Abstract
Leukemia inhibitory factor (LIF), and its receptor (LIFR), are commonly over-expressed in many solid cancers and recent studies have implicated LIF/LIFR axis as a promising clinical target for cancer therapy. LIF/LIFR activate oncogenic signaling pathways including JAK/STAT3 as immediate effectors and MAPK, AKT, mTOR further downstream. LIF/LIFR signaling plays a key role in tumor growth, progression, metastasis, stemness and therapy resistance. Many solid cancers show overexpression of LIF and autocrine stimulation of the LIF/LIFR axis; these are associated with a poorer relapse-free survival. LIF/LIFR signaling also plays a role in modulating multiple immune cell types present in tumor micro environment (TME). Recently, two targeted agents that target LIF (humanized anti-LIF antibody, MSC-1) and LIFR inhibitor (EC359) were under development. Both agents showed effectivity in preclinical models and clinical trials using MSC-1 antibody are in progress. This article reviews the significance of LIF/LIFR pathways and inhibitors that disrupt this process for the treatment of cancer.
Keywords: LIF, LIFR, LIFR inhibitor, STAT3, Targeted therapy
Abbreviations: leukemia inhibitory factor, (LIF); LIF receptor, (LIFR); programmed death-ligand 1, (PD-L1); cancer stem cells, (CSCs); tumor micro environment, (TME); oncostatin M, (OSM); ciliary neurotrophic factor, (CNTF); cardiotrophin 1, (CTF1); humanized Anti-LIF antibody, (MSC-1); breast cancer, (BCa); endometrial cancer, (ECa); ovarian cancer, (OCa); prostate cancer, (PCa); colorectal cancer, (CRC); pancreatic ductal adenocarcinoma, (PDAC); AKT, protein kinase B; HER2, human epidermal growth factor receptor 2; JAK, Janus kinase; mitogen activated protein kinase, (MAPK); mammalian target of rapamycin, (mTOR); signal transducer and activator of transcription 3, (STAT3); triple negative breast cancer, (TNBC)
Introduction
Leukemia inhibitory factor (LIF) is the most pleiotropic member of the interleukin-6 family of cytokines.1 LIF mediates its signaling using membrane receptor complex that is comprised of LIFR and glycoprotein 130 (gp130).2 LIF first binds to LIFR which leads to the formation of high affinity functional complex with gp130. The LIFR does not have an intrinsic tyrosine kinase activity. However, LIFR and gp130 constitutively associate with JAK-Tyk family of cytoplasmic tyrosine kinases, which facilitates downstream signaling.3 Tumors exhibit upregulated LIF/LIFR-JAK-STAT3 signaling via autocrine and paracrine mechanisms.4, 5, 6, 7 LIF promotes activation of mTORC1/p70S6K signaling pathway enhancing tumor growth and inhibit DNA damage responses.8 LIF/LIFR signaling promotes tumorigenesis and metastasis of breast cancer by enhancing AKT-mTOR signaling.9 LIFR regulates tumor angiogenesis through ERK/IL-8 pathway.10 LIF/LIFR signaling negatively regulates tumor-suppressor p53 through STAT3/ID1/MDM2 axis.11 LIFR signaling is complex as multiple ligands such as Oncostatin M (OSM), Ciliary Neurotrophic Factor (CNTF), and Cardiotrophin 1 (CTF1) also have the potential to interact with LIFR and activate its downstream signaling.7 Earlier studies revealed that LIF, CTF1, and OSM share an overlapping binding site located in the Ig-like domain of LIFR and different behaviors of LIF, CTF1, and OSM can be related to the different affinity of their site for LIFR.12 In this review, we summarize the emerging significance and functions of LIF/LIFR signaling in cancer. We will also discuss recent therapeutic strategies that are being developed in targeting LIF/LIFR axis in cancer.
Significance of LIF/LIFR signaling in cancer
LIF/LIFR axis is implicated in tumor growth and progression by acting on multiple aspects of cancer biology.13 LIF functions as a growth factor in pancreas carcinoma cells4 and high LIF expression in pancreatic ductal adenocarcinoma (PDAC) was associated with shorter overall survival.14 Aberrant production of LIF in the pancreas is restricted to pathological conditions and circulating LIF correlates well with tumor response to therapy.15 LIFR expression identifies highly malignant melanocytic lesions at an early stage; and LIFR is associated with unfavorable prognosis in melanoma.16 LIFR also serves as a novel prognostic biomarker for gallbladder cancer.17
Histone methyltransferase KMT2D sustains prostate carcinogenesis and metastasis by epigenetically activating LIFR.18 The LIF promoter is hypermethylated in normal breast epithelial cells, but extensively demethylated during Breast Cancer (BCa) progression.5 LIF promotes proliferation and metastasis of BCa cells, and overexpression of LIF is commonly associated with poorer relapse free survival in BCa patients.9 Triple Negative Breast Cancer (TNBC) cells exhibit a higher expression of LIF and LIFR compared to that of ER + BCa cells and the overexpression of LIF is significantly associated with a poorer relapse free survival in BCa patients.9 BCa progression increases the expression of OSM, LIF, OSMR, LIFR and gp130. The secretion of OSM and LIF by both epithelial and stromal cells may further contribute to BCa progression by autocrine and paracrine manner.20
LIFR expression is upregulated in colorectal cancer (CRC) and represents a potential therapeutic target.10 Overexpression of LIF is associated with poor prognosis in CRC.11 LIF is also frequently overexpressed in osteosarcoma, and promotes the growth and invasion by activating STAT3 pathway.21 PI3K subunit p110α controls the expression of LIFRα via c-Myc and miR-125b axis to promote Medulloblastoma cell proliferation.19 LIF/LIFR/STAT axis maintain programmed death-ligand 1 (PD-L1) protein stability and promote metastasis-associated gene expression and may serve as a prognostic biomarker and potential therapeutic target for prostrate cancer.22
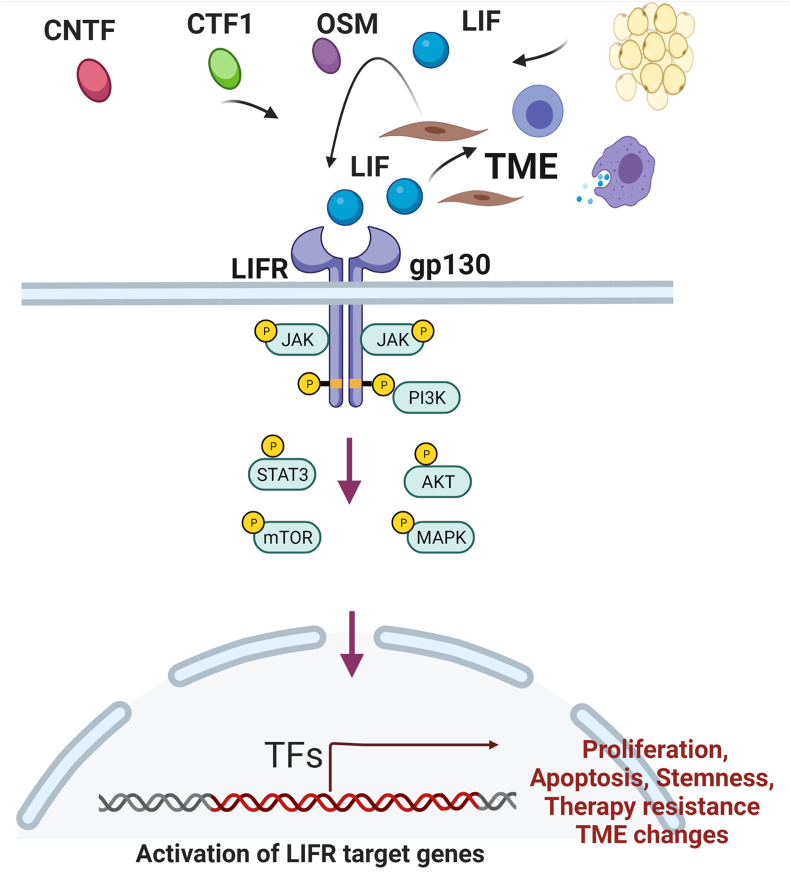
Furthermore, LIF/LIFR axis exhibits differential effects in various cell types including stimulating or inhibiting cell proliferation, differentiation and survival depending on the cell type.9,13,23 LIF promotes gastric cancer cell proliferation, migration, and invasion via the LIFR-Hippo-YAP pathway and inhibited apoptosis. LIF inhibits the activity of the Hippo pathway by reducing phosphorylation of YAP, increased YAP nuclear translocation, and increased cell proliferation.24 LIFR is also reported to function as a metastasis suppressor through the Hippo-YAP pathway25 and confer a dormancy phenotype in BCa cells disseminating to bone.26 Despite the ability of LIF to activate JAK1/STAT3, PI3K/AKT and MAPK pathways in these cells, differences in signaling outcome may in part arise from differential levels of activation of these three pathways, multiple ligands to LIFR and differences in tumor micro environment (TME).1,27 Collectively, these findings implicate that LIF binding to LIFR complex activates multiple signaling events and in many solid cancers LIF/LIFR signaling promote cancer progression (Fig. 1).
Figure 1.
LIF/LIFR oncogenic signaling network. LIF/LIFR signaling promotes tumor progression by regulating multiple signaling aspects including activation of STAT3 signaling, mTOR, AKT, MAPK. LIF/LIFR axis promotes cell survival, enhances stemness and inhibits apoptosis. Further LIF/LIFR axis regulates multiple aspects of tumor microenvironment including macrophage signaling. Further, autocrine or paracrine loops of LIF/LIFR signaling also drives cancer progression.
LIF/LIFR signaling in tumor microenvironment
LIF/LIFR signaling plays a role in modulating multiple immune cell types present in tumor micro environment (TME) including T-eff, T-reg, macrophages,28 and myeloid cells which leads to immune suppression.29 LIF regulates CXCL9 in tumor-associated macrophages and prevents CD8+ T cell tumor-infiltration impairing anti-PD1 therapy. The combination of LIF neutralizing antibodies with the inhibition of the PD1 immune checkpoint promotes tumor regression, immunological memory, and an increase in overall survival.30 LncAMPC upregulates LIF/LIFR expression to stimulate the Jak1-STAT3 pathway to maintain PD-L1 protein stability and immunosuppression.22
Ovarian Cancer (OCa) cells secrete LIF and are implicated in the development of immune deficiency in the peritoneal cavity of OCa patients.28,31 Obesity signals (such as estrogen, leptin) promote endometrial cancer (ECa)32 and function as potent inducers of LIF.33,34 LIF is an established E2-responsive gene in the uterus.35 LIF signaling promotes crosstalk between tumor cells and fibroblasts, and mediate pro-invasive activation of stromal fibroblasts.36 Obese people have unusually high levels of leptin; and leptin increases p-STAT3, LIF, LIFR, levels in cultured human endometrial cells.34
Tumors upregulate LIF/LIFR signaling via autocrine and paracrine mechanisms.4, 5, 6 LIF functions both as paracrine or autocrine growth factor for breast, kidney and prostate cancers.13 TGFβ induces the self-renewal capacity of glioma stem cells through the SMAD-dependent induction of LIF.37 Hypoxia induces LIF expression in human cancer cells.38 LIF signaling plays a role in crosstalk between tumor cells and fibroblasts, and mediate pro-invasive activation of stromal fibroblasts.36 LIF is produced by OCa associated mesenchymal stem cells, induces tumor cell JAK-STAT signaling via LIFR, increases the percentage of cancer stemlike cells, and promotes tumor growth.39 Autocrine loop involving LIF, LIF Receptor, and STAT4 drives sustained fibroblast production of inflammatory mediators.40
Role of LIF/LIFR axis in stemness and therapy resistance
The evidence for the role of LIF/LIFR axis in its contribution to therapy resistance is well supported by literature. Several studies demonstrated that cancer stem cells (CSCs) are highly tumorigenic, spared by chemotherapy, sustain tumor growth, and are implicated in tumor recurrence.41, 42, 43, 44 LIF/LIFR axis is a key regulator of CSCs13; further, LIF-STAT3 axis is implicated in stem cell self-renewal and pluripotency45 and in the regulation of the transcription factors SOX2 and NANOG that play a critical role in stemness.46 Recent studies showed that the switch from migration to proliferation was regulated by BM-MSC-secreted LIF/LIFR/p-ERK/pS727-STAT3 signaling to promote early disseminated cancer cells mesenchymal–epithelial transition and premetastatic niche formation.47 TGFβ is shown to promote BCa stem cell self-renewal through an Interleukin-like EMT Inducer (ILEI)/LIFR signaling axis.48 Glioma-initiating cells (GICs) are responsible for the initiation/recurrence of gliomas and TGFβ/LIF signaling induces the self-renewal capacity and prevents the differentiation of GICs.37
High circulating LIF levels correlate well with tumor recurrence and contribute to chemoresistance.6,49 Overexpression of LIF is an important mechanism for the attenuation of p53, which promotes chemoresistance in CRCs.11 LIF is a commonly upregulated gene in carboplatin- and paclitaxel-resistant cells and its expression correlates with poor outcome in ECa patients.50 Additionally, LIF/LIFR signaling contributes to drug-resistant OCa.51 Autocrine and paracrine LIF signaling is shown to promote chemoresistance in cholangiocarcinoma by up-regulating Mcl-1 via a novel STAT3-PI3K/AKT-dependent pathway.6 Androgen deprivation therapy (ADT) upregulates EGFR–LIFR signaling that activates succinate-CoA ligase GDP-forming beta subunit (SUCLG2), which subsequently stimulates the metabolic changes associated with neuroendocrine differentiation and aggressive prostate cancer phenotype.52
Elevated circulating LIF is a marker of poor response to neo-adjuvant treatment in oesophageal adenocarcinoma (OAC) and targeting LIF signaling enhances neoadjuvant treatment responses.53 LIFR mediated activation of STAT3 confers resistance to T-DM1 in HER2-positive breast cancer.54 LIF promotes nasopharyngeal carcinoma (NPC) tumorigenesis and serum LIF levels predict local recurrence and radiosensitivity.8 Collectively, the ability of LIF/LIFR axis to promote therapy resistance can be attributed to its propensity to enhance stemness, promoting survival and reduce apoptosis.
Structural features of LIFR for blocking ligand signaling
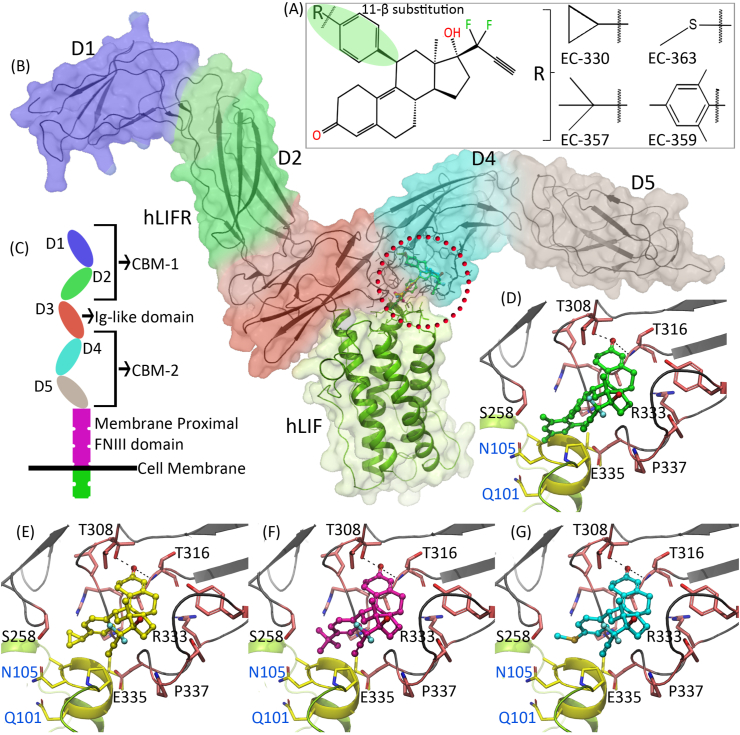
To understand the structural features of LIFR, we conducted homology modeling of the human LIFR (hLIFR) based on human LIF–mouse LIFR complex (hLIF–mLIFR). This model suggested the presence of two cytokine-binding modules (CBM-1 and 2). The CBM-1 is constituted by domains D1 and D2, whereas the CBM-2 is constituted by domains D4 and D5. The D1 and D2 domains are connected by a short linker. The two CBMs are separated by an Ig-like domain, D3 is packed across the end of the D2 domain through loops originating from the end of D2 domain. D3 domain is connected to the D4 domain through loops. Similarly, D4 domain is also connected to D5 domain through loops. Although three-membrane proximal fibronectin type-III (FNIII) domains are present in the LIFR, they were not resolved in previously reported crystal structures (PDB IDs: 2q7n, 3e0g and 1pvh). Due to the high sequence identity between the mLIFR and hLIFR, it was no surprise that our modeled hLIFR structure also adopted the extended zigzag conformation seen in mLIFR (Fig. 2B, C). A saddle-shaped protein–protein interacting (PPI) interface is constituted by a four-helix bundle from LIF, and one sheet (D3) and loops (D4) from LIFR. Potential binding pockets are found on both the LIF and LIFR in the vicinity of the PPI interface (Fig. 2B, red circle). We surmise that small molecules that could interact with these binding pockets may interfere with LIF–LIFR interactions. Our previous molecular docking studies suggested that ligands such as EC359, EC330, EC357 and EC363 are able to bind to the hLIFR at the PPI interface and block interactions with LIF (Fig. 2D–G).55,56 In silico binding energy calculations ranked the affinity of these compounds towards hLIFR in the order of EC359 > EC330 > EC357 > EC363. Similarly, our previous structure activity relationship studies suggested that the 11-β substitution in the ligands is the determining factor for the blockade of LIF–LIFR interactions. Ligands with a bulkier substitution displayed more steric clashes with LIF and exhibited higher inhibiting properties compared to those with a smaller substitution. Collectively, these modeling studies helped to design and develop potent small molecule inhibitors for targeting LIFR.
Figure 2.
Binding mode of LIFR inhibitors. (A) Structures of the compounds that preferentially block the interactions of LIF with LIFR. The 11-β position is shown in green color. (B) Three dimensional structure of hLIF–LIFR complex. Domains in hLIFR are displayed in blue (domain D1), green (domain D2), red (domain D3), cyan (domain D4) and gray (domain D5) color surfaces and as a black ribbon. The hLIF is displayed in green ribbon and white surface. The red circle in the hLIFR denotes potential binding pocket. (C) Domain representation of LIFR receptor illustrated in cartoon. (D–G) Binding modes of EC359 (represented as green ball and stick), EC330 (yellow), EC357 (violet), and EC363 (cyan). hLIFR is shown in black ribbon and pink stick, hLIF is shown in yellow ribbon and sticks.
Therapeutic strategies targeting LIF/LIFR
Analyses of LIF KO mice have revealed that many of the LIF actions are not apparent during normal development,1 indicating a potential therapeutic window for LIF/LIFR axis inhibitors in addition to less toxicity in normal adult tissues. A recent study demonstrated that LIF is a key paracrine factor from stromal cells acting on cancer cells; and LIF blockade or genetic LIFR deletion slows tumor progression, and augments the efficacy of chemotherapy to prolong survival of PDAC.15 It is suggested that LIF serves as a target to limit neural remodeling in pancreatic cancer, which contributes to poorer quality of life with increased metastatic progression and LIF-blocking antibody reduced intratumoral nerve density.57 Another study also confirmed that blockade of LIF by neutralizing antibodies represents an attractive approach to improving therapeutic outcome in KRAS driven pancreatic cancer.58
Concomitantly, antibodies to LIF significantly suppressed growth of breast, kidney and prostate cancer cells suggesting that LIF acts as either a paracrine or an autocrine growth factor for these solid cancers.13 Considering the importance of the LIF/LIFR pathway, Northern Biologics/Celgene recently developed a humanized Anti-LIF antibody (MSC-1) that blocks LIF signaling, and its utility is being tested in a phase I clinical trial to determine its safety and tolerability (ClinicalTrials.gov, NCT03490669). A recent study examined the antitumor effect of immunization against LIF and LIFR. This study found that immune-mediated blockage of LIF and LIFR delayed/prevented breast tumor growth and immunotherapy of LIFR was a more efficient approach in the inhibition of tumor growth compared to immunotherapy of LIF.59
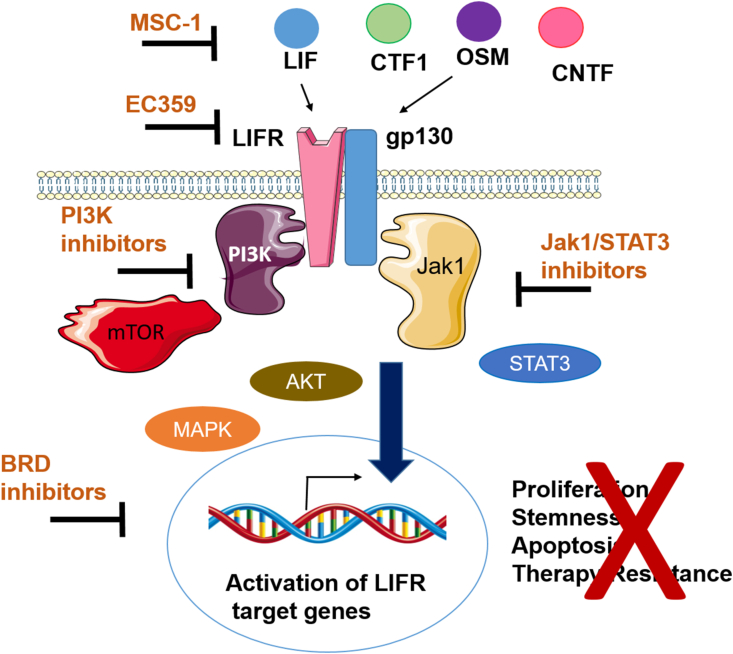
Recently, several small molecules that preferentially target LIF/LIFR axis were identified including EC330, EC357, and EC363 (US patent 10,053,485) (Fig. 2A). Of these molecules, EC330 efficiently inhibited LIF/LIFR mediated proliferation and tumor growth and molecular docking studies suggested EC330 target LIFR.55 Further optimization of EC330 to emulate the LIF binding site in LIFR and to improve PK properties resulted in the development of a first-in-class LIFR inhibitor (EC359) (Fig. 2A).56 The specificity of EC359 to target LIFR and its activity was rigorously tested using biophysical assays (SPR, MST, modeling, biotin pull down), and CRISPR KO cells. EC359 also has the ability to block signaling mediated by other cytokines (CTF1, CNTF, and OSM) that interact LIFR at LIF/LIFR interface.56 We speculate that the unique ability of EC359 to bind to common ligand binding site which blocks multiple ligands interaction with LIFR, offers an advantage over biologics or small molecules that can only target either of these ligands alone (Fig. 3). Modeling studies predicted that EC359 will interact at the LIF–LIFR binding interface and block the interaction of LIF to LIFR (Fig. 2). EC359 showed effectivity in preclinical xenograft and PDX models and has substantiated PK properties.56 Utilizing pancreatic cell line-derived organoids, EC359 is also shown to be effective in targeting oncogenic LIF/LIFR signaling in pancreatic tumor stroma.60 Since EC359 is a small, stable molecule; it is amenable for translation in cancer patients as monotherapy, or in combination with current standard of care.56
Figure 3.
Schematic depicting the drugs that inhibit LIF/LIFR downstream signaling. Anti LIF antibody MSC-1 or LIFR inhibitor EC359 can be used to directly interfere LIF/LIFR signaling. Targeting LIF/LIFR signaling using inhibitors of LIFR activated pathways including Jak1/STAT3 inhibitors, PI3K inhibitors or BRD inhibitors may also be useful to interfere LIF/LIFR signaling.
HDAC inhibition upregulates LIFR expression, activates JAK1-STAT3 signaling and JAK1 or BRD4 inhibition sensitizes BCa to HDAC inhibitors, implicating combination inhibition of HDAC with JAK1 or BRD4 as potential therapies for BCa (Fig. 3).61 Recent study showed that LIF functions in parallel with IL-6 to promote OCa growth and that therapeutic targeting of a single cytokine axis may be less effective.39 Therefore, combination therapy of LIF/LIFR inhibitors along with blockage of JAK/STAT pathway may be beneficial (Fig. 3).
Conclusion and future perspectives
In closing, LIF/LIFR signaling is implicated in the cancer progression and deregulation of LIF/LIFR axis occurs in many solid cancers. Preclinical studies using in vitro and in vivo models confirmed oncogenic functions of LIF/LIFR in tumor progression, stemness, therapy resistance, and alterations in tumor microenvironment. Humanized Anti-LIF antibody (MSC-1) therapeutics for targeting LIF are in Phase I clinical trials to test the efficacy of LIF antibody as a therapeutic in cancer (Fig. 3). However, LIFR can be activated by multiple ligands in addition to LIF, activating LIFR downstream pathways. Because cancer cells express multiple ligands that activate LIFR, it may be prudent to develop inhibitors that target common binding pocket for all the ligands in LIFR. EC359 is a rationally designed organic molecule that can emulate the LIF/LIFR binding site which functions as a first-in class LIFR inhibitor blocking its interactions with multiple ligands (Fig. 3). In preclinical studies EC359 showed effectivity; however, future studies are needed to better the understanding of LIF/LIFR signaling mechanisms in solid cancer cells using global approaches, validating efficacy of LIF/LIFR axis inhibitors in multiple solid tumor models and establishing LIF/LIFR signaling effects in modulating tumor micro environment.
Author contributions
S.V; D.K.V; K.Y.J.Z; H.B.N; R.K.V: Conceptualization, writing, reviewing and editing. All authors approved the final manuscript.
Conflict of interests
H.B.N is an employee of Evestra Inc. All other authors declare no conflicts of interest.
Funding
This work was supported by the DOD BCRP (No. W81XWH-18-1-0016 (R.K.V); W81XWH-18-1-0015 (H.B.N); NCI R44CA235991 (H.B.N)); NCI Cancer Center Support (No. P30CA054174-17); Elsa U. Pardee foundation (No. 166675-44096 (S.V), NIH (No. 1R01CA179120-01 (R.K.V)).
Acknowledgements
We thank Jessica Perry for proof reading the manuscript.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Nicola N.A., Babon J.J. Leukemia inhibitory factor (LIF) Cytokine Growth Factor Rev. 2015;26(5):533–544. doi: 10.1016/j.cytogfr.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stahl N., Boulton T.G., Farruggella T., et al. Association and activation of Jak-Tyk kinases by CNTF-LIF-OSM-IL-6 beta receptor components. Science. 1994;263(5143):92–95. doi: 10.1126/science.8272873. [DOI] [PubMed] [Google Scholar]
- 3.Taga T., Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol. 1997;15:797–819. doi: 10.1146/annurev.immunol.15.1.797. [DOI] [PubMed] [Google Scholar]
- 4.Kamohara H., Ogawa M., Ishiko T., Sakamoto K., Baba H. Leukemia inhibitory factor functions as a growth factor in pancreas carcinoma cells: involvement of regulation of LIF and its receptor expression. Int J Oncol. 2007;30(4):977–983. [PubMed] [Google Scholar]
- 5.Shin J.E., Park S.H., Jang Y.K. Epigenetic up-regulation of leukemia inhibitory factor (LIF) gene during the progression to breast cancer. Mol Cells. 2011;31(2):181–189. doi: 10.1007/s10059-011-0020-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morton S.D., Cadamuro M., Brivio S., et al. Leukemia inhibitory factor protects cholangiocarcinoma cells from drug-induced apoptosis via a PI3K/AKT-dependent Mcl-1 activation. Oncotarget. 2015;6(28):26052–26064. doi: 10.18632/oncotarget.4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi Y., Hunter S., Hunter T. Stem cell factor LIFted as a promising clinical target for cancer therapy. Mol Cancer Ther. 2019;18(8):1337–1340. doi: 10.1158/1535-7163.MCT-19-0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu S.C., Tsang N.M., Chiang W.C., et al. Leukemia inhibitory factor promotes nasopharyngeal carcinoma progression and radioresistance. J Clin Investig. 2013;123(12):5269–5283. doi: 10.1172/JCI63428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X., Yang Q., Yu H., et al. LIF promotes tumorigenesis and metastasis of breast cancer through the AKT-mTOR pathway. Oncotarget. 2014;5(3):788–801. doi: 10.18632/oncotarget.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu H.X., Cheng X., Jing X.Q., et al. LIFR promotes tumor angiogenesis by up-regulating IL-8 levels in colorectal cancer. Biochim Biophys Acta Mol Basis Dis. 2018;1864(9 Pt B):2769–2784. doi: 10.1016/j.bbadis.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Yu H., Yue X., Zhao Y., et al. LIF negatively regulates tumour-suppressor p53 through Stat3/ID1/MDM2 in colorectal cancers. Nat Commun. 2014;5 doi: 10.1038/ncomms6218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plun-Favreau H., Perret D., Diveu C., et al. Leukemia inhibitory factor (LIF), cardiotrophin-1, and oncostatin M share structural binding determinants in the immunoglobulin-like domain of LIF receptor. J Biol Chem. 2003;278(29):27169–27179. doi: 10.1074/jbc.M303168200. [DOI] [PubMed] [Google Scholar]
- 13.Kellokumpu-Lehtinen P., Talpaz M., Harris D., Van Q., Kurzrock R., Estrov Z. Leukemia-inhibitory factor stimulates breast, kidney and prostate cancer cell proliferation by paracrine and autocrine pathways. Int J Cancer. 1996;66(4):515–519. doi: 10.1002/(SICI)1097-0215(19960516)66:4<515::AID-IJC15>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 14.Wang D., Liu K., Yang Y., et al. Prognostic value of leukemia inhibitory factor and its receptor in pancreatic adenocarcinoma. Future Oncol. 2020;16(3):4461–4473. doi: 10.2217/fon-2019-0684. [DOI] [PubMed] [Google Scholar]
- 15.Shi Y., Gao W., Lytle N.K., et al. Targeting LIF-mediated paracrine interaction for pancreatic cancer therapy and monitoring. Nature. 2019;569(7754):131–135. doi: 10.1038/s41586-019-1130-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo H., Cheng Y., Martinka M., McElwee K. High LIFr expression stimulates melanoma cell migration and is associated with unfavorable prognosis in melanoma. Oncotarget. 2015;6(28):25484–25498. doi: 10.18632/oncotarget.4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao X., Xu M., Cai Z., Yuan W., Cui W., Li M.D. Identification of LIFR, PIK3R1, and MMP12 as novel prognostic signatures in gallbladder cancer using network-based module analysis. Front Oncol. 2019;9:325. doi: 10.3389/fonc.2019.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lv S., Ji L., Chen B., et al. Histone methyltransferase KMT2D sustains prostate carcinogenesis and metastasis via epigenetically activating LIFR and KLF4. Oncogene. 2018;37(10):1354–1368. doi: 10.1038/s41388-017-0026-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salm F., Dimitrova V., von Bueren A.O., et al. The phosphoinositide 3-kinase p110alpha isoform regulates leukemia inhibitory factor receptor expression via c-Myc and miR-125b to promote cell proliferation in medulloblastoma. PLoS One. 2015;10(4) doi: 10.1371/journal.pone.0123958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia-Tunon I., Ricote M., Ruiz A., Fraile B., Paniagua R., Royuela M. OSM, LIF, its receptors, and its relationship with the malignance in human breast carcinoma (in situ and in infiltrative) Cancer Investig. 2008;26(3):222–229. doi: 10.1080/07357900701638491. [DOI] [PubMed] [Google Scholar]
- 21.Liu B., Lu Y., Li Y., Liu J., Wang W. Leukemia inhibitory factor promotes tumor growth and metastasis in human osteosarcoma via activating STAT3. APMIS. 2015;123(10):837–846. doi: 10.1111/apm.12427. [DOI] [PubMed] [Google Scholar]
- 22.Zhang W., Shin X., Chen R., et al. Novel long non-coding RNA lncAMPC promotes metastasis and immunosuppression in prostate cancer by stimulating LIF/LIFR expression. Mol Ther. 2020;28(11):2473–2487. doi: 10.1016/j.ymthe.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yue X., Zhao Y., Zhang C., et al. Leukemia inhibitory factor promotes EMT through STAT3-dependent miR-21 induction. Oncotarget. 2016;7(4):3777–3790. doi: 10.18632/oncotarget.6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bian S.B., Yang Y., Liang W.Q., Zhang K.C., Chen L., Zhang Z.T. Leukemia inhibitory factor promotes gastric cancer cell proliferation, migration, and invasion via the LIFR-Hippo-YAP pathway. Ann N Y Acad Sci. 2021;1484(1):74–89. doi: 10.1111/nyas.14466. [DOI] [PubMed] [Google Scholar]
- 25.Chen D., Sun Y., Wei Y., et al. LIFR is a breast cancer metastasis suppressor upstream of the Hippo-YAP pathway and a prognostic marker. Nat Med. 2012;18(10):1511–1517. doi: 10.1038/nm.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson R.W., Finger E.C., Olcina M.M., et al. Induction of LIFR confers a dormancy phenotype in breast cancer cells disseminated to the bone marrow. Nat Cell Biol. 2016;18(10):1078–1089. doi: 10.1038/ncb3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Auernhammer C.J., Melmed S. Leukemia-inhibitory factor-neuroimmune modulator of endocrine function. Endocr Rev. 2000;21(3):313–345. doi: 10.1210/edrv.21.3.0400. [DOI] [PubMed] [Google Scholar]
- 28.Duluc D., Delneste Y., Tan F., et al. Tumor-associated leukemia inhibitory factor and IL-6 skew monocyte differentiation into tumor-associated macrophage-like cells. Blood. 2007;110(13):4319–4330. doi: 10.1182/blood-2007-02-072587. [DOI] [PubMed] [Google Scholar]
- 29.Zhao X., Ye F., Chen L., Lu W., Xie X. Human epithelial ovarian carcinoma cell-derived cytokines cooperatively induce activated CD4+CD25-CD45RA+ naïve T cells to express forkhead box protein 3 and exhibit suppressive ability in vitro. Cancer Sci. 2009;100(11):2143–2151. doi: 10.1111/j.1349-7006.2009.01286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pascual-García M., Bonfill-Teixidor E., Planas-Rigol E., et al. LIF regulates CXCL9 in tumor-associated macrophages and prevents CD8+ T cell tumor-infiltration impairing anti-PD1 therapy. Nat Commun. 2019;10(1) doi: 10.1038/s41467-019-10369-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen L.L., Ye F., Lü W.G., Lü W.G., Chen H.Z., Xie X. Evaluation of immune inhibitory cytokine profiles in epithelial ovarian carcinoma. J Obstet Gynaecol Res. 2009;35(2):212–218. doi: 10.1111/j.1447-0756.2008.00935.x. [DOI] [PubMed] [Google Scholar]
- 32.Daley-Brown D., Oprea-Ilies G.M., Lee R., Pattillo R., Gonzalez-Perez R.R. Molecular cues on obesity signals, tumor markers and endometrial cancer. Horm Mol Biol Clin Investig. 2015;21(1):89–106. doi: 10.1515/hmbci-2014-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang T.S., Gao F., Qi Q.R., et al. Dysregulated LIF-STAT3 pathway is responsible for impaired embryo implantation in a streptozotocin-induced diabetic mouse model. Biol Open. 2015;4(7):893–902. doi: 10.1242/bio.011890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gonzalez R.R., Rueda B.R., Ramos M.P., Littell R.D., Glasser S., Leavis P.C. Leptin-induced increase in leukemia inhibitory factor and its receptor by human endometrium is partially mediated by interleukin 1 receptor signaling. Endocrinology. 2004;145(8):3850–3857. doi: 10.1210/en.2004-0383. [DOI] [PubMed] [Google Scholar]
- 35.Chen J.R., Cheng J.G., Shatzer T., Sewell L., Hernandez L., Stewart C.L. Leukemia inhibitory factor can substitute for nidatory estrogen and is essential to inducing a receptive uterus for implantation but is not essential for subsequent embryogenesis. Endocrinology. 2000;141(12):4365–4372. doi: 10.1210/endo.141.12.7855. [DOI] [PubMed] [Google Scholar]
- 36.Albrengues J., Bourget I., Pons C., et al. LIF mediates proinvasive activation of stromal fibroblasts in cancer. Cell Rep. 2014;7(5):1664–1678. doi: 10.1016/j.celrep.2014.04.036. [DOI] [PubMed] [Google Scholar]
- 37.Peñuelas S., Anido J., Prieto-Sánchez R.M., et al. TGF-beta increases glioma-initiating cell self-renewal through the induction of LIF in human glioblastoma. Cancer Cell. 2009;15(4):315–327. doi: 10.1016/j.ccr.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 38.Wu L., Yu H., Zhao Y., et al. HIF-2α mediates hypoxia-induced LIF expression in human colorectal cancer cells. Oncotarget. 2015;6(6):4406–4417. doi: 10.18632/oncotarget.3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McLean K., Tan J., Bolland D.E., et al. Leukemia inhibitory factor functions in parallel with interleukin-6 to promote ovarian cancer growth. Oncogene. 2019;38(9):1576–1584. doi: 10.1038/s41388-018-0523-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nguyen H.N., Noss E.H., Mizoguchi F., et al. Autocrine loop involving IL-6 family member LIF, LIF receptor, and STAT4 drives sustained fibroblast production of inflammatory mediators. Immunity. 2017;46(2):220–232. doi: 10.1016/j.immuni.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bao B., Prasad A.S. Targeting CSC in a most aggressive subtype of breast cancer TNBC. Adv Exp Med Biol. 2019;1152:311–334. doi: 10.1007/978-3-030-20301-6_17. [DOI] [PubMed] [Google Scholar]
- 42.Creighton C.J., Li X., Landis M., et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci U S A. 2009;106(33):13820–13825. doi: 10.1073/pnas.0905718106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee K.L., Kuo Y.C., Ho Y.S., Huang Y.H. Triple-negative breast cancer: current understanding and future therapeutic breakthrough targeting cancer stemness. Cancers. 2019;11(9) doi: 10.3390/cancers11091334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Idowu M.O., Kmieciak M., Dumur C., et al. CD44(+)/CD24(-/low) cancer stem/progenitor cells are more abundant in triple-negative invasive breast carcinoma phenotype and are associated with poor outcome. Hum Pathol. 2012;43(3):364–373. doi: 10.1016/j.humpath.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 45.Cartwright P., McLean C., Sheppard A., Rivett D., Jones K., Dalton S. LIF/STAT3 controls ES cell self-renewal and pluripotency by a Myc-dependent mechanism. Development. 2005;132(5):885–896. doi: 10.1242/dev.01670. [DOI] [PubMed] [Google Scholar]
- 46.Kuphal S., Wallner S., Bosserhoff A.K. Impact of LIF (leukemia inhibitory factor) expression in malignant melanoma. Exp Mol Pathol. 2013;95(2):156–165. doi: 10.1016/j.yexmp.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 47.Lin W.H., Chang Y.W., Hong M.X., et al. STAT3 phosphorylation at Ser727 and Tyr705 differentially regulates the EMT-MET switch and cancer metastasis. Oncogene. 2021;40(4):791–805. doi: 10.1038/s41388-020-01566-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woosley A.N., Dalton A.C., Hussey G.S., et al. TGFβ promotes breast cancer stem cell self-renewal through an ILEI/LIFR signaling axis. Oncogene. 2019;38(20):3794–3811. doi: 10.1038/s41388-019-0703-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu J., Yu H., Hu W. LIF is a new p53 negative regulator. J Nat Sci. 2015;1(7) [PMC free article] [PubMed] [Google Scholar]
- 50.Hellweg R., Mooneyham A., Chang Z., et al. RNA sequencing of carboplatin- and paclitaxel-resistant endometrial cancer cells reveals new stratification markers and molecular targets for cancer treatment. Horm Cancer. 2018;9(5):326–337. doi: 10.1007/s12672-018-0337-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duan Z., Foster R., Bell D.A., et al. Signal transducers and activators of transcription 3 pathway activation in drug-resistant ovarian cancer. Clin Cancer Res. 2006;12(17):5055–5063. doi: 10.1158/1078-0432.CCR-06-0861. [DOI] [PubMed] [Google Scholar]
- 52.Lin S.R., Wen Y.C., Yeh H.L., et al. EGFR-upregulated LIFR promotes SUCLG2-dependent castration resistance and neuroendocrine differentiation of prostate cancer. Oncogene. 2020;39(44):6757–6775. doi: 10.1038/s41388-020-01468-9. [DOI] [PubMed] [Google Scholar]
- 53.Buckley A.M., Lynam-Lennon N., Kennedy S.A., et al. Leukaemia inhibitory factor is associated with treatment resistance in oesophageal adenocarcinoma. Oncotarget. 2018;9(72):33634–33647. doi: 10.18632/oncotarget.25950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang L., Wang Q., Gao M., et al. STAT3 activation confers trastuzumab-emtansine (T-DM1) resistance in HER2-positive breast cancer. Cancer Sci. 2018;109(10):3305–3315. doi: 10.1111/cas.13761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yue X., Wu F., Wang J., et al. EC330, a small-molecule compound, is a potential novel inhibitor of LIF signaling. J Mol Cell Biol. 2020;12(6):477–480. doi: 10.1093/jmcb/mjaa008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Viswanadhapalli S., Luo Y., Sareddy G.R., et al. EC359: a first-in-class small-molecule inhibitor for targeting oncogenic LIFR signaling in triple-negative breast cancer. Mol Cancer Ther. 2019;18(8):1341–1354. doi: 10.1158/1535-7163.MCT-18-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bressy C., Lac S., Nigri J., et al. LIF drives neural remodeling in pancreatic cancer and offers a new candidate biomarker. Cancer Res. 2018;78(4):909–921. doi: 10.1158/0008-5472.CAN-15-2790. [DOI] [PubMed] [Google Scholar]
- 58.Wang M.T., Fer N., Galeas J., et al. Blockade of leukemia inhibitory factor as a therapeutic approach to KRAS driven pancreatic cancer. Nat Commun. 2019;10(1) doi: 10.1038/s41467-019-11044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ghanei Z., Mehri N., Jamshidizad A., Joupari M.D., Shamsara M. Immunization against leukemia inhibitory factor and its receptor suppresses tumor formation of breast cancer initiating cells in BALB/c mouse. Sci Rep. 2020;10(1) doi: 10.1038/s41598-020-68158-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hall B.R., Cannon A., Thompson C., et al. Utilizing cell line-derived organoids to evaluate the efficacy of a novel LIFR-inhibitor, EC359 in targeting pancreatic tumor stroma. Genes Cancer. 2019;10(1–2):1–10. doi: 10.18632/genesandcancer.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zeng H., Qu J., Jin N., et al. Feedback activation of leukemia inhibitory factor receptor limits response to histone deacetylase inhibitors in breast cancer. Cancer Cell. 2016;30(3):459–473. doi: 10.1016/j.ccell.2016.08.001. [DOI] [PubMed] [Google Scholar]