Abstract
Understanding symptoms associated with COVID-19 cases requiring intensive care unit(ICU) attention is important in management of the life-threatening cases of the disease. This study aimed to determine laboratory indicators of ICU admission for COVID-19 patients. For this retrospective chart review study, data from 116 patients(ICU, n = 18, Non-ICU, n = 98) with confirmed SARS-CoV-2, managed at two hospitals in Trinidad and Tobago, from March 12th to April 12th 2020, were analyzed. The median age of non-ICU patients was 59.0(IQR = 23.5) years; ICU patients had a median age of 62.5(IR = 17.5). From univariate analysis, laboratory indicators significantly associated with ICU admission included WBC(P = 0.037), lymphocyte(P = 0.016), LDH(P = 0.002), AST(P = 0.005) and CRP(P = 0.0001). However, multivariate analysis including WBC, neutrophil, lymphocyte, PLT, AST, LDH, ALT and CRP indicated that only AST was associated with high odds of ICU admission(OR 0.002, 95% CI 0.000–0.004, P = 0.017). Statistically significant AUC were obtained for neutrophil(AUC = 0.704, P = 0.007), CRP (AUC = 0.81, p = 0.00) and LDH(AUC = 0.766, P = 0.00) and AST (AUC = 0.729, P = 0.003). The findings indicate that neutrophils, AST and LDH's ROC curves are good tests, CRP curve is a very good test, but lymphocyte curve is a poor test, in determining COVID-19 patients for ICU admission. Neutrophil, AST, LDH and CRP are suitable predictors of COVID-19 patients that should receive intensive unit care. The study provides significant insights into laboratory parameters that can be used to predict COVID-19 severity and important considerations for healthcare providers in making evidence-based decisions regarding COVID-19 patient management, especially in the context of limited ICU facilities. This study was not funded.
Keywords: Biomarkers, COVID-19, Intensive care unit ICU, SARS-CoV-2, Trinidad and Tobago
1. Introduction
COVID-19 was declared a pandemic by the World Health Organization (WHO) in March 2020 after Wuhan confirmed its first case in December 2019 and thereafter, spreading to over 180 countries by March 2020. COVID-19 is a beta-coronavirus sharing phylogenetic similarities to SARS-CoV [1]. Sequencing analysis also showed that the COVID-19 virus serology was homologous to a SARS-like coronavirus [2]. COVID-19 has emerged as the third novel coronavirus in the last eighteen (18) years [3] and differs from other coronaviruses in its class due to its longer incubation period and lower fatality rates [2]. These novel characteristics have been thought to be underlying factors in the rapid spread of the virus.
Due to the virus' extensive spread, research has been launched into the investigation of possible laboratory predictors for COVID-19. Laboratory parameters have been used previously to shed light on disease severity, defining the prognosis, aid in follow ups, guiding treatment and therapeutic monitoring [4]. Parameters such as interleukin-6 (IL-6), D-Dimer, glucose, thrombin time, fibrinogen and C-reactive protein (CRP) have shown to be indicative of severe or mild COVID -19 [5,6]. Limited research has been conducted to evaluate the accuracy of laboratory predictors for COVID-19 patients who have been tested positive via RT-PCR. Studies in Trinidad and Tobago have been limited to discussing patterns of presented symptoms forSARS-CoV-2, alluding to the unique characteristics of patients with COVID-19 in this population and the greater need for research especially in this region [7].
Patients testing positive for COVID-19 are subjected to a Complete Blood Count (CBC) which guides clinicians in caring for their overall health. Any irregularities point to a series of health issues such as anemia, leukemia and much more. In light of COVID-19 findings, CBC results were used to differentiate between patients with severe COVID-19 and those needing Intensive Care Unit (ICU) care [8]. Patients with higher blood leukocyte counts, > 10*10^9/L, were more likely to have severe COVID-19 and be admitted into ICU.
White blood cells (WBCs) fight against infections in the body. COVID-19 patients showed a normal or decrease in WBC and lymphocyte counts upon hospital admission [[9], [10], [11]]. Even though research shows that COVID-19 survivors and non-survivors had normal WBCs, non-survivors had higher WBC counts and slightly reduced lymphocyte counts [11].
Exaggerated elevation of inflammatory cytokines in the body, like IL-6, causes the onset of a cytokine storm, as seen in COVID-19 patients, which results in multiple organ failure and Acute Respiratory Distress Syndrome(ARDS) [10,[12], [13], [14], [15]]. Rapid viral replication in the body causes vigorous pro-inflammatory responses and apoptosis in the lung's epithelial tissue, leading to hypoxia and ARDS [13,16].
Studies reported elevated levels of D-dimer as a more common laboratory finding in COVID-19 patients requiring hospitalization [6,17]. D-dimer elevation was associated with a hypercoagulable state, however, its specificity on the main cause of elevation may not be known as D-dimer elevations were associated with several unfavorable events including occlusion, sepsis, micro-thrombosis, and intravascular coagulation [8,17,18].
Lactate Dehydrogenase (LDH) is an enzyme expressed in all cells including the heart, liver, muscle, kidney, lung and bone marrow [2]. Increased LDH levels indicated cell damage to cells that it is normally expressed in. Elevated LDH is associated with worse clinical outcome of COVID-19 patients [8,19]. Monitoring both LDH and lymphocyte count can differentiate between ICU and non-ICU patients.
Liver abnormalities have been reported in COVID-19 patients upon hospital admission and during hospital stay [6,20]. Elevated alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were reported, ranging from 14 to 53% [10,20,21]. ALT, AST, total Bilirubin, Creatinine, and Blood Urea Nitrogen (BUN) were within normal ranges according to Ruoqing Li et al., 2020 [9], however, these results were taken at hospital admission and not during hospitalization.
CRP, produced in the liver, shows elevated levels in COVID-19 patients which is also a key indicator of MAS [[9], [10], [11],14]. Higher levels of CRP have been associated with lung lesions and are very important in assessing COVID-19 severity [22]. Excessive inflammation was also reflected by very high CRP levels in patients with severe disease state [8].
Laboratory predictors are key components in providing faster and more accurate diagnosis of the novel COVID-19. This is important due to the virus' rapid transmission and long incubation time. Early and accurate diagnosis of COVID-19 can reduce the load on healthcare systems worldwide.
2. Methods
For this retrospective chart review study, a judgment sampling technique was employed in collecting data from one hundred and sixteen (116) adult (≥18 years) patients with confirmed SARS-CoV-2, managed at Arima General Hospital and Couva Medical and Multi-training Facility in Trinidad and Tobago, from March 12th 2020 to April 12th 2020. Cases were confirmed via real-time reverse transcription polymerase chain reaction assays by an accredited laboratory. The medical records departments of the facilities were engaged and asked to populate a Microsoft Excel sheet with the variables of interest for patients who met the inclusion criteria of the study. These variables included clinical data such as CBC reports (WBC, neutrophil, lymphocyte, PLT) and biochemistry reports (AST, LDH, ALT, CRP), demographics (age, sex) and clinical symptomology. The study included all patients who were confirmed COVID-19 positive via RT-PCR and were subsequently admitted to the two facilities during the study period. Patients were excluded if they were admitted to the facility as ‘suspected’ COVID-19 patients, that is, with pending RT-PCR results during the study period. Patients were also excluded if they were < 18 years old at the time of the study, or if no laboratory data had been recorded in their medical files during the study period. The study was approved by the Ministry of Health of Trinidad and Tobago Ethics Committee (3/13/441 Vol. II), and the North Central Regional Health Authority Ethics Committee, Trinidad. As only de-identified data were collected, participant informed consent was waived by the ethics committees.
All statistical analysis was processed using IBM SPSS 22.0 statistical software. Descriptive statistics (median and interquartile range, and frequencies) were used to analyse patient characteristics. Means and standard deviation were used to describe laboratory test outcomes. Univariate and multivariate analyses were used to determine the statistical significance of the association between laboratory indicators and ICU admission. Univariate analysis involved univariate logistic regression of one dependent variable (ICU or non-ICU) and one independent variable (individual laboratory parameters; WBC, Neutrophil, Lymphocyte, PLT, AST, LDH, ALT, or CRP). Multivariate analysis involved logistic regression of one dependent variable (ICU or non-ICU) and multiple independent variables (all laboratory parameters; WBC, Neutrophil, Lymphocyte, PLT, AST, LDH, ALT, and CRP). The univariate analysis showed the isolated effect of one independent variable while multivariate analysis showed the effect of one independent variable when other variables are controlled. Receiver operating characteristic (ROC) curve and AUC were used to analyze the optimal cut-off for prediction of ICU admission. AUC 0.9 to 1 was defined as excellent accuracy, 0.8–0.9 as very good, 0.7–0.8 as good, 0.6–0.7 as sufficient, 0.5–0.6 as bad, and < 0.5 as poor (useless test). P < 0.5 denoted statistical significance.
Data availability: The data that support the findings of the study are available from the corresponding author, CG, upon reasonable request.
3. Results
3.1. Patient characteristics
Among the patients in the study, 45(38.8%) patients were males; 71(61.2%) were females. The number of male and female patients differed significantly between ICU and non-ICU patients (p = 0.008) with 66.7% of ICU patients being male while among non-ICU patients 66.3% were females. Chi-square outcome indicated that the observed age difference between the ICU and non-ICU patients was not statistically significant (P = 0.101). Since the distribution was skewed median is used to describe the age. The median age for the non-ICU patients was 59.0 (IQR = 23.5) years while the ICU patients had a median age of 62.5 (IQR = 17.5).
3.2. Laboratory test outcomes
Independent sample t-test was carried out to evaluate if there was difference in the biochemistry laboratory parameters between the ICU and non-ICU patients. The biochemistry laboratory findings in Table 1 indicate that the AST level for non-ICU patients (M = 45.4 ± 39.84) was lower compared to ICU patients(80.6 ± 5.76). The reported difference was statistically significant(P = 0.005). Table 1 also indicates that LDH was statistically significantly higher among ICU patients (994.2 ± 423.19) compared to non-ICU patients (669.6 ± 40.74,P = 0.002). The CRP concentration outcome is the other biochemistry test outcome that was statistically significantly different between ICU(93.2 ± 20.09) and non-ICU patients (35.8 ± 5.33, P = 0.00).). However, the findings presented in Table 1 indicate that the concentration of ALT did not vary between ICU and non- ICU patients (P = 0.76). The CBC findings in Table 1 indicate that the concentration of WBC was significantly higher among ICU patients (8.26 ± 3.61) compared to non-ICU patients (6.7 ± 2.47, P = 0.037). The findings also indicate that concentration of the lymphocytes were statistically higher among non-ICU patients (2.05 ± 0.75) compared to ICU patients (1.57 ± 0.71, P < 0.016). The level of neutrophil in non-ICU (6.11 ± 18.79) did not statistically differ from ICU (6.03 ± 3.41 P = 0.986)
Table 1.
Demographic and laboratory parameters among ICU and non-ICU COVID-19 patients.
| Variables | Normal Range | Non ICU(n = 98) Number (%) | ICU(n = 18) Number (%) | Chi squared | P-value |
|---|---|---|---|---|---|
| Age Median (IQR), years | 59.0 (23.5) | 62.5 (17.5) | 0.101 | ||
| Sex, n (%) | |||||
| Male | 33 (33.7) | 12 (66.7) | 6.972 | 0.008 | |
| Female | 65 (66.3) | 6 (33.3) | |||
| LDH(U/L) | 140–280 | 669.6 (40.74) | 994.2 (423.19) | 0.002 | |
| ALT(U/L) | 7–55 | 58.6 (9.54) | 52.0 (9.93) | 0.76 | |
| AST(U/L) | 8–48 | 45.4 (39.84) | 80.6 (75.76) | 0.005 | |
| CRP(U/L) | < 10 mg/L | 35.8 (5.33) | 93.2 (20.09) | 0.00 | |
| Lymphocyte count x 1000/UL | 1–4 | 2.05 (0.75) | 1.57 (0.71) | 0.016 | |
| Neutrophil count x 1000/UL | 2.5–8 | 6.11 (18.79) | 6.03 (3.41) | 0.986 | |
| WBC count x 1000/UL | 4.5–11.0 × 109 /L | 6.7 (2.47) | 8.26 (3.61) | 0.037 |
The univariate logistic regression indicted that laboratory indicators that were significantly associated with ICU admission included WBC(P = 0.037), lymphocyte (P = 0.016), LDH (P = 0.002), AST (P = 0.005) and CRP (P = 0.0001). However, multivariate logistic regression that included WBC, neutrophil, lymphocyte, PLT, AST, LDH, ALT, and CRP indicated that only AST was associated with high odds of patients being admitted to ICU (OR 0.002, 95% CI(0.001–0.004), P = 0.017) (See Table 2).
Table 2.
Univariate and multivariate analyses for predictors of ICU admission among COVID-19 patients.
| Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| WBC | −0.027 (−0.233–0.179) | 0.037 | 0.031 (−0.001–0.064) | 0.060 |
| Neutrophil | 0.00 (−0.005–0.004) | 0.986 | −0.001 (−0.006–0.003) | 0.520 |
| Lymphocyte | −0.12 (−0.217–0.023) | 0.016 | −0.059 (−0.172–0.054) | 0.302 |
| PLT | 0.00 (−0.001–0.001) | 0.589 | 000 (−0.001–0.000) | 0.304 |
| AST | 0.002 (0.001–0.004) | 0.005 | 0.002 (0.000–0.004) | 0.017 |
| LDH | 0.00 (0.00–0.00) | 0.002 | 0.000 (0.000–0.000) | 0.346 |
| ALT | 0.00 (−0.001–0.001) | 0.076 | −0.001 (−0.002–0.000) | 0.267 |
| CRP | 0.002 (0.001–0.003) | 0.000 | 0.001 (−0.001–0.002) | 0.505 |
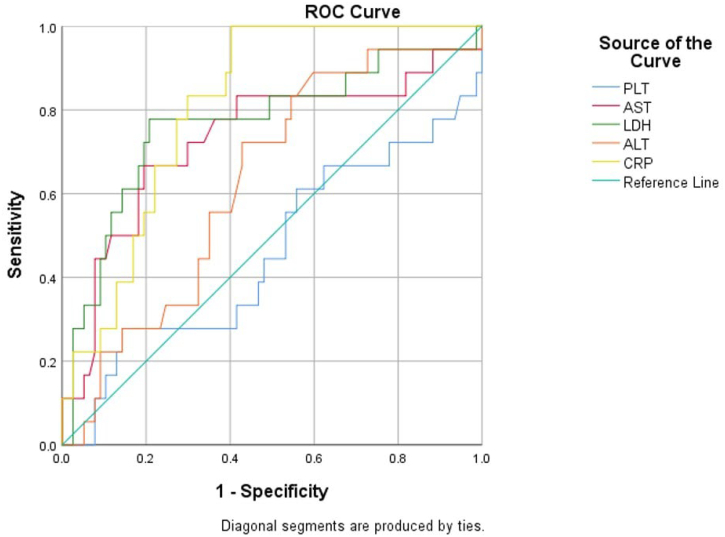
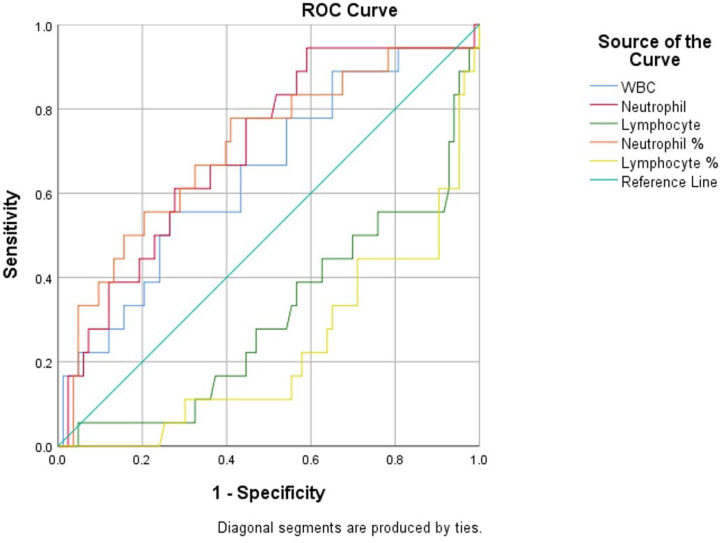
Table 3, Fig. 1 and Fig. 2 show the area under the ROC curve of the assessed biochemistry parameters(Fig. 1) and the CBC parameters(Fig. 2). The biochemistry with good accuracy was Neutrophil(AUC = 0.704, P = 0.007) while lymphocyte AUC(0.309) indicated that the parameter has no value in predicting ICU admission. The findings in Table 3 indicate that the AUC for WBC was not statistically significant. For the CBC parameters, Table 3 shows that statistically significant AUCs were obtained for CRP(AUC = 0.81,P = 0.00) and LDH(AUC = 0.766,P = 0.00) and AST(AUC = 0.729, P = 0.003). Statistically significant ROC curves were interpreted using AUC 0.9–1 as excellent accuracy, 0.8–0.9 as very good, 0.7–0.8 as good, 0.6–0.7 as sufficient, 0.5–0.6 as bad, and < 0.5 as poor (useless test). As shown in Table 3 neutrophils, AST and LDH's ROC curves are good tests while CRP curve is a very good test. However, lymphocyte curve is a poor test.
Table 3.
Area under ROC curve for laboratory parameters among ICU COVID-19 patients.
| Parameters | AUC | 95% CI | P | Interpretation |
|---|---|---|---|---|
| WBC | 0.646 | 0.501–0.790 | 0.053 | |
| Neutrophil | 0.704 | 0.574–0.835 | 0.007 | Good |
| Lymphocyte | 0.309 | 0.171–0.447 | 0.011 | Poor |
| PLT | 0.459 | 0.297–0.621 | 0.592 | |
| AST | 0.729 | 0.580–0.878 | 0.003 | Good |
| LDH | 0.766 | 0.627–0.904 | 0.000 | Good |
| ALT | 0.628 | 0.495–0.761 | 0.091 | |
| CRP | 0.810 | 0.722–0.898 | 0.000 | Very good |
Fig. 1.
ROC curve for the biochemistry test outcome for prediction of ICU patients.
Fig. 2.
ROC curve for the CBC test outcome for prediction of ICU patients.
4. Discussion
This study showed a difference in biochemical and CBC test parameters between ICU and non-ICU patients, with elevated AST, LDH, WBC, lymphocytes and CRP among ICU patients compared to non-ICU patients. Our findings show that the parameters that have good specificity and sensitivity in predicting ICU admission among COVID-19 patients include neutrophil, AST, LDH and CRP. Analysis of demographic variables revealed that ICU patients are older than non-ICU patients, which corroborates findings of the previous studies [[23], [24], [25]]. However, this age difference was smaller compared to that reported previously, noted as a > 10-year difference [24]. Noteworthy to mention is that the study sample of COVID-19 patients cared for at the facilities during the study period included more females than males, however, a significantly higher number of male ICU patients compared to female ICU patients was observed in this study which supports previous observations that indicated gender as a potential determinant of likelihood to develop serious complications that might require ICU care [23,26,27]. This observed difference could be attributed to behavioral differences between the sexes, notably that more men engage in smoking and alcohol consumption than women [28] heightening their risk of chronic diseases such as hypertension, cardiovascular disease, and chronic pulmonary disease, which have been associated with a greater likelihood of developing severe illness with COVID-19 [29]. Hormonal factors may also play a role in the observed heightened risk of developing severe COVID-19 in males. The TMPRSS2 protein, which is involved in facilitating viral entry and activation of SARS-CoV-2, is encoded by an androgen-regulated TMPRSS2 gene. This could account for the sex-specific increased susceptibility of males for developing severe COVID-19 as TMPRSS2 expression is increased in the presence of androgens [[30], [31], [32]].
Our univariate analysis findings regarding the CBC parameters contradict previous findings [2,4,33]. The observed lack of statistically significant difference in the level of neutrophils among ICU and non-ICU patients contradict previous researchers who concluded that elevated neutrophils concentration is characteristic of patients with severe cases [[34], [35], [36]]. Evidence suggests that severe COVID-19 is associated with elevated neutrophil levels, which increase inflammation via cytokine storm, and haemorrhages especially in the lungs, which occur as a result of neutrophil-induced tissue damage via their extravasation into alveolar spaces, and requires the patient to be placed in intensive care [35,36].
However, Mardani et al. [39] supports our findings regarding the reduced concentration of WBC among ICU COVID-19 patients. The univariate assessment of CBC parameters showed that among ICU patients, the increase in the concentration of lymphocytes is associated with significant reduction in the odds of ICU hospitalization. This outcome suggests that reduction of lymphocytes increases odds of ICU hospitalization. A reduced concentration of lymphocytes, or lymphopenia, has been previously associated with COVID-19 severity [27,37,38]. Wang et al. [24] documented the importance of lymphopenia in identifying COVID-19 patients requiring ICU care. Fan et al. in the assessment of the baseline characteristics of the COVID-19 patients requiring ICU care also noted that patients had significantly low levels of lymphocytes, which further corroborates the obtained findings. Wu et al. [36] also noted COVID-19 patients with lymphopenia are likely to be experiencing respiratory distress syndrome, which according to the researchers is associated with elevated levels of neutrophils. A proposed underlying cause of lymphopenia and its association with severe COVID-19 lies in the observation that COVID-19 infection can result in T-cell exhaustion. CD4+ and CD8+ T-cells have been reported to show increased expression of markers of T-cell exhaustion, such as PD-1 and Tim-3, which correlated with disease severity and intensive care requirement in COVID-19 patients [40,41]. Other proposed mechanisms of lymphopenia suggest that SARS-CoV-2 infection disrupts T-cell expansion by downregulating the expression of genes involved in their activation and function in patients with severe COVID-19 [42].
Our biochemistry test findings, which showed an elevated level of CRP, AST, LDH among ICU patients, corroborates the conclusion made by Mardani et al. [39]. Evidence suggests that the increased levels of AST among severe cases of COVID-19 is associated with liver damage [[43], [44], [45]]. Our findings regarding the lack of difference in ALT levels among ICU and non-ICU patients contradict those of previous studies [4,46]. However, it should be noted that elevated AST levels have been noted to be more common than ALT, which explains our observations [47]. The study by Fan et al. [24] also noted that COVID-19 patients requiring ICU care had higher LDH levels. Other researchers reporting higher LDH levels among these patients include [4,48]. Researchers have also noted that severe COVID-19 cases develop neutrophilia during hospitalization [4,24].
However, our multivariate analysis showed AST to be the only parameter having significant association with ICU admission of COVID-19 patients. When the effect of WBC, neutrophil, lymphocyte, PLT, AST, LDH, ALT, and CRP is controlled, the increase in the level of AST was associated with a significant increase in the odds of ICU admission, which corroborate the observation of Mardani et al. [39]. The ROC curves in this study indicated sensitivity and specificity of various parameters in identifying ICU and non-ICU COVID-19 patients. It is evident from this study that the only good predictors are neutrophil, AST, LDH and CRP. The reported excellent accuracy of CRP and neutrophils in the prediction of patients with COVID-19 corroborates findings of Mardani et al. [39]. However, unlike the findings of this study which showed good accuracy of LDH and AST, other researchers found the tests to be excellent with AUC >0.8 [39]. Although we found ALT to be of no value in predicting ICU admissions of COVID-19 patients, other researchers observed that the test offered excellent prediction accuracy [5,39,49]. This difference could be associated with the difference in patients and progression of the disease in individuals especially with respect to the integrity of the liver [43,45].
Our findings have practical implications in treatment of COVID-19 patients. As indicated by previous researchers, COVID-19 severity varies across different cases [39,45]. It is therefore important to identify and provide adequate care for patients with the likelihood of developing serious complications. Our study indicates that certain biochemical and CBC tests are important in predicting COVID-19 patients' need for ICU care. Specifically, laboratory tests that should be prioritized to determine patient risk of developing serious COVID-19 complications that might require ICU care include AST, LDH and CRP tests. CRP is most preferred as it was noted to be a very good test. The next most preferred are AST and LDH, observed to be good tests.
Limitations should be considered in the interpretation of our findings. One limitation relates to the sample used which had unequal number of males and females. Also, the study did not control for the effect of pre-existing health conditions. Future studies should determine whether the recorded observations occurred independently of the potential pre-existing health conditions such as hypertension and diabetes.
Despite its limitations, this study has provided insights into laboratory parameters that can be used to predict the severity of COVID-19 cases. Our findings show neutrophil, AST, LDH and CRP are good predictors of COVID-19 patients that should receive ICU care. It is therefore recommended that healthcare providers consider these parameters in making an evidence-based decisions regarding patient management especially where there are limited ICU facilities.
5. Conclusions
In predicting ICU admission among COVID-19 patients, neutrophils, AST and LDH's were found to be good tests while CRP was a very good test. Lymphocyte was a poor test in predicting ICU admission. Neutrophil, AST, LDH and CRP are suitable predictors of COVID-19 patients that should receive ICU care.
Contributors
CDG and DV were responsible for data analysis, with intellectual contributions from DT. CDG and DV drafted the article. All authors contributed to the conception and design of the paper, interpretation of data, and critical revisions contributing to the intellectual content and approval of the final version of the manuscript.
Funding
The authors have not received any funding from industry or elsewhere to conduct or publish this study.
Data sharing
Deidentified participant data collected for this study will be made available by the corresponding author upon reasonable request via email.
Research in context
Evidence before this study
Understanding symptoms associated with COVID-19 cases requiring intensive care unit (ICU) attention is important in the management of the life-threatening cases of the disease. Laboratory parameters have been used previously to shed light on disease severity, defining the prognosis, aid in follow ups, guiding treatment and therapeutic monitoring [4]. Parameters such as interleukin-6 (IL-6), D-Dimer, glucose, thrombin time, fibrinogen and C-reactive protein (CRP) have shown to be indicative of severe or mild COVID -19 [5,6]. Limited research has been conducted to evaluate the accuracy of laboratory predictors for COVID-19 patients who have tested positive via RT-PCR. Studies in Trinidad and Tobago have been limited to discussing patterns of presented symptoms for SARS COV-19, alluding to the unique characteristics of patients with COVID-19 in this population and the greater need for research especially in this region [7].
Added value of this study
To the best of the authors' knowledge, this study is the first in the Caribbean region to evaluate the utility of common laboratory parameters in determining COVID-19 patients' need for intensive care. The results have shown that neutrophil, AST, LDH and CRP are suitable predictors of COVID-19 patients in need of intensive unit care.
Implications of all the available evidence
The study provides significant insights into laboratory parameters that can be used to predict COVID-19 severity and important considerations for healthcare providers in making evidence-based decisions regarding COVID-19 patient management, especially in the context of limited ICU facilities.
Declaration of Competing Interest
This study was granted ethical approval by the following ethics committees:
The North Central Regional Health Authority Ethics Committee, Trinidad– approval granted.
The Ministry of Health of Trinidad and Tobago (3/13/441 Vol. II) Ethics Committee – approval granted. Participant consent was waived by the ethics committees due to the exclusive use of de-identified data, which was entered in this study-specific database, and solicited through the ethics committees. No participant identifiers (name, address, telephone or cell phone number/email/any contact information, ID numbers) were collected nor used for the purpose of this study.
References
- 1.Guan W.-j., Ni Z.-y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frater J.L., Zini G., d’Onofrio G., Rogers H.J. COVID-19 and the clinical hematology laboratory. Int J Lab Hematol. 2020;42(Suppl. 1):11–18. doi: 10.1111/ijlh.13229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azkur A.K., Akdis M., Azkur D., Sokolowska M., van de Veen W., Brüggen M.C., et al. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy. 2020;75(7):1564–1581. doi: 10.1111/all.14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lippi G., Plebani M. Laboratory abnormalities in patients with COVID-2019 infection. Clin Chem Lab Med. 2020;58(7):1131–1134. doi: 10.1515/cclm-2020-0198. [DOI] [PubMed] [Google Scholar]
- 5.Gao Y., Li T., Han M., Li X., Wu D., Xu Y., et al. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J Med Virol. 2020;92(7):791–796. doi: 10.1002/jmv.25770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lake M.A. What we know so far: COVID-19 current clinical knowledge and research. Clin Med (Lond) 2020;20(2):124–127. doi: 10.7861/clinmed.2019-coron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gopaul C., Ventour D., Trotman M., Thomas D. The epidemiology characteristics of positive COVID-19 patients in Trinidad and Tobago. MedRxiv. 2020 doi: 10.1101/2020.08.06.20148288. [Preprint] Available from: [DOI] [Google Scholar]
- 8.Zhang G., Zhang J., Wang B., Zhu X., Wang Q., Qiu S. Analysis of clinical characteristics and laboratory findings of 95 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a retrospective analysis. Respir Res. 2020;21(1):74. doi: 10.1186/s12931-020-01338-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li R., Tian J., Yang F., Lv L., Yu J., Sun G., et al. Clinical characteristics of 225 patients with COVID-19 in a tertiary Hospital near Wuhan, China. J Clin Virol. 2020;127:104363. doi: 10.1016/j.jcv.2020.104363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao Y., Nie H.-X., Hu K., Wu X.-J., Zhang Y.-T., Wang M.-M., et al. Abnormal immunity of non-survivors with COVID-19:predictors for mortality. Infect Dis Poverty. 2020;9(1):108. doi: 10.1186/s40249-020-00723-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henry B.M., de Oliveira M.H.S., Benoit S., Plebani M., Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019(COVID-19):a meta-analysis. Clin Chem Lab Med. 2020;58(7):1021–1028. doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- 13.Ye Q., Wang B., Mao J. The pathogenesis and treatment of the `Cytokine Storm’ in COVID-19. J Infect. 2020;80(6):607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGonagle D., Sharif K., O’Regan A., Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020;19(6) doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan. China Intensive Care Med. 2020;46(5):846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X., Ma X. Acute respiratory failure in COVID-19: is it “typical” ARDS? Crit Care. 2020;24(1):198. doi: 10.1186/s13054-020-02911-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang L., Yan X., Fan Q., Liu H., Liu X., Liu Z., et al. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost. 2020;18(6):1324–1329. doi: 10.1111/jth.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henry B.M., Aggarwal G., Wong J., Benoit S., Vikse J., Plebani M., et al. Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID-19) severity and mortality: A pooled analysis. Am J Emerg Med. 2020;38(9):1722–1726. doi: 10.1016/j.ajem.2020.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chai X., Hu L., Zhang Y., Han W., Lu Z., Ke A., et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. bioRxiv. 2020 doi: 10.1101/2020.02.03.931766. [DOI] [Google Scholar]
- 21.Cai Q., Huang D., Yu H., Zhu Z., Xia Z., Su Y., et al. COVID-19: Abnormal liver function tests. J Hepatol. 2020;73(3):566–574. doi: 10.1016/j.jhep.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang L. C-reactive protein levels in the early stage of COVID-19. Med Mal Infect. 2020;50(4):332–334. doi: 10.1016/j.medmal.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jain V., Yuan J.M. Predictive symptoms and comorbidities for severe COVID-19 and intensive care unit admission: a systematic review and meta-analysis. Int J Public Health. 2020;65(5):533–546. doi: 10.1007/s00038-020-01390-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan B.E., Chong V.C.L., Chan S.S.W., Lim G.H., Lim K.G.E., Tan G.B., et al. Hematologic parameters in patients with COVID-19 infection. Am J Hematol. 2020;95(6) doi: 10.1002/ajh.25774. E131-E4. [DOI] [PubMed] [Google Scholar]
- 25.Lee P.I., Hu Y.L., Chen P.Y., Huang Y.C., Hsueh P.R. Are children less susceptible to COVID-19? J Microbiol Immunol Infect. 2020;53(3):371–372. doi: 10.1016/j.jmii.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gausman J., Langer A. Sex and gender disparities in the COVID-19 pandemic. J Womens Health (Larchmt) 2020;29(4):465–466. doi: 10.1089/jwh.2020.8472. [DOI] [PubMed] [Google Scholar]
- 27.Remuzzi A., Remuzzi G. COVID-19 and Italy: what next? Lancet (London, England) 2020;395(10231):1225–1228. doi: 10.1016/S0140-6736(20)30627-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.GBD 2015 Tobacco Collaborators Smoking prevalence and attributable disease burden in 195 countries and territories, 1990-2015: a systematic analysis from the Global Burden of Disease Study 2015 [published correction appears in Lancet. 2017 Oct 7;390(10103):1644] Lancet. 2017;389(10082):1885–1906. doi: 10.1016/S0140-6736(17)30819-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Darmadi D., Pakpahan C., Ruslie R.H., Rezano A. Inflammatory laboratory findings associated with severe illness among hospitalized individuals with COVID-19 in Medan, Indonesia: a cross-sectional study [version 2; peer review: 2 approved] F1000Research. 2022;10(1246) doi: 10.12688/f1000research.74758.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lucas J.M., Heinlein C., Kim T., et al. The androgen-regulated protease TMPRSS2 activates a proteolytic cascade involving components of the tumor microenvironment and promotes prostate cancer metastasis. Cancer Discov. 2014 Nov 1;4(11):1310–1325. doi: 10.1158/2159-8290.CD-13-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wulandari L., Hamidah B., Pakpahan C., et al. Initial study on TMPRSS2 p.Val160Met genetic variant in COVID-19 patients. Hum Genomics. 2021 May 17;15(1):29. doi: 10.1186/s40246-021-00330-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strope J.D., PharmD C.H.C., Figg W.D. TMPRSS2: potential biomarker for COVID-19 outcomes. J Clin Pharmacol. 2020 Jul;60(7):801–807. doi: 10.1002/jcph.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Velavan T.P., Meyer C.G. Mild versus severe COVID-19: Laboratory markers. Int J Infect Dis. 2020;95:304–307. doi: 10.1016/j.ijid.2020.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laforge M., Elbim C., Frère C., Hémadi M., Massaad C., Nuss P., et al. Author correction: tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat Rev Immunol. 2020;20(9):579. doi: 10.1038/s41577-020-00425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan. China JAMA Intern Med. 2020;180(7):1–11. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bermejo-Martin J.F., Almansa R., Menéndez R., Mendez R., Kelvin D.J., Torres A. Lymphopenic community acquired pneumonia as signature of severe COVID-19 infection. J Infect. 2020;80(5) doi: 10.1016/j.jinf.2020.02.029. e23-e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fathi N., Rezaei N. Lymphopenia in COVID-19: Therapeutic opportunities. Cell Biol Int. 2020;44(9):1792–1797. doi: 10.1002/cbin.11403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mardani R., Ahmadi Vasmehjani A., Zali F., Gholami A., Mousavi Nasab S.D., Kaghazian H., et al. Laboratory parameters in detection of COVID-19 patients with positive RT-PCR; a diagnostic accuracy study. Arch Acad Emerg Med. 2020;8(1) [PMC free article] [PubMed] [Google Scholar]
- 40.Diao B., Wang C., Tan Y., Chen X., Liu Y., Ning L., et al. Reduction and functional exhaustion of t cells in patients with coronavirus disease 2019 (COVID-19) Front Immunol. 2020;11(827) doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.André S., Picard M., Cezar R., et al. T cell apoptosis characterizes severe Covid-19 disease. Cell Death Differ. 2022;22:1–14. doi: 10.1038/s41418-022-00936-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ouyang Y., Yin J., Wang W., Shi H., Shi Y., Xu B., et al. Down-regulated gene expression spectrum and immune responses changed during the disease progression in COVID-19 patients. Clin Infect Dis. 2020;71(16):2052–2060. doi: 10.1093/cid/ciaa462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ding Y., He L., Zhang Q., Huang Z., Che X., Hou J., et al. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol. 2004;203(2):622–630. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tan Y.J., Fielding B.C., Goh P.Y., Shen S., Tan T.H., Lim S.G., et al. Overexpression of 7a, a protein specifically encoded by the severe acute respiratory syndrome coronavirus, induces apoptosis via a caspase-dependent pathway. J Virol. 2004;78(24):14043–14047. doi: 10.1128/JVI.78.24.14043-14047.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang C., Shi L., Wang F.S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5(5):428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.D’Antiga L. Coronaviruses and immunosuppressed patients: the facts during the third epidemic. Liver Transpl. 2020;26(6):832–834. doi: 10.1002/lt.25756. [DOI] [PubMed] [Google Scholar]
- 48.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Y., Liu Y., Liu L., Wang X., Luo N., Li L. Clinical Outcomes in 55 Patients With Severe Acute Respiratory Syndrome Coronavirus 2 Who Were Asymptomatic at Hospital Admission in Shenzhen. China J Infect Dis. 2020;221(11):1770–1774. doi: 10.1093/infdis/jiaa119. [DOI] [PMC free article] [PubMed] [Google Scholar]