2 at high levels. In contrast to the situation in epithelial/carcinoma cells, some matriptase-expressing neoplastic B cells express very low or undetectable levels of HAI-1.26, 27, 28 While these neoplastic B cells do express HAI-2, observations made using several B-cell tumor lines show that, surprisingly, the levels of enzymatically active matriptase were not inversely correlated with the level of HAI-2 protein expression.26 This observation suggests that HAI-2 may not be an effective inhibitor of matriptase activity in this cellular context, even though HAI-2 is a highly potent matriptase inhibitor in solution. In the current study, we assess how HAI-1 and HAI-2 regulate matriptase expression, cell surface translocation, and the residual levels of matriptase enzymatic activity shed into the extracellular milieu from Daudi cells, which naturally express matriptase with negligible levels of HAI-2 and undetectable levels of HAI-1.
Materials and methods
Cell lines
The Burkitt Lymphoma cell line Daudi was obtained from the Tissue Culture Shared Resource (TCSR) in the Lombardi Comprehensive Cancer Center, Georgetown University (Washington, D.C.). The identity of the Daudi cell line was verified by short tandem repeat fingerprinting conducted by the TCSR, and the cells were regularly tested and shown to be free of mycoplasma contamination, also conducted by the TCSR. The cells were cultured in RPMI-1640 medium (Lonza, Walkersville, MD), supplemented with 10% fetal bovine serum (FBS) at 37 °C in a humidified atmosphere with 5% CO2.
Western blot and antibodies
The details of sample preparation have been previously described.26 In brief, the cells of equal number of approximate 5 × 105 were lysed with PBS containing 1% Triton, and 1 mM 5,5-dithio-bis-(2-nitrobenzoic acid) (DTNB), which was included in the lysis buffer to prevent the cleavage of disulfide linkages.29 Protein samples were resolved by 7.5% SDS-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose membrane, and subsequently probed with the indicated monoclonal antibodies (mAbs). Immunoreactive signals were visualized using horseradish peroxidase-labeled secondary antibodies and Western Lightning ECL pro reagent (PerkinElmer, Waltham, MA) exposed to x-ray film for variable times from 30 s to 60 min for the optimal signals. The following primary antibodies were used: the total matriptase mAb M24, the activated matriptase-specific mAb M69, the HAI-1 mAb M19, and the HAI-2 mAbs DC16 and XY9. The generation and characterization of these mAbs can be found in our previous studies.5,18,24,30
Acid-induced matriptase activation
The acid-induced matriptase zymogen activation method was initially developed and characterized using epithelial/carcinoma cells.31, 32, 33 Briefly, cells are incubated with either control buffer (PBS), or phosphate buffer (pH 6.0) for the indicated time, after which the cells and the conditioned buffer are separated and analyzed. In order to better assess the levels of active matriptase shed by neoplastic lymphocytes, a modified assay was used as previously described.26 The modifications included incubating a fixed number of cells relative to the volume of acidic buffer (5 × 105 cells to 10 μl of buffer), and the use of an incubation temperature of 37 °C for 10 min.
Matriptase amidolytic assay
The synthetic, fluorogenic substrate (N-t-Boc)-Gln-Ala-Arg-AMC was used to assess matriptase proteolytic activity. The reaction buffer with a final volume of 100 μl contained 20 mM Tris–HCl, pH 8.5 and the fluorogenic substrate at a final concentration of 10 μM. The hydrolysis of the peptide substrate was recorded every minute for a total of 30 or 40 min using a Wallac 1420 Victor 2 (PerkinElmer®) Microplate Reader, using an excitation wavelength of 355 nm and measuring emission at 460 nm.
Gelatin zymography
Samples of the pH 6 phosphate buffer removed from the cells (shed fraction) were mixed with 5X sodium dodecyl sulfate (SDS) sample buffer containing no reducing reagent and incubated at room temperature for 5 min. The proteins were resolved by SDS-PAGE on polyacrylamide gels containing 1 mg/ml gelatin, as previously described.34 The gelatin gels were washed with PBS containing 2.5% Triton X-100 to remove the SDS and then incubated in pH 8.5 Tris buffer at 37 °C overnight. The gels were stained with Coomassie Brilliant Blue R250 at room temperature for 1 h and then destained using 10% isopropanol and 10% acetic acid.
Doxycycline-inducible expression of HAI-1 and HAI-2
The Lenti-X tet-on 3G inducible expression system was obtained from Clontech Laboratories, Inc. (Mountain View, CA), and HAI-1 and HAI-2 expression constructs were prepared. Briefly, cDNAs containing the open reading frame of the HAI-1 or HAI-2 genes (Spint-1 and Spint-2) were generated by PCR amplification and ligated into the pLVX-TER-IRES expression vector, with the puromycin-resistant marker. The constructs were validated by DNA sequencing and packaged, as described previously.28 The lentiviral expression constructs were then used to infect Daudi cells, which were previously transduced and selected for the stable expression of the Tet-On 3G tetracycline-dependent transactivator protein. Transduced cells were selected in the presence of 2 μg/ml puromycin, and stable pools of resistant cells were established. The cell pools derived from this dual selection process were then cultured with different amounts of the tetracycline analogue doxycycline (0.01, 0.05, 0.25, 1, and 5 μg/ml for 24 h) to induce different levels of HAI-1 or HAI-2 expression.
The ratio of total to cell surface matriptase
The ratio of total to cell surface matriptase was determined by the biotinylation of the extracellular surface proteins of viable cells, followed by depleting the biotinylated matriptase from whole-cell lysates and comparison of the level of matriptase in the lysates before and after depletion. Five million Daudi cells were washed with PBS three times, after which the cells were incubated with 0.2 mg/ml sulfosuccinimidyl biotin (sulfo-NHS-biotin) dissolved in PBS pH 8.0 at 4 °C for 30 min, to label cell surface proteins, including cell surface matriptase. The residual biotinylation reagent was quenched and removed by washing the cells three times with 0.16 M glycine in PBS. The cells were then lysed in RIPA buffer, and the biotinylated cell surface proteins were removed by incubating the lysates with Avidin-Agarose beads mixed end-over-end in a cold room for 2 h. The levels of total and intracellular matriptase were determined by immunoblot analysis of the cell lysate before and after depletion of the cell surface proteins. The x-ray films from the immunoblots were scanned, and band intensity determined using Image J. The ratio of cell surface matriptase was calculated by the band intensity.
Results
In order to characterize and compare the roles of HAI-1 versus HAI-2 in matriptase protein expression, translocation to the cell surface, and the shedding of enzymatically active matriptase into the extracellular milieu, we made use of Daudi neoplastic B-cells as a model system. Several interesting characteristics of Daudi cells make this Burkitt lymphoma line a suitable model to sort out the complicated functional relationship between matriptase and the HAIs. The cells express no detectable HAI-1 and only very low levels of HAI-2 (less than 0.01% of the level of matriptase expression, as previously shown26 and so they provide an excellent environment to test the effects of increased HAI expression on matriptase biology. Furthermore, it has been demonstrated that co-expression of HAIs is required for normal matriptase synthesis and trafficking out of the endoplasmic reticulum (ER) and the Golgi apparatus.11,35 It is not clear, however, what is the minimal level of HAI expression that is required to support “normal” matriptase synthesis and trafficking, both of which are important parameters affecting the levels of active matriptase shed into the extracellular milieu. The very low level of HAI-2 expression in these cells likely results in sub-optimal support for matriptase protein synthesis and cell surface translocation and so these cells are an ideal test-bed to evaluate the impact of increased expression of either HAI-1 or HAI-2 on synthesis and trafficking.
Increased HAI-1 expression effectively suppresses the level of shed active matriptase
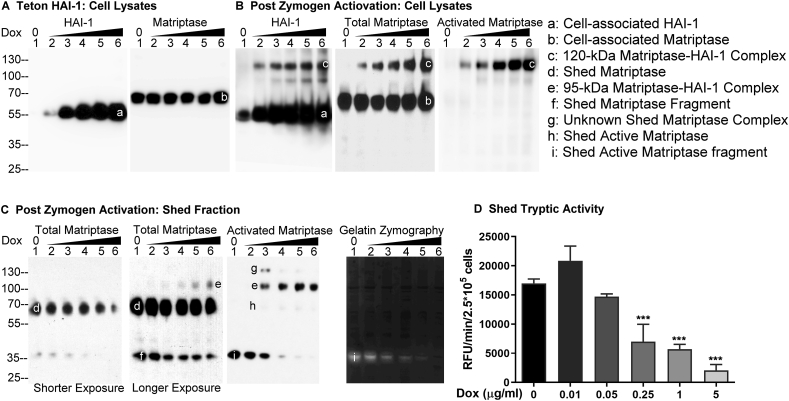
In order to assess the impact of increased HAI-1 expression on matriptase zymogen activation and the shedding of matriptase proteolytic activity, Daudi cells were engineered to express HAI-1 in a doxycycline-dependent manner, as described in the Methods section. Treating these cells with increasing concentrations of doxycycline resulted in the expression of increasing levels of HAI-1 (Fig. 1A, HAI-1, band a). Given the potential impact of HAI expression on matriptase synthesis, the same lysates were next probed to assess matriptase protein levels. Matriptase protein expression levels were unchanged by treating the engineered cells with different amounts of doxycycline (Fig. 1A, Matriptase, band b). To investigate the impact of increasing HAI-1 expression on matriptase zymogen activation and the shedding of matriptase proteolytic activity, an equal number (5 × 105) of these cells were transiently exposed to a pH 6.0 buffer to induce matriptase zymogen activation31,32 (Fig. 1B, C). The conditioned buffers were collected and concentrated to a volume equivalent to that of the cell lysates. The cell lysates at the same volume were analyzed by immunoblot for the species of matriptase and HAI-1 (Fig. 1B). The conditioned buffers at the same volume were analyzed for matriptase species by Western blot (Fig. 1C), the gelatinolytic activity by gelatin zymography (Fig. 1C), and the amidolytic activity using a synthetic fluorogenic substrate (Fig. 1D). Following the induction of robust matriptase zymogen activation, HAI-1 can be seen to rapidly inhibit the active matriptase by forming a stable 120-kDa complex (Fig. 1 B, band c), the identity of which was confirmed by using three mAbs: the HAI-1 mAb M19, the (total) matriptase mAb M24, and the activated matriptase mAb M69. It is worth noting that the M69 mAb is specific for the activated form of the enzyme and so does not interact with matriptase zymogen (mass approximately 70-kDa). As HAI-1 expression levels increased in cells treated with the higher concentrations of doxycycline, the level of cell-associated 120-kDa matriptase-HAI-1 complex increased in parallel (Fig. 1B, band c). As HAI-1 expression and cell-associated matriptase-HAI-1 complex increased, the total level of shed matriptase species, including the 70-kDa form and the 35-kDa degraded matriptase fragment (Fig. 1C, bands d and f, respectively), did not appear to be significantly changed, except for when the highest HAI-1 expression was induced (Fig. 1C, Total Matriptase, Shorter Exposure, lane 6, band d). The decrease in matriptase shedding in response to very high levels of HAI-1 expression echoed a corresponding accumulation of cell-associated matriptase (Fig. 1B, Total Matriptase, lane 6, band b). A relatively constant level of shed total matriptase was also observed for the shedding of “total” levels of activated matriptase, as represented by a combination of the 95-kDa matriptase-HAI-1 complex and enzymatically active matriptase (Fig. 1C, Activated Matriptase, bands e + h + i). The 95-kDa matriptase-HAI-1 complex was also detected by the total matriptase mAb (Fig. 1C, band e) and the HAI-1 mAb (data not shown), which represents a complex comprised of activated matriptase bound to a 40-kDa HAI-1 fragment.24,34,36 An opposite trend was, however, observed for the two activated matriptase species with a steady increase in the level of the 95-kDa activated matriptase-HAI-1 complex (Fig. 1C, Total Matriptase and Activated Matriptase, bands e) and a steady decrease in the level of enzymatically active matriptase (Fig. 1C, Activated Matriptase, bands i). Collectively, the increase in HAI-1 expression appears to result in an increase in the inhibition of active matriptase by the formation of the enzyme–inhibitor complexes, and so, as a consequence, the level of enzymatically active matriptase decreases in proportion. It is worth noting that the shed active matriptase was detected primarily in the form of a 35-kDa degradation product, which can be detected by not only the activated matriptase mAb (Fig. 1C, Activated matriptase, band i) but also the total matriptase mAb (Fig. 1C, Total Matriptase, band f) in Western blot analysis. More importantly, the proteolytic activity of the 35-kDa, degraded active matriptase was verified by the gelatinolytic activity with the corresponding sizes and in the same decreasing trend along with the increase in HAI-1 expression (Fig. 1C, Gelatin Zymography, band i). Although many attempts have been made to prevent the degradation of active matriptase from the 70-kDa form into the 35-kDa fragment, the proteolytic degradation still occurred. This degradation appears to be context dependent because enzymatically active matriptase has been detected as an intact 70-kDa form in our previous studies.28,30,34,37
Figure 1.
Increasing HAI-1 expression results in increasing suppression of the shedding of active matriptase. (A) Daudi cells were engineered to express HAI-1 in a doxycycline-regulated manner (Teton HAI-1). The cells were treated with increasing concentrations of doxycycline. Lysates prepared from the same number of cells were then analyzed by Western blot for HAI-1 (left panel) and matriptase (right panel) levels. (B–D) Teton HAI-1 cells were induced to express increasing levels of HAI-1 by treating the cells with increasing concentrations of doxycycline, after which the cells in equal numbers were induced to activate matriptase by acid buffer exposure. The cell lysates (B) and the conditioned buffer (Shed Fraction) (C) were collected and adjusted to the same volumes. An equal volume of the cell lysates (B) and the shed fraction (C) were analyzed by Western blot for HAI-1 species (B), total matriptase (B) and (C), and activated matriptase (B) and (C), and by gelatin zymography for the gelatinolytic activity (C), and by the amidolytic activity assay using a fluorogenic peptide substrate (D). The matriptase and HAI-1 species, including cell-associated HAI-1 (band a), cell-associated matriptase (band b), the 120-kDa matriptase-HAI-1 complex (band c), shed matriptase (band d), shed 95-kDa matriptase-HAI-1 complex (band e), degraded matriptase fragment (band f), as yet unidentified matriptase complex (band g), shed active matriptase (band h), and shed active matriptase fragment (band i) are indicated. Shorter and longer exposures are also indicated. These experiments to study the impact of HAI-1 expression on the shedding of active matriptase were performed at least three times, and representative data are presented. ns: nonsignificant difference; ∗∗∗: P < 0.001.
Based on the absence of corresponding 70-kDa gelatinolytic activity in the gelatin zymogram and negligible 70-kDa active matriptase band detected in Western blot using the activated matriptase mAb (Fig. 1C, band h), it appears that the vast majority of the shed 70-kDa matriptase band detected by the total matriptase mAb was matriptase zymogen (Fig. 1C, band d). In contrast, the shed 35-kDa matriptase fragment (Fig. 1C, band f) should contain shed active matriptase fragment in much higher proportion, based on the levels of the 35-kDa active matriptase fragment by Western blot analysis and the gelatinolytic activity (Fig. 1C, bands i). Because the ratio of the 95-kDa matriptase-HAI-1 complex to the shed matriptase zymogen was very low, the 95-kDa band can be visualized by the total matriptase mAb only after longer exposure (Fig. 1C, Total Matriptase, Longer Exposure, band e). In contrast, the activated matriptase mAb can detect the 95-kDa matriptase-HAI-1 complex in the absence of the interference of the 70-kDa matriptase zymogen (Fig. 1C, Activated Matriptase, band e). As the level of the 95-kDa complex increased with increased HAI-1 expression (Fig. 1C, band e), the level of the 35-kDa active matriptase fragment decreased (Fig. 1C, band f). This inverse relationship was particularly clear in the activated matriptase specific mAb M69 immunoblot (Fig. 1C, activated matriptase, bands e and i).
An activated matriptase containing complex with a molecular weight higher than the 95-kDa complex was also detected by the activated matriptase mAb (Fig. 1C, band g). The identity of this complex remains unclear, but it is interesting to note that its level decreased significantly as HAI-1 levels began to increase significantly.
A decrease in the amount of free enzymatically active matriptase shed from the cells as HAI-1 levels increased was also confirmed using a more quantitative assay for matriptase proteolytic activity (Fig. 1D), and a decreasing trend was also confirmed using the less quantitative assays of immunoblot and gelatin zymography. Matriptase tryptic activity shed from the same number of cells (2.5 × 105) decreased in parallel with increased HAI-1 expression and with the decreased 35-kDa gelatinolytic activity (Fig. 1C, Gelatin Zymography, band i) and the decreased level of the 35-kDa active matriptase fragment (Fig. 1C, Activated Matriptase, band i). It is worth noting that while there is no completely specific fluorogenic substrate for matriptase proteolytic activity, the proteolytic activity shed from these cells can be shown to predominantly be the result of matriptase enzymatic activity by specific immunodepletion of activated matriptase from the conditioned medium. Such immunodepletion using the mAb M69 linked to Sepharose results in the almost complete abrogation of tryptic activity.26,28
Increasing HAI-2 expression does not efficiently suppress the level of shed active matriptase
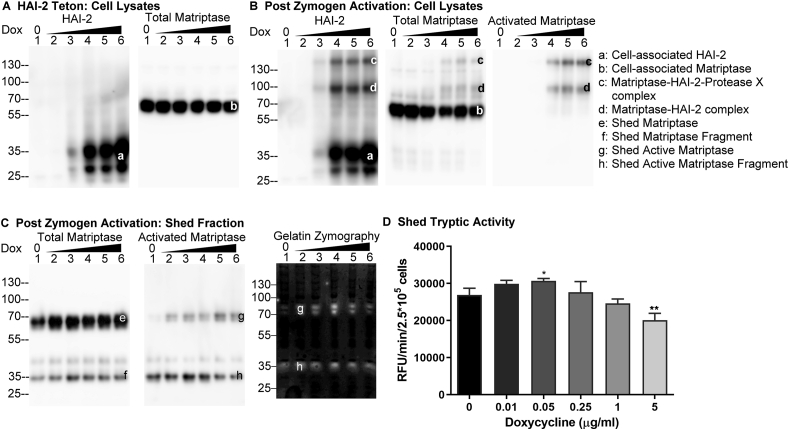
In order to further evaluate the ability of HAI-2 (or lack thereof) to control extracellular matriptase activity, Daudi cells were engineered for the inducible overexpression of HAI-2 (Fig. 2A HAI-2 Teton). Treating these cells with increasing concentrations of doxycycline resulted in the expression of increasing levels of HAI-2 (Fig. 2A, HAI-2, bands a). HAI-2 was detected as one major and one minor band likely due to the different degree of N-glycosylation, given that HAI-2 is synthesized with variable sizes of N-glycans.5,18 The level of matriptase protein expression was not affected by the treatment with different amounts of doxycycline (Fig. 2A, Total Matriptase, band b). Following induction of robust matriptase zymogen activation, increasing the expression of HAI-2 led to an increase in the amount activated matriptase-HAI-2 complexes (Fig. 2B, bands c and d), which could be detected in cell lysates by the three respective mAbs for HAI-2 - mAb DC16, total matriptase - mAb M24, and activated matriptase - mAb M69. Two different matriptase-HAI-2 complexes have been detected: the 100-kDa complex, which contains activated matriptase and HAI-2 in 1:1 stoichiometry (Fig. 2B, band d) and the 130-kDa complex (Fig. 2B, band c), in which an unidentified protease X has been proposed to bind to the Kunitz domain 2 of HAI-2 and activated matriptase bound to the Kunitz domain 1 in a 1:1:1 stoichiometry. The two HAI-2 complexes were initially detected and characterized in breast cancer cells.18 Activated matriptase-HAI-2 complexes began to be detectable in response to the appearance of low levels of HAI-2 (Fig. 2B, lanes 3, bands c and d). The appearance of matriptase-HAI-2 complexes resulted in a corresponding reduction in the levels of 70-kDa matriptase zymogen in the lysates (Fig. 2B, lanes 4 and 5, band b). The level of the matriptase-HAI-2 complexes present in the lysate soon reached a plateau (Fig. 2B, lanes 4, 5, and 6, bands c and d). The increasing levels of HAI-2 expression did, therefore, not result in a corresponding increase in the level of activated matriptase-HAI-2 complexes (Fig. 2B, comparing lanes 6 with lanes 5, bands c and d), suggesting that cell-associated activated matriptase was limited to a certain level. At least two possibilities could explain the relatively constant levels of cell-associated activated matriptase-HAI-2 complexes: 1) matriptase zymogen activation reaches a plateau when HAI-2 is expressed beyond certain levels or 2) the access of HAI-2 to the active matriptase is limited in some way. The observation that similar levels of shed total matriptase (Fig. 2C, bands e + f), activated matriptase (Fig. 2C, bands g + h), and matriptase gelatinolytic activity (Fig. 2C, Gelatin Zymography, bands g + h) were found in the conditioned buffer over a wide range of HAI-2 expression levels is consistent with the latter possibility. In contrast to the situation with HAI-1, there was no shed activated matriptase-HAI-2 complex (Fig. 2C). Using the more quantitative enzymatic assay for matriptase proteolytic activity, it can be seen that there was a slight increase in shed proteolytic activity along with the increasing doxycycline concentration up to 0.05 μg/ml in parallel with the increasing HAI-2 expression although the increase is not statistically significant (Fig. 2D). While the shed matriptase tryptic activity did decrease slightly (Fig. 2D) with the induction of high levels of HAI-2 expression (Fig. 2A, HAI-2, lanes 4, 5, and 6), the reduction was not proportional to the increase in HAI-2 expression (Figs. 2D, 0.25 and 1, and 5 μg/ml Dox). Thus, HAI-2 is generally ineffective in the control of matriptase extracellular proteolytic activity in these systems and paradoxically may promote matriptase function.
Figure 2.
Increasing HAI-2 expression only results in modest suppression of active matriptase shedding. (A) Daudi cells were engineered to express HAI-2 in a doxycycline-regulated manner (Teton HAI-2). Teton HAI-2 cells were treated with increasing amounts of doxycycline. Lysates from an equal number of cells were then analyzed by Western blot for HAI-2 (left panel) and total matriptase (right panel) levels. (B–D) Teton HAI-2 cells were treated with increasing doxycycline concentrations to induce the expression of increasing levels of HAI-2, after which an equal number of these cells were induced to activate matriptase by acid buffer exposure. The cell lysate and the conditioned buffer (shed fraction) were collected and adjusted to the same volume. An equal volume of the cell lysates (B) and the shed fraction (C) were analyzed by Western blot for HAI-2 species (B), total matriptase (B) and (C), and activated matriptase (B and C), and by gelatin zymography for matriptase gelatinolytic activity (C), and by the amidolytic activity assay using a fluorogenic peptide substrate (D). The HAI-2 and matriptase species, including cell-associated HAI-2 (band a), cell-associated matriptase (band b), matriptase-HAI-2-protease X complex (band c), matriptase-HAI-2 complex (band d), shed matriptase (band e), shed matriptase fragment (band f), shed active matriptase (band g), and shed active matriptase fragment (band h) are indicated. These experiments to evaluate the impact of HAI-2 expression on the shedding of active matriptase were performed at least three times, and representative data are presented. ns: non-significant difference; ∗∗: P < 0.01.
Low levels of HAI expression promote active matriptase shedding by increasing matriptase expression
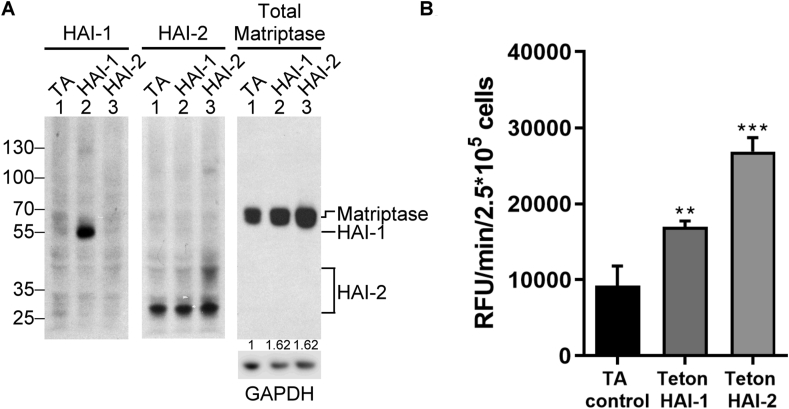
While HAI-1 and HAI-2 show differential effectiveness in preventing active matriptase shedding when expressed at high levels, the two Kunitz protease inhibitors appear to resemble one another with regard to their ability to promote active matriptase shedding when expressed at very low levels. For example, slightly increased active matriptase was detected in the conditioned buffer from HAI-2 Teton Daudi cells treated with 0.01 and 0.05 μg/ml doxycycline compared to those cells treated with no doxycycline (Fig. 2C, D). Similar data were observed with the HAI-1 Teton Daudi cells (Fig. 1C, D). Interestingly the biggest increase in active matriptase shedding was observed when comparing the HAI-1 Teton and HAI-2 Teton cells with the TA control Daudi cells, all grown in the absence of doxycycline (Fig. 3). It is not uncommon for Tet-regulated expression systems to show some “leakiness,” allowing low-level expression in the absence of doxycycline treatment. When large amounts of lysates prepared from 106 cells were analyzed using long X-ray film exposures more than 1 h for the Western blot analysis (as indicated by the minor non-specific bands), very low levels of endogenous HAI-2 could be observed in the TA control cells (Fig. 3A, HAI-2, lane 1), but even under these conditions, no HAI-1 was detected (Fig. 3A, HAI-1, lane 1). More importantly, however, low levels of HAI-1 or HAI-2 were expressed in the HAI-1 Teton cells and the HAI-2 Teton cells, respectively, compared to either the TA control cells or the cells transfected with the other Teton HAI construct (Fig. 3A, HAI-1, comparing lane 2 with lanes 1 and 3; HAI-2, comparing lane 3 with lanes 1 and 2). It is worth noting that the majority of the endogenous HAI-2 in Daudi cells is the species without extensive N-glycan branching. Furthermore, the HAI-2 species with extensive N-glycan branching is synthesized to very low in the absence of doxycycline (Fig. 3), and very high levels are induced by doxycycline in the Teton HAI-2 cells (Fig. 2). The HAI-2 species with extensive N-glycan branching represents the mature and major form of endogenous HAI-2 in the vast majority cell lines examined so far.5 When matriptase protein expression was analyzed and compared (Fig. 3A, Matriptase), the presence of “leakage”-level expression of HAI-1 and HAI-2 promoted matriptase protein expression (Fig. 3A, Matriptase, compare lane 2 or 3 with lane 1). We next examined the effect of acid-induced matriptase activation on the three transfected cell lines and compared the level of shed tryptic activity (Fig. 3B). Along with the slight increase in matriptase protein levels caused by “leakage”-level HAI expression, after acid exposure, a significantly higher level of active matriptase was shed into the conditioned buffer in the HAI-1 Teton and HAI-2 Teton cells, compared with the TA control cells (Fig. 3B).
Figure 3.
Matriptase expression and the subsequent shedding of active matriptase can be enhanced by the expression of very low levels of HAI-1 or HAI-2 in Daudi cells. (A) Daudi cells transduced with the tetracycline transactivator protein alone (TA, lanes 1), Teton HAI-1 cells (HAI-1, lanes 2), and Teton HAI-2 cells (HAI-2, lanes 3) were grown in the absence of doxycycline. The cell lysates prepared from an equal number of cells were analyzed by immunoblot assay for HAI-1, HAI-2, and matriptase, as indicated. GAPDH was used as a loading control and to estimate the ratios of matriptase expression in Teton HAI-1 cells and Teton HAI-2 cells relative to the TA control cells. The ratios are indicated. (B) These cells at the same number were induced to activate matriptase by transiently exposing the cells to a pH 6.0 buffer. The conditioned buffers were collected and assayed for matriptase proteolytic activity using the fluorogenic substrate. The data presented are representative of those obtained in more than three independent experiments. ∗∗: P < 0.01; ∗∗∗: P < 0.001.
In addition to increasing matriptase expression, HAI-1 and HAI-2 have also been shown to facilitate matriptase trafficking out of the Golgi/ER system.11,35 The role of the HAIs in promoting matriptase trafficking may also promote the shedding of enzymatically active matriptase. As shown in Figure 3, the very low level of HAI expression due to the “leakiness” of the Teton system increased matriptase protein expression by around 60% compared to that in TA control cells (Fig. 3A, Matriptase). The very low level of HAI-1 expression increased the shed matriptase tryptic activity by approximately 80% and the HAI-2 expression by more than 190% (Fig. 3B). This disproportional increase in the shed active matriptase could be, in part, attributed to increased cell surface translocation of matriptase by the very low level of HAI expression. Furthermore, the much greater increase in the shed matriptase tryptic activity in the Teton HAI-2 cells compared to the Teton HAI-1 cells is likely due to their differential subcellular distribution with HAI-1 primarily on the cell surface and HAI-2 primarily inside the cells, assuming the subcellular distribution of the HAIs in neoplastic B-cells is similar to that in epithelial/carcinoma cells.
HAI-1 and HAI-2 enhance matriptase translocation to the cell surface, even when expressed at very low levels
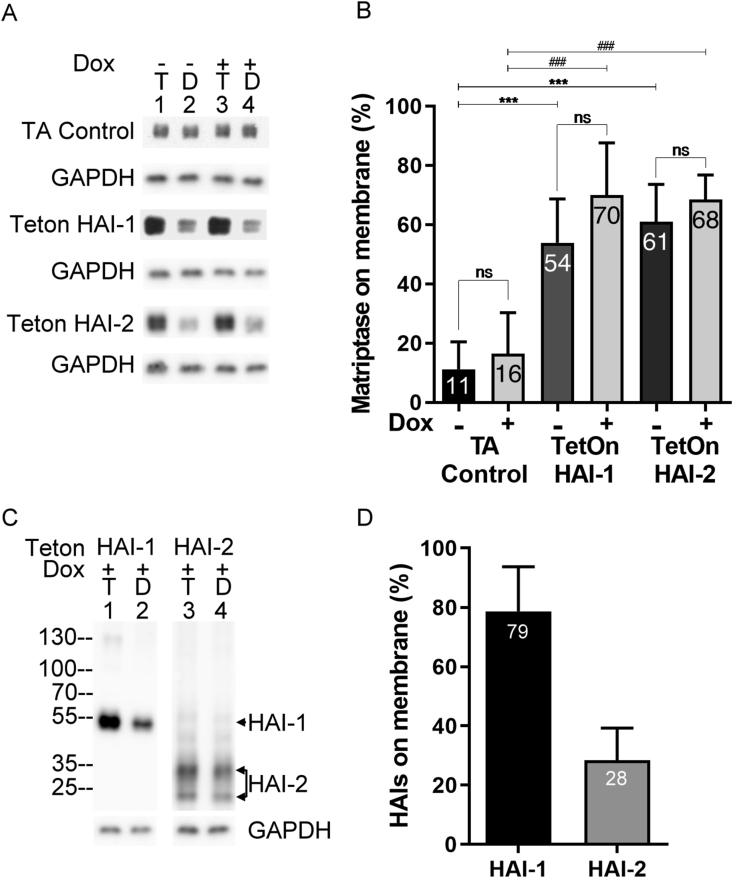
Much of what is known about the subcellular distribution of these three proteins has been determined in epithelial/carcinoma cells by immunohistochemical and immunocytochemical studies, both of which methods are semi-quantitative. We, therefore, set out to determine the subcellular distribution of HAI-1 and HAI-2, and the impact of their expression on matriptase subcellular distribution in neoplastic B-cells, using a more quantitative method: assessing the ratios of the levels of the three proteins on the cell surface relative to their total expression level (Fig. 4). Cell surface proteins, including matriptase, HAI-1, and HAI-2, were labeled with biotin in live cells using a cell impermeable surface biotinylation reagent. After quenching and removing the remaining biotinylation reagent, the cells were lysed and cell surface proteins removed from the lysate using avidin-agarose beads, which included any cell surface matriptase, HAI-1, and HAI-2. The levels of total and intracellular proteins were determined by immunoblot analysis of the lysates before and after avidin bead based-depletion, allowing the percentage of matriptase on the cell surface to be estimated.
Figure 4.
HAI-1 and HAI-2 both promote matriptase plasma membrane translocation despite their different subcellular distributions. Teton HAI-1 cells, Teton HAI-2 cells, and the TA control were grown in the absence (Dox -) or presence (Dox +) of 1ug/ml doxycycline for 24 h. These cells were then subjected to biotinylation of cell surface proteins, followed by lysis of the cells and the depletion of biotinylated cell surface proteins from the lysates using avidin-agarose beads. The lysates prior to (lanes 1 and 3, T) and after (lane 2 and 4, D) depletion of biotinylated cell surface proteins were analyzed by immunoblot for matriptase (A), HAI-1 (C, left panel), HAI-2 (C, right panel), and GAPDH as a loading control. The ratios of cell surface matriptase, HAI-1, and HAI-2 relative to their respective total expression levels are presented in panels (B) and (D). These data are representative of more than 3 independent experiments. ns: non-significant difference; ∗: P-value of comparison without Dox treatment; ∗∗∗: P < 0.001; #: P-value of comparison with Dox treatment; #: P-value < 0.001. The protein bands of HAI-1 and HAI-2 were indicated.
Using this method, we set to compare the subcellular distribution of matriptase in the TA control cells, the Teton HAI-1 cells, and the Teton HAI-2 cells in the absence and presence of Dox (Fig. 4A, B). Matriptase protein expression levels remained similar regardless of the presence of Dox in each of the three cell lines (Fig. 4A, comparing lanes 1 and lanes 3), consistent with that observed in Figure 1, Figure 2. We also observed higher matriptase protein levels in both the Teton HAI-1 and HAI-2 cells than in the TA control cells, which was also seen in Fig. 3A). When the cell surface biotinylated matriptase was removed from the TA control cells lysates using the Avidin beads, the residual intracellular matriptase level remained close to that prior to the depletion of biotinylated matriptase (Fig. 4A, TA Control, comparing lane 2 with lane 1; lane 4 with lane 3). The ratio of cell surface matriptase to total matriptase in the TA control cells was estimated to be less than 20%, and the presence of Dox did not significantly change this ratio (Fig. 4B, TA Control). In contrast, only a small amount of matriptase remained in the Teton HAI-1 and Teton HAI-2 cell lysates after the depletion of cell surface biotinylated matriptase (Fig. 4A, Teton HAI-1 and Teton HAI-2, comparing lanes 2 with lanes 1). Similar results were also observed when expressions of the HAIs were induced by doxycycline (Fig. 4A, Teton HAI-1 and Teton HAI-2, comparing lanes 4 with lanes 3). The ratios of cell surface to total matriptase in both Teton HAI cell lines were estimated to range between 50 and 60%, regardless of the presence of doxycycline (Fig. 4B, Teton HAI-1 and Teton HAI-2). These data suggest that HAI-1 and HAI-2, even when expressed at very low levels, can promote matriptase translocation to the cell surface.
The same approach was also used to examine subcellular distribution of HAI-1 and HAI-2 in neoplastic B-cells (Fig. 4C, D) and showed that while more than 70% of the HAI-1 was detected on the cell surface, only about 20% of the HAI-2 was present on the cell surface. In spite of the presence of a hydrophobic stretch in the C-terminal sequences of both HAI-1 and HAI-2, and both proteins being considered to be integral membrane proteins, these data suggest that the majority of the HAI-2 remains inside the cells under steady-state conditions. In contrast, the majority of HAI-1 is translocated to the cell surface of neoplastic B-cells, a similar situation to what is seen in epithelial/carcinoma cells. In addition to the monomer forms of the HAIs, a 120-kDa HAI-1 complex (Fig. 4C, HAI-1, lane 1) and HAI-2 complexes with sizes between 40 and 60-kDa (Fig. 4C, HAI-2, lane 1) were also seen in the two Teton cells. The appearance of these complexes indicate that matriptase zymogen activation, formation of enzyme–inhibitor complexes, and proteolytic degradation occurred during the process of surface biotinylation. Similar to their respective monomers, the HAI-1 complex was largely depleted and the HAI-2 complexes were largely not.
Taking Figure 3, Figure 4 together, matriptase protein expression caused by the “leakiness” increases by approximately 60% in both Teton HAI-1 and Teton HAI-2 cells (from 1 in TA control to 1.62 in Teton HAI-1 or HAI-2). The increase in matriptase protein expression is attributed to the chaperone activity of the HAIs. The increase in the level of shed matriptase proteolytic activity is much greater than the increase in matriptase protein: approximate 1.8-fold in Teton HAI-1 and 2.9-fold in Teton HAI-2 cells (Fig. 3B). The chaperone activity of the HAIs also promotes matriptase translocation to the cell surface (Fig. 4). Because the proportion of cell surface-matriptase rises from 10% in TA control to slightly more than 50% in Teton HAI-1 cells and Teton HAI-2 cells (Fig. 4, in the absence of doxycycline), one might expect that the level of cell surface-matriptase on the Teton HAI cells would be 8 fold higher than the TA control cells (1.6∗0.5/1∗0.1 = 0.8/0.1 = 8). Following the induction of matriptase zymogen activation, a proportion of cell surface-matriptase is, however, converted into active matriptase, the majority of which will be inhibited by HAI-1 in the Teton HAI-1 cells. As a result, the shed active matriptase activity in Teton HAI-1 cells (16,985 RFU/min/2.5∗105) is approximately 1.8-fold higher than for the TA control cells (9218 RFU/min/2.5∗105). A greater increase (2.9-fold) in the shed active matriptase proteolytic activity in the Teton HAI-2 cells (26,816 RFU/min/2.5∗105) is due to the fact that only a small proportion (30%) of HAI-2 is translocated to cell surface (Fig. 4D). The higher level of active matriptase shed from the Teton HAI-2 cells compared to the Teton HAI-1 cells is consistent with and likely due to the differential subcellular distribution of the HAIs. This is a case of the “butterfly effect”, in which trace amount of doxycycline in fetal bovine serum causes a slight increase in HAI expression, which subsequently causes a modest increase in protein expression and significant cell surface translocation of matriptase, leading to a high level of shed active matriptase.
Discussion
Our previous study revealed that HAI-2 represents the predominant inhibitor/regulator for matriptase for some hematological cancers, some of which either do not express HAI-1 or express it at extremely low levels: particularly the Burkitt lymphoma subtype.26 HAI-2 expression levels in hematological cancer are quite variable with HAI-2:matriptase protein molar ratios ranging from approximately 2 to less than 0.01, and it is likely that HAI-2 relatively ineffective with respect to the suppression of matriptase proteolytic activity. This conclusion that HAI-2 is not an effective matriptase inhibitor in this context is based on a study of seven different neoplastic B-cell lines that exhibit a wide range of endogenous HAI-2 expression levels, for which there was no correlation between the high-level HAI-2 expression and the level of active matriptase generated and shed.26 In the current study, through the use of a controllable model system using one cell line, we demonstrated that the levels of residual active matriptase are not be suppressed in proportion to the increasing level of HAI-2 (and therefore the ratio of HAI-2:matriptase) (Fig. 2). This conclusion is consistent with and supportive of our previous observation among different neoplastic B-cell lines with endogenous matriptase and HAI-2. The ineffectiveness of HAI-2 is in stark contrast to the proportional reduction in the levels of residual active matriptase observed with increasing levels of HAI-1 protein expression (Fig. 1). The effective control of matriptase activity by HAI-1 follows the conventional relationship between a given protease and its cognate protease inhibitor. Thus, although HAI-2, like HAI-1, is a specific and potent enzymatic inhibitor against matriptase in solution, HAI-2 appears to be unlike HAI-1, which is an effective matriptase inhibitor in cells. While the HAIs are different from one another concerning their effectiveness in governing matriptase enzymatic activity in cells, the HAIs resemble one another in their ability to promote matriptase protein expression and translocation to the plasma membrane (Figure 3, Figure 4). Matriptase, like other serine proteases, is synthesized as a zymogen with only limited intrinsic zymogen activity due to the lack of a well-formed substrate binding pocket.36,38, 39, 40 The limited intrinsic zymogen activity is too weak for meaningful proteolytic processing of the substrates activated by the mature active serine proteases. Matriptase zymogen cannot form stable complexes with its canonical protease inhibitors HAI-1 and HAI-2 due to unfavorably kinetics. This low-level intrinsic zymogen activity does, however, confer on matriptase the unusual ability to undergo autoactivation in order to acquire its full enzymatic activity.41 The enzymatically active matriptase with a well-formed substrate binding pocket can then act on substrates and form very stable complexes with the HAIs.
When anchored on lipid bilayer biomembranes, matriptase autoactivation can occur spontaneously at a physiological pH, and the rate of autoactivation can be enhanced by reducing the pH.31,32,34,42 Matriptase autoactivation can also be enhanced by the oxidative potential in living cells.33 The secretory pathway is characterized by a decreasing pH gradient toward the plasma membrane and with different redox environments within different elements along the pathway. Low level, largely undetectable, premature matriptase zymogen activation probably occurs during the synthesis, posttranslational modification, and trafficking through the secretory pathway. Suppression of the resultant undesired proteolytic activity is probably important to maintain the normal function of the secretory pathway and to allow matriptase synthesis and translocation to the plasma membrane to occur. Co-expression with HAI-1 and/or HAI-2 apparently fulfills this need in matriptase-expressing cells by suppressing the undesired matriptase proteolysis in the secretory pathway. This hypothesis has been supported by observations of the very limited expression of matriptase protein, primarily trapped in the endoplasmic reticulum and the Golgi apparatus (ER/Golgi) with some secretion of enzymatically active protein when matriptase is expressed in cells in the absence of either of the HAIs.11,12,35,41,43 When co-expressed with the HAIs, matriptase is easily expressed at high levels and traffics out of the ER/Golgi. Furthermore, consistent with this hypothesis are the two findings 1) that matriptase variants with mutations at the active site triads can be expressed at normal levels in cells in the absence of the HAIs, and 2) that a HAI-1 variant with a mutation at Arg-260 in the reactive loop as the P1 site loses its ability to chaperone matriptase in the secretory pathway. Only very low levels of the HAIs are required to support normal matriptase synthesis and intracellular trafficking (Figure 3, Figure 4). This type of function is in contrast to the anti-protease activity of the HAIs, which appear to require cells to express stoichiometrically high levels of the HAIs relative to matriptase in order to effectively regulate matriptase proteolytic activity (Figure 1, Figure 2). Among the seven matriptase-expressing neoplastic B-cells examined in our previous work,26 the level of HAI-2 in Daudi cells is the second-lowest with HAI-2:matriptase ratio less than 0.01. Interestingly, a significant proportion of matriptase protein was detected intracellularly in Daudi cells. The increase in matriptase protein expression and translocation to the plasma membrane can be induced by only a slight increase in the expression of either HAI-1 or HAI-2 (Figure 3, Figure 4). Thus, the chaperone activity of the HAIs requires only very low levels of these proteins.
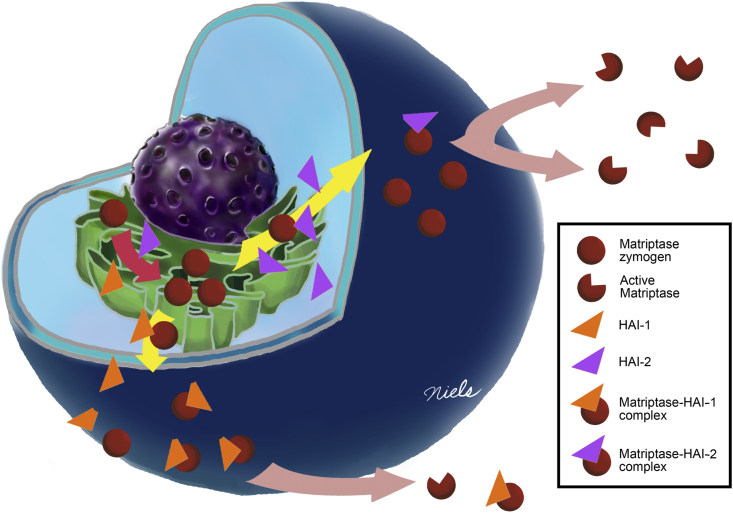
Once on the cell surface, matriptase zymogen can be converted into the active enzyme, which is rapidly shed into the extracellular milieu.44 Given the intrinsic ability of HAI-1 to translocate to the plasma membrane and the tendency of HAI-2 to remain largely inside the cells, the two Kunitz-type serine protease inhibitors exhibit distinct differences concerning the control of pericellular matriptase activity. The cell surface HAI-1 has direct access to the active matriptase both before and after it is shed. In contrast, the intracellular HAI-2 does not have ready access to the pericellular active matriptase. As a consequence, when HAI-1 is expressed at very high levels, pericellular active matriptase is largely inhibited. In contrast, even when HAI-2 is expressed at very high levels, only a small proportion of the HAI-2 is present on the cell surface, resulting in the ineffective inhibition of pericellular active matriptase. In neoplastic B-cells, co-expression of HAI-2 with matriptase could, paradoxically, promote rather than suppress matriptase function. The functional relationship between matriptase and HAIs is summarized in Figure 5.
Figure 5.
Schematic summery of the functional relationship between matriptase and its cognate inhibitors HAI-1 and HAI-2 in B-cell malignancies. The regulations of matriptase expression and plasma membrane translocation by HAI-1 versus HAI-2 are summarized schematically. In the presence of extremely low levels of the HAIs, matriptase can only be synthesized to relatively low levels, most of which remains inside the cells. With a modest increase in HAI expression, matriptase synthesis and translocation to the plasma membrane is significantly increased. The majority of the HAI-2 remains inside the cells in vesicle-like structures. As a consequence, HAI-2 can’t effectively control cell surface matriptase proteolysis. In contrast, the majority of HAI-1 is targeted to the plasma membrane, where HAI-1 can rapidly inhibit active matriptase by forming a stable protease–protease inhibitor complex. As a consequence, along with the increase in HAI-1 expression, the formation of activated matriptase-HAI-1 complex increases and the shedding of free active matriptase decreases proportionally.
In summary, HAI-1 and HAI-2 resemble one another with respect to their overall protein domain structure and the potency and specificity against serine proteases. The HAIs have been identified to be the primary endogenous protease inhibitors for the membrane-associated serine proteases matriptase and prostasin. In addition to their canonical protease inhibitory function, the HAIs are also important for normal synthesis and trafficking of matriptase out of the secretory pathway. In the current study, the chaperone-like activity for matriptase synthesis and translocation to the cell surface has been shown to require only a very low level of the HAIs. This activity is in stark contrast to the much higher levels of the HAIs required for the control of matriptase proteolytic activity. Due to the intrinsic tendency for intracellular retention, the majority of the HAI-2, however, has no access to the extracellular active matriptase, resulting in the relative ineffectiveness of HAI-2 in the control of matriptase proteolytic activity. In contrast, the co-localization of HAI-1 with matriptase on the cell surface makes HAI-1 more like a classic protease inhibitor. This differential subcellular distributions could render HAI-1 as a suppressor and HAI-2 as a conditional enhancer of matriptase.
Conflict of interests
CYL is an inventor on US patents #6,077,938 (Title: Monoclonal antibody to an 80-kDa protease) and #6,677,377 (Title: Structure based discovery of inhibitors of matriptase for the cancer diagnosis and therapy by detection and inhibition of matriptase activity) and MDJ and CYL are inventors on US patent #7,355,015 (Title: Matriptase, a serine protease and its applications).
Funding
This study was supported by Cancer Institute Grant R01 CA 123223 (to MDJ and CYL), Grant (MAB-108-079) from the Ministry of Defense Medical Affairs Bureau, and Grants (CMNDMC10705; CMNDMC10813) from Chi-Mei Medical Center (to J.-K. Wang), Grant (TSGH-E-109213-003) from Tri-Service General Hospital (to Y.-Y. Wu), and Grants (108-2311-B-016-001-; 109-2320-B-016-004-) from Ministry of Science and Technology (to Y.-L. Chiu).
Acknowledgements
We acknowledge the assistance provided by the Microscopy and Imaging Shared Resource, and the Tissue Culture Shared Resource, which are supported in part by the Lombardi Comprehensive Cancer Center support grant (NIH/NCI grant P30-CA051008) and a CTSA pilot project grant. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Khee-Siang Chan, Email: kheesiangchan@gmail.com.
Michael D. Johnson, Email: johnsom@georgetown.edu.
Chen-Yong Lin, Email: lincy@georgetown.edu.
References
- 1.Shimomura T., Denda K., Kitamura A., et al. Hepatocyte growth factor activator inhibitor, a novel Kunitz-type serine protease inhibitor. J Biol Chem. 1997;272(10):6370–6376. doi: 10.1074/jbc.272.10.6370. [DOI] [PubMed] [Google Scholar]
- 2.Kawaguchi T., Qin L., Shimomura T., et al. Purification and cloning of hepatocyte growth factor activator inhibitor type 2, a Kunitz-type serine protease inhibitor. J Biol Chem. 1997;272(44):27558–27564. doi: 10.1074/jbc.272.44.27558. [DOI] [PubMed] [Google Scholar]
- 3.Wlodawer A., Nachman J., Gilliland G.L., Gallagher W., Woodward C. Structure of form III crystals of bovine pancreatic trypsin inhibitor. J Mol Biol. 1987;198(3):469–480. doi: 10.1016/0022-2836(87)90294-4. [DOI] [PubMed] [Google Scholar]
- 4.Laskowski M., Jr., Kato I. Protein inhibitors of proteinases. Annu Rev Biochem. 1980;49:593–626. doi: 10.1146/annurev.bi.49.070180.003113. [DOI] [PubMed] [Google Scholar]
- 5.Lai Y.J., Chang H.H., Lai H., et al. N-glycan branching affects the subcellular distribution of and inhibition of matriptase by HAI-2/placental bikunin. PLoS One. 2015;10(7):e0132163. doi: 10.1371/journal.pone.0132163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai C.H., Lai Y.J., Chou F.P., et al. Matriptase complexes and prostasin complexes with HAI-1 and HAI-2 in human milk: significant proteolysis in lactation. PLoS One. 2016;11(4) doi: 10.1371/journal.pone.0152904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szabo R., Molinolo A., List K., Bugge T.H. Matriptase inhibition by hepatocyte growth factor activator inhibitor-1 is essential for placental development. Oncogene. 2007;26(11):1546–1556. doi: 10.1038/sj.onc.1209966. [DOI] [PubMed] [Google Scholar]
- 8.Szabo R., Kosa P., List K., Bugge T.H. Loss of matriptase suppression underlies Spint1 mutation-associated ichthyosis and postnatal lethality. Am J Pathol. 2009;174(6):2015–2022. doi: 10.2353/ajpath.2009.090053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szabo R., Hobson J.P., Christoph K., Kosa P., List K., Bugge T.H. Regulation of cell surface protease matriptase by HAI2 is essential for placental development, neural tube closure and embryonic survival in mice. Development. 2009;136(15):2653–2663. doi: 10.1242/dev.038430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathias J.R., Dodd M.E., Walters K.B., et al. Live imaging of chronic inflammation caused by mutation of zebrafish Hai 1. J Cell Sci. 2007;120(Pt 19):3372–3383. doi: 10.1242/jcs.009159. [DOI] [PubMed] [Google Scholar]
- 11.Oberst M.D., Chen L.Y., Kiyomiya K.I., et al. HAI-1 regulates activation and expression of matriptase, a membrane-bound serine protease. Am J Physiol Cell Physiol. 2005;289(2):C462–C470. doi: 10.1152/ajpcell.00076.2005. [DOI] [PubMed] [Google Scholar]
- 12.Larsen B.R., Steffensen S.D., Nielsen N.V., et al. Hepatocyte growth factor activator inhibitor-2 prevents shedding of matriptase. Exp Cell Res. 2013;319(6):918–929. doi: 10.1016/j.yexcr.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu M., Yuan C., Jensen J.K., et al. The crystal structure of a multidomain protease inhibitor (HAI-1) reveals the mechanism of its auto-inhibition. J Biol Chem. 2017;292(20):8412–8423. doi: 10.1074/jbc.M117.779256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong Z., De Meulemeester L., Jacobi A., et al. Crystal structure of a two-domain fragment of hepatocyte growth factor Activator inhibitor-1: functional interactions between the kunitz-type inhibitor domain-1 and the neighboring polycystic kidney disease-like domain. J Biol Chem. 2016;291(27):14340–14355. doi: 10.1074/jbc.M115.707240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong Z., Nowakowski M., Spronk C., et al. The solution structure of the MANEC-type domain from hepatocyte growth factor activator inhibitor-1 reveals an unexpected PAN/apple domain-type fold. Biochem J. 2015;466(2):299–309. doi: 10.1042/BJ20141236. [DOI] [PubMed] [Google Scholar]
- 16.Kojima K., Tsuzuki S., Fushiki T., Inouye K. Roles of functional and structural domains of hepatocyte growth factor activator inhibitor type 1 in the inhibition of matriptase. J Biol Chem. 2008;283(5):2478–2487. doi: 10.1074/jbc.M709073200. [DOI] [PubMed] [Google Scholar]
- 17.Kojima K., Tsuzuki S., Fushiki T., Inouye K. Role of the stem domain of matriptase in the interaction with its physiological inhibitor, hepatocyte growth factor activator inhibitor type I. J Biochem. 2009;145(6):783–790. doi: 10.1093/jb/mvp036. [DOI] [PubMed] [Google Scholar]
- 18.Chang H.H., Xu Y., Lai H., et al. Differential subcellular localization renders HAI-2 a matriptase inhibitor in breast cancer cells but not in mammary epithelial cells. PLoS One. 2015;10(3):e0120489. doi: 10.1371/journal.pone.0120489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shiao F., Liu L.O., Huang N., et al. Selective inhibition of prostasin in human enterocytes by the integral membrane kunitz-type serine protease inhibitor HAI-2. PLoS One. 2017;12(1):e0170944. doi: 10.1371/journal.pone.0170944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee S.P., Kao C.Y., Chang S.C., et al. Tissue distribution and subcellular localizations determine in vivo functional relationship among prostasin, matriptase, HAI-1, and HAI-2 in human skin. PLoS One. 2018;13(2):e0192632. doi: 10.1371/journal.pone.0192632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirchhofer D., Peek M., Li W., et al. Tissue expression, protease specificity, and Kunitz domain functions of hepatocyte growth factor activator inhibitor-1B (HAI-1B), a new splice variant of HAI-1. J Biol Chem. 2003;278(38):36341–36349. doi: 10.1074/jbc.M304643200. [DOI] [PubMed] [Google Scholar]
- 22.Itoh H., Kataoka H., Hamasuna R., Kitamura N., Koono M. Hepatocyte growth factor activator inhibitor type 2 lacking the first Kunitz-type serine proteinase inhibitor domain is a predominant product in mouse but not in human. Biochem Biophys Res Commun. 1999;255(3):740–748. doi: 10.1006/bbrc.1999.0268. [DOI] [PubMed] [Google Scholar]
- 23.Friis S., Tadeo D., Le-Gall S.M., et al. Matriptase zymogen supports epithelial development, homeostasis and regeneration. BMC Biol. 2017;15(1):46. doi: 10.1186/s12915-017-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin C.Y., Anders J., Johnson M., Dickson R.B. Purification and characterization of a complex containing matriptase and a Kunitz-type serine protease inhibitor from human milk. J Biol Chem. 1999;274(26):18237–18242. doi: 10.1074/jbc.274.26.18237. [DOI] [PubMed] [Google Scholar]
- 25.Tseng I.C., Chou F.P., Su S.F., et al. Purification from human milk of matriptase complexes with secreted serpins: mechanism for inhibition of matriptase other than HAI-1. Am J Physiol Cell Physiol. 2008;295(2):C423–C431. doi: 10.1152/ajpcell.00164.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiu Y.L., Wu Y.Y., Barndt R.B., et al. Aberrant regulation favours matriptase proteolysis in neoplastic B-cells that co-express HAI-2. J Enzyme Inhib Med Chem. 2019;34(1):692–702. doi: 10.1080/14756366.2019.1577831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao L., Liu M., Dong N., et al. Matriptase is highly upregulated in chronic lymphocytic leukemia and promotes cancer cell invasion. Leukemia. 2013;27(5):1191–1194. doi: 10.1038/leu.2012.289. [DOI] [PubMed] [Google Scholar]
- 28.Chou F.P., Chen Y.W., Zhao X.F., et al. Imbalanced matriptase pericellular proteolysis contributes to the pathogenesis of malignant B-cell lymphomas. Am J Pathol. 2013;183(4):1306–1317. doi: 10.1016/j.ajpath.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee M.S., Kiyomiya K.I., Benaud C., Dickson R.B., Lin C.Y. Simultaneous activation and HAI-1-mediated inhibition of matriptase induced at activation foci in immortal human mammary epithelial cells. Am J Physiol Cell Physiol. 2005;288(4):C932–C941. doi: 10.1152/ajpcell.00497.2004. [DOI] [PubMed] [Google Scholar]
- 30.Benaud C., Dickson R.B., Lin C.Y. Regulation of the activity of matriptase on epithelial cell surfaces by a blood-derived factor. Eur J Biochem. 2001;268(5):1439–1447. doi: 10.1046/j.1432-1327.2001.02016.x. [DOI] [PubMed] [Google Scholar]
- 31.Lee M.S., Tseng I.C., Wang Y., et al. Autoactivation of matriptase in vitro: requirement for biomembrane and LDL receptor domain. Am J Physiol Cell Physiol. 2007;293(1):C95–C105. doi: 10.1152/ajpcell.00611.2006. [DOI] [PubMed] [Google Scholar]
- 32.Tseng I.C., Xu H., Chou F.P., et al. Matriptase activation, an early cellular response to acidosis. J Biol Chem. 2010;285(5):3261–3270. doi: 10.1074/jbc.M109.055640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang J.K., Teng I.J., Lo T.J., et al. Matriptase autoactivation is tightly regulated by the cellular chemical environments. PLoS One. 2014;9(4):e93899. doi: 10.1371/journal.pone.0093899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin C.Y., Wang J.K., Torri J., Dou L., Sang Q.A., Dickson R.B. Characterization of a novel, membrane-bound, 80-kDa matrix-degrading protease from human breast cancer cells. Monoclonal antibody production, isolation, and localization. J Biol Chem. 1997;272(14):9147–9152. [PubMed] [Google Scholar]
- 35.Nonboe A.W., Krigslund O., Soendergaard C., et al. HAI-2 stabilizes, inhibits and regulates SEA-cleavage-dependent secretory transport of matriptase. Traffic. 2017;18(6):378–391. doi: 10.1111/tra.12482. [DOI] [PubMed] [Google Scholar]
- 36.Lin C.Y., Anders J., Johnson M., Sang Q.A., Dickson R.B. Molecular cloning of cDNA for matriptase, a matrix-degrading serine protease with trypsin-like activity. J Biol Chem. 1999;274(26):18231–18236. doi: 10.1074/jbc.274.26.18231. [DOI] [PubMed] [Google Scholar]
- 37.Ihara S., Miyoshi E., Ko J.H., et al. Prometastatic effect of N-acetylglucosaminyltransferase V is due to modification and stabilization of active matriptase by adding beta 1-6 GlcNAc branching. J Biol Chem. 2002;277(19):16960–16967. doi: 10.1074/jbc.M200673200. [DOI] [PubMed] [Google Scholar]
- 38.Morgan P.H., Robinson N.C., Walsh K.A., Neurath H. Inactivation of bovine trypsinogen and chymotrypsinogen by diisopropylphosphorofluoridate. Proc Natl Acad Sci U S A. 1972;69(11):3312–3316. doi: 10.1073/pnas.69.11.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim M.G., Chen C., Lyu M.S., et al. Cloning and chromosomal mapping of a gene isolated from thymic stromal cells encoding a new mouse type II membrane serine protease, epithin, containing four LDL receptor modules and two CUB domains. Immunogenetics. 1999;49(5):420–428. doi: 10.1007/s002510050515. [DOI] [PubMed] [Google Scholar]
- 40.Takeuchi T., Shuman M.A., Craik C.S. Reverse biochemistry: use of macromolecular protease inhibitors to dissect complex biological processes and identify a membrane-type serine protease in epithelial cancer and normal tissue. Proc Natl Acad Sci U S A. 1999;96(20):11054–11061. doi: 10.1073/pnas.96.20.11054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oberst M.D., Williams C.A., Dickson R.B., Johnson M.D., Lin C.Y. The activation of matriptase requires its noncatalytic domains, serine protease domain, and its cognate inhibitor. J Biol Chem. 2003;278(29):26773–26779. doi: 10.1074/jbc.M304282200. [DOI] [PubMed] [Google Scholar]
- 42.Shi Y.E., Torri J., Yieh L., Wellstein A., Lippman M.E., Dickson R.B. Identification and characterization of a novel matrix-degrading protease from hormone-dependent human breast cancer cells. Cancer Res. 1993;53(6):1409–1415. [PubMed] [Google Scholar]
- 43.Désilets A., Béliveau F., Vandal G., McDuff F.O., Lavigne P., Leduc R. Mutation G827R in matriptase causing autosomal recessive ichthyosis with hypotrichosis yields an inactive protease. J Biol Chem. 2008;283(16):10535–10542. doi: 10.1074/jbc.M707012200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tseng C.C., Jia B., Barndt R., et al. Matriptase shedding is closely coupled with matriptase zymogen activation and requires de novo proteolytic cleavage likely involving its own activity. PLoS One. 2017;12(8):e0183507. doi: 10.1371/journal.pone.0183507. [DOI] [PMC free article] [PubMed] [Google Scholar]