Abstract
Cancer is one of those leading diseases worldwide, which takes millions of lives every year. Researchers are continuously looking for specific approaches to eradicate the deadly disease, ensuring minimal adverse effects along with more therapeutic significance. Targeting of different aberrantly regulated signaling pathways, involved in cancer, is surely one of the revolutionary chemotherapeutic approach. In this instance, GSK3 and PI3K signaling cascades are considered as important role player for both the oncogenic activation and inactivation which further leads to cancer proliferation and metastasis. In this review, we have discussed the potential role of GSK3 and PI3K signaling in cancer, and we further established the crosstalk between PI3K and GSK3 signaling, through showcasing their cross activation, cross inhibition and convergence pathways in association with cancer. We also exhibited the effect of GSK3 on the efficacy of PI3K inhibitors to overcome the drug resistance and preventing the cell proliferation, metastasis in a combinatorial way with GSK3 inhibitors for a better treatment strategy in clinical settings.
Keywords: Cancer, Chemotherapy, Drug resistance, GSK3, PI3K
Introduction
Cancer is the leading cause of morbidity and mortality across the world. In 2020, approximately 19.3 million new cancer cases were diagnosed worldwide, with over 10.0 million cancer deaths.1 In India the projected cancer incidence among males is 679,421 (94.1 per 100,000) and among females 712,758 (103.6 per 100,000).2 The actual cause of cancer cannot be summarized as it is induced by a variety of pharmacological variables, including genetic changes, modifications of signaling pathways and the production of numerous proteins essential for the regulation of normal cellular activities.
The recent treatment strategies for the cancer include chemotherapy, radiotherapy and surgery which come with a number of side effects such as cancer relapse, drug resistance and indiscriminate destruction of non-cancerous cells. So the advance strategies mainly focused on the targeted therapy towards specific proteins to eliminate nonspecific lethality and cytotoxicity towards the healthy cells. The targeted cancer chemotherapy has gained popularity since the approval of imatinib, a tyrosine kinase inhibitor, used for the treatment of chronic myelogenous leukemia (CML).3 Following this innovation every attempt to introduce a novel molecule for targeted therapy resulted in unavoidable adverse effect, particularly drug resistance.4 This event has sparkled an interest in exploring new sites for targeted chemotherapy that do not acquire drug resistance, which demands a detailed understanding of the targets as well as the signaling pathways that may adapt to build resistance to that specific protein of interest.
The incidence of cancer progression is associated with the oncogenic mutations which causes the over expression of mutated genes along with the deregulation of several mutated proteins. Among these large number of cancer modulated signaling pathways and proteins, phosphatidylinositol-3 kinase (PI3K), a functional protein, over expressed in most of the human cancers, which is directly involved in the regulation of cellular growth and cell survival.5,6 Numerous studies have been conducted on preclinical models of human cancers using the particular PI3K inhibitor, with good anticancer efficacy, but only a few of them were allowed for chemotherapeutic study as clinical trials, with disappointing outcomes due to drug resistance.7,8 This network seems to be regulated through a number of signaling pathways among which GSK3 singling is important in the cancer chemotherapeutic study and also act as a potential target in the treatment of cancer.
Glycogen synthase kinase-3 (GSK3) is ubiquitously expressed serine/threonine kinase, and a multifunctional monomeric protein. This is responsible for the regulation of various cellular functions such as differentiation, survival, glycogen metabolism, protein synthesis, immune responses and cell death. This is an intermediate protein component of several signaling pathways including Wnt/β-catenin, Hedgehog, nuclear factor κB (NF-κB), and transforming growth factor-β (TGF-β) signaling pathways and also plays important role as a key mediator of Insulin/Insulin receptor (IR), Insulin-like growth factor (IGF)/IGF receptor (IGFR).9 As a multifunctional protein, the role of GSK3 in cancer has been assessed through a number of preclinical chemotherapeutic studies, which suggest the dual role of GSK3 in cancer. The GSK3β, a pivotal isoform of GSK3, is a key regulator of PI3K and Wnt signaling pathway which are directly involved in the cancer development. On the other hand, activation of GSK3β caused ubiquitin mediated proteosomal degradation of phosphorylated β-catenin10 and thus showing anticancer activity through the inhibition of Wnt-β-catenin singling pathway.
Further research instigated the modulation of mTOR signaling via GSK3 signaling pathway in the context of targeted cancer chemotherapy. The influence of GSK3 activation and inactivation on drug resistance to mTOR inhibitors was revealed, which included a thorough molecular interaction between these two signaling pathways.11 In the regulation of mTOR signaling, PI3K plays a pivotal role which is also associated with the cancer cell survival and proliferation but there is lack of crosstalk between PI3K signaling and other signaling pathway in relation to the cancer chemotherapeutics.
On the basis of recent findings, the proposed study emphasized the impact of GSK3 inducer and inhibitor on the efficacy of PI3K inhibitor through cross activation and cross inhibition of the protein component related to both of the signaling pathways for effective targeted therapy of cancer.
PI3K
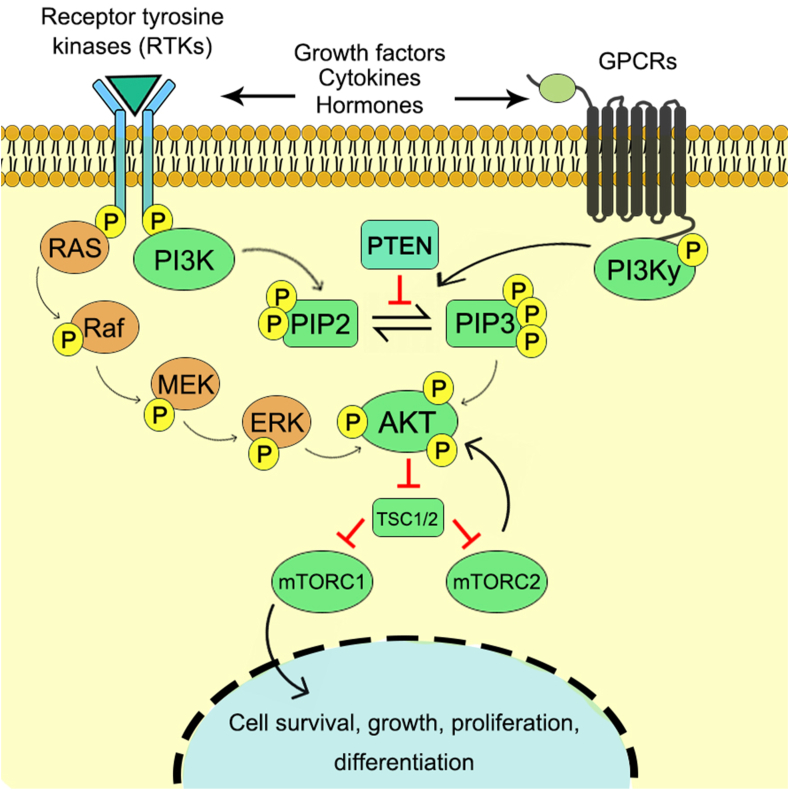
The PI3K is an important component of the PI3K/Akt/mTOR pathway which is a crucial intracellular signaling, mainly responsible for the cellular growth, cell differentiation, cell survival, cellular metabolism and reorganization of cytoskeleton12 (Fig. 1). This signaling pathway is stimulated by a number of oncogenic proteins and growth factor receptors which include insulin receptor tyrosine kinase (InsR), the related insulin-like growth factor 1 receptor (IGF-1R), epidermal growth factor (EGF), platelet-derived growth factor receptors (PDGF-R).13
Figure 1.
PI3K/Akt/mTOR signaling cascade towards cell growth, proliferation and differentiation.
Structure and activation
PI3K is an intracellular phosphatidylinositol kinase. The PI3K family of enzymes can be divided into three classes which are based on their structure and preference of substrates. The class I PI3Ks are the heterodimers which consists of regulatory and catalytic subunits that can be further classified into class IA and class IB according to the type of subunits.14 Class II PI3Ks are basically monomers which are composed of one of three different 110-kDa catalytic isoforms such as C2α, C2β and C2γ. Class III PI3Ks are composed of heterodimeric structure which consists of a regulatory subunit Vps15/p150 and a catalytic subunit Vps34.15 The main substrate of PI3K is phosphatidylinositol (PtdIns) lipid matrix such as phosphatidylinositol-4-phosphate (PIP) and phosphatidylinositol-4,5-diphosphate (PIP2).16 After the activation of PI3K through various extracellular stimuli, such as growth factors, cytokines, and hormones,17 it causes the phosphorylation of PIP2 to PIP3 and recruits several lipid binding domains to the cell membrane for downstream cascade. This in turn activates other signaling proteins such as kinases AKT and PDK1, which binds to the lipid products of PI3K and thus activates cell growth and cell survival pathways.18 Phosphatase and tensin homologue deleted on chromosome 10 (PTEN) is an important protein component for the regulation of PI3K signaling pathway through the dephosphorylation of PIP3 to PIP2 and inhibits the activation of downstream kinases.19
Oncogenic activation
Several oncoproteins and tumor suppressors are involved in cellular metabolism and regulate various signaling pathways in conjunction with PI3K signaling to maintain cellular equilibrium, which is hampered in several cancer microenvironments due to the activation and inactivation of these interrelated proteins.18,20 The alteration of PI3K signaling pathway is one of the primary reasons for cancer initiation due to oncogenic mutation and inactivation of tumor suppressor proteins through genetic remodulations.21
Mutation of PI3K
The mutation in the PI3K, more specifically at the PIK3CA gene encoding site that encodes for the catalytic subunit of Class IA PI3K, p110α, is a major reason for the alteration of PI3K signaling and cancer propagation.22,23 Generally most of the mutation in PI3K appears around two hotspots: E545K (exon 9) in the helical phosphatidylinositol kinase homology domain, which suppresses the p85 mediated inhibition of p110α; H1047 (exon 20) near the end of the catalytic domain enhancing the interaction between p110α and lipid membranes.24 Along with that, mutation on the C2 domain is also significant for the PIK3CA mutation.25 On the other hand mutations on the other catalytic subunits such as p110β, p110γ and p110δ are rare, which are also responsible for the induction of oncogenic phenotype.26
PTEN inactivation
PTEN, a tumor suppressor protein, regulates the cell survival and cellular metabolism. Inactivation of this negative regulatory protein has been associated with both heritable and sporadic malignances such as breast cancer, and prostate cancer.27,28 Any alteration in the expression of PTEN showed a major impact in the normal cellular function. According to the research conducted by Papa and his co researchers, a mutation on PTEN causes significant inhibition of PTEN lipid–phosphatase activity which leads to the enhanced activity of PI3K signaling and ultimately tumorigenesis. The carcinogenic outcome corresponds to the over activity of Akt due to the inhibition of PTEN lipid–phosphatase activity.29
Regulation of non-coding RNA (ncRNAs) and other factors
It has been observed that the PI3K signaling pathway is activated through the lncRNA CRNDE and thus promotes cell proliferation with high expression of the ncRNA in various cancer patients with non-small cell lung cancer, colorectal cancer, gastric cancer, cervical cancer, heptaocellular carcinoma and gallbladder cancer.30,31 The ncRNA is also shown to have inhibitory effect on the PI3K signaling pathway. In the tumor microenvironment the lncRNA GAS5 expression is lower and the over-expression of it inhibits cancer cell proliferation and migration.32
Shaping of tumor microenvironment (TME)
The tumor microenvironment is composed of heterogeneous cell population such as endothelial cells, pericytes, cancer associated fibroblasts (CAFs), stromal cells and host immune cells including tumor-associated myeloid cells.33 These large numbers of cellular components interact with each other via a complicated network that consists of cytokines, mitogens and growth factors which facilitates tumor growth and proliferation. In this regard, PI3K signaling plays a significant role in the oncogenic activation through proliferation, differentiation and migration of these cells thus facilitating the modulation of TME.34
PI3K in angiogenic activation
In the process of tumor growth and metastasis, angiogenesis plays an important role through the sufficient blood supply at the TME. The process of angiogenesis in the TME is regulated by a number of signaling pathways in which the cancer cells can elicit the vascular growth through the modulation of the angiogenic equilibrium maintained by several signaling molecules. In the activation of angiogenic response, PI3K signaling plays a multifaceted role in both malignant and nonmalignant cells.35 The activation of the angiogenic response in the TME is stimulated by hypoxia and nutrient deprivation due to rapid tumor growth which causes the secretion of proangiogenic growth factors and cytokines. As per the previous studies, the activation of PI3K signaling is important for the hypoxia-inducible factor (HIF)-1α-mediated secretion of VEGF.36 Inactivation of PI3K signaling through the expression of PTEN or a dominant-negative mutant Akt can downregulate the HIF-1 and VEGF expression in TME.37 According to a murine study, it has been showed that activation of PI3K p110γ promotes the myeloid cell trafficking to tumors, which facilitates tumor growth, invasion, angiogenesis and metastasis.38
PI3K in remodeling of extracellular matrix (ECM)
In the TME, the ECM is mainly secreted by cancer associated fibroblasts (CAFs) which provides structural scaffolding with biochemical and biomechanical cues surrounding cells thus leads to the cell differentiation, proliferation and migration. CAF is an essential component of ECM which is responsible for the cancer progression and metastasis.39 Various PI3K associated pathways including TGF-β, HGF, EGF and PDGF signaling pathways are closely related with the stromal cell differentiation into CAFs and CAF activity.40,41 Another essential factor for the ECM remodeling is matrix metalloproteinase (MMP), which are also associated with the activation of PI3K signaling.42
PI3K in autophagy regulation
The process of autophagy can be defined as a recycling process that is associated with the breakdown of the proteins and cellular organelles to their macromolecular precursor. TE cells undergoes autophagy in response to the cellular stress and which may lead to both the cellular death or cell survival depending upon the circumstance.43 The PI3K pathway has been identified as the major regulatory pathway for autophagy.44 The mTORC2 protein is directly involved in the regulation of autophagic proteins which is a direct downstream regulatory component of PI3K signaling axis.45 Besides that, the PI3K pathway also plays an important role in the integration of the cellular signals in association with several other pathways including Ras/Raf/MEK/ERK and MAPK/JNK pathways to regulate the autophagic responses.46 The initiation of autophagy occurs in two ways by the action of Unc-51-like kinase (ULK) complex. First, in the vicinity of membraned organelles the ULK complex gets accumulated. Second, the ULK complex binds with the ubiquitinated cellular components. Both the pathways ultimately lead to the accumulation and activation of ULK complex and triggers autophagy.47 The mTOR protein involved in the inhibition of this complex and I3K pathway regulates the fate of autophagic response extensively.48 The PI3K pathway is also involved in the ROS mediated regulation of autophagy which maintains ROS homeostasis subjected to cellular growth and proliferation.49
Regulation of cancer stemness
In the TME, among various types of cells, cancer stem cells (CSCs) are very unique subpopulation, which are self-renewable and responsible for tumor recurrence, metastasis, and progression.50 Dedifferentiation and regulation of stemness is mostly linked to the expression of PIK3CA cancer hotspot variant, H1047R, in various cancer model systems such as breast,51 lung,52 colorectal cancers.53 The activation of EMT, epithelial-to-mesenchymal transition, is also linked with the oncogenic activation of PI3Kα.54 The development of autocrine signaling loops, particularly the TGFβ pathway mediated pro-tumorigenic action, is thought to constitute a link between cancer stemness and EMT.55,56 In cancer-relevant cell models, there is substantial evidence for a connection between TGFβ and PI3K signaling in the stemness regulation.57
Thus, the role of PI3K signaling in the cancer microenvironment is obvious from the aforementioned findings, suggesting this protein as an important target for targeted chemotherapeutic research. Though PI3K has some anticancer immune responsive function, the impact on suppressive immune cell subsets outweighs the benefits of a stronger immune response against cancer and certain infectious pathogens.58 Considering this, a substantial number of PI3K inhibitors have already received approval for clinical trials.59
The PI3K pathway and drug resistance
The PI3K signaling pathway and its downstream signaling components have been identified as a important targets in the development of chemoresistance against cancer therapy in a number of malignancies.60,61 Abnormal activation of this signaling pathway causes inhibition of chemotherapy induced apoptosis via distinct mechanisms such as activation of anti-apoptotic genes and inactivation of pro-apoptotic genes thus develops drug resistance.62 Activation of Akt triggers PAK1 and phosphorylates Bad protein which released from mitochondrial membrane to cytoplasm and on the other hand prevents the translocation of Bax from cytoplasm to mitochondria. Therefore, abnormal activation of Akt stimulates the cell survival through the stimulation of Bcl2 and inhibition of Bax.63,64 The alteration of PI3K signaling pathway also stimulates the expression of X-linked inhibitor of apoptosis (XIAP), a downstream effector of Akt, which inhibit apoptosis through the regulation of cytosolic p53 level.65 The overactivation of NF-κB system by the alteration of PI3K signaling network also leads to tumor growth and apoptosis inhibition. The NF-κB pathway stimulates the process of cell cycle and thus causes development of multidrug resistance in cancer cells.66,67 The inactivation of FOX factors due to abnormal activation of PI3K signaling cascade also leads to drug resistance.68 The overactivation of Akt1 stimulates HER2/PI3K signaling which is responsible for chemoresistance in breast cancer.69 The deregulation of micro-RNA also exerts a significant effect in the development of drug resistance.70
GSK3
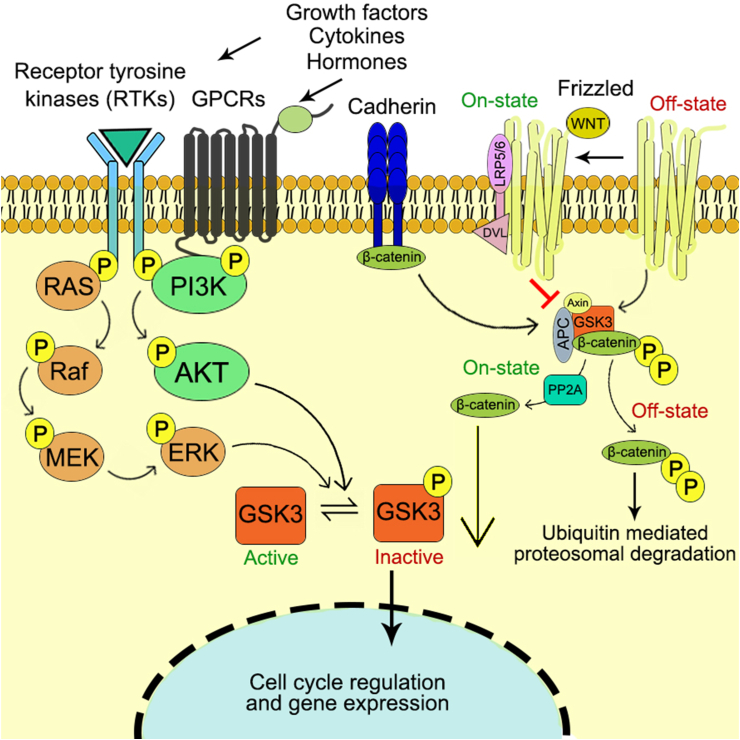
The first ever identified GSK3 was introduced in 1980s for their function in insulin signaling, since then GSK3 has been studied and found to be mediated by different molecular pathways such as Notch signaling, Wnt signaling, phospinositide 3-kinase signaling pathway.71,72 The schematic diagram representing the detailed GSK3 signaling cascade has been depicted in Fig. 2. GSK3 has significant involvement in different cellular pathways including PI3K/Akt/mTORC1, Wnt and Hedgehog signaling. Thus any alteration of GSK3 leads to different disease conditions such as diabetes, cardiovascular diseases and cancer.73,74
Figure 2.
GSK3 signaling cascade towards cell cycle regulation and gene expression profiling.
Structure and regulation of GSK3
GSK3 is a member of CMGC family, a reserved serine/threonine kinase. In eukaryotes GSK3α (51 Da) and GSK3β (47 kDa) are encoded by GSK-3A and GSK-3B genes.75 GSK3 is made up of two domains: a strand with seven anti parallel β-strands interrupted by a short helix of highly conserved regions and α-helix. A hinge region and a glycine-rich loop surround the catalytic site at the interface of the two domains.76 These two isoforms share 98% similarity in their kinase domain and 36% in their C-terminal. Additionally, GSK3α is comprised of a glycine contained extensions at their amino terminal which lacks in GSK3β. These isoforms can be divided based on their cellular functions, patterns and substrate of interest.9
GSK3 activation and inactivation
The activation and inactivation of GSK3 is controlled by the post translational, site specific phosphorylation of serine and tyrosine residues. In normal resting cells, GSK3α and GSK3β remain active. They enhance the catalytic action at the position of Y279 and Y216, which has a constitutive function in resting cells.77 Additionally, it has been shown that Y216 phosphorylation is also induced by apoptotic stimulus.78 Sometimes, proline rich tyrosine kinase 2 and Fyn tyrosine kinase helps in the phosphorylation and causing the activation of GSK3.79,80
On the other hand, activation of insulin mediated phosphatidylinositol 3-kinase (PI3K), PDGF and EGF helps in the inactivation of GSK3. Besides that, upregulatory kinases phosphorylate GSK3β at S9 residue and inactivate GSK3 including protein kinase A, protein kinase B/Akt, mitogen activated kinase.81,82 Followed by the activation, GSK3 phosphorylate different downstream substrates. GSK3α and GSK3β have greater affinity towards the substrates which are already phosphorylated by different protein kinases (priming kinases). Additionally, the substrate which contains priming phosphate gets phosphorylated by GSK3 easily than that which lacks priming phosphate.72
GSK3 in cancer
GSK3 signaling has been found to be associated with several intracellular pathways which attribute to different modes of oncogenic regulation. GSK3 involves in the process of cell survival through the regulation of NF-κB pathway and repress Wnt/β–Catenin, showing tumor suppressor activity. GSK3 is also associated with Wnt and Hedgehog pathway, promoting variety of cancers such as melanoma, hepatocellular carcinoma, prostate, colorectal cancer.83,84
Induction and suppression of malignancy
GSK3 over expression has been found to be associated with tumor promoting activity in several cancer models. As per the earlier studies, over expression of GSK3 promotes cell proliferation, survival, invasion, and angiogenesis. Activation of GSK3β induces kinase activity and tumor dedifferentiation resulting in brain cancer, along with that; it also decreases the patient survival rate in glioblastoma multiforme.85 Low level of pSer9 GSK3β expression along with the active GSK3 induces cellular growth in pancreatic, renal and colorectal cancer. Inactivation of GSK3β triggers apoptosis and control proliferation in these cancers.86 It has been also found that, GSK3 up regulation is also associated with a worse survival rate in endometrial cancer and it is positively correlated with prognosis, dedifferentiation, and myometrial infiltrate level.87 In blood cancer, splicing of GSK3β mRNA up regulate the β-Catenin level and activate stem cells.88 Hence, the inhibition of GSK3 possesses a novel approaches in the cancer chemotherapy.
In contrast, tumor suppressive function of GSK3β is also instigated through the inactivation of the related genes which causes cancer. In dermal malignancy, the over expression of pSer9 GSK3β and low level of pTyr216 GSK3β was observed.89 The mutation or inhibition of GSK3β is also correlated with the induction of breast carcinoma.90 The sequential expression of pS21 GSK3α and pS9 GSK3β also contribute in the oral cancer progression.91
Cell cycle regulation
Cyclins, cyclin dependent kinase inhibitors (CDK) and check point inhibitors are important protein which has a crucial role in cell cycle regulation. GSK3 is associated with the phosphorylation of these proteins and shown to have crucial effect on the cell cycle.91 The phosphorylation of cyclin D1 at T286 and cyclin E at T380 is regulated by GSK3, which is essential for nuclear export and break down.92,93 Additionally, the suppression of cyclin D1/CDK4/6 complex mediated phosphorylation of Rb tumour suppressor protein leads to the inhibition of tumour GSK3β activity.93 GSK3 influences the RNA synthesis of cell cycle checkpoint blockers such as GADD45 and GADD153 through the expression of the tumour suppressor TP53, which increases in response to DNA damage.94
Role in apoptosis
Several proteins are involved in the cellular apoptosis which act as a potential target for GSK3β. Among them the anti-apoptotic protein such as, few Bcl-2 sub types has been found to have great affinity towards GSK3β.78 Two major transcription factor, CREB factor and c-Myb get phosphorylated by the GSK3β, which alter the Bcl-2 expression.95 GSK3β mediated inhibition of ubiquitin-dependent destruction of c-Myb and transcriptional activation enhancement of the Bcl-2 promotes cellular proliferation and survival of the blood cancer cell.96 Besides that, GSK3β also promotes the phosphorylation of Bax that leads to mitochondrial localization and trigger the proapoptotic activity in the neuronal cells.97 From these above findings, the multi-disciplinary role of GSK3β can be found in cellular apoptosis in different cancer model systems.
Role in autophagy
Autophagy is a complex cellular phenomenon which involved diverse molecular machinery including mTOR, a key component in the autophagy regulation.98 mTOR senses the intracellular amino acids, ATP, hormones and eventually inhibits the process of autophagy. GSK3 also possessed a vital role in the autophagy regulation through the activation of mTORC1 and thus inhibits autophagy.99 Additionally, the increased expression of GSK3 subunits associated with the activation of MTORC1 through the phosphorylation of mTOR associated scaffold protein Raptor at Ser859 and leads to the inhibition of autophagic responses. The inactivation of GSK3 causes inhibition of mTOR and raptor via reduced phosphorylation of both p70S6K1, ULK-1 and triggers autophagic responses.100 The overexpression of GSK3 also causes an increase in the number of autophagosome through the inhibition of autophagic flux via inhibition of lysosomal acidification.101 Moreover, GSK3 regulates the transcription factor EB (TFEB), a key regulator of autophagy and lysosomal biogenesis.102 In prostate cancer inhibition of GSK3β induces autophagy through the increase in AMP/ATP ratio, which triggers the activation of AMPK.103 Along with that, GSK3 inhibits mTOR through the phosphorylation of TSC2 in an AMPK-priming phosphorylation-dependent manner.104
Role in tumour invasion
The epithelial–mesenchymal transition (EMT) can be distinguished by the loss of function of E-cadherin, which is one of the initial steps in tumour invasion. GSK3 suppresses EMT by blocking Snail, which works as an E-cadherin transcriptional repressor. GSK3 mediated phosphorylation of Snail leads to the nuclear transportation, proteosomal degradation followed by elevated E-cadherin and decreased matrix metalloproteinase (MMP) expression.105 Further studies showed that inhibition of both GSK3 isoforms triggered Snail expression, whereas inhibition of either isoform alone did not exhibit any effect indicating that activity of the both isoforms is necessary for the maintenance of epithelial morphology.106 Besides that, GSK3 has also been demonstrated to suppress Snail transcription through the suppression of NF-κB and β-catenin signalling molecules. Thus, the role of GSK3 in the regulation of EMT through various signalling pathways has been identified.9
miRNAs and GSK3
OncomiRs
miR-26a is a direct target of GSK3, which increase the metastasis in lung cancer through the inactivation of GSK3β, which leads to the β-catenin nuclear translocation and enhance the regulation of C-myc and cyclin D1.107 On the other hand, the over activation of GSK3β can alternate the oncogenic activity of miR-26a. In mammary carcinoma, GSK3β get targeted by the over activated miR-1229 and increase proliferation and trigger wnt/β-catenin pathway.108 There are various oncomiRs which play significant roles in different ways.109,110
Tumour suppressor miRs
According to dual-luciferase and xenograft reports from the bioinformatics study, GSK3β might be a potential target of miR-129 in the endometrial carcinoma. The GSK3β inhibitor AZD1080 protect the endometrial cells from the uncontrolled growth, invasion and regulate the apoptosis via GSK3β targeted genes.87 In oral cancer, both miR-99b-3p expression and GSK3β silencing are crucial for the prevention of oral carcinoma through the suppression of GSK3β.111
Stemness
Stem-cell-like phenotype promotion is one of the functions of overexpressed miR-744 in case of pancreatic cancer cells. These miR-44 overexpression targets GSK-3β to enhance Wnt/β-catenin signaling.112 In case of CD133 liver cancer stem cells, miR-1246 can promote the Wnt/β-catenin pathway and reduce GSK3β activity which is important for the enzyme mediated self-regulation and uncontrolled growth of cancer cells. These incidences provide new opportunities for the development of novel therapeutic approaches.113
Role in drug resistance
GSK3 singling pathway has been demonstrated to be involved in the development of chemoresistance by the action of GSK3β particularly. In glioma, the nuclear GSK3β is associated with induction of DNA double strand break repair through the phosphorylation of 53BP1 and thus promotes chemoresistance.114 Temozolomide is a first line chemotherapeutic drug to treat glioblastoma. GSK3β causes methylation of O6-methylguanine DNA methyltransferase promoter which sensitizes the cancer cells against temozolomide.115 Additionally, GSK3β phosphorylates the Akt and PTEN which further initiates chemoresistance in breast cancer.116 In colorectal cancer, the overexpression of NF-κB mediated through the GSK3β activity modulates the regulation of cancer stem cells to develop drug resistance.117 The function of GSK3β is associated with the activation of ATR-interacting protein TopBP1 and activation of ATR in single strand DNA damage repair which desensitizes the pancreatic ductal to chemotherapy and promotes chemoresistance.118 The overexpression of GSK3β associated with the development of acquired resistance in ovarian carcinoma.119 GSK3β also promotes epithelial to mesenchymal and cancer stem cell properties by acting on the TGFβ which promotes chemoresistance in the ovarian carcinoma.120 Moreover, GSK3β regulates the p53 expression and phosphorylation of Ser9 of GSK3β (pGSK3β Ser9) confer the cisplatin resistance of ovarian carcinomas.121 In renal carcinoma, GSK3β causes the phosphorylation of 4EBP1 and activation of mTORC1 which triggers the renal carcinoma cells to acquire resistance against mTORC1 inhibitors.122
PI3K inhibitor
In order to develop the PI3K inhibitors tremendous attempts has been made which slows tumor progression and modifies immunosuppressive tumor microenvironment. The chemotherapeutic activity of PI3K inhibitors have also been established in clinical settings (Table 1). These PI3K inhibitors are identified as the potential strategy to combat cancer which can be classified based on their selectivity such as pan-PI3K inhibitors, selective isoform PI3K inhibitors and dual PI3K/mTOR inhibitors.135
Table 1.
Summary of trials, outcomes and adverse effects associated with PI3K inhibitors in various phases of clinical studies.
| Treatment and reference | Phase and NCT | Clinical outcomes | Adverse effects |
|---|---|---|---|
| BKM120/Buparlisib | |||
| Buparlisib in combination with Everolimus (Eve) in patients with advanced solid tumors123 | I, NCT01470209 | The combination was well tolerated and safe in these patients. The MTD and RP2D for Eve and Bup was 5 and 60 mg, respectively, when on continuous daily schedule. There was no evidence of drug–drug interaction with concurrent administration of Eve and Bup. Paired skin biopsies for baseline and cycle 1 patients demonstrated target engagement with modulation of mTOR/PI3K signaling pathway biomarkers. There was a marked reduction in pS6 and p4EBP1 levels in cycle 1 biopsies compared to baseline. | Diarrhea, nausea, hyperglycemia, hypokalemia, muscular pain, anorexia, fatigue and elevated ALT/AST. 7 patients had additional DLTs such as mucositis, acute kidney injury and urinary tract infection. Gr 4 and 5 adverse effects were rarely observed. |
| Buparlisib in combination with MEK162/Binimetinib in patients with advanced solid tumors124 | I, NCT01363232 | The combination showed promising activity in patients with ovarian cancer with RAS/BRAF mutation. The MTD for Bup and MEK162 was established at 90 mg/day and 45 mg twice daily dose, respectively. The RP2D for Bup was determined as 80 mg/day and MEK162 45 mg twice daily dose. Other dosing strategies such as pulsatile dosing should be adopted for further trials as continuous dosing led to intolerable toxicities. | Central serous retinopathy, diarrhea, stomatitis, pneumonia, vomiting, nausea, maculopapular rash, increase in ALT and elevation in blood creatine phosphokinase. |
| Buparlisib in combination with Temozolamide (Tem) and Radiation Therapy in newly diagnosed125 glioblastoma patients | I, NCT01473901 | Due to challenging safety profile and inability to achieve the MTD, the sponsor decided not to pursue the use of Bup in newly diagnosed glioblastoma patients. | |
| Buparlisib in combination with mAb targeting EGFR, Panitumumab (Pani) in patients with metastatic/advanced RAS-WT colorectal cancer126 | Ib, NCT01591421 | The combination of Bup (given 5 days a week) and Pani (6 mg/kg by IV route biweekly) was well tolerated (n = 19). Both drugs were administrated in 3 DLs. The median PFS was 2 months. 9 patients had PD and 7 patients exhibited SD (the median duration was 5.4 months [range: 3.7–8.4 months]). The phase II study was stopped, as the predefined futility rule for response was not achieved. | mucositis, fatigue, palmar-plantar erthrodyesthesia, rash, acneiform, hypomagnesemia and increased AST/ALT. |
| Buparlisib in combination with tyrosine kinase inhibitor, Imatinib in patients with gastrointestinal stromal tumor for whom treatment failed prior to Imatinib and Sunitinib therapy127 | Ib, NCT01468688 | The combination failed to provide additional benefits compared to current therapies that are available for these patients (n = 60). Further development of this combination was terminated due to lack of objective response. | |
| Buparlisib in combination Carboplatin or Lomustine in patients with recurrent glioblastoma multiforme128 | Ib/II, NCT01934361 | The combination did not demonstrate sufficient anti-tumor efficacy compared to single-agent Lom or Carbo. The study did not proceed to phase II trial. | |
| Buparlisib in R/R CLL patients129 | II, NCT02340780 | Bup demonstrated significant toxicities and further testing of Bup in these patients was ceased (n = 14). The data also indicated that basal raptor expression in CLL patients correlated with clinical response to Bup. | |
| Buparlisib in TNBC patients130 | II, NCT01790932 and NCT01629615 | No confirmed objective responses were observed and Bup was not associated with a strong clinical efficacy in TNBC patients as a single agent (n = 50). | |
| Buparlisib in combination with LGX818/Encorafenib (BRAF inhibitor) and MEK162/Binimetinib (MEK inhibitor) in patients with advanced BRAFV600-mutant melanoma (LOGIC-2)131 | II, NCT02159066 | The triple therapy was feasible depending on genetic alterations but low clinical activity was observed (n = 6). Further exploration is needed to identify the patterns of resistance susceptible to use this combination in these patients. | |
| Copanlisib/BAY 80-6946/Aliqopa | |||
| Copanlisib plus the MEK inhibitor, Refametinib (Ref) in advanced cancer patients132 | I, NCT01392521 | In this dose-escalation (n = 49) and expansion (n = 15) study, the MTD was defined at Cop 0.4 mg/kg weekly and Ref 30 mg twice daily dose. There was no drug–drug interaction observed. MEK-ERK inhibition and decreased tumor FDG uptake were reported during treatment. Best response was SD in n = 21 patients. | Fatigue, diarrhea, nausea and acneiform rash. DLTs included oral mucositis increased ALT/AST, rash acneiform, hypertension and diarrhea. |
| Copanlisib in patients with solid tumors and NHL patients133 | I, NCT02155582 | PRP pAKT levels demonstrated sustained reductions from baseline post-Cop treatment [median inhibition: 0.4 mg/kg, 73.8% (range-94.9 to 144.0); 0.8 mg/kg, 79.6% (range-96.0 to 408.0)]. Tumor pAKT was lowered versus baseline with Cop 0.8 mg/kg in paired biopsy samples. Dose-related transient plasma glucose elevations were seen. Cop plasma exposure significantly correlated with alterations in plasma pAKT levels and glucose metabolism markers. | Hyperglycemia, fatigue and hypertension. |
| Copanlisib in Chinese patients134 | I, NCT03498430 | Cop PK exposure profiles were in the same range as of those from previous studies of Cop in non-Chinese patients in clinical dose of 60 mg. The ORR was 50% (95% CI: 21.1, 78.9) with 6 patients achieving a best response of a PR in 1 patient and SD in 6 patient. | Hyperglycemia, transient hypertension both Gr 3. However, no Gr 4 or Gr 5 adverse events observed. |
Pan-PI3K inhibitors
Pan-PI3K inhibitors are ATP-competitive inhibitors, targeting all four isoforms of PI3K class I which leads to numerous side effects due to nonselective blockage of PI3K. In the cases of PTEN inactivation or aberrant upstream PI3K signaling they are often utilized as they effectively block the PI3K pathway.136 Some of the pan-PI3K inhibitors are Buparlisib, PX-866, voxtalisib, pilaralisib, pictilisib, Copanlisib, and ZSTK474.
Selective PI3K isoform inhibitors
Selective PI3K isoform inhibitors are the significant approach to develop ATP-competitive which is associated with minor side effects such as immunosuppression and glucose intolerance. Though they are responsible for the inhibition of selective enzymes, but they have very narrow therapeutic window due to the lack of selectivity on the mutant isoforms.137 It can be divided based on the isoforms to 4 classes which includes selective PI3Kα, PI3Kβ, PI3K γ, and PI3Kδ inhibitors.
PI3K/mTOR dual inhibitors
It has also been shown that few PI3K inhibitors also cause the inhibition of mTOR protein due to their binding affinity to the catalytic domain of mTOR which resembles high degree of sequence homology with p110 catalytic subunit of the class I PI3Ks. The primary advantages of these dual inhibitors are mainly targeting both the protein components at the same time with a single agent which reduces the unnecessary side effects and also improves the therapeutic outcome.138 Some of the PI3K/mTOR dual inhibitors are currently under clinical trial program such as GSK2126458, XL-765, GDC-0980, PKI-587, PF-06491502, BEZ235 and BGT226, among which GSK2126458, BEZ235 and BGT226 showed excellent chemotherapeutic outcome in vitro and in vivo.139
Toxicity and adverse reactions
Besides all the potent clinical achievements the PI3K inhibitors comes with potentially fatal adverse reactions. The most frequent adverse events that have been noticed during the treatment with PI3K inhibitors include nausea, vomiting, fatigue, cough, fever, headache and anorexia which are not at all serious adverse effects sufficient for termination of the therapy and can be managed clinically. The serious adverse effects for the patients treated with idelalisib plus rituximab were pneumonia, diarrhoea, pyrexia, sepsis, and febrile neutropenia which lead to discontinuation of the therapy. The most common side effects for the termination of the therapy are hepatotoxicity and diarrhoea/colitis.140
GSK3 inhibitor
There are different types of GSK3 inhibitors such as ATP-competitive GSK3 inhibitor, non-ATP-competitive GSK3 inhibitor, substrate competitive GSK3 inhibitor, isoform-specific GSK3 inhibitor, combination with GSK3 inhibitor, and multikinase inhibitor.9
ATP-competitive GSK3 inhibitor
ATP-competitive GSK3 inhibitors are more available than the others. They are non-selective to two types of isoforms of GSK3.141 Lithium is the first approved GSK3 inhibitor, it inhibit GSK3 directly and indirectly with the help of magnesium and Akt activation, respectively.142 One of the bis-indole compounds indirubin is considered as dual inhibitor of CDKs and GSK3β through ATP-competitive process. The indirubin derivative 6-bromoindirubine-3′-monoxime scaffolds are crucial for developing the inhibitors of GSK3β. It also helps to downregulate androgen receptor-V7 which acts as a part of prostate cancer.143
Non-ATP-competitive GSK3 inhibitors
Heterocyclic thiadiazolidinone classified small molecule, tideglusib (TDZD-8) are ATP non-competitive GSK3 inhibitors which contain thiadiazolidindione scaffold.144 The novel substituent of benzothiazinones are reported to inhibit GSK3β through non-ATP competitive inhibition and termed as a potent candidate for the treatment of ovarian cancer.145
Substrate-competitive peptide GSK3 inhibitors
Short phosphorylated peptides which are developed from pre-phosphorylated substrates function as selective substrate-competitive inhibitors. A heat shock factor-1 (HSF-1) derivative, peptide L803, is also an effective inhibitor of GSK3. Further, in the modified version of L803 (L803-mts), myristic acid was introduced to N-terminus for increasing the permeability. L803-mts is found to be more specific towards GSK3 with more reducing potential of phosphorylation of the GSK3 substrates without interfering with other protein kinases.146
Isoform-specific GSK3 inhibitors
Some of the compounds have the ability to inhibit both the isoforms of GSK3. In case of acute myeloid leukemia, ‘scorpion shaped’ GSK3α inhibitor are used for more specific activity which reduces unnecessary side effects.147 Additionally, AR-A014418GSK3 is a specific inhibitor which can decrease the telomerase activity.148
Combination therapy with GSK3 inhibitors
The demand of the combination therapy is getting more hyped and GSK3 inhibitors are not excluded from the list. GSK3β specified inhibitors in a low dose with the combination of the different therapeutic agents are claimed to posses’ synergistic effects.115 As an example, metformin, pioglitazone and lithium comprise a triple agent dose regime which is suggested as adjuvant for metabolic activity.149 GSK3 inhibitors such as BIO acetoxime, TDZD-8, L803-mts combine with some autophagy modulators like chloroquine, 3-methyladine which might be potential for treatment of malignant gliomas.150
Multikinase inhibitors
Multiple kinases inhibiting compounds are being developed for better chemotherapeutic outcome. Compounds which are comprised of a central pyrazole and benzimidazole scaffold causes the inhibition or modulation of GSK3, Aurora, CDK kinases.150
Toxicity and adverse effects
No such documented adverse effects are found in case of the GSK3 inhibitors as most of the inhibitors are under clinical trial process (Table 2).151 The specificity and safety against other kinases is a big question for the researchers. As most of the GSK3 inhibitors has a tendency to compete with ATP to get bind with GSK3, there can be a chance of the off target binding. Additionally, as GSK3 is present at the center of different crossed signaling pathways, inhibition of GSK3 can interfere with other signaling pathways which lead to undesirable output. β-Catenin activation is a topic of concern which can cause transformation of the healthy to malignant phenotype through activating different genes.9 In order to eradicate all probable adverse reactions an in-depth investigation of GSK3 in both normal cell and cancerous microenvironment is encouraged.
Table 2.
GSK-3 activity in clinical trials/studies.
| Treatment and reference | Outcome | Clinical trial | NCT |
|---|---|---|---|
| The protein kinase C beta inhibitor Enzastaurin results in inhibition of AKT which leads to activation of GSK-3. The effects of Enzataurin and the vascular endothelial growth factor A (VEGFa) inhibitor Bevacizumab were examined in advanced or metastatic cancer patients.152 | Finished Phase I study. 67 patients were evaluable for safety and efficacy. Good results with patients with ovarian cancers. 50.4% of ovarian cancer patients remained without disease progression after 6 months. | Enzastaurin and Bevacizumab in Treating Patients With Locally Advanced or Metastatic Cancer | NCT00550927 |
| To determine effects of combination of enzataurin and bevacizumab in adults with glioma.153 | Finished Phase II study with 81 patients with glioblastomas (GBM, n = 40) and anaplastic gliomas (AG, n = 41). Early response was associated with longer progression free survival for glioblastomas. Combined treatment was well tolerated, and survival time was similar to that observed in patients treated with bevacizumab. | Phase II study with enzastaurin (LY317615) in combination with bevacizumab in adults with recurrent malignant gliomas. | NCT00586508 |
| Combining the EGFR/HER2 inhibitor with the proteasomal inhibitor bortezomib. Lapatinib should inhibit AKT activity which will lead to GSK-3 activity.154 | Phase I study was terminated due to withdrawal of sponsor support | A Phase I Study of the HER1, HER2 Dual Kinase Inhibitor, Lapatinib Plus the Proteasomal Inhibitor Bortezomib in Patients With Advanced Malignancies | NCT01497626 |
| Effects of Trametinib MEK inhibitor and pan AKT inhibitor (GSK2141795) treatment in melanoma. Suppression of AKT should result in increased.155 | Completed, Phase II clinical study did not reveal any clinical benefit of trametinib and GSK2141795 treatment in melanoma patients with NRAS mutations or wild-type melanoma. GSK 2141795 inhibited phosphorylation of GSK3β | Trametinib With GSK2141795 in BRAF Wild-type Melanoma | NCT01941927 |
| Treatment of humans and mouse model of recurrent GBM with temozolomide (TMZ) and other drugs which suppress GSK-3β (cimetidine, lithium, olanzapine, and valproate, (CLOVA) cocktail. The safety and efficacy of the CLOVA cocktail) in combination with TMZ were performed to human and murine studies.156 | Inhibition of active GSK-3β in the tumor resulted in increased patient survival. The combination of TMZ and the CLOVA cocktail significantly inhibited cell invasion and TMZ increased survival compared to patients treated with TMZ alone. Active GSK-3β was associated with a poor prognosis | Clinical study in Japan completed with 7 GBM patients. |
Crosstalk between PI3K and GSK3
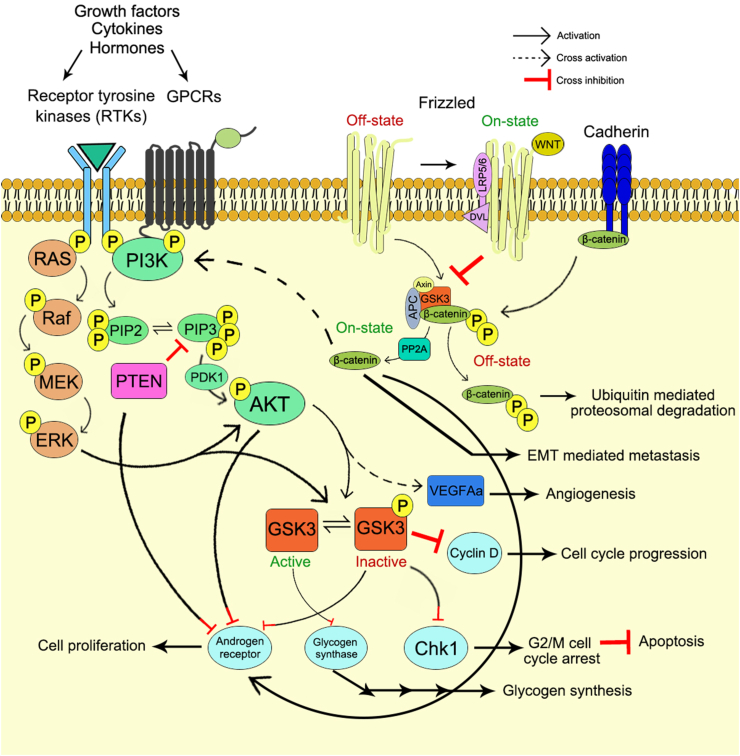
The PI3K and GSK3 proteins are linked to each other through various complex and imprecise signaling networks. The crosstalk between PI3K and GSK3 is associated with the unwinding of the intricate intracellular signaling by means of cross-activation, cross-inhibition and pathway convergence on substrates (Fig. 3, 4).
Figure 3.
Interplay between PI3K and GSK3 signaling cascade through cross activation and cross inhibition.
Figure 4.
The pathway convergence between PI3K and GSK3 signaling axis.
Cross-inhibition
The PI3K signaling pathway negatively regulates the activity of GSK3. Phosphatase and tensin homolog (PTEN) is an important negative regulator of PI3K signaling pathway. Mutation of PTEN is very commonly associated with the gliomas,155,156 which leads to the loss of inhibitory control over the glycolysis and thus caused the tumor invasion. The loss of negative regulation on PI3K signaling caused the phosphorylation mediated deactivation of GSK3 and allows the activation of glycogen synthase (GS). There are various other kinases that can phosphorylate and inactivate GSK3 activity such as mitogen-activated protein kinase (MAPK, ERK1/2).157 However, an intimate relation between MAPK/ERK cascade and PI3K signaling justifies the effect of PI3K on GSK3 through MAPK mediated pathway. Both PI3K/Akt and MAPK plays an important role in the cell proliferation and regulation of cell cycle through the phosphorylation of various downstream effector molecules.158 Mutations in the RAS genes results in the activation of both PI3K/Akt and MAPK pathways which leads to phosphorylation of GSK3 and thus inactivation. Furthermore, the mutation of RAS also linked with the activation of Akt in thyroid tumors development.159,160 The occurrence of prostate cancer have been distinguished by observing the progression from androgen-dependent phenotype to androgen independent (AI) and lethal. The PI3K and androgen receptor (AR) signaling pathways plays important role in the development of prostate cancer through the inhibition of apoptotic events associated with androgen withdrawal therapy. The loss of PTEN and GSK3 activity is readily associated with the induction of AR activity and PI3K/Akt gain-of-function as PTEN is a negative regulator of AR and PI3K signaling. So the loss of PTEN activity leads to accumulation of the phospholipid and results in the phosphorylation of Akt at the submembranous position161 which further causes phospho-inhibition of GSK3β and inactivation of apoptotic factors. Additionally, several evidences have been suggested the role of GSK3 in the regulation of AR oncogenic function in the process of prostate cancer development. The phosphorylation of the AR hinge and ligand binding regions by GSK3β inhibited the activation of AR-genes targets and cell proliferation.162,163 These evidences strongly suggesting the GSK3 regulation in the prostate cancer cells and serves as a vital pharmacological target.164 Along with that, in prostate cancer the enhanced autocrine production of IGF-1 and increased IGF1-R levels have also been observed due to increased activity of PI3K signaling165,166 which increase the nuclear localization of β-catenin and enhanced β-catenin/AR interactions.167 These findings firmly suggested the PTEN mediated regulation of IGF-1R.168
Cross-activation
The activation of PI3K signaling axis by the action of GSK3 has also been elucidated in several literatures. Sometimes, the GSK3β plays an important role in the embryonic angiogenesis of zebrafish through a positive feedback loop of PI3K/Akt and Wnt/β-catenin signaling cascade. The study has demonstrated that, in gsk3b-mRNA-overexpressed embryos there is increased level of phosphorylated GSK3β, which in turn prevents the proteosomal degradation of β-catenin and thus up regulated the phosphorylated Akt activity in intersegmental vessels that leads to the up regulation of VegfAa expression.169
Pathway convergence
The PI3K and GSK3 signaling cascade often acts on same protein to regulate several cellular modalities. A diverse array of proteins have been identified as a substrate of GSK3 which are being phosphorylated by GSK3 and partly regulated by the Akt mediated phosphorylation of GSK3. The RNA binding protein and suppressor of p53 translation, RNPC1 has been identified as an important substrate of GSK3.170 GSK3 mediated phosphorylation of RNPC1 derepressed the p53 translation stimulated by PI3K/Akt activity. On the other hand, pharmacological inhibition of Akt showed reduced phosphorylation of GSK3 and increased the RNPC1 phosphorylation causing increased translation of p53. Similarly, there are several proteins recognized as the substrate of PI3K/Akt signaling that also get phosphorylated by the action of GSK3. The Akt activator RICTOR is acted upon by GSK3 through the phosphorylation of TORC2 in a phosphodegron sequence.171 Furthermore, GSK3α can bind and phosphorylate Akt1 on Thr 312 and leads to the inhibition of Akt kinase activity.172 PI3K dependent activation of Akt caused phospho-inhibition of TSC2 leads to the downstream activation of mTOR and promote anabolic metabolism. In this instance, GSK3 has been found to mediate the phosphorylation of TSC2 in distinct inhibitory site along with Wnt ligands which promoted the Wnt stimulated mTOR activation.104,173 GSK3β and Akt both involved in a complex interplay of phosphor-regulation and direct regulation of AR activity. GSK3β directly phosphorylated the AR hinge and ligand binding regions which leads to the deactivation of AR target genes and triggers cell proliferation.162,163 Additionally, Akt has a complex relationship with AR as it preferentially phosphorylated hAR at Ser210 and Ser790174,175 which causes both induction176 and inhibition of AR transactivation.174,175
Effect of GSK3 on the activity of PI3K inhibitors
Involvement of GSK3 in the resistance
Dual PI3K/mTOR inhibitor
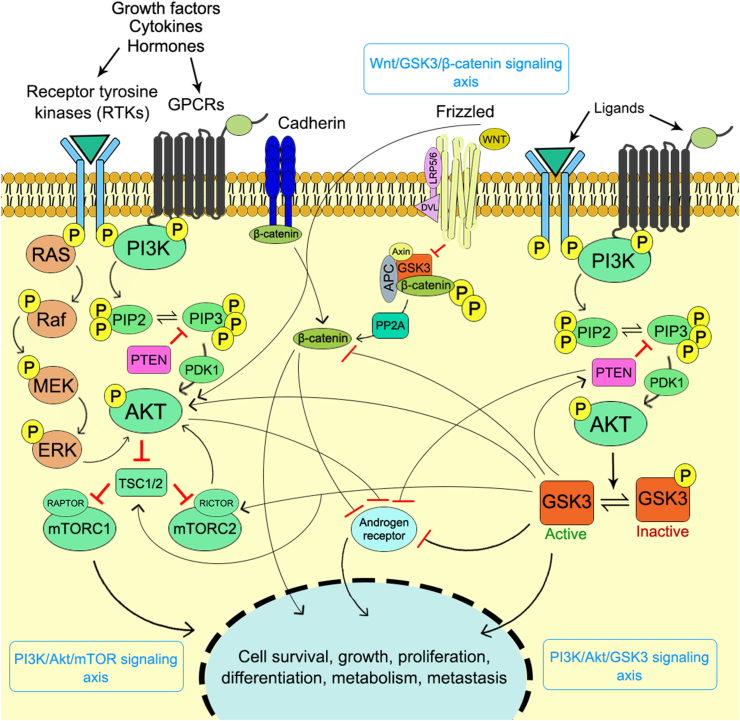
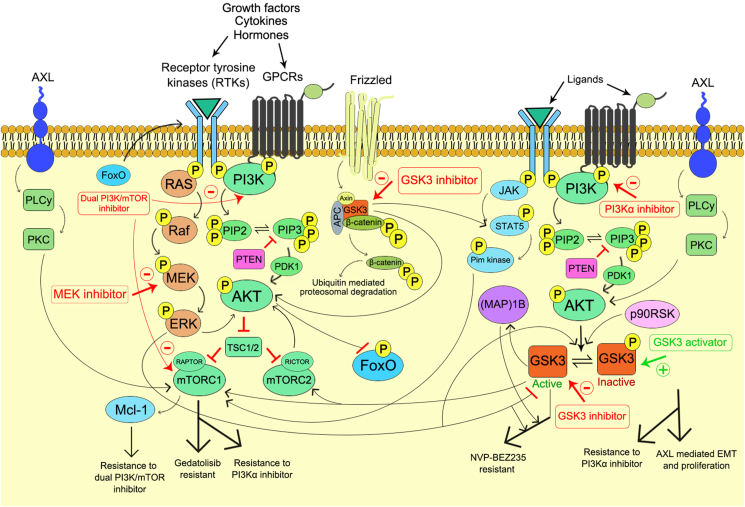
It has been frequently observed that the long term exposure of PI3K inhibitors associated with acquired resistance which in turn limits the therapeutic efficacy of the drug. In order to prevent the therapeutic resistance towards the cancer cells an in depth mechanism of resistance is urgently needed to improve the chemotherapeutic efficacy of the drugs targeting PI3Ks. The detailed mechanism for resistance against the PI3K inhibitors has been showcased in Figure 5. The Wnt/β-catenin signaling pathway is negatively regulated by the GSK3. The GSK3/Wnt/β-catenin signaling axis has been found to be involved in the development of mTOR inhibitor resistance in the human colorectal cancer (CRC) cells. The development of resistance against dual PI3K/mTOR inhibitor such as PF-05212384 (gedatolisib) has been demonstrated in all CRC cell lines which is responsible for the frameshift mutation (c.465_466insC; H155 fs∗) in T-cell factor/lymphoid enhancer-binding factor (TCF/LEF) 7 (TCF7). In the resistant cell line an increase level of active Tyr216 p-GSK3β and decrease level of inactive Ser9 p-GSK3β was observed due to TCF7 frameshift mutation. The active GSK3β caused increased association of mTOR with Raptor and enhanced the mTORC1 activity in the gedatolisib resistant cells.177 Hence, the down regulation of active GSK3β expression through the pharmacological inhibition of GSK3β re-established the sensitivity of the resistant cells to gedatolisib via inhibition of mTORC1 activity.177 Therefore, the combination treatment with GSK3 inhibitor along with the dual PI3K/mTOR inhibitor might be the novel approach in the treatment of resistant cells which is characterized with high expression of active GSK3β in CRC. In the human primary glioblastoma (GBM) cells the involvement of GSK3β in the dual PI3K/mTOR inhibitor (NVP-BEZ235) resistance has been well documented which is primarily responsible for the chronic exposure of the drug itself.178 In this instance, the combination treatment with selective GSK3β inhibitor CHIR99021 resensitizes the resistant cell lines. Moreover, the downregulation of GSK3β through the shRNA strategy also markedly increases the sensitivity of GBM cells to NVP-BEZ235.178 The knockdown of Rictor, an mTORC2 component, via shRNA prevented the development of resistance to NVP-BEZ235, which suggested the involvement of mTORC2. The MEK inhibitor AZD6244 decreases the resistance against NVP-BEZ235 which justified the involvement of MEK/ERK signaling cascade. The phosphorylation of Akt at Ser473 by mTORC2 is essential for the inhibition of nuclear localization of Forkhead box O (FoxO) protein.4 Thus, the long term inhibition of Akt activity by the exposure of NVP-BEZ235 leads to increased activity of FoxO protein which upregulated the expression of several receptor tyrosine kinases (RTKs).179 This enhanced expression of RTKs activated the MEK/ERK signaling cascade.180 Increased ERK activation caused the phosphorylation of GSK3β at Thr43 which leads to p90RSK (downstream target of ERK) mediated subsequent phosphorylation of GSK3β at Ser9, that causes the inactivation of GSK3β.181 Among various RTKs, the human epidermal growth factor receptor 2 (HER2) and HER3 exhibited significant expression, which play the pivotal role in the pathophysiology of human GBM.182 The MEK/ERK/p90RSK/GSK3β signaling axis plays an important role in several human tumors, such as breast, stomach, kidney and liver cancer.181 Further studies have identified the microtubule-associated protein (MAP)1B, a downstream target of MEK/ERK/p90RSK/GSK3β signaling axis that develops resistance to NVP-BEZ235 in human GBM cells. As MAP1B is a downstream target of GSK3β, the pharmacological inhibition of GSK3β or the shRNA approach to downregulate the GSK3β expression caused decreased level of p-MAP1B. Whereas, the exact mechanism for the MAP1B mediated development of resistant against NVP-BEZ235 is still unknown. The rapamycin treated GBM cells also developed the GSK3β/MAP1B dependent drug resistance due to long term exposure of the drug which dampens the mTORC2 activity in various cancer model systems.178 These outcomes provides sufficient evidence to outline the mechanism for the development of GSK3 mediated resistance against dual PI3K/mTOR inhibitor which correlates the mTORC2 inhibition and MEK/ERK activation through the MEK/ERK/p90RSK/GSK3β signaling axis. Additionally, the findings also suggested the GSK3 as a prognostic factor in several cancer model systems which act in the other way to enhance the patient outcome.85 Thus, the pharmacological inhibition of GSK3β may attribute to the combinations therapy in the resistant cell lines while it may also negatively impact the other aspects of GBM pathophysiology. The development of PI3K/Akt inhibitor resistance in the cancer cells is also associated with the increased expression of Pim kinases through the activation of STAT5 which enhanced the mTORC1/Mcl-1 pathway.183 The dual PI3K/mTOR inhibitor triggered a positive feedback loop to increase the activation of JAK2/STAT5 and IL-8 secretion which contributes to the drug resistance.184 Moreover, the GSK3β also contributes to the activation of STAT5 through the tyrosine phosphorylation at Tyr694 by either IFNα or IFNγ,185,186 whereas the GSK3α responsible for different regulatory activities. The combination therapy of GSK3β inhibitor with dual PI3K/mTOR inhibitor might result in sensitization of resistant cells towards the PI3K inhibitor through the downregulation of STAT5 activation which leads to the inhibition of STAT5 mediated mTORC1/Mcl-1 pathway.
Figure 5.
Schematic representation of resistance developed on cancer against PI3K inhibitors through GSK3 modulation.
Selective PI3K isoform inhibitors
Other mechanisms of resistance development include the activation of AXL. In the head, neck and oesophageal squamous cell carcinomas (OSCC) the resistance against PI3Kα develops through the activation of receptor tyrosine kinase AXL which promotes resistance via induction of phospholipase C (PLCγ) and protein kinase C (PKC) and results in PI3K independent mTOR activation.187 The sensitivity towards the PI3Kα inhibitor could be restored through the repression of AXL.188 Additionally, the activation of AXL is associated with the epithelial–mesenchymal transition (EMT) which further leads to oncogenic metastasis of the cancer cells.189,190 In this instance, the repression of AXL expression caused decreased proliferation, invasion and migration of OSCC cells. Furthermore, the repression of AXL decreased the Akt activation and subsequent inhibition of NF-κB transcriptional activity. The inactivation of Akt further activates the GSK3β which in turn results in the inhibition of EMT through the upregulation of epithelial markers. Thus, it can be hypothesized that the induction of GSK3β activity decreased the AXL mediated EMT and proliferation of cancer cells and sensitizes the cancer cells towards PI3Kα inhibitor.191
Conclusion
In various cancer model systems, the abnormal activation of PI3K signaling pathway is the major cause for the initiation and progression of malignancy with therapeutic resistance towards the chemotherapeutic management due to long term exposure of the drugs. The development of the resistance against the PI3K inhibitor has become the major concern in the treatment of cancer which is associated with the treatment failure and cancer relapse as observed in various cancer incidences. Therefore, the elimination of the resistance towards PI3K inhibitors through in depth understanding of the mechanism for specific resistance is the primary objective in the field of cancer chemotherapy.
The function of GSK3 in cancer is very conflicting and ambiguous. GSK3 involves in several signaling pathways through complicated regulatory networks which directly contributes to cancer initiation and differentiation. However, the dual role of GSK3 in cancer demonstrated its function as either cancer promoter or inhibitor as per the cell type.
Indeed, the PI3K and GSK3 signaling axis are interconnected through a complex regulatory downstream cascade which plays the key role in several malignancies via the manipulation of cell survival strategies which includes disruption of apoptosis, increased angiogenesis, EMT mediated metastasis. In various clinical settings, the up-regulated activity of GSK3 plays the pivotal role in the development of therapeutic resistance against PI3K inhibitor. Therefore, it can be hypothesized that the combination therapy of GSK3 inhibitor with PI3K inhibitor might be a significant approach to sensitize the cancer cells to chemotherapeutic treatment by suppressing the GSK3 mediated drug resistance. On the other hand, in some other cancer phenotypes the activation of GSK3 is directly related to the suppression of drug resistance and for those incidences the specific GSK3 activators possessed the vital role to elevate the function of PI3K inhibitor through their sensitization towards the target cells. However, the development of such specific GSK3 activators has not been reported till date.
The paradoxical activity of GSK3 in various cancer cell types abolished the development of the targeted GSK3 therapy in cancer chemotherapy. Although, extensive investigation on the mechanism of GSK3 is readily required to unravel the relation between the activation of GSK3 with the cancer prevention through the PI3K inhibition and sensitization of cancer cells against the PI3K inhibitor. Besides, this approach will also deliver an insight into the components of GSK3 signaling axis which might be further targeted for therapeutic interventions.
In conclusion, the role of specific GSK3 activator or inhibitor subjected to cancer phenotypes might be a significant approach which not only improves the activity of PI3K inhibitor but also targets several GSK3 substrates to unleash new treatment modalities against neoplastic transformation.
Author contributions
Abhijit Das: Article writing, processing of illustration, research design; Barshana Bhattacharya: Data collection, article writing, conceptualization; Souvik Roy: Article revision, final approval.
Conflict of interests
Authors declare no conflict of interests.
Acknowledgements
The authors are thankful to Department of Pharmacy, NSHM Knowledge Campus, Kolkata- Group of Institutions, for their sincere support and cooperation throughout the study.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020:GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Mathur P., Sathishkumar K., Chaturvedi M., et al. Cancer statistics, 2020:report from national cancer registry programme, India. JCO Glob Oncol. 2020;6:1063–1075. doi: 10.1200/GO.20.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen M.H., Williams G., Johnson J.R., et al. Approval summary for imatinib mesylate capsules in the treatment of chronic myelogenous leukemia. Clin Cancer Res. 2002;8(5):935–942. [PubMed] [Google Scholar]
- 4.Chiarini F., Evangelisti C., Lattanzi G., McCubrey J.A., Martelli A.M. Advances in understanding the mechanisms of evasive and innate resistance to mTOR inhibition in cancer cells. Biochim Biophys Acta Mol Cell Res. 2019;1866(8):1322–1337. doi: 10.1016/j.bbamcr.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 5.Jiang W., Ji M. Receptor tyrosine kinases in PI3K signaling: the therapeutic targets in cancer. Semin Cancer Biol. 2019;59:3–22. doi: 10.1016/j.semcancer.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Revathidevi S., Munirajan A.K. Akt in cancer: mediator and more. Semin Cancer Biol. 2019;59:80–91. doi: 10.1016/j.semcancer.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Alzahrani A.S. PI3K/Akt/mTOR inhibitors in cancer: at the bench and bedside. Semin Cancer Biol. 2019;59:125–132. doi: 10.1016/j.semcancer.2019.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Song M., Bode A.M., Dong Z., Lee M.H. AKT as a therapeutic target for cancer. Cancer Res. 2019;79(6):1019–1031. doi: 10.1158/0008-5472.CAN-18-2738. [DOI] [PubMed] [Google Scholar]
- 9.Nagini S., Sophia J., Mishra R. Glycogen synthase kinases: moonlighting proteins with theranostic potential in cancer. Semin Cancer Biol. 2019;56:25–36. doi: 10.1016/j.semcancer.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 10.MacDonald B.T., Tamai K., He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17(1):9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evangelisti C., Chiarini F., Paganelli F., Marmiroli S., Martelli A.M. Crosstalks of GSK3 signaling with the mTOR network and effects on targeted therapy of cancer. Biochim Biophys Acta Mol Cell Res. 2020;1867(4):118635. doi: 10.1016/j.bbamcr.2019.118635. [DOI] [PubMed] [Google Scholar]
- 12.Liu P., Cheng H., Roberts T.M., Zhao J.J. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8(8):627–644. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fruman D.A., Chiu H., Hopkins B.D., Bagrodia S., Cantley L.C., Abraham R.T. The PI3K pathway in human disease. Cell. 2017;170(4):605–635. doi: 10.1016/j.cell.2017.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fritsch R., Downward J. SnapShot: class I PI3K isoform signaling. Cell. 2013;154(4):940.e1. doi: 10.1016/j.cell.2013.07.045. [DOI] [PubMed] [Google Scholar]
- 15.Vanhaesebroeck B., Guillermet-Guibert J., Graupera M., Bilanges B., Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol. 2010;11(5):329–341. doi: 10.1038/nrm2882. [DOI] [PubMed] [Google Scholar]
- 16.Vanhaesebroeck B., Ali K., Bilancio A., Geering B., Foukas L.C. Signalling by PI3K isoforms: insights from gene-targeted mice. Trends Biochem Sci. 2005;30(4):194–204. doi: 10.1016/j.tibs.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Guo H., German P., Bai S., et al. The PI3K/AKT pathway and renal cell carcinoma. J Genet Genomics. 2015;42(7):343–353. doi: 10.1016/j.jgg.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manning B.D., Cantley L.C. AKT/PKB signaling: navigating downstream. Cell. 2007;129(7):1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hennessy B.T., Smith D.L., Ram P.T., Lu Y., Mills G.B. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4(12):988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 20.Altomare D.A., Testa J.R. Perturbations of the AKT signaling pathway in human cancer. Oncogene. 2005;24(50):7455–7464. doi: 10.1038/sj.onc.1209085. [DOI] [PubMed] [Google Scholar]
- 21.Courtney K.D., Corcoran R.B., Engelman J.A. The PI3K pathway as drug target in human cancer. J Clin Oncol. 2010;28(6):1075–1083. doi: 10.1200/JCO.2009.25.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qiu W., Schönleben F., Li X., et al. PIK3CA mutations in head and neck squamous cell carcinoma. Clin Cancer Res. 2006;12(5):1441–1446. doi: 10.1158/1078-0432.CCR-05-2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murugan A., Hong N., Fukui Y., Munirajan A., Tsuchida N. Oncogenic mutations of the PIK3CA gene in head and neck squamous cell carcinomas. Int J Oncol. 2008;32(1):101–111. [PubMed] [Google Scholar]
- 24.Jiang W., He T., Liu S., et al. The PIK3CA E542K and E545K mutations promote glycolysis and proliferation via induction of the β-catenin/SIRT3 signaling pathway in cervical cancer. J Hematol Oncol. 2018;11(1):139. doi: 10.1186/s13045-018-0674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Croessmann S., Sheehan J.H., Lee K.M., et al. PIK3CA C2 domain deletions hyperactivate phosphoinositide 3-kinase (PI3K), generate oncogene dependence, and are exquisitely sensitive to PI3K α inhibitors. Clin Cancer Res. 2018;24(6):1426–1435. doi: 10.1158/1078-0432.CCR-17-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang S., Bader A.G., Vogt P.K. Phosphatidylinositol 3-kinase mutations identified in human cancer are oncogenic. Proc Natl Acad Sci USA. 2005;102(3):802–807. doi: 10.1073/pnas.0408864102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liaw D., Marsh D.J., Li J., et al. Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat Genet. 1997;16(1):64–67. doi: 10.1038/ng0597-64. [DOI] [PubMed] [Google Scholar]
- 28.Chen C.Y., Chen J., He L., Stiles B.L. PTEN: tumor suppressor and metabolic regulator. Front Endocrinol. 2018;9:338. doi: 10.3389/fendo.2018.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haddadi N., Lin Y., Travis G., Simpson A.M., Nassif N.T., McGowan E.M. PTEN/PTENP1:'Regulating the regulator of RTK-dependent PI3K/Akt signalling', new targets for cancer therapy. Mol Cancer. 2018;17(1):37. doi: 10.1186/s12943-018-0803-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen S., Liu H., Wang Y., et al. Long non-coding RNA CRNDE promotes gallbladder carcinoma carcinogenesis and as a scaffold of DMBT1 and C-IAP1 complexes to activating PI3K-AKT pathway. Oncotarget. 2016;7(45):72833–72844. doi: 10.18632/oncotarget.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu X.X., Xiong H.P., Huang J.S., Qi K., Xu J.J. Highly expressed long non-coding RNA CRNDE promotes cell proliferation through PI3K/AKT signalling in non-small cell lung carcinoma. Clin Exp Pharmacol Physiol. 2017;44(8):895–902. doi: 10.1111/1440-1681.12780. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y., Kong D. LncRNA GAS5 represses osteosarcoma cells growth and metastasis via sponging miR-203a. Cell Physiol Biochem. 2018;45(2):844–855. doi: 10.1159/000487178. [DOI] [PubMed] [Google Scholar]
- 33.Hanahan D., Coussens L.M. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21(3):309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 34.Sasaki T., Irie-Sasaki J., Jones R.G., et al. Function of PI3Kgamma in thymocyte development, T cell activation, and neutrophil migration. Science. 2000;287(5455):1040–1046. doi: 10.1126/science.287.5455.1040. [DOI] [PubMed] [Google Scholar]
- 35.Liu X., Xu Y., Zhou Q., et al. PI3K in cancer: its structure, activation modes and role in shaping tumor microenvironment. Future Oncol. 2018;14(7):665–674. doi: 10.2217/fon-2017-0588. [DOI] [PubMed] [Google Scholar]
- 36.Xia C., Meng Q., Cao Z., Shi X., Jiang B.H. Regulation of angiogenesis and tumor growth by p110 alpha and AKT1 via VEGF expression. J Cell Physiol. 2006;209(1):56–66. doi: 10.1002/jcp.20707. [DOI] [PubMed] [Google Scholar]
- 37.Fang J., Ding M., Yang L., Liu L.Z., Jiang B.H. PI3K/PTEN/AKT signaling regulates prostate tumor angiogenesis. Cell Signal. 2007;19(12):2487–2497. doi: 10.1016/j.cellsig.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmid M.C., Avraamides C.J., Dippold H.C., et al. Receptor tyrosine kinases and TLR/IL1Rs unexpectedly activate myeloid cell PI3Kγ, A single convergent point promoting tumor inflammation and progression. Cancer Cell. 2011;19(6):715–727. doi: 10.1016/j.ccr.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu P., Ken T., Weaver V.M., Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harbor Perspect Biol. 2011;3(12):a005058. doi: 10.1101/cshperspect.a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhowmick N.A., Chytil A., Plieth D., et al. TGF-ß signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303(5659):848–851. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- 41.Wu X., Chen X., Zhou Q., et al. Hepatocyte growth factor activates tumor stromal fibroblasts to promote tumorigenesis in gastric cancer. Cancer Lett. 2013;335(1):128–135. doi: 10.1016/j.canlet.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 42.Zhou R., Xu L., Ye M., Liao M., Du H., Chen H. Formononetin inhibits migration and invasion of MDA-MB-231 and 4T1 breast cancer cells by suppressing MMP-2 and MMP-9 through PI3K/AKT signaling pathways. Horm Metab Res. 2014;46(11):753–760. doi: 10.1055/s-0034-1376977. [DOI] [PubMed] [Google Scholar]
- 43.Levy J., Towers C.G., Thorburn A. Targeting autophagy in cancer. Nat Rev Cancer. 2017;17(9):528–542. doi: 10.1038/nrc.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karim M.R., Fisher C.R., Kapphahn R.J., Polanco J.R., Ferrington D.A. Investigating AKT activation and autophagy in immunoproteasome-deficient retinal cells. PLoS One. 2020;15(4) doi: 10.1371/journal.pone.0231212. e0231212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang H., Liu Y., Wang D., et al. The upstream pathway of mTOR-mediated autophagy in liver diseases. Cells. 2019;8(12):1597. doi: 10.3390/cells8121597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang B., Zhong Y., Li Q., Cui L., Huang G. Autophagy of macrophages is regulated by PI3k/Akt/mTOR signalling in the development of diabetic encephalopathy. Aging. 2018;10(10):2772–2782. doi: 10.18632/aging.101586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Turco E., Fracchiolla D., Martens S. Recruitment and activation of the ULK1/Atg1 kinase complex in selective autophagy. J Mol Biol. 2020;432(1):123–134. doi: 10.1016/j.jmb.2019.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Torii S., Yoshida T., Arakawa S., Honda S., Nakanishi A., Shimizu S. Identification of PPM1D as an essential Ulk1 phosphatase for genotoxic stress-induced autophagy. EMBO Rep. 2016;17(11):1552–1564. doi: 10.15252/embr.201642565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kma L., Baruah T.J. The interplay of ROS and the PI3K/Akt pathway in autophagy regulation. Biotechnol Appl Biochem. 2022;69(1):248–264. doi: 10.1002/bab.2104. [DOI] [PubMed] [Google Scholar]
- 50.Dubrovska A., Kim S., Salamone R.J., et al. The role of PTEN/Akt/PI3K signaling in the maintenance and viability of prostate cancer stem-like cell populations. Proc Natl Acad Sci USA. 2009;106(1):268–273. doi: 10.1073/pnas.0810956106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koren S., Reavie L., Couto J.P., et al. PIK3CA(H1047R) induces multipotency and multi-lineage mammary tumours. Nature. 2015;525(7567):114–118. doi: 10.1038/nature14669. [DOI] [PubMed] [Google Scholar]
- 52.van Veen J.E., Scherzer M., Boshuizen J., et al. Mutationally-activated PI30-kinase-α promotes de-differentiation of lung tumors initiated by the BRAFV600E oncoprotein kinase. eLife. 2019;8:e43668. doi: 10.7554/eLife.43668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Riemer P., Rydenfelt M., Marks M., et al. Oncogenic β-catenin and PIK3CA instruct network states and cancer phenotypes in intestinal organoids. J Cell Biol. 2017;216(6):1567–1577. doi: 10.1083/jcb.201610058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chakrabarty A., Surendran S., Bhola N.E., et al. The H1047R PIK3CA oncogene induces a senescence-like state, pleiotropy and acute HSP90 dependency in HER2+ mammary epithelial cells. Carcinogenesis. 2019;40(10):1179–1190. doi: 10.1093/carcin/bgz118. [DOI] [PubMed] [Google Scholar]
- 55.Shibue T., Weinberg R.A. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol. 2017;14(10):611–629. doi: 10.1038/nrclinonc.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scheel C., Eaton E.N., Li S.H.J., et al. Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell. 2011;145(6):926–940. doi: 10.1016/j.cell.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Katsuno Y., Meyer D.S., Zhang Z., et al. Chronic TGF-β exposure drives stabilized EMT, tumor stemness, and cancer drug resistance with vulnerability to bitopic mTOR inhibition. Sci Signal. 2019;12(570):eaau8544. doi: 10.1126/scisignal.aau8544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaneda M.M., Cappello P., Nguyen A.V., et al. Macrophage PI3Kγ drives pancreatic ductal adenocarcinoma progression. Cancer Discov. 2016;6(8):870–885. doi: 10.1158/2159-8290.CD-15-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spangle J.M., Roberts T.M., Zhao J.J. The emerging role of PI3K/AKT-mediated epigenetic regulation in cancer. Biochim Biophys Acta Rev Cancer. 2017;1868(1):123–131. doi: 10.1016/j.bbcan.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rahmani F., Ziaeemehr A., Shahidsales S., et al. Role of regulatory miRNAs of the PI3K/AKT/mTOR signaling in the pathogenesis of hepatocellular carcinoma. J Cell Physiol. 2020;235(5):4146–4152. doi: 10.1002/jcp.29333. [DOI] [PubMed] [Google Scholar]
- 61.Soltani A., Torki S., Ghahfarokhi M.S., Jami M.S., Ghatrehsamani M. Targeting the phosphoinositide 3-kinase/AKT pathways by small molecules and natural compounds as a therapeutic approach for breast cancer cells. Mol Biol Rep. 2019;46(5):4809–4816. doi: 10.1007/s11033-019-04929-x. [DOI] [PubMed] [Google Scholar]
- 62.Rocha G., Oliveira R.R., Kaplan M.A.C., Gattass C.R. 3β-Acetyl tormentic acid reverts MRP1/ABCC1 mediated cancer resistance through modulation of intracellular levels of GSH and inhibition of GST activity. Eur J Pharmacol. 2014;741:140–149. doi: 10.1016/j.ejphar.2014.07.054. [DOI] [PubMed] [Google Scholar]
- 63.Choi E., Kim E., Kim J.H., et al. AKT1-targeted proapoptotic activity of compound K in human breast cancer cells. J Ginseng Res. 2019;43(4):692–698. doi: 10.1016/j.jgr.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Adams J.M., Cory S. The BCL-2 arbiters of apoptosis and their growing role as cancer targets. Cell Death Differ. 2018;25(1):27–36. doi: 10.1038/cdd.2017.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang X., Wu Z., Mei Y., Wu M. XIAP inhibits autophagy via XIAP-mdm2-p53 signalling. EMBO J. 2013;32(16):2204–2216. doi: 10.1038/emboj.2013.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen P., Zhang J.Y., Sha B.B., et al. Luteolin inhibits cell proliferation and induces cell apoptosis via down-regulation of mitochondrial membrane potential in esophageal carcinoma cells EC1 and KYSE450. Oncotarget. 2017;8(16):27471–27480. doi: 10.18632/oncotarget.15832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eberle J. Countering TRAIL resistance in melanoma. Cancers. 2019;11(5):656. doi: 10.3390/cancers11050656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kumar S., Kushwaha P.P., Gupta S. Emerging targets in cancer drug resistance. Cancer Drug Resist. 2019;2:161–177. doi: 10.20517/cdr.2018.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Knuefermann C., Lu Y., Liu B., et al. HER2/PI-3K/Akt activation leads to a multidrug resistance in human breast adenocarcinoma cells. Oncogene. 2003;22(21):3205–3212. doi: 10.1038/sj.onc.1206394. [DOI] [PubMed] [Google Scholar]
- 70.Si W., Shen J., Zheng H., Fan W. The role and mechanisms of action of microRNAs in cancer drug resistance. Clin Epigenet. 2019;11(1):25. doi: 10.1186/s13148-018-0587-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yao H.B., Shaw P.C., Wong C.C., Wan D.C.C. Expression of glycogen synthase kinase-3 isoforms in mouse tissues and their transcription in the brain. J Chem Neuroanat. 2002;23(4):291–297. doi: 10.1016/s0891-0618(02)00014-5. [DOI] [PubMed] [Google Scholar]
- 72.Sutherland C. What are the Bona fide GSK3 substrates? Int J Alzheimer's Dis. 2011;2011:505607. doi: 10.4061/2011/505607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McCubrey J.A., Fitzgerald T.L., Yang L.V., et al. Roles of GSK-3 and microRNAs on epithelial mesenchymal transition and cancer stem cells. Oncotarget. 2017;8(8):14221–14250. doi: 10.18632/oncotarget.13991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Luo J. Glycogen synthase kinase 3beta (GSK3beta) in tumorigenesis and cancer chemotherapy. Cancer Lett. 2009;273(2):194–200. doi: 10.1016/j.canlet.2008.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Woodgett J.R. Molecular cloning and expression of glycogen synthase kinase-3/factor A. EMBO J. 1990;9(8):2431–2438. doi: 10.1002/j.1460-2075.1990.tb07419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stamos J.L., Chu M.L.H., Enos M.D., Shah N., Weis W.I. Structural basis of GSK-3 inhibition by N-terminal phosphorylation and by the Wnt receptor LRP6. eLife. 2014;3:e01998. doi: 10.7554/eLife.01998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goc A., Al-Husein B., Katsanevas K., et al. Targeting Src-mediated Tyr216 phosphorylation and activation of GSK-3 in prostate cancer cells inhibit prostate cancer progression in vitro and in vivo. Oncotarget. 2014;5(3):775–787. doi: 10.18632/oncotarget.1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jacobs K.M., Bhave S.R., Ferraro D.J., Jaboin J.J., Hallahan D.E., Thotala D. GSK-3β: a bifunctional role in cell death pathways. Int J Cell Biol. 2012;2012:930710. doi: 10.1155/2012/930710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McCubrey J.A., Steelman L.S., Bertrand F.E., et al. GSK-3 as potential target for therapeutic intervention in cancer. Oncotarget. 2014;5(10):2881–2911. doi: 10.18632/oncotarget.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sayas C.L., Ariaens A., Ponsioen B., Moolenaar W.H. GSK-3 is activated by the tyrosine kinase Pyk2 during LPA1-mediated neurite retraction. Mol Biol Cell. 2006;17(4):1834–1844. doi: 10.1091/mbc.E05-07-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fang X., Yu S.X., Lu Y., Bast R.C., Jr., Woodgett J.R., Mills G.B. Phosphorylation and inactivation of glycogen synthase kinase 3 by protein kinase A. Proc Natl Acad Sci USA. 2000;97(22):11960–11965. doi: 10.1073/pnas.220413597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tsujio I., Tanaka T., Kudo T., et al. Inactivation of glycogen synthase kinase-3 by protein kinase C delta: implications for regulation of tau phosphorylation. FEBS Lett. 2000;469(1):111–117. doi: 10.1016/s0014-5793(00)01234-5. [DOI] [PubMed] [Google Scholar]
- 83.Takahashi-Yanaga F. Activator or inhibitor? GSK-3 as a new drug target. Biochem Pharmacol. 2013;86(2):191–199. doi: 10.1016/j.bcp.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 84.Cervello M., Augello G., Cusimano A., et al. Pivotal roles of glycogen synthase-3 in hepatocellular carcinoma. Adv Biol Regul. 2017;65:59–76. doi: 10.1016/j.jbior.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 85.Furuta T., Sabit H., Dong Y., et al. Biological basis and clinical study of glycogen synthase kinase- 3β-targeted therapy by drug repositioning for glioblastoma. Oncotarget. 2017;8(14):22811–22824. doi: 10.18632/oncotarget.15206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pal K., Cao Y., Gaisina I.N., et al. Inhibition of GSK-3 induces differentiation and impaired glucose metabolism in renal cancer. Mol Cancer Therapeut. 2014;13(2):285–296. doi: 10.1158/1535-7163.MCT-13-0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen S., Sun K.X., Liu B.L., Zong Z.H., Zhao Y. The role of glycogen synthase kinase-3β (GSK-3β) in endometrial carcinoma: a carcinogenesis, progression, prognosis, and target therapy marker. Oncotarget. 2016;7(19):27538–27551. doi: 10.18632/oncotarget.8485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Abrahamsson A.E., Geron I., Gotlib J., et al. Glycogen synthase kinase 3beta missplicing contributes to leukemia stem cell generation. Proc Natl Acad Sci USA. 2009;106(10):3925–3929. doi: 10.1073/pnas.0900189106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ma C., Wang J., Gao Y., et al. The role of glycogen synthase kinase 3beta in the transformation of epidermal cells. Cancer Res. 2007;67(16):7756–7764. doi: 10.1158/0008-5472.CAN-06-4665. [DOI] [PubMed] [Google Scholar]
- 90.Farago M., Dominguez I., Landesman-Bollag E., et al. Kinase-inactive glycogen synthase kinase 3beta promotes Wnt signaling and mammary tumorigenesis. Cancer Res. 2005;65(13):5792–5801. doi: 10.1158/0008-5472.CAN-05-1021. [DOI] [PubMed] [Google Scholar]
- 91.Mishra R., Nagini S., Rana A. Expression and inactivation of glycogen synthase kinase 3 alpha/beta and their association with the expression of cyclin D1 and p53 in oral squamous cell carcinoma progression. Mol Cancer. 2015;14:20. doi: 10.1186/s12943-015-0300-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mishra R. Glycogen synthase kinase 3 beta: can it be a target for oral cancer. Mol Cancer. 2010;9:144. doi: 10.1186/1476-4598-9-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Welcker M., Singer J., Loeb K.R., et al. Multisite phosphorylation by Cdk2 and GSK3 controls cyclin E degradation. Mol Cell. 2003;12(2):381–392. doi: 10.1016/s1097-2765(03)00287-9. [DOI] [PubMed] [Google Scholar]
- 94.Kitano A., Shimasaki T., Chikano Y., et al. Aberrant glycogen synthase kinase 3β is involved in pancreatic cancer cell invasion and resistance to therapy. PLoS One. 2013;8(2):e55289. doi: 10.1371/journal.pone.0055289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Grimes C.A., Jope R.S. CREB DNA binding activity is inhibited by glycogen synthase kinase-3 beta and facilitated by lithium. J Neurochem. 2001;78(6):1219–1232. doi: 10.1046/j.1471-4159.2001.00495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhou F., Zhang L., van Laar T., Dam H.V., Ten Dijke P. GSK3β inactivation induces apoptosis of leukemia cells by repressing the function of c-Myb. Mol Biol Cell. 2011;22(18):3533–3540. doi: 10.1091/mbc.E11-06-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Linseman D.A., Butts B.D., Precht T.A., et al. Glycogen synthase kinase-3beta phosphorylates bax and promotes its mitochondrial localization during neuronal apoptosis. J Neurosci. 2004;24(44):9993–10002. doi: 10.1523/JNEUROSCI.2057-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Klionsky D.J., Abdelmohsen K., Abe A., et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition) Autophagy. 2016;12(1):1–222. doi: 10.1080/15548627.2015.1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mancinelli R., Carpino G., Petrungaro S., et al. Multifaceted roles of GSK-3 in cancer and autophagy-related diseases. Oxid Med Cell Longev. 2017;2017:4629495. doi: 10.1155/2017/4629495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stretton C., Hoffmann T.M., Munson M.J., et al. GSK3-mediated raptor phosphorylation supports amino-acid-dependent mTORC1-directed signalling. Biochem J. 2015;470(2):207–221. doi: 10.1042/BJ20150404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Azoulay-Alfaguter I., Elya R., Avrahami L., Katz A., Eldar-Finkelman H. Combined regulation of mTORC1 and lysosomal acidification by GSK-3 suppresses autophagy and contributes to cancer cell growth. Oncogene. 2015;34(35):4613–4623. doi: 10.1038/onc.2014.390. [DOI] [PubMed] [Google Scholar]
- 102.Marchand B., Arsenault D., Raymond-Fleury A., Boisvert F.M., Boucher M.J. Glycogen synthase kinase-3 (GSK3) inhibition induces prosurvival autophagic signals in human pancreatic cancer cells. J Biol Chem. 2015;290(9):5592–5605. doi: 10.1074/jbc.M114.616714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sun A., Li C., Chen R., et al. GSK-3β controls autophagy by modulating LKB1-AMPK pathway in prostate cancer cells. Prostate. 2016;76(2):172–183. doi: 10.1002/pros.23106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Inoki K., Ouyang H., Zhu T., et al. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126(5):955–968. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 105.Doble B.W., Woodgett J.R. Role of glycogen synthase kinase-3 in cell fate and epithelial-mesenchymal transitions. Cells Tissues Organs. 2007;185(1–3):73–84. doi: 10.1159/000101306. [DOI] [PubMed] [Google Scholar]
- 106.Bachelder R.E., Yoon S.O., Franci C., de Herreros A.G., Mercurio A.M. Glycogen synthase kinase-3 is an endogenous inhibitor of Snail transcription: implications for the epithelial-mesenchymal transition. J Cell Biol. 2005;168(1):29–33. doi: 10.1083/jcb.200409067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lin G., Liu B., Meng Z., et al. miR-26a enhances invasive capacity by suppressing GSK3β in human lung cancer cells. Exp Cell Res. 2017;352(2):364–374. doi: 10.1016/j.yexcr.2017.02.033. [DOI] [PubMed] [Google Scholar]
- 108.Tan Z., Zheng H., Liu X., et al. microRNA-1229 overexpression promotes cell proliferation and tumorigenicity and activates Wnt/β-catenin signaling in breast cancer. Oncotarget. 2016;7(17):24076–24087. doi: 10.18632/oncotarget.8119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Qiu H.J., Lu X.H., Yang S.S., Weng C.Y., Zhang E.K., Chen F.C. miR-769 promoted cell proliferation in human melanoma by suppressing GSK3B expression. Biomed Pharmacother. 2016;82:117–123. doi: 10.1016/j.biopha.2016.04.052. [DOI] [PubMed] [Google Scholar]
- 110.Yang H.W., Liu G.H., Liu Y.Q., et al. Over-expression of microRNA-940 promotes cell proliferation by targeting GSK3β and sFRP1 in human pancreatic carcinoma. Biomed Pharmacother. 2016;83:593–601. doi: 10.1016/j.biopha.2016.06.057. [DOI] [PubMed] [Google Scholar]
- 111.He K., Tong D., Zhang S., et al. miRNA-99b-3p functions as a potential tumor suppressor by targeting glycogen synthase kinase-3β in oral squamous cell carcinoma Tca-8113 cells. Int J Oncol. 2015;47(4):1528–1536. doi: 10.3892/ijo.2015.3135. [DOI] [PubMed] [Google Scholar]
- 112.Zhou W., Li Y., Gou S., et al. miR-744 increases tumorigenicity of pancreatic cancer by activating Wnt/β-catenin pathway. Oncotarget. 2015;6(35):37557–37569. doi: 10.18632/oncotarget.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chai S., Ng K.Y., Tong M., et al. Octamer 4/microRNA-1246 signaling axis drives Wnt/β-catenin activation in liver cancer stem cells. Hepatology. 2016;64(6):2062–2076. doi: 10.1002/hep.28821. [DOI] [PubMed] [Google Scholar]
- 114.Yang Y., Lei T., Du S., et al. Nuclear GSK3β induces DNA double-strand break repair by phosphorylating 53BP1 in glioblastoma. Int J Oncol. 2018;52(3):709–720. doi: 10.3892/ijo.2018.4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pyko I.V., Nakada M., Sabit H., et al. Glycogen synthase kinase 3β inhibition sensitizes human glioblastoma cells to temozolomide by affecting O6-methylguanine DNA methyltransferase promoter methylation via c-Myc signaling. Carcinogenesis. 2013;34(10):2206–2217. doi: 10.1093/carcin/bgt182. [DOI] [PubMed] [Google Scholar]
- 116.Gao C., Yuan X., Jiang Z., et al. Regulation of AKT phosphorylation by GSK3β and PTEN to control chemoresistance in breast cancer. Breast Cancer Res Treat. 2019;176(2):291–301. doi: 10.1007/s10549-019-05239-3. [DOI] [PubMed] [Google Scholar]
- 117.Fitzgerald T.L., Lertpiriyapong K., Cocco L., et al. Roles of EGFR and KRAS and their downstream signaling pathways in pancreatic cancer and pancreatic cancer stem cells. Adv Biol Regul. 2015;59:65–81. doi: 10.1016/j.jbior.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 118.Ding L., Madamsetty V.S., Kiers S., et al. Glycogen synthase kinase-3 inhibition sensitizes pancreatic cancer cells to chemotherapy by abrogating the TopBP1/ATR-mediated DNA damage response. Clin Cancer Res. 2019;25(21):6452–6462. doi: 10.1158/1078-0432.CCR-19-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fu Y., Hu D., Qiu J., Xie X., Ye F., Lu W.G. Overexpression of glycogen synthase kinase-3 in ovarian carcinoma cells with acquired paclitaxel resistance. Int J Gynecol Cancer. 2011;21(3):439–444. doi: 10.1097/IGC.0b013e31820d7366. [DOI] [PubMed] [Google Scholar]
- 120.Matsumoto T., Yokoi A., Hashimura M., Oguri Y., Akiya M., Saegusa M. TGF-β-mediated LEFTY/Akt/GSK-3β/Snail axis modulates epithelial-mesenchymal transition and cancer stem cell properties in ovarian clear cell carcinomas. Mol Carcinog. 2018;57(8):957–967. doi: 10.1002/mc.22816. [DOI] [PubMed] [Google Scholar]
- 121.Cai G., Wang J., Xin X., Ke Z., Luo J. Phosphorylation of glycogen synthase kinase-3 beta at serine 9 confers cisplatin resistance in ovarian cancer cells. Int J Oncol. 2007;31(3):657–662. [PubMed] [Google Scholar]
- 122.Ito H., Ichiyanagi O., Naito S., et al. GSK-3 directly regulates phospho-4EBP1 in renal cell carcinoma cell-line: an intrinsic subcellular mechanism for resistance to mTORC1 inhibition. BMC Cancer. 2016;16:393. doi: 10.1186/s12885-016-2418-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Owonikoko T.K., Harvey R.D., Carthon B., et al. A phase I study of safety, pharmacokinetics, and pharmacodynamics of concurrent everolimus and buparlisib treatment in advanced solid tumors. Clin Cancer Res. 2020;26(11):2497–2505. doi: 10.1158/1078-0432.CCR-19-2697. [DOI] [PubMed] [Google Scholar]
- 124.Bardia A., Gounder M., Rodon J., et al. Phase ib study of combination therapy with MEK inhibitor binimetinib and phosphatidylinositol 3-kinase inhibitor buparlisib in patients with advanced solid tumors with RAS/RAF alterations. Oncol. 2020;25(1):e160–e169. doi: 10.1634/theoncologist.2019-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wen P.Y., Rodon J.A., Mason W., et al. Phase I, open-label, multicentre study of buparlisib in combination with temozolomide or with concomitant radiation therapy and temozolomide in patients with newly diagnosed glioblastoma. ESMO Open. 2020;5(4):e000673. doi: 10.1136/esmoopen-2020-000673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Goodwin R., Jonker D., Chen E., et al. A phase Ib study of a PI3Kinase inhibitor BKM120 in combination with panitumumab in patients with KRAS wild-type advanced colorectal cancer. Invest N Drugs. 2020;38(4):1077–1084. doi: 10.1007/s10637-019-00814-3. [DOI] [PubMed] [Google Scholar]
- 127.Gelderblom H., Jones R.L., George S., et al. Imatinib in combination with phosphoinositol kinase inhibitor buparlisib in patients with gastrointestinal stromal tumour who failed prior therapy with imatinib and sunitinib: a Phase 1b, multicentre study. Br J Cancer. 2020;122(8):1158–1165. doi: 10.1038/s41416-020-0769-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rosenthal M., Clement P.M., Campone M., et al. Buparlisib plus carboplatin or lomustine in patients with recurrent glioblastoma: a phase Ib/II, open-label, multicentre, randomised study. ESMO Open. 2020;5(4):e000672. doi: 10.1136/esmoopen-2020-000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Assouline S., Amrein L., Aloyz R., et al. IND.216:a phase II study of buparlisib and associated biomarkers, raptor and p70S6K, in patients with relapsed and refractory chronic lymphocytic leukemia. Leuk Lymphoma. 2020;61(7):1653–1659. doi: 10.1080/10428194.2020.1734594. [DOI] [PubMed] [Google Scholar]
- 130.Garrido-Castro A.C., Saura C., Barroso-Sousa R., et al. Phase 2 study of buparlisib (BKM120), a pan-class I PI3K inhibitor, in patients with metastatic triple-negative breast cancer. Breast Cancer Res. 2020;22(1):120. doi: 10.1186/s13058-020-01354-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dummer R., Sandhu S.K., Miller W.H., et al. A phase II, multicenter study of encorafenib/binimetinib followed by a rational triple-combination after progression in patients with advanced BRAF V600-mutated melanoma (LOGIC2) J Clin Oncol. 2020;38(15_suppl):10022. [Google Scholar]
- 132.Ramanathan R.K., von Hoff D.D., Eskens F., et al. Phase ib trial of the PI3K inhibitor copanlisib combined with the allosteric MEK inhibitor refametinib in patients with advanced cancer. Targeted Oncol. 2020;15(2):163–174. doi: 10.1007/s11523-020-00714-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Morschhauser F., Machiels J.P., Salles G., et al. On-target pharmacodynamic activity of the PI3K inhibitor copanlisib in paired biopsies from patients with malignant lymphoma and advanced solid tumors. Mol Cancer Therapeut. 2020;19(2):468–478. doi: 10.1158/1535-7163.MCT-19-0466. [DOI] [PubMed] [Google Scholar]
- 134.Song Y., Liu W., Niu Y., et al. 260P a phase I study of copanlisib, a pan-class I phosphatidylinositol 3-kinase (PI3K) inhibitor, in Chinese patients with relapsed indolent non-Hodgkin lymphoma (iNHL) Ann Oncol. 2020;31:S1344. [Google Scholar]
- 135.Elmenier F.M., Lasheen D.S., Abouzid K.A.M. Phosphatidylinositol 3 kinase (PI3K) inhibitors as new weapon to combat cancer. Eur J Med Chem. 2019;183:111718. doi: 10.1016/j.ejmech.2019.111718. [DOI] [PubMed] [Google Scholar]
- 136.Scott W.J., Hentemann M.F., Rowley R.B., et al. Discovery and SAR of novel 2, 3-dihydroimidazo[1, 2-c]quinazoline PI3K inhibitors: identification of copanlisib (BAY 80-6946) ChemMedChem. 2016;11(14):1517–1530. doi: 10.1002/cmdc.201600148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rodon J., Dienstmann R., Serra V., Tabernero J. Development of PI3K inhibitors: lessons learned from early clinical trials. Nat Rev Clin Oncol. 2013;10(3):143–153. doi: 10.1038/nrclinonc.2013.10. [DOI] [PubMed] [Google Scholar]
- 138.Han F., Lin S., Liu P., et al. Discovery of a novel series of thienopyrimidine as highly potent and selective PI3K inhibitors. ACS Med Chem Lett. 2015;6(4):434–438. doi: 10.1021/ml5005014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang J., Lv X., Ma X., Hu Y. Discovery of a series of N-(5-(quinolin-6-yl)pyridin-3-yl)benzenesulfonamides as PI3K/mTOR dual inhibitors. Eur J Med Chem. 2017;127:509–520. doi: 10.1016/j.ejmech.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 140.Curigliano G., Shah R.R. Safety and tolerability of phosphatidylinositol-3-kinase (PI3K) inhibitors in oncology. Drug Saf. 2019;42(2):247–262. doi: 10.1007/s40264-018-0778-4. [DOI] [PubMed] [Google Scholar]
- 141.Walz A., Ugolkov A., Chandra S., et al. Molecular pathways: revisiting glycogen synthase kinase-3β as a target for the treatment of cancer. Clin Cancer Res. 2017;23(8):1891–1897. doi: 10.1158/1078-0432.CCR-15-2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Beurel E., Blivet-Van Eggelpoël M.J., Kornprobst M., et al. Glycogen synthase kinase-3 inhibitors augment TRAIL-induced apoptotic death in human hepatoma cells. Biochem Pharmacol. 2009;77(1):54–65. doi: 10.1016/j.bcp.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 143.Zhang X., Castanotto D., Nam S., Horne D., Stein C. 6BIO enhances oligonucleotide activity in cells: a potential combinatorial anti-androgen receptor therapy in prostate cancer cells. Mol Ther. 2017;25(1):79–91. doi: 10.1016/j.ymthe.2016.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Domínguez J.M., Fuertes A., Orozco L., del Monte-Millán M., Delgado E., Medina M. Evidence for irreversible inhibition of glycogen synthase kinase-3β by tideglusib. J Biol Chem. 2012;287(2):893–904. doi: 10.1074/jbc.M111.306472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gao Y., Ye D.Y., Zhou W.C., Chu Y. The discovery of novel benzothiazinones as highly selective non-ATP competitive glycogen synthase kinase 3β inhibitors for the treatment of ovarian cancer. Eur J Med Chem. 2017;135:370–381. doi: 10.1016/j.ejmech.2017.04.039. [DOI] [PubMed] [Google Scholar]
- 146.Eldar-Finkelman H., Licht-Murava A., Pietrokovski S., Eisenstein M. Substrate competitive GSK-3 inhibitors - strategy and implications. Biochim Biophys Acta. 2010;1804(3):598–603. doi: 10.1016/j.bbapap.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 147.Neumann T., Benajiba L., Göring S., Stegmaier K., Schmidt B. Evaluation of improved glycogen synthase kinase-3α inhibitors in models of acute myeloid leukemia. J Med Chem. 2015;58(22):8907–8919. doi: 10.1021/acs.jmedchem.5b01200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mai W., Kawakami K., Shakoori A., et al. Deregulated GSK3{beta} sustains gastrointestinal cancer cells survival by modulating human telomerase reverse transcriptase and telomerase. Clin Cancer Res. 2009;15(22):6810–6819. doi: 10.1158/1078-0432.CCR-09-0973. [DOI] [PubMed] [Google Scholar]
- 149.Elmaci İ., Altinoz M.A. A metabolic inhibitory cocktail for grave cancers: metformin, pioglitazone and lithium combination in treatment of pancreatic cancer and glioblastoma multiforme. Biochem Genet. 2016;54(5):573–618. doi: 10.1007/s10528-016-9754-9. [DOI] [PubMed] [Google Scholar]
- 150.Palomo V., Martinez A. Glycogen synthase kinase 3 (GSK-3) inhibitors: a patent update (2014-2015) Expert Opin Ther Pat. 2017;27(6):657–666. doi: 10.1080/13543776.2017.1259412. [DOI] [PubMed] [Google Scholar]
- 151.Nwankwo N., Zhang Z., Wang T., et al. Phase I study of enzastaurin and bevacizumab in patients with advanced cancer: safety, efficacy and pharmacokinetics. Invest N Drugs. 2013;31(3):653–660. doi: 10.1007/s10637-012-9850-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Odia Y., Iwamoto F.M., Moustakas A., et al. A phase II trial of enzastaurin (LY317615) in combination with bevacizumab in adults with recurrent malignant gliomas. J Neuro Oncol. 2016;127(1):127–135. doi: 10.1007/s11060-015-2020-x. [DOI] [PubMed] [Google Scholar]
- 153.Lynce F., Wang H., Petricoin E.F., et al. A phase I study of HER1, HER2 dual kinase inhibitor lapatinib plus the proteasome inhibitor bortezomib in patients with advanced malignancies. Cancer Chemother Pharmacol. 2019;84(5):1145–1151. doi: 10.1007/s00280-019-03947-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Algazi A.P., Esteve-Puig R., Nosrati A., et al. Dual MEK/AKT inhibition with trametinib and GSK2141795 does not yield clinical benefit in metastatic NRAS-mutant and wild-type melanoma. Pigment Cell Melanoma Res. 2018;31(1):110–114. doi: 10.1111/pcmr.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Fan X., Aalto Y., Sanko S.G., Knuutila S., Klatzmann D., Castresana J.S. Genetic profile, PTEN mutation and therapeutic role of PTEN in glioblastomas. Int J Oncol. 2002;21(5):1141–1150. [PubMed] [Google Scholar]
- 156.Zundel W., Schindler C., Haas-Kogan D., et al. Loss of PTEN facilitates HIF-1-mediated gene expression. Genes Dev. 2000;14(4):391–396. [PMC free article] [PubMed] [Google Scholar]
- 157.Stambolic V., Woodgett J.R. Mitogen inactivation of glycogen synthase kinase-3 beta in intact cells via serine 9 phosphorylation. Biochem J. 1994;303(Pt 3):701–704. doi: 10.1042/bj3030701. Pt 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.McCubrey J.A., Steelman L.S., Abrams S.L., et al. Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT pathways in malignant transformation and drug resistance. Adv Enzym Regul. 2007;1773(8):1263–1284. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Abubaker J., Jehan Z., Bavi P., et al. Clinicopathological analysis of papillary thyroid cancer with PIK3CA alterations in a Middle Eastern population. J Clin Endocrinol Metab. 2008;93(2):611–618. doi: 10.1210/jc.2007-1717. [DOI] [PubMed] [Google Scholar]
- 160.Vasko V., Saji M., Hardy E., et al. Akt activation and localisation correlate with tumour invasion and oncogene expression in thyroid cancer. J Med Genet. 2004;41(3):161–170. doi: 10.1136/jmg.2003.015339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hemmings B.A. Akt signaling: linking membrane events to life and death decisions. Science. 1997;275(5300):628–630. doi: 10.1126/science.275.5300.628. [DOI] [PubMed] [Google Scholar]
- 162.Salas T.R., Kim J., Vakar-Lopez F., et al. Glycogen synthase kinase-3β is involved in the phosphorylation and suppression of androgen receptor activity. J Biol Chem. 2004;279(18):19191–19200. doi: 10.1074/jbc.M309560200. [DOI] [PubMed] [Google Scholar]
- 163.Wang Q., Horiatis D., Pinski J. Interleukin-6 inhibits the growth of prostate cancer xenografts in mice by the process of neuroendocrine differentiation. Int J Cancer. 2004;111(4):508–513. doi: 10.1002/ijc.20286. [DOI] [PubMed] [Google Scholar]
- 164.Mulholland D.J., Dedhar S., Wu H., Nelson C.C. PTEN and GSK3β: key regulators of progression to androgen-independent prostate cancer. Oncogene. 2006;25(3):329–337. doi: 10.1038/sj.onc.1209020. [DOI] [PubMed] [Google Scholar]
- 165.Nickerson T., Chang F., Lorimer D., Smeekens S.P., Sawyers C.L., Pollak M. In vivo progression of LAPC-9 and LNCaP prostate cancer models to androgen independence is associated with increased expression of insulin-like growth factor I (IGF-I) and IGF-I receptor (IGF-IR) Cancer Res. 2001;61(16):6276–6280. [PubMed] [Google Scholar]
- 166.Krueckl S.L., Sikes R.A., Edlund N.M., et al. Increased insulin-like growth factor I receptor expression and signaling are components of androgen-independent progression in a lineage-derived prostate cancer progression model. Cancer Res. 2004;64(23):8620–8629. doi: 10.1158/0008-5472.CAN-04-2446. [DOI] [PubMed] [Google Scholar]
- 167.Verras M., Sun Z. Β-catenin is involved in insulin-like growth factor 1-mediated transactivation of the androgen receptor. Mol Endocrinol. 2005;19(2):391–398. doi: 10.1210/me.2004-0208. [DOI] [PubMed] [Google Scholar]
- 168.Zhao H., Dupont J., Yakar S., Karas M., LeRoith D. PTEN inhibits cell proliferation and induces apoptosis by downregulating cell surface IGF-IR expression in prostate cancer cells. Oncogene. 2004;23(3):786–794. doi: 10.1038/sj.onc.1207162. [DOI] [PubMed] [Google Scholar]
- 169.Lee H.C., Lin Y.Z., Lai Y.T., et al. Glycogen synthase kinase 3 beta in somites plays a role during the angiogenesis of zebrafish embryos. FEBS J. 2014;281(19):4367–4383. doi: 10.1111/febs.12942. [DOI] [PubMed] [Google Scholar]
- 170.Zhang M., Zhang J., Chen X., Cho S.J., Chen X. Glycogen synthase kinase 3 promotes p53 mRNA translation via phosphorylation of RNPC1. Genes Dev. 2013;27(20):2246–2258. doi: 10.1101/gad.221739.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Koo J., Wu X., Mao Z., Khuri F.R., Sun S.Y. Rictor undergoes glycogen synthase kinase 3 (GSK3)-dependent, FBXW7-mediated ubiquitination and proteasomal degradation. J Biol Chem. 2015;290(22):14120–14129. doi: 10.1074/jbc.M114.633057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gulen M.F., Bulek K., Xiao H., et al. Inactivation of the enzyme GSK3α by the kinase IKKi promotes AKT-mTOR signaling pathway that mediates interleukin-1-induced Th17 cell maintenance. Immunity. 2012;37(5):800–812. doi: 10.1016/j.immuni.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mak B.C., Kenerson H.L., Aicher L.D., Barnes E.A., Yeung R.S. Aberrant beta-catenin signaling in tuberous sclerosis. Am J Pathol. 2005;167(1):107–116. doi: 10.1016/s0002-9440(10)62958-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lin H.K., Hu Y.C., Yang L., et al. Suppression versus induction of androgen receptor functions by the phosphatidylinositol 3-kinase/Akt pathway in prostate cancer LNCaP cells with different passage numbers. J Biol Chem. 2003;278(51):50902–50907. doi: 10.1074/jbc.M300676200. [DOI] [PubMed] [Google Scholar]
- 175.Lin H.K., Yeh S., Kang H.Y., Chang C. Akt suppresses androgen-induced apoptosis by phosphorylating and inhibiting androgen receptor. Proc Natl Acad Sci USA. 2001;98(13):7200–7205. doi: 10.1073/pnas.121173298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wen Y., Hu M.C., Makino K., et al. HER-2/neu promotes androgen-independent survival and growth of prostate cancer cells through the Akt pathway. Cancer Res. 2000;60(24):6841–6845. [PubMed] [Google Scholar]
- 177.Park Y.L., Kim H.P., Cho Y.W., et al. Activation of WNT/beta-catenin signaling results in resistance to a dual PI3K/mTOR inhibitor in colorectal cancer cells harboring PIK3CA mutations. Int J Cancer. 2019;144(2):389–401. doi: 10.1002/ijc.31662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Laks D.R., Oses-Prieto J.A., Alvarado A.G., et al. A molecular cascade modulates MAP1B and confers resistance to mTOR inhibition in human glioblastoma. Neuro Oncol. 2018;20(6):764–775. doi: 10.1093/neuonc/nox215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chandarlapaty S., Sawai A., Scaltriti M., et al. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell. 2011;19(1):58–71. doi: 10.1016/j.ccr.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhuang G., Yu K., Jiang Z., et al. Phosphoproteomic analysis implicates the mTORC2-FoxO1 axis in VEGF signaling and feedback activation of receptor tyrosine kinases. Sci Signal. 2013;6(271):ra25. doi: 10.1126/scisignal.2003572. [DOI] [PubMed] [Google Scholar]
- 181.Ding Q., Xia W., Liu J.C., et al. Erk associates with and primes GSK-3beta for its inactivation resulting in upregulation of beta-catenin. Mol Cell. 2005;19(2):159–170. doi: 10.1016/j.molcel.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 182.Berezowska S., Schlegel J. Targeting ErbB receptors in high-grade glioma. Curr Pharmaceut Des. 2011;17(23):2468–2487. doi: 10.2174/138161211797249233. [DOI] [PubMed] [Google Scholar]
- 183.Okada K., Nogami A., Ishida S., et al. FLT3-ITD induces expression of Pim kinases through STAT5 to confer resistance to the PI3K/Akt pathway inhibitors on leukemic cells by enhancing the mTORC1/Mcl-1 pathway. Oncotarget. 2017;9(10):8870–8886. doi: 10.18632/oncotarget.22926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Britschgi A., Andraos R., Brinkhaus H., et al. JAK2/STAT5 inhibition circumvents resistance to PI3K/mTOR blockade: a rationale for cotargeting these pathways in metastatic breast cancer. Cancer Cell. 2012;22(6):796–811. doi: 10.1016/j.ccr.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 185.Platanias L.C. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005;5(5):375–386. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- 186.Mui A.L., Wakao H., O'Farrell A.M., Harada N., Miyajima A. Interleukin-3, granulocyte-macrophage colony stimulating factor and interleukin-5 transduce signals through two STAT5 homologs. EMBO J. 1995;14(6):1166–1175. doi: 10.1002/j.1460-2075.1995.tb07100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Elkabets M., Pazarentzos E., Juric D., et al. AXL mediates resistance to PI3Kα inhibition by activating the EGFR/PKC/mTOR axis in head and neck and esophageal squamous cell. Cancer Cell. 2015;27(4):533–546. doi: 10.1016/j.ccell.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Badarni M., Prasad M., Balaban N., et al. Repression of AXL expression by AP-1/JNK blockage overcomes resistance to PI3Ka therapy. JCI Insight. 2019;5(8):e125341. doi: 10.1172/jci.insight.125341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Gjerdrum C., Tiron C., Høiby T., et al. Axl is an essential epithelial-to-mesenchymal transition-induced regulator of breast cancer metastasis and patient survival. Proc Natl Acad Sci USA. 2010;107(3):1124–1129. doi: 10.1073/pnas.0909333107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Vuoriluoto K., Haugen H., Kiviluoto S., et al. Vimentin regulates EMT induction by Slug and oncogenic H-Ras and migration by governing Axl expression in breast cancer. Oncogene. 2011;30(12):1436–1448. doi: 10.1038/onc.2010.509. [DOI] [PubMed] [Google Scholar]
- 191.Paccez J.D., Duncan K., Vava A., et al. Inactivation of GSK3β and activation of NF-κB pathway via Axl represents an important mediator of tumorigenesis in esophageal squamous cell carcinoma. Mol Biol Cell. 2015;26(5):821–831. doi: 10.1091/mbc.E14-04-0868. [DOI] [PMC free article] [PubMed] [Google Scholar]